Abstract
Background:
Preconception health may have intergenerational influences. We have formed the PrePARED (Preconception Period Analysis of Risks and Exposures influencing health and Development) research consortium to address methodological, conceptual, and generalisability gaps in the literature.
Objectives:
The consortium will investigate the effects of preconception exposures on four sets of outcomes: 1) fertility and miscarriage; 2) pregnancy-related conditions; and 3) perinatal and child health, and 4) adult health outcomes.
Population:
A study is eligible if it has data measured for at least one preconception time point, has a minimum of selected core data, and is open to collaboration and data harmonization.
Design:
The included studies are a mix of studies following women or couples intending to conceive, general-health cohorts that cover the reproductive years, and pregnancy/child cohort studies that have been linked with preconception data. The majority of the participating studies are prospective cohorts, but a few are clinical trials or record linkages.
Methods:
Data analysis will begin with harmonization of data collected across cohorts. Initial areas of interest include nutrition and obesity; tobacco, marijuana, and other substance use; and cardiovascular risk factors.
Preliminary results:
23 cohorts with data on almost 200,000 women have combined to form this consortium, begun in 2018. Twelve studies are of women or couples actively planning pregnancy, 6 are general-population cohorts that cover the reproductive years; the remainder have some other design. The primary focus for four was cardiovascular health, eight was fertility, one was environmental exposures, three was child health, and the remainder general women’s health. Among other cohorts assessed for inclusion, the most common reason for ineligibility was lack of prospectively collected preconception data.
Conclusions:
The consortium will serve as a resource for research in many subject areas related to preconception health, with implications for science, practice, and policy.
Keywords: Preconception care, cohort studies, fertility, birthweight, pregnancy, research design, common data elements
Social media quote
The PrePARED consortium combines data on almost 200,000 women from over 20 studies of perinatal health to study the preconception period.
Background
In recent years, there has been growing interest in the investigation of preconceptional exposures as determinants of long-term health outcomes for mothers and their children.1–4 Preconception health may have intergenerational influences on human health.5–7 According to the U.S. Centers for Disease Control and Prevention, “Eliminating disparities in preconception health can potentially reduce disparities in two of the leading causes of death [for women] in early and middle adulthood (i.e., heart disease and diabetes).”8 In the U.S., Black and Hispanic women are at higher risk for adverse preconception exposures (e.g., poor nutrition, cigarette smoking, racial discrimination, depression/anxiety/stress, environmental chemicals) adverse health conditions.9 and limited access to care.10–12
The preconception period is also important for understanding and improving infant and child health outcomes. A conference on the Louisiana Birth Outcomes Initiative addressed preconception health, emphasizing that “[P]renatal care may be too little too late. … If we want to do something about preventing [adverse birth outcomes] in this country, we really need to start taking care of women’s health long before they get pregnant.”13
“Preconception” can be construed narrowly, for instance, as the time period when pregnancy is planned, or broadly, as any point in the life span prior to pregnancy.4, 14, 15 Exposures from both time periods have been found to affect pregnancy and infant health (e.g., folic acid supplementation16 or adverse childhood experiences17). Many adverse health behaviors, including smoking, sedentary lifestyle, and poor diet, are apparent preconceptionally, meaning that pregnancy begins under less than optimal conditions and the stage is set for long-term chronic diseases.4 In addition, socioeconomic status and exposure to stress and trauma over the life course influence both biology and behavior, and may be crucial to understanding women’s experience of pregnancy and fertility decisions. Thus, the preconception period is an important time window for health interventions that could positively shape women’s and offspring health. Although the traditional focus for preconception research has been women, such interventions should also target men, given not only their direct biological influences on fertility14 but also their role on child health and development18 and family well-being.19
To examine the impact or preconception exposures in women, men, and their children, large-scale analysis may be required. While the importance of some preconception factors has been established, the effect of timing and duration of many exposures (e.g., environmental toxicants) have yet to be investigated in humans, and may have small effect sizes. Rare exposures also require a large sample size to identify associations, to assess potential for etiologic heterogeneity and disease subtypes, and to allow for meaningful subgroup analysis (e.g., by race and ethnicity). Single cohorts often lack the precision to address less common complications and effect modification by factors such as age and parity. Finally, large datasets allow for more fine-grained stratification which is useful to adequately adjust for confounders.
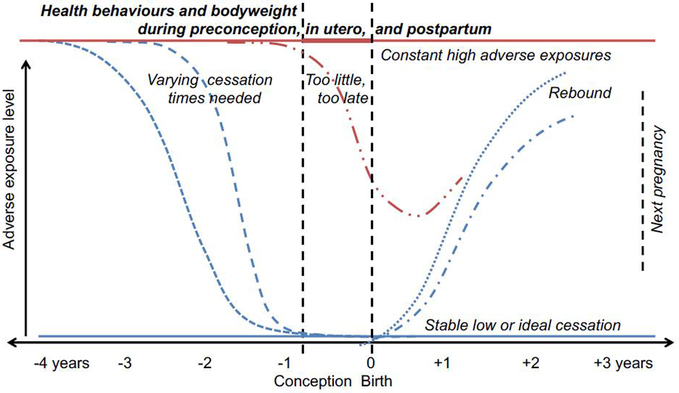
Assessing mechanisms of effect is another important goal. Preconception studies can not only provide insight into child health and developmental programming but may also help to predict risk of pregnancy complications and chronic conditions. Women with chronic hypertension who become pregnant are at higher risk of preterm birth, small-for-gestational-age, stillbirth, in-hospital mortality, and severe forms of hypertensive diseases of pregnancy,20–22 and some studies indicate that preconception cardiovascular health outside clinical disease also has an impact on pregnancy health.23–25 Women with low HDL-cholesterol, impaired fasting glucose, or high fasting insulin prior to conception have a two to four-fold higher odds of developing gestational diabetes independent of pre-pregnancy BMI.26–28 Similarly, preconception depression often carries through pregnancy and postpartum.29 Preconception risk factors could lead to poorer birth outcomes by affecting placentation in the first trimester,30, 31 increasing inflammation,32 or producing epigenetic changes that carry into pregnancy.33, 34 Often studies lack information about the time period prior to pregnancy that is necessary to clearly place pregnancy health within the life course, or lack sufficient follow-up time post-pregnancy to estimate associations with later-life health (Figure 1). Few studies have information on both preconception and during-pregnancy exposures that allow for distinguishing risks between these time periods.35, 36 Pregnancy cohorts usually have limited, retrospectively-collected information on a few exposures and diagnosed preconception conditions, if any information at all.
Figure 1.
Various relationships between preconception exposures, pregnancy health, and post-pregnancy health.
Studies of preconception health may be prone to selection and recall biases. Those that recruit pregnant women and measure data retrospectively produce an achieved-pregnancy bias, if there is a common cause for both the outcome under study and fertility or miscarriage.37, 38 These biases may be exacerbated in birth cohort studies that recruit mothers and infants after delivery. Studies including only the most recent pregnancy may be confounded by factors that predict both miscarriage and use of effective contraception,39 while pregnancy/birth cohort studies that rely on retrospectively obtained self-reported data (e.g., preconception nutrition) may be susceptible to recall bias and left truncation.40 For these reasons, prospective studies of preconception health are of particular value. However, studies of couples attempting to conceive may be susceptible to selection bias, as they are likely to over- or under-recruit fertile couples, if couples are excluded because they seek fertility treatment,41 or are included after a period of failing to conceive (even if this is not an explicit criterion for study entry).42 Moreover, targeted preconception recruitment usually excludes unintended pregnancies, which are a non-negligible proportion of pregnancies in the United States.43 To capture preconception health of unintended pregnancies, ongoing cohort studies aimed at chronic health conditions can be used or a population of women of reproductive age and potential can be followed prospectively for changes in risk of pregnancy and actual occurrence of pregnancy (if resources allow).44 However, cohort studies of cardiovascular, metabolic, or other general health tend to focus on older populations (at higher risk for most conditions) and frequently either exclude pregnant women, or, in the case of younger populations, assess health status outside pregnancy only. Studies assessing preconception health care are often geared towards women with clinical conditions or who are at otherwise high risk.45–47
For these reasons, we have formed a research consortium of studies (PrePARED, Preconception Period Analysis of Risks and Exposures influencing health and Development) addressing preconception health. The goal of the consortium is to use the large number of studies with preconception data to: 1) identify meaningful (at a clinical or population level) effect sizes of preconception exposures; 2) determine the relative importance of pre-, during-, inter-, and post-pregnancy effects; 3) distinguish selection effects due to differential fertility and pregnancy loss; 4) identify plausible mechanisms of effect; 5) examine rare-prevalence exposures and outcomes; and 6) examine effect modification in associations between preconception exposures and health outcomes across subgroups of interest (e.g., age, race/ethnicity, socioeconomic status).
Methods
Overview, structure, and operations
PrePARED is a consortium of studies incorporating information on preconception and pregnancy health, both studies of couples actively planning pregnancy and more general studies that cover the reproductive years. Participating studies are ongoing and completed studies from around the world. The organizing committee is formed of investigators contributing data.
Eligibility criteria
A study is eligible for inclusion in the consortium if it meets three criteria: 1) The study has data measured for at least one preconception time point (i.e., preconception exposures should not be entirely retrospectively reported.) In other words, studies where the only information on the preconception period is what the woman reports during pregnancy are not eligible. 2) A minimum set of data (listed in table 1) is available. This set of data was chosen as necessary for analysis of almost any pregnancy-related outcome (fertility, miscarriage, pregnancy complications, birth outcomes), for comparing across cohorts, and for assessing the definition of the preconception period for a given analysis. 3) The study is open to collaboration and data harmonization. All studies have information beyond the minimum, and can opt in or out of a given analysis. Preconception subcohorts of larger pregnancy or general-population studies are eligible.
Table 1.
Minimum data required for consortium participation
| parity |
| gravidity |
| pregnancy outcome(s) |
| age at pregnanc(ies) |
| measure of socioeconomic status |
| pre-pregnancy BMI |
| time between measure and pregnancy |
| calendar year of measure |
Procedure for adding new cohorts
To date, cohorts have been selected through word of mouth, literature searches, and web searches. Additional cohorts may join this open consortium, which will serve as a rich resource for identifying preconception factors with perinatal and potentially intergenerational long-term influences. Interested cohorts interested should contact the corresponding authors for more information.
Ethical issues, data sharing, and security
No analysis will be conducted that is not permissible under the studies’ and institutions’ informed consent or data use policies. Data harmonization consists of two phases: 1) Developing and maintaining a database at each project site (largely already done by the individual studies); and 2) Establishing a data repository to integrate and share data sources across study sites. Data capture and management will be centralized at Tulane’s Global Research Data Center. While the preference is for (de-identified, limited) data to be centrally stored and analyzed, this may not be possible for all topics or all studies, so procedures will be flexible to the exigencies of a given case. Data sharing agreements will be required for initial participation, and the principal and lead investigators from each study will approve all other requests for reports or datasets before dissemination. When consistent with the studies’ data use agreements, data will be released after publication of the primary results. Metadata templates will be designed and coding guides will be developed for all study variables.
Human subject data will be uploaded onto secured computers only, with no unique identifiers attached. All study personnel will be required to use password-protected computers to access the project data, and permission will be required for any data reuse. Data will be regularly backed up to a secure server and made available only to key project personnel, and analysis will generally be performed on this central server. Digital signatures and encryption will prevent unauthorized changes to datasets or documents as well as identifying who created the document and verifying time and date of any change.
This description of study design and measures is not human subjects research and does not require Institutional Review Board review.
Results
Study populations
A description of each cohort is provided in Tables 2 and 3. The consortium brings together 23 studies with data on almost 200,000 women. Eighteen of the participating studies are based in the U.S., with 2 in China, and 1 each in Canada, Australia, and Denmark. All include data collected during the reproductive years (approximately ages 15–44), but some have followed the participants before or after. Studies have also enrolled over 6500 male partners. 26,000 children were enrolled as part of a mother-child dyad; two studies (Bogalusa Heart Study and GUTS) also include children (n=28603) of the original study participants. Twelve studies are of women or couples actively planning pregnancy, 6 are general-population cohorts that cover the reproductive years. and the remainder have some other design.
Table 2.
Participant life stages represented in PrePARED consortium studies
| Name | Reproductive-aged women (women enrolled regardless of pregnancy planning status) | Fecundability/fertility (follows women intending to get pregnant) | Paternal (couples/fathers enrolled, not just reported on) | Mother-child (information collected on preconception and pregnancy of mother, as well as on child) |
|---|---|---|---|---|
| Australian Longitudinal Study on Women’s Health (ALSWH)50, 51 | X | X | X | |
| Bogalusa Heart Study | X | subset | ||
| Coronary Artery Risk Development in Young Adults (CARDIA)52 | X | |||
| Canadian Assisted Reproductive Technology Cohort Study (CARTS)53 | X | X | X | |
| Chinese Preconception Cohort Study (CPCS) | X | X | X | |
| CoLab Prepregnancy and Early Pregnancy Consortium54 | X | X | X | X |
| Effects of Aspirin in Gestation and Reproduction (EAGeR)55 | X | |||
| Early Pregnancy Study (EPS)56 | X | |||
| Hispanic Community Health Study/Study of Latinos (HCHS/SOL)57 | X | subset | ||
| Home Observation of Periconceptional Exposures (HOPE) Study58 | X | X | ||
| Longitudinal Investigation of Fertility and the Environment (LIFE)59 | X | X | ||
| NHLBI Growth and Health Study (NGHS)60 | X | |||
| Nurses Health Study 3/Growing Up and Today Study (GUTS)61 | X | subset | subset | X |
| Preconceptional Offspring Trajectory Study (PLOTS) | X | X | X | |
| Pregnancy Study Online (PRESTO)62 | X | X | X | |
| Retrospective cohort study of assisted reproductive technology53 | X | |||
| Shanghai Birth Cohort63 | X | X | X | |
| Snart Gravid/Snart Foraeldre64 | X | X | X | |
| Strong Healthy Women and SMART Strong Healthy Women65 | X | X | ||
| Time to Conceive | X | Tentative | ||
| Time to Pregnancy in Couples of Proven Fecundity66 | X | |||
| University of Iowa Women’s Health Tissue Repository67 | X | X | X | X |
| Upstate KIDS59 | X |
Table 3.
Study populations, the PrePARED Consortium
| STUDY | AGE RANGE | STUDY FOCUS | STUDY POPULATION DESCRIPTION | N |
|---|---|---|---|---|
| Reproductive-aged women | ||||
| Australian Longitudinal Study on Women’s Health | 18–27 for the 1989–95 cohort (baseline in 2012/13) 18–42 for the 1973–78 cohort (baseline in 1996) | General women’s health | Representative sample of Australian women | 21,032 |
| Bogalusa Heart Study | 3–80 (when studied) 1st gen 30–70, 2nd gen 12–45 (now) | Cardiovascular | Black and white, semi-rural men and women | 4049 women, 3500 with pregnancy data, 917 generation 2 |
| CARDIA | Age 18–30 years at baseline (1985–1986), 48–60 years in 2015–2016 Multi-center USA | Cardiovascular | 50% Black and 50% White men and women, multiple exams every 2 to 5 years during the 30 years of follow up; | 2787 women enrolled; gravidity and parity at exams; (1,392 women who had 2,510 births after baseline during 30-y follow up; 1,951 women gave birth before and/or after baseline) |
| CoLab Prepregnancy and Early Pregnancy Consortium | > 18 y/o (Adults) and associated children | General Women’s Health with Pregnancy Focus | National and international collaboration to share biosamples and clinical data in the peripregnancy time frame. | 13478 to date (enrollment ongoing) |
| Growing Up Today Study | 7–16 (2 enrollment waves 1996 and 2004) now 20–36 | Child health | Children of women in Nurses’ Health Study II | 27, 706 (13,000 in active follow-up) |
| Hispanic Community Health Study/Study of Latinos | 18–74 baseline (2008–2011), 24–80 (2014–2017), 3rd 2020–2023 | Cardiovascular | Largest Hispanic health study in the US | 16,415; 9835 women, 3801 aged 18–41; 440 mom-child dyads (ancillary; 2019–2021) |
| NHLBI Growth and Health Study | 9–28 (when studied) 40–42 (now) | Cardiovascular | Girls enrolled in 1987 at age 9–10, 50% black 50% white, followed annually or biannually first 20 years | 871 enrolled, approx. 500 in follow-up |
| Nurses’ Health Study 3 | 18–52 (mothers) | General women’s health | Nurses in N. America | 45,066 enrolled, 6,623 pregnancies (at least 20 wks) to date |
| Women or couples attempting pregnancy | ||||
| Canadian Assisted Reproductive Technology Cohort Study (CARTS) | Couples (30–45 years) who underwent IVF/ICSI treatment | Long-term outcomes in mothers and their offspring child | 18000 couples who under infertility treatment in Canadian province of Ontario with success pregnancy; follow-up through provincial health record linkage is possible | 18000 mother-baby pairs (up to December 2018) |
| Chinese Preconception Cohort Study (CPCS) | Couples (18–35 years) plan to have baby soon (< 6 months) | Long-term outcomes in mothers and their offspring child | 5000 couples who planned to have a baby soon in Liuyang, China; follow-up at 8–12 years after childbirth was planned | 2000 mother-baby pairs |
| CoLab Prepregnancy and Early Pregnancy Consortium | > 18 y/o | General Women’s Health with Fertility/Pregnancy Focus | National and international collaboration to share biosamples and clinical data in the peripregnancy time frame. | 516 to date (enrollment ongoing) |
| EAGeR | 18–40 (when studied) 24–46 (now) | Fertility | Women with 1–2 prior pregnancy losses attempting pregnancy | 1228 |
| Early Pregnancy Study | Median age is 29, range ~ 22–40 | Early loss | Women from the Triangle area of North Carolina, enrolled in 1982–86, | 221 |
| HOPE Study | Preconception (women ages 18–35, men ages 18–40 upon enrollment) | Environment | Heterosexual couples trying to conceive, greater Salt Lake City area, primarily white | 183 couples, 170 with follow-up information |
| LIFE | 18–40 (when studied), 27–53 (now) | Fertility | Couples attempting pregnancy | 501 |
| Time to Pregnancy in Couples of Proven Fecundity | Women age 18–35 | Fertility awareness instruction | 143 women attempting to conceive, prior pregnancy, greater Salt Lake City area, primarily white | 140 |
| Preconceptional Offspring Trajectory Study (PLOTS) | 20s (two years follow up now) | Environmental, social determinants and life style etc. on Fertility, pregnancy outcomes, child health | Preconception couples followed up to conception and childbirth, continue to follow offspring | at least 3000 couples |
| Pregnancy Study Online (PRESTO) | Men ≥21 years; women aged 21–45 years | Fertility, pregnancy, child’s health, environment | Preconception cohort of North American (50 US states and 10 Canadian provinces) couples attempting pregnancy | 11,000 North American women; 2,500 North American men |
| Retrospective cohort study of assisted reproductive technology | Mothers 20–40 (at time of birth), all stillbirths and live births (with follow up to one year of age) | Infertility treatment and child health | All births between April 1, 2012 and October 31, 2015 in Ontario captured by BORN, the provincial perinatal registry | 17,907 assisted pregnancies |
| Shanghai Birth Cohort | ≥ 20 years | Pregnancy | Women in two preconception care clinics | 1180 |
| Snart Gravid (“Soon Pregnant”), Snart Foraeldre (“Soon Parents”) | Men ≥21 years; women 21–49 years | Fertility, pregnancy, child’s health, environment | Preconception cohort of Danish couples attempting pregnancy | 13,500 women (mothers); 1,200 Danish men (fathers) |
| Strong Healthy Women and SMART Strong Healthy Women (adapted for mHealth delivery) | 18–35 (when studied) 28–50 (now) | General women’s health and pregnancy | Preconception women residing in rural and urban communities in Central PA | 692* (*includes N=45 women who became pregnant during 12-month follow-up) |
| Time to Conceive | 30–44 at enrollment in 2008–2015 | Biomarkers of fertility | Women with no history of infertility, over age 30, from the Triangle area of North Carolina | 750 |
| University of Iowa Women’s Health Tissue Repository | 18–50 | General Women’s Health, Fertility, Reproductive Endocrinology, pregnancy and long-term outcomes | Individuals and couples seeking care in the Reproductive Endocrinology & Infertility clinic at the University of Iowa | 377 to date (enrollment ongoing) |
| Upstate KIDS | 0–3 years (now 8 years ongoing) | Child health | Population based cohort oversampled on conceptions by infertility treatment in upstate NY | 5034 mothers, 6171 children (twins and higher order multiples included) |
CARDIA, Coronary Artery Risk Development in Young Adults; EAGeR, Effects of Aspirin in Gestation and Reproduction; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; HOPE, Home Observation of Periconceptional Exposures; LIFE, Longitudinal Investigation of Fertility and the Environment; NCS, National Children’s Study; NGHS, National Growth and Health Study; GUTS, Growing Up and Today Study; PLOTS, Preconceptional Offspring Trajectory Study; PRESTO, Pregnancy Study Online; SMART, KIDS
What does it cover?
The studies in the consortium are designed to address questions of health behaviors, environmental risk factors, fertility, or cardiometabolic health status (Tables 2, 4). The primary focus for four was cardiovascular health, eight was fertility, one was environmental exposures, three was child health, and the remainder general women’s health or no single focus. All have some information on pregnancy health and outcomes. The consortium will investigate the effects of preconception exposures on four broad sets of outcomes: 1) fertility, fecundability, and miscarriage; 2) pregnancy-related conditions (e.g., gestational diabetes, gestational hypertension, preeclampsia, and placental disorders, such as intrauterine growth restriction, placenta previa and placental abruption), 3) perinatal and child health outcomes (e.g., fetal growth, gestational age, child development); and 4) effects of pregnancy course, outcomes, and lactation on women’s health and disease over the life course. Individual studies generally have a strength in one of these areas.
Table 4.
Data included in cohorts for major exposures and outcomes of interest, the PrePARED Consortium
| preconception | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI | tobacco/substance use | mood/depression | endocrine conditions | diet | medication | physical activity | stress | cardiovascular | |
| Australian Longitudinal Study on Women’s Health | x | x | x | x | x | x | x | x | |
| Bogalusa Heart Study | x | x | xsubset | subset | x | x | x | subset | x |
| CARDIA | x | x | x | x | x | x | x | x | x |
| Canadian Assisted Reproductive Technology Cohort Study (CARTS) | x | x | x | x | x | x | x | ||
| Chinese Preconception Cohort Study (CPCS) | x | x | x | x | x | ||||
| CoLab Prepregnancy and Early Pregnancy Consortium | x | x | x | x | subset | x | subset | subset | x |
| Growing Up Today Study (GUTS) | x | x | x | x | x | x | x | x | x |
| Hispanic Community Health Study/Study of Latinos (HCHS/SOL) | x | x | x | x | x | x | x | x | |
| NHLBI Growth and Health Study | x | x | x | x | x | x | x | x | |
| Nurses Health Study 3 | x | x | x | x | x | x | x | x | x |
| EAGeR | x | x | x | x | x | x | |||
| Early Pregnancy Study (EPS) | x | x | |||||||
| HOPE Study | x | x | x | x | x | x | x | ||
| LIFE | x | x | x | x | x | x | x | ||
| Preconceptional Offspring Trajectory Study (PLOTS) | x | x | x | x | x | x | x | x | |
| PRESTO | x | x | x | x | x | x | x | x | x |
| Retrospective cohort study of assisted reproductive technology | x | x | x | ||||||
| Shanghai Birth Cohort | x | x | x | x | x | x | x | x | x |
| Snart Gravid, Snart Foraeldre | x | x | x | x | x | x | x | x | x |
| Strong Healthy Women and SMART Strong Healthy Women | x | x | x | x | x | x | x | ||
| Time to Conceive | x | x | x | x | x | x | |||
| Time to Pregnancy in Couples of Proven Fecundity | x | x | x | x | |||||
| University of Iowa Women’s Health Tissue Repository | x | x | subset | x | subset | x | subset | subset | x |
| Upstate KIDS | x | x | x | ||||||
| pregnancy | post-pregnancy | child | male partner | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| fertility | birth outcomes | pregnancy complications | depression | cardiovas-cular health | asthma | growth/adiposity | |||||
| Australian Longitudinal Study on Women’s Health | x | x | x | x | x | x | x | ||||
| Bogalusa Heart Study | x | x | x | subset | subset | x | x | subset enrolled | |||
| CARDIA | x | x | x | x | x | ||||||
| Canadian Assisted Reproductive Technology Cohort Study (CARTS) | x | x | x | x | x | x | x | x | |||
| Chinese Preconception Cohort Study (CPCS) | x | x | x | x | x | x | x | ||||
| EAGeR | x | x | x | ||||||||
| Early Pregnancy Study | x | x | |||||||||
| Growing Up Today Study | x | x | x | x | x | x | reported | ||||
| Hispanic Community Health Study/Study of Latinos | x | x | x | x | x | x | |||||
| HOPE Study | x | x | x | enrolled | |||||||
| LIFE | x | x | x | enrolled | |||||||
| National Children’s Study | x | x | x | x | x | reported | |||||
| NHLBI Growth and Health Study | x | x | subset | x | |||||||
| Nurses Health Study 3 | x | x | x | x | x | subset enrolled, others reported | |||||
| Preconceptional Offspring Trajectory Study (PLOTS) | x | x | x | x | x | x | reported | ||||
| Preconceptional cohort study of pre-eclampsia and gestational diabetes | |||||||||||
| PRESTO, Snart Gravid, Snart Foraeldre | x | x | x | x | x | x | enrolled | ||||
| Retrospective cohort study of assisted reproductive technology | x | x | |||||||||
| Shanghai Birth Cohort | x | x | x | x | x | x | x | ||||
| Snart Gravid, Snart Foraeldre | x | x | x | x | x | x | enrolled | ||||
| Strong Healthy Women and SMART Strong Healthy Women | |||||||||||
| Time to Conceive | x | x | x | ||||||||
| Time to Pregnancy in Couples of Proven Fecundity | x | x | x | reported | |||||||
| Upstate KIDS | x | x | x | x | x | x | x | reported | |||
x=routinely measured; subset=specifically measured in a subset (not just missing data)
BHS, Bogalusa Heart Study; CARDIA, Coronary Artery Risk Development in Young Adults; EAGeR, Effects of Aspirin in Gestation and Reproduction; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; HOPE, Home Observation of Periconceptional Exposures; LIFE, Longitudinal Investigation of Fertility and the Environment; NCS, National Children’s Study; NGHS, National Growth and Health Study; GUTS, Growing Up and Today Study; PLOTS, Preconceptional Offspring Trajectory Study; PRESTO, Pregnancy Study Online; KIDS
CARDIA, Coronary Artery Risk Development in Young Adults; EAGeR, Effects of Aspirin in Gestation and Reproduction; HOPE, Home Observation of Periconceptional Exposures; LIFE, Longitudinal Investigation of Fertility and the Environment; NCS, NGHS, National Growth and Health Study; GUTS, Growing Up and Today Study; PRESTO, Pregnancy Study Online; KIDS
How often have they been followed up and what is the anticipated attrition?
Among other cohorts approached for inclusion, the most common reasons for declining or ineligibility were a) lack of time by the investigators, and b) lack of prospectively collected preconception data. Attrition within the cohorts is quite variable and tends to track with the length of time the cohort has been followed; however, many of the long-term studies have impressive rates of follow-up.48 An eligibility criterion is that all studies should include at least some follow-up for pregnancy outcomes. Studies also vary in their ability to recontact participants. Some studies also include participants who were never pregnant or delivered a live birth, allowing for comparisons that address selective fertility and pregnancy loss.
What are the major areas of research?
Data analysis will consist of determining how existing data corresponds across cohorts, and harmonizing key exposures, outcomes and covariates. Initial preconception exposures of interest include nutrition, diet, and obesity; marijuana, tobacco and other substance use; cardiometabolic risk factors; and male partners’ exposures. These topics were chosen because of their relevance to current public health concerns and the fact that they are measured across many of the cohorts, allowing for establishing data harmonization protocols. In addition, the harmonized variables developed in the course of these projects are likely to be important confounders and mediators of analyses on other topics of interest.
Comment
The large numbers will allow for subgroup analysis. Most of the studies are quite detailed in at least one area, and often have extensive measures of cardiovascular risk, diet, fertility, and/or environmental factors. The included studies are diverse in their populations and in study design, which will increase generalisability, as well as allowing the description of the interrelationships between the natural history of pregnancy and women’s health over the life course. Findings that indicate systematic similarities or differences across cohort studies can provide insights into the behavioural, social, or biological factors at play.
Weaknesses include the fact that many of the studies are complete, with wide variability in the types of measures and biological samples available. Some of the studies are limited to pregnancy planners, who tend to be healthier and older than the average pregnant woman, while general population studies do not have a consistent time frame between the measures and the pregnancy, and may lack data collected during pregnancy itself. Some studies of reproductive-aged populations, such as the Coronary Artery Risk Development in Young Adults (CARDIA) Study, use a mix of data collection methods, conducting assessments in women both before and after pregnancy, with pregnancy data validated with medical records. Other studies were conducted among women at risk of pregnancy loss or using of infertility treatment, as such groups are more prone to be followed prospectively. The combination of study designs means that in some cases, subsamples from some cohorts may be needed to establish a consistent time frame of measurement (either age or relative to pregnancy) or degree of validation (e.g., medical record confirmation). A range of calendar time is covered by the studies, both a potential strength and limitation, as it allows for examining whether an effect is constant over time, and thus more likely to be causal, but also increases the possibility of inconclusive findings due to heterogeneity. Cohort effects will most commonly be addressed, by adjustment or interaction analysis, where possible. If there is reason to believe that results will be relevant to a particular time period, analyses will be limited to studies with data from that period only.
Preconception health can be defined as including men’s as well as women’s health.49 Several of the cohorts include male participants, either as partners in couples planning pregnancy, or as participants in general-population studies. Pregnancy health is a major focus of the consortium and so participating cohorts are required to have at least some information on female health and pregnancy. Cohorts that focus on male fertility are eligible to participate as long as they also include this information.
Conclusions
The consortium welcomes proposals to collaborate from qualified researchers. The corresponding authors should be contacted for more information. Persons interested in requesting data should contact the corresponding author for more details on procedure. At a minimum, a data use agreement will be required, and costs may be required to be shared. Updated information on the consortium can be found at the consortium website (https://sph.tulane.edu/prepared-consortium). Beyond the topics identified, the consortium will serve as a resource for work in many subject areas related to the preconception period, with implications for science, practice, and policy.
Synopsis.
Study question: Can studies covering the preconception period be combined to allow for better understanding of this life period?
What’s already known: Several risk factors for adverse pregnancy outcomes begin prior to pregnancy.
What this study adds: By combining efforts from several studies and study designs, gaps in understanding such as the effect of timing and duration of exposures, rare exposures, small effect sizes, etiologic heterogeneity, disease subtypes, selection issues, and meaningful subgroup analysis (e.g., by race and ethnicity) can be addressed.
Funding:
Planning for the PrePARED consortium was supported in part by a grant from the Louisiana Clinical and Translational Science Center, U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Declarations
Competing interests: The authors declare that they have no competing interests.
References
- 1.Ashton DM, Lawrence HC 3rd, Adams NL 3rd, Fleischman AR. Surgeon General’s Conference on the Prevention of Preterm Birth. Obstetrics and Gynecology. 2009; 113:925–930. [DOI] [PubMed] [Google Scholar]
- 2.Kroelinger CD, Okoroh EM, Boulet SL, Olson CK, Robbins CL. Making the Case: The Importance of Using 10 Key Preconception Indicators in Understanding the Health of Women of Reproductive Age. Journal of Women’s Health. 2018; 27:739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Posner SF, Broussard DL, Sappenfield WM, Streeter N, Zapata LB, Peck MG. Where are the data to drive policy changes for preconception health and health care? Women’s Health Issues. 2008; 18:S81–86. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson J, Heslehurst N, Hall J, Schoenaker D, Hutchinson J, Cade JE, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeler E, Nobile ZL, Homanics GE. Paternal Preconception Every-Other-Day Ethanol Drinking Alters Behavior and Ethanol Consumption in Offspring. Brain Sciences. 2019; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graus-Nunes F, Dalla Corte Frantz E, Lannes WR, da Silva Menezes MC, Mandarim-de-Lacerda CA, Souza-Mello V. Pregestational maternal obesity impairs endocrine pancreas in male F1 and F2 progeny. Nutrition. 2015; 31:380–387. [DOI] [PubMed] [Google Scholar]
- 7.Gray SAO, Jones CW, Theall KP, Glackin E, Drury SS. Thinking Across Generations: Unique Contributions of Maternal Early Life and Prenatal Stress to Infant Physiology. Journal of the American Academy of Child and Adolescent Psychiatry. 2017; 56:922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins C, Boulet SL, Morgan I, D’Angelo DV, Zapata LB, Morrow B, et al. Disparities in Preconception Health Indicators - Behavioral Risk Factor Surveillance System, 2013–2015, and Pregnancy Risk Assessment Monitoring System, 2013–2014. MMWR: Surveillance Summaries. 2018; 67:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preconception health indicators among women--Texas, 2002–2010. MMWR: Morbidity and Mortality Weekly Report. 2012; 61:550–555. [PubMed] [Google Scholar]
- 10.Denny CH, Floyd RL, Green PP, Hayes DK. Racial and ethnic disparities in preconception risk factors and preconception care. Journal of Women’s Health. 2012; 21:720–729. [DOI] [PubMed] [Google Scholar]
- 11.Hogan VK, Culhane JF, Crews KJ, Mwaria CB, Rowley DL, Levenstein L, et al. The impact of social disadvantage on preconception health, illness, and well-being: an intersectional analysis. American Journal of Health Promotion. 2013; 27:eS32–42. [DOI] [PubMed] [Google Scholar]
- 12.Strutz KL, Hogan VK, Siega-Riz AM, Suchindran CM, Halpern CT, Hussey JM. Preconception stress, birth weight, and birth weight disparities among US women. American Journal of Public Health. 2014; 104:e125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gee RE, Johnson KA. Louisiana Birth Outcomes Initiative: improving birth outcomes with interventions before, during, and after pregnancy. Journal of the Louisiana State Medical Society. 2012; 164:6–9. [PubMed] [Google Scholar]
- 14.Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker M, Dombrowski SU, Colbourn T, Fall CHD, Kriznik NM, Lawrence WT, et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet. 2018; 391:1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toivonen KI, Lacroix E, Flynn M, Ronksley PE, Oinonen KA, Metcalfe A, et al. Folic acid supplementation during the preconception period: A systematic review and meta-analysis. Preventive Medicine. 2018; 114:1–17. [DOI] [PubMed] [Google Scholar]
- 17.Racine N, Plamondon A, Madigan S, McDonald S, Tough S. Maternal Adverse Childhood Experiences and Infant Development. Pediatrics. 2018; 141. [DOI] [PubMed] [Google Scholar]
- 18.de Kluiver H, Buizer-Voskamp JE, Dolan CV, Boomsma DI. Paternal age and psychiatric disorders: A review. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2017; 174:202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisacane A, Continisio GI, Aldinucci M, D’Amora S, Continisio P. A controlled trial of the father’s role in breastfeeding promotion. Pediatrics. 2005; 116:e494–498. [DOI] [PubMed] [Google Scholar]
- 20.Odell CD, Kotelchuck M, Chetty VK, Fowler J, Stubblefield PG, Orejuela M, et al. Maternal hypertension as a risk factor for low birth weight infants: comparison of Haitian and African-American women. Maternal and Child Health Journal. 2006; 10:39–46. [DOI] [PubMed] [Google Scholar]
- 21.Sibai BM, Caritis SN, Hauth JC, MacPherson C, VanDorsten JP, Klebanoff M, et al. Preterm delivery in women with pregestational diabetes mellitus or chronic hypertension relative to women with uncomplicated pregnancies. The National institute of Child health and Human Development Maternal-Fetal Medicine Units Network. American Journal of Obstetrics and Gynecology. 2000; 183:1520–1524. [DOI] [PubMed] [Google Scholar]
- 22.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. American Journal of Obstetrics and Gynecology. 2012; 206:134.e131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romundstad PR, Davey Smith G, Nilsen TI, Vatten LJ. Associations of prepregnancy cardiovascular risk factors with the offspring’s birth weight. American Journal of Epidemiology. 2007; 166:1359–1364. [DOI] [PubMed] [Google Scholar]
- 24.Catov JM, Ness RB, Wellons MF, Jacobs DR, Roberts JM, Gunderson EP. Prepregnancy Lipids Related to Preterm Birth Risk: the Coronary Artery Risk Development in Young Adults Study. Journal of Clinical Endocrinology and Metabolism. 2010; 95:3711–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology. 2011; 22:724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunderson EP, Quesenberry CP Jr., Jacobs DR Jr., Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: The CARDIA study. American Journal of Epidemiology. 2010; 172:1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstetrics and Gynecology Clinics of North America. 2009; 36:317–332, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunderson EP, Jacobs DR Jr., Chiang V, Lewis CE, Tsai A, Quesenberry CP Jr., et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. American Journal of Obstetrics and Gynecology. 2009; 201:177.e171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leigh B, Milgrom J. Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry. 2008; 8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorp JM. Placental vascular compromise: Unifying the etiologic pathways of perinatal compromise. Current Problems in Obstetrics and Gynecology. 2001; 24:197–220. [Google Scholar]
- 31.Kroener L, Wang ET, Pisarska MD. Predisposing Factors to Abnormal First Trimester Placentation and the Impact on Fetal Outcomes. Seminars in Reproductive Medicine. 2016; 34:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDade TW, Borja JB, Largado F, Adair LS, Kuzawa CW. Adiposity and Chronic Inflammation in Young Women Predict Inflammation during Normal Pregnancy in the Philippines. Journal of Nutrition. 2016; 146:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St Fleur M, Damus K, Jack B. The future of preconception care in the United States: multigenerational impact on reproductive outcomes. Upsala Journal of Medical Sciences. 2016:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Human Reproduction Update. 2013; 19:640–655. [DOI] [PubMed] [Google Scholar]
- 35.Cassina M, Johnson DL, Robinson LK, Braddock SR, Xu R, Jimenez JL, et al. Pregnancy outcome in women exposed to leflunomide before or during pregnancy. Arthritis and Rheumatism. 2012; 64:2085–2094. [DOI] [PubMed] [Google Scholar]
- 36.Armentl VT, Wilson GA, Radomski JS, Moritz MJ, McGrory CH, Coscia LA. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clinical Transplants. 1999:111–119. [PubMed] [Google Scholar]
- 37.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. International Journal of Epidemiology. 2015; 44:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallmen M, Bonde JP, Lindbohm ML, Kristensen P. Selection bias due to parity-conditioning in studies of time trends in fertility. Epidemiology. 2015; 26:85–90. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg CR, Baird DD, Wilcox AJ. Bias in retrospective studies of spontaneous abortion based on the outcome of the most recent pregnancy. Annals of the New York Academy of Sciences. 1994; 709:280–286. [DOI] [PubMed] [Google Scholar]
- 40.Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatric and Perinatal Epidemiology. 2013; 27:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hvidman HW, Bang AK, Priskorn L, Scheike T, Birch Petersen K, Nordkap L, et al. Anti-Mullerian hormone levels and fecundability in women with a natural conception. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2017; 217:44–52. [DOI] [PubMed] [Google Scholar]
- 42.Tielemans E, Burdorf A, te Velde E, Weber R, van Kooij R, Heederik D. Sources of bias in studies among infertility clients. American Journal of Epidemiology. 2002; 156:86–92. [DOI] [PubMed] [Google Scholar]
- 43.Finer LB, Zolna MR. Declines in Unintended Pregnancy in the United States, 2008–2011. New England Journal of Medicine. 2016; 374:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker D, Park C, Sweeney C, McCormack L, Durkin M, Brenner R, et al. Recruitment of women in the National Children’s Study Initial Vanguard Study. American Journal of Epidemiology. 2014; 179:1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Beek C, Hoek A, Painter RC, Gemke R, van Poppel MNM, Geelen A, et al. Women, their Offspring and iMproving lifestyle for Better cardiovascular health of both (WOMB project): a protocol of the follow-up of a multicentre randomised controlled trial. BMJ Open. 2018; 8:e016579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Velasquez MM, von Sternberg KL, Floyd RL, Parrish D, Kowalchuk A, Stephens NS, et al. Preventing Alcohol and Tobacco Exposed Pregnancies: CHOICES Plus in Primary Care. American Journal of Preventive Medicine. 2017; 53:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tieu J, Middleton P, Crowther CA, Shepherd E. Preconception care for diabetic women for improving maternal and infant health. Cochrane Database Syst Rev. 2017; 8:Cd007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Funkhouser E, Wammack J, Roche C, Reis J, Sidney S, Schreiner P. Where are they now? Retention strategies over 25 years in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Contemp Clin Trials Commun. 2018; 9:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotelchuck M, Lu M. Father’s Role in Preconception Health. Maternal and Child Health Journal. 2017; 21:2025–2039. [DOI] [PubMed] [Google Scholar]
- 50.Loxton D, Tooth L, Harris ML, Forder PM, Dobson A, Powers J, et al. Cohort Profile: The Australian Longitudinal Study on Women’s Health (ALSWH) 1989–95 cohort. International Journal of Epidemiology. 2018; 47:391–392e. [DOI] [PubMed] [Google Scholar]
- 51.Dobson AJ, Hockey R, Brown WJ, Byles JE, Loxton DJ, McLaughlin D, et al. Cohort Profile Update: Australian Longitudinal Study on Women’s Health. International Journal of Epidemiology. 2015; 44:1547,1547a–1547f. [DOI] [PubMed] [Google Scholar]
- 52.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology. 1988; 41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 53.Wen SW, Leader A, White RR, Leveille MC, Wilkie V, Zhou J, et al. A comprehensive assessment of outcomes in pregnancies conceived by in vitro fertilization/intracytoplasmic sperm injection. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2010; 150:160–165. [DOI] [PubMed] [Google Scholar]
- 54.Myers JE, Myatt L, Roberts JM, Redman C. COLLECT, a collaborative database for pregnancy and placental research studies worldwide. BJOG: An International Journal of Obstetrics and Gynaecology. 2019; 126:8–10. [DOI] [PubMed] [Google Scholar]
- 55.Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatric and Perinatal Epidemiology. 2013; 27:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. New England Journal of Medicine. 1988; 319:189–194. [DOI] [PubMed] [Google Scholar]
- 57.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Annals of Epidemiology. 2010; 20:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porucznik CA, Cox KJ, Schliep KC, Wilkins DG, Stanford JB. The Home Observation of Periconceptional Exposures (HOPE) study, a prospective cohort: aims, design, recruitment and compliance. Environmental Health: A Global Access Science Source. 2016; 15:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buck Louis GM, Hediger ML, Bell EM, Kus CA, Sundaram R, McLain AC, et al. Methodology for establishing a population-based birth cohort focusing on couple fertility and children’s development, the Upstate KIDS Study. Paediatric and Perinatal Epidemiology. 2014; 28:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. American Journal of Public Health. 1992; 82:1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chavarro JE, Rich-Edwards JW, Gaskins AJ, Farland LV, Terry KL, Zhang C, et al. Contributions of the Nurses’ Health Studies to Reproductive Health Research. American Journal of Public Health. 2016; 106:1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wise LA, Rothman KJ, Mikkelsen EM, Stanford JB, Wesselink AK, McKinnon C, et al. Design and Conduct of an Internet-Based Preconception Cohort Study in North America: Pregnancy Study Online. Paediatric and Perinatal Epidemiology. 2015; 29:360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Tian Y, Wang W, Ouyang F, Xu J, Yu X, et al. Cohort profile: the Shanghai Birth Cohort. International Journal of Epidemiology. 2019. [DOI] [PubMed] [Google Scholar]
- 64.Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort profile: the Danish Web-based Pregnancy Planning Study--’Snart-Gravid’. International Journal of Epidemiology. 2009; 38:938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Downs DS, Feinberg M, Hillemeier MM, Weisman CS, Chase GA, Chuang CH, et al. Design of the Central Pennsylvania Women’s Health Study (CePAWHS) strong healthy women intervention: improving preconceptional health. Maternal and Child Health Journal. 2009; 13:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanford JB, Smith KR, Varner MW. Impact of instruction in the Creighton model fertilitycare system on time to pregnancy in couples of proven fecundity: results of a randomised trial. Paediatric and Perinatal Epidemiology. 2014; 28:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santillan MK, Leslie KK, Hamilton WS, Boese BJ, Ahuja M, Hunter SK, et al. “Collection of a lifetime: a practical approach to developing a longitudinal collection of women’s healthcare biological samples”. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2014; 179:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]