Abstract
Compared with conventional cancer therapies, the spectrum of toxicities observed with checkpoint inhibitors is unique and can affect any organ system. Arthralgia and myalgia were by far the most commonly reported rheumatic immune-related adverse events in clinical trials, and there is now a growing number of case series and reports describing clinical features of de novo rheumatic immune-related adverse events, which will be the focus of this review. Some patients develop genuine classic rheumatic and musculoskeletal diseases, but a number of rheumatic immune-related adverse events mimic rheumatic and musculoskeletal diseases with atypical features, mainly polymyalgia rheumatica, rheumatoid arthritis and myositis, as well as several systemic conditions, including sicca syndrome, vasculitis, sarcoidosis, systemic sclerosis and lupus.
Keywords: checkpoint inhibitors, immune-related adverse events, rheumatic and musculoskeletal diseases
Rheumatology key messages
Polymyalgia-rheumatica and rheumatoid-arthritis-like syndromes are frequent with checkpoint inhibitors therapy.
Myositis is a potentially life-threatening complication.
Other systemic manifestations, including sicca syndrome, vasculitis, sarcoidosis, systemic sclerosis and lupus, are described.
Introduction
Although the concept of immunotherapy in cancer is far from new, the monoclonal antibodies ‘checkpoint inhibitors’ (CPI) targeting CTLA-4 or PD-1/PD-L1 pathway clearly marked a turning point in the success of this approach [1]. By enhancing antitumour T-cell activity, some unprecedented long-lasting tumour responses were observed in patients with unresectable or metastatic disease [2]. The clinical value of these CPI, as single agent or in combination, is being investigated in various solid tumours and hematological malignancies, and their use is expanding rapidly. As anticipated, the T-cell activation induced by such therapies promotes inflammatory side effects, known as immune-related adverse events (irAEs). Compared with conventional cancer therapies, this spectrum of toxicities is unique and can affect any organ system, with a particular tropism for the gastrointestinal tract, endocrine glands, skin and liver [3]. Arthralgia and myalgia were by far the most commonly reported rheumatic irAEs in clinical trials, with a prevalence ranging from 1% to 43% and from 2% to 21%, respectively [4]. To date, a growing number of case series and reports is describing clinical features of de novo rheumatic irAEs, which will be the focus of this review. Of note, some of them have the appearance of classic rheumatic and musculoskeletal diseases (RMDs) and others mimic RMDs, therefore representing potentially new clinical variants.
Rheumatic manifestations
While the two major clinical entities observed are PMR-like syndromes [5–7] and RA-like syndromes [8–10], the broad spectrum of rheumatic irAEs includes arthralgia [11], monoarthritis and oligoarthritis [12], polyarthritis [13, 14], PsA [15–17], reactive arthritis [8], RS3PE [18–20], tenosynovitis [21], enthesitis [22], non-inflammatory musculoskeletal conditions [6] and osteoarthritis [11]. These are all the clinical patterns found in the literature regarding rheumatic irAEs, in addition to arthritis or inflammatory arthritis, which are the terms often used [8, 23, 24].
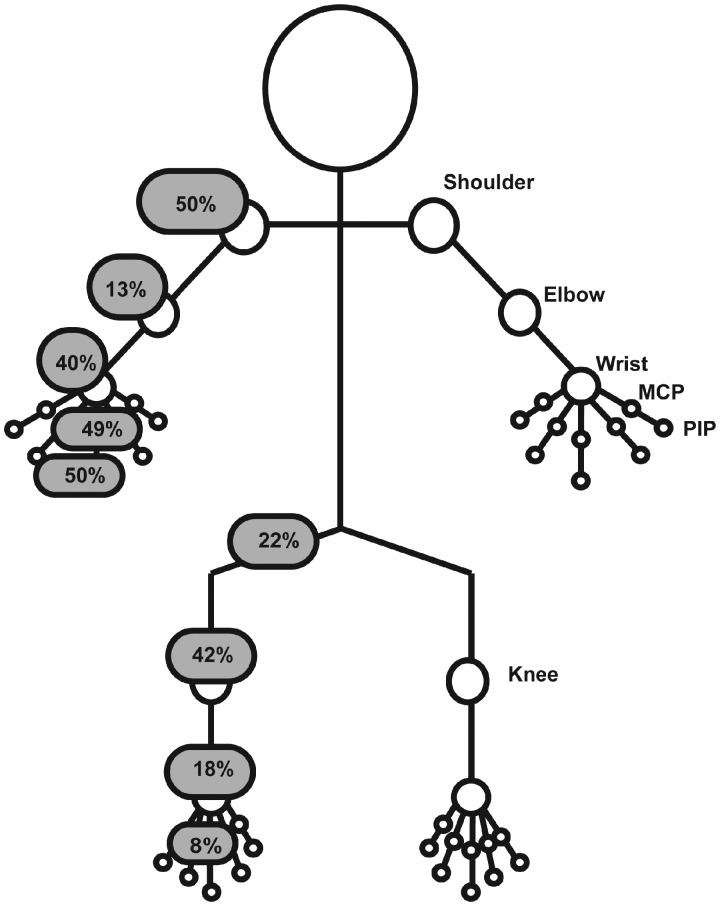
The joints involved most frequently are the shoulders, MCP and PIP joints of the hands (around 50%), followed by the knees and wrists (40%). The hips, elbows, ankles and feet are also affected in some patients, as described in Fig. 1 (unpublished personal data).
Fig. 1.
Frequency and type of joints involvement in patients experiencing rheumatic irAEs with CPI therapy CPI: checkpoint inhibitors; irAEs: immune-related adverse events.
Except for osteoarthritis cases, synovial fluid analysis revealed a clear inflammatory reaction with predominant polymorphonuclear cells [23, 25, 26] but lymphocytic component is also described [27]. When reported, inflammatory markers are elevated for two-thirds of patients with a median CRP value of 58 mg/l (from 6 to 332 mg/l). Importantly, the search for antibodies is negative for a large proportion of patients or with isolated ANA positivity >1/160. This striking preponderance of seronegative diseases is observed by several groups, which is an important message for clinicians [4–8, 10–12, 23, 24]. Indeed, only a few patients are tested positive for RF and/or anti-CCP antibodies [28]. Plain radiographs are inconsistently reported and often considered as normal, but osteoarthritis lesions, joint space narrowing and erosions may be visualized [23, 29]. Ultrasound data available in the literature include mostly the presence of synovitis (31%), tenosynovitis (24%) or bursitis (15%), also frequently reported with PET-CT or MRI, as illustrated in Fig. 2.
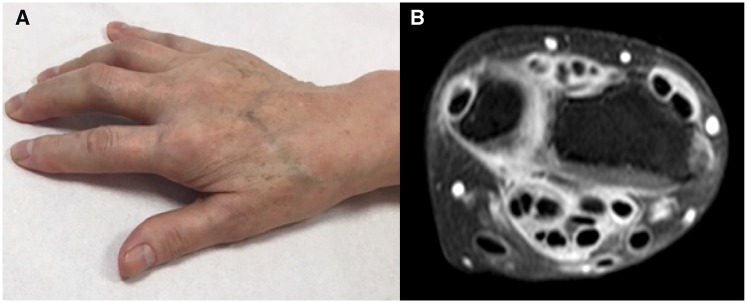
Fig. 2.
CPI-induced tenosynovitis(A) Clinical presentation and (B) MRI findings on T1-SPIR weighted sequence with gadolinium-enhanced tenosynovitis. CPI: checkpoint inhibitors; SPIR: spectral presaturation with inversion recovery.
Overall, on the basis of case series and case reports, around 20% of patients fulfilled classification criteria of RA (55/271) or PMR (11/52). This percentage is higher (55%) for PsA (6/11). Rheumatologists should be aware that patients might present with atypical features, such as PMR clinical phenotype with no increase of inflammatory markers or RA-like symptoms without autoantibodies.
Myositis
Several cases of myositis have been reported as a potentially life-threatening complication in patients treated with CPI, presenting with remarkably homogeneous and unique clinicopathologic features (Fig. 3) [30–32]. Symptoms onset is dominated by acute or subacute myalgia (38%) and proximal muscle weakness (50%) including some patients presenting with dropped head syndrome. Furthermore, up to 25% of patients may present with oculomotor (ptosis/diplopia) and/or bulbar (dysphagia/dysarthria) symptoms. Dyspnoea should alert on a possible concurrent myocarditis, which is frequently reported as critical complication [33]. Therefore, cardiac evaluation is needed in all patients presenting with CPI-induced myositis, including troponin, electrocardiography and echocardiogram if myocarditis is suspected. Associated myasthenia gravis is also frequently encountered (15%) and should be considered with weakness, diplopia or bulbar symptoms [32, 34, 35]. Most described cases have been associated with the presence of anti-acetylcholine receptor antibodies and decremental response was sometimes found on electromyogram. Finally, fatigue is reported in 7% of patients presenting with myositis and typical skin rash of dermatomyositis is described in few patients [36, 37].
Fig. 3.
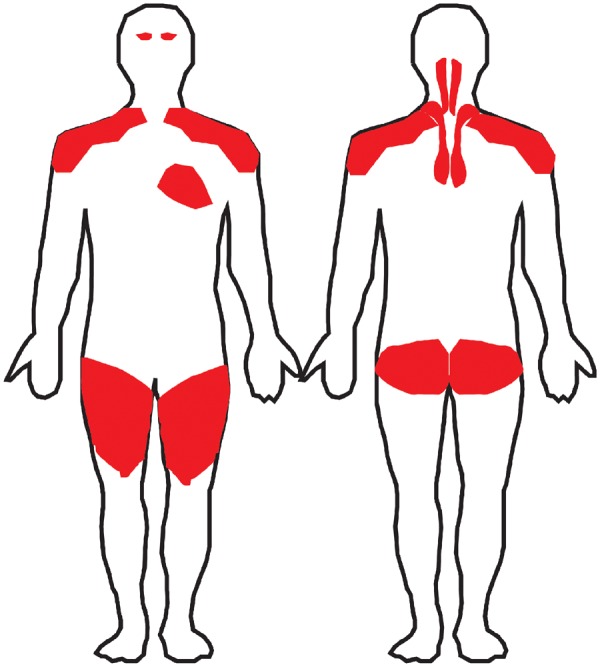
Distribution of muscle involvement in patients experiencing CPI-induced myositis: limb-girdle, cervical, eyes and heartCPI: checkpoint inhibitors.
A strong increase in creatine kinase levels is reported in almost all patients presenting with myositis, with a median of 2650 UI/l (ranging from 335 to 20 270 UI/l). Of note, creatine kinase levels are usually within the normal range in patients presenting with myalgia [32, 38]. Myositis-associated autoantibodies are mostly negative, with the exception of rare case reports with positive ANA, anti-striated antibodies, anti-PM/Scl, anti-SM, anti-TIF1gamma, aPL-7, aPL12, anti-Jo1 or anti-signal recognition part [24, 32, 39, 40]. Of interest, anti-acetylcholine receptor and anti-striated muscle antibodies were detected in serum samples obtained prior to CPI therapy and associated with the development of myositis in thymoma patients [41]. Myopathic pattern is usually found on electrodiagnostic studies and musculature enhancement observed on MRI. Fasciitis is also reported on MRI findings [23, 42, 43]. Skeletal muscle biopsy is often performed and reveals variable degrees of inflammatory and necrotic changes [30].
Importantly, because of the frequent limb-girdle myalgia and weakness that may mimic PMR-like conditions, a high index of myositis suspicion is needed among rheumatologists with a low threshold for creatine kinase dosage in the diagnostic work-up of rheumatic irAEs. Furthermore, it is noteworthy to mention that median exposure time to CPI is usually shorter for myositis than for other rheumatic irAEs, with symptoms’ onset occurring during the first month of treatment in half of the cases.
Sicca syndrome
Clinical characteristics of sicca syndrome described with CPI include mostly dry mouth, in almost 80%, dry eyes being reported in half of cases and associated arthralgia in around 10% [5, 7, 8, 10, 44–46]. Parotid swelling and painful parotid gland are both reported in one patient and neurological symptoms occurred in three patients (two with paresthaesia, one reported as neuro-Sjögren) [47]. Of note, severe salivary hypofunction was documented by scintigraphy in two cases [10].
Only one case report mentioned a hypergammaglobulinaemia and one a normal serum protein electrophoresis; the data is missing for the others. Some, but not all, patients tested positive for ANA, SSA and/or SSB and/or RF.
The largest series including histopathological data of 20 patients experiencing sicca syndrome related to ICI has been published recently [48]. Interestingly, it showed a predominant T-cell infiltrate, composed mainly of CD3+ T cells with a slight predominance of CD4+ over CD8+ T cells, distinct from what is observed in classical Sjögren’s syndrome with mainly CD20+ B cells and variable germinal centre formation.
Vasculitis
Patients experiencing CPI-induced vasculitis presented with various clinical manifestations, including arthralgia, arthritis, myalgia, purpura, digital necrosis, fever, fatigue and abdominal pain. The corresponding diagnosis are cutaneous leucocytoclastic vasculitis [49], acral vasculitis [10, 50, 51], granulomatosis with polyangiitis [52], eosinophilic granulomatosis with polyangiitis [53], cryoglobulinemic vasculitis [44], giant cell arthritis [54–56] and a large proportion of cases are reported as vasculitis [7, 57, 58].
Acute phase reactants accompany symptoms’ onset in one-third of patients. The search for ANA, ANCA, cryoglobulin and RF is often requested but rarely positive. Whenever possible, biopsy (i.e. skin, temporal artery) is performed and was contributive in all but one case.
Other systemic manifestations
In the last two years, the broad spectrum of irAEs is rapidly expanding, with descriptions of other various systemic manifestations.
Sarcoidosis or sarcoid-like reactions are commonly described [59, 60]. Up to 25% of patients might be asymptomatic, the diagnosis being suspected on imaging (usually PET-CT with new hilar lymphadenopathy or pulmonary nodules), then documented on biopsy with noncaseating epithelioid granuloma or granulomatous reaction in all but one case. In such situations, biopsy is worthwhile in order to distinguishing this phenomenon from progressive stage-4 cancer disease and to not discontinue CPI treatment. Other symptoms include frequent cutaneous manifestations (nodules, rash), cough/dyspnea and arthralgia/arthritis. Uveitis, parotiditis, hypercalcemia and neurological symptoms are rarely reported [61, 62]. Of note, serum angiotensin I-converting enzyme level might be elevated or normal.
Some patients experienced systemic sclerosis [7] or scleroderma-like reaction [63–65], all presenting with skin thickening but only one with associated recent Raynaud phenomenon. None tested positive for specific autoantibodies.
Subacute cutaneous lupus erythematosus with typical erythematous eruption on trunk and/or limbs are described [66–68], as well as systemic lupus erythematosus with high titre of ANA and positivity of either anti-dsDNA or anti-SSA/SSB [66, 69]. One patient experienced CPI-induced lupus nephritis [70] and one developed Jaccoud arthropathy [71].
Differential diagnoses
In the context of advanced cancer, metastases should always be considered as a differential diagnosis of rheumatic irAEs, notably in the case of localized articular symptoms or the lack of improvement with adequate treatment [72]. Recently, non-malignant resorptive lesions (shoulder, hand and clavicle) and rapid bone loss leading to multiple fractures with vertebral compression have also been described under CPI treatment, making the differential diagnosis with metastases even more challenging and raising the question of the potential influence of immune activation on bone metabolism [73].
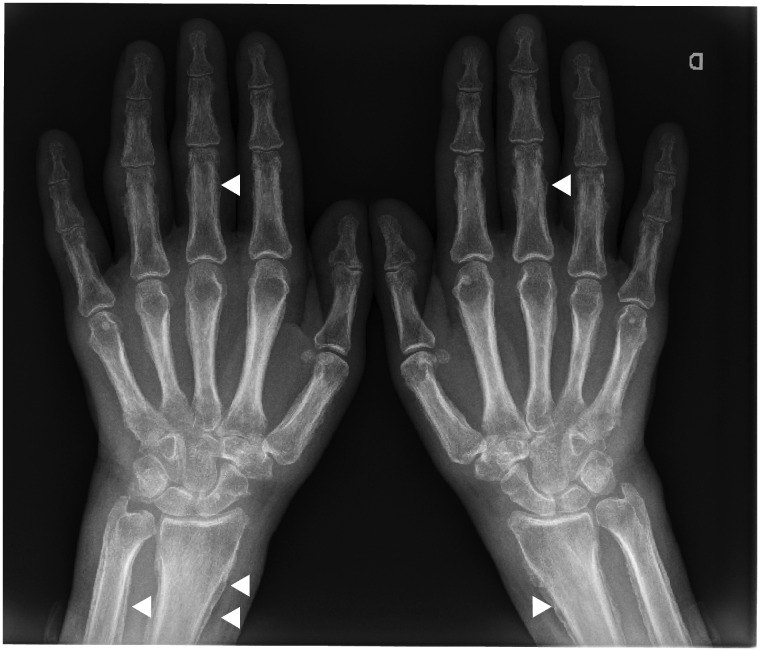
In lung cancer patients, paraneoplastic syndromes such as hypertrophic osteoarthropathy occurring after CPI-onset while not present or asymptomatic at cancer diagnosis have also recently been noticed. Patients usually presented with inflammatory pain in extremities, swollen joints (with non-inflammatory synovial fluid analysis) and nail clubbing. Plain radiographs show periostitis, as illustrated with Fig. 4.
Fig. 4.
Representative image of periostitis in a patient developing hypertrophic osteoarthropathy after CPI onsetCPI: checkpoint inhibitors.
Finally, in case of fatigue and diffuse pain or weakness, central adrenal insufficiency should be suspected as it is a possible immune-related complication of CPI [74]. The diagnosis can be challenging, confirmed by the low dosage of cortisol and low Adreno CorticoTrophic Hormone. The presence of associated hypotension and hyponatremia may help to suspect an underlying endocrinopathy.
Conclusion
Rheumatic, musculoskeletal and systemic irAEs represent a new clinical entity within the field, as numbers of patients presenting with rheumatic irAE do not fulfil the traditional classification criteria of RMDs. On the other hand, it seems that some patients develop genuine classic RMDs, with possible influence of immunogenetic framework [75]. Owing to the rapid development and dissemination of CPI therapy, rheumatologists should be aware of the wide spectrum of rheumatic irAEs, with their respective clinical characteristics.
Funding: This paper was published as part of a supplement funded by an educational grant from BMS.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Wei SC, Duffy CR, Allison JP.. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–86. [DOI] [PubMed] [Google Scholar]
- 2. Wolchok JD, Chiarion-Sileni V, Gonzalez R. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Postow MA, Sidlow R, Hellmann MD.. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 4. Cappelli LC, Gutierrez AK, Bingham CO, Shah AA.. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res 2017;69:1751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calabrese C, Kirchner E, Kontzias K, Velcheti V, Calabrese LH.. Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open 2017;3:e000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kostine M, Rouxel L, Barnetche T. et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 2018;77:393–8. [DOI] [PubMed] [Google Scholar]
- 7. Richter MD, Crowson C, Kottschade LA. et al. Rheumatic syndromes associated with immune checkpoint inhibitors: a single-center cohort of sixty-one patients. Arthritis Rheumatol 2019;71:468–75. [DOI] [PubMed] [Google Scholar]
- 8. Cappelli LC, Gutierrez AK, Baer AN. et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le Burel S, Champiat S, Mateus C. et al. Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: a single-centre pharmacovigilance database analysis. Eur J Cancer 2017;82:34–44. [DOI] [PubMed] [Google Scholar]
- 10. Narváez J, Juarez-López P, LLuch J. et al. Rheumatic immune-related adverse events in patients on anti-PD-1 inhibitors: fasciitis with myositis syndrome as a new complication of immunotherapy. Autoimmun Rev 2018;17:1040–5. [DOI] [PubMed] [Google Scholar]
- 11. Buder-Bakhaya K, Benesova K, Schulz C. et al. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol Immunother 2018;67:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leipe J, Christ LA, Arnoldi AP. et al. Characteristics and treatment of new-onset arthritis after checkpoint inhibitor therapy. RMD Open 2018;4:e000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lidar M, Giat E, Garelick D. et al. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev 2018;17:284–9. [DOI] [PubMed] [Google Scholar]
- 14. Kim ST, Tayar J, Trinh VA. et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 2017;76:2061–4. [DOI] [PubMed] [Google Scholar]
- 15. Law-Ping-Man S, Martin A, Briens E, Tisseau L, Safa G.. Psoriasis and psoriatic arthritis induced by nivolumab in a patient with advanced lung cancer. Rheumatology 2016;55:2087–9. [DOI] [PubMed] [Google Scholar]
- 16. Elosua-González M, Pampín-Franco A, Mazzucchelli-Esteban R. et al. A case of de novo palmoplantar psoriasis with psoriatic arthritis and autoimmune hypothyroidism after receiving nivolumab therapy. Dermatol Online J 2017;23:8. [PubMed] [Google Scholar]
- 17. Sapalidis K, Kosmidis C, Michalopoulos N. et al. Psoriatic arthritis due to nivolumab administration a case report and review of the literature. Respir Med Case Rep 2018;23:182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amini-Adle M, Piperno M, Tordo J. et al. Remitting seronegative symmetrical synovitis with pitting edema (RS3PE) associated with partial melanoma response under anti-CTLA-4 and anti-PD-1 combination. Arthritis Rheumatol 2018;70:1358. [DOI] [PubMed] [Google Scholar]
- 19. Gauci M-L, Baroudjian B, Laly P. et al. Remitting seronegative symmetrical synovitis with pitting edema (RS3PE) syndrome induced by nivolumab. Semin Arthritis Rheum 2017;47:281–7. [DOI] [PubMed] [Google Scholar]
- 20. Ngo L, Miller E, Valen P, Gertner E.. Nivolumab induced remitting seronegative symmetrical synovitis with pitting edema in a patient with melanoma: a case report. J Med Case Rep 2018;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan MMK, Kefford RF, Carlino M, Clements A, Manolios N.. Arthritis and tenosynovitis associated with the anti-PD1 antibody pembrolizumab in metastatic melanoma. J Immunother 2015;38:37–9. [DOI] [PubMed] [Google Scholar]
- 22. Inamo J, Kaneko Y, Takeuchi T.. Inflammatory tenosynovitis and enthesitis induced by immune checkpoint inhibitor treatment. Clin Rheumatol 2018;37:1107–10. [DOI] [PubMed] [Google Scholar]
- 23. Mooradian MJ, Nasrallah M, Gainor JF. et al. Musculoskeletal rheumatic complications of immune checkpoint inhibitor therapy: a single center experience. Semin Arthritis Rheum 2019;48:1127–32. [DOI] [PubMed] [Google Scholar]
- 24. Mitchell EL, Lau PKH, Khoo C. et al. Rheumatic immune-related adverse events secondary to anti-programmed death-1 antibodies and preliminary analysis on the impact of corticosteroids on anti-tumour response: a case series. Eur J Cancer 2018;105:88–102. [DOI] [PubMed] [Google Scholar]
- 25. Spathas N, Economopoulou P, Cheila M. et al. Inflammatory arthritis induced by pembrolizumab in a patient with head and neck squamous cell carcinoma. Front Oncol 2018;8:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuswanto WF, MacFarlane LA, Gedmintas L. et al. Rheumatologic symptoms in oncologic patients on PD-1 inhibitors. Semin Arthritis Rheum 2018;47:907–10. [DOI] [PubMed] [Google Scholar]
- 27. Dasanu CA, Jen T, Skulski R.. Late-onset pericardial tamponade, bilateral pleural effusions and recurrent immune monoarthritis induced by ipilimumab use for metastatic melanoma. J Oncol Pharm Pract 2017;23:231–4. [DOI] [PubMed] [Google Scholar]
- 28. Belkhir R, Burel SL, Dunogeant L. et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis 2017;76:1747–50. [DOI] [PubMed] [Google Scholar]
- 29. Haikal A, Borba E, Khaja T, Doolittle G, Schmidt P.. Nivolumab-induced new-onset seronegative rheumatoid arthritis in a patient with advanced metastatic melanoma: a case report and literature review. Avicenna J Med 2018;8:34–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Touat M, Maisonobe T, Knauss S. et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018;91:e985–94. [DOI] [PubMed] [Google Scholar]
- 31. Shah M, Tayar JH, Abdel-Wahab N, Suarez-Almazor ME.. Myositis as an adverse event of immune checkpoint blockade for cancer therapy. Semin Arthritis Rheum 2019;48:736–40. [DOI] [PubMed] [Google Scholar]
- 32. Moreira A, Loquai C, Pföhler C. et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer 2019;106:12–23. [DOI] [PubMed] [Google Scholar]
- 33. Moslehi JJ, Salem J-E, Sosman JA, Lebrun-Vignes B, Johnson DB.. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gonzalez NL, Puwanant A, Lu A, Marks SM, Živković SA.. Myasthenia triggered by immune checkpoint inhibitors: new case and literature review. Neuromuscul Disord 2017;27:266–8. [DOI] [PubMed] [Google Scholar]
- 35. Suzuki S, Ishikawa N, Konoeda F. et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017;89:1127–34. [DOI] [PubMed] [Google Scholar]
- 36. Kudo F, Watanabe Y, Iwai Y. et al. Advanced lung adenocarcinoma with nivolumab-associated dermatomyositis. Intern Med 2018;57:2217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sheik Ali S, Goddard AL, Luke JJ. et al. Drug-associated dermatomyositis following ipilimumab therapy: a novel immune-mediated adverse event associated with cytotoxic T-lymphocyte antigen 4 blockade. JAMA Dermatol 2015;151:195–9. [DOI] [PubMed] [Google Scholar]
- 38. Zimmer L, Goldinger SM, Hofmann L. et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:210–25. [DOI] [PubMed] [Google Scholar]
- 39. Liewluck T, Kao JC, Mauermann ML.. PD-1 Inhibitor-associated myopathies: emerging Immune-mediated Myopathies. J Immunother 2018;41:208–11. [DOI] [PubMed] [Google Scholar]
- 40. Bilen MA, Subudhi SK, Gao J. et al. Acute rhabdomyolysis with severe polymyositis following ipilimumab-nivolumab treatment in a cancer patient with elevated anti-striated muscle antibody. J Immunother Cancer 2016;4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mammen AL, Rajan A, Pak K. et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis 2019;78:150–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parker MJ, Roberts ME, Lorigan PC, du Plessis DG, Chinoy H.. Autoimmune fasciitis triggered by the anti-programmed cell death-1 monoclonal antibody nivolumab. BMJ Case Rep 2018;2018;doi:10.1136/bcr-2017-223249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khoja L, Maurice C, Chappell M. et al. Eosinophilic fasciitis and acute encephalopathy toxicity from pembrolizumab treatment of a patient with metastatic melanoma. Cancer Immunol Res 2016;4:175–8. [DOI] [PubMed] [Google Scholar]
- 44. Le Burel S, Champiat S, Routier E. et al. Onset of connective tissue disease following anti-PD1/PD-L1 cancer immunotherapy. Ann Rheum Dis 2018;77: 468–70. [DOI] [PubMed] [Google Scholar]
- 45. Takahashi S, Chieko X, Sakai T, Hirose S, Nakamura M.. Nivolumab-induced sialadenitis. Respirol Case Rep 2018;6:e00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Teyssonneau D, Cousin S, Italiano A.. Gougerot-Sjogren-like syndrome under PD-1 inhibitor treatment. Ann Oncol 2017;28:3108. [DOI] [PubMed] [Google Scholar]
- 47. Ghosn J, Vicino A, Michielin O. et al. A severe case of neuro-Sjögren’s syndrome induced by pembrolizumab. J Immunother Cancer 2018;6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Warner BM, Baer AN, Lipson EJ. et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist 2019;doi:10.1634/theoncologist. 2018-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tomelleri A, Campochiaro C, De Luca G, Cavalli G, Dagna L.. Anti-PD1 therapy-associated cutaneous leucocytoclastic vasculitis: a case series. Eur J Intern Med 2018;57:e11–2. [DOI] [PubMed] [Google Scholar]
- 50. Comont T, Sibaud V, Mourey L, Cougoul P, Beyne-Rauzy O.. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer 2018;6:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Padda A, Schiopu E, Sovich J. et al. Ipilimumab induced digital vasculitis. J Immunother Cancer 2018;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van den Brom RRH, Abdulahad WH, Rutgers A. et al. Rapid granulomatosis with polyangiitis induced by immune checkpoint inhibition. Rheumatology 2016;55:1143–5. [DOI] [PubMed] [Google Scholar]
- 53. Roger A, Groh M, Lorillon G. et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) induced by immune checkpoint inhibitors. Ann Rheum Dis 2018;doi:10.1136/annrhuemdis-2018/213857. [DOI] [PubMed] [Google Scholar]
- 54. Goldstein BL, Gedmintas L, Todd DJ.. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheumatol 2014;66:768–9. [DOI] [PubMed] [Google Scholar]
- 55. Hid Cadena R, Abdulahad WH, Hospers GAP. et al. Checks and balances in autoimmune vasculitis. Front Immunol 2018;9:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Micaily I, Chernoff M.. An unknown reaction to pembrolizumab: giant cell arteritis. Ann Oncol 2017;28:2621–2. [DOI] [PubMed] [Google Scholar]
- 57. Kang A, Yuen M, Lee DJ.. Nivolumab-induced systemic vasculitis. JAAD Case Rep 2018;4:606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tsukamoto J, Monteiro M, Vale S. et al. Thromboembolic events related to treatment with checkpoint inhibitors: report of two cases. Case Rep Oncol 2018;11:648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Firwana B, Ravilla R, Raval M, Hutchins L, Mahmoud F.. Sarcoidosis-like syndrome and lymphadenopathy due to checkpoint inhibitors. J Oncol Pharm Pract 2017;23:620–4. [DOI] [PubMed] [Google Scholar]
- 60. Lomax AJ, McGuire HM, McNeil C. et al. Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: case series and immunophenotypic analysis. Int J Rheum Dis 2017;20:1277–85. [DOI] [PubMed] [Google Scholar]
- 61. Dunn-Pirio AM, Shah S, Eckstein C.. Neurosarcoidosis following immune checkpoint inhibition. Case Rep Oncol 2018;11:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tan I, Malinzak M, Salama AKS.. Delayed onset of neurosarcoidosis after concurrent ipilimumab/nivolumab therapy. J Immunother Cancer 2018;6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tjarks BJ, Kerkvliet AM, Jassim AD, Bleeker JS.. Scleroderma-like skin changes induced by checkpoint inhibitor therapy. J Cutan Pathol 2018;45:615–8. [DOI] [PubMed] [Google Scholar]
- 64. Shenoy N, Esplin B, Barbosa N. et al. Pembrolizumab induced severe sclerodermoid reaction. Ann Oncol 2017;28:432–3. [DOI] [PubMed] [Google Scholar]
- 65. Barbosa NS, Wetter DA, Wieland CN. et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc 2017;92:1158–63. [DOI] [PubMed] [Google Scholar]
- 66. Michot J-M, Fusellier M, Champiat S. et al. Drug-induced lupus erythematosus following immunotherapy with anti-programmed death-(ligand) 1. Ann Rheum Dis 2019;78:e67. [DOI] [PubMed] [Google Scholar]
- 67. Zitouni NB, Arnault J-P, Dadban A. et al. Subacute cutaneous lupus erythematosus induced by nivolumab: two case reports and a literature review. Melanoma Res 2019;29:212–5. [DOI] [PubMed] [Google Scholar]
- 68. Liu RC, Sebaratnam DF, Jackett L, Kao S, Lowe PM.. Subacute cutaneous lupus erythematosus induced by nivolumab. Australas J Dermatol 2018;59:e152–4. [DOI] [PubMed] [Google Scholar]
- 69. de Chabot G, Justeau G, Pinquié F. et al. [Unexpected adverse events of immunotherapies in non-small cell lung cancer: about 2 cases]. Rev Pneumol Clin 2017;73:326–30. [DOI] [PubMed] [Google Scholar]
- 70. Fadel F, El Karoui K, Knebelmann B.. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 2009;361:211–2. [DOI] [PubMed] [Google Scholar]
- 71. de Velasco G, Bermas B, Choueiri TK.. Autoimmune arthropathy and uveitis as complications of programmed death 1 inhibitor treatment. Arthritis Rheumatol 2016;68:556–7. [DOI] [PubMed] [Google Scholar]
- 72. Albayda J, Bingham CO, Shah AA, Kelly RJ, Cappelli L.. Metastatic joint involvement or inflammatory arthritis? A conundrum with immune checkpoint inhibitor-related adverse events. Rheumatology 2018;57:760–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moseley KF, Naidoo J, Bingham CO. et al. Immune-related adverse events with immune checkpoint inhibitors affecting the skeleton: a seminal case series. J Immunother Cancer 2018;6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nagasaka M, Abdallah N, Samantray J, Sukari A.. Is this really just ‘fatigue’? A case series of immune-related central adrenal insufficiency secondary to immune checkpoint inhibitors. Clin Case Rep 2018;6:1278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van der Vlist M, Kuball J, Radstake TRD, Meyaard L.. Immune checkpoints and rheumatic diseases: what can cancer immunotherapy teach us? Nat Rev Rheumatol 2016;12:593–604. [DOI] [PubMed] [Google Scholar]