Abstract
Background
Preclinical and clinical studies have shown that the extract of Cynanchum paniculatum (bunge) kitag and the fukeqianjin formulation have beneficial effects in pelvic inflammatory disease (PID). This study aimed to compare the effects of Cynanchum paniculatum and fukeqianjin with a new decoction, xiaoyuningkun, consisting of Melia toosendan, Angelica biserrata, and Cynanchum paniculatum, in a mouse model of PID.
Material/Methods
The mouse model of PID included injection of the upper genital tract with hydrochloric acid (HCl) and lipopolysaccharide (LPS). The control group underwent sham treatment with 0.9% physiological saline. Cynanchum paniculatum, fukeqianjin, and xiaoyuningkun decoction were administered orally for 15 days. Acetic acid-induced writhing and thermal nociception hot plate tests evaluated the analgesic effects of treatment. Mouse uterus and Fallopian tubes were examined histologically to evaluate the degree of inflammation. Immunohistochemistry was used to measure the protein expression of intercellular adhesion molecule-1 (ICAM-1) and vascular endothelial growth factor (VEGF). Enzyme-linked immunosorbent assay (ELISA) measured serum levels of inflammatory cytokines.
Results
Treatment with xiaoyuningkun decoction significantly reduced the pain threshold in the mouse model of PID and the degree of inflammation in the uterus and Fallopian tubes compared with Cynanchum paniculatum and fukeqianjin. Cynanchum paniculatum decoction significantly reduced the serum levels of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), ICAM-1, and VEGF, and the expression of ICAM-1 and VEGF in the mouse uterus and Fallopian tubes.
Conclusions
The new xiaoyuningkun decoction had analgesic and anti-inflammatory effects in the mouse model of PID, possibly by inhibiting ICAM-1, VEGF, and inflammatory cytokines.
MeSH Keywords: Analgesics, Inflammation, Pelvic Inflammatory Disease
Background
Pelvic inflammatory disease (PID) is due to infection of the upper genital tract in women and includes endometritis, salpingitis, and peritonitis [1]. PID can have serious complications, including chronic pelvic pain, infertility, endometriosis, and ectopic pregnancy [2]. The clinical findings of PID on examination include adnexal tenderness, pain on moving the cervix, and uterine tenderness on bimanual pelvic examination [3]. In PID, inflammatory cells (neutrophils and lymphocytes) infiltrate the upper genital tract and can be detected in endometrial biopsy samples [4]. Inflammation is associated with the release of proinflammatory cytokines, including interleukin-1β (IL-1β) and IL-6 [5]. Currently, clinical treatment of PID includes the use of antibiotics and hormones, which may not always be effective and may be associated with side effects that include antimicrobial resistance [6]. Therefore, alternative or adjunctive treatment for PID or the symptoms of PID, such as pain, which include natural compounds with fewer side effects require further study.
Decoction is one of the most widely used treatments in traditional Chinese medicine (TCM), which has been practiced in China for more than 3,000 years and is also widely practiced in other Asian nations. The effective ingredients are extracted from the components of the decoction and the quality of the final decoction depends on the method of extraction. For the first time, this study reports the effects of a new decoction, xiaoyuningkun decoction, consisting of Melia toosendan, Angelica biserrata, and Cynanchum paniculatum (Bunge) Kitagawa, which have possible antimicrobial and anti-inflammatory activities. Preclinical and clinical studies have shown that extracts of Cynanchum paniculatum and the fukeqianjin formulation have beneficial effects in pelvic inflammatory disease, and Melia toosendan shows antimicrobial activity and modulates the activation of nuclear factor-κB (NF-κB), a proinflammatory signaling protein [7,8]. The compounds in Cynanchum paniculatum have previously been shown to reduce the production of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β in mice pulmonary microvascular endothelial cells studied in vitro [9]. C57BL/6J mice have previously been studied as an animal model of chronic pelvic pain, where they have shown increased expression levels of inflammatory cytokines and adhesion molecules [10]. Therefore, in this study, female C57BL/6J mice were used to establish the mouse model of PID, as previously described [11].
Cynanchum paniculatum is the principal component of xiaoyuningkun decoction. However, the pharmacological effects of this new decoction in PID have not previously been reported. Therefore, this study aimed to use a C57BL/6J mouse model of PID to compare the effects of Cynanchum paniculatum and fukeqianjin with the xiaoyuningkun decoction that consists of Cynanchum paniculatum, Melia toosendan, and Angelica biserrata.
Material and Methods
Material and reagents
Lipopolysaccharide (LPS) and pregnant mare serum gonadotropin (PMSG) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Hydrochloric acid (HCl) was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from HyClone (Logan, UT, USA). The enzyme-linked immunosorbent assay (ELISA) kit for interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), ICAM-1, and vascular endothelial growth factor (VEGF) was obtained from R&D Systems (Minneapolis, MN, USA).
Animals
Female C57BL/6J mice, weighing between 18–20 gm, were purchased from Shanghai SLRC Laboratory Animal Co., Ltd. (Shanghai, China). The mice were housed in standard conditions with a 12-hourly dark and light cycles, at a temperature of 22–24°C, and relative humidity of 60–65%. The experimental procedures were approved by the Ethics Committee for Animal Experimentation of Shanghai Gongli Hospital. All experimental procedures were approved by the Guidelines for Animal Experimentation of Shanghai Gongli Hospital.
Preparation of the xiaoyuningkun decoction and the Cynanchum paniculatum decoction
The xiaoyuningkun decoction, consisting of Melia toosendan, Angelica biserrata, and Cynanchum paniculatum (Bunge) Kitagawa, is a new decoction that was prepared from 12 gm of Melia toosendan, 12 gm of Angelica biserrata, and 15 gm of Cynanchum paniculatum. Distilled water was added at 10 times the volume of the compound, which was then soaked for 20 minutes, boiled, and decocted at low temperature for 20 min. The decoction was homogenized and concentrated by heating until the concentration of the crude compound was 1.5 g/mL. For the Cynanchum paniculatum decoction, 50 g of Cynanchum paniculatum was soaked in 10 times the volume of distilled water and decocted by heating until the concentration of the crude drug was 1.5 g/mL. The two concentrated solutions were stored at 4°C.
The mouse model of pelvic inflammatory disease (PID)
The mouse model of PID was established as previously described [12]. Briefly, the mice were anesthetized with an intraperitoneal injection of 3% pentobarbital sodium (50 mg/kg). The uterus was exposed with an incision along the midline of the abdomen, and a needle was inserted into the uterine cavity longitudinally, followed by administration of hydrochloric acid (HCl) (1N). Four doses of lipopolysaccharide (LPS) (50 mg/kg) were injected intraperitoneally starting at 2 hours after the injection of HCl with one dose every two hours. In the PID groups, pregnant mare’s serum gonadotropin (PMSG) (7.5 IU per mouse) was administered by intraperitoneal injection two days before the proprioception experiments. Mice that received the sham operation and 0.9% physiological saline represented the control group. From an initial 76 mice, 16 mice died within 48 h after induction of PID induction, which left 60 mice, which were divided into five study groups.
Experimental design and study groups
Female C57BL/6J mice (N=60) were randomly divided into five groups: the PID group (N=12) underwent induction of PID and received gavage of 0.9% normal saline; the fukeqianjin group (N=12) underwent induction of PID and received gavage of fukeqianjin; the xiaoyuningkun decoction group (N=12) underwent induction of PID and received gavage of xiaoyuningkun decoction (1.5 gm) for 15 days; the Cynanchum paniculatum group (N=12) underwent induction of PID and received gavage of Cynanchum paniculatum decoction for 15 days; the control group (N=12) underwent sham operation and received gavage with 0.9% normal saline.
Acetic acid-induced writhing test
Forty mice were randomly divided into four groups and received physiological saline, indomethacin (2 mg/kg), Xiaoyuningkun decoction, and Cynanchum paniculatum decoction. On the 4th day, the mice were administrated 0.7% acetic acid by intraperitoneal injection (10 mL/kg), and their writhe reactions were observed, and the number of writhes was counted and recorded within 20 min [13]. The analgesic percentage was calculated as follows:
Inhibition rate (%)=number of writhes (control)–number of writhes (treated)/number of writhes (control)×100%.
Thermal nociception hot plate test
After administration of physiological saline, indomethacin (2 mg/kg), xiaoyuningkun decoction, and Cynanchum paniculatum decoction for four days, the female mice underwent the thermal nociception hot plate test (55.5±0.5°C). The antinociceptive effects were evaluated by the activities of lifting, licking the hind paws, and jumping. The pain threshold was defined as the duration before the mice first exhibited one of the antinociceptive indicators after staying on the hot plate (latency period), with the maximal duration of 30 seconds. The pain threshold of the mice in each group was measured at 30, 60, 90, and 120 min after the oral administration of indomethacin, xiaoyuningkun decoction, or Cynanchum paniculatum decoction [13].
Histology of the mouse tissues
After oral administration of xiaoyuningkun decoction for 15 days, the mice in the model of PID were euthanized by cervical dislocation, and the left half of the uterus and the left Fallopian tube were fixed in 4% paraformaldehyde for 4 hours, paraffin-embedded, sectioned at 2 μm, stained with hematoxylin and eosin (H&E), and examined by light microscopy. The inflammatory cells in the mouse tissue were semi-quantified histologically at high-power magnification (×100), randomly from three areas on each slide, as previously described [14]. The degree of inflammation in the uterus and Fallopian tube of the mice in each study group was graded from 0–3.
Immunohistochemistry
The right half of the uterus and right Fallopian tube of mice in each study group were fixed in 4% paraformaldehyde for 4 hours, paraffin-embedded, and sectioned at 2 μm onto glass slides. The tissue sections were deparaffinized and rehydrated. Following antigen retrieval, the tissue sections were incubated in 3% H2O2 to block endogenous peroxidase activity. The tissue sections were incubated with primary antibodies to ICAM-1 and VEGF (1: 100) overnight at 4°C. On the following day, the tissue sections were incubated with biotinylated horseradish-peroxidase (HRP)-conjugated secondary antibody (1: 100) at room temperature for 1 h, and stained with diaminobenzidine (DAB), and counterstained with hematoxylin. The immunostained tissue sections were examined by light microscopy. Five high-power fields were randomly selected for photomicroscopy and image analysis. Image-Pro Plus software was used to calculate the integrated optical density (IOD) values of ICAM-1 and VEGF immunostaining, which was normalized to the controls [14].
Enzyme-linked immunosorbent assay (ELISA)
Serum samples from the mice in each study group were collected, weighed, and mixed with physiologic saline at a ratio of 1: 5 (w/v) and then mixed. The levels of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), intercellular adhesion molecule-1 (ICAM-1) and vascular endothelial growth factor (VEGF) were measured using ELISA kits (Nanjing Jiancheng, Nanjing, China), according to the manufacturer’s instructions.
Statistical analysis
Data were presented as the mean±standard error of measurement (SEM), and analyzed using SPSS version 18.0 statistical software (IBM, Chicago, IL, USA). The differences between groups were evaluated using one-way analysis of variance (ANOVA), followed by Bonferroni’s post hoc analysis for comparison between groups. A P-values <0.05 was considered to indicate a statistically significant difference.
Results
Treatment with xiaoyuningkun decoction reduced inflammation in the mouse model of pelvic inflammatory disease (PID)
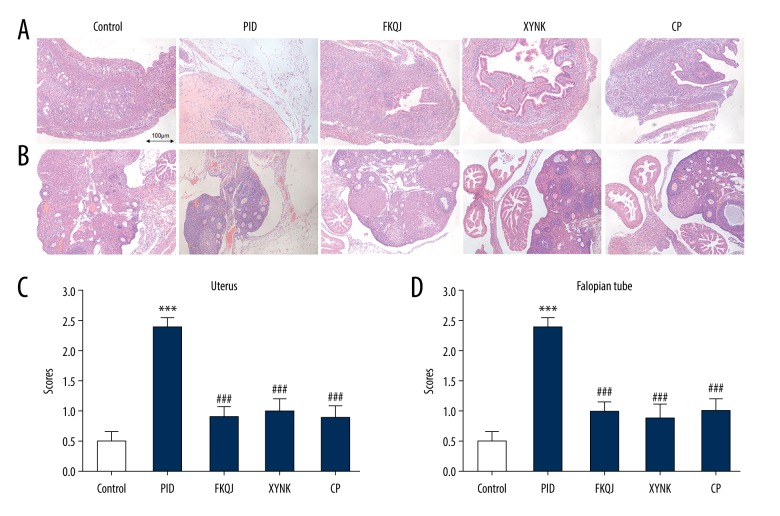
The xiaoyuningkun decoction, consisting of Melia toosendan, Angelica biserrata, and Cynanchum paniculatum (Bunge) Kitagawa, was a new decoction that was prepared for this study. Histopathology of the uterus and Fallopian tissue of the mice in each study group was performed after 15 days of treatment. In the PID group, inflammatory cells, consisting of neutrophils and lymphocytes, were observed histologically to infiltrate the uterus and Fallopian tubes, supporting that an inflammatory response was present in the upper genital tract. Compared with the PID group, the fukeqianjin, xiaoyuningkun decoction, and Cynanchum paniculatum groups all showed significantly reduced degrees of inflammation in the uterus and Fallopian tube (Figure 1).
Figure 1.
(A–D) The effect of xiaoyuningkun decoction on inflammation of upper genital tract in the mouse model of pelvic inflammatory disease (PID). (A) Photomicrograph of representative areas of the mouse uterus. Hematoxylin and eosin (H&E). Magnification ×100. (B) Photomicrograph of representative areas of the mouse Fallopian tube. H&E. Magnification ×100. Histological semi-quantitative scores of inflammatory cells, including neutrophils and lymphocytes. Each bar represents the mean±SEM (N=12). One-way analysis of variance (ANOVA) followed with Bonferroni’s post hoc test were performed. *** P<0.001 vs. control group; ### P<0.001 vs. the PID group. PID – pelvic inflammatory disease; FKQJ – fukeqianjin formulation; XYNK – xiaoyuningkun decoction; CP – Cynanchum paniculatum.
The xiaoyuningkun decoction inhibited the inflammatory response in the mouse model of PID
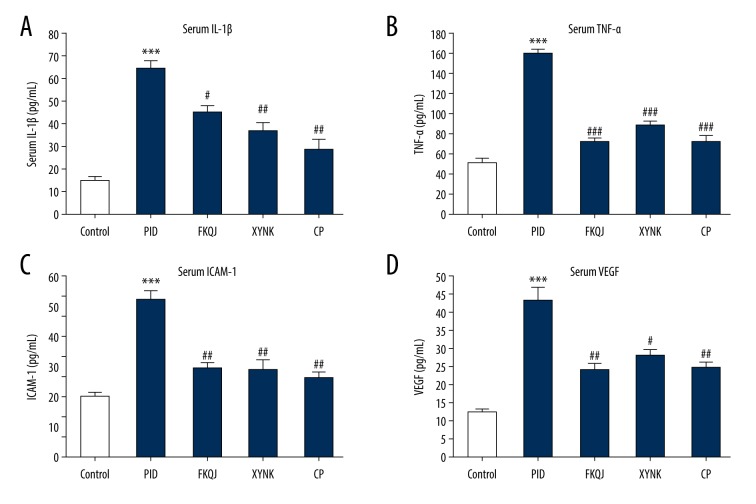
Enzyme-linked immunosorbent assay (ELISA) showed that serum levels of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), intercellular adhesion molecule-1 (ICAM-1), and vascular endothelial growth factor (VEGF) in the mouse model of PID were significantly increased compared with the control group, and that treatment with fukeqianjin, the xiaoyuningkun decoction, and Cynanchum paniculatum significantly inhibited the production of these cytokines in the mouse model (Figure 2A–2D). These results indicated that there was an inflammatory response in the mouse model of PID and that the xiaoyuningkun decoction had anti-inflammatory effects. The findings also indicated that the xiaoyuningkun decoction might also suppress cell adhesion and angiogenesis in the uterus and Fallopian tube of the mouse model of PID.
Figure 2.
The effect of xiaoyuningkun decoction on the inflammatory response in the mouse model of pelvic inflammatory disease (PID). Enzyme-linked immunosorbent assay (ELISA) shows that xiaoyuningkun decoction reversed the increase in serum levels of interleukin-1β (IL-1β) (A), tumor necrosis factor-α (TNF-α) (B), intercellular adhesion molecule-1 (ICAM-1) (C), and vascular endothelial growth factor (VEGF) (D) in the upper genital tract of the mouse model of PID. Data are presented as the means±standard error (N=12). One-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test were performed. *** P<0.001 vs. the control group; # P<0.05, ## P<0.01, ### P<0.001 vs. the PID group.
The xiaoyuningkun decoction reduced pain perception in the mouse model of PID
The analgesic effects of xiaoyuningkun decoction were evaluated using the writhing test and hot plate test. Mice received physiological saline, indomethacin (2 mg/kg), xiaoyuningkun decoction, and Cynanchum paniculatum decoction for 4 days. Compared with the control group, the number of writhes in the mice treated with indomethacin, xiaoyuningkun decoction, and Cynanchum paniculatum decoction were significantly reduced (P<0.001), and the inhibition rates in the xiaoyuningkun decoction and Cynanchum paniculatum groups (41.8%, 36.3%) were all lower than that in the indomethacin-treated group (53.9%) (Table 1). These findings indicated that xiaoyuningkun decoction and its main component, Cynanchum paniculatum, had an analgesic effect in the mouse model of PID.
Table 1.
The effects of xiaoyuningkun decoction on the acetic acid-induced writhing test in the mouse model of pelvic inflammatory disease (PID).
| Groups | Number of writhes | Inhibition (%) |
|---|---|---|
| Control | 47.9±1.65 | – |
| Indomethacin | 21.8±1.91*** | 53.9±4.43 |
| PID-XYNK | 27.6±0.99*** | 41.8±2.70 |
| PID-CP | 30.3±1.10*** | 36.3±2.74 |
Each value is shown as the mean±SEM (n=10).
P<0.001 vs. the control group.
PID – pelvic inflammatory disease; FKQJ – fukeqianjin formulation; XYNK – xiaoyuningkun decoction; CP – Cynanchum paniculatum decoction.
The hot plate test was performed to evaluate the pain threshold of the mice for heat. Compared with the control group, the pain thresholds (latency period) in the model groups were all significantly increased by indomethacin, xiaoyuningkun decoction, and Cynanchum paniculatum at different time points (P<0.001) (Table 2). These results indicated that xiaoyuningkun decoction and its main component, Cynanchum paniculatum, could significantly prolong the time standing on a hot plate and could improve the heat tolerance of the mice, and provided an analgesic effect.
Table 2.
Effects of xiaoyuningkun decoction on the hot plate test in the mouse model of pelvic inflammatory disease (PID).
| Groups | Latency period at different time points | |||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | |
| Control | 3.25±0.13 | 3.42±0.17 | 3.47±0.13 | 3.54±0.23 |
| Indomethacin | 4.64±0.24*** | 4.69±0.28** | 4.87±0.24*** | 5.08±0.21*** |
| XYNK | 4.06±0.31* | 4.54±0.29** | 5.32±0.24*** | 5.78±0.30*** |
| CP | 3.87±0.10** | 4.37±0.20** | 5.33±0.23*** | 6.15±0.23*** |
Each value is presented as the mean±SEM (n=10).
P<0.05,
P<0.01,
P<0.001.
P<0.001 vs. control group.
PID – pelvic inflammatory disease; FKQJ – fukeqianjin formulation; XYNK – Xiaoyuningkun decoction; CP – Cynanchum paniculatum decoction.
The xiaoyuningkun decoction reduced the expression of ICAM-1 and VEGF in the mouse model of PID
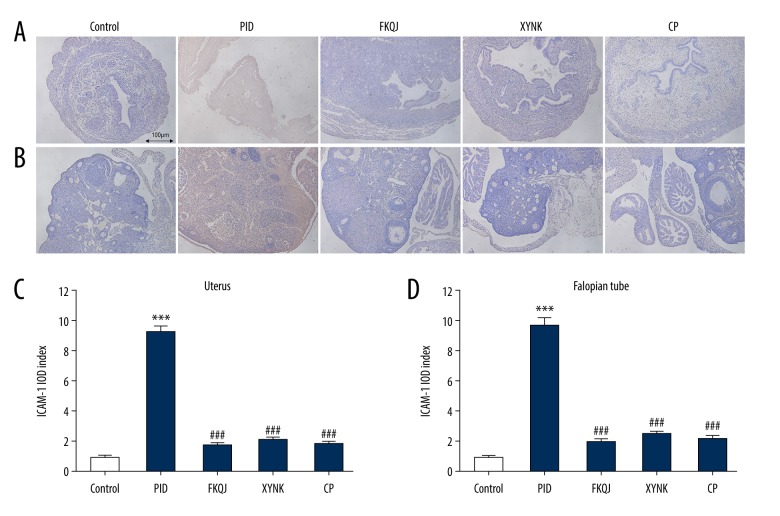
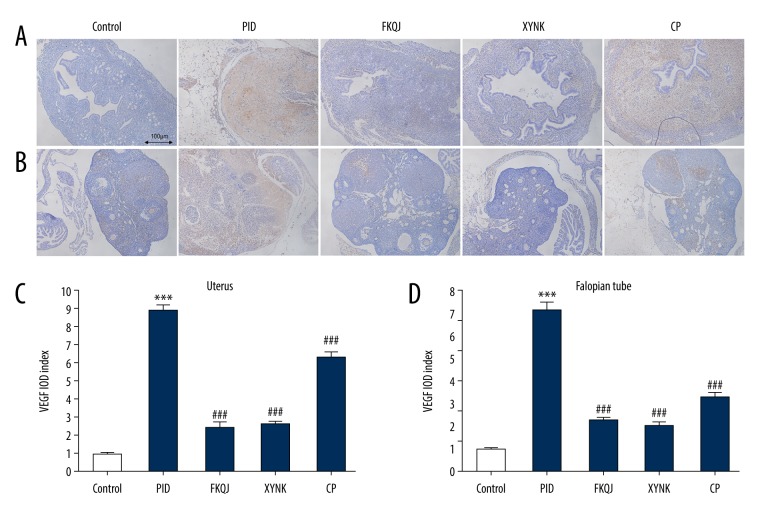
To investigate the detailed mechanisms underlying analgesic and anti-inflammatory effects induced by xiaoyuningkun decoction, immunohistochemistry was performed to measure the protein expression of ICAM-1 and VEGF. ICAM-1 expression was significantly increased in the mouse model of PID. However, mice in xiaoyuningkun decoction group, and the Cynanchum paniculatum group showed significantly reduced immunostaining for ICAM-1 in the uterus and Fallopian tube (Figure 3A, 3B). The semi-quantitative analysis showed that the ICAM-1 expression was significantly reduced by treatment with xiaoyuningkun decoction and Cynanchum paniculatum decoction (Figure 3C, 3D). VEGF expression was increased in the uterus and Fallopian tube of the mouse model of PID, but was significantly reduced in the uterus and Fallopian tube of the mice treated with xiaoyuningkun decoction and Cynanchum paniculatum (Figure 4). Also, treatment with xiaoyuningkun decoction reversed the increase in VEGF expression, but its main component, Cynanchum paniculatum, could only partially reverse the increased expression levels of VEGF, particularly in the mouse uterus. These findings indicate that other components in the xiaoyuningkun decoction might also have had an antiangiogenic effect in the mouse model of PID.
Figure 3.
Effect of xiaoyuningkun decoction on the expressions of intercellular adhesion molecule-1 (ICAM-1) in the mouse model of pelvic inflammatory disease (PID). Representative photomicrographs of the histology of the immunohistochemistry findings in the mouse uterus (A) and Fallopian tube (B). Magnification ×100. Semi-quantitative evaluation of ICAM-1 expression is represented by the integrated optical density (IOD) index for the mouse uterus (C) and Fallopian tube (D). Each bar represents the mean±SEM (N=12). One-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test were performed. *** P<0.001 vs. the control group; ### P<0.001 vs. the PID group.
Figure 4.
The effect of xiaoyuningkun decoction on the expressions of vascular endothelial growth factor (VEGF) in the mouse model of pelvic inflammatory disease (PID). Representative photomicrographs of the histology of the immunohistochemistry findings in the mouse uterus (A) and Fallopian tube (B). Magnification ×100. Semi-quantitative evaluation of VEGF expression is represented by the integrated optical density (IOD) index for the mouse uterus (C) and Fallopian tube (D). Each bar represents the mean±SEM (N=12). One-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test were performed. *** P<0.001 vs. the control group; ### P<0.001 vs. the PID group.
Discussion
For the first time, this study reported the effects of the xiaoyuningkun decoction, consisting of Melia toosendan, Angelica biserrata, and Cynanchum paniculatum (Bunge) Kitagawa, on inflammation and pain perception in a mouse model of pelvic inflammatory disease (PID). The mouse model of PID was developed by injection of the upper genital tract with hydrochloric acid (HCl) and lipopolysaccharide (LPS). The results showed that treatment of the mice with PID with an oral preparation of xiaoyuningkun decoction significantly reduced inflammation in the mouse uterus and Fallopian tube and also had an analgesic effect by improving the pain threshold when compared with the non-treated controls and with Cynanchum paniculatum and fukeqianjin alone. Xiaoyuningkun decoction also reduced the serum levels of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), intercellular adhesion molecule-1 (ICAM-1), and vascular endothelial growth factor (VEGF) in the mouse model of PID. Xiaoyuningkun decoction treatment reduced ICAM-1 and VEGF expression in the mouse uterus and Fallopian tube. The findings from this study showed that xiaoyuningkun decoction has significant analgesic and anti-inflammatory activity in the mouse model of PID.
The mice in the model of PID developed inflammation of the upper genital tract, consisting of neutrophils and lymphocytes, which could be quantified by light microscopy. Clinically, patients with PID show inflammation of the upper genital tract following infection, which includes the recruitment of neutrophils and lymphocytes from the circulation that cross the endothelial barrier [15]. In the present study, the mouse model of PID was developed using an injection of the upper genital tract with hydrochloric acid (HCl) and lipopolysaccharide (LPS). A recent study showed that LPS promoted neutrophil infiltration into the endometrium of a mouse model of endometritis [16], which is consistent with the findings in the present study. Neutrophils at the site of infection release inflammatory cytokines, reactive oxygen species (ROS), and proteolytic enzymes, which have bactericidal effects. However, the effects of inflammation will also cause damage to the tissue of the upper genital tract. In chronic PID, chronic inflammation that includes lymphocyte infiltration is a response to infection [14]. This finding is supported the significantly increased neutrophil to lymphocyte ratio (NLR) before treatment compared with that after treatment in PID [17]. Tissue damage is also associated with lymphocyte infiltration of tissues [18]. Therefore, following treatment of the infectious cause of PID, approaches to reduce neutrophils and lymphocytes in the upper genital tract may reduce tissue damage that can result in infertility [19]. Chronic pelvic pain is the main symptom of PID [20], and natural treatments that reduce pain may have a role in the management of women with PID.
In the present study, the mice in the model of PID showed significantly increased serum levels of IL-1β and TNF-α, which were reduced following treatment with xiaoyuningkun decoction. IL-1β and TNF-α are two major proinflammatory cytokines, and play essential roles in initiating and propagating the inflammatory response following bacterial infection [21]. Clinically, in patients with PID, serum levels of IL-1β and TNF-α are significantly increased before antibiotic treatment, and the levels decrease after treatment [5]. These proinflammatory cytokines promote the proliferation and activation of leukocytes in the upper genital tract and enhance immune cell recruitment. Recruited neutrophils and lymphocytes release inflammatory cytokines and chemokines to enhance the inflammatory response [22]. Also, IL-1β promotes the survival and function of neutrophils [23], which release inflammatory cytokines and chemokines, to further increase the inflammatory response. In this study, increased levels of IL-1β and TNF-α in the serum of mice in the model of PID were significantly reduced after treatment with xiaoyuningkun decoction.
Treatment of the mice in the model of PID showed that xiaoyuningkun decoction reduced the serum levels and tissue levels of ICAM-1 and VEGF. ICAM-1 is an adhesion molecule that stimulates leukocyte adhesion and transmigration in response to proinflammatory cytokines [24]. Clinically, patients with PID have an increased risk of developing endometriosis [25], and when compared with healthy women, they have significantly higher serum and peritoneal fluid ICAM-1 levels [26]. ICAM-1 expression can be induced by TNF-α in human endometriotic stromal cells, thus modulating cell proliferation, adhesion, activation, motility, and implantation [27]. Further studies are required to investigate the effects of xiaoyuningkun decoction on the expression of cell adhesion molecules such as ICAM-1 and cell adhesion.
VEGF is a proinflammatory factor that is associated with angiogenesis and neovascularization. The danzhi decoction formulation contains Morus alba L., Salvia miltiorrhiza Bge., Dipsacus asper Wall, Liguisticum chuanxiong Hort., Litchi chinensis Sonn., Forsythia suspensa Vahl, Corydalis turtschaninovii, and Cyperus rotundus L. [28]. The findings from a previously published study showed that in the upper genital tract in a mouse model of PID, treatment with danzhi decoction accelerated pelvic blood flow and reduced the expression of VEGF [28]. Vascular endothelial injury in PID is associated with increased expression of VEGF [29]. The findings from the present study showed increased of VEGF levels in serum, uterus, and Fallopian tube of the mouse model of PID, which was developed using LPS treatment. A previously published study in a mouse model of LPS-induced inflammation also showed increased expression of VEGF mRNA [30]. Further studies are required to investigate the effects of xiaoyuningkun decoction on the endothelium in inflammation.
In the mouse model of PID, used in the present study, inflammation was induced chemically and did not follow infection. This model was used in a preliminary study to investigate the effects of xiaoyuningkun decoction. The fukeqianjin formulation was used as a positive control, and Cynanchum paniculatum was also studied as it is the main component of xiaoyuningkun decoction. The findings showed that xiaoyuningkun decoction completely reversed the increase in VEGF in the mouse model of PID, but its main compound, Cynanchum paniculatum, only partially reversed the increase in VEGF in the mouse uterus. This finding may indicate that there may be components in the xiaoyuningkun decoction that have an inhibitory effect on VEGF expression and angiogenesis, which requires further study.
Conclusions
There have been no previously published studies on the effects of the new xiaoyuningkun decoction, which is prepared from Melia toosendan, Angelica biserrata, and Cynanchum paniculatum (Bunge) Kitagawa. In a mouse model of pelvic inflammatory disease (PID), when compared with xiaoyuningkun decoction showed significant analgesic and anti-inflammatory effects, reduced neutrophil and lymphocyte infiltration, and inhibited the production of cytokines and the expression of vascular endothelial growth factor (VEGF). Cynanchum paniculatum, the main component of xiaoyuningkun decoction showed similar effects. Further studies are needed to evaluate and compare the effects of Cynanchum paniculatum and xiaoyuningkun decoction.
Footnotes
Source of support: This study was supported by the Key Subjects of Traditional Chinese Medicine Gynecology in Pudong New District (No. PWZxk2017-03) and a Project of Discipline Leader in Pudong New District Health Bureau (grant no. PWRD2016-03)
References
- 1.Soper DE. Pelvic inflammatory disease. Obstet Gynecol. 2010;116(2 Pt 1):419–28. doi: 10.1097/AOG.0b013e3181e92c54. [DOI] [PubMed] [Google Scholar]
- 2.Chayachinda C, Rekhawasin T. Reproductive outcomes of patients being hospitalised with pelvic inflammatory disease. J Obstet Gynaecol. 2017;37(2):228–32. doi: 10.1080/01443615.2016.1234439. [DOI] [PubMed] [Google Scholar]
- 3.Risser WL, Risser JM, Risser AL. Current perspectives in the USA on the diagnosis and treatment of pelvic inflammatory disease in adolescents. Adolesc Health Med Ther. 2017;8:87–94. doi: 10.2147/AHMT.S115535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vicetti Miguel RD, Chivukula M, Krishnamurti U, et al. Limitations of the criteria used to diagnose histologic endometritis in epidemiologic pelvic inflammatory disease research. Pathol Res Pract. 2011;207(11):680–85. doi: 10.1016/j.prp.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SA, Tsai HT, Ou HC, et al. Plasma interleukin-1beta, -6, -8 and tumor necrosis factor-alpha as highly informative markers of pelvic inflammatory disease. Clin Chem Lab Med. 2008;46(7):997–1003. doi: 10.1515/CCLM.2008.196. [DOI] [PubMed] [Google Scholar]
- 6.Mari G, Iacono E, Toni F, et al. Evaluation of the effectiveness of intrauterine treatment with formosulphathiaxole of clinical endometritis in postpartum dairy cows. Theriogenology. 2012;78(1):189–200. doi: 10.1016/j.theriogenology.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Zhao K, Li J, Shen M, et al. Actinobacteria associated with Chinaberry tree are diverse and show antimicrobial activity. Sci Rep. 2018;8(1):11103. doi: 10.1038/s41598-018-29442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu GY, Bai LP, Liu L, Jiang ZH. Limonoids from the fruits of Melia toosendan and their NF-κB modulating activities. Phytochemistry. 2014;107:175–81. doi: 10.1016/j.phytochem.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Wei P, Zhang T, Dong H, et al. Anti-inflammatory and antiviral activities of cynanversicoside A and cynanversicoside C isolated from Cynanchun paniculatum in influenza A virus-infected mice pulmonary microvascular endothelial cells. Phytomedicine. 2017;36:18–25. doi: 10.1016/j.phymed.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Breser ML, Motrich RD, Sanchez LR, Rivero VE. Chronic pelvic pain development and prostate inflammation in strains of mice with different susceptibility to experimental autoimmune prostatitis. Prostate. 2017;77:94–104. doi: 10.1002/pros.23252. [DOI] [PubMed] [Google Scholar]
- 11.Oh Y, Kwon YS, Jung BD. Anti-inflammatory effects of the natural compounds cortex Phellodendri and Humulus japonicus on pelvic inflammatory disease in mice. Int J Med Sci. 2017;14:729–34. doi: 10.7150/ijms.19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh Y, Lee J, Kim HC, et al. Establishment of HCl-LPS-induced pelvic inflammatory disease model. J Vet Sci. 2016;17(3):413–19. doi: 10.4142/jvs.2016.17.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G, Hu Z, Song X, et al. Analgesic and anti-inflammatory activities of resveratrol through classic models in mice and rats. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/5197567. 5197567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou W, Xiao Z, Wen X, et al. The anti-inflammatory effect of Andrographis paniculata (Burm. f.) Nees on pelvic inflammatory disease in rats through down-regulation of the NF-κB pathway. BMC Complement Altern Med. 2016;16(1):483. doi: 10.1186/s12906-016-1466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebordão M, Carneiro C, Alexandre-Pires G, et al. Neutrophil extracellular traps formation by bacteria causing endometritis in the mare. J Reprod Immunol. 2014;106:41–49. doi: 10.1016/j.jri.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Lv X, Fu K, Li W, et al. TIIA attenuates LPS Induced mouse endometritis by suppressing the NF-κB signaling pathway. Can J Physiol Pharmacol. 2015;93(11):967–71. doi: 10.1139/cjpp-2015-0003. [DOI] [PubMed] [Google Scholar]
- 17.Akopuz A, Turan V, Ozcan A, et al. A novel marker for the assessment of the treatment result in pelvic inflammatory disease. Minerva Ginecol. 2016;68(2):117–23. [PubMed] [Google Scholar]
- 18.Patton DL, Askienazy-Elbhar M, Henry-Suchet J, et al. Detection of Chlamydia trachomatis in Fallopian tube tissue in women with postinfectious tubal infertility. Am J Obstet Gynecol. 1994;171(1):95–101. doi: 10.1016/s0002-9378(94)70084-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhao WH, Hao M. Pelvic inflammatory disease: A retrospective clinical analysis of 1,922 cases in North China. Gynecol Obstet Invest. 2014;77(3):169–75. doi: 10.1159/000358393. [DOI] [PubMed] [Google Scholar]
- 20.Chung SD, Chang CH, Hung PH, et al. Correlation between bladder pain syndrome/interstitial cystitis and pelvic inflammatory disease. Medicine. 2015;94(46):e1878. doi: 10.1097/MD.0000000000001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tortorella C, Piazzolla G, Matteo M, et al. Interleukin-6, interleukin-1β, and tumor necrosis factor α in menstrual effluents as biomarkers of chronic endometritis. Fertil Steril. 2014;101(1):242–47. doi: 10.1016/j.fertnstert.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 22.Gasperini S, Zambello R, Agostini C, et al. Impaired cytokine production by neutrophils isolated from patients with AIDS. Aids. 1998;12(4):373–79. doi: 10.1097/00002030-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Prince LR, Allen L, Jones EC, et al. The role of interleukin-1beta in direct and toll-like receptor 4-mediated neutrophil activation and survival. Am J Pathol. 2004;165(5):1819–26. doi: 10.1016/s0002-9440(10)63437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61(1):22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 25.Bedaiwy MA, Falcone T, Sharma RK, et al. Prediction of endometriosis with serum and peritoneal fluid markers: A prospective controlled trial. Hum Reprod. 2002;17(2):426–31. doi: 10.1093/humrep/17.2.426. [DOI] [PubMed] [Google Scholar]
- 26.Li R, Qiu Y. Diagnostic value of serum ICAM-1 for endometriosis: A meta-analysis. Medicine. 2018;97(31):e11760. doi: 10.1097/MD.0000000000011760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KH, Lee EN, Park JK, et al. Curcumin attenuates TNF-α-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother Res. 2012;26(7):1037–47. doi: 10.1002/ptr.3694. [DOI] [PubMed] [Google Scholar]
- 28.Bu X, Liu Y, Lu Q, Jin Z. Effects of “Danzhi Decoction” on chronic pelvic pain, hemodynamics, and proinflammatory factors in the murine model of sequelae of pelvic inflammatory disease. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/547251. 547251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhasmana D, Hathorn E, McGrath R, et al. The effectiveness of nonsteroidal anti-inflammatory agents in the treatment of pelvic inflammatory disease: A systematic review. Syst Rev. 2014;3:79. doi: 10.1186/2046-4053-3-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azuma Y, Taniguchi F, Nakamura K, et al. Lipopolysaccharide promotes the development of murine endometriosis-like lesions via the nuclear factor-kappa B pathway. Am J Reprod Immunol. 2017;77(4) doi: 10.1111/aji.12631. [DOI] [PubMed] [Google Scholar]