Abstract
Background
Depression is a major mood disorder. Some patients have been reported to improve following acupuncture. This study aimed to investigate the effects of acupuncture on behaviors associated with depression in the chronic unpredictable mild stress (CUMS) rat model. The expression of signaling pathway components of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) in the rat hippocampus and plasma were also measured.
Material/Methods
Male Sprague-Dawley rats (N=40) were divided into the control group (N=10), the model group (N=10), the acupuncture group (N=10), and the non-acupuncture group (N=10). The rat model was established by orphaning combined with chronic unpredictable mild stress (CUMS) for six weeks. The acupuncture group was given 21 days of treatment using acupoints (AP) or non-acupoints (NP). Rat behaviors associated with depression were tested using the sucrose preference test (SPT), the open field test (OFT), and the elevated plus maze (EPM) test. Enzyme-linked immunoassay (ELISA) and reverse transcription-polymerase chain reaction (RT-PCR) were used to detect the expression of inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS), and the N-methyl-D-aspartate (NMDA) receptor subunits, NR1, NR2A, and NR2B in the rat plasma and hippocampus.
Results
Acupuncture reversed the behaviors associated with depression in the CUMS rat model and reduced the expression of components of the NO and cGMP pathway in the rat hippocampus and plasma.
Conclusions
In the CUMS rat model, treatment with acupuncture reduced behaviors associated with depression, and these effects were associated with changes in the NO and cGMP signaling pathway.
MeSH Keywords: Acupuncture Therapy, Behavior and Behavior Mechanisms, Cyclic GMP-Dependent Protein Kinases, Depression, Nitric Oxide, Wnt Signaling Pathway
Background
Depression is a mood disorder characterized by a persistent depressed mood, a feeling of helplessness, and can be associated with anxiety [1]. Severe depression can be associated with symptoms of delusion, including hallucinations, and suicidal thoughts [1]. Chronic depression impairs quality of life. Currently, antidepressant drugs have variable efficacy and can have serious side effects [2]. Therefore, new and safer approaches to the treatment of depression are needed.
Acupuncture is a traditional Chinese medicine that is effective in some cases of clinical depression and has few side effects [3–5]. Acupuncture has been practiced in China for at least 3,000 years and has become of increasing interest in Western medical practice, supported by clinical research [6]. However, the mechanisms for the effects of acupuncture in the treatment of depression remain to be determined.
Previous studies have shown that the nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) signaling pathway of the central nervous system has a role in depression and other mental disorders [7,8]. The NO-cGMP signaling pathway includes inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS), and the N-methyl-D-aspartate (NMDA) receptor subunits, NR1, NR2A, and NR2B. NR1 is the essential subunit of ion channels, and NR2 is the regulatory subunit [7,8]. Functional NMDA receptors contain the NR1 subunit and two NR2 subunits, which can form the active compound [7,8]. The NMDA receptor is an excitatory receptor that binds to glutamate, resulting in the activation of the Glu-NMDA-nNOS-cGMP signaling pathway to increase levels of NO [7]. A high concentration of NO in cells can further release Glu through NO-cGMP pathway, resulting in a neuronal cytotoxic effect, reducing synaptic plasticity of neurons [7]. These changes associated with the NO-cGMP signaling pathway is associated with depression [7]. The pharmacological effects of the nitric oxide, cycloguanosine monophosphate, protein kinase G (NO-cGMP-PKG) pathway can inhibit neuronal transmission in the central nervous system [7].
Previous studies have shown that the mechanism of the effect of acupuncture on depression may be related to the NO-cGMP pathway [8–10]. Electro-acupuncture can increase the expression of nNOS and the content of cGMP in the hippocampus of rats in the models and maintains the regular activity of the NO-cGMP signaling pathway [8]. Acupuncture and acupoint stimulation are effective in animal models and clinically [9,10].
In traditional Chinese medicine (TCM) 13 ‘ghost’ acupoints are commonly for the treatment of mental illness [11]. Historically, in China, mental illness was believed to be caused by ghosts or spirits, and in TCM, each acupoint is termed a ‘ghost,’ and the number used is ‘13’ [12,13]. Recent clinical research also supports the therapeutic effects of acupuncture in patients with depression and other psychiatric illnesses [14,15]. However, previous studies have investigated clinical efficacy, and there have been few studies that have investigated the mechanisms of the effects of acupuncture.
Therefore, this study aimed to to investigate the effects of acupuncture on behaviors associated with depression in the chronic unpredictable mild stress (CUMS) rat model. In this study, two of the 13 ‘ghost’ acupoints were selected, Shangxing (DU23) and Daling (PC7). The expression of signaling pathway components of NO and cGMP in the rat hippocampus and plasma were also measured.
Material and Methods
Animal care and initial evaluation
Forty specific pathogen-free (SPF) male Sprague-Dawley rats weighing 120–150 g were provided by Shanghai Slake Laboratory Animal Co., Ltd. (Shanghai, China). The animal experiments were approved and undertaken under the animal license number SCXK 2017-0005 (Shanghai, China). Animal care and all experimental procedures were approved by the Animal Care and Use Committee of Xiamen University (License No. XMULAC20170376) and implemented in accordance with the international guidelines on ethical laboratory animal treatment.
The rats were given one week to adapt to the laboratory environment before the study began, and were given free access to food and water. Then, the body weight was measured, and the behavior of the rats was tested. The baseline of body weight and the baseline behavior of all rats were normal at the start of the study.
The rats (N=40) were divided into the control group (N=10), the model group (N=10), the acupuncture group (N=10), and the non-acupuncture group (N=10). The rats in the normal group were fed together, and the rats in the other groups were fed and housed separately in single cages.
Establishment of the rat model of chronic unpredictable mild stress (CUMS)
The rat model was established by orphaning combined with chronic unpredictable mild stress (CUMS) for six weeks, as previously described [16,17]. Briefly, the rats fasted for 24 h and then underwent a series of stressful stimuli each day. The stimuli included: water deprivation for 24 h; cold water stimulation at 4°C for 5 min; concussion for 30 min; restraint for 3 h; noise stimulation for 3 h at 80 dB; tail clamping for 2 min; day and night inversion for 12 h; and cage tilting at 45° for 24 h.
Behavioral testing was conducted on day 0, day 21, and day 42 during the CUMS modeling period. Rat behaviors that were tested included the sucrose preference test (SPT), the open field test (OFT), and the elevated plus maze (EPM) test. The criteria for the successful establishment of the model included a significant reduction in the behavioral score, reduced sucrose consumption in the SPT, reduced crossing, rearing, grooming numbers in the OFT.
Acupuncture treatment
Acupuncture treatment commenced on the third week of the six-week study, once every day for three weeks. Before the intervention, rats were confined to minimize their activity. In this study, two of the 13 ‘ghost’ acupoints were selected, Shangxing (DU23), and Daling (PC7) [18].
Acupuncture was performed at the two acupoints by vertical insertion of the acupuncture needle into the rat skin to a depth of 1–3 mm. The needles used were disposable sterile acupuncture needles, 0.25×25 mm (Changchun Aikang Medical Devices Co., Ltd., Changchun, China). At the Shangxing point (DU23), the needle was inserted vertically to a depth of 2–3 mm; at the Daling (PC7), the needle was inserted vertically to a depth of 1–2 mm. And acupuncture sustained for 30 minutes, for 2 min each time, once a day, for three weeks. Acupuncture needle manipulation (twirling) was performed by rotation of the needle clockwise (360°) and counter-clockwise (360°).
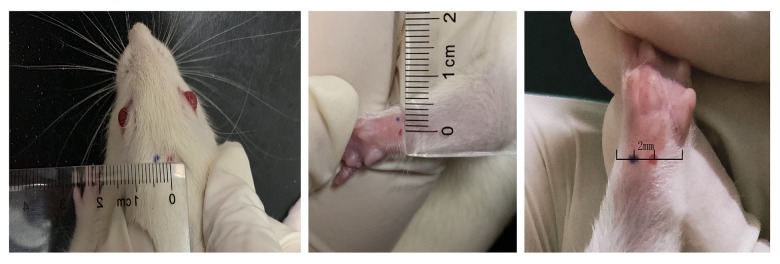
In the sham (non-acupoint) group, the rats were given acupuncture at non-acupoints, located at 2–5 mm adjacent to the two main acupoints, according to the physical characteristics of the rats, 5 mm adjacent to DU23 and 2 mm adjacent to PC7, as shown in Figure 1. The needle depth was less than in the treatment group, and only penetrated the surface of the skin, but did not reach the acupoint depth of 0.5–1.0 mm. The duration of needle retention and the operating frequencies were the same as the treated group.
Figure 1.
The location of the acupoints and the non-acupoints used during acupuncture in the male Sprague-Dawley rats. The location of acupoints (red) and non-acupoints (blue). Shangxing (DU23) and Daling (PC7) are the acupoints. In the sham group (non-acupoint), rats were given acupuncture at non-acupoints located 2–5 mm adjacent to the acupoints according to different body physique (5 mm adjacent to DU23 and 2 mm adjacent to PC7).
The behavioral sucrose preference test (SPT)
A revised version of the SPT was used to measure rat behavior, as previously described [19]. Sucrose adaptation was performed for 48 hours before the experiment. The rats had a choice of two drinking bottles, one containing 1% sucrose and the other containing pure water. The drinking bottles were placed in the rat cage and were changed every 12 hours. The rats fasted and were deprived of water for 24 hours before the SPT was performed. Fluid intake from the two bottles was recorded during 12 h to determine the sucrose preference. The following calculation was used for the SPT:
Sucrose preference =[sucrose fluid consumption (ml)/sucrose fluid+pure water consumption (ml)]×100.
The behavioral open field test (OFT)
A modified version of the OFT was used to measure rat behavior, as previously described [20]. Before the experiment, rats were placed in the test room to adapt at least 15 minutes. The room noise he was controlled to <30 dB, and the light was controlled to <60 Lux. During the test, the rats were placed in the central area of a black square device (100×100×40 cm), which was divided into a 5×5 cm grid. A video camera was used to record the rat behavior, and SMART software was used to analyze the activities of the rats during five minutes. The incidence of the total crossing counts, rearing counts, and grooming counts were evaluated. After each rat test, 75% ethanol was used to clean the open field board.
The elevated plus-maze (EPM) behavioral test
A modified version of the EPM test was used to measure rat behavior, as previously described [21,22]. The experimental apparatus consisted of two open arms (50×10×40 cm), a central area (5×5 cm) and two closed arms (50×10×40 cm). The maze was elevated 100 cm above the ground, and the test was performed in a quiet and dim environment. The rats were placed on the central platform facing the connection between the closed arm and the open arm. The autonomous movement between the open arm and the closed arm was analyzed by video recording and SMART software for 5 minutes. The total distance of movement and the duration time in the centered and open arms were measured. The apparatus was cleaned with 75% ethanol between each test.
Blood and brain sampling
The rats fasted and were deprived of water for 12 hours before they were euthanized. Blood samples were taken from the abdominal aorta, and the brain from each rat was removed. The blood was centrifuged to obtain the plasma. The hippocampus was separated from the brain rapidly and under ice and stored at −80° for further examination.
Enzyme-linked immunoassay (ELISA) to detect the protein components of the rat nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) signaling pathway
Tissue from the rat hippocampus was lysed by adding phosphate-buffered saline (PBS) at pH 7.4 (Solarbio Science & Technology Co., Ltd., Beijing, China), placed in a centrifuge at 4°C and centrifuged at 12000 rpm for 10 min to obtain the supernatant. The rat blood was treated in the same way to obtain the plasma. Enzyme-linked immunoassay (ELISA) was used to detect the protein components of the NO and cGMP signaling pathway. A rat cGMP enzyme-linked immunosorbent assay kit (H163) (Shanghai Nanjing Institute of Bioengineering Research) and a nitric oxide (NO) assay kit (A102) (Shanghai Nanjing Institute of Bioengineering) were used. The absorbance was measured at 450 nm and 550 nm using a GloMax microplate reader (Promega, Madison, WI, USA), and the cGMP and NO contents in hippocampus and plasma were calculated.
Reverse transcription-polymerase chain reaction (RT-PCR) to detect mRNA of genes in the NO and cGMP signaling pathway
The mRNA coding sequences of iNOS, nNOS, NR1, NR2A, and NR2B were identified from the Consensus Coding Sequence (CCDS) database of the National Center for Biotechnology Information (NCBI). The details of the primer sequences for iNOS, nNOS, NR1, NR2A, NR2B, and the β-Actin control are shown in Table 1. The PCR primers were developed. RNA extraction was performed using 100 mg of rat hippocampal tissue that was homogenized in liquid nitrogen followed by RNA extraction using TRIzol and an RNA extraction kit. Tissue was pyrolyzed with 1 ml TRIzol at room temperature for 5 min and centrifuged at 12,000 rpm for 10 min. The supernatant was removed and mixed with 0.2 ml of chloroform and centrifuged at 12,000 rpm for 10 min. Then, 500 μl of the aqueous phase was absorbed and augmented with 250 μl absolute ethyl alcohol, and run in an adsorption column for 45s. The adsorption column was washed with 500 μl of buffer and eluted with 80 μl of H2O. The quality of RNA was determined using 1% agarose gel electrophoresis, and the concentration of RNA was detection by spectrophotometry.
Table 1.
Primers used for rat iNOS, nNOS, NR1, NR2A, NR2B, and β-actin.
| mRNA primers Rat (Rattus sp.) | Sequence (5′-3′) |
|---|---|
| β-actin, forward | 5′ CTGGCTCCTAGCACCATGAA 3′ |
| β-actin, reverse | 5′ AAAACGCAGCTCAGTAACAGTC 3′ |
| nNOS, forward | 5′ GGCGTCCGTGACTACTGT 3′ |
| nNOS, reverse | 5′ TTGAGCATCTCCTGGTGG 3′ |
| NR1, forward | 5′ GGAGCGGGTAAACAACAG 3′ |
| NR1, reverse | 5′ CAGCAGGTACAGCATCACA 3′ |
| iNOS, forward | 5′ AACTACTGCTGGTGGTGAC 3′ |
| iNOS, reverse | 5′ GAACTGAGGGTACATGCTG 3′ |
| NR2A, forward | 5′ AGGACTGTAGCGATGTTGA 3′ |
| NR2A, reverse | 5′ ATTCTCAGGCAGGGTTAT 3′ |
| NR2B, forward | 5′ TGGGCTCTATGACTGTGAC 3′ |
| NR2B, reverse | 5′ ACTGCTGTTTCCTCCTCTT 3′ |
RNA reverse transcription was performed by transcribing RNA into cDNA using an FSQ-101 Reverse Ace reverse transcription kit (Toyobo, Osaka, Japan). The cDNA was amplified by PCR using an ETC811 Gene Amplifier (Suzhou Taomsun Scientific Instruments Co. Ltd., Jiangsu, China). The amplification procedure was performed using 30 PCR cycles as follows: 94°C for 5 min; 94°C for 30 s; 60°C for 30 s; 72°C for 30 s. The last cycle was performed at 72°C for 10 min. The PCR products were detected by agarose gel electrophoresis using a JY-SPFT electrophoresis chamber (Junyi Dongfang Electrophoresis Equipment Co., Ltd., Beijing, China). The gels were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The results were expressed as the mean±standard error of the mean (SEM) of each group. Data were analyzed using SPSS version 19.0 software (IBM, Chicago, IL, USA). Statistical comparisons were performed using one-way analysis of variance (ANOVA) followed by Fisher’s post hoc test for multiple comparisons. A P-value <0.05 was considered to be statistically significant.
Results
The effect of acupuncture on the sucrose preference test (SPT) in the rat model of chronic unpredictable mild stress (CUMS)
As shown in Figure 2, the consumption of 1% sucrose by rats in the model group was significantly reduced after CUMS procedure compared with the control group (P<0.05). After acupuncture intervention, the 1% sucrose consumption of rats in the acupoint group was significantly improved compared with the model group (P<0.05), while there was no significant difference in non-acupoint acupuncture group (P>0.05).
Figure 2.
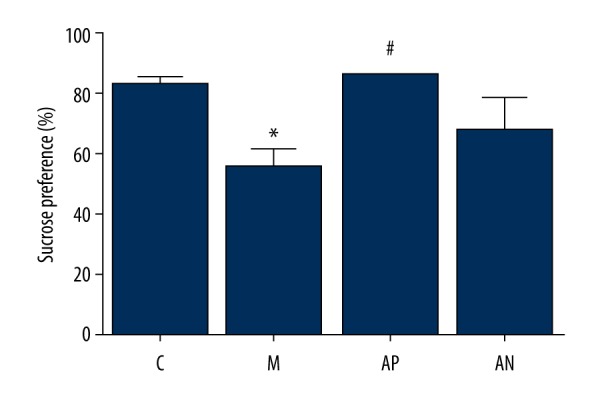
The effects of acupuncture on the sucrose preference test (SPT) of the Sprague-Dawley rats in the chronic unpredictable mild stress (CUMS) model. The percentage of 1% sucrose consumed by the rats. * Mean significant difference vs. control (P<0.05). # Mean significant difference vs. the model (P<0.05).
The effect of acupuncture treatment on locomotor activity in the open-field test (OFT) in the rat model of CUMS
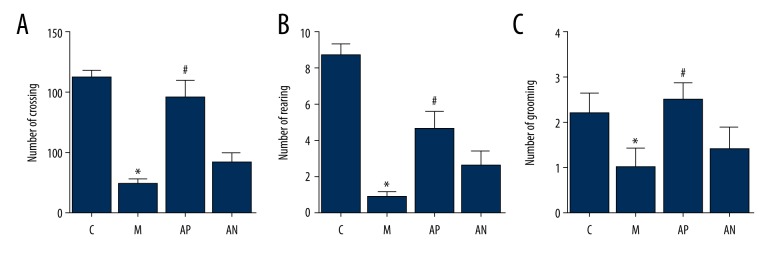
As shown in Figure 3, the CUMS procedure significantly reduced the total count of lattice crossing, the count of leg rearing, and the count of fur grooming in the model group compared with the control group (P<0.05). After acupuncture treatment, the total count of lattice crossing, leg rearing, and the fur grooming in the acupoint rats were significantly improved, compared with the model rats (P<0.05). There was no significant difference in the rats in the non-acupoint group (P>0.05).
Figure 3.
The effects of acupuncture on the open field test (OFT) of the Sprague-Dawley rats in the chronic unpredictable mild stress (CUMS) model. (A) The lattice crossing counts. (B) The leg rearing counts. (C) The fur grooming counts. * Mean significant difference vs. control (P<0.05). # Mean significant difference vs. the model (P<0.05).
The effect of acupuncture treatment on locomotor activity in the elevated plus-maze (EPM) test in the rat model of CUMS
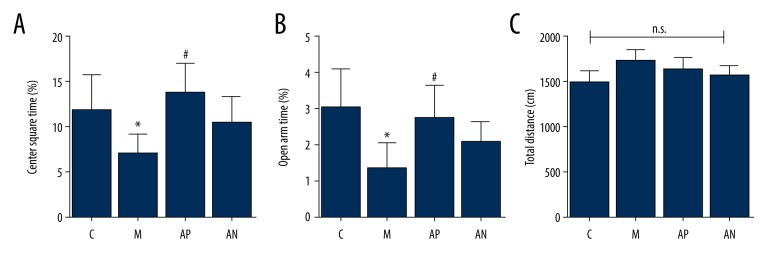
As shown in Figure 4, the model rats show significantly fewer open arm and center duration retention ratio after the CUMS procedure compared with the control rats (P<0.05). Compared with the model group, the retention ratio of center and open arm in the acupoint group were significantly increased (P<0.05). The total elevated distance of rats in each group showed no statistical difference (P>0.05).
Figure 4.
The effects of acupuncture on the elevated plus maze (EPM) test of the Sprague-Dawley rats in the chronic unpredictable mild stress (CUMS) model. (A) The center square time. (B) The open arm time. (C) Total distance. * Mean significant difference vs. the control (P<0.05). # Mean significant difference vs. the model (P<0.05).
The effects of acupuncture treatment nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) in the hippocampus and plasma in the rat model of CUMS
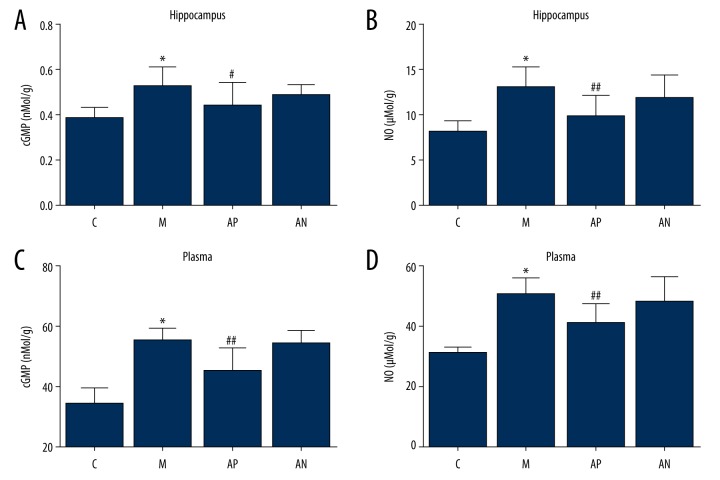
In the rat model, the expression of cGMP and NO in the hippocampus and plasma of the model group were significantly increased compared with the control group (P<0.05). Acupuncture significantly decreased cGMP and NO expression in the hippocampus and plasma in the acupoint group compared with the model group (P<0.05). There was no significant difference for the non-acupoint acupuncture group (P>0.05) (Figure 5).
Figure 5.
The effect of acupuncture on cyclic guanosine monophosphate (cGMP) and nitric oxide (NO) in the rat hippocampus and plasma measured by enzyme-linked immunoassay (ELISA). (A) cGMP in the rat hippocampus. (B) cGMP in rat plasma. (C) NO in the rat hippocampus. (D) NO in rat plasma. The results were quantified by ELISA in the hippocampus and plasma. * Mean significant difference vs. the control (P<0.05). #, ## Mean significant difference vs. the model (P<0.05).
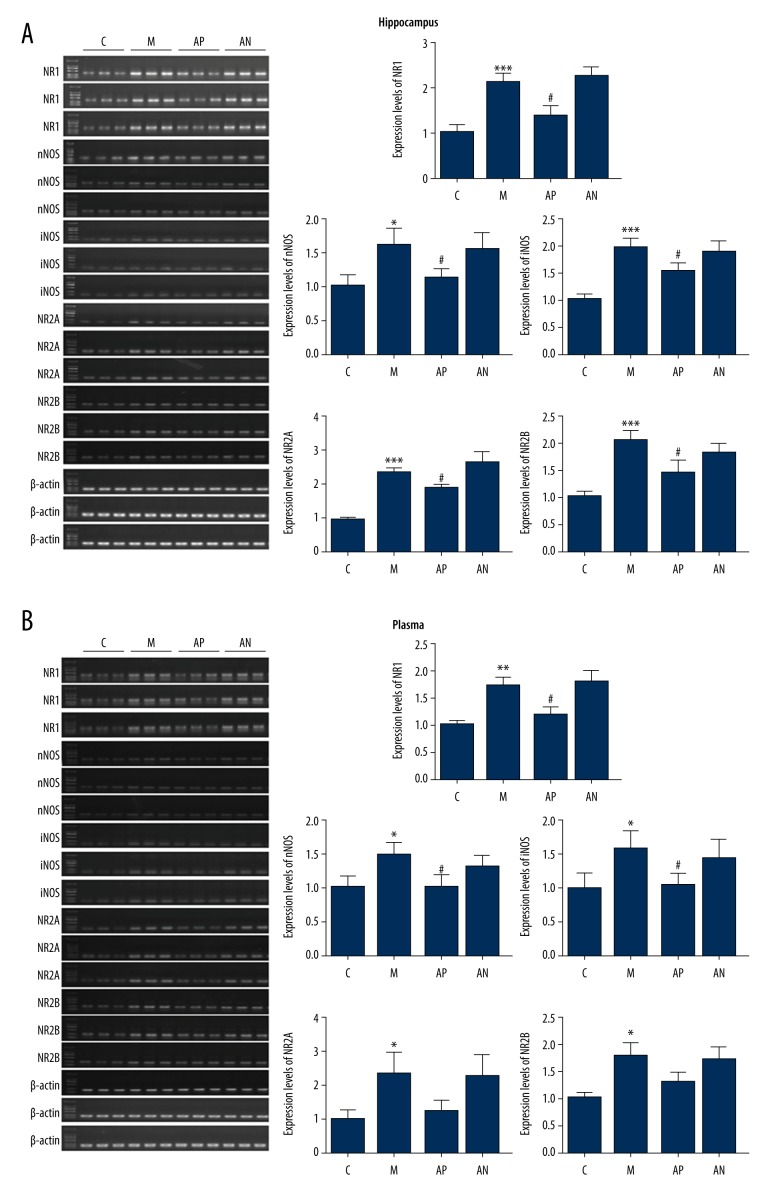
The effects of acupuncture treatment on the expression of mRNA of the NO-cGMP pathway genes (iNOS, nNOS, NR1, NR2A, NR2B) in the hippocampus and plasma in the rat model of CUMS
There were significantly increased levels of iNOS, nNOS, NR1, NR2A, and NR2B mRNA expression in the plasma and hippocampus of the model group compared with the control group (P<0.05). Acupuncture significantly reduced the expression level of iNOS, nNOS, NR1, NR2A, and NR2B mRNA in the plasma and hippocampus in acupoint group compared with the model group (P<0.05). There was no significant difference for the non-acupoint acupuncture group (P>0.05) (Figure 6).
Figure 6.
The effect of acupuncture on the cyclic guanosine monophosphate (cGMP) and nitric oxide (NO) pathway mRNA for iNOS, nNOS, NR1, NR2A, NR2B in the rat hippocampus and plasma measured by reverse transcription-polymerase chain reaction (RT-PCR). (A) mRNA expression of iNOS, nNOS, NR1, NR2A, and NR2B in the rat hippocampus, using RT-PCR. (B) mRNA expression of iNOS, nNOS, NR1, NR2A, and NR2B) in the rat plasma, using RT-PCR. *** Mean significant difference vs. the control (P<0.05). ### Mean significant difference vs. the model (P<0.05).
Discussion
In the chronic unpredictable mild stress (CUMS) rat model, treatment using the acupoints Daling (PC7) and Shangxing (DU23) reversed the behavioral changes evaluated by the sucrose preference test (SPT), the open field test (OFT), and the elevated plus maze (EPM) test. Acupuncture also maintained the balance of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) protein and mRNA expression, when compared with the use of non-acupoints. The use of this rat model in the evaluation of behavior that may be associated with depression has been supported by previous studies [23,24]. The findings from this study showed that prolonged acupuncture treatment reduced behaviors in the CUMS rat model that may be associated with depression, as shown by increased sucrose consumption in the SPT, the scores from the OFT, and the behavior scores from the EPM test, which are supported by findings from previous studies [4,25,26]. However, in this study, the mechanism associated with the effects of acupuncture were investigated at the protein and mRNA level. This study included enzyme-linked immunoassay (ELISA) and reverse transcription-polymerase chain reaction (RT-PCR) were to detect the expression of inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS), and the N-methyl-D-aspartate (NMDA) receptor subunits, NR1, NR2A, and NR2B in the rat plasma and hippocampus.
Previous studies have shown that NO-cGMP signaling may be involved in depression [27–19]. NO is an essential neuronal messenger that has an important regulatory role in the central nervous system, and changes in NO levels affect the levels of cGMP. The central neurotransmitter, dopamine, which has a role in depression, is also dependent on cGMP levels [27–29]. Increased levels of NO can stimulate the release of glutamate, which acts on the N-methyl-D-aspartate (NMDA) receptor to increase calcium ions and activate NOS, to maintain normal neuronal function, learning, and memory. When levels of the NMDA receptor subunits NR1, NR2A, and NR2B are increased, they can cause neuro-excitatory toxicity and neuropsychiatric symptoms [27–29]. Chronic stress causes hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis, and increased release of glucocorticoid, which promotes the release of excitatory amino acids, including glutamate. The imbalance of excitatory and inhibitory amino acids can lead to depressive behavior [27–29].
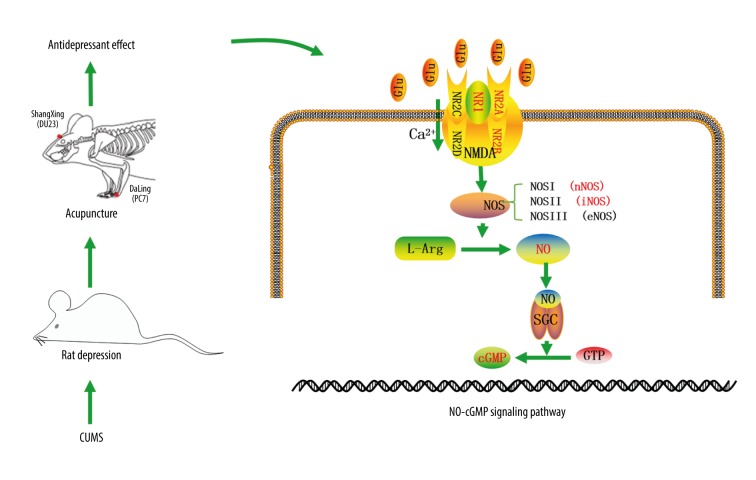
Figure 7 is a schematic diagram of the proposed mechanism of the effect of acupuncture in the CUMS rat model that is based on the findings from the present study. Acupuncture might have an antidepressant effect through the NO-cGMP signaling pathway. Glutamate acts on NMDA receptors to affect learning and memory, as they affect the survival of neurons, regulate the development of neuronal dendrites and axons, and participate in the formation of synaptic plasticity [30,31]. Because NMDA receptors are activated in different brain regions, increased intracellular Ca2+ levels, combined with Ca2+-conjugated proteins, activate nNOS, to increase the level of NO in the central nervous system [32,33]. The NO derived from iNOS and nNOS) has neurotoxic effects [34,35]. Also, NO is a neurotransmitter with biological activity that is mediated by soluble guanosine cyclase (sGC), and cGMP is produced by intracellular guanosine triphosphate (GTP) under the action of sGC. The overexpression of cGMP can mediate protein phosphorylation to produce physiological and pathological effects, damage DNA and neurons, which may lead to depression [36,37]. In 1997, Lev-Ram et al. reported that the effects of NO and cGMP induction of Purkinje neurons were similar to the long-term inhibition of hippocampal CA1 neurons [37]. Therefore, the NO-cGMP signaling pathway may be associated with synaptic plasticity of neurons in the brain, and with the occurrence of depression and other mental disorders.
Figure 7.
The schematic diagram of the mechanism of the effect of acupuncture in the chronic unpredictable mild stress (CUMS) rat model. In the chronic unpredictable mild stress (CUMS) rat model, the effect of acupuncture may have been associated with the inhibition of N-methyl-D-aspartate (NMDA) receptors, nitric oxide synthase (NOS), cyclic guanosine monophosphate (cGMP), and the glutamatergic system, through activation of the NO-cGMP signaling pathway.
Because there have been few previous studies to investigate the mechanism associated with the effects of acupuncture, in the present study, the CUMS rat model was investigated not only in terms of the behavioral changes following acupuncture treatment, but plasma and brain tissue were examined. The results showed that when the levels of nNOS in the hippocampus and plasma decreased in the model group, cGMP also decreased. When the expression of nNOS in the rat hippocampus and plasma increased after acupuncture treatment, the levels of cGMP also significantly increased. This finding supported the view that the antidepressant effect of acupuncture might be achieved via the protein phosphorylation process mediated by NO-cGMP signal transduction pathway, which is a finding that is supported by a previous study [8].
From the 13 acupuncture ‘ghost’ points, or acupoints, which have been used in traditional Chinese medicine (TCM) for the treatment of depression and mental disease, the Chinese literature has shown that the use of one or two acupoints can be effective. Therefore, in this animal study, we selected Shangxing (DU23) and Daling (PC7) as treatment acupoints. Daling (PC7) is the original acupoint of the pericardium meridian that, according to the meridian theory of TCM can have an effect on psychiatric disorders [38,39]. Shangxing (DU23) is an acupoint located in the governing meridian that is associated with mental wellbeing. A previously reported clinical study showed that the use of the 13 ‘ghost’ points combined with conventional acupuncture treatment reduced the symptoms of depression and anxiety in patients with somatization disorder [40]. In 2012, Wang et al. showed that the use of acupuncture using the 13 ‘ghost’ acupoints resulted in a therapeutic effect that was significantly better than fluoxetine treatment but with fewer side effects [41]. In 2015, Yu et al. showed that needling the 13 ‘ghost’ acupoints could reduce the symptoms of postpartum depression and improve the quality of life for patients [42]. In 2017, Li et al. treated patients with anxiety and depression following an acute stroke with acupuncture of the 13 ghost acupoints and showed a significant improvement in symptoms [43]. Currently, clinical research on the effects of acupuncture using the 13 ‘ghost’ acupoints in patients with depression has been aimed at validating clinical efficacy, and there have been few studies on the specific mechanisms involved [44].
At the end of the last century, Western countries began to undertake controlled clinical studies on the effects of acupuncture using scoring systems for its effects to distinguish outcomes from placebo [45]. Therefore, in the present study, both acupoints and non-acupoints were used, and the effects were directly compared. When the findings from the use of acupoints and non-acupoints were compared, the use of acupuncture in the CUMS rat model resulted in significant improvement in behavior when assessed by the SPT, OFT, and EPM test. Also, the use of acupuncture using the acupoints significantly reduced the expression of components of the NO and cGMP pathway in the rat hippocampus and plasma.
This study had several limitations. Although the CUMS rat model is well-established, it is an animal model, and caution should be used when extrapolating the findings from an animal study to a chronic and complex human disease, such as depression. The 13 ‘ghost’ acupoints that have been used to treat depression have been used for centuries in humans, and this study has assumed that the effects in the rat will be the same. In the rat model, only two acupoints were used, and future studies should include more acupoints. In this study, although the whole rat brain was removed, only the rat hippocampus was studied, as this was assumed to be an important brain area associated with depression. However, the pathophysiology of clinical depression involves several brain regions. In this study, the NO-cGMP signaling pathway was studied, but clinical depression involves several genes and molecular pathways that have yet to be investigated in this animal model.
Conclusions
This study aimed to investigate the effects of acupuncture on behaviors associated with depression in the chronic unpredictable mild stress (CUMS) rat model. The expression of signaling pathway components of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) in the rat hippocampus and plasma were also measured. In the CUMS rat model, treatment with acupuncture reduced behaviors associated with depression, and these effects were associated with changes in the NO and cGMP signaling pathway.
Acknowledgments
The department of Traditional Chinese Medicine in Medical College of Xiamen University is thanked for the invaluable contribution to the study. The authors would also like to thank the participants for volunteering their time to the study.
Footnotes
Source of support: This study was supported by grants from the Province Natural Science Foundation of Fujian (No. 2018J01135)
Conflict of interest
None.
References
- 1.Schmidt PJ. Mood, depression, and reproductive hormones in the menopausal transition. Am J Med. 2005;118(Suppl 12B):54–58. doi: 10.1016/j.amjmed.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 2.Nemeroff CB, Owens MJ. Treatment of mood disorders. Nat Neurosci. 2002;5(Suppl):1068–70. doi: 10.1038/nn943. [DOI] [PubMed] [Google Scholar]
- 3.Cai W, Stewart R, Mueller C, et al. Poststroke depression and risk of stroke recurrence and mortality: Protocol of a meta-analysis and systematic review. BMJ Open. 2018;8:e26316. doi: 10.1136/bmjopen-2018-026316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao X, Zhang J, Jin Y, et al. Acupuncture for perimenopausal depression: A protocol for a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e14073. doi: 10.1097/MD.0000000000014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KR, Patel JN, Symanowski JT, et al. Acupuncture for cancer pain and symptom management in a palliative medicine clinic. Am J Hosp Palliat Care. 2019;36:326–32. doi: 10.1177/1049909118804464. [DOI] [PubMed] [Google Scholar]
- 6.Pyne D, Shenker NG. Demystifying acupuncture. Rheumatology (Oxford) 2008;47:1132–36. doi: 10.1093/rheumatology/ken161. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong GA, Rodgers CI, Money TG, Robertson RM. Suppression of spreading depression-like events in locusts by inhibition of the NO/cGMP/PKG pathway. J Neurosci. 2009;29:8225–35. doi: 10.1523/JNEUROSCI.1652-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han YJ, Li WX, Jia BH, et al. [Effect of electroacupuncture on hippocampal NO-cGMP signaling pathway in depression rats]. Zhen Ci Yan Jiu. 2009;34:236–41. [in Chinese] [PubMed] [Google Scholar]
- 9.Davila-Hernandez A, Zamudio SR, Martinez-Mota L, et al. Antidepressant effects of acupoint stimulation and fluoxetine by increasing dendritic arborization and spine density in CA1 hippocampal neurons of socially isolated rats. Neurosci Lett. 2018;675:48–53. doi: 10.1016/j.neulet.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, Zhang X, Lu J, et al. Antidepressant-like effects of acupuncture. Insights from DNA methylation and histone modifications of brain-derived neurotrophic factor. Front Psychiatry. 2018;9:102. doi: 10.3389/fpsyt.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun SM, Tang D. [A golden prescription for emergencies]. Beijing, China: Medical Science and Technology Publishers; 2011. [in Chinese] [Google Scholar]
- 12.Ding DZ. [Clinical application of sun zhenren’s thirteen ghost points in psychiatric diseases]. Chinese Journal of Traditional Chinese Medicine. 2014;29:2250–52. [in Chinese] [Google Scholar]
- 13.Zhang LC. [Exploration of guimen 13-needle acupuncture and moxibustion academic thought]. Chinese Journal of Basic Medicine of Traditional Chinese Medicine. 2012;18:1014–29. [in Chinese] [Google Scholar]
- 14.Li AD, Wu JM, Hu J, et al. [An overview of ancient literature and modern research on thirteen ghost points for the treatment of psychiatric diseases]. Asia-Pacific Traditional Medicine. 2017;13:68–70. [in Chinese] [Google Scholar]
- 15.Wang CW, Sun SG, Qin XH. [Clinical observation of sun simiao’s “thirteen ghost points” in the treatment of depression]. Western Traditional Chinese Medicine. 2012;25:73–75. [in Chinese] [Google Scholar]
- 16.Lu J, Shao RH, Hu L, et al. Potential anti-inflammatory effects of acupuncture in a chronic stress model of depression in rats. Neurosci Lett. 2016;618:31–38. doi: 10.1016/j.neulet.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Timberlake MN, Prall K, Dwivedi Y. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:99–109. doi: 10.1016/j.pnpbp.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li ZR. Experimental acupuncture and moxibustion. Beijing, China: Traditional Chinese Medicine Publishers; 2007. [in Chinese] [Google Scholar]
- 19.Liu MY, Yin CY, Zhu LJ, et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13:1686–98. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- 20.Kraeuter AK, Guest PC, Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. 2019;1916:99–103. doi: 10.1007/978-1-4939-8994-2_9. [DOI] [PubMed] [Google Scholar]
- 21.Walia V, Garg C, Garg M. NO-sGC-cGMP signaling influence the anxiolytic like effect of lithium in mice in light and dark box and elevated plus maze. Brain Res. 2019;1704:114–26. doi: 10.1016/j.brainres.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Kraeuter AK, Guest PC, Sarnyai Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol Biol. 2019;1916:69–74. doi: 10.1007/978-1-4939-8994-2_4. [DOI] [PubMed] [Google Scholar]
- 23.Song Y, Sun R, Ji Z, et al. Perilla aldehyde attenuates CUMS-induced depressive-like behaviors via regulating TXNIP/TRX/NLRP3 pathway in rats. Life Sci. 2018;206:117–24. doi: 10.1016/j.lfs.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R, Guo L, Ji Z, et al. Radix scutellariae attenuates CUMS-induced depressive-like behavior by promoting neurogenesis via cAMP/PKA pathway. Neurochem Res. 2018;43:2111–20. doi: 10.1007/s11064-018-2635-3. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Jiang H, Meng H, et al. Antidepressant mechanism research of acupuncture: Insights from a genome-wide transcriptome analysis of frontal cortex in rats with chronic restraint stress. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/1676808. 1676808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H, Zhang X, Lu J, et al. Antidepressant-like effects of acupuncture-insights from dna methylation and histone modifications of brain-derived neurotrophic factor. Front Psychiatry. 2018;9:102. doi: 10.3389/fpsyt.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son H, Jung S, Shin JH, et al. Anti-stress and anti-depressive effects of spinach extracts on a chronic stress-induced depression mouse model through lowering blood corticosterone and increasing brain glutamate and glutamine levels. J Clin Med. 2018;7(11) doi: 10.3390/jcm7110406. pii: E406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madeira C, Vargas-Lopes C, Brandao CO, et al. Elevated glutamate and glutamine levels in the cerebrospinal fluid of patients with probable Alzheimer’s disease and depression. Front Psychiatry. 2018;9:561. doi: 10.3389/fpsyt.2018.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haroon E, Chen X, Li Z, et al. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl Psychiatry. 2018;8:189. doi: 10.1038/s41398-018-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian Z, Wu X, Qiao Y, et al. Downregulation of mGluR2/3 receptors during morphine withdrawal in rats impairs mGluR2/3- and NMDA receptor-dependent long-term depression in the nucleus accumbens. Neurosci Lett. 2019;690:76–82. doi: 10.1016/j.neulet.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Farber NB. NMDA antagonists for treatment-resistant depression. Handb Exp Pharmacol. 2019;250:287–305. doi: 10.1007/164_2018_165. [DOI] [PubMed] [Google Scholar]
- 32.Gegg ME, Beltran B, Salas-Pino S, et al. Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: Implications for neuroprotection/neurodegeneration? J Neurochem. 2003;86:228–37. doi: 10.1046/j.1471-4159.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- 33.Moncada S, Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–89. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- 34.Amsterdam JD, Shults J. Fluoxetine monotherapy of bipolar type II and bipolar NOS major depression: A double-blind, placebo-substitution, continuation study. Int Clin Psychopharmacol. 2005;20:257–64. doi: 10.1097/01.yic.0000171519.64080.c9. [DOI] [PubMed] [Google Scholar]
- 35.Amsterdam JD, Shults J, Brunswick DJ, Hundert M. Short-term fluoxetine monotherapy for bipolar type II or bipolar NOS major depression – low manic switch rate. Bipolar Disord. 2004;6:75–81. doi: 10.1046/j.1399-5618.2003.00083.x. [DOI] [PubMed] [Google Scholar]
- 36.Khan MI, Ostadhadi S, Zolfaghari S, et al. The involvement of NMDA receptor/NO/cGMP pathway in the antidepressant like effects of baclofen in mouse forced swimming test. Neurosci Lett. 2016;612:52–61. doi: 10.1016/j.neulet.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Lev-Ram V, Jiang T, Wood J, et al. Synergies and coincidence requirements between NO, cGMP, and Ca2+ in the induction of cerebellar long-term depression. Neuron. 1997;18:1025–38. doi: 10.1016/s0896-6273(00)80340-2. [DOI] [PubMed] [Google Scholar]
- 38.Chen P. Beijing University of Traditional Chinese Medicine, 2007, Master’s Degree. p. 68. [in Chinese] [Google Scholar]
- 39.Guo ZY. Guangzhou University of Traditional Chinese Medicine, 2012, Ph.D. Degree. p. 94. [in Chinese] [Google Scholar]
- 40.Yao L. [Clinical observation of thirteen ghost points combined with conventional acupuncture in the treatment of somatization disorder]. Shanghai Journal of Acupuncture and Moxibustion. 2018;37:1386–89. [in Chinese] [Google Scholar]
- 41.Wang CW, Sun SG, Qin XH. [Clinical observation of sun simiao’s “thirteen ghost points” in the treatment of depression]. Western Chinese Medicine. 2012;25:73–75. [in Chinese] [Google Scholar]
- 42.Yu SJ, Li XQ, Feng XM, Cao WF. [Effects of acupuncturing thirteen ghost points on the clinical efficacy and quality of life of postpartum depression]. Shanghai Acupuncture and Moxibustion Journal. 2015;34:14–16. [in Chinese] [Google Scholar]
- 43.Duan JY, Ding BY, Zong L. [Clinical observation on acupuncture of thirteen ghost points for the treatment of symptoms of stroke, anxiety and depression]. Shanghai Acupuncture and Moxibustion Journal. 2014;33:536–38. [in Chinese] [Google Scholar]
- 44.Li AD, Wu JM, Hu J, et al. [An overview of ancient literature and modern research on the treatment of mental disorders at 13 ghost points]. Asia-Pacific Traditional Medicine. 2017;13:68–70. [in Chinese] [Google Scholar]
- 45.Scharf HP, Mansmann U, Streitberger K, et al. Acupuncture and knee osteoarthritis: A three-armed randomized trial. Ann Intern Med. 2006;145:12–20. doi: 10.7326/0003-4819-145-1-200607040-00005. [DOI] [PubMed] [Google Scholar]