Abstract
Background
Breast cancer is a common malignant tumor worldwide. Despite the huge advances in modern medicine, many patients still face a high risk of recrudescent and metastatic breast cancer. Berberine was widely implemented in clinic treatment of breast cancer. This study was performed to contribute to a better understanding on the mechanisms underlying berberine affecting breast cancer.
Material/Methods
We mined survival data of metadherin (MTDH) in breast cancer patients through Kaplan-Meier Plotter and analyzed the transcriptional and posttranscriptional expression profile of MTDH in several breast cancer cell lines. The cell viability and MTDH mRNA level were detected under the si-MTDH vector and different concentrations of berberine. The MTDH-expression vector was transfected into MCF-7 and MDA-MB-231 cells, and the changes of cell viability and apoptosis were determined after berberine (50 μM) treatment.
Results
High MTDH expression was related to worse relapse-free survival (RFS) of breast cancer (P-value=6.2e-08). High-expressed MTDH is common in breast cancer cells, compared with that in normal breast cells (P<0.01). MTDH knockout could inhibit the viabilities of MCF-7 and MDA-MA-231 cells (P<0.01). When the concentration was higher than 10 μM, the suppressive effects of berberine on viability and MTDH reached significant level. As MTDH expression increased, the enhanced apoptosis rates of breast cancer cells by berberine were remarkably inhibited.
Conclusions
High-expressed MTDH was helpful to cell proliferation and survival in breast cancer. The anti-cancer ability of berberine in breast cancer may be partially dependent on the regulation of MTDH.
MeSH Keywords: Apoptosis, Cell Survival, Flow Cytometry, Prognosis
Background
Breast cancer is a frequent malignancy in women, especially in middle-aged or elder women aged between 40 and 60 years old. However, recent researches showed that the incidence of breast cancer was increasing at 2.7% per year and the age distribution became younger in China [1–3]. At present, surgery in combination with radiation therapy and medicine chemotherapy was the main therapeutic approach to treat breast cancer, however, chemotherapeutics have many severe side effects on healthy cells [4]. Although the combined therapy of breast cancer was gradually optimized, a considerable portion of the patients was still at the high risk of recurrence and metastasis. Therefore, it is highly urgent and necessary to find safer and more effective therapeutic targets for the improvement of recurrence and metastasis of breast cancer. Berberine, a plant-derived compound, could be extracted from many Chinese herbal medicines. Berberine has been demonstrated to contain a widely pharmacological effect, including antibacterial infection [5], anti-inflammatory action [6], and anti-hyperglycemia [7]. Studies showed that berberine also contained anti-cancer ability, which had a promoting effect on cell apoptosis while inhibiting cell proliferation in several types of tumors [8–10]. In 2016, Ma et al. revealed that berberine treatment obviously suppressed the abilities of breast cancer cells to proliferate and migrate via disturbing ephrin-B2 [11]. Meanwhile, Xie et al. demonstrated that berberine could promote the reactive oxygen species (ROS) generation and activate mitochondrion pathway of apoptosis [12]. However, the detailed mechanism underlying the anti-cancer ability of berberine in breast cancer is still incompletely understood.
Metadherin (MTDH) was highly expressed in many types of cancers including colorectal [13], glioma [14], and breast cancers [15]. MTDH was involved in tumorigenesis and tumor progression in multiple aspects. In the previous study, the overexpression of MTDH played a vital role in carcinogenesis, development, metastasis, and chemoresistance of breast cancer [16–18]. MTDH was believed to act as an oncogene, which enhanced the abilities of tumor cell invasion and migration, resulting in an aggressive phenotype and a poor prognosis, thus, MTDH may have the potential to serve as a biomarker of breast cancer [19]. Accumulated evidence has demonstrated that MTDH knockdown could notably suppress the progression of breast cancer [10,20], and endogenous MTDH silencing contributed to enhancing the sensibility of breast cancer cells to tumor necrosis factor-associated apoptosis induces ligand (TRAIL)-mediated apoptosis [21]. These research studies suggested the potential of MTDH as a therapeutic target for breast cancer. Thus, in this study, we aimed to investigate the molecular mechanism underlying the therapeutic action of berberine on breast cancer.
Material and Methods
Cell culture
The normal breast cells (Hs 578Bst, #HTB-125) and human breast cancer cell lines (MCF-7, MCF-10A, MDA-MB-231, and T47D) (#HTB-22, #CRL-10317, #HTB-26, and #HTB-133™) were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI 1640 medium (#11875119, ThermoScientific, Hudson, NH, USA) that supplemented with 10% fetal bovine serum (FBS, #10099141, ThermoScientific) in 5% CO2 at 37°C.
The Kaplan-Meier plotter
Kaplan-Meier plotter (KMplotter; http://kmplot.com/analysis/) [22] was applied to assess the prognostic value of MTDH expression in breast cancer. KMplotter is an online database that provides assessment of the effect of 54 675 genes on survival using 10 293 cancer samples derived from GEO (Gene Expression Omnibus), TCGA (The Cancer Genome Atlas), and EGA (European Genome-phenome Atlas) databases, including 22 277 genes in 4142 patients with breast cancer. In our analysis, patients were divided into high and low expression groups by median values of mRNA expression level of MTDH and survival analyses were performed without follow-up restrictions. The number of cases, hazard ratio (HR) and log rank P-value were obtained from the webpage of the KM plotter.
Plasmid construction and transfection
The sequences of siRNA specific targeting the MTDH transcripts were purchased from Wuhan Genesil Biotechnology Co., Ltd. (Wuhan, China). For MTDH-siRNA (si-MTDH): 5′-AACAGAAGAAGAAGAACCGGA-3′, the MTDH overexpression vector and negative control (NC) were imported into a pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA). After being incubated into 6-well plates (3×105 cell/well) for 24 hours, cells were transfected with MTDH, si-MTDH and negative control vectors (100 nM) using Lipofectamine 2000 (Invitrogen) and incubated in 5% CO2 at 37°C with high humidity for 6 hours. Finally, the cells were transferred to complete medium with 10% FBS for further culture.
Cell Counting Kit-8 (CCK-8) assay
The cell viability was determined using Cell Counting Kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan). Cells were cultured with 200 μL medium in 96-well plates (4×103 cells/well) containing berberine (0, 1, 10, 50, 100, and 200 μM, #BP1108, Sigma-Aldrich) and maintain at 37°C in 5% CO2. After 24 and 48 hours, cells were transferred to culture with CCK-8 reagent for 2 hours. The absorbance was examined on a microplate reader at 450 nm (CANY, Shanghai, China). The concentration of berberine that caused 50% inhibition of breast cancer cell activity was defined as IC50. The inhibition rate was calculated using the following formula: Inhibition rate (%)=(1–ODtreated/ODcontrol)×100%.
Flow cytometer for apoptosis detection
MCF-7 and MDA-MB-231 cells were seeded into 96-well plates (4×103 cells per well) for incubation. After removing culture medium, the cells were washed and then resuspended with a binding buffer consisting of propidium iodide (PI) and annexin V-fluorescein isothiocyanate (V-FITC). The stained cells were blocked in the dark for 15 minutes. Apoptosis rates were subsequently determined by flow cytometry on a Cytomics FC 500 flow cytometer (Beckman Coulter, Inc., Brea, CA, USA)
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from all treated cells were extracted following the instructions of TRIzol reagent (Invitrogen). The purified RNA was used as the template for cDNA synthesis by PrimeScript RT reagent kit (Takara), and the reaction parameter was as follows: firstly, at 65°C for 5 minutes, secondly, at 30°C for 6 minutes and finally at 50°C for 60 minutes. The MTDH expression levels in breast cell lines and normal breast cells were determined by SYBR green detection (Takara) using ABI 7500 Fast Real-Time PCR System (Foster City, CA, USA). All primers were shown in Table 1 and the reaction mixture contained 1 μL forward and reverse primers (10 μM), 10 μL SYBR fluorescent dye, 2 μL cDNA and RNase Free dH2O. The reaction was conducted at 94°C for 3 minutes, subsequently at 94°C for 30 seconds, at 55°C for 30 seconds and at 72°C for 2 minutes for 40 cycles. The mRNA level of GAPDH served as an internal control. The 2–ΔΔCt method was employed in calculating relative mRNA levels.
Table 1.
Primers for qRT-PCR.
| Gene name | Primer sequences |
|---|---|
| MTDH | Forward: 5′-GAGAAGCCCAAACCAAAT-3′ |
| Reverse: 5′-ATCAGTCAGCACCTTATCAC-3′ | |
| GAPDH | Forward: 5′-ACCACAGTCCATGAAATCAC-3′ |
| Reverse: 5′-AGGTTTCTCCAGGCGGCATG-3 |
qRT-PCR – quantitative real time polymerase chain reaction; MTDH – metadherin.
Western blot
MCF-7 and MDA-MB-231 cells were solubilized in RIPA lysis buffer. Protein samples were subjected by 10% SDS/PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (ThermoScientific), and then blocked in 1×TBST buffer, followed by culturing with primary antibodies. After that, membranes were subsequently incubated with the second antibody (anti-rabbit IgG, HRP-linked antibody, #7074, 1: 2000, Cell Signaling Technology, CST, Danvers, MA, USA) for 1 hour at room temperature. The signals were detected using a chemiluminescent detection system, and densities of bands were quantified by ImageJ software. GAPDH was examined as a loading control. All primary antibodies, including anti-GAPDH (1: 1000, #2118), Metadherin (1: 1000, #14065), anti-B-cell lymphoma 2 (Bcl-2) (#4223) and anti-Bax (#5023), were obtained from CST.
Statistical analysis
All data were shown as means±SEM. Statistical significance was analyzed by one-way analysis of variance or Student’s t-test using SPSS. Statistical significance was determined as P<0.05.
Results
High-expressed MTDH worsened relapse-free survival (RFS) in breast cancer
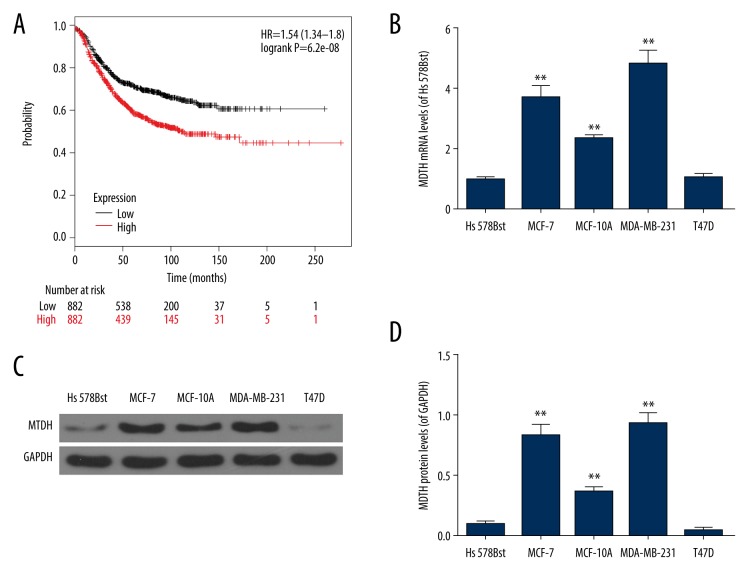
To investigate the prognostic significance of MTDH, the Kaplan-Meier survival analysis was applied for the determination of the relapse-free survival (RFS) of patients. And our results showed that high MTDH expression trended toward shorter RFS than that in low MTDH expression group (P=6.2e-08, Figure 1A). These results indicated a significant association between the high-expressed MTDH and worse RFS.
Figure 1.
The ectopic expression of MTDH showed a possible association with worse RFS in breast cancer. (A) The prognostic value of MTDH in breast cancer patients for RFS (OS). (B–D) The relative levels of MTDH in several breast cancer cell lines (MCF-7, MCF-10A, MDA-MB-231, and T47D) and normal breast cell (Hs 578Bst cells) were determined by qRT-PCR and western blot. Each value was represented as mean±SEM (n=3). GAPDH served as an internal control. ** P<0.01 versus Hs 578Bst cells. MTDH – metadherin; RFS – relapse-free survival; OS – overall survival; qRT-PCR – quantitative real time polymerase chain reaction; SEM – standard error of the mean.
High MTDH expression was frequent in breast cancer cells
According to the results in Figure 1B, 1C and 1D, the expressions of MTDH in MCF-7, MCF-10A, and MDA-MB-231 cells were greatly higher than those in normal breast cells (Hs 578Bst) (P<0.01). However, there was no significant difference in the expression of MTDH between T47D and Hs 578Bst cells. Considering that the relative level of MTDH was the highest in MCF-7 and MDA-MB-231 cells (P<0.01) (Figure 1B–1D), both were chosen for subsequent experiments.
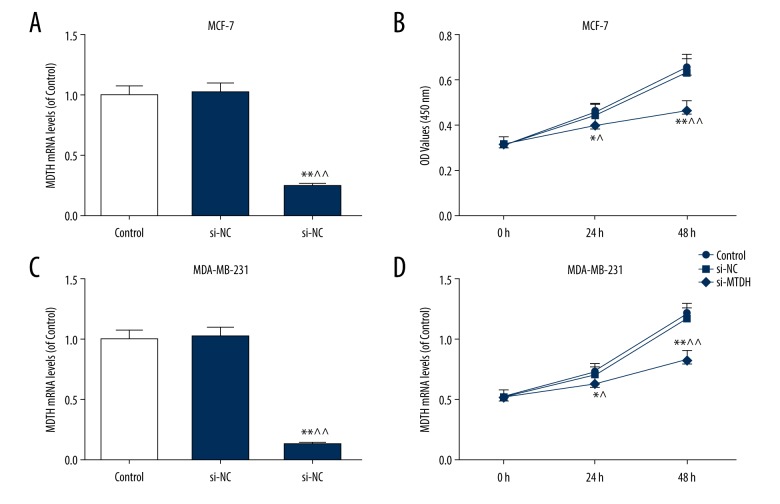
MTDH knockdown obviously suppressed the viabilities of MCF-7 and MDA-MB-231 cells
After transfection with si-MTDH, we measured the changes in breast cancer cell viability. Si-MTDH could significantly inhibit the MTDH expression in MCF-7 and MDA-MB-231 cells (Figure 2A, 2C). In the meanwhile, MTDH inhibition could effectively reduce the cell viabilities in MCF-7 and MDA-MB-231 cells at 24 h (p<0.05), and such a negative effect was strengthened over time (p<0.01) (Figure 2B, 2D).
Figure 2.
Knockdown of MTDH suppressed the viabilities of MCF-7 and MDA-MB-231 cells. To investigate the role of MTDH in breast cancer, si-MTDH was transfected into MCF-7 and MDA-MB-231 cells. (A) The transfection efficiency of si-MTDH in MCF-7 cells was measured by qRT-PCR. (B) The changes in MCF-7 cell viability were performed by CCK-8 kit. (C) The transfection efficiency of si-MTDH in MDA-MB-231 cells was evaluated by qRT-PCR. (D) The changes in MDA-MB-231 cell viability were detected by CCK-8 kit. Each value represents mean±SEM (n=3). GAPDH served as an internal control. * P<0.05, ** P<0.01 versus control group; ^ P<0.05, ^^ P<0.01 versus si-NC group. MTDH – metadherin; RFS – relapse-free survival; OS – overall survival; qRT-PCR – quantitative real time polymerase chain reaction; SEM – standard error of the mean; CCK-8 – Cell Counting Kit-8.
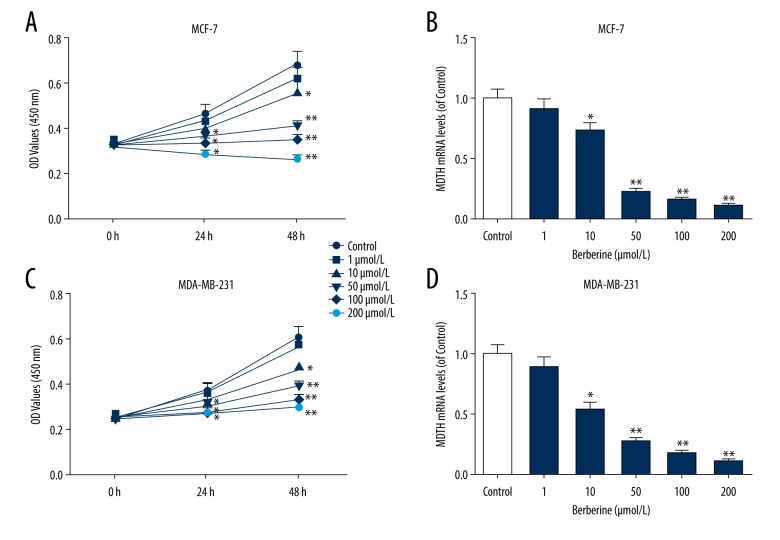
Berberine inhibited MCF-7 and MDA-MB-231 cell viabilities in concentration- and time-dependent manners
MCF-7 and MDA-MB-231 cells were subjected to various concentrations of berberine treatment (0, 1, 10, 50, 100, and 200 μM). Our preliminary experiments showed that MCF-7 cells had an IC50 value of 102.34±27.4 μM, while MDA-MB-231 had an IC50 value of 158.64±38.59 μM. As shown in Figure 3A and 3C, MCF-7 cell viability was much decreased as the concentration of berberine increased, similarly, in MDAMB-231 cells, the cell viability was also obviously decreased. We also measured the mRNA levels of MTDH in MCF-7, and MDA-MB-231 cells treated with berberine, and found that berberine affected slightly the cell viability when the concentration was less than 10 μM, however, the inhibitory effect continued to increase when the concentration was higher than 50 μM (P<0.01) (Figure 3B, 3D). Importantly, although the apoptosis rate in 100 μM treatment group was slightly higher than that in 50 μM berberine group, microscope observation showed that the proportion of cell rupture in 100 μM group was greatly more than the 50 μM group. Therefore, considering the purpose of this study, we suggested that 50 μM of berberine was more appropriate to be the experimental concentration.
Figure 3.
Berberine induced inhibition of MCF-7 and MDA-MB-231 cell viabilities in a concentration-manner. (A) The viabilities of MCF-7 cells were shown by CCK-8 kit under the treatment of various concentrations of berberine (0, 1, 10, 50, 100, and 200 μM). (B) The changes in MTDH mRNA levels in MCF-7 cells were determined by qRT-PCR. (C) The viabilities of MDA-MB-231 cells were detected by CCK-8 kit under the treatment of various concentrations of berberine. (D) The changes in MTDH mRNA expression of MDA-MB-231 cells were investigated by qRT-PCT. Each value was represented by mean±SEM (n=3). GAPDH served as an internal control. * P<0.05, ** P<0.01 versus control group. MTDH –metadherin; qRT-PCR – quantitative real time polymerase chain reaction; SEM – standard error of the mean.
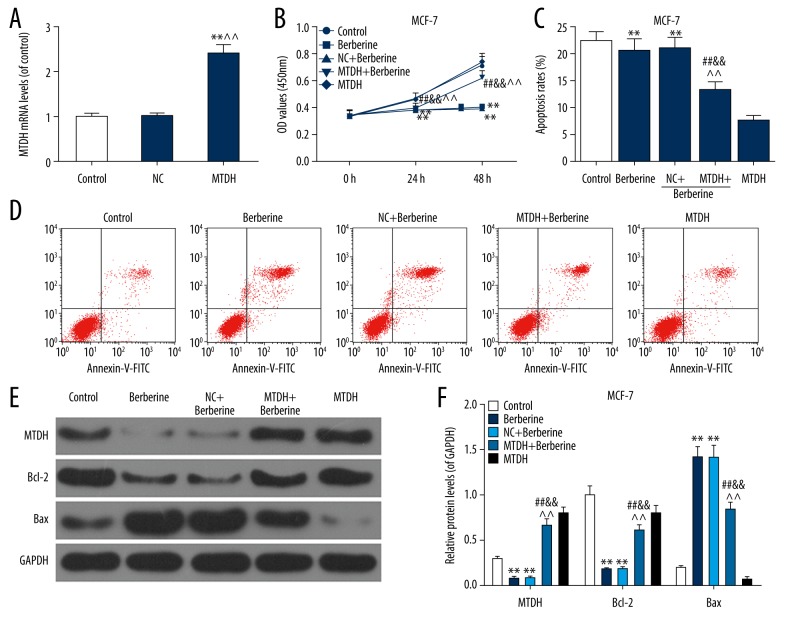
Overexpressed MTDH reversed the inhibitory effects of berberine on MCF-7 and MDA-MB-231 cells
As demonstrated in Figure 4A, the MTDH vector has stably exerted its role in MCF-7 cells. The result of cell viability detection showed that MTDH overexpression could notably offset the negative effect of berberine on MCF-7 cell viabilities, compared to berberine and NC+berberine groups (P<0.01, Figure 4B). As shown in Figure 4C and 4D, the treatment of berberine markedly enhanced the apoptosis rate. Meanwhile, the apoptosis rate of MTDH+berberine group was reduced from 20% to 14% (P<0.01) in berberine and NC+berberine groups.
Figure 4.
Overexpressed MTDH partially reversed the inhibitory effects of berberine (50 μM) on MCF-7 cells. In order to study whether the anti-cancer ability of berberine in breast cancer was associated with the regulation of MTDH, MTDH overexpression vector was transfected into MCF-7 cells before the treatment of berberine. ** P<0.01 versus control group; ^^ P<0.01 versus NC group. (A) The transfection efficiency was assessed by qRT-PCR. (B) The changes in viability of MCF-7 cells were determined by CCK-8 kit. (C, D) The effects of MTDH overexpression on the berberine-induced increased apoptosis rate of MCF-7 cells were detected by flow cytometry. (E, F) The MTDH protein level and apoptotic factors (Bcl-2 and Bax) were detected by western blot. Each value was represented by mean±SEM (n=3). GAPDH served as an internal control. ** P<0.01 versus control group; ## P<0.01 versus berberine group; && P<0.01 versus NC+berberine group; ^^ P<0.01 versus MTDH group. MTDH – metadherin; qRT-PCR – quantitative real time polymerase chain reaction; CCK-8 – Cell Counting Kit-8; SEM – standard error of the mean.
To further confirm the effects of MTDH on MCF-7 cell apoptosis, we also detected Bcl-2 and Bax protein levels. As listed in Figure 4E and 4F, berberine had a notably inhibitory effect at MTDH and Bcl-2 protein levels (P<0.01) and it enhanced the Bax expression (P<0.01). The overexpressed MTDH partially rescued the effects of berberine on Bcl-2 and Bax. In addition, the effects of MTDH overexpression on cell viability, apoptosis rate and apoptosis-associated protein levels of MDA-MB-231 cells were shown in Figure 5A–5F, which was basically consistent with the changes in MCF-7 cells. Therefore, our findings suggested that overexpressed MTDH reversed the inhibitory effects of berberine and enhanced the apoptosis rate of MCF-7 and MDA-MB-231 cells.
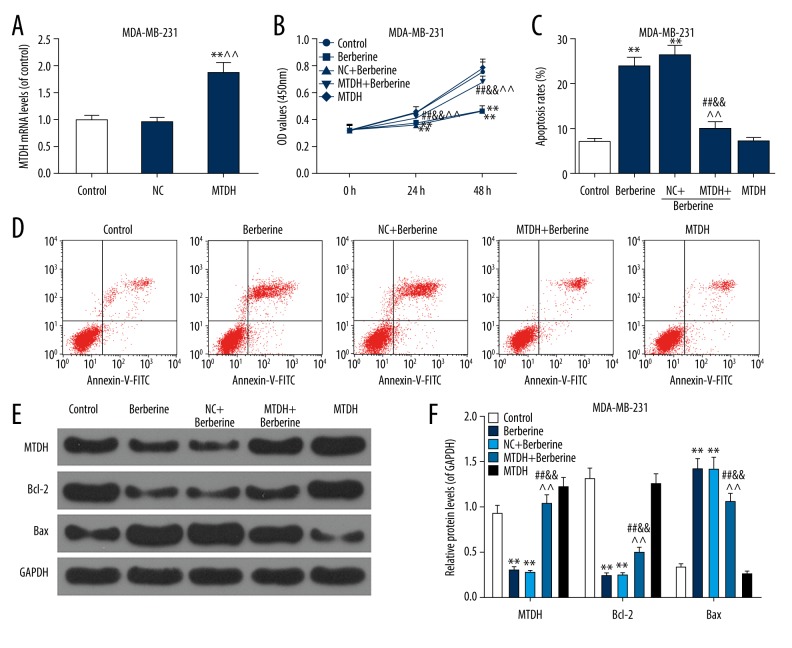
Figure 5.
Overexpressed MTDH partially reversed the inhibitory effects of berberine (50 μM) on MDA-MB-231 cells. In order to study whether the anti-cancer ability of berberine in breast cancer was associated with the regulation of MTDH, MTDH overexpression vector was transfected into MDA-MB-231 cells before the treatment of berberine. (A) The transfection efficiency of MTDH overexpression vector in MDA-MB-231 cells was assessed by qRT-PCR. ** P<0.01 versus control group; ^^ P<0.01 versus NC group (B) The changes in viability of MDA-MB-231 cells were measured by CCK-8. (C, D) The effects of MTDH overexpression on the berberine-induced increased apoptosis rate of MDA-MB-231 cells were performed by flow cytometry. (E, F) The MTDH protein level and apoptotic factors (Bcl-2 and Bax) were detected by western blot. Each value was represented by mean±SEM (n=3). GAPDH served as an internal control. ** P<0.01 versus control group; ## P<0.01 versus berberine group; && P<0.01 versus NC+berberine group; ^^ P<0.01 versus MTDH group. MTDH – metadherin; qRT-PCR – quantitative real time polymerase chain reaction; CCK-8 – Cell Counting Kit-8; SEM – standard error of the mean.
Discussion
Breast cancer seriously affects female health and is also a common malignant tumor worldwide. The incidence of breast cancer had an approximately 10-fold increase in China during the last 10 years [23]. MTDH, a recognized cancer gene, is widely involved in the pathogenesis and progression of multiple cancers and plays a vital role in the epithelial-mesenchymal transition (EMT) process of tumor cell [24]. Numerous researches demonstrated that berberine could be widely implemented in clinic treatment and it contributed to impeding the development of a variety of cancers [11,25]. Berberine also had the ability to increase cancer therapy sensitization to assist chemotherapy [26]. However, there are currently limited researches about the mechanisms underlying the anti-cancer ability of berberine in breast cancer. In this study, our results indicated that the ability of berberine to inhibit cell viability and induce apoptosis in breast cancer may be dependent on impeding MTDH expression.
The ectopic expression of MTDH could induce several biological behaviors of breast cancer cells such as proliferation, angiogenesis, invasion and metastasis and drug resistance, ultimately resulting in a poor prognosis [27]. In our results, high levels of MTDH were found in MCF-7, MCF-10A, and MDA-MA-231 cells. Meanwhile, the Kaplan-Meier survival analysis indicated that a high expression of MTDH exhibited a close relation with a poor prognosis. Previous studies demonstrated that the inhibition of MTDH by microRNAs could suppress the cell proliferation and motility of breast cancer, and subsequently, inhibit EMT process to prevent tumor progression [20,28], which was consistent with our results that the knockdown of MTDH induced remarkable inhibitions in cell viabilities of MCF-7 and MDA-MA-231 cells. Therefore, our data suggested that high-expressed MTDH might be highly associated with the poor prognosis in breast cancer.
To further investigate the functions of berberine in breast cancer, the cell viability and MTDH mRNA level in breast cancer were determined under various concentrations of berberine. Several types of research proved that the concentration of berberine could negatively regulate the invasive and migratory abilities of MCF-7 cells [29]. At this point, the downregulation of cell viability and MTDH levels in MCF-7 and MDA-MA-231 cells suggested that the therapeutic effects of berberine were positively correlated with the concentration. In addition, we observed the duration of berberine treatment is also a key factor in the suppressive effects on tumor development. Thus, our findings indicated that berberine reduced the viabilities of MCF-7 and MDA-MA-231 cells and MTDH mRNA level in the concentration- and time-dependent manner.
Finally, MTDH overexpression vector was transfected into MCF-7 and MDA-MA-231 cells. As the expression of MTDH increased, berberine (50 μM) could have markedly inhibited the viabilities of MCF-7 and MDA-MA-231 cells, indicating that berberine inhibiting breast cancer development might be mediated through regulating MTDH expression. Both Bcl-2 and Bax have been reported to work as the most important apoptotic indexes [30]. To further verify the changes of apoptosis rate in the breast cancer cells, we measured the Bcl-2 and Bax levels. Previous studies identified that the downregulated Bcl-2 expression and upregulated Bax expression contributed to cell apoptosis [31]. In the current study, the results of flow cytometer were consistent with these researches, and we found that overexpression of MTDH can reverse the berberine-induced elevated apoptosis rate and decreased viability in MCF-7 and MDA-MA-231 cells. Based on these results, the suppressive ability of berberine on the cell viability and apoptosis was suggested to be highly associated with the regulation of MTDH expression in breast cancer cells. In addition, the previous study indicated that berberine could effectively inhibit tumor growth in the MDA-MB-231 xenograft mouse model [32]. However, our study did not perform the experiments in vivo. Due to the complex and changeable tumor microenvironment in vivo, whether berberine could also repress MTDH to inhibit the growth and development of breast cancer in vivo still requires further investigation.
There are some limitations in our study. For instance, although our study demonstrated that the treatment of berberine could significantly suppress breast cancer cell proliferation and survival in vitro through repressing MTDH expression, but whether the inhibitory effects of berberine on MTDH can be able to be applied to solid tumors in body still need to be further verified.
Conclusions
We observed that the ectopic expression of MTDH may be associated with poor prognosis of breast cancer. Berberine showed inhibitory effects on cell viabilities and MTDH mRNA levels in MCF-7, and MDA-MA-231 cell in a concentration-manner. We further transfected MTDH overexpression vector into breast cancer cells and found that the elevated MTDH expression could significantly reverse the suppressive effects of berberine on cell proliferation and survival in breast cancer. These data indicated that the anti-cancer effects of berberine on breast cancer may partially rely on the regulation of MTDH. In addition, MTDH might be considered as a valuable prognostic marker of breast cancer prognosis.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Levaggi A, Poggio F, Lambertini M. The burden of breast cancer from China to Italy. J Thorac Dis. 2014;6(6):591–94. doi: 10.3978/j.issn.2072-1439.2014.06.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. A Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. A Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Shao D. Berberine reverses hypoxia-induced chemoresistance in breast cancer through the inhibition of AMPK-HIF-1α. Int J Biol Sci. 2017;13(6):794–803. doi: 10.7150/ijbs.18969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu HH, Kim KJ, Cha JD, et al. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8(4):454–61. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 6.Wei X, Wang C, Hao S, et al. The therapeutic effect of berberine in the treatment of nonalcoholic fatty liver disease: A meta-analysis. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/3593951. 3592951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Li XD, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93(7):2559–65. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 8.Mantena SK, Sharma SD, Katiyar SK. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther. 2006;5(2):296–308. doi: 10.1158/1535-7163.MCT-05-0448. [DOI] [PubMed] [Google Scholar]
- 9.Tian Y, Zhao L, Wang Y, et al. Berberine inhibits androgen synthesis by interaction with aldo-keto reductase 1C3 in 22Rv1 prostate cancer cells. Asian J Androl. 2016;18(4):607–12. doi: 10.4103/1008-682X.169997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gali-Muhtasib H, Hmadi R, Kareh M, et al. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis. 2015;20(12):1531–62. doi: 10.1007/s10495-015-1169-2. [DOI] [PubMed] [Google Scholar]
- 11.Ma W, Zhu M, Zhang D, et al. Berberine inhibits the proliferation and migration of breast cancer ZR-75-30 cells by targeting Ephrin-B2. Phytomedicine. 2017;25:45–51. doi: 10.1016/j.phymed.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Xie J, Xu Y, Huang X, et al. Berberine-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species generation and mitochondrial-related apoptotic pathway. Tumour Biol. 2015;36(2):1279–88. doi: 10.1007/s13277-014-2754-7. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Wu B, Li D, et al. Knockdown of astrocyte elevated gene-1 inhibits tumor growth and modifies microRNAs expression profiles in human colorectal cancer cells. Biochem Biophys Res Commun. 2014;444(3):338–45. doi: 10.1016/j.bbrc.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Kochanek DM, Wells DG. CPEB1 regulates the expression of MTDH/AEG-1 and glioblastoma cell migration. Mol Cancer Res. 2013;11(2):149–60. doi: 10.1158/1541-7786.MCR-12-0498. [DOI] [PubMed] [Google Scholar]
- 15.Wan L, Kang Y. Chapter four pleiotropic roles of AEG-1/MTDH/LYRIC in breast cancer. Adv Cancer Res. 2013;120(120):113–34. doi: 10.1016/B978-0-12-401676-7.00004-8. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar D, Park ES, Emdad L, et al. Molecular basis of nuclear factor-κB activation by astrocyte elevated gene-1. Cancer Res. 2008;68(5):1478–84. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- 17.Thirkettle HJ, Girling J, Warren AY, et al. LYRIC/AEG-1 is targeted to different subcellular compartments by ubiquitinylation and intrinsic nuclear localization signals. Clin Cancer Res. 2009;15(9):3003–13. doi: 10.1158/1078-0432.CCR-08-2046. [DOI] [PubMed] [Google Scholar]
- 18.Yu C, Chen K, Zheng H, et al. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30(5):894–901. doi: 10.1093/carcin/bgp064. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Kong X, Li X, et al. Knockdown of metadherin inhibits angiogenesis in breast cancer. Int J Oncol. 2015;46(6):2459–66. doi: 10.3892/ijo.2015.2973. [DOI] [PubMed] [Google Scholar]
- 20.Zhou CX, Wang CL, Yu AL, et al. MiR-630 suppresses breast cancer progression by targeting metadherin. Oncotarget. 2016;7(2):1288–99. doi: 10.18632/oncotarget.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang N, Wang X, Huo Q, et al. The oncogene metadherin modulates the apoptotic pathway based on the tumor necrosis factor superfamily member TRAIL (Tumor Necrosis Factor-related Apoptosis-inducing Ligand) in breast cancer. J Biol Chem. 2013;288(13):9396–407. doi: 10.1074/jbc.M112.395913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szasz AM, Lanczky A, Nagy A, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7(31):49322–33. doi: 10.18632/oncotarget.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashim D, Boffetta P, La Vecchia C, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol. 2016;27(5):926–33. doi: 10.1093/annonc/mdw027. [DOI] [PubMed] [Google Scholar]
- 24.Yu C, Liu Y, Qin Z. Metadherin contributes to epithelial-mesenchymal transition and paclitaxel resistance induced by acidic extracellular pH in nasopharyngeal carcinoma. Oncol Lett. 2018;15(3):3858–63. doi: 10.3892/ol.2018.7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YX, Pang WQ, Zeng QX, et al. Synthesis and biological evaluation of new berberine derivatives as cancer immunotherapy agents through targeting IDO1. Eur J Med Chem. 2018;143:1858–68. doi: 10.1016/j.ejmech.2017.10.078. [DOI] [PubMed] [Google Scholar]
- 26.Fan LX, Liu CM, Gao AH, et al. Berberine combined with 2-deoxy-d-glucose synergistically enhances cancer cell proliferation inhibition via energy depletion and unfolded protein response disruption. Biochim Biophys Acta. 2013;1830(11):5175–83. doi: 10.1016/j.bbagen.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Haug S, Schnerch D, Halbach S, et al. Metadherin exon 11 skipping variant enhances metastatic spread of ovarian cancer. Int J Cancer. 2015;136(10):2328–40. doi: 10.1002/ijc.29289. [DOI] [PubMed] [Google Scholar]
- 28.Dong R, Liu X, Zhang Q, et al. miR-145 inhibits tumor growth and metastasis by targeting metadherin in high-grade serous ovarian carcinoma. Oncotarget. 2014;5(21):10816–29. doi: 10.18632/oncotarget.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen C, Wu L, Fu L, et al. Berberine enhances the anti-tumor activity of tamoxifen in drug-sensitive MCF-7 and drug-resistant MCF-7/TAM cells. Mol Med Rep. 2016;14(3):2250–56. doi: 10.3892/mmr.2016.5490. [DOI] [PubMed] [Google Scholar]
- 30.Groeger AM, Esposito V, De Luca A, et al. Prognostic value of immunohistochemical expression of p53, bax, Bcl-2 and Bcl-xL in resected non-small-cell lung cancers. Histopathology. 2004;44(1):54–63. doi: 10.1111/j.1365-2559.2004.01750.x. [DOI] [PubMed] [Google Scholar]
- 31.Sabzichi M, Mohammadian J, Mohammadi M, et al. Vitamin D-loaded nanostructured lipid carrier (NLC): A new strategy for enhancing efficacy of doxorubicin in breast cancer treatment. Nutr Cancer. 2017;69(6):840–48. doi: 10.1080/01635581.2017.1339820. [DOI] [PubMed] [Google Scholar]
- 32.Su K, Hu P, Wang X, et al. Tumor suppressor berberine binds VASP to inhibit cell migration in basal-like breast cancer. Oncotarget. 2016;7(29):45849–62. doi: 10.18632/oncotarget.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]