Abstract
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) (RES) is a naturally-derived phytoestrogen found in the skins of red grapes and berries and has potential as a novel and effective therapeutic agent. In the current study, we investigated the role of microRNA (miRNA) in RES-mediated attenuation of experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis. Administration of RES effectively decreased disease severity, including inflammation and central nervous system immune cell infiltration. miRNA microarray analysis revealed an altered miRNA profile in encephalitogenic CD4+ T cells from EAE mice exposed to RES treatment. Additionally, bioinformatics and in silico pathway analysis suggested the involvement of RES-induced miRNA in pathways and processes that regulated cellular proliferation. Additional studies confirmed that RES affected cell cycle progression and apoptosis in activated T cells, specifically in the brain.RES treatment significantly upregulated miR-124 during EAE, while suppressing associated target gene, sphingosine kinase 1 (SK1), and this too was specific to mononuclear cells in the brains of treated mice, as peripheral immune cells remained unaltered upon RES treatment. Collectively, these studies demonstrate that RES treatment leads to amelioration of EAE development through mechanism(s) potentially involving suppression of neuroinflammation via alteration of the miR-124/SK1 axis, thereby halting cell-cycle progression and promoting apoptosis in activated encephalitogenic T cells.
Keywords: Experimental autoimmune encephalitis (EAE), Resveratrol (RES), CD4+ T cells, microRNA 124 (miR-124), Sphingosine kinase 1 (SK1), Multiple sclerosis (MS)
Introduction
Multiple sclerosis (MS) is a neurodegenerative disease caused largely by T cell-mediated autoimmune destruction of the protective myelin sheaths surrounding neurons in the central nervous system (CNS). While there is no cure for MS, there are modestly effective disease-modifying treatments. However, because such treatments are immunosuppressive, they often predispose patients to opportunistic infections and in many cases, patients are non-responsive or develop tolerance. Thus, the importance for developing better treatments for MS is immediately obvious. Experimental autoimmune encephalomyelitis (EAE) is a laboratory mouse model of multiple sclerosis and its pathogenesis is characterized by proinflammatory Th1 and Th17 CNS infiltration and subsequent demyelination and inflammation. Adoptive transfer of encephalitogenic CD4+ T cells from diseased mice induces EAE in recipient mice, indicating a pivotal role for CD4+ T cells in mediating disease (Batoulis et al. 2011).
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) (RES) is a naturally occurring, plant-derived phytoestrogen initially drawing interest for its ability to promote disease resistance in plants (Gautam and Jachak 2009). In addition to its fungitoxic and protective properties, other pharmacological actions have been assigned including antioxidant, anticarcinogenic and anti-inflammatory (Weiskirchen and Weiskirchen 2016). While RES is extensively studied in cancer and cardiovascular disease, it has recently emerged as a potential therapeutic for the treatment of various inflammatory diseases. RES acts as an anti-inflammatory agent through its antioxidant properties and through inhibition of lipoxygenase and cyclo-oxygenase formation (Gautam and Jachak 2009). Moreover, RES regulates specific cell functions including apoptosis and cell cycle progression (Quoc Trung et al. 2013). In models of MS, RES has well-established neuroprotective effects (Pallas et al. 2013) and it negatively regulates pro-inflammatory immune cell function. RES attenuates EAE severity by altering T cell phenotype (Imler Jr. and Petro 2009), and by aryl hydrocarbon and estrogen receptor-mediated T cell apoptosis (Singh et al. 2007), although the mechanisms of apoptosis are unclear. RES and other polyphenolic compounds regulate pro- and anti-inflammatory miRNA expression and considering the established neuroprotective effects, along with its rapidly growing anti-inflammatory properties, RES is emerging as an ideal candidate for the treatment of neuroinflammatory diseases like MS.
Epigenetic regulation of gene expression is a major mechanism by which numerous cellular processes occur and drives both normal physiological and pathophysiological events. MicroRNAs are small non-coding RNAs that influence gene expression by binding to complimentary sequences in target genes and, in most cases, negatively regulating their expression. miRNAs are involved in basic cell functions including metabolism, molecule transport and signaling, as well as in coordinated events such as differentiation, growth and proliferation and apoptosis. miRNAs regulate T cell differentiation by directing expression of cytokines and transcription factors. For example, miR-31 negatively regulates generation of anti-inflammatory T regulatory cells (Fayyad-Kazan et al. 2010) and let-7e positively regulates pro-inflammatory Th1 and Th17 differentiation (Guan et al. 2013). Likewise, miRNAs have a role in regulating macrophage polarization and function, as miR-146a drives M2 macrophage polarization by inhibition of Notch1 signaling (Wang et al. 2016) and miR-155 drives M1 polarization in a model of viral myocarditis (Zhang et al. 2016). Furthermore, identifying miRNAs involved in processes key for immune cell differentiation, activation and function will shed light on the still unclear epigenetic mechanisms by which immune cell plasticity is regulated. In addition to roles in immune cell function, miRNAs, have been identified in several pathologies including cancer and autoimmune diseases (Lekka and Hall 2018). miRNA expression studies carried out in MS patients and in EAE animals have identified miRNAs that differ in disease vs normal states, classifying them as potential bio-markers of disease (Thamilarasan et al. 2012).
In this study, we hypothesized that treatment with naturally-occurring RES prevents neuroinflammation via miRNA-mediated modulation of activated CD4+ T cell survival. Our data demonstrated that treatment of EAE mice with RES diminished disease severity with concurrent alterations in the expression of a number of miRNAs in brain infiltrating T cells. Furthermore, we found that RES altered the miR-124/SPHK1 axis, as well as induced apoptosis specifically in immune cells found in the brains but not in peripheral lymphoid organs of diseased mice. Overall, our studies indicate that RES attenuates neuroinflammation seen in EAE potentially through modulation of key miRNAs involved in cell cycle progression and apoptosis in activated encephalitogenic T cells.
Results
Resveratrol Diminishes EAE Severity
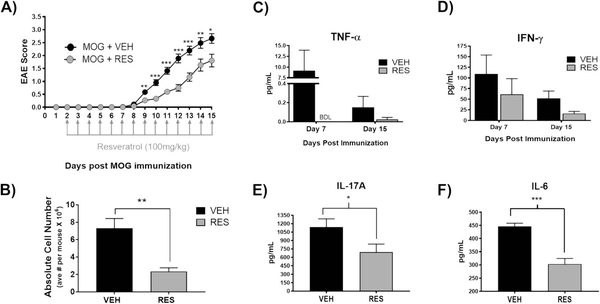
In this study, we evaluated the role of microRNAs (miRNAs) in mediating the protective effects of the natural plant-derived phytoalexin resveratrol (RES) on myelin oligodendrocyte peptide (MOG35–55)-induced autoimmune encephalomyelitis (EAE), a mouse model of the neuroinflammatory disease, multiple sclerosis (MS). EAE was induced in C57BL/6 mice using MOG35–55 as previously described (Chitrala et al. 2017). Vehicle (VEH) or RES at 100 mg/kg was given daily, beginning on day 2 following immunization. Mice were evaluated daily for disease severity and clinical scores were assigned. As evidenced by clinical scores, mice given RES exhibited significantly less-severe motor impairment relative to VEH-treated mice (Fig. 1a). Furthermore, mononuclear cell infiltration into the brains of EAE mice was significantly reduced in RES-treated mice when compared to VEH-treated mice (Fig. 1b). Additionally, serum samples taken from VEH-and RES-treated mice on days 7 and 15 post-immunization indicated lower levels of circulating pro-inflammatory cytokines TNFα and IFNγ, a hallmark cytokine of EAE, in RES-treated mice relative to VEH-treated mice (Fig. 1c and d). Moreover, upon ex vivo MOG restimulation of brain infiltrating mononuclear cells, those derived from RES-treated mice produced significantly less pro-inflammatory cytokines IL-6 and IL-17A, also hallmark EAE cytokines (Damsker et al. 2010), when compared to VEH-treated mice (Fig. 1e and f).
Fig. 1. Resveratrol diminishes EAE severity and pro-inflammatory cytokines.
MOG35–55-immunized mice were treated daily, starting on day 2, with vehicle (VEH) or resveratrol (RES) at 100 mg/kg by oral gavage. A) EAE clinical scores of VEH- and RES-treated mice (n > 9). B) Absolute number of brain-infiltrating mononuclear cells (n = 4). Data are representative (n > 9). (C) TNF-α and D) IFNγ serum cytokine levels detected by ELISA on days 7 & 15 post immunization. Brain mononuclear cells were isolated from VEH- or RES-treated mice and stimulated ex vivo with 30μg/mL MOG for 72 h. Supernatants were analyzed for E) IL-17A and F) IL-6. Data presented as the mean ± S.E.M. of triplicates. Significance assessed using two-tailed student’s T test: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. BDL, below detection level
Resveratrol Alters Immune Cell Distribution and Activation in EAE
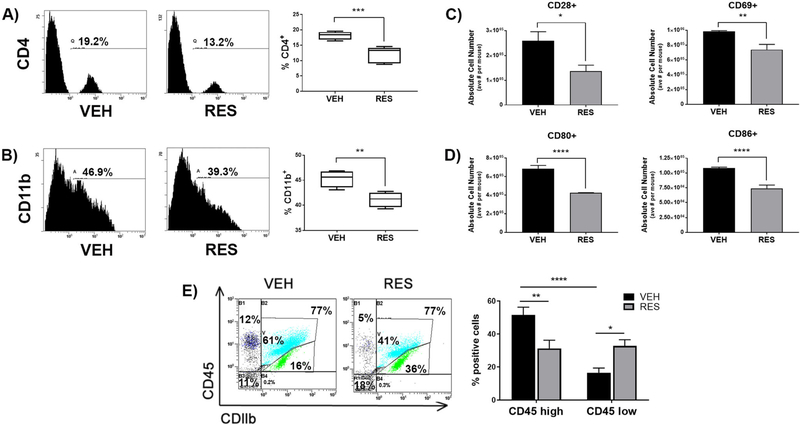
To evaluate the effect of RES on brain resident or infiltrating immune cells, mononuclear cells were isolated from brains of VEH- or RES-treated EAE mice and stained with antibodies representative of varying immune cells and activation states. There was a significant decrease in infiltrating CD4+ T helper cells, as well as CD11b + resident microglia or infiltrating macrophages (Fig. 2a and b). Additionally, mononuclear cells derived from the brains of RES-treated mice had significantly lower expression of the T cell co-stimulatory molecule CD28 and the T cell activation marker CD69 (Fig. 2c). Important in antigen presenting cell-mediated activation of T cells, co-stimulatory molecules CD80 and CD86 were also significantly decreased in cells derived from brains of RES-treated EAE mice relative to VEH-treated EAE mice (Fig. 2d).
Fig. 2. Resveratrol alters immune cell distribution and activation in the brains of EAE mice.
Infiltrating mononuclear cells were isolated from brains of VEH- or RES-treated EAE mice and stained for the indicated cell surface markers and analyzed by flow cytometry. Representative histograms and bar graphs of combined experiments (n > 3) displayed as percent positive cells of A) CD4 and B) CD11b. C) Absolute cell numbers of T cell co-stimulatory molecule CD28 and activation marker CD69 and D) co-stimulatory molecules CD80 and CD86. E) Representative dot plots of CD11b+/CD45hi and CDllb+/CD45lo mononuclear cells and bar graphs of combined experiments (n > 3). Data is presented as the mean ± S.E.M. Statistical significance (A-D) evaluated using Student’s T test or (E) One-way ANOVA with Tukey’s multiple comparisons: *, p < 0.05; ** p < 0.01; ***, p < 0.001; ****, p < 0.0001
Next, in an effort to distinguish between brain-resident microglial cells and infiltrating monocytes/macrophages, cells from the brains of VEH- or RES-treated EAE mice were stained with antibodies against CD11b and CD45. CD11b+/CD45 low cells are representative of brain-resident resting microglia, while CD11b+/CD45 high cells represent activated infiltrating monocytes/macrophages and may also include activated resident microglia (Ponomarev et al. 2006). Activated infiltrating monocytes/macrophages or activated resident microglia made up a large portion of cells derived from brains of VEH-treated EAE mice with approximately 50% being CD11b+/CD45 high, while substantially fewer cells (p < 0.0001) from VEH-treated mice represented resting microglia, with <20% being CD11b+/CD45 low (Fig. 2e). On the other hand, cells derived from the brains of RES-treated EAE mice were represented by nearly equal proportions of CD45 high and CD45 low cells, with approximately 30% each of resting microglia and activated microglia or activated infiltrating monocytes/macrophages. Additionally, the proportion of activated monocytes/macrophages (CD45 high) from the brains of VEH-treated mice (51.5 ± 4.7%) was significantly higher (p < 0.001) than the proportion from RES-treated mice (31.2 ± 5.0%); meanwhile, RES-treated mice (32.8 ± 3.8%) had a significantly higher proportion (p < 0.05) of resting microglia (CD45 low) relative to VEH-treated mice (16.5 ± 2.9%). Taken together, these data indicated that RES diminishes clinical symptoms of EAE as well as decreases immune cell infiltration and activation in the brains of EAE mice.
Resveratrol Treatment Leads to Cell-Cycle Arrest and Apoptosis
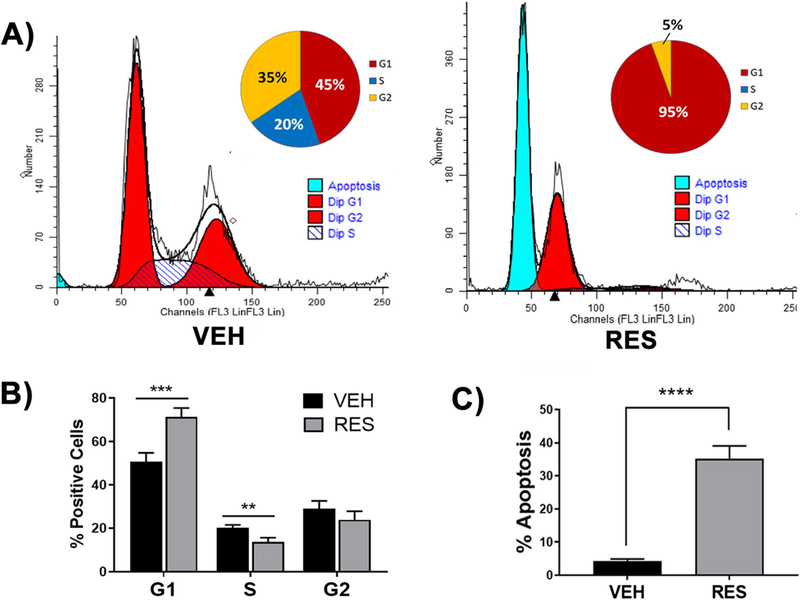
Given that RES-treatment significantly reduced brain mononuclear cells in numbers and percentages in EAE (Figs.1 and 2), (Singh et al. 2007) we evaluated the effect of RES on brain mononuclear cell apoptosis and cell cycle. To this end, brain mononuclear cells from VEH- or RES-treated EAE mice were isolated and stained with a propidium iodide/RNase solution and subjected to flow cytometry (Fig. 3a). Cells derived from VEH-treated mice had detectable G0/G1, S and G2/M phases at 45%, 20% and 35%, respectively, when analyzed using ModFit LTsoftware; meanwhile, only G0/G1 and G2/M were detected at 95% and 5%, respectively, in RES-treated mice, with no cells detected in S phase (Fig. 3a). Furthermore, when data from multiple experiments were combined and analyzed for cell cycle, there was a significant increase in the proportion of cells in G0/G1 phase (p < 0.001) and a decrease in cells in S phase (p < 0.01) and G2/M phase relative to VEH-treated mice (Fig. 3b), indicating that RES-treatment led to G0/G1 arrest of brain mononuclear cells in EAE. Moreover, cells derived from RES-treated EAE brains underwent significantly (p < 0.0001) more apoptosis than those derived from VEH-treated EAE mice with 35.2% (±12%) apoptosis in RES-treated mice and 4.2% (±2.2%) in VEH-treated mice (Fig. 3c).
Fig. 3. Resveratrol induces apoptosis in brain-infiltrating cells.
Brain-derived mononuclear cells from VEH- or RES-treated EAE mice were stained with a PI/Rnase solution and cell cycle analysis evaluated using flow cytometry. A) Flow cytometry data from a representative experiment was analyzed and stages of the cell cycle modeled using MODFit LT software. Inset, percentage of cells in stages of cell cycle displayed as pie charts, data are representative. C) Percentage of cells in stages of cell cycle and D) apoptotic cells as indicated by ModFit LT analysis from combined experiments (n > 3). Data represented as the mean ± S.E.M. Significance evaluated using Student’s T test: **, p < 0.01; ***, p < 0.001; ****, p < 0.0001
Resveratrol Alters microRNA Expression in Encephalitogenic CD4+ T Cells
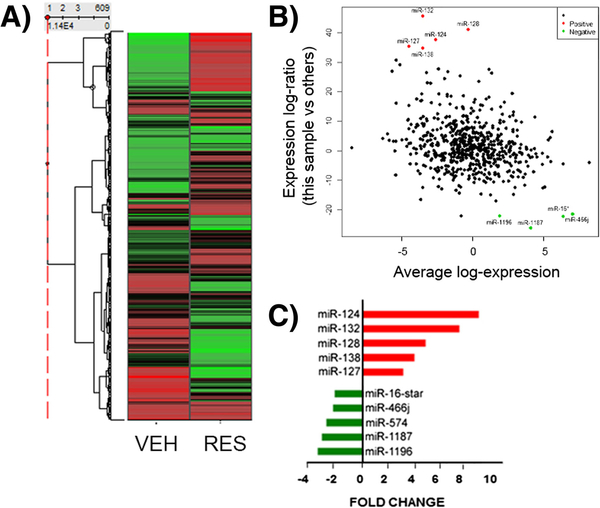
There is ample evidence to support a role for miRNA in the regulation of many cellular processes, including apoptosis and proliferation, as well as in driving T cell phenotype (Guan et al. 2013) and in pathogenesis of clinical disorders, such as MS and EAE. We therefore hypothesized that RES-mediated protection from EAE may result from miRNA-mediated modulation of encephalitogenic CD4+ T cell function. In order to investigate the role of miRNAs in inhibiting EAE by RES, miRNA expression in CD4+ T helper cells from the brains of VEH- or RES-treated EAE mice was evaluated using an Affymetrix miRNA1.0 array. miRNA values are expressed as fold change (RES- vs VEH-treated EAE mice) and data for 609 miRNAs are presented in a heat map (Fig. 4a). We identified 13 significantly down-regulated and 52 significantly up-regulated miRNAs with a greater than 1.5-fold change with RES treatment (Fig. 4b). The top five up- and down-regulated miRNAs detected by array analysis upon RES treatment were miR-124, miR-132, miR-128, miR-138, miR-127 and miR-16*, miR-466j, miR-574, miR-1187, miR-1196, respectively, and are shown as fold change in Fig. 4c and indicated on MA plot in Fig. 4b. In multiple independent experiments, qRT-PCR was performed to validate the top up-regulated miRNAs detected by microarray analysis (Supp. Fig. 1). These data indicated that RES differentially regulates expression of miRNA in encephalitogenic CD4+ T in EAE.
Fig. 4. Resveratrol alters miRNA expression in brain-derived CD4+ T cells.
CD4+ T cells were purified from the brain as described in Fig. 2 and analyzed for miRNA expression. A) Heatmap representing miRNA fold change in enecphalitogenic CD4+ T cells from VEH- or RES- treated EAE mice. B) Array analysis indicated 13 down- and 52 up-regulated miRNAs in RES- vs VEH-treated EAE mice, changes greater than ± 1.5 fold-change, visualized on an MA plot, where the data was transformed onto M (log ratio) and A (mean average) scales. C) The top 5 up-and down-regulated miRNAs determined by array analysis
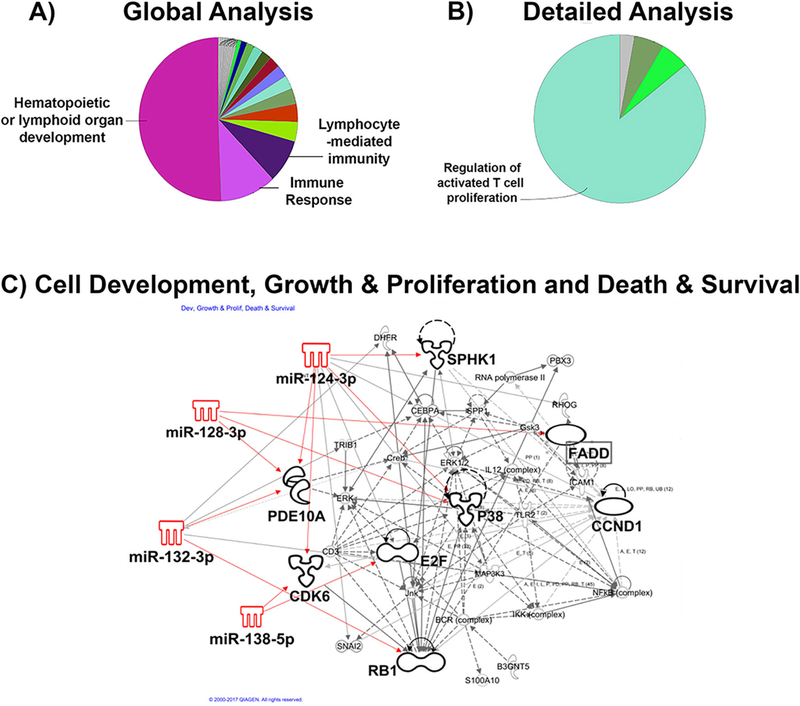
Ontology and Pathway Analysis of Genes Targeted by Resveratrol-Altered miRNAs
Because the miRNAs which were up-regulated with RES treatment were more differentially expressed (4–10-fold increased) than the miRNAs that were down-regulated with RES treatment (<3-fold decreased), highly-predicted and previously-validated target mRNAs were indexed for the 5 most-highly up-regulated miRNAs and used for gene ontology enrichment and biological function analyses. In order to gain insight into the potential functional roles of the target genes and using bioinformatics software, Cytoscape, gene ontology enrichment analysis of target genes was performed and mapped for the specific biological process, Immune System Processing. A global analysis revealed significant overlap between highly-predicted and previously-validated miRNA target genes and pathways involved in Hematopoiesis or Lymphoid Organ Development (p = 1.1 × 10−5), Immune Response (p = 1.6 × 10−4 ), and Lymphocyte-mediated Immunity (p = 0.02) with 37%, 24% and 18% associated genes, respectively (Fig. 5a). A detailed analysis of target genes revealed significant overlap with pathways related to Regulation of Activated T Cell Proliferation (p = 0.03), with 28% of target genes associated with the biological function (Fig. 5b).
Fig. 5. Gene Ontology and Pathway Analysis.
Gene ontology enrichment analysis of target genes was performed and mapped for Biological Process: Immune System Process using Cytoscape bioinformatics software. A) Global analysis revealed significant overlap between target genes and pathways involved in Hematopoiesis or Lymphoid Organ Development, Immune Response and Lymphocyte-mediated Immunity. B) A more detailed analysis using Cytoscape revealed overlap between target genes and Regulation of Activated T cell Proliferation. Statistical significance assessed using Enrichment/Depletion (Two-sided Hypergeometric test) with Benjamini-Hochberg correction. C) Ingenuity Pathway Analysis (IPA) software was used to evaluate pathway analysis of the top 5 up-regulated miRNA target genes; Top Network: Cell Morphology, Development and Growth & Proliferation (p = 4.3 × 10–5–2.0 × 10–2); Functional and Canonical Pathway Analysis statistical significance assessed using Fisher’s exact Test. (SPHK1, sphingosine kinase 1; p38, p38 MAP kinase; PDE10A, phosphodiesterase 10A; CDK6, cyclin-dependent kinase 6; E2F, E2F transcription factor; RB1, retinoblastoma protein; FADD, FAS-associated protein with death domain; CCND1, cyclin-D1)
Using Ingenuity Pathway Analysis software, further pathway analysis of the top five RES-induced miRNAs and associated highly-predicted or previously-validated target genes revealed significant overlap with molecular and cellular processes involved in Cellular Development (p = 2.5 × 10−15–2.6 × 10−4), Cellular Growth and Proliferation (p = 2.9 × 10−16-2.6 × 10−4) and Cellular Death and Survival (p = 4.5 × 10−12-3.0 × 10−4), with 885, 798 and 690 target genes identified within each biological process, respectively (Fig. 5c). Importantly, these results support a role for RES-up-regulated miRNAs in the negative regulation of T cell proliferation, which may result in cell-cycle arrest and apoptosis, like that shown previously in Fig. 3.
Resveratrol Alters the miR-124/Sphingosine Kinase 1 Axis Specifically in the Brain
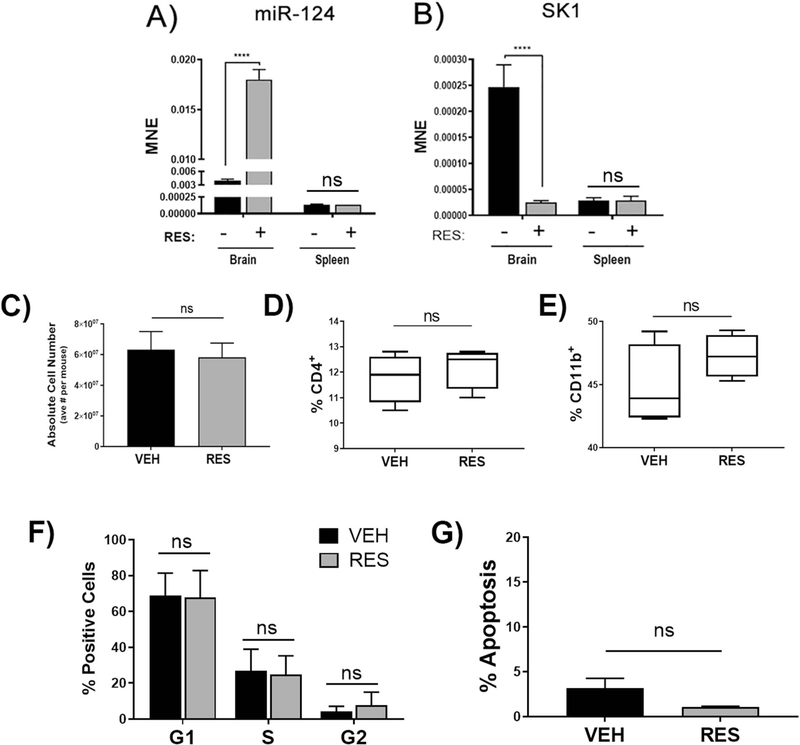
Because miR-124 was the most highly up-regulated miRNA (+8.3 fold-change) in encephalitogenic CD4+ T cells in EAE mice treated with RES relative to VEH-treated mice, and given that miR-124 is the most abundantly expressed miRNA in the brain (Mishima et al. 2007) and its dysregulation has been linked to central nervous system (CNS) disorders such as EAE (Ponomarev et al. 2011), we focused our further efforts on miR-124. In order to validate miRNA array findings, we isolated CD4+ T helper cells from the brains and spleens of VEH- or RES-treated EAE mice and evaluated miR-124 expression using qRT-PCR. Interestingly, we found a robust and significant increase in miR-124 in CD4+ Tcells from the brains of RES-treated EAE mice when compared to VEH-treated mice; however, there was no difference in miR-124 expression in CD4+ cells from the spleens of RES- and VEH-treated mice, suggesting a potential CNS micro-environmental component involved in RES-mediated up-regulation of miR-124 in encephalitogenic CD4+ T cells (Fig. 6a).
Fig. 6. Resveratrol alters the miR-124/SK1 axis specifically in the brain.
Spleen- or brain-derived CD4+ cells from VEH- or RES-treated EAE mice were evaluated for levels of A) miR-124 and B) target gene SK1 by qRT-PCR (n > 6). Splenocytes were isolated from VEH- or RES-treated EAE mice and analyzed for C) absolute cell number (n = 3), D) percent CD4+ T cells (and F) percent CD11b + monocytes/macrophages. Data representative of >3 independent experiments. Splenocytes were also evaluated for cell-cycle and apoptosis using PI/Rnase staining and ModFit LT software (n > 4). MNE, mean normalized expression. Statistical significance was evaluated using Student’s T test: p < 0.0001
Next, we sought to identify miR-124 target genes that may be responsible for RES-induced cell-cycle arrest and apoptosis of encephalitogenic CD4+ cells in EAE. SK1 was identified in our in silico analysis (Fig. 5c) and because it has been previously identified as a bona fide target of miR-124 and has a well-established role in cellular proliferation (Zhao et al. 2017; Xia et al. 2012; Zhou et al. 2017), we chose to further evaluate this gene in our model. CD4+ T cells derived from VEH- or RES-treated EAE brains and spleens were isolated and SK1 expression assessed using qRT-PCR. In line with miR-124 expression levels detected in brains of VEH- and RES-treated EAE mice, SK1 was significantly down-regulated in encephalitogenic CD4+ cells isolated from RES-treated EAE mice when compared to VEH-treated EAE mice (Fig. 6b). Furthermore, and also in agreement with spleen miR-124 expression, there was no detectable difference in SK1 mRNA in CD4+ cells isolated from spleens of VEH- and RES-treated EAE mice, again indicating a potential brain-specific effect of RES.
In order to further determine if RES-protective effects occur primarily through miRNA-mediated apoptosis of brain infiltrating immune cells, we evaluated spleens from VEH-and RES-treated EAE mice for cellular composition, cell cycle and apoptosis. There was no difference in absolute cell number in spleens from VEH- and RES-treated mice, primarily indicating that RES does not induce apoptosis of splenic cells (Fig. 6c). Furthermore, unlike the decrease in CD4+ and CD11b + cells which was observed in the brains of RES-treated mice, there was no significant difference in the percentages of these cell types in spleens from VEH- and RES-treated mice (Fig. 6d and e). In order to further confirm that RES was not affecting peripheral immune cells, mononuclear cells were isolated and stained with PI/RNase in order to assess cell-cycle and apoptosis. Interestingly, there was no difference in any of phases of the cell-cycle (Fig. 6f) or in apoptosis (Fig. 6g) in splenocytes derived from EAE mice treated with RES relative to VEH. Taken together, these data suggest that the protective effects of RES may be mediated by alteration of the miR-124/Sphingosine Kinase 1 axis, specifically in activated encephalitogenic T cells.
Discussion
Multiple Sclerosis (MS) is a neurodegenerative disease characterized by immune cell-mediated destruction of the myelin sheaths insulating neuronal axons in the central nervous system (CNS) (Compston and Coles 2002) and can lead to the loss or impairment of vision, hearing, cognition, locomotion and acute or chronic pain. The etiology of MS remains unclear; however, a combination of factors is thought to contribute: immunologic, environmental, infectious and genetic. Microarray studies from MS patients and laboratory models have identified genetic components, with overwhelming evidence for alterations in T cell-mediated immune mechanisms (International Multiple Sclerosis Genetics, C 2011). Experimental autoimmune encephalitis (EAE) is a well-established, T cell-mediated animal model of neuroinflammatory demyelinating disease, whereby immunization with myelin oligodendrocyte peptide (MOG35– 55) and pertussis toxin-mediated breakdown of the blood-brain barrier lead to CD4+ T helper cell-mediated inflammation and consequent demyelination. In the current study, we investigated the mechanism by which naturally-occurring,plant-derived resveratrol protects from an experimental model of MS and identified a potential role for miRNA in mediating RES-conferred protection from EAE.
We found that RES-treatment led to attenuation in clinical parameters of disease including reduced clinical scores reflective of motor impairment, reduced brain mononuclear cell infiltration and decreased circulating pro-inflammatory cytokines, consistent with our previous findings (Singh et al. 2007)(Fig. 1). Additionally, EAE mice treated with RES exhibited decreased proportions of CD4+ and CD11b + cells in the brain, as well as decreased markers of T cell activation and co-stimulatory molecules (Fig. 2). RES-induced changes in cell numbers and proportions of CD4+ and CD11b + cells were specific to the brain, as there were no differences observed in these parameters in the spleens of RES- and VEH-treated mice (Fig. 6). Furthermore, RES significantly decreased the proportion of activated infiltrating monocytes/macrophages/activated resident microglia in the brains of EAE mice, while considerably increasing the proportion of resting brain-resident microglia (Fig. 2). We found that the decreases in cellular populations in the brains of RES-treated mice were likely due to G0/G1 cell-cycle arrest and apoptosis, as RES significantly increased the positive proportion of cells in these categories relative to VEH-treated EAE mice (Fig. 3). RES-induced apoptosis was found to occur specifically in brain mononuclear cells from diseased mice, as the proportions of cells in the phases of the cell cycle and those undergoing apoptosis in the spleens of RES-treated mice were unchanged when compared to those derived from spleens of VEH-treated mice (Fig. 6). These data are consistent with our previous studies in which we found that RES induces enhanced apoptosis in activated T cells when compared to naïve T cells (Singh et al. 2007).
In silico analysis of the differentially up-regulated miRNAs detected by array analysis and their respective highly predicted and experimentally-validated target genes in encephalitogenic CD4+ T cells from the brains of RES-treated mice revealed significant overlap with cellular and biological functions including Regulation of Activated T Cell Proliferation, Cell Development, Cell Growth and Development, and Cell Survival and Death (Figs. 4 and 5). Upon further evaluation of the most significantly up-regulated miRNA by RES treatment, we identified miR-124, whose expression was significantly increased in mononuclear cells isolated from the brains of EAE mice treated with RES with a concomitant decrease in target gene, SK1 (Fig. 6). These findings were specific to the brains of RES-treated mice, the site of active inflammation, as spleens from VEH- and RES-treated diseased mice showed no differences in expression of miR-124 or SK1 (Fig. 6).
RES is a naturally-occurring, non-flavonoid polyphenolic compound that is important in plant defense against microbial pathogens (Gautam and Jachak 2009). RES has numerous beneficial effects on human health and understanding the mechanisms by which RES mediates these effects can lead to novel, innovative therapeutic approaches. RES has been shown to possess anti-inflammatory properties in numerous inflammatory and autoimmune diseases, including MS, and while complex and pleiotropic, the mode of action of RES’s anti-inflammatory effects have been attributed to inhibition of immune cell function through various mechanisms. Studies from our laboratory have shown that RES protects mice from EAE by inducing aryl hydrocarbon (AhR) and estrogen receptor-mediated apoptosis, primarily in activated T cells (Singh et al. 2007). The current study has identified a potential epigenetic-mediated mechanism involving microRNA by which RES may induce immune cell apoptosis and suppress inflammation in the CNS of mice with EAE. RES and other polyphenolic compounds have been shown to directly and indirectly modulate AhR activity, inhibiting downstream transcriptional events as well as leading to apoptosis. In one study, RES was found to induce apoptosis is EL4 cells through AhR-mediated upregulation of FAS and FASL (Singh et al. 2011), while in others, RES promoted apoptosis of breast cancer (Venkatadri et al. 2016) and prevented lung cancer progression (Yu et al. 2013) via regulation of miRNA expression. Moreover, many AhR effects are known to occur through regulation of miRNA. In a model of delayed-type hypersensitivity, our group identified AhR-mediated regulation of miRNA expression as the mechanism of protection by indoles (Singh et al. 2016), while another group found that dioxin-mediated alleviation of EAE occurred through AhR up-regulation of miR-132 and associated down-regulation of acetylcholinesterase and cholinergic inflammation (Hanieh and Alzahrani 2013). Thus, it is plausible that RES induces miR-124 expression in brain infiltrating mononuclear cells through modulation of AhR activity. Furthermore, there are numerous reports indicating that AhR inhibition prevents EAE and other forms of CNS inflammation. For example, a study from our lab demonstrated that dietary indoles I3C and DIM protected from EAE via AhR-mediated inhibition of Th17 T helper cells and promotion of regulatory T cells (Rouse et al. 2013). Other studies have found that the drug Laquinimod, currently under investigation for the treatment of MS and other neuroinflammatory diseases, prevented development of EAE and this was dependent on AhR, as the protective effects were abolished in AhR knockout mice (Berg et al. 2016). Because AhR activation in antigen-primed T cells is also known to induce apoptosis (Singh et al. 2012a), it is likely that RES, which is known to act as AhR ligand, may attenuate EAE by inducing apoptosis in activated immune cells as seen in the current study.
RES has many well-established effects on diseases of the CNS. However, the mechanism(s) by which RES acts in this context is not completely understood. In CNS inflammatory conditions, RES is neuro- and glioprotective and this has been attributed to numerous functions including stabilization of the blood-brain barrier (Wang et al. 2016), inhibition of neuronal damage (Shindler et al. 2010), promotion of neurogenesis and increased expression of neuronal SIRT1 (Shindler et al. 2007). RES-activation of SIRT1 is a crucial step in its neuroprotective abilities (Cao et al. 2018), as activation of SIRT1 promotes neuronal growth and differentiation as well as prevents neuronal apoptosis (Gomes et al. 2018). On the other hand, RES-induced SIRT1 has been shown to inhibit NFκB and T cell activation in DSS-induced colitis (Singh et al. 2010), as well as deacetylate c-jun, preventing activation of T cells in a collagen-induced arthritis model (Zou et al. 2013); therefore, we cannot rule out a role for SIRT1 in neurons or T cells in RES-mediated protection from EAE. Furthermore, RES is known to lower the Michaelis constant of SIRT1 for acetylated substrates (Howitz et al. 2003), including p53 (Vaquero et al. 2004). There is evidence that acetylation of SK1 increases its stability and function (Yu et al. 2012), therefore, the potential SIRT1-mediated deacetylation of SK1 cannot be ruled out as mechanism by which RES protects from EAE, in the current study. As an additional example of its direct effects on cells in the CNS, RES was shown to inhibit LPS-induced NO, TNF-α, IL-1β, IL-6, MCP-1 and C reactive protein in primary mouse astrocytes (Wight et al. 2012). Independent of RES’s beneficial effects on cells of the CNS in neuroinflammatory diseases, it is also known to act directly on cells of the immune system. RES has been shown to epigenetically regulate the macrophage inflammatory response, as well as survival and apoptosis, as levels of miR-Let7a were increased in human THP-1 macrophages upon RES treatment and LPS-induced production of pro-inflammatory signaling molecules TNF-α and IL-6 were reduced, while anti-inflammatory molecules IL-4 and IL-10 were increased upon RES treatment (Song et al. 2016). Furthermore, RES has been shown to inhibit LPS-induced microglial activation and proliferation, while promoting apoptosis, and drive the anti-inflammatory M2 microglial phenotype in conditions of neuroinflammatory injury (Yang et al. 2017), as well as promote tolerogenic dendritic cell differentiation (Svajger et al. 2010). The aforementioned studies are in line with our data indicating that RES-treatment leads to decreases in activated infiltrating monocytes/macrophages and increases in resting microglia. While RES has been shown to protect from Staphylococcal Enterotoxin B (SEB)-induced acute lung injury and an IL10-knockout-model of chronic colitis by indirect inhibition of T cell function in part by up-regulation of myeloid-derived suppressor cells (MDSCs) (Singh et al. 2012b), we found RES had no effect on MDSCs in the CNS in our model (data not shown). In addition to its effects on the innate immune system, RES can directly regulate T cell function. Treatment with RES was shown to inhibit CD4+ T cell proliferation and induce apoptosis in a CD4+ T cell line (Goldhahn et al. 2016), while the RES metabolite, piceatannol, inhibited T cell receptor signaling, preventing T effector cell function in primary murine splenocytes (Kim et al. 2015). Other mechanisms of action of RES on T cells include downregulation of NFKB K(Rieder et al. 2012) and alteration of the CD28/CTLA-4 and CD80 co-stimulatory pathway (Sharma et al. 2007), which is in line with our findings of RES-mediated decrease in the proportions of CD28+, CD80+, and CD86+ cells in the brains of EAE mice.
miR-124 is the most abundant miRNA in the brain and regulates neuronal differentiation by targeting a negative regulator of neural gene expression programs (Jiang et al. 2016). While miR-124 function is perhaps best understood in the context of the brain, it has also been reported to be expressed in some immune cells, particularly in brain-resident microglial cells. miR-124 has been shown to be a critical regulator of microglial function by contributing to microglial maturity (Svahn et al. 2016), modulating microglial polarization (Hamzei Taj et al. 2016), and by targeting CEBPα, a master transcription factor in myeloid cell development (Ponomarev et al. 2011). Alternatively activated M2 microglial cells are driven by miR-124 expression (Yu et al. 2017), while miR-124 inversely correlates with classically activated, proinflammatory M1 microglial markers. For example, during the active inflammation in the CNS during EAE, microglial cells (and infiltrating macrophages) up-regulate M1 markers and this occurs in parallel with a decrease in miR-124 expression (Ponomarev et al. 2007). Conversely, in normal CNS or in the CNS during recovery phases of EAE, there is an increase in miR-124 with a concomitant decrease in M1 markers (Ponomarev et al. 2011). Furthermore, Ponomerav, et. al. found that miR-124 expression in microglia or peripheral macrophages, although miR-124 expression in peripheral myeloid cells was absent or very low, resulted in a senescent phenotype and that systemic administration of miR-124 in EAE led to marked suppression of EAE (Ponomarev et al. 2011). Furthermore, miR-124 has also been found to be protective in other CNS neuroinflammatory diseases including models of stroke (Wang et al. 2017), Parkinson’s disease (PD) (Dong et al. 2017) and Huntington’s disease (HD) (Lee et al. 2017). In fact, treatment with miR-124-bearing nano-particles or –containing exosomes has emerged as a potential therapeutic modality for the treatment of PD (Saraiva et al. 2016) and HD (Lee et al. 2017). Our findings align nicely with what is currently known about miR-124 and microglial function in models of CNS inflammatory diseases, as we found that RES-treatment increased miR-124 expression, while it decreased activated microglia and/or infiltrating macrophages along with markers of activation and co-stimulatory molecules, including CD45 and MHCII (data not shown), as well as pro-inflammatory cytokines TNFα and IL-6.
Experimentally-validated target genes of miR-124 include CCAAT/enhancer-binding protein-α (CEBPα), small Cterminal domain phosphatase 1 (SCP1), Polypyrimidine tract-binding protein 1 (PTBP1), Toll-Like Receptor 4 (TLR4), a BH3-only protein (BIM) and Sphingosine Kinase 1 (SK1) (Zhao et al. 2017; Xia et al. 2012; Zhou et al. 2017; Gao et al. 2017), among others. In this study, we chose to focus on target gene SK1, as it was identified in our in silico analyses and it plays a critical role in cellular growth and proliferation (Gandy and Obeid 2013). SK1 is the enzyme responsible for phosphorylation of sphingolipid molecule, sphingosine, to sphingosine 1-phosphate (S1P). Bioactive lipid-signaling molecule S1P has a well-established role in driving pro-survival functions, including cell proliferation and migration (Gandy and Obeid 2013; Hannun and Obeid 2008), as inhibition of SK1 (and subsequently it’s product, S1P) by genetic or pharmacologic approaches leads to apoptosis and senescence (Hannun and Obeid 2008) (Supp. Fig. 2). Furthermore, inhibition of SK1 results in a flux in the sphingolipid metabolic pathway, driving the production of pro-apoptotic ceramide (Hannun and Obeid 2008) (Supp. Fig. 2). In fact, the role of SK1 in cell survival and proliferation is so well established that SK1 is considered an oncogene because it is overexpressed in numerous cancers and its expression is even used as a biomarker and prognostic indicator of disease and treatment outcome (Cai et al. 2017). Complimentary to SK1’s role as an oncogene, miR-124 has been shown to be dysregulated in many cancers with decreased levels correlating with poor prognosis and metastasis and is therefore considered a tumor suppressor (Zhao et al. 2017). It is well-established that inhibition of SK1 prevents cell proliferation and survival, as it has been extensively shown to result in cell-cycle arrest and apoptosis, as well as senescence (Taha et al. 2006) (Supp. Fig. 2). Thus, it is likely that RES-mediated down-regulation of SPHK1 via miR-124 may serve as the mechanism by which apoptosis occurs in encephalitogenic cells in EAE, in our study. Interestingly, RES is also known to directly inhibit the catalytic activity of SK1 by occupying the substrate binding pocket (Lim et al. 2014), as well as by preventing localization to the plasma membrane (Tian and Yu 2015), where it is able to access its sphingosine substrate. RES-inhibition of SK1 has also been shown to decrease its expression and result in apoptosis (Abdin 2013). Furthermore, studies have shown that SK1 is downstream of AhR and that AhR is a bona fide target of miR-124, as well (Huang et al. 2011). This, along with our data, indicates that RES may employ multiple mechanisms to negatively regulate AhR and SK1 function and it will be interesting to evaluate the RES/miR-124/AhR/SK1 axis in mediating apoptosis and subsequent protection from inflammatory diseases and other pathologies that possess an important SK1 component, of which there are many.
RES is known to alter miRNA expression in cancer, yet there have been no direct observations of RES-regulation of miR-124, or other up-regulated miRNAs detected in our study, in the context of cancer. Although RES has not been shown to regulate miR-124 in cancer, it has been shown to downregulate several oncogenic miRNAs and up-regulate tumor suppressor miRNAs (miR-663) in human colon cancer cells (Tili et al. 2010). Furthermore, RES was shown to inhibit tumorigenesis in a mouse model of colorectal cancer by suppressing oncogenic KRAS (Saud et al. 2014), which is also known to activate the SK1/S1P pathway in cancer (Gault et al. 2012). Given these studies, RES has the potential to affect tumor suppressor miR-124, along with oncogenic SK1 in cancers, as it does in our EAE studies. Moreover, there is evidence that RES suppresses the growth and proliferation of glioblastomas, leading to cell-cycle arrest and subsequent apoptosis (Clark et al. 2017; Zielinska-Przyjemska et al. 2017). Interestingly, miR-124 has also been shown to inhibit proliferation and promote differentiation of glioblastoma (Silber et al. 2008; Qiao et al. 2017); while, targeting SK1 induces apoptosis and suppresses the growth of human glioblastoma cells and xenografts (Kapitonov et al. 2009), supporting the ability of RES to alter the miR-124/SK1 axis in cancer, as it does in our study.
Our findings indicate that RES alters the miR-124/SK1 axis specifically in the brains of EAE mice, as there were no differences observed in spleens derived from VEH- or RES-treated mice. The simplest explanation of the brain-specific effects of RES in our study is that RES may accumulate at higher levels in the brain, relative to other organs. It is known that RES accumulates in both the brain (Rege et al. 2014) and spleen (Asensi et al. 2002); however, at what levels, in our model, remain to be determined. Additionally, while RES was shown to induce apoptosis in both unactivated and ConA- and antigen-activated splenocytes, the proportion of cells undergoing apoptosis in activated Tcells was significantly elevated when compared to that in unactivated cells (Singh et al. 2007). Because the brain is the target organ in EAE and the source of the antigen, there are many more activated cells present in the CNS of EAE relative to other organs. Furthermore, studies from our lab have shown that other natural products induce apoptosis, specifically in activated Tcells (Elliott et al. 2016). Thus, it is feasible that the apoptotic effects of RES in our model are carried out on brain-localized, activated cells.
Interestingly, while in vitro RES treatment of splenocytes leads to apoptosis (Singh et al. 2007), it does not induce miR-124 in peripherally-derived immune cells (Supp. Fig. 3); therefore in vitro RES-induced apoptosis in peripherally-derived immune cells may involve miRNAs other than miR-124 and/or mechanisms distinct from regulation of miRNA expression. One additional intriguing explanation for the brain-specific effects of RES in our model is based on the well-established neuronal effects of RES. RES is known to act directly onneurons and to promote neurogenesis, a process that is regulated by miR-124 (Jiang et al. 2016; Liu et al. 2016a). It is possible, therefore, that RES acts on cells in the CNS, particularly neurons, to drive miR-124 expression and immune cells in the brains of EAE mice may acquire miR-124 by virtue of being bystanders. Neurons are known to shed miRNA-containing exosomes and micro vesicles (Pinto et al. 2017; Xu et al. 2017), and infiltrating macrophages and/or activated brain-resident microglia (Pinto et al. 2017) are capable of taking-up these structures; moreover, there is evidence for exosome transfer during T cell activation (Domenis et al. 2017; Muller et al. 2016; Liu et al. 2016b), suggesting that the three dominant immune cell types that drive EAE are capable of acquiring miRNA-containing exosomes or vesicles that may potentially be released from RES-stimulated-neurons or-some-other CNS-specific cell type. Likewise, neuronal-derived miR-124-rich exosomes have been shown to drive microglial phenotype to that of senescence (Pinto et al. 2017). Additionally, miR-124-loaded nano-particles and exosomes have been evaluated for their therapeutic potential in mouse models of Parkinson’s (Saraiva et al. 2016) and Huntington’s disease (Lee et al. 2017), with favorable outcomes. While further in-depth studies of RES-mediated miR-124 expression and exosomal release from neurons, as well as immune cell uptake in EAE are required, our results suggest a necessary CNS microenvironmental component in mediating RES-induced miR-124 and cell-cycle arrest and apoptosis in brain mononuclear cells in EAE.
In conclusion, the current study identifies a potential miRNA-mediated mechanism of RES’s protective effects on EAE. We observed that RES alters miR-124/SK1 axis specifically in encephalitogenic CD4+ T cells in the brains of EAE mice. Furthermore, we show that RES treatment leads to cell-cycle arrest and apoptosis of mononuclear cells and that this occurs in a brain-specific manner, as well. These findings provide insight into how the naturally-occurring, plant-derived RES may be used to prevent CNS autoimmune and inflammatory diseases.
Materials and Methods
Animal Use and Care
Female, 8–10-week-old C57BL/6 were purchased from The Jackson Laboratory (Bar Harbor, ME) and were maintained at Association for Assessment and Accreditation of Laboratory Animal Care (AALAC)–accredited University of South Carolina, School of Medicine Animal Facility. Mice were kept in conventional housing under National Institutes of Health guidelines and according to Institutional Animal Care and Use Committee-approved protocols.
EAE Induction and Assessment and Resveratrol Treatment Regimen
EAE was induced in 8–10-week-old female C57BL/6 mice as previously described (Rouse et al. 2013). Briefly,on day 0, mice were immunized via subcutaneous injection in each hind flank of 75 μg myelin oligodendrocyte peptide (MOG35–55) (for a total of 150 μg MOG35–55) emulsified in 50 μL complete Freund’s adjuvant (CFA) (Difco, Detroit, MI) containing 6 mg/Ml killed Mycobacterium tuberculosis H37Ra(Difco).Mice were given 200 ng and 400 ng pertussis toxin (List Biologicals, Campbell, CA) via intraperitoneal injection on day 0 and day 2, respectively. Following immunization, mice were monitored daily and assigned disease scores based on the severity of disease symptoms: 0 = no symptoms; 1 = partial loss of tail tonicity; 2 = complete tail atony,clumsygait;3 = hind limb weakness, partial paralysis; 4 = complete hind limb paralysis, fore limb weakness; and 5 = tetraplegia, moribund. Myelin oligodendrocyte glycol-protein (MOG35–55) peptide and H-MEVGWYRSP FSRVVHLYRNGK-OH from PolyPeptide Laboratories San Diego (San Diego,CA). Additional measures were taken to ensure accessibility to fresh food and water for paralytic animals. Moribund animals were euthanized, as indicated in IACUC- and AALAS-approved protocols, by inhalant anesthetic isoflurane over dose. Death was not used as an index for clinical scores. Beginning on day two post-immunization, 100 mg/kg RES was administered as a suspension in 0 .2mL of water, and given daily for the duration of the experiement, as previously described. (Singh et al. 2007)
Antibodies and Flow Cytometry
In order to evaluate cell surface markers, cells were stained with fluorescently-conjugated antibodies and analyzed using the Beckman Coulter FC500 (Indianapolis, IN) The following antibodies from Bio Legend (San Diego, CA) were used: allophycocyanin (APC)-conjugated anti-CD11b (clone: M1/70), fluorescein isothiocyanate–conjugated anti-CD45 (clone: 30-F11), anti-CD11b (clone: M1/70) and anti-CD80 (clone: 16–10A1), phycoerthyrin (PE)-conjugated anti-CD4 (clone: GK 1.5), anti-CD69 (clone: H1.2F3) and anti-CD86 (clone: GL-1) and PE-Cy5-conjugated anti-CD28 (clone: 37.51).
Isolation of Brain Mononuclear Cells and MOG Restimulation
At peak of disease (approximately day 15 post-immunization), mice were perfused with 10 mL heparinized PBS via direct cardiac puncture and brains isolated. Brains from at least 10 mice were pooled and homogenized using Seward stomacher and mononuclear cells from brains isolated using a 30% percoll (GE Healthcare Life Sciences; Pittsburgh, PA) gradient. Where stated, CD4+ T cells were isolated using Stem Cell Technologies (Cambridge, MA) EasySep™ Mouse PE Positive Selection Kit and phycoerthyrin (PE)-conjugated anti-CD4 (clone: GK 1.5) (purity >90%). Where indicated, total brain mononuclear cells were cultured for 72 h in the presence of 30 μg/mL MOG. Cultures were maintained in complete RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum, 10 mM l-glutamine, 10 mM HEPES, 50 μM β-mercaptoethanol, and 100 μg/ml penicillin/streptomycin at 37 °C and 5% CO2.
Cytokine Detection
Mice were bled prior to sacrifice and serum isolated following centrifugation of blood. Cytokine levels for TNFα and IFN-γ were measured in serum and IL-17A and IL-6 were detected in cell culture supernatants following MOG restimulation. All cytokines were measured using Biolegend ELISA Max kits (San Diego, CA) per manufacturer’s protocol.
Cell Cycle Analysis
Brain mononuclear cells or splenocytes were isolated from VEH- or RES-treated mice at peak of disease. Cells were harvested and fixed with 70% ice cold ethanol overnight at 4 °C. After washing with PBS, cells were incubated in a PI/ RNase solution (4087, Cell Signaling Technologies) for 20min at 37°C. Cell-cycle analysis was performed using flow cytometry in which samples were gated on live cells. Listmode (LMD) files were further analyzed using ModFit LT (Verity Software House, Topsham, ME).
miRNA and mRNA Isolation, PCR and miRNA Array
Total RNA was collected from CD4+ T cells isolated from brain mononuclear cells or splenocytes from VEH- or RES-treated EAE mice using miRNeasy minikit (Qiagen, Valencia, CA), and quality and abundance of RNA was confirmed spectrophotometrically (ThermoFisher Nanodrop 2000). Next, we performed expression profiling of miRNAs using the Affymetrix Gene Chip miRNA 1.0 array platform (Affymetrix, Santa Clara, CA). The array included 609 murine-specific probes from Sanger miRBase (v11). A heat map was generated using a normalized log 2 expression value, i.e., normalized expression = [log2Exp – average (log2Exp)]/average (log2Exp) *100, and hierarchical clustering was performed. To visualize the differentially regulated miRNAs, we transformed the data onto M (log ratio) and A (mean average) scales in an MA plot, generated in R. To validate miRNA expression, the miScript cDNA synthesis kit (Qiagen) was used followed by quantitative real-time polymerase chain reaction (qRT-PCR) using the miScript SYBR Green PCR kit (Qiagen). Mean normalized expression (MNE) of miRNA and mRNAwas determined using Q-gene processing software and expressed relative to Snord96a (MS00033733, Qiagen) or β-Actin, respectively. MNE is directly proportional to the amount of RNA of the target gene relative to the amount of RNA of the reference gene. For target mRNA validation, iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) was used to carry out qRT-PCR. miR-124–3p primers were purchased from Qiagen (MS00029211) and β-Actin and SK1 primers purchased from Integrated DNA Technologies: sequences SK1:(Forward, 5′ TAT GCT GGG TAC GAG CAG GT 3′) (Reverse, 5’ CAG GTT CAT GGG TGA CAG GC 3′); β-Actin (Forward, GGC TGT ATT CCC CTC CAT G 3′) (Reverse, 5′ GTT GGT AAC AAT GCC ATG T 3′).
Bioinformatics and Pathway Analysis
The commercially available analysis software, Ingenuity Pathway Analysis (IPA) (Qiagen) was used to analyze and sort a total of609 miRNAs. Pathways and mRNA targets were selected and only strongly predicted or experimentally validated interactions in published literature were used to create miRNA-mRNA pathways as previously described (Hegde et al. 2013). In addition, in silico analysis of miRNA target genes was conducted using the Ingenuity knowledge base that combines data from miRBase (http://www.mirbase.org/), TarBase (http://diana.cslab.ece.ntua.gr/tarbase/), and Target Scan Human (http://www.targetscan.org/) target prediction softwares. Gene ontology enrichment analysis was performed using Cytoscape (http://www.cytoscape.org/), an open-source bioinformatics software.
Statistical Analysis
GraphPad Prism 7.03 was used for statistical analysis. Data are represented as mean values ± S.E.M. Where appropriate, a two-tailed Student’s t test or one-way ANOVA with Tukey’s multiple comparisons was used to assess statistical significance. Brains pooled from a minimum of 5 mice were used for each experiment, with n ≥ 3, as indicated in the figure legends. Statistical significance for Gene Ontology analysis was assessed using Enrichment/Depletion (Two-sided Hypergeometric test) with Benjamini-Hochberg correction. p < 0.05 was considered to be significant.
Supplementary Material
Acknowledgments
The studies were supported in part by NIH grants F32AT008539, P01AT003961, R01AT006888, R01AI123947, R01AI129788, R01MH094755, and P20GM103641.
Footnotes
Data Availability The datasets generated and/or analyzed during the current study are included in this published article or are available from the corresponding author upon reasonable request.
Compliance with Ethical Standards
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11481–019-09842–5) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Competing Interests The authors declare no financial or non-financial competing interests.
References
- Abdin AA (2013) Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol 718(1–3): 145–153 [DOI] [PubMed] [Google Scholar]
- Asensi M, Medina I, Ortega A, Carretero J, Baño MC, Obrador E, Estrela JM (2002) Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med 33(3):387–398. 10.1016/S0891-5849(02)00911-5 [DOI] [PubMed] [Google Scholar]
- Batoulis H et al. (2011) Experimental autoimmune encephalomyelitis– achievements and prospective advances. APMIS 119(12):819–830. 10.1111/j.1600-0463.2011.02794.x [DOI] [PubMed] [Google Scholar]
- Berg J, Mahmoudjanlou Y, Duscha A, Massa MG, Thöne J, Esser C, Gold R, Haghikia A (2016) The immunomodulatory effect of laquinimod in CNS autoimmunity is mediated by the aryl hydrocarbon receptor. J Neuroimmunol 298:9–15. 10.1016/j.jneuroim.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Cai H, Xie X, Ji L, Ruan X, Zheng Z (2017) Sphingosine kinase 1: a novel independent prognosis biomarker in hepatocellular carcinoma. Oncol Lett 13(4):2316–2322. 10.3892/ol.2017.5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Dou Y, Li A (2018) Resveratrol boosts cognitive function by targeting SIRT1. Neurochem Res 43(9):1705–1713. 10.1007/s11064-018-2586-8 [DOI] [PubMed] [Google Scholar]
- Chitrala KN, Guan H, Singh NP, Busbee B, Gandy A, Mehrpouya-Bahrami P, Ganewatta MS, Tang C, Chatterjee S, Nagarkatti P, Nagarkatti M (2017) CD44 deletion leading to attenuation of experimental autoimmune encephalomyelitis results from alterations in gut microbiome in mice. Eur J Immunol 47(7):1188–1199. 10.1002/eji.201646792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PA et al. (2017) Resveratrol targeting of AKT and p53 in glioblastoma and glioblastoma stem-like cells to suppress growth and infiltration. J Neurosurg 126(5):1448–1460. 10.3171/2016.1.JNS152077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A (2002) Multiple sclerosis. Lancet 359(9313): 1221–1231. 10.1016/S0140-6736(02)08220-X [DOI] [PubMed] [Google Scholar]
- Damsker JM, Hansen AM, Caspi RR (2010) Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci 1183:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenis R, Cesselli D, Toffoletto B, Bourkoula E, Caponnetto F, Manini I, Beltrami AP, Ius T, Skrap M, di Loreto C, Gri G (2017) Systemic T cells immunosuppression of glioma stem cell-derived exosomes is mediated by Monocytic myeloid-derived suppressor cells. PLoS One 12(1):e0169932 10.1371/journal.pone.0169932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong RF et al. (2017) The neuroprotective role of miR-124–3p in a 6Hydroxydopamine-induced cell model of Parkinson’s disease via the regulation of ANAX5. J Cell Biochem [DOI] [PubMed] [Google Scholar]
- Elliott DM, Nagarkatti M, Nagarkatti PS (2016) 3,39-Diindolylmethane ameliorates staphylococcal enterotoxin B-induced acute lung injury through alterations in the expression of MicroRNA that target apoptosis and cell-cycle arrest in activated T cells. J Pharmacol Exp Ther 357(1):177–187. 10.1124/jpet.115.226563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyad-Kazan H, Rouas R, Merimi M, el Zein N, Lewalle P, Jebbawi F, Mourtada M, Badran H, Ezzeddine M, Salaun B, Romero P, Burny A, Martiat P, Badran B (2010) Valproate treatment of human cord blood CD4-positive effector T cells confers on them the molecular profile (microRNA signature and FOXP3 expression) of natural regulatory CD4-positive cells through inhibition of histone deacetylase. J Biol Chem 285(27):20481–20491. 10.1074/jbc.M110.119628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy KA, Obeid LM (2013) Regulation of the sphingosine kinase/ sphingosine 1-phosphate pathway. Handb Exp Pharmacol 216: 275–303 [DOI] [PubMed] [Google Scholar]
- Gao M, Chang Y, Wang X, Ban C, Zhang F (2017) Reduction of COX-2 through modulating miR-124/SPHK1 axis contributes to the antimetastatic effect of alpinumisoflavone in melanoma. Am J Transl Res 9(3):986–998 [PMC free article] [PubMed] [Google Scholar]
- Gault CR, Eblen ST, Neumann CA, Hannun YA, Obeid LM (2012) Oncogenic K-Ras regulates bioactive sphingolipids in a sphingosine kinase 1-dependent manner. J Biol Chem 287(38):31794–31803. 10.1074/jbc.M112.385765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam R, Jachak SM (2009) Recent developments in anti-inflammatory natural products. Med Res Rev 29(5):767–820. 10.1002/med.20156 [DOI] [PubMed] [Google Scholar]
- Goldhahn K et al. (2016) Antiproliferative and pro-apoptotic activities of a novel resveratrol prodrug against Jurkat CD4+ T-cells. Anticancer Res 36(2):683–689 [PubMed] [Google Scholar]
- Gomes BAQ et al. (2018) Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: role of SIRT1. Oxidative Med Cell Longev 2018:8152373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Fan D, Mrelashvili D, Hao H, Singh NP, Singh UP, Nagarkatti PS, Nagarkatti M (2013) MicroRNA let-7e is associated with the pathogenesis of experimental autoimmune encephalomyelitis. Eur J Immunol 43(1):104–114. 10.1002/eji.201242702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei Taj S, Kho W, Aswendt M, Collmann FM, Green C, Adamczak J, Tennstaedt A, Hoehn M (2016) Dynamic modulation of microglia/macrophage polarization by miR-124 after focal cerebral ischemia. J NeuroImmune Pharmacol 11(4):733–748. 10.1007/s11481-016-9700-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanieh H, Alzahrani A (2013) MicroRNA-132 suppresses autoimmune encephalomyelitis by inducing cholinergic anti-inflammation: a new Ahr-based exploration. Eur J Immunol 43(10):2771–2782. 10.1002/eji.201343486 [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9(2):139–150. 10.1038/nrm2329 [DOI] [PubMed] [Google Scholar]
- Hegde VL, Tomar S, Jackson A, Rao R, Yang X, Singh UP, Singh NP, Nagarkatti PS, Nagarkatti M (2013) Distinct microRNA expression profile and targeted biological pathways in functional myeloid-derived suppressor cells induced by Delta9-tetrahydrocannabinol in vivo: regulation of CCAAT/enhancer-binding protein alpha by microRNA-690. J Biol Chem 288(52):36810–36826. 10.1074/jbc.M113.503037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425(6954):191–196. 10.1038/nature01960 [DOI] [PubMed] [Google Scholar]
- Huang TC, Chang HY, Chen CY, Wu PY, Lee H, Liao YF, Hsu WM, Huang HC, Juan HF (2011) Silencing of miR-124 induces neuro-blastoma SK-N-SH cell differentiation, cell cycle arrest and apoptosis through promoting AHR. FEBS Lett 585(22):3582–3586. 10.1016/j.febslet.2011.10.025 [DOI] [PubMed] [Google Scholar]
- Imler TJ Jr, Petro TM (2009) Decreased severity of experimental auto-immune encephalomyelitis during resveratrol administration is associated with increased IL-17+IL-10+ T cells, CD4(−) IFN-gamma+cells, and decreased macrophage IL-6 expression. Int Immunopharmacol 9(1):134–143. 10.1016/j.intimp.2008.10.015 [DOI] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics, C et al. (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476(7359):214–219. 10.1038/nature10251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, du J, Zhang X, Zhou W, Zong L, Dong C, Chen K, Chen Y, Chen X, Jiang H (2016) miR-124 promotes the neuronal differentiation of mouse inner ear neural stem cells. Int J Mol Med 38(5):1367–1376. 10.3892/ijmm.2016.2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, Zipkin RE, Dent P, Kordula T, Milstien S, Spiegel S (2009) Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res 69(17):6915–6923. 10.1158/0008-5472.CAN-09-0664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Lee YG, Park HJ, Lee JA, Kim HJ, Hwang JK, Choi JM (2015) Piceatannol inhibits effector T cell functions by suppressing TcR signaling. Int Immunopharmacol 25(2):285–292. 10.1016/j.intimp.2015.01.030 [DOI] [PubMed] [Google Scholar]
- Lee ST, Im W, Ban JJ, Lee M, Jung KH, Lee SK, Chu K, Kim M (2017) Exosome-based delivery of miR-124 in a Huntington’s disease model. J Mov Disord 10(1):45–52. 10.14802/jmd.16054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekka E, Hall J (2018) Noncoding RNAs in disease. FEBS Lett 592(17): 2884–2900. 10.1002/1873-3468.13182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KG, Gray AI, Anthony NG, Mackay SP, Pyne S, Pyne NJ (2014) Resveratrol and its oligomers: modulation of sphingolipid metabolism and signaling in disease. Arch Toxicol 88(12):2213–2232. 10.1007/s00204-014-1386-4 [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang Q, Cai Y, Sun D, He X, Wang L, Yu D, Li X, Xiong X, Xu H, Yang Q, Fan X (2016a) Resveratrol counteracts lipopolysaccharide-induced depressive-like behaviors via enhanced hippocampal neurogenesis. Oncotarget 7(35):56045–56059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Gao W, Yuan J, Wu C, Yao K, Zhang L, Ma L, Zhu J, Zou Y, Ge J (2016b) Exosomes derived from dendritic cells improve cardiac function via activation of CD4(+) T lymphocytes after myocardial infarction. J Mol Cell Cardiol 91:123–133 [DOI] [PubMed] [Google Scholar]
- Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T, Takizawa T, RT-PCR-based analysis of microRNA (miR-1 and −124) expression in mouse CNS. Brain Res, 2007. 1131(1): p. 37–43, 10.1016/j.brainres.2006.11.035 [DOI] [PubMed] [Google Scholar]
- Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL (2016) Tumor-derived exosomes regulate expression of immune function related genes in human T cell subsets. Sci Rep 6:20254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas M et al. (2013) Resveratrol: new avenues for a natural compound in neuroprotection. Curr Pharm Des 19(38):6726–6731. 10.2174/1381612811319380005 [DOI] [PubMed] [Google Scholar]
- Pinto S, Cunha C, Barbosa M, Vaz AR, Brites D (2017) Exosomes from NSC-34 cells transfected with hSOD1-G93A are enriched in miR-124 and drive alterations in microglia phenotype. Front Neurosci 11: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Dittel BN (2006) CD40 expression by microglial cells is required for their completion of a two-step activation process during central nervous system autoimmune inflammation. J Immunol 176(3):1402–1410. 10.4049/jimmunol.176.3.1402 [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Maresz K, Tan Y, Dittel BN (2007) CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci 27(40):10714–10721. 10.1523/JNEUROSCI.1922-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL (2011) MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alphaPU.1 pathway. Nat Med 17(1):64–70. 10.1038/nm.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao W, Guo B, Zhou H, Xu W, Chen Y, Liang Y, Dong B (2017) miR-124 suppresses glioblastoma growth and potentiates chemosensitivity by inhibiting AURKA. Biochem Biophys Res Commun 486(1):43–48. 10.1016/j.bbrc.2017.02.120 [DOI] [PubMed] [Google Scholar]
- Quoc Trung L, Espinoza JL, Takami A, Nakao S (2013) Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS One 8(1):e55183 10.1371/journal.pone.0055183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege SD et al. (2014) Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci 6:218 10.3389/fnagi.2014.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SA, Nagarkatti P, Nagarkatti M (2012) Multiple anti-inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin B-induced lung injury. Br J Pharmacol 167(6): 1244–1258. 10.1111/j.1476-5381.2012.02063.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse M, Singh NP, Nagarkatti PS, Nagarkatti M (2013) Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br J Pharmacol 169(6):1305–1321. 10.1111/bph.12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva C, Ferreira L, Bernardino L (2016) Traceable microRNA-124 loaded nanoparticles as a new promising therapeutic tool for Parkinson’s disease. Neurogenesis (Austin) 3(1):e1256855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saud SM, Li W, Morris NL, Matter MS, Colburn NH, Kim YS, Young MR (2014) Resveratrol prevents tumorigenesis in mouse model of Kras activated sporadic colorectal cancer by suppressing oncogenic Kras expression. Carcinogenesis 35(12):2778–2786. 10.1093/carcin/bgu209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Chopra K, Kulkarni SK, Agrewala JN (2007) Resveratrol and curcumin suppress immune response through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin Exp Immunol 147(1):155–163. 10.1111/j.1365-2249.2006.03257.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Rex TS, Elliott P, Rostami A (2007) SIRT1 activation confers neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci 48(8):3602–3609. 10.1167/iovs.07-0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A (2010) Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol 30(4):328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF, Bergers G, Weiss WA, Alvarez-Buylla A, Hodgson JG (2008) miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P (2007) Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol 72(6):1508–1521. 10.1124/mol.107.038984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS (2010) Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther 332(3):829–839. 10.1124/jpet.109.160838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Hegde VL, Guan H, Hofseth L, Nagarkatti M, Nagarkatti PS (2011) Resveratrol (trans-3,5,4′-trihydroxystilbene) suppresses EL4 tumor growth by induction of apoptosis involving reciprocal regulation of SIRT1 and NF-kappaB. Mol Nutr Food Res 55(8):1207–1218. 10.1002/mnfr.201000576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Guan H, Nagarkatti P, Nagarkatti M (2012a) Prenatal exposure to TCDD triggers significant modulation of microRNA expression profile in the thymus that affects consequent gene expression. PLoS One 7(9):e45054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UP, Singh NP, Singh B, Hofseth LJ, Taub DD, Price RL, Nagarkatti M, Nagarkatti PS (2012b) Role of resveratrol-induced CD11b(+) gr1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(−/−) mice. Brain Behav Immun 26(1):72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Rouse M, Zhang J, Chatterjee S, Nagarkatti PS, Nagarkatti M (2016) Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from Proinflammatory Th17 cells to anti-inflammatory regulatory T cells through regulation of MicroRNA. J Immunol 196(3):1108–1122. 10.4049/jimmunol.1501727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Jun M, Ahn MR, Kim OY (2016) Involvement of miR-Let7A in inflammatory response and cell survival/apoptosis regulated by resveratrol in THP-1 macrophage. Nutr Res Pract 10(4):377–384. 10.4162/nrp.2016.10.4.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svahn AJ, Giacomotto J, Graeber MB, Rinkwitz S, Becker TS (2016) miR-124 contributes to the functional maturity of microglia. Dev Neurobiol 76(5):507–518. 10.1002/dneu.22328 [DOI] [PubMed] [Google Scholar]
- Svajger U, Obermajer N, Jeras M (2010) Dendritic cells treated with resveratrol during differentiation from monocytes gain substantial tolerogenic properties upon activation. Immunology 129(4):525–535. 10.1111/j.1365-2567.2009.03205.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha TA, Kitatani K, el-Alwani M, Bielawski J, Hannun YA, Obeid LM (2006) Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J 20(3):482–484. 10.1096/fj.05-4412fje [DOI] [PubMed] [Google Scholar]
- Thamilarasan M, Koczan D, Hecker M, Paap B, Zettl UK (2012) MicroRNAs in multiple sclerosis and experimental autoimmune encephalomyelitis. Autoimmun Rev 11(3):174–179. 10.1016/j.autrev.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Tian H, Yu Z (2015) Resveratrol induces apoptosis of leukemia cell line K562 by modulation of sphingosine kinase-1 pathway. Int J Clin Exp Pathol 8(3):2755–2762 [PMC free article] [PubMed] [Google Scholar]
- Tili E, Michaille JJ, Alder H, Volinia S, Delmas D, Latruffe N, Croce CM (2010) Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFbeta signaling pathway in SW480 cells. Biochem Pharmacol 80(12):2057–2065. 10.1016/j.bcp.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D (2004) Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell 16(1):93–105. 10.1016/j.molcel.2004.08.031 [DOI] [PubMed] [Google Scholar]
- Venkatadri R, Muni T, Iyer AKV, Yakisich JS, Azad N (2016) Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis 7:e2104 10.1038/cddis.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Li SP, Fu JS, Zhang S, Bai L, Guo L (2016) Resveratrol defends blood-brain barrier integrity in experimental autoimmune encephalomyelitis mice. J Neurophysiol 116(5):2173–2179. 10.1152/jn.00510.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wei Z, Jiang G, Liu H (2017) Neuroprotective mechanisms of miR-124 activating PI3K/Akt signaling pathway in ischemic stroke. Exp Ther Med 13(6):3315–3318. 10.3892/etm.2017.4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskirchen S, Weiskirchen R (2016) Resveratrol: how much wine do you have to drink to stay healthy? Adv Nutr 7(4):706–718. 10.3945/an.115.011627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight RD, Tull CA, Deel MW, Stroope BL, Eubanks AG, Chavis JA, Drew PD, Hensley LL (2012) Resveratrol effects on astrocyte function: relevance to neurodegenerative diseases. Biochem Biophys Res Commun 426(1):112–115. 10.1016/j.bbrc.2012.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Wu Z, Yu C, He W, Zheng H, He Y, Jian W, Chen L, Zhang L, Li W (2012) miR-124 inhibits cell proliferation in gastric cancer through down-regulation of SPHK1. J Pathol 227(4):470–480. 10.1002/path.4030 [DOI] [PubMed] [Google Scholar]
- Xu B, Zhang Y, du XF, Li J, Zi HX, Bu JW, Yan Y, Han H, du JL (2017) Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res 27:882–897. 10.1038/cr.2017.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Xu S, Qian Y, Xiao Q (2017) Resveratrol regulates microglia M1/M2 polarization via PGC-1alpha in conditions of neuroinflammatory injury. Brain Behav Immun 64:162–172. 10.1016/j.bbi.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Yu H, Shao Y, Gao L, Zhang L, Guo K, Wu C, Hu X, Duan H (2012) Acetylation of sphingosine kinase 1 regulates cell growth and cell-cycle progression. Biochem Biophys Res Commun 417(4):1242–1247. 10.1016/j.bbrc.2011.12.117 [DOI] [PubMed] [Google Scholar]
- Yu YH, Chen HA, Chen PS, Cheng YJ, Hsu WH, Chang YW, Chen YH, Jan Y, Hsiao M, Chang TY, Liu YH, Jeng YM, Wu CH, Huang MT, Su YH, Hung MC, Chien MH, Chen CY, Kuo ML, Su JL (2013) MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene 32(4):431–443. 10.1038/onc.2012.74 [DOI] [PubMed] [Google Scholar]
- Yu A, Zhang T, Duan H, Pan Y, Zhang X, Yang G, Wang J, Deng Y, Yang Z (2017) MiR-124 contributes to M2 polarization of microglia and confers brain inflammatory protection via the C/EBP-alpha pathway in intracerebral hemorrhage. Immunol Lett 182:1–11. 10.1016/j.imlet.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang M, Li X, Tang Z, Wang X, Zhong M, Suo Q, Zhang Y, Lv K (2016) Silencing MicroRNA-155 attenuates cardiac injury and dysfunction in viral myocarditis via promotion of M2 phenotype polarization of macrophages. Sci Rep 6:22613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ling Z, Hao Y, Pang X, Han X, Califano JA, Shan L, Gu X (2017) MiR-124 acts as a tumor suppressor by inhibiting the expression of sphingosine kinase 1 and its downstream signaling in head and neck squamous cell carcinoma. Oncotarget 8(15):25005–25020. 10.18632/oncotarget.15334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Han Y, Zhang Z, Shi Z, Zhou L, Liu X, Jia X (2017) MicroRNA-124 upregulation inhibits proliferation and invasion of osteosarcoma cells by targeting sphingosine kinase 1. Hum Cell 30(1):30–40. 10.1007/s13577-016-0148-4 [DOI] [PubMed] [Google Scholar]
- Zielinska-Przyjemska M et al. (2017) The effect of resveratrol, its naturally occurring derivatives and tannic acid on the induction of cell cycle arrest and apoptosis in rat C6 and human T98G glioma cell lines. Toxicol in Vitro 43:69–75. 10.1016/j.tiv.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Zou T, Yang Y, Xia F, Huang A, Gao X, Fang D, Xiong S, Zhang J (2013) Resveratrol inhibits CD4+ T cell activation by enhancing the expression and activity of Sirt1. PLoS One 8(9):e75139. 10.1371/journal.pone.0075139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.