Abstract
Immunotherapy has an increasing role in the management of cancer, both in metastatic disease and as an adjuvant therapy. However, sensitization of the immune system with checkpoint inhibitors comes with a unique side effect profile. Full appreciation of this can take some time to emerge as some adverse events are rare, or can be subtle and potentially overlooked. Clinician awareness of these side effects can be particularly important in patients with pre-existing autoimmune conditions. Here we describe common symptoms and diagnostic strategies for organ-specific side effects of anti-CTLA-4 and anti-PD-1/PD-L1 immunotherapy agents.
Keywords: checkpoint inhibitor, adverse event
Rheumatology key messages
The onset of checkpoint inhibitor side effects may follow a predictable temporal pattern.
Checkpoint inhibitor side effects can be diverse and serious; a multi-disciplinary approach is advised.
Introduction
Checkpoint inhibitors (CPIs) have now become a mainstay of treatment for a range of cancers, including melanoma, bladder cancer, non-small cell lung cancer and Hodgkin’s lymphoma [1].
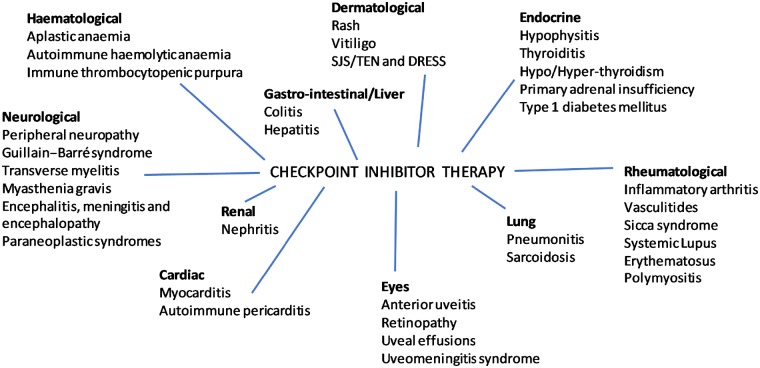
Currently there are six approved therapies. Ipilimumab is a cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) fully human IgG1 antibody, licensed for use in advanced melanoma [2]. Pembrolizumab and nivolumab are both IgG4 monoclonal antibodies that block the programmed cell death (PD-1) protein on T cells. Both are licensed for use in melanoma, non-small cell lung cancer and mismatch repair deficient (dMMR) and microsatellite instability high colorectal cancers [2]. Three monoclonal antibodies targeting the protein programmed death-ligand 1 (PD-L1) have been approved. Atezolizumab and durvalumab are both licensed for use in non-small cell lung cancer and urothelial carcinoma. Avelumab is also used to treat urothelial carcinoma [1, 2]. Durable disease responses can result from treatment with these drugs, but potentially come at a cost of wide-ranging side effects. Immune-related adverse events (irAEs) are caused by activation of the immune system with the development of auto-antibodies and can affect almost any organ system, as summarized in Fig. 1.
Fig. 1.
Immune-related adverse events
DRESS: drug rash with eosinophilia and systemic symptoms; SJS: Stevens–Johnson syndrome; TEN: toxic epidermal necrolysis.
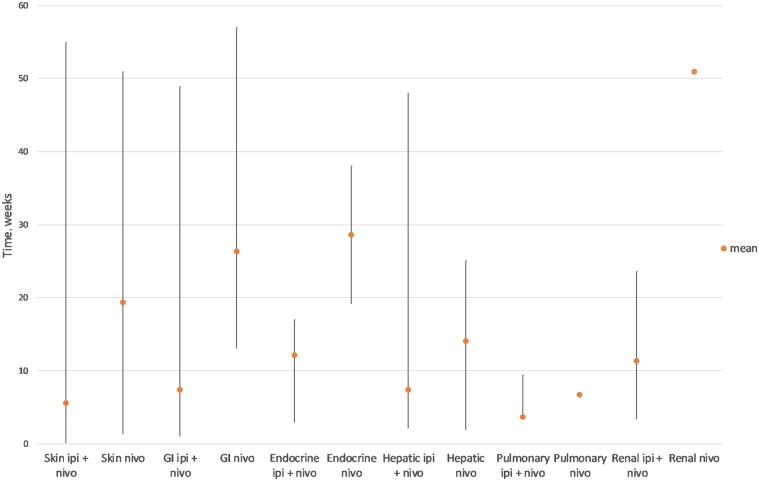
Immune-related adverse events may affect a single organ system, but some patients can develop multiple side effects, not necessarily simultaneously. In a small study of 78 patients receiving either anti-CTLA-4 therapy (32%) or anti-PD-1 therapy (68%), over half developed irAEs, with 12 patients (15%) developing more than one [3]. These are often mild, but a small number can be life-threatening; in a randomized, multicentre phase 3 trial (Checkmate 067) involving 296 patients receiving either combination ipilimumab/nivolumab or monotherapy with either agent, grade 3 or 4 drug-related adverse events occurred in 32 (11%) patients, with three (1%) treatment-related deaths [4]. Urgent management may therefore be required which is beyond the scope of this review but has been extensively reviewed elsewhere [6]. Meta-analysis of CPI-related side effects has shown that these profiles follow a specific temporal pattern (Fig. 2), which is consistent across solid tumour types [5].
Fig. 2.
Time to onset of grade 3–4 immunotherapy-related adverse events (amended from [6])
Adverse effects have been reported more frequently with CTLA-4 inhibitors compared with PD-1/PD-L1 inhibitors; a meta-analysis suggested all-grade irAEs of 75% with ipilimumab (24–43% grade 3 or above) [7], compared with up to 37% of patients receiving anti-PD-1/PD-L1 agents experiencing irAEs of any grade (10–20% grade 3 or above) [4, 5, 8]. Patient and drug factors, including dose and treatment setting (adjuvant vs metastatic) potentially influence the incidence of irAEs to CPIs [9]. Accurately estimating the incidence of specific side effects can be difficult, as there may be significant under-reporting, particularly with the less specific effects such as fatigue. Distinguishing new irAEs from a flare of pre-existing co-morbidities may add to the diagnostic challenge, as patients with pre-existing auto-immune or inflammatory diseases have often been excluded from trials involving CPI therapy [10]. The side effects reported with checkpoint therapy are discussed below, in order of prevalence, including common symptoms and suggested diagnostic approaches.
Gastrointestinal
Gastrointestinal irAEs are a significant side effect of CPIs, and occur in 44% of patients receiving combination anti-CTLA-4/anti-PD-1, 23–33% of patients receiving anti-CTLA-4 therapy and <20% of patients receiving single-agent anti-PD-1 therapy [1]. Symptoms, including bloody diarrhoea, abdominal pain and sometimes pyrexia, occur on average after three infusions, although they can occur earlier in treatment or even months after stopping checkpoint therapy [2]. In a phase 3 melanoma trial of 511 patients receiving ipilimumab, five (1%) developed intestinal perforation and 26 (5%) were hospitalized for severe enterocolitis [8]. Although any part of the colon can be involved, the descending colon has been most commonly reported to be affected, possibly because the proximal colon is viewed less frequently by endoscopy [11, 12]. Colonoscopy is useful to visualize the mucosa, which may show mild erythema or severe inflammation with friability and ulceration [13, 14]. The presence of ulceration is associated with steroid-refractory disease so mucosal appearances can be helpful in guiding treatment [15].
Similar to the endoscopic appearance of colitis from inflammatory bowel disease, these appearances may be diffuse or occur segmentally [16]. Although a full mechanism has not yet been elucidated, at least two typical histological appearances have been reported: neutrophilic infiltration into micro-abscesses and epithelial cell atrophy causing crypt atrophy, or lymphocytic infiltration into the epithelium as a response to epithelial injury [17, 18]. The small bowel can be affected rarely, and cases of enteritis have been confirmed with CT after combination therapy has been given. The upper GI tract can also be affected, although less commonly so. Most obvious in terms of appearance, mucositis can present with inflamed lips or mouth, which if severe, can affect oral intake and may necessitate nutritional supplementation. Cases of oesophagitis and gastritis can present non-specifically with nausea and anorexia, with confirmation by endoscopy [6].
General treatment strategies include treatment interruption, fluid replacement and usually glucocorticoids. In the phase 3 Checkmate 067 study, this was sufficient for resolution of three cases of grade 3–4 diarrhoea [4]. A confirmed diagnosis with a detailed history and endoscopic analysis is very important before commencing treatment, as the management of upper GI pathology such as gastritis from non-immunotherapy related causes would not normally include steroids. Rarely, escalation to other immunosuppressive agents or even surgical intervention is required.
Hepatic
Immune-related hepatitis is the most common hepatic adverse event, affecting ∼5% of patients receiving anti-PD-1 therapy, 5–15% patients receiving ipilimumab monotherapy (dose dependent) and a third of patients receiving combination therapy [2]. In most cases, asymptomatic elevation of liver enzymes is noted, which occurs with median onset 6–14 weeks after receiving therapy [19].
Occasionally patients may present with symptoms that can include fatigue, fever and jaundice and, in very rare cases, death may occur. The radiological appearance is similar between checkpoint agents, with ultrasound and CT findings such as hepatomegaly, oedema and lymphadenopathy [20, 21].
Liver biopsy may not change patient management, unless alternative diagnoses are suspected such as drug- and infection-related liver injury. Both anti-CTLA-4 and anti-PD-1/PD-L1 agents can cause histopathological appearances in keeping with either hepatocyte injury with endothelial inflammation, central hepatic vein damage and discrete areas of necrosis, or bile duct injury with portal vein inflammation. Additionally, in cases caused by anti-CTLA-4 treatment, confluent necrosis and histiocytic aggregates has been reported [16].
General management strategies include withholding immunotherapy until improvement is seen in hepatic enzyme blood parameters, with steroid as necessary. Immunotherapy should usually be stopped permanently in cases of grade 3 hepatic toxicity (aspartate transaminase or alanine transaminase >5 times upper limit of normal or total bilirubin >3 times upper limit of normal). These patients will receive immunosuppressive therapies such as intravenous methylprednisolone or mycophenolate mofetil, in discussion with hepatology specialists.
Endocrine
Endocrine-related irAEs are more common with anti-CTLA-4 antibody treatment than anti-
PD-1/PD-L1 therapy. Side effects include hypophysitis (13% vs 1%) and hypo-/hyperthyroiditis (∼6% vs 4% for anti-CTLA-4 antibody treatment vs anti-PD-1/PD-L1 therapy, respectively).
Other endocrinopathies such as primary adrenal insufficiency, type 1 diabetes mellitus (T1DM), hypercalcaemia and hypoparathyroidism are rare but can present acutely. They typically occur after three cycles of combination treatment (Fig. 2), are often a later side effect of CPI monotherapy and are usually irreversible, requiring life-long hormone replacement [1].
Pre-treatment blood tests measuring thyroid function by thyroid-stimulating hormone and free thyroxine (fT4), and early morning adrenal function by adrenocorticotropic hormone (ACTH) and cortisol can act as a baseline to help identify changes. Many centres choose to monitor these routinely ahead of treatment to detect early changes and reduce the risk of an acute presentation. Due to the delayed nature of onset, there is some evidence to recommend continued monitoring even after treatment completion [2].
Hypophysitis
Inflammation of the pituitary gland can cause hypopituitarism, leading to central hypothyroidism, central adrenal insufficiency and hypogonadotropic hypogonadism. These endocrine abnormalities can be difficult to detect, especially in early stages as patients can experience non-specific symptoms such as fatigue, nausea or loss of appetite. If patients have already been commenced on steroids for other irAEs, interpretation of blood results potentially becomes more complex.
Severe cases can cause headache or postural hypotension, although visual disturbances are rare. Blood tests may demonstrate hyponatraemia with low thyroid-stimulating hormone, fT4 and ACTH; usually luteinizing hormone, follicle-stimulating hormone and prolactin are unaffected [22]. By the time clinical and biochemical changes are noted, changes on MRI are usually seen, such as enlargement and heterogeneous enhancement of the pituitary gland. These appearances often resolve after treatment of hypophysitis [23].
Thyroid dysfunction
Like patients with pituitary dysfunction, patients with thyroid dysfunction are often asymptomatic and the diagnosis is usually only initially by biochemistry. The pathophysiology is reported to be dissimilar to autoimmune diseases of the thyroid, with CPI-induced cases appearing to be part of a spectrum induced by T cell cytotoxicity, starting with acute thyroiditis (with raised fT4 and low thyroid-stimulating hormone) typically after 1 month of therapy and progressing to hypothyroidism after a further month [22]. Thyrotoxicosis, secondary to thyroiditis or Grave’s disease, is a rare complication of CPI therapy. Testing for antibodies such as anti-thyroid peroxidase and thyroid-stimulating immunoglobulin can be useful. Rarely, patients may have symptoms of acute hyperthyroidism (tachycardia, palpitations, diarrhoea or tremor) or hypothyroidism (constipation, weight gain, lethargy) [23].
Adrenal
Primary adrenal insufficiency is a rare irAE. It can present with non-specific symptoms such as fatigue, nausea or weight loss, although occasionally skin hyperpigmentation from raised
ACTH is noted. Diagnosis is therefore generally made from biochemistry results, with low cortisol and normal or high levels of ACTH. Anti-21-hydroxylase and adrenal cortex antibody levels should be checked to detect auto-antibodies induced by CPI therapy [23].
Diabetes mellitus type 1
This is a rare but potentially life-threatening immunotherapy-induced endocrinopathy, such that data for its incidence are scarce. Patients typically present acutely with weight loss, nausea, fatigue, polyuria and polydipsia. In addition to new cases, CPIs can cause worsening of pre-existing T1DM. Where suspected, management is like that for non-irAE-induced T1DM, including the management of acute ketoacidotic crises. The pathophysiology has not yet been fully elucidated but there is likely involvement of CD8+ T cell response to T1DM antigen and T1DM-specific autoantibodies (GAD65) [24].
Skin
Cutaneous irAEs are common with both anti-CTLA-4 (experienced by up to 50% of patients) and anti-PD-1/PD-L1 agents (affecting 30–40% of patients) [8]. Symptoms can occur early on in treatment, especially with anti-CTLA-4 therapy, and range from a mild rash and intermittent pruritus to life-threatening Stevens–Johnson syndrome. Rash is the most common irAE with both PD-1 and CTLA-4 inhibitors; a systematic review suggests up to 20% of patients taking anti-PD-1 treatments develop a rash. Most of these are pruritic erythematous macules or papules [25]. Histological analysis of skin biopsies demonstrates a T cell heavy infiltrate [26]. Treatment usually consists of topical emollient, topical steroid, oral anti-histamine creams and occasionally oral steroids.
Vitiligo affects up to 10% patients receiving PD-1/PD-L1 therapy for melanoma, although interestingly is not seen with the same incidence in patients receiving CPIs for small cell lung cancer. Like the immune-related rashes, vitiligo lesions are usually symmetrical [27, 28].
Similar to the observation that cetuximab-related skin rash correlates with better outcomes in patients with colorectal cancer, the presence of vitiligo in patients with melanoma also seems to have a positive correlation [28, 29]. Although most dermatological irAEs are not severe, they can have psychological impact on patients due to the effect on appearance. Life-threatening skin manifestations such as Stevens–Johnson syndrome/toxic epidermal necrolysis, and drug rash with eosinophilia and systemic symptoms are very rare even when combination therapy is used [19, 30]. These high grade side effects require permanent cessation of immunotherapy and specialist dermatology input to help with diagnosis and management. In patients with pre-existing skin disorders such as psoriasis, specialist opinion should be sought prior to treatment.
Rheumatological
Approximately 15% of patients treated with anti-PD-1/PD-L1 agents suffer with mild arthralgia and myalgia. These symptoms often occur later than non-rheumatological irAEs, and do not usually require dose interruption [31].
Inflammatory signs such as joint swelling, stiffness, tenderness and erythema suggest arthritis. This occurs after a median of months of treatment and can persist beyond 2 years, even after immunotherapy is stopped [19]. It is an important adverse effect as it can lead to rapid joint damage. Inflammatory arthritis is more common in patients treated with anti-PD-1/PD-L1 agents and combination therapy (up to 10% of patients) compared with anti-CTLA-4 monotherapy (<1% of patients). It usually occurs in patients who have already experienced at least one other organ irAE [31–33]. Symptoms fall broadly into three classes and reflect the range of the rheumatological spectrum of disease. The ‘reactive’ group mirrors reactive arthritis, with large joints affected, often with associated eye inflammation (conjunctivitis and uveitis). A second class reflects RA, with small joint polyarthritis of the hands. Like true RA, this group can have erosive changes on radiographs and may have positive serology for RF and ACPA. A ‘seronegative’ group present with synovitis of medium and large joints. An algorithm to aid the diagnosis of inflammatory arthritis has been published, as per Table 1 [34].
Table 1.
An algorithm to aid the diagnosis of inflammatory arthritis
| Grade | Clinical exam | Lab testing | Imaging |
|---|---|---|---|
| 1 | Joint examination and functional assessment | ||
| 2/3 | Joint examination and functional assessment | ANA, RF, anti-CCP ESR, CRP | Consider plain X-ray, MRI and/or ultrasound of affected joints |
Other rheumatological syndromes because of immunotherapy are less common (<1%). These include vasculitides such as giant cell arteritis and polymyalgia rheumatica, Sicca syndrome (presenting with eye and mouth dryness) and systemic lupus erythematosus [2]. Polymyositis is seen in <1% of patients treated with anti-PD-1/PD-L1 agents and can present as a spectrum, from mild myalgia and weakness (with minimal associated creatinine kinase rise) to life-threatening myasthenia, rhabdomyolysis and myocarditis. These patients clearly require specialist input to co-ordinate myositis antibody panel testing, muscle biopsy and EMG [35].
Neurological (central/peripheral)
Neurological irAEs are uncommon, but are more common in patients receiving anti-PD-1/PD-L1 therapy (6%) than with anti-CTLA-4 treatment (4% in EORTC 18071 trial) [36]. With combination treatment, the incidence of all-grade neurological adverse events is ∼12%), and most symptoms are mild and non-specific, such as headache and vertigo [2]. Events of grade 3 and above are very rare (<1%) [2]. It is therefore important to rule out other causes of symptoms, such as disease progression (brain or leptomeningeal metastasis), infection and metabolic disturbance. It is also vital to consider managing patients with severe neurological irAEs in an intensive care setting, as patients with Guillain–Barré syndrome, encephalitis or myasthenia gravis may require respiratory support.
Peripheral neuropathy is one of the more common irAEs described in the literature and should be considered if patients describe sensory changes or paraesthesia. When accompanied by features such as ascending weakness and eye involvement (diplopia, reduced acuity), demyelination such as Guillain–Barré syndrome and transverse myelitis should be considered. These have a median onset of 4 weeks from commencing therapy [22]. Diagnostic tests include MRI of the spine, which can show uptake of contrast by nerve fibres, and EMG, which typically demonstrate reduced conduction velocity [37]. Lumbar puncture should be performed to test for the presence of anti-ganglioside antibodies. Rarely, nerve biopsies may be required; in cases of transverse myelitis these have shown necrosis and lymphocyte infiltration [38].
Production of antibodies to the acetylcholine receptor, which are pathognomonic of myasthenia gravis, can be enhanced by administration of CPIs. Ipilimumab has shown worsening of pre-existing myasthenia gravis and de novo cases have been reported. Patients present with fatigable weakness, which can affect limbs, as well as recti muscles to affect vision, and intercostal muscles to affect respiration. Serum samples can demonstrate the presence of anti-acetylcholine receptor and anti-muscle-specific kinase antibodies, to help make the diagnosis [39].
Central nervous system effects of CPIs are also seen; encephalitis, meningitis and encephalopathy can present with confusion, fever, seizures, upper motor neurone signs such as spasticity and cerebellar signs such as nystagmus [40, 41]. MRI of the brain shows diffuse dural enhancement with parenchymal sparing. Lumbar puncture is used to check for anti-NMDA receptor antibodies and for cerebrospinal fluid analysis, which would typically show increased protein and mononuclear white cell count, but normal glucose [42].
Paraneoplastic syndromes can present with a variety of central and peripheral neurological symptoms. They occur due to autoantibodies against tumour antigens, which are released in response to treatment. In patients with ipilimumab-treated small cell lung cancer, almost half developed antineuronal antibodies (e.g. anti-Hu and anti-Yo). The antibody-positive patients were more likely to develop paraneoplastic syndromes but interestingly also had significantly longer median progression-free survival [31, 43]. Imaging of the central nervous system, lumbar puncture for cerebrospinal fluid analysis and EMG for nerve conduction study are helpful for diagnosis [22].
Associations between the development of neurological irAEs and favourable response rate has also been observed; a case series of 40 patients with neurological side effects from CPI therapy has found a 90% overall response rate [44]. In a phase III trial, overall response rate was up to 30% (and median OS 11.2 months), compared with overall response rate of 70% (and median OS 45.7 months) in patients with a neurological irAE [44].
Renal
irAEs affecting the kidney occur rarely with anti-PD-1/PD-L1 therapy, anti-CTLA-4 therapy (2%) and combination checkpoint therapy (5%) [45]. However, as lower grades of adverse event are often symptomless, and only detected on blood tests, the true incidence could be higher. Patients who present with symptoms may have oliguria, haematuria or peripheral oedema, and up to 10% patients with immunotherapy-induced nephritis develop rash and fever [46]. The time of onset appears to be class-dependent, with onset at around two months following anti-CTLA-4 treatment, and between three and ten months with combination CPI [47]. In a case series of 13 patients who developed renal irAEs from checkpoint therapy; six had already experienced effects on other organs, and 12 of the 13 had acute interstitial nephritis, as confirmed by renal biopsy, with inflammatory infiltrate in the renal cortex and interstitial oedema [48]. Blood tests in patients with acute interstitial nephritis usually demonstrate acutely raised creatinine, eosinophilia and hyponatraemia [49]. Non-tubular renal irAEs are seen less commonly but cases of nephrotic syndrome have been reported, with either focal segmental sclerosis, membranous nephropathy or minimal change seen on biopsies [50].
Given the effects of CPIs on the immune system, clinicians have been understandably reluctant to use these treatments for patients with renal transplants, and therefore there are few data in this setting. Case reports of six such patients have had significant tumour response to CPIs. Four of these patients received anti-PD-1 therapy and developed acute rejection of the transplanted kidney [50]. This was not observed in the two transplant patients who received ipilimumab [51].
Pulmonary
A meta-analysis found the overall incidence of pneumonitis in patients receiving anti-PD-1/PD-L1 therapy is <5% [2]. Similar incidence is seen with anti-CTLA-4 inhibitor therapy. Although pneumonitis cases of grade 3 and above are rare (1–2%), it is one of the most common causes of immunotherapy-related death [2].
Common symptoms include new or increasing breathlessness, dry cough, wheeze or chest pain. Patients may describe reduced exercise tolerance, or even require supplementary oxygen. The onset of these symptoms is dependent on tumour type; patients with lung cancer experience symptoms earlier [median 2.1 (range 0.2–27.4) months] than patients with melanoma [median 5.2 (0.2–18.1) months] [52]. Monitoring oxygen saturation at rest and on ambulation may help identify affected patients. CT imaging of pneumonitis can show cryptogenic organizing pneumonia, non-specific interstitial pneumonitis, hypersensitivity pneumonitis, or usual interstitial pneumonitis/pulmonary fibrosis [2].
There have been case reports of CPI therapy causing pulmonary sarcoidosis; this can be symptom-less or present with breathlessness and dry cough. CT imaging demonstrates intrathoracic lymphadenopathy and irregular densities in keeping with granulomatous reactions [2].
Cardiology
Less than 1% of CPI-related adverse events are cardiac in nature [2]. Patients can present with a range of symptoms, from non-specific fatigue or muscle pain to chest pain, shortness of breath, palpitations or oedema. Side effects usually occur early, in the first few weeks of treatment [53]. As cardiac complications of CPI therapy are rare, most data so far come from pre-clinical models. Dilated cardiomyopathy has been described in genetically engineered mice; knocking out PD-1 caused sudden congestive heart failure, and in mice lacking PD-L1, autoimmune myocarditis was observed [54, 55].
Two cases of myocarditis and myositis occurred in a clinical trial of patients with melanoma receiving ipilimumab with nivolumab [53]. Literature suggests myocarditis occurs in <1% of patients receiving combination therapy, and the first presentation is potentially with isolated rhythm disturbance. A baseline ECG may make identification of subsequent change more straightforward. Serum troponin and brain natriuretic peptide are usually elevated [22]. Serial ECGs and cardiac imaging with MRI can be useful in diagnosis and monitoring. Myocardial biopsy demonstrates T cell and macrophage infiltration of the myocardium, cardiac sinus and atrioventricular nodes [53].
Autoimmune pericarditis is an extremely rare complication of CPI therapy. It presents similarly to infective pericarditis, with fever and chest pain that improves on sitting forward. A pericardial friction rub may be heard on auscultation. Aspiration of pericardial fluid for analyses typically demonstrates lymphocyte infiltration, but with no evidence of malignant cells or infective organisms [22].
Ophthalmological adverse events
The eye is an immune-privileged site, and so immune-related events are rare (<1% of patients). The most common ophthalmic irAE is anterior uveitis, for which there are case reports in patients receiving anti-PD-1/PD-L1 treatment [8, 56]. This can present with a painful red eye (conjunctival injection), blurred vision or photophobia, between 3 and 8 weeks after commencing CPI therapy. Ophthalmic assessment, including fundoscopy, is warranted and should include both eyes as presentation is often asymmetrical [2]. As ophthalmic irAEs are so uncommon, it is unlikely that a non-eye specialist will have seen many (if any) complications and be able to diagnose with certainty, so specialist referral is encouraged.
Retinopathy and uveal effusions have been reported with anti-PD-1/PD-L1 agents. Both of these present with blurred vision, and uveal effusions can additionally cause a painful red eye [57, 58]. There have been case reports of patients who have received nivolumab developing Vogt–Koyanagi–Harada-like syndrome (uveomeningitis syndrome) [59]. This has ophthalmic symptoms of blurred vision and bilateral uveitis, and can cause localized retinal detachment in addition to extra-ophthalmic symptoms in the skin and brain.
Haematological
Unlike with chemotherapy treatment, CPI therapy is not commonly known to cause myelosuppression or cytopenia. There are case reports of aplastic anaemia (bone marrow) and autoimmune haemolytic anaemia (peripheral blood) and immune thrombocytopenic purpura with anti-PD-1 agents, which seem to occur within 12 weeks of starting therapy [60–62]. However, given the rarity of these cases, patients with cytopenia should be evaluated for common causes too, such as cancer progression, gastrointestinal bleeding and drugs.
In cases of nivolumab-induced aplastic anaemia, blood work should include a Coombs test, reticulocyte count and haemolysis assays (lactate dehydrogenase, bilirubin and haptoglobin). Blood smears demonstrate pancytopenia with scattered lymphocytes. Bone marrow has a paucity of haematopoietic elements, with CD8+ T cells accounting for around 50% of the aspirate on flow cytometry [62].
Autoimmune haemolytic anaemia is investigated in a similar way, with results showing a positive direct Coombs test, raised reticulocyte count and haemolysis assay of raised lactate dehydrogenase and bilirubin, and reduced haptoglobin. Blood smears show spherocytosis [61]. The development of immune thrombocytopenic purpura is likely related to raised PD-1 expression on B cells. Blood results show decreased platelet count, with anti-platelet antibodies present. Bone marrow biopsy demonstrates an abundance of megakaryocytes with immature platelets [63].
Activation of pre-existing autoimmune diseases
Autoimmune diseases encompass a spectrum of over 80 separate disorders, and can be localized to a specific organ, or multi-systemic. The prevalence globally is thought to be between 3 and 8% [64]. However, despite being relatively common, most patients with autoimmune diseases were excluded from clinical trials involving CPIs. Initial evidence for CPI use in these patients is therefore from case studies.
Anti-CTLA-4 therapy (ipilimumab)
A 56-year-old gentleman with relapsing and remitting multiple sclerosis (MS) was diagnosed with metastatic melanoma 15 years after his MS diagnosis, at which point he had good control of the neurological disorder with methotrexate and glatiramer acetate. These drugs were stopped on commencement of ipilimumab, and the patient subsequently experienced severe clinical relapses of his MS. The patient, however, responded to ipilimumab and the authors concluded that ipilimumab can be used with caution in patients with MS; however, each individual case requires as assessment or risk vs benefit [65].
In a series of seven patients with RA who developed metastatic melanoma and were treated with ipilimumab, four had a partial response, with two of these undergoing surgical resection for complete response. Of note, three of these four responders were sero-positive. Four of the seven patients had a flare of RA; three patients had grade 1 flares, managed with non-steroidal anti-inflammatory agents) and two patients had grade 3 flares, requiring prednisolone. New irAEs were also seen in addition to flares of existing autoimmune disease; four patients had grade 3 colitis requiring hospitalization [66]. This high response rate, coupled with manageable exacerbations of autoimmune disease is promising for the use of ipilimumab in patients with pre-existing RA. This is supported by a retrospective review of 30 patients who received ipilimumab for metastatic melanoma who had pre-existing autoimmune disease, including RA, psoriasis, inflammatory bowel disease, systemic lupus erythematosus, MS and autoimmune thyroiditis. Thirteen patients (43%) were receiving immunosuppressive therapy (prednisolone or hydroxychloroquine) at the time of commencing ipilimumab. Eight patients (27%) had an exacerbation of their autoimmune disease, managed with corticosteroids. In addition, 10 patients (33%) had new irAEs of grade 3 or above [67]. Notably, 15 patients (50%) had neither a flare of existing autoimmune disease nor new irAEs, and six patients (20%) had an objective response [64].
Anti PD-1 therapy
A German study of patients with melanoma receiving anti-PD-1 therapy included 19 patients with pre-existing autoimmunity, including psoriasis, RA, vasculitis, polymyalgia rheumatica, sarcoidosis, inflammatory bowel disease, Guillain–Barré syndrome, MS and autoimmune thyroiditis. Before anti-PD-1 therapy commenced, all patients had well-controlled autoimmune disease; six patients (32%) were taking anti-inflammatory therapies, six patients (32%) were receiving hormone replacement for autoimmune disease and seven patients (37%) were not on treatment. However, within 20 weeks of treatment, eight patients (42%) had suffered a flare of their autoimmune disease, the majority being in those with pre-existing rheumatological disorders (five of nine patients, 55%). These flares did not correlate with response; eight patients (42%) had a response but only three of these had experienced a flare of pre-existing autoimmune disease [68].
Similar results were seen in a larger, multicentre trial involving 52 patients with autoimmune disease who received anti-PD-1 treatment for advanced melanoma. Twenty patients (38%) experienced a flare of autoimmune disease, particularly those with RA, polymyalgia rheumatica and Sjögren’s syndrome. Interestingly, in this study, no patients with gastrointestinal or neurological autoimmune disorders experienced a flare (n = 6 and 5, respectively). Fifteen patients (29%) experienced other irAEs unrelated to their autoimmune disease, for which four (8%) stopped treatment, compared with two patients (4%) who stopped due to flare of pre-existing autoimmune disease. The overall response rate was 33% and was similar in those that did and did not have a flare of autoimmune disease (7/20, 35% vs 10/32, 31%) [69].
It seems, therefore, that anti-PD-1 agents may cause particular flare of existing rheumatological autoimmune disease, but anti-melanoma activity is also evident. Such patients should not be denied such treatments, but require an individual assessment and discussion of risks vs benefits.
Conclusions
Immunotherapy has heralded a new frontier in the adjuvant and metastatic treatment of some cancers. Clinicians must be alert to the range of adverse effects that have been seen with these drugs and vigilant for novel side effects. Overlapping symptoms in multiple organs can add to the challenge, as can the flare of autoimmune disease that was well controlled prior to CPI therapy. The timing of onset may follow a predictable pattern and this can assist in diagnosis. As we gain more experience, longer-term data will emerge that may help predict at-risk patients. In the meantime, a collaborative approach between specialties is advised.
Acknowledgements
L.S. is supported by the National Institute for Health Research (NIHR).
Funding: This paper was published as part of a supplement funded by an educational grant from BMS.
Disclosure statement: M.P. has received speaker’s fees, honoraria and support with travel from BMS, MSD, Amgen and Novartis. N.C. has received speaker’s fees from BMS. The other author has declared no conflicts of interest.
References
- 1. Cousin S, Seneschal J, Italiano A.. Toxicity profiles of immunotherapy. Pharmacol Ther 2018;181:91–100. [DOI] [PubMed] [Google Scholar]
- 2. Puzanov I, Diab A, Abdallah K. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kartolo A, Sattar J, Sahai V, Baetz T, Lakoff JM.. Predictors of immunotherapy-induced immune-related adverse events. Curr Oncol 2018;25:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Topalian SL, Hodi FS, Brahmer JR. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer NIH public access. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maughan BL, Bailey E, Gill DM, Agarwal N.. Incidence of immune-related adverse events with program death receptor-1- and program death receptor-1 ligand-directed therapies in genitourinary cancers. Front Oncol 2017;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haanen J, Carbonnel F, Robert C. et al. Management of toxicities from immunotherapy: eSMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–42. [DOI] [PubMed] [Google Scholar]
- 7. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T.. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Villadolid J, Amin A.. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 2015;4:560–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin Z, Chen X, Li Z. et al. PD-1 antibody monotherapy for malignant melanoma: a systematic review and meta-analysis. PLoS One 2016;11:e0160485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson DB, Sullivan RJ, Ott PA. et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2019;2:234–40. [DOI] [PubMed] [Google Scholar]
- 11. Oble DA, Mino-Kenudson M, Goldsmith J. et al. α-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol 2008;32:1130–7. [DOI] [PubMed] [Google Scholar]
- 12. Gupta A, De Felice KM, Loftus EV, Khanna S.. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther 2015;42:406–17. [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez RS, Salaria SN, Bohannon CD. et al. PD-1 inhibitor gastroenterocolitis: case series and appraisal of ‘immunomodulatory gastroenterocolitis’. Histopathology 2017;70:558–67. [DOI] [PubMed] [Google Scholar]
- 14. Karamchandani DM, Chetty R.. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists’ perspective. J Clin Pathol 2018;71:665–71. [DOI] [PubMed] [Google Scholar]
- 15. Jain A, Lipson EJ, Sharfman WH, Brant SR, Lazarev MG.. Colonic ulcerations may predict steroid-refractory course in patients with ipilimumab-mediated enterocolitis. World J Gastroenterol 2017;23:2023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim KW, Ramaiya NH, Krajewski KM. et al. Ipilimumab-associated colitis: cT findings. Am J Roentgenol 2013;200:468–74. [DOI] [PubMed] [Google Scholar]
- 17. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2007;24:2283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berman D, Parker SM, Siegel J. et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun 2010;10:11. [PMC free article] [PubMed] [Google Scholar]
- 19. Spain L, Diem S, Larkin J.. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44:51–60. [DOI] [PubMed] [Google Scholar]
- 20. Cramer P, Bresalier RS.. Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Curr Gastroenterol Rep 2017;19:3. [DOI] [PubMed] [Google Scholar]
- 21. Mekki A, Dercle L, Lichtenstein P.. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur J Cancer 2018;96:91–;104. [DOI] [PubMed] [Google Scholar]
- 22. Baraibar I, Melero I, Sarvise MP, Castanon E.. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf 2019;42:281. [DOI] [PubMed] [Google Scholar]
- 23. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M.. Cancer immunotherapy – immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 2017;13:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hughes J, Vudattu N, Sznol M. et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 2015;38:e55–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antinori A, Arendt G, Becker JT. et al. Characterization and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer 2015;69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naidoo J, Page DB, Li BT. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sibaud V. Dermatologic reactions to immune checkpoint inhibitors. Am J Clin Dermatol 2018;19:345–61. [DOI] [PubMed] [Google Scholar]
- 28. Hua C, Boussemart L, Mateus C. et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016;152:45–51. [DOI] [PubMed] [Google Scholar]
- 29. Orditura M, De Vita F, Galizia G. et al. Correlation between efficacy and skin rash occurrence following treatment with the epidermal growth factor receptor inhibitor cetuximab: A single institution retrospective analysis. Oncol Rep 2009;21:1023–8. [DOI] [PubMed] [Google Scholar]
- 30. Larkin J, Chiarion-Sileni V, Gonzalez R. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lidar M, Giat E, Garelick D. et al. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev 2018;17:284–9. [DOI] [PubMed] [Google Scholar]
- 32. Cappelli LC, Gutierrez AK, Baer AN. et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Belkhir R, Burel SL, Dunogeant L. et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis 2017;76:1747–50. [DOI] [PubMed] [Google Scholar]
- 34. Naidoo J, Cappelli LC, Forde PM. et al. Inflammatory arthritis: a newly recognized adverse event of immune checkpoint blockade. Oncologist 2017;22:627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Touat M, Maisonobe T, Knauss S, Ben O, Salem H.. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018;91:e985–94. [DOI] [PubMed] [Google Scholar]
- 36. Eggermont AMM, Chiarion-sileni V, Grob J. et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522–30. [DOI] [PubMed] [Google Scholar]
- 37. Nukui T, Nakayama Y, Yamamoto M. et al. Nivolumab-induced acute demyelinating polyradiculoneuropathy mimicking Guillain-Barré syndrome. J Neurol Sci 2018;390:115–6. [DOI] [PubMed] [Google Scholar]
- 38. Tanaka R, Maruyama H, Tomidokoro Y. et al. Nivolumab-induced chronic inflammatory demyelinating polyradiculoneuropathy mimicking rapid-onset Guillain-Barré syndrome: a case report. Jpn J Clin Oncol 2016;46:875–8. [DOI] [PubMed] [Google Scholar]
- 39. Gonzalez NL, Puwanant A, Lu A, Marks SM, Živkovic SA.. Myasthenia triggered by immune checkpoint inhibitors: new case and literature review. Neuromuscul Disord 2017;27:266–8. [DOI] [PubMed] [Google Scholar]
- 40. Levine JJ, Somer RA, Hosoya H, Squillante C.. Atezolizumab-induced encephalitis in metastatic bladder cancer: a case report and review of the literature. Cli Genitourin Cancer 2017;15:e847–9. [DOI] [PubMed] [Google Scholar]
- 41. Burke M, Hardesty M, Downs W.. A case of severe encephalitis while on PD-1 immunotherapy for recurrent clear cell ovarian cancer. Gynecol Oncol Reports 2018;24:51–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams TJ, Benavides DR, Patrice K. et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol 2016;73:928–33. [DOI] [PubMed] [Google Scholar]
- 43. Feng S, Coward J, Mccaffrey E. et al. Pembrolizumab-induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J Thorac Oncol 2017;12:1626–35. [DOI] [PubMed] [Google Scholar]
- 44. Smith JL, Menzies AM, Cohen JV. et al. Neurotoxicity associated with anti-PD1 therapy: a multi-center case series. J Clin Oncol 2017;35:e21641. [Google Scholar]
- 45. Izzedine H, Gueutin V, Gharbi C. et al. Kidney injuries related to ipilimumab. Invest New Drugs 2014;32:769–73. [DOI] [PubMed] [Google Scholar]
- 46. Belliere J, Meyer N, Mazieres J. et al. Acute interstitial nephritis related to immune checkpoint inhibitors. Br J Cancer 2016;115:1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wanchoo R, Karam S, Uppal NN. et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017;45:160–9. [DOI] [PubMed] [Google Scholar]
- 48. Cortazar FB, Marrone KA, Troxell ML. et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Belliere J, Meyer N, Mazieres J. et al. Acute interstitial nephritis related to immune checkpoint inhibitors. Br J Cancer 2016;115:1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lefebvre J, Glezerman IG.. Kidney toxicities associated with novel cancer therapies. Adv Chronic Kidney Dis 2017;24:233–40. [DOI] [PubMed] [Google Scholar]
- 51. Lipson EJ, Bodell MA, Kraus ES, Sharfman WH.. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol 2014;32:e69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Delaunay M, Cadranel J, Lusque A. et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 2017;50:1700050. [DOI] [PubMed] [Google Scholar]
- 53. Johnson DB, Balko JM, Compton ML. et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nishimura H, Okazaki T, Tanaka Y.. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001;291:319–23. [DOI] [PubMed] [Google Scholar]
- 55. Lucas JA, Menke J, Rabacal WA. et al. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol 2008;181:2513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kumar V, Chaudhary N, Garg M. et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 2017;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kanno H, Ishida K, Yamada W. et al. Uveitis induced by programmed cell death protein 1 inhibitor therapy with nivolumab in metastatic melanoma patient. J Infect Chemother 2017;23:774–7. [DOI] [PubMed] [Google Scholar]
- 58. Thomas M, Armenti ST, Ayres B, Demirci H.. Uveal effusion after immune checkpoint inhibitor therapy. JAMA Ophthalmol 2018;136:553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arai T,, Harada K,, Usui Y,, Irisawa R,, Tsuboi R.. Case of acute anterior uveitis and Vogt–Koyanagi–Harada syndrome-like eruptions induced by nivolumab in a melanoma patient. J Dermatol 2017;44:975–6. [DOI] [PubMed] [Google Scholar]
- 60. Atwal D, Joshi KP, Ravilla R, Mahmoud F.. Pembrolizumab-induced pancytopenia: a case report. Perm J 2017;21:17-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Michot J, Vargaftig J, Leduc C. et al. Immune-related bone marrow failure following anti-PD1 therapy. Eur J Cancer 2017;80:1–4. [DOI] [PubMed] [Google Scholar]
- 62. Comito RR, Badu LA, Forcello N.. Nivolumab-induced aplastic anemia: a case report and literature review. J Oncol Pharm Pract 2019;25:221–5. [DOI] [PubMed] [Google Scholar]
- 63. Pföhler C, Burgard B, Müller CSL.. A case of immune thrombocytopenia as a rare side effect of an immunotherapy with PD1-blocking agents for metastatic melanoma. Transfus Med Hemother 2017;44:426–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Donia M, Pedersen M, Svane IM.. Cancer immunotherapy in patients with preexisting autoimmune disorders. Semin Immunopathol 2017;39:333–7. [DOI] [PubMed] [Google Scholar]
- 65. Gettings EJ, Hackett CT, Scott TF.. Severe relapse in a multiple sclerosis patient associated with ipilimumab treatment of melanoma. Mult Scler 2015;21:670. [DOI] [PubMed] [Google Scholar]
- 66. Lee B, Wong A, Kee D. et al. The use of ipilimumab in patients with rheumatoid arthritis and metastatic melanoma. Ann Oncol 2016;27:1174–7. [DOI] [PubMed] [Google Scholar]
- 67. Johnson DB, Sullivan RJ, Ott PA. et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016;2:234–40. [DOI] [PubMed] [Google Scholar]
- 68. Gutzmer R, Koop A, Meier F. et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur J Cancer 2017;75:24–32. [DOI] [PubMed] [Google Scholar]
- 69. Menzies AM, Johnson DB, Ramanujam S. et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–76. [DOI] [PubMed] [Google Scholar]