Abstract
Aims
The growing number of genomically-targeted therapies has made genomic testing an important part of the care for patients with non-small cell lung cancer. However, limited tissue availability, cost, and long turn-around times can create barriers to efficient genomic testing and subsequent treatment. Effective approaches to reduce these barriers are needed.
Methods
302 advanced lung adenocarcinomas from consecutive patients seen at University Hospitals Cleveland Medical Center (UHCMC) were tested in-house using a hybrid DNA/RNA NGS panel. Sample testing was reflexed from pathology for all stage III or IV tumors. Genomic alterations were tiered according to their clinical relevance and reported with guideline-recommended therapies. Clinical implications of genomic testing results was assessed by manual chart review.
Results
With a sample cohort consisting of 64% biopsies, 16% excisions/resections, and 20% fine-needle aspirations, the assay was reliable with a 95% success rate. The average turn-around time from receipt of unstained FFPE slides to reporting was 4.8±2.1 days, half of the recommended 10 days and similar to single gene testing. Alterations with FDA-approved or NCCN guideline-recommended targeted therapies were found in 18% of cases. Within this group, 60% of patients went on genomically-driven therapies.
Conclusions
We found our reflexed in-house NGS assay to be reliable, cost-effective, and efficient. Incorporation of reflex testing with our NGS assay led to an expansion of successful genomic profiling for all guideline recommended alterations and by including an expanded number of alterations within our panel, we obtained clinically useful information outside the guidelines without changing cost or efficiency. This approach has enabled UHCMC clinicians to efficiently initiate genomically-driven therapies for patients with lung adenocarcinoma.
Introduction
Lung cancer remains the leading cause of cancer-related death in the United States, with adenocarcinoma being the most common form of lung cancer1. However, the introduction of genotype-directed targeted therapies for lung adenocarcinoma has transformed the care for these patients and dramatically improved survival2-7. This new era of targeted therapy started with FDA approval of tyrosine kinase inhibitors (TKIs) as first line therapy for patients with activating EGFR mutations3. This was followed by approval of crizotinib for patients with ALK fusions in 20134, and for patients with ROS1 fusions in 20165,8. In 2017, the FDA approved a targeted combination therapy for patients with the V600E BRAF mutation7,9, approved osimertinib for patients with EGFR-T790M mutations6,10, and approved immunotherapy for patients with PDL-1 expression11,12. In addition, there are a host of other genotype-directed targeted therapies under early and late-stage clinical investigation. Given the improvement in survival with targeted therapies, molecular testing of these tumors has become critical to guiding therapy decisions for clinicians and their patients.
The College of American Pathologist, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology recently published a 2018 update of their 2013 consensus guidelines for molecular testing in lung cancer13. Their recommendations include testing all advanced-stage (stage IIIB and IV) lung cancer for targetable molecular alterations. The minimal acceptable testing is single gene testing for alterations in EGFR, ALK, and ROS1. If these are negative, patients can be tested for an additional set of targetable alterations using a multiplexed genetic sequencing panel. These include BRAF, MET, RET, ERBB2 (HER2), and KRAS. First and second line genetic alterations can also be tested at the same time in a panel rather than testing sequentially.
Given the increasing number of targetable alterations, coupled with the widespread adoption and maturation of next generation sequencing (NGS) in clinical laboratories, the National Cancer Center Network’s (NCCN) current guidelines recommend EGFR mutation testing as part of a broad molecular profiling upfront with multiplex mutation screening assays or NGS rather than as a separate test14.
While the field and guidelines are rapidly moving towards upfront profiling of all advanced lung cancers with NGS, there are still practical issues with NGS that can limit clinical testing and prevent or delay patients being started on targeted therapies. These barriers to therapy include assay failure due to insufficient sampling or sequencing failure, cost, and significantly increased turn-around times. Effective approaches to reduce these barriers are needed.
Here we demonstrate an efficient, reliable, and cost-effective in-house molecular testing system that includes alterations for all FDA-approved and NCCN guideline-recommended therapies, as well as other emerging markers for lung adenocarcinoma. Using this assay as a reflex test for all advanced stage lung adenocarcinoma has enabled clinicians within our health system to efficiently initiate genotype-directed targeted therapies for patients with lung adenocarcinoma.
Materials and Methods
We implemented the Oncomine™ Focus Assay as our base assay. The Oncomine™ Focus Assay is a NGS oncology assay designed to simultaneously analyze hundreds of variants across 52 genes relevant to solid tumors. This assay enables concurrent analysis of DNA and RNA in a single workflow to detect hotspots, single nucleotide variants (SNVs), indels, copy number variants (CNVs), and gene fusions in various types of solid tumors. It is designed to detect alterations that have clinical utility in prognosis, diagnosis or therapeutic implications in various solid tumors. The biomarkers included were selected based on information in the Oncomine Knowledgebase15,16 and confirmed with industry-leading pharmaceutical partners. Important in our selection was that this assay can be reliably performed on low input, low quality, and formalin-fixed paraffin embedded (FFPE) tissue, including fine needle aspirate (FNA) samples. All of our samples were from FFPE blocks.
We used the Ion Torrent system for NGS (Ion Personal Genome Machine™ (PGM) and Ion Chef, ThermoFisher Scientific), as it enables a faster and cheaper sequencing than hybridization-capture based NGS (Illumina platform) with our throughput. The Ion Torrent PGM is a NGS platform that uses semi-conductor technology to detect the pH change in a microenvironment by the release of a proton upon nucleotide addition to an extending DNA template. This technology allows accurate multiplexing of patient samples, while maintaining individual sample identification through unique sequence barcode identifiers.
We validated the Oncomine™ Focus Assay from ThermoFisher Scientific for use at University Hospital Cleveland Medical Center’s (UHCMC) Clinical Laboratory Improvement Amendments (CLIA)-certified translational laboratory (UHTL). We then expanded this assay to include 17 new biomarkers after 168 cases. We refer to this as our expanded Solid Tumor Focus Assay (see Supplemental Figure 1).
We implemented an institution-wide reflex to NGS testing for any patient diagnosed with stage III or IV lung adenocarcinoma by pathology. A specimen was reflexed automatically from pathology when the site of the specimen was a distant metastasis or a N2/N3 lymph node (typically mediastinal or subcarinal lymph nodes). This was the majority of cases. For specimens from the lung that needed further clinical staging, the molecular testing was requested by the oncology team after clinical staging demonstrated stage III or IV lung adenocarcinoma.
During a 15 month period, starting in December 2016, 302 consecutive patients diagnosed with stage III or IV lung adenocarcinoma at UHCMC or UH affiliated community hospitals patients had their tumors reflexed to in-house NGS testing with the Oncomine™ Focus Assay (first 168 patients) or our expanded Solid Tumor Focus Assay (next 134 patients). For variant calling, Ion Torrent Suite™ alignment and variant caller were utilized with an in-house pipeline to call DNA variants. Ion Reporter™ was utilized for fusion calls from RNA. Genomic alterations were reviewed by a molecular pathologist, tiered according to their clinical relevance, and reported with accompanying NCCN-therapeutic guidelines. We retrospectively reviewed the medical charts of the 302 patients who had undergone NGS to determine the clinical implications of their NGS results. This study was performed and consents were obtained in accordance with UHCMC Institutional Review Board guidelines.
RESULTS
Validation of Molecular Assay
We validated the Oncomine™ Focus Assay on the Ion Torrent system in our UHTL laboratory (see Appendix 1 for full validation results). As part of our validation, we tested 30 cases that were previously tested by the FDA approved EGFR Therascreen® CDx assay (Qiagen), 6 ALK positive samples tested by FDA-approved Ventana ALK (D5F3) CDx assay (Ventana Medical Systems), and 29 cases that were previously tested by FoundationOne® assay from Foundation Medicine.16 Of the alterations included on our panel, our assay was able to detect all alterations that had been detected by EGFR Therascreen®, Ventana ALK IHC, and FoundationOne® assays (see Appendix 1 in the supplemental data). We later expanded the DNA portion of the Oncomine™ Focus Assay to include alterations in 17 other genes (Supplemental Figure 1), and refer to this as our in-house Solid Tumor Focus Assay. This expanded assay was validated in a similar fashion (see Appendix 1 in supplemental data for summary).
Assay Performance on Clinical Application
Based on these positive validation results, we implemented an institution-wide reflex to this NGS assay for any advanced stage (III or IV) lung adenocarcinoma. This prevented delays in ordering follow-up testing and ensured all samples received appropriate assessment. The empiric performance data from the first 302 consecutive samples assayed using this system are consistent with our validation data and demonstrate the assay is reliable, efficient, and cost-effective (Figure 1A-D).
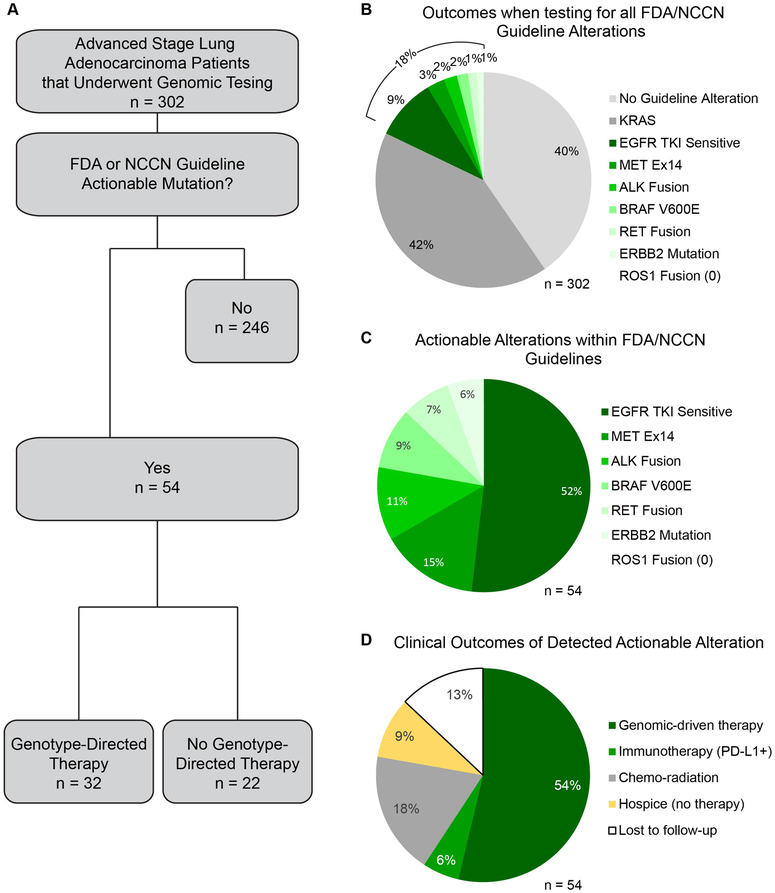
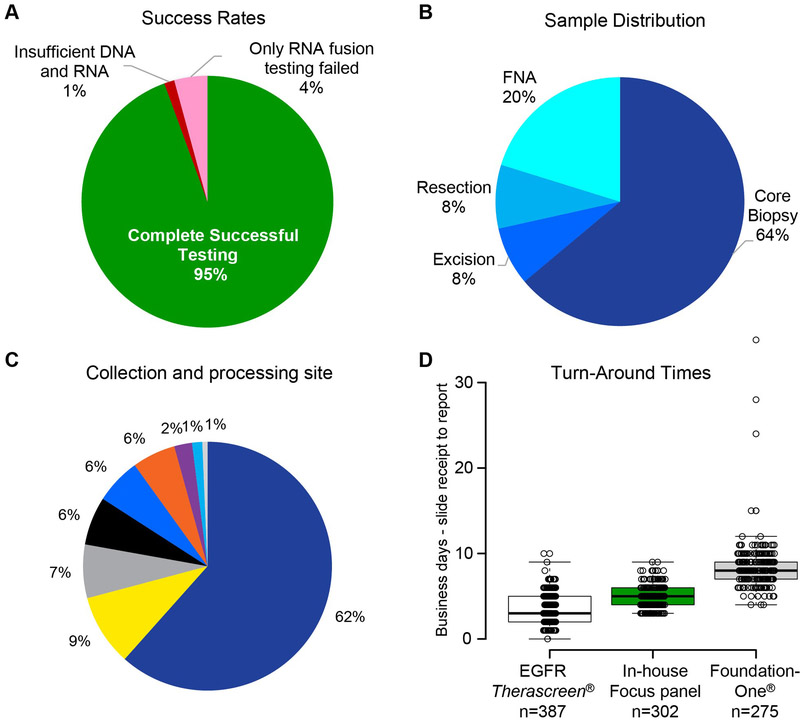
Figure 1. Assay Performance.
A) Success rate and reasons for assay failure. B) Distribution of sample types. C) Distribution of samples collection sites within the University Hospital System. D) Turn-around times three different assays used to determine EGFR mutation status, or EGFR mutation status and other alterations. EGFR Therascreen® and FoundationOne® data were collected from 2014-2016. Data for the in-house Focus Panel, which includes both the Oncomine™ Focus Assay and the expanded Solid Tumor Focus Assay, were collected from 2016-2018. n = 302 for all pie charts.
Within this clinical cohort of 302 patients, the assay had a success rate of 95%S (Figure 1A). There were only 3 samples (1%) that failed for DNA. Two samples had severe deamination artifact that did not allow variant calls to be made and one sample did not amplify. For RNA, samples failed due to degraded RNA that did not amplify enough reads to pass QC. Typically, samples that failed had significantly lower read lengths as well as insufficient read number. This low failure rate was particularly promising given that tumor tissue was from FFPE slides and obtained from a wide variety of sources, including 20% FNAs (Figure 1B). A large proportion of the samples were of limited material, with successful NGS with as little as 9 ng DNA and 33 ng RNA. The assay was also robust in regards to tissue preparation technique, as samples were collected from 9 different locations, each with independent histopathological processing (Figure 1C).
Our assay had an average turn-around time of 4.8 days, half of the guideline recommended 10 days. This was from the time we received the FFPE slides to report. By comparison, within our system from 2014 to 2016, the turn-around time for the single gene EGFR Therascreen® assay was 3.6 days, and FoundationOne® testing, which includes all genes tested by our focused assay as well as others, had an average turn-around time of 8.5 days (Figure 1D).
The cost of the assay was competitive with single-gene testing and cheaper than the broader FoundationOne® assay. While the cost of the assay may vary across molecular pathology departments, the cost to insurance and the individual can be assessed across institutions by the reimbursement rate set by the Centers for Medicare and Medicaid Services (CMS). CMS reimburses $324.58 for the EGFR single gene test (CPT81235), $597.91 for the targeted genomic sequencing of the Solid Tumor Focus Assay (CPT81445), and $2919.60 for the comprehensive genomic sequencing of the FoundationOne® assay (CPT81455).
Clinical Implications of Testing
The most important parameter of a clinical assay is how the results are used by clinicians to impact patient care. To understand this, we first reviewed results from all 302 patients who had undergone sequencing using our in-house assay and charted the genetic alteration data for each patient. We found that using our assay we detected FDA or NCCN guideline genetic alterations in 60% of our patients (Figure 2A, B). Nearly 42% of total patients had a KRAS mutation, which is higher than the roughly 25% of other studies17,18 and reflective of the higher smoking prevalence in our patient population. We detected FDA or NCCN guideline alterations with genetically-directed therapies in 18% of all patients (Figure 2A-C). This mutation rate was nearly the same to FoundationOne® testing within our system from 2014-2016, with 39% of patients have KRAS mutations and 21% of patients having one of the FDA or NCCN guideline alterations with genetically-directed therapies (Supplemental Figure 2A, B). The percent of tumors with EGFR tyrosine kinase inhibitor (TKI)-sensitive alterations was also similar within the 3 assays (Supplemental Figure 2C).
Figure 2. Clinical Outcome Data.
A) Flow chart of clinical outcomes based on patient tumor genomic alteration status. B) Alteration data for all 302 tumors assayed. C) Distribution of only actionable alterations according to FDA/NCCN guidelines. D) Clinical outcomes for patients with tumors containing actionable alterations.
Next, we sought to understand how the reported alterations with genetically-directed therapies were being used by our clinicians to guide therapy in practice. We manually reviewed the charts for the 302 patients who had undergone sequencing with our in-house assay to determine the clinical implication of their sequencing results. We found that 60% were put on genetically-directed therapies. However, 13% were lost to follow-up, which may be due to changing care to another institution, so the number of patients receiving genetically-targeted therapy may be slightly higher. The other 27% were continued on chemo-radiation or admitted to hospice (Figure 2A, D and Table 1). The distribution of which alterations were treated with genetically-directed therapy was fairly even across alterations (Table 1)
Table 1.
Clinical outcomes broken down by alteration.
| Clinical Action |
EGFR mutation |
ALK fusion |
BRAF V600E |
MET ex.14 skip |
ERBB2 mutation |
RET fusion |
Total (%) |
|---|---|---|---|---|---|---|---|
| Genetically targeted therapy | 19 | 5 | 3 | 2 | 29 (54) | ||
| Immunotherapy (PD-L1+) | 1 | 2 | 3 (6) | ||||
| Chemo-radiation | 3 | 1 | 2 | 2 | 2 | 10 (18) | |
| Hospice (no therapy) | 4 | 1 | 5 (9) | ||||
| Lost to follow-up | 2 | 1 | 1 | 1 | 2 | 7 (13) | |
| Total | 28 | 6 | 5 | 8 | 3 | 4 | 54 (100) |
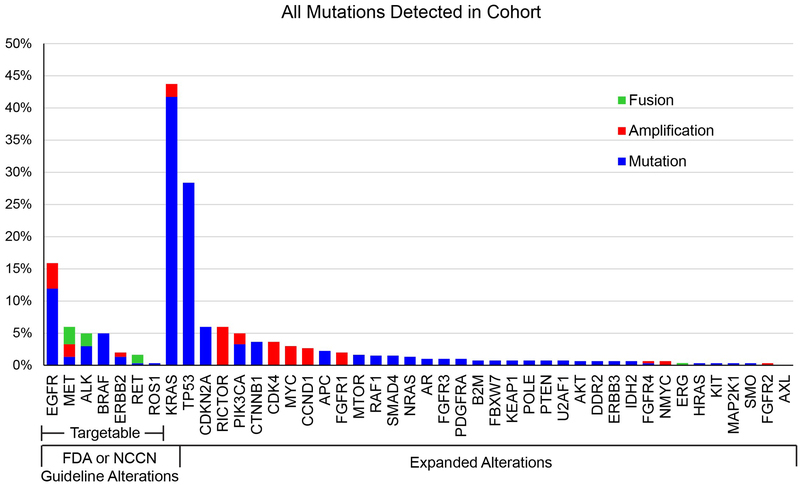
In addition to the FDA and NCCN guideline alterations, the Solid Tumor Focus Assay analyzes over 60 other alterations, including emerging lung adenocarcinoma targets as well as important alterations for other types of solid tumors. Within our patient cohort, we found a number of alterations that were outside of the FDA and NCCN guidelines (Figure 3), some of which have clinical trials targeting the specific alterations, have prognostic indications, or may be used to understand implications of the mutations in future retrospective research studies. When including all alterations tested, we found nearly 80% of patients had tumors with at least one alteration when using the Oncomine™ Focus Assay and 96% had at least one alteration when using our expanded Solid Tumor Focus Assay, compared to 60% when only reporting the FDA and NCCN guideline alterations.
Figure 3. Alteration Rates.
Rates of alterations of all detected alterations within the cohort of 302 lung adenocarcinomas assayed at UHCMC. Percent reflects rate of alterations in all 302 patients for alterations assessed by the Oncomine™ Focus Assay, or in 134 patients for alterations only assessed by the expanded Solid Tumor Focus Assay.
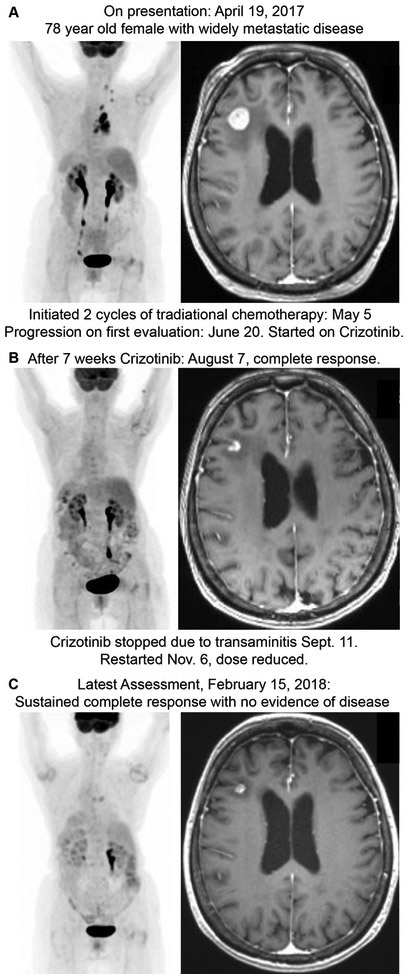
By conducting a larger panel upfront, we are able to provide clinicians and patients with alteration data for the standard genes included in FDA and NCCN guidelines in about the same amount of time and same reliability as a single gene in-house EGFR assay. Additionally, with the same assay, we are able to provide clinicians with information on a large number of other alterations, including the expanded FDA and NCCN guideline alterations and alterations outside these guidelines. This information could be used to start patients on newly approved genomically-driven therapies, enroll patients in current or future clinical trials, start patients on therapies through off-label use, inform management of immunotherapy, or change overall treatment strategy. Indeed, we saw examples of these scenarios in our patient cohort (Table 2). An exemplary case of this can be seen in Figure 4. A 78 year old woman with widely metastatic lung adenocarcinoma was found to have MET exon 14 deletion by our Solid Tumor Focus Assay and was treated with Crizotinib. She had a complete sustained response seen within 7 weeks and continues at 8 months (Figure 4). While MET alteration are part of the expanded target guidelines, many centers do not test for MET and ERBB2 alterations as part of their standard upfront workup.
Table 2.
Different types of guidance that can be provided by the using an upfront expanded assay that includes both secondary alterations to assess via the FDA/NCCN guidelines as well as alterations not included in the guidelines.
| Case studies of clinical management change based on alterations found from expanded testing | ||
|---|---|---|
| Class of guidance | Alteration | Clinical Outcome |
| Extended Targets | MET exon 14 deletion | 78 yr old female, never-smoker: Complete response of widely metastatic disease, including brain metastasis on crizotinib. Initially started on routine chemotherapy-2 cycles of carboplatin and pemetrexed with gamma-knife to brain lesion, but was progressing on first assessment. Started on crizotinib and had a complete response, including brain lesions on first assessment. No evidence of disease after 2 months of crizotinib. Stopped crizotinib after transaminitis, but still no evidence of disease 6 months later (Figure 4). |
| Potential Immunotherapy guidance | B2M p.L15Ffs*41 | 69 year old male: Presented with widely metastatic disease. Negative for all guideline mutations except tumor had a KRAS activating mutation, indicating resistance to TKIs. Immunotherapy was discussed, but tumor had a B2M loss-of-function, which is associated with poor immunotherapy response. Referred to hospice. |
| General treatment strategy: (Change in diagnosis) | TMPRSS-ERG fusion | 81 yr old male with 18 year remote history of treated prostate cancer controlled with androgen deprivation therapy. New onset shortness of breath and other symptoms brought him in and he was diagnosed with widely metastastic lung adenocarcinoma and was started on traditional chemotherapy. Found to have TMPRSS-ERG fusion that is almost exclusively found in prostate cancer. Progressed on chemotherapy and then reassessed for primary prostate and lost to follow-up. |
Figure 4. Case Example.
PET CT and T1-weighted MRI of the brain at A) presentation, B) after 7 weeks of crizotinib, and C) 8 months after starting crizotinib. Patient was discovered to have a MET exon 14 deletion using the Solid Tumor Focus Assay and clinical management and outcome was changed based on this result.
Discussion
Our goal was to create an efficient and cost-effective molecular testing system for our institution that enabled clinicians to initiate guided therapy for our patients quickly. To accomplish this, we instituted reflex testing for all advanced stage lung adenocarcinoma to a NGS focused panel that included alterations for all FDA-approved and NCCN guideline-recommended therapies, as well as other emerging markers for lung adenocarcinoma that could add valuable clinical information in a subset of cases. The testing used an ion torrent system, which enabled a reliable, cost effective assay with turn-around times similar to standard singe-gene EGFR testing.
Our success rate was 95%, which is a significant improvement over other reported assays. For comparison, the Lung Cancer Mutation Consortium reported a 71% success rate17. Completed in 2015, they used a system of sequential testing, rather than a single upfront NGS panel, and they used assays that required higher amounts of input material. The majority of the failures reported in the Lung Cancer Mutation study were due to inadequate sample, which can stem from doing multiple assays or from assays needing more material than is available. Single assay mutation panels can also need significantly more input material than our focused panel. The largest commercial provider for NGS panel testing is Foundation Medicine and their FoundationOne® assay, which is a broader assay but requiring five times more input material than our Solid Tumor Focus Assay16. The small percentage of samples that failed in our assay typically only failed the RNA component of the assay and these failures were nearly all due to poor quality of the starting RNA. In 99% of cases, we were able to report out DNA alteration data. Of the 1% of samples in our cohort that failed the DNA component of the assay, two cases were due to severe deamination artifact and one case did not amplify a sufficient library. When an assay fails or there is insufficient material, the patient either loses the ability to get genomically-driven targeted therapy or has to undergo another biopsy, losing time and leading to increased cost for the health system. This provides further rationale that testing for all mutations in a single assay with low input requirements is beneficial for patients and clinicians.
Our turn-around time was 4.8 days, significantly faster than broader assays used for lung adenocarcinoma. In our experience at our institution, FoundationOne® testing had a turn-around time of 8.5 days. The MSK-IMPACT panel of 341 cancer associated genes had a reported turn-around time of 17 days in a recent large-scale study of lung adenocarcinoma tumors18. However, this study analyzed both match normal blood and tumor, which may allow for a larger number of called variants18.
This study demonstrates that the Oncomine™ Focus Assay is not only a reliable assay to run in-house, but it also allows for simple expansion to a larger number of targets as the field evolves. It is also cost effective without sacrificing clinically relevant information. Our targeted Solid Tumor Focus Assay provides nearly all of the actionable information of more comprehensive sequencing while costing significantly less. It is also cost effective compared to sequential single-gene testing, as the guideline workup using single gene testing requires 3 individual assays. With this assay we are able to discover actionable alterations that may not be commonly tested in the community that can be used to impact patient care (See Figure 3 and Table 2). We believe it’s the right balance between cost and information gained.
Most importantly, we demonstrate this system allowed our clinicians to efficiently initiate genotype-directed targeted therapies for patients with lung adenocarcinoma in our health system. Given the increased response rates to genotype directed therapies, we hope that this leads to increased utilization of these therapies and better outcomes for our patients.
Supplementary Material
Take Home Messages.
Genomic testing is an integral component in the treatment for patients with advanced lung adenocarcinoma. Time to appropriate treatment depends on quick, reliable, and accurate testing.
We demonstrate that implementing in-house genomic sequencing of these tumors is reliable, cost-effective, and significantly reduces time to report of genomic data. In fact, the cost and turn-around time was similar to single gene testing of EGFR, in our experience.
By using a focused panel of alterations upfront, we were able to provide clinicians with all alterations in the FDA and NCCN guidelines that allowed them to more efficiently initiate genotype directed targeted therapies in our health system.
Footnotes
Licence for Publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in JCP and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
Competing Interest
Please list Competing Interests if they exist if not please include the following statement; Competing Interest: None declared.
DISCLAIMERS: The authors have no disclaimers to report.
REFERENCES
- 1.Noone AM, Cronin KA, Altekruse SF, et al. Cancer Incidence and Survival Trends by Subtype Using Data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2017;26(4):632–41. doi: 10.1158/1055-9965.EPI-16-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenthal GM, Karuri SW, Zhang H, et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33(9):1008–14. doi: 10.1200/JCO.2014.59.0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. The New England journal of medicine 2010;363(18):1693–703. doi: 10.1056/NEJMoa1006448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. The New England journal of medicine 2013;368(25):2385–94. doi: 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 5.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. The New England journal of medicine 2014;371(21):1963–71. doi: 10.1056/NEJMoa1406766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. The New England journal of medicine 2017;376(7):629–40. doi: 10.1056/NEJMoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. The Lancet Oncology 2016;17(7):984–93. doi: 10.1016/S1470-2045(16)30146-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazandjian D, Blumenthal GM, Luo L, et al. Benefit-Risk Summary of Crizotinib for the Treatment of Patients With ROS1 Alteration-Positive, Metastatic Non-Small Cell Lung Cancer. The oncologist 2016;21(8):974–80. doi: 10.1634/theoncologist.2016-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odogwu L, Mathieu L, Blumenthal G, et al. FDA Approval Summary: Dabrafenib and Trametinib for the Treatment of Metastatic Non-Small Cell Lung Cancers Harboring BRAF V600E Mutations. The oncologist 2018. doi: 10.1634/theoncologist.2017-0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khozin S, Weinstock C, Blumenthal GM, et al. Osimertinib for the Treatment of Metastatic EGFR T790M Mutation-Positive Non-Small Cell Lung Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23(9):2131–35. doi: 10.1158/1078-0432.CCR-16-1773 [DOI] [PubMed] [Google Scholar]
- 11.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine 2016;375(19):1823–33.doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 12.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine 2015;373(17):1627–39. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. The Journal of molecular diagnostics : JMD 2018. doi: 10.1016/j.jmoldx.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 14.Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN 2017;15(4):504–35. [DOI] [PubMed] [Google Scholar]
- 15.Hovelson DH, McDaniel AS, Cani AK, et al. Development and validation of a scalable next-generation sequencing system for assessing relevant somatic variants in solid tumors. Neoplasia 2015;17(4):385–99. doi: 10.1016/j.neo.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature biotechnology 2013;31(11):1023–31. doi: 10.1038/nbt.2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10(5):768–77. doi: 10.1097/JTO.0000000000000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan EJ, Kim HR, Arcila ME, et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer discovery 2017;7(6):596–609. doi: 10.1158/2159-8290.CD-16-1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.