Abstract
Background:
S1400D is a biomarker-driven therapeutic sub-study of Lung-MAP evaluating the fibroblast growth factor receptor (FGFR) inhibitor AZD4547 in patients with FGF pathway-activated squamous cell non-small cell lung cancer (SqNSCLC). This is the first phase II trial to evaluate AZD4547 as a targeted approach in patients with previously treated FGFR-altered SqNSCLC and is the first demonstration of successful implementation and conduct of a national umbrella protocol in this disease setting.
Methods:
Eligible patients had tumoral FGFR alteration or mutation and had progressive disease after at least 1 line of platinum-based systemic therapy. Patients received AZD4547 80 mg twice daily orally. Primary endpoint was response by RECIST 1.1; secondary endpoints included progression-free survival (PFS), overall survival (OS) and duration of response (DoR).
Results:
Ninety-two patients were assigned to S1400D, 43 were enrolled, and 27 AZD4547-treated patients were evaluable. Evaluable patients were predominantly white (n=24, 89%), median age 66 y (49–88), female (n=7, 26%). FGFR alterations included FGFR1 amplification (n=23; 85%); FGFR3 amplification (n=2; 7%); FGFR3 S249C (n=2; 7%); and FGFR3 fusion (n=1; 4%).
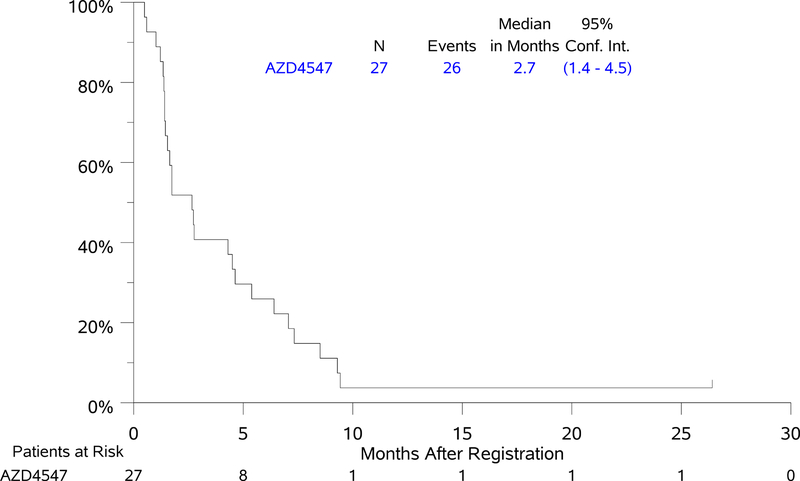
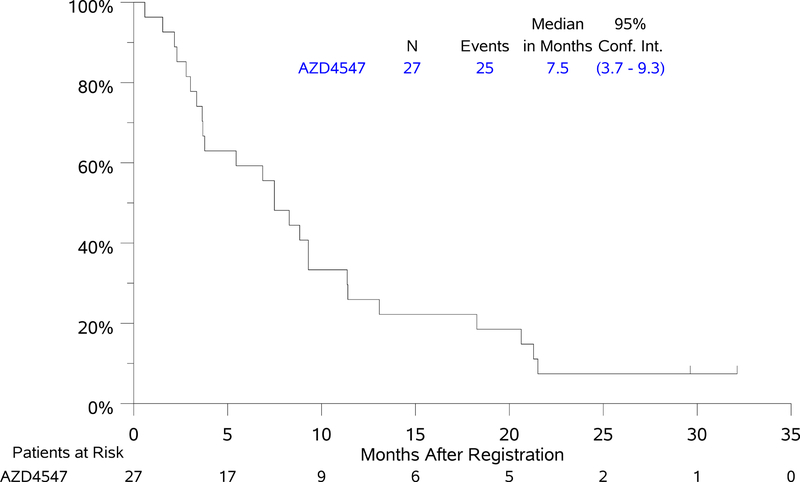
Treatment with ADZ4547 was well tolerated, grade 3 adverse events (AEs) were seen in six patients; one patient had Grade 4 sepsis. Of 27 response evaluable patients, one patient with FGFR3 S249C had unconfirmed partial response with DoR of 1.5 months and one patient with FGFR1 amplification had a confirmed partial response with DoR of 2.9 months (7%, 95% CI 0–17%). Median PFS and OS for the AZD4547–treated cohort were 2.7 mos (95% CI 1.4 – 4.5 mos) and 7.5 mos (95% CI 3.7–9.3 mos).
Conclusions:
AZD4547 had an acceptable safety profile but minimal activity in this predominantly FGFR 1/3 amplified cohort. Evaluation of other targeted agents in Lung-MAP is currently ongoing.
ClinicalTrials.gov Identifier:
Introduction
Squamous cell non-small cell lung cancer (SqNSCLC) comprises about 20–30% of all cases of NSCLC [1]. In contrast to the therapeutic advances seen in non-squamous NSCLC, including molecular characterization and use of targeted therapy for relevant actionable oncogenic driver mutations (e.g., EGFR, ALK), there has been limited progress in the personalized medicine approaches for SqNSCLC [2]. Platinum-based combination therapy currently remains the backbone of first line systemic palliative therapy for advanced SqNSCLC, with modest improvement in outcomes seen with the addition of agents such as the EGFR monoclonal antibody necitumumab, VEGF targeting with ramucirumab, and afatinib in molecularly-unselected patients. In the second line setting, the paradigm of therapy for SqNSCLC has changed significantly with the approval of immune checkpoint inhibitors.
Molecular genotyping has led to the potential application of targeted agents for prevalent mutations in SqNSCLC. The Lung Master Protocol (Lung-MAP, SWOG S1400) is an umbrella protocol which contains a next generationsequencing (NGS) screening component and multiple independently conducted and analyzed treatment sub-studies [3]. The overarching goal of the umbrella master protocol is to genomically screen a large population of previously treated SqNSCLC patients to evaluate targeted therapies (or combinations) in biomarker-driven sub-studies and immunotherapy combinations that can lead to approval of efficacious regimens. Here we report on results of SWOG S1400D, a phase II biomarker-driven therapeutic sub-study of Lung-MAP evaluating the FGFR inhibitor AZD4547 in patients with FGFR pathway-activated SqNSCLC after failure of platinum based therapy [4]. Amplifications of the FGFR1 have been described in up to 20% of SqNSCLC with mutations and fusions in FGFR 2 and 3 occurring at a lower incidence (each < 4%).
In preclinical studies, AZD4547 is active against tumor cell lines and tumors bearing a broad range of FGFR amplifications/mutations to [5], and AZD4547 induces tumor regression or stasis in patient derived explant xenograft models carrying FGFR1 gene amplification [4]. In a small clinical study in SqNSCLC there was at least 1 PR in the 14 patients treated with AZD4547, and the patient carried FGFR1 amplification [6]. These preclinical and early clinical data coupled with the lack of significant long-term safety concerns for the drug administered as monotherapy, led to the development of S1400D using AZD4547 as potential targeted therapy for FGFR positive SqNSCLC.
Patients and Methods
Patients with previously-treated and histologically-proven SqNSCLC were eligible for the Lung-MAP protocolas previously described eligibility criteria [3]. Sub-study eligibility was determined based on an NGS-based mutational analysis of the patient’s tumor. Mutational analysis, including determination of tumor mutational burden (TMB), was performed on archival formalin-fixed paraffin-embedded (FFPE) tumor specimens using FoundationOne® (Foundation Medicine, Cambridge, MA). The full list of alterations evaluated is included as a supplementary table 1. The FGFR alterations required for eligibility to S1400D were FGFR 1/2/3 amplifications, fusions or substitutions. Amplification was defined as ≥ 6 estimated copies (or ≥ 7 for triploid or ≥8 for tetraploid samples). In addition, eligibility to S1400D included age ≥ 25 years, Zubrod PS of 0–2, measurable disease by RECIST 1.1, adequate hematologic, hepatic, cardiac, renal and ophthalmological function, and no impairment of gastrointestinal function or gastrointestinal disease that could significantly alter absorption of AZD4547. Exclusion criteria included leptomeningeal disease, symptomatic, untreated brain metastases, and chemotherapy within 21 days prior to registration. Written informed consent was required from all patients prior to enrollment in the master protocol and a separate consent was required for treatment on the specific sub-study.
AZD4547 was administered orally at 80 mg bid. Treatment cycles were 21 days. Fasting was not required; however, AZD4547 was not administered with foods known to modulate CYP3A4 or CYP2D6 enzyme activity. Disease assessment occurred every 2 cycles, and treatment could continue until progression. Dose reductions and adjustments had to be discussed with the study chair and were followed as specified in the protocol.
Statistical Considerations
The primary objective for S1400D was to evaluate the response rate (RR) (confirmed and unconfirmed, complete and partial) in patients treated with AZD4547. The sample size (n=40) was based on a design with 91% power to rule out a RR of 15% at the 1-sided 5% level, if the true rate was 35%. A key secondary objective was an investigator assessment of median PFS (mPFS). If the RR was less than 25% but the mPFS was at least 4.5 months, this would be considered sufficient evidence to continue to the follow-on phase III. With 40 patients, this design had 90% power to rule out a median PFS of 3 months or less, if the true mPFS was 6 months, at the 5% 1-sided level. Binary proportions and associated 95% confidence intervals were estimated. Survival distributions were estimated using the Kaplan-Meier method and the Brookmeyer-Crowley method was used to estimate confidence intervals.
Results
Baseline patient characteristics for the 27 evaluable patients are displayed in Table 1. Frequency of FGFR alterations among evaluable patients were as follows: FGFR1 amplification (n=23, 85%); FGFR3 amplification (n=2, 7%); FGFR3 S249C (n=2, 7%); and FGFR3 fusion (n=1, 4%) (Table 2). All but one patient had one FGFR alteration detected; one patient had two FGFR alterations. The median and range TMB scores were 10.88 (2.42–21.77), with 13 (52%) patients having TMB scores ≥10.
Table 1:
Patient Demographics and Characteristics
| N(%) N=27 |
|
|---|---|
| Age Median (Range) | 66.3(49–88) |
| Male Gender | 20(74%) |
| Performance Status | |
| 0 | 4(15%) |
| 1 | 23(85%) |
| 2 | 0 (0%) |
| Race/Ethnicity | |
| White | 24(89%) |
| Black | 3(11%) |
| Asian | 0 (0%) |
| Hispanic | 2(7%) |
| Number of Prior Lines of Therapy for Stage IV Disease | |
| 0 | 2(7%) |
| 1 | 22(82%) |
| 2 or more | 3(11%) |
| Smoking Status | |
| Current Smoker | 11(41%) |
| Former Smoker | 16(59%) |
| Never Smoker | 0(0%) |
Table 2.
Gene Alterations Detected on FMI NGS Screening*
| N (%) (n=27) |
|
|---|---|
| Study Eligibility Alterations (FGFR+) | |
| FGFR1 amplification | 23(85%) |
| FGFR3 S249C | 2(7%) |
| FGFR3 amplification | 2(7%) |
| FGFR3 fusion | 1(4%) |
| Number of FGFR Gene Alterations | |
| 1 | 26(96%) |
| 2 | 1(4%) |
| Tumor Mutation Burden Score (N=25)** | |
| Median | 10.88 |
| Range | 2.42–21.77 |
| Interquartile Range | 8.46–15.72 |
| <10 | 12 (48%) |
| ≥10 | 13 (52%) |
| Other Concomitant Gene Alterations | |
| Short Variants | |
| TP53 | 26(96%) |
| MLL2 | 5(19%) |
| CDKN2A, NF1, NFE2L2 | 3(11%) |
| FBXW7, LRP1B, PAX5, SMAD4 | 2(7%) |
| BRCA2, CREBBP, DAXX, | 1(4%) |
| EP300, GATA2, HRAS, KDM6A, | |
| MYD88, NOTCH1, PALB2, | |
| PIK3R1, PTCH1, PTEN, RB1, | |
| RNF43, STAG2, TSC1 | |
| Copy Number Alterations | |
| ZNF703 | 18(67%) |
| MYST3 | 10(37%) |
| SOX2 | 9(33%) |
| PIK3CA, RICTOR | 6(22%) |
| MYC | 5(19%) |
| CDKN2A, CDKN2B | 4(15%) |
| AKT2, FGF10 | 3(11%) |
| CTNNB1, FGF12, GNAS, IRS2, | 2(7%) |
| KDM5A, KDM6A, NF1, PTEN | |
| AKT1, ARFRP1, ARID1A, | 1(4%) |
| AURKA, AXL, BAP1, BCL2L2, | |
| CCND1, CDK8, ERBB2, ERBB3, | |
| FGF19, FGF3, FGF4, FLT3, JUN, | |
| KDR, KIT, KRAS, MCL1, | |
| MTOR, NFKBIA, NKX2–1, | |
| NRAS, PDGFRA, PRSS8, RB1, | |
| RPTOR, SRC, TSC1, ZNF217 | |
| Rearrangements | |
| LRP1B | 2(7%) |
| ARID1A, BRCA2, CDKN2A, | 1(4%) |
| PRDM1, PTEN, ROS1 |
Full list of alterations is included as Supplementary Table
TMB was calculated as the number of somatic, coding, short variants, excluding known driver mutations, per megabase of the genome interrogated; TMB score was not evaluable for 2 patients.
Overall, treatment with AZD 4547 was well tolerated (Table 3). Treatment related Grade 3 AEs were seen in 6 patients. One patient had Grade 4 sepsis possibly related to study drug. There were no Grade 5 AEs. Patients received a median of 2 cycles (range = 1–12, IQR=2–3) of AZD4547.
Table 3.
Adverse Events Attributed to Treatment
| Adverse event | Grade of AE | ||
|---|---|---|---|
| N = 27 | |||
| 3 | 4 | 5 | |
| Dyspnea | 1(4%) | ||
| Fatigue | 1(4%) | ||
| Hyponatremia | 1(4%) | ||
| Lung infection | 1(4%) | ||
| Lymphocyte count decreased | 1(4%) | ||
| Mucositis oral | 1(4%) | ||
| Retinopathy | 1(4%) | ||
| Sepsis | 1(4%) | ||
| Maximum grade of any AE | 6(22%) | 1(4%) | 0 |
Of 27 response evaluable patients, two patients had PR (7%, 95% CI 0–17%), one with FGFR3 S249C gene mutation and one with FGFR1 amplification, with DoR of 1.5 and 2.9 months, respectively. The magnitude of response for the 27 evaluable patients by sub-mutation is depicted in the waterfall plot in Figure 1. Median PFS was 2.7 months (95% CI 1.4 – 4.5 months). Median OS was 7.5 months (95% CI 3.7–9.3 months) (Figure 2). The 1- and 2-year OS estimates were 25.9% and 7.4%. Forest plot analyses for PFS and OS demonstrated a significantly worse OS, but not PFS, for current versus former smokers (notably there were no never smokers in this study) (Supplementary Figure). Current smokers had a 4-fold increased risk of death over former smokers (HR [95% CI] = 4.12 [1.53–11.10], p = 0.005). However, no subgroup derived significant benefit from treatment with AZD 4547.
Figure 1. Waterfall plot of response to AZD4547.
Each vertical bar represents a patient’s best percent decrease in tumor burden when compared to baseline as defined by RECIST 1.1. Only patients with measurable disease at baseline are presented in the plot. Patients who did not have follow up tumor disease assessment were presented at the very left of the plot marked with ‘NA’. Patients who had new lesions appear at their first follow-up assessment or who expired prior to the first scheduled the disease assessment and the death can reasonably be assumed to be due to disease progression are represented graphically as a 100% increase in tumor burden. Patients who had symptomatic deterioration at first disease assessment are coded as “Symptomatic deterioration”. Patients who expired prior to disease assessment, but the death was not due to disease were coded as ‘Early death’. Negative numbers represent decrease in tumor burden from baseline while positive numbers represent increase in tumor burden from baseline.
Figure 2: Kaplan-Meier Curves of Progression-free Survival (PFS) and Overall Survival (OS).
Discussion
Unfortunately, despite early promise, AZD4547 demonstrated minimal antitumor activity in this previously treated, genomically selected SqNSCLC patient population with FGFR pathway-activation. In this cohort, FGFR1 amplification was the most common alteration followed by FGFR3 amplification. Two patients had short lived partial responses, one of which was seen in a patient with FGFR3 S249 C gene mutation, and the other one in a patient with FGFR1 amplification, consistent with previous reports of activity of this drug in an amplified tumor. Very few patients had FGFR mutations or fusions, so conclusions regarding efficacy in this subpopulation should await further investigation. AZD4547 was overall well tolerated, and no significant safety signals were noted.
We believe there are several reasons why this trial was not successful. First, the biology of SqNSCLC remains complex. Unlike oncogene addicted adenocarcinomas, such as EGFR mutant, or ALK rearranged NSCLC, squamous cell lung cancers often have multiple mutations, without a single driver mutation. They are characterized by a high overall mutational burden, at a rate of 8.1 mutations per megabase. Almost all patients display somatic mutations of TP53, and the most commonly observed alterations are in pathways associated with tumor growth, proliferation and survival. Since the growth and signaling pathways are complex, we hypothesize that one drug may not be adequate to effectively inhibit the growth of the FGFR altered tumors studied in this trial. Additionally, FGFR amplifications are biologically different from FGFR fusions or mutations in that they represent a potentially heterogeneous aberration, with different response to targeted therapies and potentially different resistance mechanisms. It is entirely possible that as we target the initial FGFR amplification there may be complex resistance pathways in place to enable tumor growth and survival. It is also possible that AZD4547 was just not effective, however selection of the drug was based on preclinical activity and evidence of clinical activity in a phase I study.
While this was a negative study, there are several positive take aways. The Lung-MAP study was broadly designed to include patients with previously treated squamous cell carcinoma. The protocol was swiftly amended to alter randomization when standard of care was changed, and multiple sub-studies such as S1400D were designed that could open and close autonomously and allow for rapid identification and evaluation of targeted agents. Each sub-study was then re-designed as a phase II study, and futility analyses were built in to enable early termination as in this study. Importantly, S1400D represents a successful and extensive collaboration between industry, academia and national organizations such as FNIH and the National Cancer Institute, and evaluation of other targeted agents in Lung-MAP is currently ongoing.
Supplementary Material
Forest Plots of Potential Prognostic Factors for Progression-Free Survival (PFS) and Overall Survival (OS)
A: PFS
B: OS
Funding:
this research supported in part by NIH/NCI grants CA180888, CA180819, CA180820, CA180821, CA180868, CA189858, CA189861, CA189830, CA189821, CA180858, CA189971, CA189972, CA180826, CA189822, CA180798; and by Amgen, AstraZeneca, Bristol-Myers Squibb Company, Genentech and Pfizer through the Foundation for the National Institutes of Health, in partnership with Friends of Cancer Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interests: CA reports other from Bristol Myers Squibb, Celgene, Roche/Genentech, Astra Zeneca, outside the submitted work; PNL reports consulting for Exelixis, Pfizer, AstraZeneca, Bayer, Genentech/Roche, Janssen, Bristol Myers Squibb, Abbvie, Turnstone Bio, Foundation Medicine, Merck, CellMax Life, Nektar, and research funding from: Millennium, Polaris, GlaxoSmithKline, Genentech/Roche, Aragon Pharmaceuticals, Janssen Biotech, Heat Biologics, TRACON Pharma, Merck, Pharmacyclics, Incyte; Dr. Borghaei reports grants and personal fees from BMS, Lilly, Merck, and, Celgene, grants from Millennium, personal fees from EMD-Serono, Pfizer, Genentech, Boerhinger-Ingelheim, Astra Zeneca, Genmab, Novartis, Regeneron, BioNTech, Cantargia AB, Amgen, Abbvie, Axiom, Takeda, outside the submitted work; JDB reports personal fees and non-financial support from AstraZeneca, Inc, grants, personal fees and other from Mevion Medical Systems, Inc, personal fees and other from ViewRay, Inc, outside the submitted work; MJF reports personal fees from Boehringer Ingelheim, Lilly Pharmaceuticals, Bristol Myers Squibb, outside of submitted work; PCM reports personal fees from AstraZeneca, Guardant Health, Pfizer, grants from Boehringer Ingelheim, outside the submitted work; VAP reports personal fees from Nektar Therapeutics, Astra Zeneca Pharmceuticals, Arrys Therapeutics, Merck, LOXO Oncology, ARAXES Pharma, F Hoffman-La Roche, Janssen Research Foundation, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, Novartis, Takeda, Abbvie, Tesaro Exelixis, Gritstone, outside the submitted work and Research support to my institution: Eli Lilly, Novartis, Merck, AstraZeneca, F Hoffman-La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte; RSH reports personal fees from Abbvie Pharmaceuticals, Biodesix, Bristol-Myers Squibb, EMD Serrano, Genentech/Roche, Heat Biologics, Jun Shi Pharmaceuticals, Loxo Oncology, Nektar, NextCure, Novartis, Pfizer, Sanofi, Seattle Genetics, Shire PLC, Spectrum Pharmaceuticals, Symphogen, TESARO, Neon Therapeutics, Infinity Pharmaceuticals, grants and personal fees from AstraZeneca, Eli Lilly and Company, Merck and Company, outside the submitted work; KK reports personal fees and other from AstraZeneca, outside the submitted work; DRG reports grants and other from AstraZeneca during the conduct of the study;
No Conflicts to report: MWR, KM, JM, PH, AJN
References
- 1.Siegel RL, Miller KD, and Jemal A, Cancer Statistics, 2017. CA Cancer J Clin, 2017. 67(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network, Comprehensive genomic characterization of squamous cell lung cancers. Nature, 2012. 489(7417): p. 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbst RS, et al. , Lung Master Protocol (Lung-MAP)-A Biomarker-Driven Protocol for Accelerating Development of Therapies for Squamous Cell Lung Cancer: SWOG S1400. Clin Cancer Res, 2015. 21(7): p. 1514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavine PR, et al. , AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res, 2012. 72(8): p. 2045–56. [DOI] [PubMed] [Google Scholar]
- 5.Wu YM, et al. , Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov, 2013. 3(6): p. 636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss J, et al. , Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med, 2010. 2(62): p. 62ra93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest Plots of Potential Prognostic Factors for Progression-Free Survival (PFS) and Overall Survival (OS)
A: PFS
B: OS