Abstract
Objective:
In adolescence, sensation seeking is associated with earlier onset of alcohol use, which is a risk factor for a variety of negative consequences later in life. Individual differences in sensation seeking are related to brain function in the nucleus accumbens (NAcc), a brain region that undergoes considerable structural development during adolescence. Therefore, the goal of this study was to determine whether NAcc volume in alcohol-naive adolescents was associated with future sensation seeking and alcohol use and whether these associations differed by sex.
Method:
High-resolution magnetic resonance imaging was used to measure NAcc volume at baseline in 514 alcohol-naive adolescents (50.2% female) from the National Consortium on Alcohol & Neurodevelopment in Adolescence study. Direct effects of NAcc volume on adolescent drinking 2 years after baseline, and indirect effects mediated through sensation seeking 1 year after baseline, were assessed.
Results:
An indirect effect of NAcc volume on subsequent drinking through sensation seeking was significant for males, but not females. This effect was driven by a positive association between NAcc volume and sensation seeking observed in male, but not female, participants. A direct effect of NAcc volume on subsequent alcohol use was detected in females, but not males. In females, no association between NAcc volume and sensation seeking was detected, but NAcc volume was positively associated with future alcohol use.
Conclusions:
These findings suggest that delayed structural maturation of the NAcc may be a risk factor for alcohol use in adolescence; however, the mechanism by which the structure of the NAcc confers risk differs by sex.
Problematic alcohol use, including heavy episodic drinking and alcohol dependence, remains pervasive worldwide with devastating consequences (Grant et al., 2015; Hingson et al., 2009; Shield et al., 2013). More often than not, the development of problem drinking is rooted in adolescence, as the vast majority of individuals diagnosed with alcohol dependence initiate alcohol use during their teenage years or earlier (Grant & Dawson, 1997). Although some alcohol use during adolescence may be normative, it is clear that adolescents who start drinking sooner and at heavier levels are at higher risk for numerous problems, including personal injury, legal repercussions, and chronic medical conditions (Hingson et al., 2009; Kuntsche et al., 2013; Merline et al., 2008; Patrick & Schulenberg, 2011). Delaying the initiation of alcohol use and reducing use among those who have started drinking would likely curb the development of alcohol use disorders. Although public health initiatives aimed at reducing alcohol use in adolescents have had modest success, such interventions may be more impactful if specifically targeted toward those at higher risk for problematic use.
A variety of personality characteristics have been examined in relation to alcohol use. Sensation-seeking tendencies have been among the strongest and most consistent predictors of alcohol consumption during adolescence (Hittner & Swickert, 2006; Magid et al., 2007). Sensation seeking is a personality trait that is characterized by a propensity for pursuing novel and intense experiences, often without regard for potential consequences (Zuckerman & Neeb, 1979). In addition to its positive association with quantity and frequency of alcohol consumption (Fernandez-Artamendi et al., 2018), individuals high in sensation seeking are more likely to initiate alcohol use at earlier ages and progress to alcohol use disorder (Ball et al., 1994). A study in adolescents demonstrated that baseline levels of sensation seeking and larger increases in sensation seeking over time were prospectively associated with subsequent alcohol use (MacPherson et al., 2010). Therefore, identifying neurobiological factors that are associated with sensation seeking may also be useful for predicting problematic substance use.
Individual differences in sensation seeking have been associated with the function of the ventral striatum, a key region in mesolimbic circuitry implicated in reward processing. For example, in early adolescence (∼ages 10–12) and young adulthood (∼ages 17–25), reward-related ventral striatal activation has been associated with individual differences in sensation seeking in the 3-year period following the baseline scan (Hawes et al., 2017). Similarly, among 18- to 22-year-olds, anticipatory reward-related functional connectivity of the nucleus accumbens (NAcc) to the sensorimotor cortex and precuneus is positively correlated with sensation seeking (Weiland et al., 2013). Consistent with the hypothesis that there is overlap in the neurobiological factors that underlie individual differences in sensation seeking and alcohol use, greater NAcc activation during reward-based decision making in adolescents without a significant history of substance use is correlated with earlier initiation of binge drinking (Morales et al., 2018). Developmental studies have demonstrated that NAcc volume declines during adolescence and young adulthood (Tamnes et al., 2013; Wierenga et al., 2014); however, it remains unclear whether individual differences in the structural development of the NAcc are related to future sensation seeking and alcohol use.
In the current study, we measured NAcc volume in alcohol-naive 12- to 18-year-olds to determine its association with levels of sensation seeking 1 year later and total alcohol use 2 years later. Based on prior work indicating that college-aged binge drinkers have greater NAcc volume than control participants (Howell et al., 2013), we hypothesized that larger NAcc volume would be associated with greater future alcohol use. Given longitudinal evidence that individual differences in NAcc neurobiology are associated with future sensation seeking (Hawes et al., 2017), we also anticipated that the association between NAcc volume and alcohol use would be mediated by greater sensation seeking. Furthermore, since males exhibit higher levels of sensation seeking than females (Shulman et al., 2015), we examined whether the associations between NAcc volume, sensation seeking, and future alcohol use varied by sex. Prior work demonstrated a stronger association between sensation seeking and risky decision making involving rewards in males than females (Harden et al., 2018); therefore, we anticipated that associations between NAcc volume, sensation seeking, and alcohol use would be stronger in males than females.
Method
Participants
The National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) study is designed to follow adolescents, between ages 12 and 21 at study entry, annually for up to 9 years. Data from baseline and the first two follow-up annual visits were available and included in the following analyses. A total of 831 adolescents were enrolled across five sites in the United States (University of California at San Diego [UCSD], SRI International, Duke University Medical Center, University of Pittsburgh Medical Center, and Oregon Health & Science University [OHSU]). Assessment procedures were consistent across each study site (for full study description, see Brown et al., 2015). Because we were interested in predicting alcohol use during adolescence, participants who had not yet consumed alcohol (self-reported lifetime alcohol use on the Customary Drinking and Drug Use Record) and who were between ages 12 and 18 at study enrollment were selected and analyzed, resulting in a total sample of 516 youth. At each site, adult participants or the parents of minor participants provided written informed consent before participation in the study, and minor participants provided informed assent before participation. The Institutional Review Boards of each site approved this study.
MRI acquisition and analysis
T1-weighted images were collected on two scanners: 3T General Electric (GE) Discovery MR750 at 3 sites (129 from UCSD, 101 from SRI, 119 from Duke) and 3T Siemens TIMTRIO scanners at 2 sites (71 from University of Pittsburgh, 96 from OHSU). The GE sites acquired an Inversion Recovery-Spoiled Gradient Recalled echo sequence (TR = 5.904 ms, TI = 400 ms, TE = 1.932 ms, flip angle = 11°, NEX = 1, matrix = 256 × 256, FOV = 24 cm, slice dimensions = 1.2 × 0.9375 × 0.9375 mm, 146 slices) using an 8-channel head coil and Array Spatial Sensitivity Encoding Technique for parallel and accelerated imaging. The Siemens sites acquired an MPRAGE sequence (TR = 1900 ms, TI = 900 ms, TE = 2.92 ms, flip angle = 9°, NEX = 1, matrix = 256 × 256, FOV= 24 cm, slice dimensions = 1.2 × 0.9375 × 0.9375 mm, 160 slices) with a 12-channel head coil and iPAT for parallel imaging and temporal acceleration.
Image preprocessing was described in detail in a prior publication (Nichols & Pohl, 2015; Pfefferbaum et al., 2016). Briefly, T1-weighted images were skull-stripped using the Robust Brain Extraction (ROBEX) method (Iglesias et al., 2011) and FSL BET (Smith, 2002). Skull-stripped images were further processed with FreeSurfer (Dale et al., 1999; Fischl et al., 2004), and total NAcc volume (right and left summed) was extracted. Intracranial volume (ICV) was calculated using the SRI24 atlas-based analysis pipeline (Rohlfing et al., 2010, 2014).
Participant characterization
Sensation seeking.
The short version of the UPPS-P Impulsivity Scale (SUPPS; Cyders et al., 2014) consists of 20 items that assess five dimensions of impulsivity, including positive and negative urgency, lack of premeditation, lack of perseverance, and sensation seeking. Items were rated on a four-point scale from 1 (disagree strongly) to 4 (agree strongly), and the scale has been shown to have acceptable psychometric properties (Cyders et al., 2014). The items of each subscale were summed, with higher scores indicating greater impulsivity. The sensation-seeking scores were of primary interest based on a priori hypotheses about the associations between sensation seeking, reward-related circuitry, and alcohol use. The four items of the sensation-seeking subscale assess the tendency to pursue novel, exciting, and potentially dangerous experiences (e.g., “I quite enjoy taking risks”), and have been found to be associated with alcohol involvement (Coskunpinar & Cyders, 2013; Cyders et al., 2014).
Puberty and drinking behavior.
Pubertal development was measured using participant self-report on the Pubertal Development Scale (Petersen et al., 1988). Participants completed the Customary Drinking and Drug Use Record (Brown et al., 1998) to assess alcohol use. At each study visit, participants were asked to indicate the number of days in the past year that they consumed any amount of alcohol, as well as the average number of drinks consumed in the average 24-hour period when they were drinking during the past year. The product of these two items was used as a composite score of the total number of drinks consumed during the past year. Participants also answered questions about how many times they engaged in binge drinking (defined as 5+ drinks for males and 4+ drinks for females per occasion), the number of days they used marijuana, and how many cigarettes they smoked in the past year.
Statistical analysis
Direct and indirect effects of baseline individual differences in NAcc volume on subsequent alcohol use were assessed with path analysis using Mplus Version 8.0 (Muthén & Muthén, 2017). NAcc volume measured at the baseline assessment was entered as the independent variable, sensation seeking assessed at the year 1 follow-up visit was entered as the single mediating variable, and alcohol use measured at the year 2 follow-up visit was entered as the dependent variable. ICV and MRI scanner type were included as covariates on NAcc volume, and age and pubertal development were included as covariates on all variables. Bootstrapping with replacement was used in the analysis to account for nonnormality that is common among alcohol use variables from data that includes nondrinkers. The statistical significance of the indirect effect of NAcc volume on alcohol use through sensation seeking was assessed using 10,000 bootstrap samples to calculate bias-corrected confidence intervals. Bias-corrected bootstrapping is recommended for models assessing multiple comparisons (e.g., mediation models), as the approach achieves higher power while maintaining reasonable control over type I errors (MacKinnon et al., 2002) and does not make assumptions about the normality of the indirect effect. Sex differences in these direct and indirect effects were further examined using the multigroup command in Mplus (Muthén & Muthén, 2017). Goodness of model fit was evaluated using the following criteria: comparative fit index (CFI) greater than .95 (Hu & Bentler, 1999), root mean square error of approximation (RMSEA) less than .08 (Browne & Cudeck, 1992), and standardized root mean square residual (SRMR) less than .08 (Hu & Bentler, 1999). Although our primary interest was in sensation seeking, post hoc exploratory analyses using the same analytic strategy were conducted to determine whether other UPPS-P subscales (i.e., lack of premeditation, lack of perseverance, positive urgency, negative urgency) were related to NAcc volume and future alcohol use. Lastly, to determine the specificity of our findings, we examined whether NAcc volume and sensation seeking were associated with binge drinking occasions and days of marijuana use in year 2.
Results
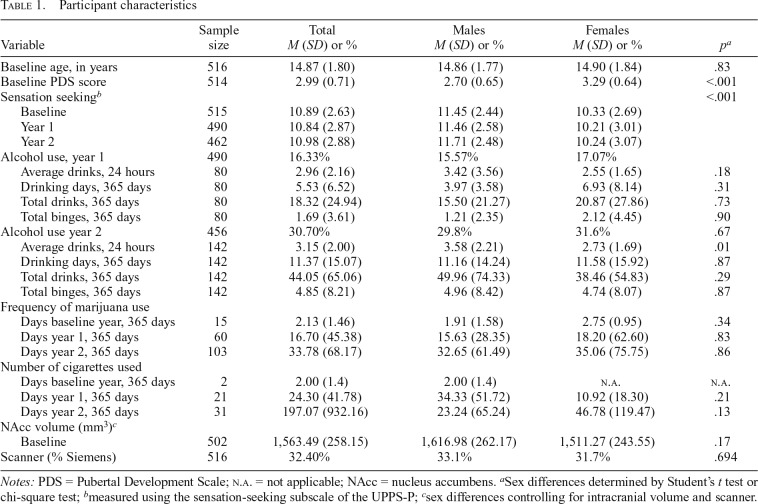
Participant characteristics (Table 1)
Table 1.
Participant characteristics
| Variable | Sample size | Total M (SD) or % | Males M (SD) or % | Females M (SD) or % | pa |
| Baseline age, in years | 516 | 14.87 (1.80) | 14.86 (1.77) | 14.90 (1.84) | .83 |
| Baseline PDS score | 514 | 2.99 (0.71) | 2.70 (0.65) | 3.29 (0.64) | <.001 |
| Sensation seekingb | <.001 | ||||
| Baseline | 515 | 10.89 (2.63) | 11.45 (2.44) | 10.33 (2.69) | |
| Year 1 | 490 | 10.84 (2.87) | 11.46 (2.58) | 10.21 (3.01) | |
| Year 2 | 462 | 10.98 (2.88) | 11.71 (2.48) | 10.24 (3.07) | |
| Alcohol use, year 1 | 490 | 16.33% | 15.57% | 17.07% | |
| Average drinks, 24 hours | 80 | 2.96 (2.16) | 3.42 (3.56) | 2.55 (1.65) | .18 |
| Drinking days, 365 days | 80 | 5.53 (6.52) | 3.97 (3.58) | 6.93 (8.14) | .31 |
| Total drinks, 365 days | 80 | 18.32 (24.94) | 15.50 (21.27) | 20.87 (27.86) | .73 |
| Total binges, 365 days | 80 | 1.69 (3.61) | 1.21 (2.35) | 2.12 (4.45) | .90 |
| Alcohol use year 2 | 456 | 30.70% | 29.8% | 31.6% | .67 |
| Average drinks, 24 hours | 142 | 3.15 (2.00) | 3.58 (2.21) | 2.73 (1.69) | .01 |
| Drinking days, 365 days | 142 | 11.37 (15.07) | 11.16 (14.24) | 11.58 (15.92) | .87 |
| Total drinks, 365 days | 142 | 44.05 (65.06) | 49.96 (74.33) | 38.46 (54.83) | .29 |
| Total binges, 365 days | 142 | 4.85 (8.21) | 4.96 (8.42) | 4.74 (8.07) | .87 |
| Frequency of marijuana use | |||||
| Days baseline year, 365 days | 15 | 2.13 (1.46) | 1.91 (1.58) | 2.75 (0.95) | .34 |
| Days year 1, 365 days | 60 | 16.70 (45.38) | 15.63 (28.35) | 18.20 (62.60) | .83 |
| Days year 2, 365 days | 103 | 33.78 (68.17) | 32.65 (61.49) | 35.06 (75.75) | .86 |
| Number of cigarettes used | |||||
| Days baseline year, 365 days | 2 | 2.00 (1.4) | 2.00 (1.4) | n.a. | n.a. |
| Days year 1, 365 days | 21 | 24.30 (41.78) | 34.33 (51.72) | 10.92 (18.30) | .21 |
| Days year 2, 365 days | 31 | 197.07 (932.16) | 23.24 (65.24) | 46.78 (119.47) | .13 |
| NAcc volume (mm3)c | |||||
| Baseline | 502 | 1,563.49 (258.15) | 1,616.98 (262.17) | 1,511.27 (243.55) | .17 |
| Scanner (% Siemens) | 516 | 32.40% | 33.1% | 31.7% | .694 |
Notes: PDS = Pubertal Development Scale; n.a. = not applicable; NAcc = nucleus accumbens.
Sex differences determined by Student’s t test or chi-square test;
measured using the sensation-seeking subscale of the UPPS-P;
sex differences controlling for intracranial volume and scanner.
On average, participants were 14.87 (SD = 1.80) years old and scored 2.99 (SD = 0.71) on the pubertal development scale when they entered the study. Although there were no sex differences in age, t(514) = 0.22, p = .83, females had higher pubertal development scores, t(512) = 10.31, p < .001. After we controlled for intracranial volume and scanner, there were no sex differences in NAcc volume at baseline, F(3, 498) = 0.16, p = .69. At the year 1 follow-up visit, males reported greater levels of sensation seeking than females, t(488) = -4.94, p < .001. On average, 30.70% of participants reported any alcohol use at the 2-year follow-up visit, and there were no sex differences in the proportion of participants reporting alcohol use, χ2(1, 456) = 0.18, p = .67. Among those who endorsed some alcohol use, the average number of alcoholic beverages consumed in the past year was 43.6 (SD = 65.32), the number of binge drinking episodes was 4.85 (SD = 8.21), and there were no differences in either measure by sex (ps > .2). Furthermore, 22.59% of participants reported any marijuana use during the year 2 follow-up visit. Among those reporting some marijuana use, 33.78 (SD = 68.17) was the average number of days used over the past year, and there were no sex differences in frequency of use, t(101) = 0.18, p = .86.
Path analysis of associations between NAcc, sensation seeking, and alcohol use
Two participants were excluded from the analyses described below (n = 514) because of missing data. Results of the model not accounting for sex differences indicated poor fit, χ2(6) = 62.50, p < .001, CFI = 0.86, RMSEA = 0.14, SRMR = 0.06. Before we examined potential sex differences in the estimated model, a baseline multiple group model was tested in which all direct paths were constrained to equality across the male and female groups. This fully constrained baseline model was compared with a model in which all paths were freely estimated to determine whether the strength of the associations differed by sex. A chi-square difference test indicated that the constrained model resulted in a significantly worse fit than the model in which path coefficients were freely estimated across the two groups, Δχ2(12) = 26.91, p = .008, suggesting that significant sex differences were evident for the magnitude of the path coefficients.
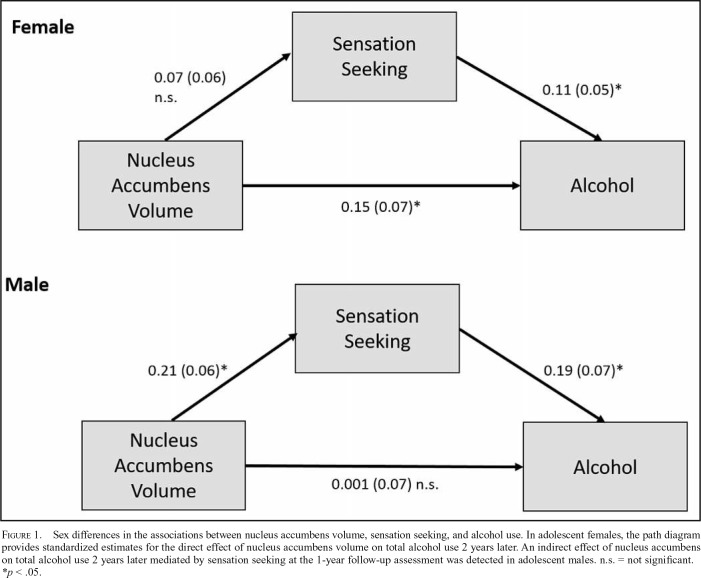
Results of the freely estimated multiple group model indicated that the model provided a good overall fit, χ2(12) = 18.19, p = .11; CFI = 0.98, RMSEA = 0.05, SRMR = 0.03 (Figure 1). The path from NAcc volume to sensation seeking was significant for males (β = .21, SE = .06, p < .001), but not for females (β = .07, SE = .06, p = .26). Males with a larger NAcc volume at baseline reported significantly greater sensation-seeking tendencies 1 year later. The association between sensation seeking and total alcohol use in year 2 was significant for both males (β = .19, SE = .07, p = .01) and females (β = .11, SE = .05, p = .03), with greater sensation seeking predicting greater alcohol consumption. The direct effect of NAcc volume on total alcohol use was significant for females (β = .15, SE = .07, p = .03), but not for males (β = .001, SE = .07, p = .99). Although NAcc volume did not directly predict total alcohol use among males, the indirect effect through sensation seeking was significant (β = .04, SE = .02, p = .046, 95% CI [.01, .09]) and accounted for almost 100% of the total effect of NAcc volume on alcohol consumption (β = .04, SE = .07, p = .57, 95% CI [-.12, .16]). Among females, the indirect effect of NAcc volume on alcohol use through sensation seeking was not significant (β = .008, SE = .008, p = .35, 95% CI [-.004, .03]) and accounted for only 5% of the total effect (β = .15, SE = .07, p = .02, 95% CI [.004, .27]). Similar patterns of results were observed when binge drinking occasions and days of marijuana use in year 2 were used as outcome variables (Supplementary Table 1 and 2). However, in the unconstrained multigroup models, there was no direct effect of NAcc volume on binge drinking or frequency of marijuana use in females (Supplementary Table 2).
Figure 1.
Sex differences in the associations between nucleus accumbens volume, sensation seeking, and alcohol use. In adolescent females, the path diagram provides standardized estimates for the direct effect of nucleus accumbens volume on total alcohol use 2 years later. An indirect effect of nucleus accumbens on total alcohol use 2 years later mediated by sensation seeking at the 1-year follow-up assessment was detected in adolescent males. n.s. = not significant. *p < .05.
Post hoc exploratory analyses not accounting for sex differences indicated that models using other dimensions from the UPPS-P as potential mediators (e.g., premeditation, lack of perseverance, positive urgency, and negative urgency) were a poor fit, and other dimensions from the UPPS-P were not significantly associated with NAcc volume or total drinks in year 2 (all ps > .05). Furthermore, chi-square difference tests indicated that there were no differences between the constrained and freely estimated models by sex (ps > .05), suggesting that there were no significant sex differences for the magnitude of the path coefficients.
Discussion
Our findings provide novel evidence that individual differences in NAcc volume during adolescence are associated with future sensation seeking and alcohol use. Furthermore, sex differences were evident for indirect effects, suggesting that the influence of the NAcc on adolescent alcohol use is differentially transmitted for males and females. Among adolescent males, the influence of NAcc volume on subsequent drinking was largely explained by the shared association with sensation seeking; however, no association between NAcc volume and sensation seeking was observed in females. As direct effects of NAcc volume on future alcohol use were evident in females, more research is needed to determine whether the relationship between NAcc volume and alcohol use in females is mediated by other facets of personality, cognition, or affect that are linked to the NAcc and confer greater risk for future alcohol use.
Our findings extend prior cross-sectional work demonstrating that college-aged binge drinkers have greater volume in the NAcc than age-matched controls (Howell et al., 2013) and that frequency of alcohol use is positively correlated with NAcc volume in adolescents (Thayer et al., 2012), by providing evidence that greater NAcc volume during adolescence is a pre-existing risk factor for alcohol use. Consistent with prior work demonstrating that young marijuana users have greater NAcc volumes than control participants (Gilman et al., 2014) and that sensation seeking predicts other substance use (Castellanos-Ryan et al., 2013), we also demonstrate that the association between greater NAcc volume and future marijuana use is mediated by sensation seeking in adolescent males. These findings suggest that NAcc volume during adolescence may be a risk factor for using a variety of substances; however, more research is needed to determine if NAcc volume relates to cigarette or other illicit drug use. In contrast to our findings, a previous study demonstrated that adolescents with smaller NAcc volume at baseline were more likely to initiate various forms of substance use 2 years later (Urošević et al., 2015). A variety of factors could contribute to these discrepant findings. For example, Uroševic; and colleagues only studied 14 participants who initiated substance use over the course of 2 years; therefore, it is possible that participant characteristics that present potential confounds could not be adequately addressed (e.g., sex differences, pubertal stage).
In adolescent males, but not females, we demonstrated that greater NAcc volume at baseline is associated with greater sensation seeking 1 year later, suggesting that the structural development of other brain regions may be useful for explaining individual differences in sensation seeking and alcohol use in females. A cross-sectional study in adults demonstrated that greater sensation seeking was associated with less cortical thickness in the anterior cingulate, middle frontal gyrus, and supramarginal gyrus (Holmes et al., 2016). However, longitudinal studies examining the associations between prefrontal brain structure and future alcohol use during adolescence have produced mixed results. Although some studies have demonstrated that lower gray-matter volume or thickness in the anterior cingulate and lateral prefrontal cortex at baseline are associated with the initiation and escalation of alcohol use (Brumback et al., 2016; Cheetham et al., 2014; Squeglia et al., 2014, 2017), a large multisite study found no associations between gray-matter volume in the anterior cingulate, orbitofrontal cortex, or medial prefrontal cortex at age 14 and heavy drinking at age 19 (Seo et al., 2019). Furthermore, task-based (Hawes et al., 2017; Weiland et al., 2013) and resting-state functional MRI (Sharkey et al., 2019) have been linked to sensation seeking, but little is known about how individual differences in gray-matter volume relate to brain function during development.
More research is needed to determine the genetic and environmental influences that affect individual differences in sensation seeking and NAcc volume. A recent longitudinal study in young adults determined that the association between a polygenic risk score and future alcohol use problems was mediated by sensation seeking (Li et al., 2017). The emergence of large-scale international genetic neuroimaging consortiums (Mackey et al., 2016) will facilitate the ability to conduct similar analyses to determine whether brain structure and function mediate the association between genetic variability and alcohol use. Environmental influences may also play a critical role in shaping risk factors for alcohol use. For example, a longitudinal study during adolescence found that different parenting styles affect the trajectory of NAcc volume across adolescence (Whittle et al., 2016). Better understanding of the factors that affect risk for alcohol use may be useful for identifying and targeting interventions for those who are at higher risk for adolescent alcohol use. For example, interventions targeting sensation seeking have shown moderate success at reducing adolescent binge drinking (Conrod et al., 2011, 2013). Our results suggest that these interventions may be improved by taking into account sex differences in the neurobiological underpinnings of sensation seeking.
This study extends our understanding of the role that NAcc volume and sensation seeking play in alcohol use during adolescence; however, it is not without limitations. The sex differences observed in this study might be attributable, in part, to sex hormones. For example, studies in adolescents and young adults found that testosterone levels were correlated with sensation seeking and with future alcohol use (Braams et al., 2016; Campbell et al., 2010), and fluctuations in testosterone and estradiol have been linked to changes in brain structure (Herting et al., 2014). In this study, age and pubertal development were included as covariates in statistical models; however, it is possible that sex hormones would help further explain individual variability in brain structure, sensation seeking, and alcohol use that cannot be captured by assessing age or pubertal stage (Herting et al., 2014). Furthermore, although earlier initiation of alcohol use is a risk factor for problem alcohol use later in life (Hingson et al., 2009), most adult consumers of alcohol report an adolescent onset of alcohol use, with few if any lifetime consequences, suggesting that early initiation is not sufficient for explaining the rates of alcohol dependence (King & Chassin, 2007). Therefore, longer prospective studies may be useful for identifying additional risk factors for escalating patterns of use.
This study demonstrates that greater NAcc volume in adolescence may be a risk factor for future substance use. In adolescent males, greater nucleus accumbens volume was associated with higher levels of sensation seeking, a known risk factor for substance use. In adolescent females, although NAcc volume was directly associated with future alcohol use, more work is needed to identify the risk factors that mediate this association and to identify neurobiological underpinnings of sensation seeking.
Acknowledgments
The data presented here are based on the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) Data Release NCANDA_DATA_00010_V1, 1-Sep-2015 as described in Pfefferbaum et al. (2016) and the NIH grants 1U01AA021695-01, 1U01AA021692-01, 1U01AA021696-01, 1U01AA021697-01, 1U01AA021690-01, 1U01AA021681-01, and 1U01AA0216.
Footnotes
This work was supported by the U.S. National Institute on Alcohol Abuse and Alcoholism with co-funding from the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Child Health and Human Development (National Consortium on Alcohol & Neurodevelopment in Adolescence grant numbers: AA021697, AA021695, AA021692, AA021696, AA021681, AA021690, AA021691).
References
- Ball S. A., Carroll K. M., Rounsaville B. J. Sensation seeking, substance abuse, and psychopathology in treatment-seeking and community cocaine abusers. Journal of Consulting and Clinical Psychology. 1994;62:1053–1057. doi: 10.1037//0022-006x.62.5.1053. doi:10.1037/0022-006X.62.5.1053. [DOI] [PubMed] [Google Scholar]
- Braams B. R., Peper J. S., van der Heide D., Peters S., Crone E. A. Nucleus accumbens response to rewards and testosterone levels are related to alcohol use in adolescents and young adults. Developmental Cognitive Neuroscience. 2016;17:83–93. doi: 10.1016/j.dcn.2015.12.014. doi:10.1016/j.dcn.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. A., Brumback T., Tomlinson K., Cummins K., Thompson W. K., Nagel B. J., Tapert S. F. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A multisite study of adolescent development and substance use. Journal of Studies on Alcohol and Drugs. 2015;76:895–908. doi: 10.15288/jsad.2015.76.895. doi:10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. A., Myers M. G., Lippke L., Tapert S. F., Stewart D. G., Vik P. W. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. doi:10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Browne M., Cudeck R. Alternative ways of assessing model fit. Sociological Methods & Research. 1992;21:230–258. doi:10.1177/0049124192021002005. [Google Scholar]
- Brumback T. Y., Worley M., Nguyen-Louie T. T., Squeglia L. M., Jacobus J., Tapert S. F. Neural predictors of alcohol use and psychopathology symptoms in adolescents. Development and Psychopathology. 2016;28:1209–1216. doi: 10.1017/S0954579416000766. doi:10.1017/S0954579416000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. C., Dreber A., Apicella C. L., Eisenberg D. T. A., Gray P. B., Little A. C., Lum J. K. Testosterone exposure, dopaminergic reward, and sensation-seeking in young men. Physiology & Behavior. 2010;99:451–456. doi: 10.1016/j.physbeh.2009.12.011. doi:10.1016/j.physbeh.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N., O’Leary-Barrett M., Sully L., Conrod P. Sensitivity and specificity of a brief personality screening instrument in predicting future substance use, emotional, and behavioral problems: 18-month predictive validity of the Substance Use Risk Profile Scale. Alcoholism: Clinical and Experimental Research. 2013;37(Supplement 1):E281–E290. doi: 10.1111/j.1530-0277.2012.01931.x. doi:10.1111/j.1530-0277.2012.01931.x. [DOI] [PubMed] [Google Scholar]
- Cheetham A., Allen N. B., Whittle S., Simmons J., Yücel M., Lubman D. I. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology. 2014;231:1731–1742. doi: 10.1007/s00213-014-3483-8. doi:10.1007/s00213-014-3483-8. [DOI] [PubMed] [Google Scholar]
- Conrod P. J., Castellanos-Ryan N., Mackie C. Long-term effects of a personality-targeted intervention to reduce alcohol use in adolescents. Journal of Consulting and Clinical Psychology. 2011;79:296–306. doi: 10.1037/a0022997. doi:10.1037/a0022997. [DOI] [PubMed] [Google Scholar]
- Conrod P. J., O’Leary-Barrett M., Newton N., Topper L., CastellanosRyan N., Mackie C., Girard A. Effectiveness of a selective, personality-targeted prevention program for adolescent alcohol use and misuse: A cluster randomized controlled trial. JAMA Psychiatry. 2013;70:334–342. doi: 10.1001/jamapsychiatry.2013.651. doi:10.1001/jamapsychiatry.2013.651. [DOI] [PubMed] [Google Scholar]
- Coskunpinar A., Cyders M. A. Impulsivity and substance-related attentional bias: A meta-analytic review. Drug and Alcohol Dependence. 2013;133:1–14. doi: 10.1016/j.drugalcdep.2013.05.008. doi:10.1016/j.drugalcdep.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Cyders M. A., Littlefield A. K., Coffey S., Karyadi K. A. Examination of a short English version of the UPPS-P Impulsive Behavior Scale. Addictive Behaviors. 2014;39:1372–1376. doi: 10.1016/j.addbeh.2014.02.013. doi:10.1016/j.addbeh.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A. M., Fischl B., Sereno M. I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. doi:10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Fernández-Artamendi S., Martínez-Loredo V., Grande-Gosende A., Simpson I. C., Fernández-Hermida J. R. What predicts what? Self-reported and behavioral impulsivity and high-risk patterns of alcohol use in Spanish early adolescents: A 2-year longitudinal study. Alcoholism: Clinical and Experimental Research. 2018;42:2022–2032. doi: 10.1111/acer.13852. doi:10.1111/acer.13852. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D. H., van der Kouwe A. J., Makris N., Ségonne F., Quinn B. T., Dale A. M. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Supplement 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. doi:10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Gilman J. M., Kuster J. K., Lee S., Lee M. J., Kim B. W., Makris N., Breiter H. C. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. Journal of Neuroscience. 2014;34:5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. doi:10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. F., Dawson D. A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. doi:10.1016/S0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant B. F., Goldstein R. B., Saha T. D., Chou S. P., Jung J., Zhang H., Hasin D. S. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. doi:10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden K. P., Mann F. D., Grotzinger A. D., Patterson M. W., Steinberg L., Tackett J. L., Tucker-Drob E. M. Developmental differences in reward sensitivity and sensation seeking in adolescence: Testing sex-specific associations with gonadal hormones and pubertal development. Journal of Personality and Social Psychology. 2018;115:161–178. doi: 10.1037/pspp0000172. doi:10.1037/pspp0000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes S. W., Chahal R., Hallquist M. N., Paulsen D. J., Geier C. F., Luna B. Modulation of reward-related neural activation on sensation seeking across development. NeuroImage. 2017;147:763–771. doi: 10.1016/j.neuroimage.2016.12.020. doi:10.1016/j.neuroimage.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M. M., Gautam P., Spielberg J. M., Kan E., Dahl R. E., Sowell E. R. The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study. Human Brain Mapping. 2014;35:5633–5645. doi: 10.1002/hbm.22575. doi:10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R. W., Zha W., Weitzman E. R. Magnitude of and trends in alcohol-related mortality and morbidity among U.S. college students ages 18-24, 1998-2005. Journal of Studies on Alcohol and Drugs, Supplement. 2009;16:12–20. doi: 10.15288/jsads.2009.s16.12. doi:10.15288/jsads.2009.s16.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittner J. B., Swickert R. Sensation seeking and alcohol use: A meta-analytic review. Addictive Behaviors. 2006;31:1383–1401. doi: 10.1016/j.addbeh.2005.11.004. doi:10.1016/j.addbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Holmes A. J., Hollinshead M. O., Roffman J. L., Smoller J. W., Buckner R. L. Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. Journal of Neuroscience. 2016;36:4038–4049. doi: 10.1523/JNEUROSCI.3206-15.2016. doi:10.1523/JNEUROSCI.3206-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N. A., Worbe Y., Lange I., Tait R., Irvine M., Banca P., Voon V. Increased ventral striatal volume in college-aged binge drinkers. PLoS One. 2013;8(9):e74164. doi: 10.1371/journal.pone.0074164. doi:10.1371/journal.pone.0074164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L.-T., Bentler P. M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. doi:10.1080/10705519909540118. [Google Scholar]
- Iglesias J. E., Liu C.-Y., Thompson P. M., Tu Z. Robust brain extraction across datasets and comparison with publicly available methods. IEEE Transactions on Medical Imaging. 2011;30:1617–1634. doi: 10.1109/TMI.2011.2138152. doi:10.1109/TMI.2011.2138152. [DOI] [PubMed] [Google Scholar]
- King K. M., Chassin L. A prospective study of the effects of age of initiation of alcohol and drug use on young adult substance dependence. Journal of Studies on Alcohol and Drugs. 2007;68:256–265. doi: 10.15288/jsad.2007.68.256. doi:10.15288/jsad.2007.68.256. [DOI] [PubMed] [Google Scholar]
- Kuntsche E., Rossow I., Simons-Morton B., Bogt T. T., Kokkevi A., Godeau E. Not early drinking but early drunkenness is a risk factor for problem behaviors among adolescents from 38 European and North American countries. Alcoholism: Clinical and Experimental Research. 2013;37:308–314. doi: 10.1111/j.1530-0277.2012.01895.x. doi:10.1111/j.1530-0277.2012.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Savage J. E., Kendler K. S., Hickman M., Mahedy L., Macleod J., Dick D. M. Polygenic risk, personality dimensions, and adolescent alcohol use problems: A longitudinal study. Journal of Studies on Alcohol and Drugs. 2017;78:442–451. doi: 10.15288/jsad.2017.78.442. doi:10.15288/jsad.2017.78.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S., Kan K. J., Chaarani B., Alia-Klein N., Batalla A., Brooks S., Conrod P. Chapter 10 - Genetic imaging consortium for addiction medicine: From neuroimaging to genes. Progress in Brain Research. 2016;224:203–223. doi: 10.1016/bs.pbr.2015.07.026. doi:10.1016/bs.pbr.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D. P., Lockwood C. M., Hoffman J. M., West S. G., Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. doi:10.1037/1082-989X.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L., Magidson J. F., Reynolds E. K., Kahler C. W., Lejuez C. W. Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcoholism: Clinical and Experimental Research. 2010;34:1400–1408. doi: 10.1111/j.1530-0277.2010.01223.x. doi:10.1111/j.1530-0277.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magid V., Maclean M. G., Colder C. R. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addictive Behaviors. 2007;32:2046–2061. doi: 10.1016/j.addbeh.2007.01.015. doi:10.1016/j.addbeh.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merline A., Jager J., Schulenberg J. E. Adolescent risk factors for adult alcohol use and abuse: Stability and change of predictive value across early and middle adulthood. Addiction. 2008;103(Supplement 1):84–99. doi: 10.1111/j.1360-0443.2008.02178.x. doi:10.1111/j.1360-0443.2008.02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A. M., Jones S. A., Ehlers A., Lavine J. B., Nagel B. J. Ventral striatal response during decision making involving risk and reward is associated with future binge drinking in adolescents. Neuropsychopharmacology. 2018;43:1884–1890. doi: 10.1038/s41386-018-0087-8. doi:10.1038/s41386-018-0087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L., Muthén B. Mplus user’s guide. Los Angeles, CA: Authors; 2017. [Google Scholar]
- Nichols B. N., Pohl K. M. Neuroinformatics software applications supporting electronic data capture, management, and sharing for the neuroimaging community. Neuropsychology Review. 2015;25:356–368. doi: 10.1007/s11065-015-9293-x. doi:10.1007/s11065-015-9293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick M. E., Schulenberg J. E. How trajectories of reasons for alcohol use relate to trajectories of binge drinking: National panel data spanning late adolescence to early adulthood. Developmental Psychology. 2011;47:311–317. doi: 10.1037/a0021939. doi:10.1037/a0021939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A. C., Crockett L., Richards M., Boxer A. A selfreport measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. doi:10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Rohlfing T., Pohl K. M., Lane B., Chu W., Kwon D., Sullivan E. V. Adolescent development of cortical and white matter structure in the NCANDA sample: Role of sex, ethnicity, puberty, and alcohol drinking. Cerebral Cortex. 2016;26:4101–4121. doi: 10.1093/cercor/bhv205. doi:10.1093/cercor/bhv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T., Cummins K., Henthorn T., Chu W., Nichols B. N. N-CANDA data integration: Anatomy of an asynchronous infrastructure for multi-site, multi-instrument longitudinal data capture. Journal of the American Medical Informatics Association. 2014;21:758–762. doi: 10.1136/amiajnl-2013-002367. doi:10.1136/amiajnl-2013-002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T., Zahr N. M., Sullivan E. V., Pfefferbaum A. The SRI24 multichannel atlas of normal adult human brain structure. Human Brain Mapping. 2010;31:798–819. doi: 10.1002/hbm.20906. doi:10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S., Beck A., Matthis C., Genauck A., Banaschewski T., Bokde A. L. W., Obermayer K. Risk profiles for heavy drinking in adolescence: Differential effects of gender. Addiction Biology. 2019;24:787–801. doi: 10.1111/adb.12636. doi:10.1111/adb.12636. [DOI] [PubMed] [Google Scholar]
- Sharkey R. J., Bourque J., Larcher K., Mišic B., Zhang Y., Altınkaya A., Dagher A. Mesolimbic connectivity signatures of impulsivity and BMI in early adolescence. Appetite. 2019;132:25–36. doi: 10.1016/j.appet.2018.09.019. doi:10.1016/j.appet.2018.09.019. [DOI] [PubMed] [Google Scholar]
- Shield K., Parry C., Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Research: Current Reviews. 2013;35:155–173. [PMC free article] [PubMed] [Google Scholar]
- Shulman E. P., Harden K. P., Chein J. M., Steinberg L. Sex differences in the developmental trajectories of impulse control and sensation-seeking from early adolescence to early adulthood. Journal of Youth and Adolescence. 2015;44:1–17. doi: 10.1007/s10964-014-0116-9. doi:10.1007/s10964-014-0116-9. [DOI] [PubMed] [Google Scholar]
- Smith S. M. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. doi:10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Ball T. M., Jacobus J., Brumback T., McKenna B. S., Nguyen-Louie T. T., Tapert S. F. Neural predictors of initiating alcohol use during adolescence. American Journal of Psychiatry. 2017;174:172–185. doi: 10.1176/appi.ajp.2016.15121587. doi:10.1176/appi.ajp.2016.15121587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Rinker D. A., Bartsch H., Castro N., Chung Y., Dale A. M., Tapert S. F. Brain volume reductions in adolescent heavy drinkers. Developmental Cognitive Neuroscience. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. doi:10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C. K., Walhovd K. B., Dale A. M., Østby Y., Grydeland H., Richardson G., Fjell A. M. & the Alzheimer’s Disease Neuroimaging Initiative. Brain development and aging: Overlapping and unique patterns of change. NeuroImage. 2013;68:63–74. doi: 10.1016/j.neuroimage.2012.11.039. doi:10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer R. E., Crotwell S. M., Callahan T. J., Hutchison K. E., Bryan A. D. Nucleus accumbens volume is associated with frequency of alcohol use among juvenile justice-involved adolescents. Brain Sciences. 2012;2:605–618. doi: 10.3390/brainsci2040605. doi:10.3390/brainsci2040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S., Collins P., Muetzel R., Schissel A., Lim K. O., Luciana M. Effects of reward sensitivity and regional brain volumes on substance use initiation in adolescence. Social Cognitive and Affective Neuroscience. 2015;10:106–113. doi: 10.1093/scan/nsu022. doi:10.1093/scan/nsu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland B. J., Welsh R. C., Yau W. Y., Zucker R. A., Zubieta J. K., Heitzeg M. M. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug and Alcohol Dependence. 2013;128:130–139. doi: 10.1016/j.drugalcdep.2012.08.019. doi:10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Dennison M., Schwartz O., Simmons J. G., Sheeber L., Allen N. B. Observed measures of negative parenting predict brain development during adolescence. PLoS One. 2016;11(1):e0147774. doi: 10.1371/journal.pone.0147774. doi:10.1371/journal.pone.0147774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L., Langen M., Ambrosino S., van Dijk S., Oranje B., Durston S. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage. 2014;96:67–72. doi: 10.1016/j.neuroimage.2014.03.072. doi:10.1016/j.neuroimage.2014.03.072. [DOI] [PubMed] [Google Scholar]
- Zuckerman M., Neeb M. Sensation seeking and psychopathology. Psychiatry Research. 1979;3:255–264. doi: 10.1016/0165-1781(79)90007-6. doi:10.1016/0165-1781(79)90007-6. [DOI] [PubMed] [Google Scholar]