Abstract
Environmental DNA (eDNA) is a powerful tool for monitoring the distribution of aquatic macro-organisms. However, environmental factors, including the water temperature and water quality, can affect the inhibition and/or degradation of eDNA, which complicates accurate estimations of eDNA concentrations and the detection of the presence/absence of species in natural habitats. Further very few eDNA studies have been conducted for reptiles, especially with respect to estimating their biomass and/or abundances. Here we examined the relationship between the visually-observed number of red-eared sliders (Trachemys scripta elegans) and eDNA concentrations across 100 ponds. Additionally, we evaluated the effect of water quality on red-eared slider eDNA concentration in these ponds. We found that there was a significant positive correlation between the observed number of red-eared sliders and the eDNA concentration in the ponds. On comparing various water quality indicators, including dissolved nitrogen, dissolved phosphorous, organic matter, and chlorophyll a (Chl. a), we found that only Chl. a had a negative correlation with the red-eared slider eDNA concentration, while we did not find any inhibition in the quantitative PCR. We conclude that concentrations of eDNA can potentially be used for estimating the abundance of the red-eared slider. Additionally, Chl. a might indirectly influence the degradation of eDNA through the microorganisms bonded to the phytoplankton in the ponds, as microbial activity is thought to decrease eDNA persistence.
Keywords: eDNA, Species distribution, DNA degradation, Reptile
Introduction
Environmental DNA (eDNA) methods for monitoring the distribution of aquatic species have recently been developed (Ficetola et al., 2008; Rees et al., 2014; Goldburg, Strickler & Pilliod, 2015). The eDNA consists of DNA fragments released through the mucus, urine, gametes, or feces of species in the environment. In 2008, Ficetola et al. (2008) first used the eDNA method for a macro-organism, the American bullfrog (Rana catesbeiana), to detect their presence in ponds using water samples. It is possible to analyze DNA fragments of target species from a few liters or less of water (Ficetola et al., 2008; Rees et al., 2014; Goldberg & Strickler, 2015; Deiner et al., 2017). As we just sample the water in the field, eDNA methods are non-invasive, take a shorter time, and cost less compared with traditional monitoring methods, capture or visual survey (Thomsen & Willerslev, 2015).
The eDNA analysis has been applied to various aquatic taxa, for example, fish (Minamoto et al., 2012; Thomsen et al., 2012a; Takahara et al., 2012; Takahara, Minamoto & Doi, 2013; Eichmiller, Miller & Sorensen, 2016a), amphibians (Ficetola et al., 2008; Pilliod et al., 2013; Fukumoto, Ushimaru & Minamoto, 2015; Katano et al., 2017), mollusks (Goldberg et al., 2013), crustaceans (Tréguier et al., 2014), insects (Thomsen et al., 2012b; Doi et al., 2017b), trematodes (Huver et al., 2015; Hashizume et al., 2017), and aquatic plants (Fujiwara et al., 2016). However, there are few studies for reptiles, especially on the eDNA quantification of turtles (e.g., Davy, Kidd & Wilson, 2015; De Souza et al., 2016; Lacoursière-Roussel et al., 2016).
Here, we conducted research to determine the distribution and abundance of a turtle species, the red-eared slider (Trachemys scripta elegans). The red-eared slider is listed in the top 100 of the world’s worst invasive species by the World Conservation Union IUCN (Global Invasive Species Database, http://www.issg.org/database). In Japan, red-eared sliders were imported from USA as a pet in the 1950s, and since then it has been released into the local natural habitats (Oi et al., 2011). This species is an omnivore and it has gained some attention as they affect indigenous animals (Lever, 2003). In 2013, The Nature Conservation Society of Japan (NACS-J) conducted a visual survey in 41 of the 47 prefectures in Japan (N = 4,146), and reported that 64% of the turtle individuals observed were identified as red-eared sliders (NACS-J, 2013). Red-eared sliders disrupt pond ecosystems and have expanded their distribution into numerous ponds in Japan (Taniguchi et al., 2017). It is important to rapidly elucidate the distribution of invasive species to conserve the ecosystems in which they reside (Pyšek & Richardson, 2010); thus, methods to easily detect red-eared sliders, which are one of the common invasive species in Japan, should be developed. However, the effective sampling methods for turtles depends on the target species. Sterrett et al. (2010), suggested that we might underestimate the abundance of red-eared sliders by using visual observations. Thus, eDNA methods would be a useful tool to replace visual observations for evaluating the turtle distribution.
Despite the merits of eDNA methods for turtle surveying, previous eDNA studies in freshwater systems suggest that a number of environmental factors affect the probability of eDNA detection; for example, the water temperature and water quality, including the pH, suspended solid (SS), total phosphorous (TP), total nitrogen (TN), biological oxygen demand (BOD), and chlorophyll a (Chl. a) (e.g., Barnes et al., 2014; Strickler, Fremier & Goldberg, 2014; Eichmiller, Best & Sorensen, 2016b; Song, Small & Casman, 2017). These factors can lead to false negative detections, which prevents the accurate evaluation of eDNA concentrations and detection of the distribution/quantification of a species. To improve the eDNA evaluation of distribution and biomass/abundance in natural habitats, we should understand the relationship between eDNA detection rate/concentration and these environmental factors.
Two main environmental factors that negatively influence eDNA detection rate are ‘inhibition’ and ‘degradation’. In fact, humic acids are known to inhibit DNA polymerase in PCR (Matheson et al., 2010), and therefore, it could directly impact on eDNA detection. Mesocosm studies have found that a number of factors are important to decreasing the degradation of eDNA, such as low water temperatures, low UV-B levels, alkaline (high-pH), and increased BOD, increased Chl. a concentration, and increased total eDNA concentration (Barnes et al., 2014; Strickler, Fremier & Goldberg, 2014). Although these studies are based on laboratory experiments, in rivers, eDNA detection rate has been known to decrease with increasing Chl. a, but increase with increasing water temperature and pH (Song, Small & Casman, 2017). Because the effect of Chl. a was different in laboratory vs. in situ experiments, more evidence is needed to understand the effects of water quality on eDNA degradation; however, there are few studies on this conducted in the field.
We hypothesized that eDNA would be a suitable method to determine the distribution of red-eared sliders and that water quality would influence/inhibit eDNA measurement by quantitative real-time PCR (qPCR). In this study, our aim was to compare the eDNA concentrations of the target species, by using qPCR paired with visual observations of the turtles. Specifically, we evaluated the species abundance with the measurements of eDNA concentrations in 100 study ponds. From the eDNA concentration and water quality data, we examined the relationships between the water quality and eDNA concentrations of the red-eared slider, to consider the water quality effects that influence eDNA degradation and PCR inhibition in the ponds.
Materials & Methods
Study site
We conducted the field survey in 100 ponds that were located in Himeji, Japan (34°47′–34°54′N, 134°35′–134°45′E, Fig. 1) between July 21 and November 16, 2016. We randomly selected ponds located in each area category, comprising city, rural, and mountain areas (see Tables 1A, 1B, Fig. S1 in SEM). There are few ponds in the southern (city area) and northern areas (mountain) because the distribution of the ponds is biased. We conducted statistical analysis among the three area categories and eDNA concentration, but did not find remarkable patterns of eDNA concentration and detection among the pond locations. The field survey and pond sampling was permitted by the land owners, if needed.
Figure 1. Study sites represented by red points.
Table 1. Sampling date, location, the detection ofred-eared slidereDNA,and the number of red-eared slidersvisually observed in the study ponds.
Observed by both eDNA and visual observation is ⊚. Observed by only eDNA and by only visual observation are ∘ and •, respectively.
| (A) | |||||
|---|---|---|---|---|---|
| Pond No. | Date | Latitude | Longtitude | eDNA detected | Visual observation |
| 1 | 2016∕7∕21 | 34°51′27″71 | 134°40′36″11 | ⊚ | 10 |
| 2 | 2016∕7∕29 | 34°51′15″84 | 134°41′12″84 | • | 2 |
| 3 | 2016∕7∕29 | 34°51′16″92 | 134°41′12″84 | ∘ | – |
| 4 | 2016∕8∕4 | 34°51′02″88 | 134°41′00″96 | – | – |
| 5 | 2016∕8∕4 | 34°50′57″12 | 134°40′46″92 | ⊚ | 1 |
| 6 | 2016∕8∕8 | 34°51′36″36 | 134°40′57″57 | ⊚ | 2 |
| 7 | 2016∕8∕8 | 34°51′30″96 | 134°40′49″08 | ∘ | – |
| 8 | 2016∕8∕10 | 34°51′02″88 | 134°40′32″16 | – | – |
| 9 | 2016∕8∕10 | 34°51′50″27 | 134°40′36″26 | – | – |
| 10 | 2016∕8∕16 | 34°51′38″88 | 134°40′39″39 | ⊚ | 10 |
| 11 | 2016∕8∕16 | 34°51′42″12 | 134°40′27″84 | • | 1 |
| 12 | 2016∕8∕19 | 34°50′24″24 | 134°41′42″42 | – | – |
| 13 | 2016∕8∕19 | 34°50′26″88 | 134°41′30″84 | ⊚ | 3 |
| 14 | 2016∕8∕26 | 34°51′52″92 | 134°40′35″04 | ⊚ | 1 |
| 15 | 2016∕10∕7 | 34°52′15″97 | 134°41′11″45 | – | – |
| 16 | 2016∕10∕7 | 34°52′21″91 | 134°41′10″54 | ⊚ | 1 |
| 17 | 2016∕10∕7 | 34°52′19″39 | 134°41′12″48 | – | – |
| 18 | 2016∕10∕7 | 34°52′24″05 | 134°41′10″23 | – | – |
| 19 | 2016∕10∕7 | 34°52′06″21 | 134°41′48″00 | • | 1 |
| 20 | 2016∕10∕7 | 34°52′02″02 | 134°41′48″27 | ∘ | – |
| 21 | 2016∕10∕14 | 34°51′36″40 | 134°42′38″65 | – | – |
| 22 | 2016∕10∕14 | 34°51′52″94 | 134°42′04″27 | – | – |
| 23 | 2016∕10∕14 | 34°51′47″99 | 134°42′14″86 | ∘ | – |
| 24 | 2016∕10∕14 | 34°51′44″67 | 134°42′14″22 | – | – |
| 25 | 2016∕10∕14 | 34°51′42″18 | 134°41′59″00 | – | – |
| 26 | 2016∕10∕14 | 34°51′44″11 | 134°41′52″91 | – | – |
| 27 | 2016∕10∕14 | 34°51′40″18 | 134°41′22″60 | – | – |
| 28 | 2016∕10∕14 | 34°51′38″16 | 134°41′17″16 | • | 1 |
| 29 | 2016∕10∕14 | 34°51′50″62 | 134°40′57″02 | – | – |
| 30 | 2016∕10∕14 | 34°51′48″96 | 134°40′56″23 | • | 3 |
| 31 | 2016∕10∕17 | 34°51′54″45 | 134°40′37″75 | – | – |
| 32 | 2016∕10∕17 | 34°51′57″51 | 134°40′28″63 | – | – |
| 33 | 2016∕10∕17 | 34°51′52″84 | 134°40′48″37 | – | – |
| 34 | 2016∕10∕19 | 34°51′43″21 | 134°35′35″21 | ∘ | – |
| 35 | 2016∕10∕19 | 34°51′43″34 | 134°35′26″03 | • | 1 |
| 36 | 2016∕10∕19 | 34°51′46″64 | 134°35′17″99 | – | – |
| 37 | 2016∕10∕19 | 34°51′41″91 | 134°35′46″99 | – | – |
| 38 | 2016∕10∕19 | 34°51′53″15 | 134°35′51″31 | – | – |
| 39 | 2016∕10∕19 | 34°52′06″27 | 134°35′47″93 | – | – |
| 40 | 2016∕10∕19 | 34°52′16″58 | 134°35′44″54 | – | – |
| 41 | 2016∕10∕19 | 34°51′31″47 | 134°35′53″56 | ⊚ | 4 |
| 42 | 2016∕10∕19 | 34°51′32″04 | 134°36′02″88 | ⊚ | 1 |
| 43 | 2016∕10∕19 | 34°51′28″49 | 134°36′06″63 | • | 5 |
| 44 | 2016∕10∕19 | 34°51′27″27 | 134°36′02″16 | – | – |
| 45 | 2016∕10∕19 | 34°51′29″60 | 134°35′20″10 | – | – |
| 46 | 2016∕10∕19 | 34°51′33″48 | 134°35′25″09 | ∘ | – |
| 47 | 2016∕10∕19 | 34°51′40″47 | 134°35′24″34 | – | – |
| 48 | 2016∕10∕21 | 34°51′55″82 | 134°36′13″86 | – | – |
| 49 | 2016∕10∕21 | 34°52′00″86 | 134°36′09″34 | – | – |
| 50 | 2016∕10∕21 | 34°52′19″20 | 134°36′07″22 | – | – |
Field survey and sampling
We recorded the presence/absence and number of red-eared sliders, based on visual observations from the shore line for three minutes by an expert (A. Kakuda). We performed surveys during the daytime (1000–1300 h) each day, except for rainy days and one day after rain. From a point within each pond, 500 mL of surface water was collected for eDNA and SS analysis, and a further 100 mL of surface water was collected for Chl. a and water quality analysis. In this study, we conducted a field survey to compare the convenience of the eDNA method with visual surveys; thus, we decided to increase the number of sampling ponds rather than increase the replications at each pond. We selected a water sampling point in the middle section of the ponds, far from the water outflows/inflows. We directly sampled the eDNA using a bleached bottle and added 0.5 mL of benzalkonium chloride (BAC) to avoid a reduction of the eDNA concentration in the samples (Yamanaka et al., 2016). Samples were stored in a cooler box with a ‘cooler blank’. The ‘cooler blank’ contained 500 mL of DNA-free water, which we brought to the field, and it was treated identically to the other water samples, except that it was not opened at the field sites.
Water preparation
Within six hours after water sampling, the samples were filtered onto a GF/F glass filter (47 mm diameter and 0.7 µm pore size, GE Healthcare Japan, Tokyo, Japan). We used separate filters for the eDNA, SS (from 500 mL water), and Chl. a (from 100 mL water) analysis. The filter was then wrapped in commercial aluminum foil and stored at −20 °C until eDNA extraction, or SS/ Chl. a measurement. For eDNA samples, the ‘cooler blank’ and a ‘filter blank’ consisting of DNA-free distilled water were filtered in the same way as the samples. To avoid contamination, each piece of equipment that was used in the water sampling or filtration was soaked in a 10% commercial bleach solution (approximately 0.6% sodium hypochlorite) and rinsed using DNA-free distilled water prior to reuse. The 80 mL of the filtrated samples were stored at −20 °C until further water quality analyses. We lost some of the samples for water quality measurements (see the missing values of Tables S1A, S1B in SEM).
DNA extraction from the filters
The eDNA was extracted from each filter using a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) based on the method described by Uchii, Doi & Minamoto (2016). Each filtrate was soaked in 400 µL of buffer AL and 40 µL of protease K in a Salivette tube (Sarstedt, Nümbrecht, Germany) and incubated at 56 °C for 30 min. After centrifugation at 5,000 g for 5 min, 220 µL of TE buffer (pH 8.0) was added to each filter, and the tubes were centrifuged again in the same way after being kept for a minute. The 200 µL of buffer AL and 600 µL of 100% EtOH were then added to each filtrate and mixed by pipetting. The mixture was applied to a DNeasy Mini spin column and prepared according to the manufacture’s manual. The sample solution (100 µL of buffer AE) was stored in a 1.5 mL microtube at −20 °C until qPCR analysis.
Quantitative real-time PCR (qPCR)
The eDNA was measured with four PCR replicates using a PikoReal Real-Time PCR System (Thermo Scientific, Waltham, MA, USA). To detect and quantify the DNA of the red-eared slider using qPCR, the mitochondrial cytochrome b gene fragments were amplified and quantified with the following primers and probe: Tse-Kako-A-F (5′-CCTCCAACATCTCTGCTTGA -3′), Tse-Kako-A-R (5′-ATTGTACGTCTCGGGTGATG-3′), and Tse-Kako-A-MGB-P (5′-FAM- CGGAATTTTCTTGGCTATAC -MGB-3′). We used the consensus sequence of Trachemy scripta elegans with the following accession numbers: FJ770617, FR717131, EU787024, AF207750, and HQ442420 in NCBI (http://www.ncbi.nlm.nih.gov/). From the consensus sequence, we artificially synthesized a standard gene, which included the amplicon region and 20 bases each of the upper and lower sequences (5′- ATTCATTGATCTACCAAGCCCCTCCAACATCTCTGCTTGATGGAACTTTGGATCCTTATTAGGTACTTGCCTAATCCTACAAATCCTTACCGGAATTTTCTTGGCTATACACTACTCCCCAGACATTTCACTAGCATTCTCATCAGTAGCCCACATCACCCGAGACGTACAATACGGATGACTTATTCGTAAT-3′). The specificity of the probe and primers was confirmed by Primer-BLAST and testing on Japanese turtles (Mauremys japonica, Mauremys reevesii, and Pelodiscus sinensis). Each TaqMan reaction contained 900 nM of each primer, 125 nM of TaqMan probe, 5 µL qPCR master mix (TaqMan Environmental Master Mix 2.0, Thermo Scientific, Waltham, MA, USA), 0.2 µL AmpErase® Uracil N-Glycosylase (UNG, Thermo Scientific, Waltham, MA, USA), and 2 µL of the DNA solution. The total volume of each reaction mixture was 10 µL and we performed four replicates for PCR. The PCR conditions were as follows: 2 min at 50 °C, 10 min at 95 °C, and 55 cycles of 15 s at 95 °C and 60 s at 60 °C. These cycles are based on various previously described methods (Thomsen et al., 2012a; Tréguier et al., 2014; Katano et al., 2017; Doi et al., 2017b). The qPCR results were analyzed using PikoReal software ver. 2.2.248.601 (Thermo Fisher Scientific, Waltham, MA, USA), and the standard curves were also automatically estimated by this software. The R2 values of the standard curves ranged from 0.960 to 0.989, and PCR efficiency ranged from 73.09 to 118.56%. For the R2 values, there is a possibility that we measured the eDNA in the qPCR system with order-level differences because of the high variance in eDNA concentration, from 0.21 to 48,016.44 (copies/L). We defined the limit of detection (LOD) for red-eared slider DNA as one copy per reaction based on a qPCR assay of the four replicates. Each real-time PCR assay included four no template controls (NTCs) and we also measured the cooler and filter blanks with four replicates. We used the average value of the four replicates for each eDNA concentration. All of the above qPCR procedures were based on the MIQE checklist for qPCR (Bustin et al., 2009). We performed the PCR set up and real-time PCR in two separate rooms to avoid contamination.
PCR inhibition test
We compared the Ct shift between the samples and controls with the same number of known target DNA copies, based on the method by Doi et al. (2017b), to confirm the degree of PCR inhibition. Ct is defined as the number of cycles required for enough amplified PCR product to accumulate that it surpasses a threshold recognized by the real-time PCR instrumentation. The Ct is inversely related to the starting quantity of the target DNA in a reaction and is used to calculate this quantity. The presence of PCR inhibitors will shift (delay) the Ct for a given quantity of the template DNA. To confirm the effects of water quality factors on eDNA inhibition, we did not use a PCR inhibition removal kit (e.g., OneStep PCR Inhibitor Removal Kit, Zymo Research) for the qPCR preparation. To test for PCR inhibition in the DNA samples, 1 µL of plasmid including the cytochrome b gene from Trachurus japonicus (1.5 × 104 copies), which is a marine fish that does not inhabit the sampled ponds, was added to the PCR tempelate with 1 µL of DNA-free distilled water. We used the primer and probe set that was reported by Yamamoto et al. (2016): forward primer: 5′-CAGATATCGCAACCGCCTTT-3′; reverse primer: 5′-CCGATGTGAAGGTAAATGCAAA-3′; and probe: 5′-FAM-TATGCACGCCAACGGCGCCT-TAMRA-3′. The PCR conditions were the same as above. Each real-time PCR assay included three no template controls. We used the average value of the replicates for each Ct value. ΔCt ≥ 3 cycles were considered to be evidence of inhibition (Hartman, Coyne & Norwood, 2005).
Water quality analysis
We measured phosphate (PO4-P), nitrate (NO3-N), total phosphorus (TP), total nitrogen (TN), dissolved organic matter (DOM), and total organic matter (TOM) from the filtrate, according to the methods of Saijo & Mitamura (1995), using a spectrophotometer (HITACHI U-5100, Hitachi, Tokyo, Japan). The absorbance of DOM was read at 254 nm, using samples not in an autoclave.
SS measurement
We used the GF/F glass filter, which had been burned and dried before the weight was measured by electric balance (Sartorius CPA2252), prior to the SS analysis. The filtrate was dried in the 60 °C automatic oven (Yamato DX402, Yamato, Japan) over 12 h before the weight was measured. After that, we burned the dried filter at 450 °C for 2 h using an electric muffle furnace (Yamato FO410), then measured the weight again in the same way. The SS content was calculated as follows; (450 °C burned weight)–(60 °C dried weight).
Chl. a measurement
We extracted the Chl. a of the filter by immersing it in 99.5% ethanol over 12 h. The extracts were measured at 630, 645, 663, and 750 nm absorbances by the spectrophotometer (HITACHI U-5100). The Chl. a concentration was determined according to the following equation (UNECSO 1969):
where, k: ethanol for extraction (mL); E663, E645, and E630: each absorbance at 663, 645, 630 nm, excluding the absorbance at 750 nm; and V: water samples (L).
Statistical analysis
We used a linear model (LM) to evaluate the relationship between the red-eared slider eDNA concentration and the number of red-eared sliders observed visually. For the eDNA concentration and the number of red-eared sliders reported by visual observation, we used the data of the sites where red-eared sliders were detected, regardless of the eDNA detection (11 ponds in which red-eared sliders were detected by both methods and 10 ponds in which they were detected only by visual observation; N = 21; Tables S1A, S1B). In addition, we used a multiple linear regression to evaluate the relationship between the eDNA concentration and the following environmental factors: Chl. a, SS, PO4-P, NO3-N, TP, TN, DOM, TOM, and estimated number of red-eared sliders (animals/km2; from the result of eDNA concentration and the number of red-eared sliders observed visually). We used the data of all sites in which eDNA was detected (11 ponds in which red-eared sliders were detected by both methods and nine ponds in which they were detected by only eDNA, N = 20, Tables S1A, S1B). Prior to the multiple linear regression, we used a variance inflation factor (VIF) to check the collinearity of the factors. The maximum VIF was 59.685, indicating that co-linearity among the factors would influence the results of the multiple linear regression. Thus, we removed the factors with a VIF >5 to reduce the collinearity effect on the multiple linear regression, resulting in the Chl. a, TP, and number of red-eared sliders remaining in the final linear model (LM) analysis. All statistical analysis and graphics were conducted in R ver. 3.4.1 (R Core Team, 2018) with the “ggplot2” and “car” packages.
Results
The relationships between eDNA measurements and visual observations
Of the 100 surveyed ponds, we detected red-eared sliders in 30 ponds: they were detected in 11 ponds by both visual and eDNA surveys, 10 ponds by only visual survey, and 9 ponds by only the eDNA survey (Tables 1A, 1B). All successful amplifications were considered to be red-eared sliders, because the probe and primers were confirmed to detect only red-eared sliders by Primer-BLAST and were tested by real-time PCR using DNA extracted from Japanese turtles (Mauremys japonica, Mauremys reevesii, and Pelodiscus sinensis) living in the same region. There was a significant positive correlation between the eDNA concentrations in all ponds in which eDNA was detected and the number of red-eared sliders identified by visual observations (LM, R2 = 0.48, p < 0.001, N = 20, Fig. 2).
Figure 2. Relationship between the visual observation number of red-eared sliders (Trachemys scripta elegans) per km2 and their eDNA concentrations in the ponds.
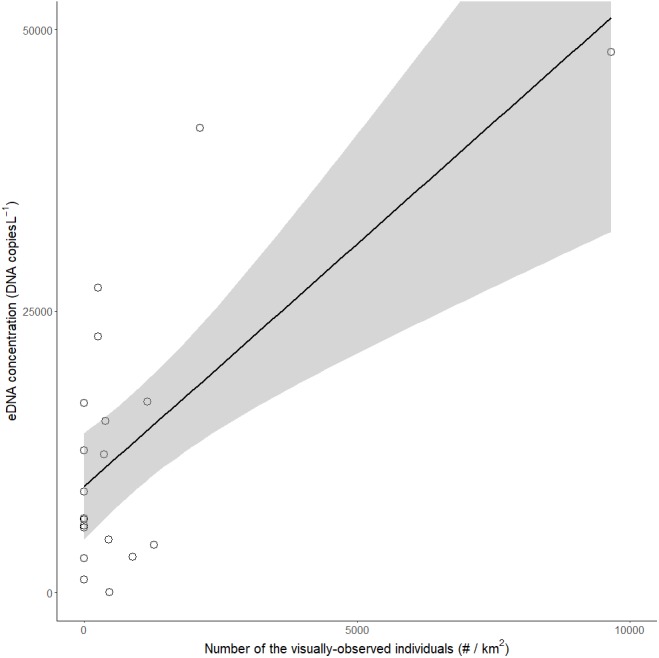
The grey area represents the limits of the 95% confidence interval for the slope of the linear regression (R2 = 0.48, p < 0.001).
The ΔCt values in 99 of the 100 ponds, except pond No. 33, were lower than 3 (0.27 ± 0.22, mean ± SD), which means that they were lower than the inhibition criteria (Hartman, Coyne & Norwood, 2005). Thus, PCR inhibition was not significant for all samples, but pond No. 33 showed no amplification in the PCR inhibition test. We did not detect any amplifications in the negative controls and equipment blanks, including the cooler and filter blanks.
The relationship between eDNA concentration and water quality
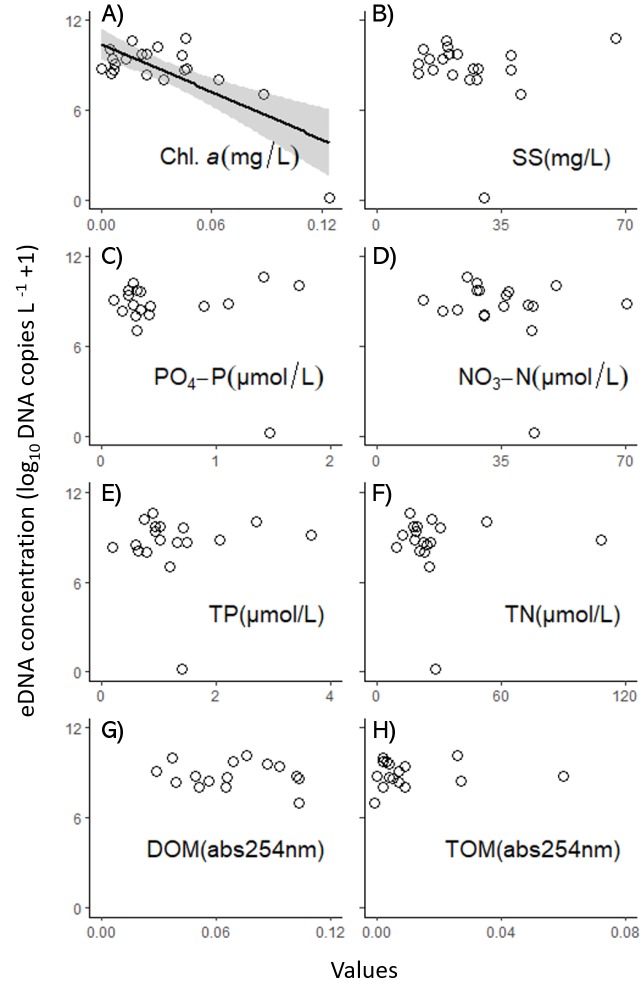
The results of the water quality analysis are shown in Tables S1A, S1B of the SEM [Chl. a: 0.04 ± 0.10 µg L−1, SS: 35.0 ± 44.34 µg L−1, PO4-P: 0.78 ± 0.87 µmol L−1, NO3-N: 38.93 ± 18.25 µmol L−1, TP: 1.00 ± 0.99 µmol L−1, TN: 29.53 ± 30.77 µmol L−1, DOM: 0.07 ± 0.04 (abs = 254 nm), and TOM: 0.00 ± 0.01 (abs = 254 nm), mean ± 1 SD]. The VIFs of the LM for each factor are shown in Table S2 in the SEM, of which the VIFs of the Chl. a (4.391), TP (1.870), and estimated number of red-eared sliders (1.801) were lower than 5. The LM, without the factors and with a VIF of >5, showed that Chl. a was negatively related with the eDNA concentration (LM, p < 0.001, Tables 2, 3), while there was no significant relationship between the eDNA concentration and other factors (Fig. 3, Tables S1A, S1B).
Table 2. Linear regression slopes with a ±95% confidence interval, SE, t values, and p values for the relationships between Chl. a, TP, and eDNA concentrations in the ponds.
Factors with a VIF > 5 were removed.
| (A) | ||||
|---|---|---|---|---|
| Factors | Slope | SE | t value | p value |
| Chl. a | −48.550 | 10.740 | −4.519 | 0.000 |
| TP | −0.345 | 0.392 | −0.882 | 0.393 |
| Turtles | 0.000 | 0.000 | 2.075 | 0.057 |
| Intercept | 10.450 | 0.754 | 13.865 | 0.000 |
Table 3. The table represents the n, F value, p value of the F value, R2, and adjusted R2 for the linear regression.
| F value | 12.73 |
| p value | 0.000 |
| R2 | 0.732 |
| Adjusted R2 | 0.674 |
Figure 3. Relationships between each water-quality factor and the eDNA concentration of the red-eared slider in the ponds (A: Chl. a, B: SS, C: PO 4-P, D: NO3-N, E: TP, F: TN, G: DOM, H: TOM).
The regression curve of Chl. a was drawn by linear regression with 95% confidence intervals for the slope (R2 = 0.54, p < 0.001).
Discussion
We detected the eDNA of the red-eared slider in the surface water samples of the ponds. In this study, we designed the red-eared slider-specific primers and probe and showed that we can use them to detect the red-eared sliders in field samples. The red-eared slider is a common invasive species in Japan, but we found them in only a few sites. This is probably because we randomly selected the study ponds from each region, including less invasive sites in the mountain area. When comparing with the visual observations, we detected the red-eared slider by both the eDNA and visual surveys in 11 ponds. In the nine ponds where only eDNA detected turtles, we suspect that turtles were too rare to be observed by visual observation. In addition, eDNA methods have often been cited to detect rare or cryptic species (Barnes & Turner, 2016). Our results in nine ponds support this phenomenon as the turtles were detected not by visual observation, but by eDNA. While, in the ten ponds detected by only visual observation, we sampled the eDNA at one point per pond as per the survey design. This result suggests that it was necessary to sample at several points at each study site, to decrease the false-negative eDNA detections (e.g., Tréguier et al., 2014; Thomsen et al., 2012a). It would be necessary to compare eDNA sampling at several points at each study site with other surveys in future studies. In summary, we can detect red-eared sliders using eDNA for two thirds of the total detections, by sampling only 500 mL of water at a point in the pond. However, we concluded that it might be appropriate to use both a visual survey and eDNA analysis for red-eared sliders, as eDNA produced false-negative detections when red-eared sliders were present.
We also found a significant positive correlation between the eDNA concentration and number of red-eared sliders detected by visual observation. Others, such as Takahara et al. (2012) have found positive correlations between eDNA concentrations and the biomass using both aquaria and outdoor experiments. The field tests for the relationship between eDNA concentration and biomass of amphibians and fish also support the positive correlation (e.g., Pilliod et al., 2013; Doi et al., 2017b). Although few studies examining the effects of biomass or number of individuals on eDNA have used reptiles, Lacoursière-Roussel et al. (2016) found eDNA detection rate was highly correlated to the relative abundance of wood turtles. In our study, likewise, a positive relationship between the eDNA concentration and the abundance of visual detections was observed. Thus, we can possibly use the eDNA concentration to estimate the number of red-eared sliders, especially for the ponds with a high species abundance.
From the relationships between water quality and eDNA concentration, Chl. a seemed to influence the degradation of eDNA. One of our hypotheses was that water quality would influence/inhibit qPCR for eDNA detection, however, we could not find any inhibition of PCR by inhibition tests, except for a single pond (No. 33). Only pond No. 33 could be inhibited, because it showed no amplification of DNA. It is considered that one of the inhibitors of the PCR for pond No. 33 was humic acid from the decomposition of leaves, which is known to inhibit PCR detection (Opel, Chung & McCord, 2009), because it was surrounded by forest and the water color was black (Fig. S2 in SEM). Thus, Chl. a might not be directly related to PCR inhibition; however, it might have an influence with respect to decreasing the eDNA concentration in the water through DNA degradation. For the other water quality characteristics in our study, Eichmiller, Best & Sorensen (2016b) measured the effects of Chl. a, TN, TP, and SS on the decay rate of carp eDNA in laboratory experiments, however, these variables were not significantly correlated with the eDNA decay rate (Eichmiller, Best & Sorensen, 2016b). This result of non-correlations with TN, TP, and SS to eDNA degradation was the same as our results, however, we showed the negative relationship with Chl. a and eDNA.
Our result that only Chl. a had a significant effect, might suggest that Chl. a influences the degradation of eDNA in the surface water of ponds. However, this phenomenon seems to be in debate. For example, the eDNA decay rate has a negative relationship with Chl. a in a mesocosm experiment for goldfish (Barnes et al., 2014), i.e., the eDNA degradation was less in the higher Chl. a. On the other hand, the eDNA detection rate has a negative relationship with Chl. a in a field survey for silver carp (Song, Small & Casman, 2017). In our study, the eDNA concentrations have a negative relationship with Chl. a, which supports the results of (Song, Small & Casman, 2017). As abiotic environmental factors indirectly influence the increase of microbial activities, eDNA may be decomposed by microorganisms (Barnes et al., 2014). Thus, the eDNA degradation by microorganisms bonded to phytoplankton, for example, indirectly increases microbial activities by providing basal resources (Lennon, 2007), although we did not directly evaluate the microbial activity. Further discussion on the “Chl. a hypothesis” on eDNA degradation is required for understanding the mechanisms of eDNA degradation and for developing eDNA methods, especially for eDNA surveys in highly-productive water bodies. In this study, we can provide the hypothesis from the field data, but further field and laboratory experiments controlling the DNA concentration and water conditions, including the water quality and planktonic community, are required for understanding the mechanisms. In addition, we detected eDNA in only 20 ponds (11 ponds by both eDNA and visual observation and 9 ponds by only eDNA) by a single sampling from each pond. In future field studies, more replications would be needed.
Conclusion
In conclusion, we detected red-eared sliders using eDNA at a similar performance to that using visual observations, and evaluated the abundance using the eDNA concentration obtained from a single sampling point at each pond. In future field studies, more replications at each sites would be needed, as other studies (e.g., Barnes & Turner, 2016) reported, which suggested that multiple water sampling points at a pond would increase the ability to evaluate the distribution and abundance by eDNA. Likewise, to prevent the spread of red-eared sliders, further developments of the eDNA method to easily detect them will be necessary in the future. We also provide the “Chl. a hypothesis” for eDNA degradation for comparing the water quality of the ponds. For eDNA surveys, we should pay attention to the potential for false-negative detections, probably because of the state of primary production with reference to the Chl. a concentration. Understanding the mechanisms in eDNA degradation would provide us with the tools for easy and accurate eDNA methods to evaluate the distribution of aquatic organisms.
Supplemental Information
Acknowledgments
We thank A. Sumi and D. Togaki for their helps on our sampling and experiments.
Funding Statement
This study was supported by the Environment Research and Technology Development Fund (4-1602) of the Ministry of the Environment, Japan and JST-CREST (JPMJCR13A2) for Hideyuki Doi and Toshifumi Minamoto and JSPS KAKENHI (15K00596, 15K07233) for Izumi Katano and Toshifumi Minamoto, respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Hideyuki Doi, Email: hideyuki.doi@icloud.com.
Izumi Katano, Email: katano@cc.nara-wu.ac.jp, katanon2003@yahoo.co.jp.
Additional Information and Declarations
Competing Interests
Rio Souma is an employee of IDEA Consultants Inc.
Author Contributions
Aozora Kakuda conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Hideyuki Doi performed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Rio Souma and Mariko Nagano performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Toshifumi Minamoto performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Izumi Katano conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data is available as a Supplemental File.
References
- Barnes & Turner (2016).Barnes MA, Turner CR. The ecology of environmental DNA and implications for conservation genetics. Cross Mark. 2016;17:1–17. doi: 10.1007/s10592-015-0775-4. [DOI] [Google Scholar]
- Barnes et al. (2014).Barnes MA, Turner CR, Jerde CL, Renshaw MA, Chadderton WL, Lodge DM. Environmental conditions influence eDNA persistence in aquatic systems. Environmental Science & Technology. 2014;48:1819–1827. doi: 10.1021/es404734p. [DOI] [PubMed] [Google Scholar]
- Bustin et al. (2009).Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele SJ, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Davy, Kidd & Wilson (2015).Davy CM, Kidd AG, Wilson CC. Development and validation of environmental DNA (eDNA) markers for detection of freshwater turtles. PLOS ONE. 2015;10:e0130965. doi: 10.1371/journal.pone.0130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza et al. (2016).De Souza LS, Godwin JC, Renshaw RA, Larson E. Environmental DNA (eDNA) detection probability is influenced by seasonal activity of organisms. PLOS ONE. 2016;11:1–15. doi: 10.1371/journal.pone.0165273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner et al. (2017).Deiner K, Bik HM, Mächler EM, Seymour M, Lacoursière-Roussel A, Altermatt F, Creer S, Bista I, Lodge DM, Vere N, Pfrender ME, Bernatchez L. Environmental DNA metabarcoding: transforming how we survey animal and plant communities. Molecular Ecology. 2017;26:5872–5895. doi: 10.1111/mec.14350. [DOI] [PubMed] [Google Scholar]
- Doi et al. (2017b).Doi H, Katano I, Sakata Y, Souma R, Kosuge T, Nagano M, Ikeda K, Yano K, Tojo K. Detection of an endangered aquatic heteropteran using environmental DNA in a wetland ecosystem. Royal Society Open Science. 2017b;4 doi: 10.1098/rsos.170568. Article 170568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmiller, Best & Sorensen (2016b).Eichmiller JJ, Best SE, Sorensen PW. Effects of temperature and trophic state on degradation of environmental DNA in lake water. Environmental Science & Technology. 2016b;50:1859–1867. doi: 10.1021/acs.est.5b05672. [DOI] [PubMed] [Google Scholar]
- Eichmiller, Miller & Sorensen (2016a).Eichmiller JJ, Miller LM, Sorensen PW. Optimizing techniques to capture and extract environmental DNA for detection and quantification of fish. Molecular Ecology Resources. 2016a;16:56–68. doi: 10.1111/1755-0998.12421. [DOI] [PubMed] [Google Scholar]
- Ficetola et al. (2008).Ficetola GF, Miaud C, Pompanon F, Taberlet P. Species detection using environmental DNA from water samples. Biology Letters. 2008;4:423–425. doi: 10.1098/rsbl.2008.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara et al. (2016).Fujiwara A, Matsuhashi S, Doi H, Yamamoto S, Minamoto T. Use of environmental DNA to survey the distribution of an invasive submerged plant in ponds. Freshwater Science. 2016;35:748–754. doi: 10.1086/685882. [DOI] [Google Scholar]
- Fukumoto, Ushimaru & Minamoto (2015).Fukumoto S, Ushimaru A, Minamoto T. A basin-scale application of environmental DNA assessment for rare endemic species and closely related exotic species in rivers: a case study of giant salamanders in Japan. Journal of Applied Ecology. 2015;52:358–365. doi: 10.1111/1365-2664.12392. [DOI] [Google Scholar]
- Goldberg et al. (2013).Goldberg CS, Sepulveda A, Ray A, Baumgardt J, Waits LP. Environmental DNA as a new method for early detection of New Zealand mudsnails (Potamopyrgus antipodarum) Freshwater Science. 2013;32:792–800. doi: 10.1899/13-046.1. [DOI] [Google Scholar]
- Goldburg, Strickler & Pilliod (2015).Goldburg CS, Strickler KM, Pilliod DS. Moving environmental DNA methods from concept to practice for monitoring aquatic macroorganisms. Biological Conservation. 2015;183:1–3. doi: 10.1016/j.biocon.2014.11.040. [DOI] [Google Scholar]
- Hartman, Coyne & Norwood (2005).Hartman LJ, Coyne SR, Norwood DA. Development of a novel internal positive control for TaqMan® based assays. Molecular and Cellular Probes. 2005;19:51–59. doi: 10.1016/j.mcp.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Hashizume et al. (2017).Hashizume H, Sato M, Sato MO, Ikeda S, Yoonuan T, Sanguankiat S, Pongvongsa T, Moji K, Minamoto T. Application of environmental DNA analysis for the detection of Opisthorchis viverrini DNA in water samples. Acta Tropica. 2017;169:1–7. doi: 10.1016/j.actatropica.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Huver et al. (2015).Huver JR, Koprivnikar J, Johnson PTJ, Whyard S. Development and application of an eDNA method to detect and quantify a pathogenic parasite in aquatic ecosystems. Ecological Applications. 2015;25(4):991–1002. doi: 10.1890/14-1530.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano et al. (2017).Katano I, Harada K, Doi H, Souma R, Minamoto T. Environmental DNA method for estimating salamander distribution in headwater streams, and a comparison of water sampling methods. PLOS ONE. 2017;12(5):e0176541. doi: 10.1371/journal.pone.0176541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoursière-Roussel et al. (2016).Lacoursière-Roussel A, Dubois Y, Normandeau E, Bernatchez L. Improving herpetological surveys in eastern North America using the environmental DNA method. Genome. 2016;59(11):991–1007. doi: 10.1139/gen-2015-0218. [DOI] [PubMed] [Google Scholar]
- Lennon (2007).Lennon JD. Diversity and metabolism of marine bacteria cultivated on dissolved DNA. Applied and Environmental Microbiology. 2007;73:2799–2805. doi: 10.1128/AEM.02674-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever (2003).Lever C. Naturalized reptiles and amphibians of the world. Oxford University Press; Oxford: 2003. p. 17. [Google Scholar]
- Matheson et al. (2010).Matheson CD, Gurney C, Esau N, Lehto R. Assessing PCR inhibition from humic substances. The Open Enzyme Inhibition Journal. 2010;3:38–45. doi: 10.1371/journal.pone.0114639. [DOI] [Google Scholar]
- Minamoto et al. (2012).Minamoto T, Yamanaka H, Takahara T, Honjo MN, Kawabata Z. Surveillance of fish species composition using environmental DNA. Limnology. 2012;13:193–197. doi: 10.1007/s10201-011-0362-4. [DOI] [Google Scholar]
- Oi et al. (2011).Oi M, Araki J, Matsumoto J, Nogami S. Helminth fauna of a turtle species introduced in Japan, the red-eared slider turtle (Trachemys scripta elegans) Veterinary Science. 2011;93:826–830. doi: 10.1016/j.rvsc.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Opel, Chung & McCord (2009).Opel KL, Chung D, McCord BR. A study of PCR inhibition mechanisms using real time PCR. Journal of Forensic Sciences. 2009;55:25–33. doi: 10.1111/j.1556-4029.2009.01245.x. [DOI] [PubMed] [Google Scholar]
- Pilliod et al. (2013).Pilliod DS, Goldberg CS, Arkle RS, Waits LP. Estimating occupancy and abundance of stream amphibians using environmental DNA from filtered water samples. Canadian Journal of Fisheries and Aquatic Sciences. 2013;70:1123–1130. doi: 10.1139/cjfas-2013-0047. [DOI] [Google Scholar]
- Pyšek & Richardson (2010).Pyšek P, Richardson DM. Invasive species, environmental change and management, and health. Annual Review of Environment and Resources. 2010;35:25–55. doi: 10.1146/annurev-environ-033009-095548. [DOI] [Google Scholar]
- R Core Team (2018).R Core Team . R Foundation for Statistical Computing; Vienna: 2018. [15 May 2018]. [Google Scholar]
- Rees et al. (2014).Rees HC, Maddison BC, Middleditch DJ, Patmore JR, Gough KC. REVIEW: the detection of aquatic animal species using environmental DNA—a review of eDNA as a survey tool in ecology. Journal of Applied Ecology. 2014;51:1450–1459. doi: 10.1111/1365-2664.12306. [DOI] [Google Scholar]
- Saijo & Mitamura (1995).Saijo Y, Mitamura O. Shinpen Kosyo tyosaho method in limnological research (in Japanese) Kodansya; Tokyo: 1995. [Google Scholar]
- Song, Small & Casman (2017).Song JW, Small MJ, Casman EA. Making sense of the noise: the effects of hydrology on silver carp eDNA detection in the Chicago area waterway system. Science of the Total Environment. 2017;605–606:713–720. doi: 10.1016/j.scitotenv.2017.06.255. [DOI] [PubMed] [Google Scholar]
- Sterrett et al. (2010).Sterrett SC, Smith LL, Schweizer SH, Maerz JC. An assessment of two methods for sampling river turtle assemblages. Herpetological Conservation and Biology. 2010;5:490–497. [Google Scholar]
- Strickler, Fremier & Goldberg (2014).Strickler KM, Fremier AK, Goldberg CS. Quantifying effects of UV-B, temperature, and pH on eDNA degradation in aquatic microcosms. Biological Conservation. 2014;183:85–92. doi: 10.1016/j.biocon.2014.11.038. [DOI] [Google Scholar]
- Takahara, Minamoto & Doi (2013).Takahara T, Minamoto T, Doi H. Using environmental DNA to estimate the distribution of an invasive fish species in ponds. PLOS ONE. 2013;8:e56584. doi: 10.1371/journal.pone.0056584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara et al. (2012).Takahara T, Minamoto T, Yamanaka H, Doi H, Kawabata Z. Estimation of fish biomass using environmental DNA. PLOS ONE. 2012;7:e35868. doi: 10.1371/journal.pone.0035868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi et al. (2017).Taniguchi M, Lovich JE, Mine K, Ueno S, Kamezaki N. Unusual population attributes of invasive red-eared slider turtles (Trachemys scripta elegans) in Japan: do they have a performance advantage? Aquatic Invasions. 2017;12(1):97–108. doi: 10.3391/ai.2017.12.1.10. [DOI] [Google Scholar]
- NACS-J (2013).The Nature Conservation Society of Japan (NACS-J) The research report of turtles in Japan (in Japanese) The Nature Conservation Society of Japan document No. 53 2013
- Thomsen et al. (2012a).Thomsen PF, Kielgast J, Iversen LL, Wiuf C, Rasmussen M, Gilbert MTP, Orland L, Willerslev E. Monitoring endangered freshwater biodiversity using environmental DNA. Molecular Ecology. 2012a;21:2565–2573. doi: 10.1111/j.1365-294X.2011.05418.x. [DOI] [PubMed] [Google Scholar]
- Thomsen et al. (2012b).Thomsen PF, Kielgast J, Iversen LL, Møller PR, Rasmussen M, Willerslev E. Detection of a diverse marine fish fauna using environmental DNA from seawater samples. PLOS ONE. 2012b;7:e41732. doi: 10.1371/journal.pone.0041732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen & Willerslev (2015).Thomsen PF, Willerslev E. Environmental DNA—an emerging tool in conservation for monitoring past and present biodiversity. Biological Conservation. 2015;183:4–18. doi: 10.1016/j.biocon.2014.11.019. [DOI] [Google Scholar]
- Tréguier et al. (2014).Tréguier A, Paillisson JM, Dejean T, Valentini A, Schlaepfer MA, Roussel JM. Environmental DNA surveillance for invertebrate species: advantages and technical limitations to detect invasive crayfish Procambarus clarkii in freshwater ponds. Journal of Applied Ecology. 2014;51:871–879. doi: 10.1111/1365-2664.12262. [DOI] [Google Scholar]
- Uchii, Doi & Minamoto (2016).Uchii K, Doi H, Minamoto T. A novel environmental DNA approach to quantify the cryptic invasion of non-native genotypes. Molecular Ecology Resources. 2016;16:415–422. doi: 10.1111/1755-0998.12460. [DOI] [PubMed] [Google Scholar]
- Yamamoto et al. (2016).Yamamoto S, Minami K, Fukaya K, Takahashi K, Sawada H, Murakami H, Tsuji S, Hashizume H, Kubonaga S, Horiuchi T, Hongo M, Nishida J, Okugawa Y, Fujiwara A, Fukuda M, Hidaka S, Suzuki KW, Miya M, Araki H, Yamanaka H, Maruyama A, Miyashita K, Masuda R, Minamoto T, Kondoh M. Environmental DNA as a Snapshot of fish distribution: a case study of Japanese Jack Mackerel in Maizuru Bay, Sea of Japan. PLOS ONE. 2016;11:e0149786. doi: 10.1371/journal.pone.0149786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka et al. (2016).Yamanaka H, Minamoto T, Matsuura J, Sakurai S, Tsuji S, Motozawa H, Hongo M, Sogo Y, Kakimi N, Teramura I, Sugita M, BabYa M, Kondo A. A simple method for preserving environmental DNA in water samples at ambient temperature by addition of cationic surfactant. Limnology. 2016;18:233–241. doi: 10.1007/s10201-016-0508-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available as a Supplemental File.