Abstract
BACKGROUND:
Patients with platinum-refractory, recurrent or metastatic squamous cell carcinoma of the head and neck (RM-SCCHN) have limited options. Activin receptor-like kinase 1 (ALK1) is a type I receptor of the transforming growth factor β superfamily expressed on activated endothelial cells. Dalantercept is an ALK1 receptor fusion protein that acts as a ligand trap to block signaling through ALK1 and inhibits stages of angiogenesis involved in blood vessel maturation and stabilization. In a phase 1 study, dalantercept demonstrated clinical activity in patients with RM-SCCHN. The objective of the current study was to evaluate the activity of dalantercept in RM-SCCHN.
METHODS:
Forty-six patients received dalantercept at doses of 80mg (n52), 0.6mg/kg (n513), or 1.2mg/kg (n531) subcutaneously every 3 weeks. The primary endpoint was the overall response rate according to Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). Secondary endpoints included progression-free survival and overall survival, safety and tolerability, and pharmacokinetic and pharmacodynamic assessments.
RESULTS:
Forty patients were evaluable for response (13 who received dalantercept 0.6mg/kg and 27 who received dalantercept 1.2mg/kg). The overall response rate was 5% (n52), and 35% of patients had stable disease; 44% of patients who received 1.2mg/kg and 30.8% of those who received 0.6mg/kg achieved disease control (partial response or stable disease). The median progression-fee survival was 1.4 months (95% confidence interval, 1.3–2.2 months), and the median overall survival was 7.1 months (95% confidence interval, 5.5–11.1 months). Drug-related adverse events (>15%) were anemia, fatigue, peripheral edema, headache, and hyponatremia.
CONCLUSIONS:
In an unselected, heavily pretreated population of patients with RM-SCCHN, dalantercept monotherapy resulted in a favorable safety profile but only modest dose-dependent activity, and it did not meet the primary efficacy objective of the study.
Keywords: activin receptor-like kinase 1 (ALK1), angiogenesis, bone morphogenetic protein 9 (BMP9), dalantercept. squamous cell carcinoma of the head and neck (SCCHN)
INTRODUCTION
Squamous cell carcinoma of the head and neck (SCCHN) is among the few cancers with an increasing incidence in the United States (59,340 vs 39,250 new cases in 2015 vs 2005), with local relapses after primary therapy and the appearance of distant metastases accounting for 12,290 US deaths in 2015.1,2 This is the combined result of increases in tobacco-related laryngeal SCCHN (13,560 vs 9880 in 2015 vs 2005) and oral cavity and oropharyngeal SCCHN (45,780 vs 29,370 in 2015 vs 2005).1,2 The most common etiologic factors in SCCHN are alcohol use, tobacco use and human papillomavirus (HPV) infection.3 The rising incidence of HPV infection has been associated with an increased incidence of oropharyngeal SCCHN and with a favorable prognosis compared with HPV-negative SCCHN.4
Recurrent, unresectable, or metastatic SCCHN (RM-SCCHN) remains incurable. Palliative systemic therapies include combination regimens of platinum, antimetabolites, and the EGFR inhibitor cetuximab; single agents (platinum, docetaxel, methotrexate, or cetuximab); or other combination regimens. Cetuximab is the only approved targeted agent in platinum-refractory RM-SCCHN and is associated with an overall response rate (ORR) of 13% and a median overall survival (OS) of< 6 months.5 Like many solid tumors, SCCHN relies on neoangiogenesis to grow and disseminate. Vascular endothelial growth factor (VEGF) levels and expression in SCCHN have been associated with lymph node metastases, decreased apoptosis, and resistance to chemoradiation therapy.6 On the basis of compelling preclinical rationale, several attempts at inhibiting the VEGF signaling pathway using agents like, sorafenib, and bevacizumab have been evaluated.6,7 Single-agent antiangiogenic agents like sorafenib and sunitinib in unselected populations of patients with RM-SCCHN have not demonstrated substantial improvements in antitumor activity or survival.8,9 Given the biologic relevance of angiogenesis in SCCHN and the limited activity of VEGF inhibitors, the investigation of antiangiogenic agents with alternative mechanisms of action is justified.
Activin receptor-like kinase-1 (ALK1) is a type I receptor in the transforming growth factor-β (TGFβ) superfamily that is expressed on activated endothelial cells and binds to the ligands bone morphogenetic protein 9 (BMP9) and BMP10.10 ALK1 signaling leads to phosphorylation of the SMAD family member 1 (SMAD1), SMAD5, and SMAD8 proteins, resulting in the activation of cellular programs distinct from VEGF that are involved in blood vessel maturation and stabilization.10 Mutations in this pathway cause hereditary hemorrhagic telangiectasia, which results in impaired vascular development, including arterial and venous malformations.11 In addition, the BMP9/BMP10/ALK1 pathway regulates the development of lymphatic vessels, which could play a role in the metastatic spread of tumor cells through lymphatic vasculature.12 ALK1 is present in the vasculature of many human tumor types, including SCCHN.13,14 Moderate to high BMP9 expression identified by immunohistochemistry has been documented in archived samples of SCCHN.15
Dalantercept is a soluble receptor fusion protein consisting of the extracellular domain of human ALK1 linked to the Fc portion of human immunoglobulin G1 (IgG1). Dalantercept binds to BMP9 and BMP10 and inhibits the activation of endogenous ALK1.16 Dalantercept has demonstrated antiangiogenic and antitumor activity in a variety of preclinical models through a mechanism that may be complementary to the VEGF pathway.16,17 In a preclinical mouse xenograft model of SCCHN (from the RPMI 2650 cell line), dalantercept demonstrated both single-agent efficacy and additive activity combined with cisplatin.15 In a phase 1 study of patients with solid tumors (N=37), dalantercept at doses ranging from 0.1 to 4.8mg/kg demonstrated antitumor activity, including 1 partial response and 27% prolonged stable disease (≥12 weeks) in evaluable patients.18 Two of 3 patients with SCCHN in that study derived clinical benefit: 1 had a partial response with 33% tumor shrinkage and received 10 cycles (30 weeks) of dalantercept 0.4mg/kg; and the other received dalantercept 1.6mg/kg, had prolonged stable disease with 29% tumor shrinkage, and completed 11 cycles (33 weeks) of dalantercept. In total, 8 patients across the higher dose levels developed telangiectasias, which is an on-target pharmacodynamic effect of dalantercept inhibition of the ALK1 pathway. The side-effect profile of dalantercept was distinct from that of VEGF pathway inhibitors and consisted of peripheral edema, fatigue, and anemia, which occurred more frequently at or above the 1.6mg/kg dose level.18 On the basis of those results, the recommended phase 2 dose level was 1.2mg/kg (approximately 80% of the maximum tolerated dose). Because of concern that patients with SCCHN would be at risk of lymphedema and fluid retention, in the phase 2 study, dalantercept doses of 0.6 and 1.2mg/kg were explored.
Together, these preclinical and clinical data supported further exploration of dalantercept in RM-SCCHN. Here, we report the results of an open-label, phase 2 study assessing the antitumor activity and safety of dalantercept in patients with RM-SCCHN.
MATERIALS AND METHODS
Eligibility Criteria
This study (clinicaltrials.gov identifier NCT01458392) was approved by local institutional review boards and conducted in accordance with national and local regulations. Written informed consent was obtained before the initiation of study-related procedures. Eligible patients were men and nonpregnant/lactating women aged ≥18 years who had histologically and/or cytologically confirmed RM-SCCHN of mucosal origin (oral cavity, oropharynx, hypopharynx, or larynx). Incurability (disease not amenable to surgery, radiation, or reirradiation) and ineligibility for platinum (refractory or platinum contraindicated) were required. Patients must not have received prior antiangiogenic therapy, were required to have measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,19 and were required to have an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients with unknown primary SCCHN presumed to be of head and neck mucosal origin were eligible if they met all other entry criteria.
Study Design
This was a phase 2, multicenter, open-label, multiple-dose study of dalantercept in RM-SCCHN. Initially, a fixed dose of 80mg delivered as a subcutaneous injection every 3 weeks for a 21-day cycle was evaluated in 2 patients. The protocol was amended to use weight-based dosing (mg/kg), and 2 dose levels were evaluated (0.6mg/kg and 1.2mg/kg) every 3 weeks. The maximum dose that could be administered was capped based on a weight of 100kg. Tumor response imaging was evaluated using RECIST version 1.1 before every other cycle (approximately 6 weeks) and was assessed by the investigator at each site.
Study Objectives
The primary objective of the study was to evaluate the objective response rate (ORR) associated with dalantercept in patients with RM-SCCHN. Secondary objectives included to evaluate safety and tolerability, progression-free survival (PFS), the disease control rate, and overall survival (OS) and to examine the pharmacokinetic profile and explore potential pharmacodynamic biomarkers in sera and in archived and fresh tumor specimens.
Statistical Analysis
The efficacy analyses used a modified intent-to-treat population consisting of patients who had received at least 1 dose of dalantercept (either 0.6 or 1.2mg/kg) and had undergone at least 1 on-treatment tumor assessment. The response rate was determined using RECIST version 1.1 and was assessed at the individual sites. A sample size of 29 evaluable patients was required to provide at least 80% power to differentiate an ORR of interest (Ha) of 30% from a minimal ORR of 10% (Ho) at a 1-sided type 1 error of 5% in a single-stage design. The 95% exact confidence interval for the ORR was calculated based on the binomial distribution. Safety analyses included all patients who had received at least 1 dose of dalantercept (80mg, 0.6mg/kg, and 1.2mg/kg).
Evaluation of Safety
Safety endpoints included evaluation of adverse events, physical examination, vital signs, electrocardiogram, echocardiogram, hematology, chemistry, urinalysis, and antidrug antibodies. Testing for antidrug antibodies and neutralizing antibodies was performed using an enzyme-linked immunosorbent assay at baseline, during dosing, and at the end of the study. A data monitoring committee comprised of 2 independent oncologists and a cardiologist met to review the safety data during the study.
Pharmacokinetics
Serum samples for determination of pharmacokinetic parameters were collected predose in cycles 1 through 3, on day 8 during the first 2 cycles, and on day 15 of the first cycle. Serum dalantercept concentrations were determined by enzyme-linked immunosorbent assay, and pharmacokinetic parameters were estimated by noncompartmental analysis of dalantercept concentration data, using actual collection times, with WinNonlin Professional (Pharsight, Mountain View, Calif). Concentrations that were below the limit of quantification were set to 0 for noncompartmental analysis.
Evaluation of Exploratory Biomarkers
Serum samples were collected on day 1 of each cycle, on day 8 of cycles 2 through 4, and at the final visit. Select serum biomarkers associated with angiogenesis (BMP9, soluble endoglin, VEGF, placental growth factor, VEGF receptor [VEGFR2], and VEGFR3) were evaluated using a Luminex quantitative multiplex immunoassay platform (Luminex Corporation, Austin, Tex) developed by Myriad RBM (Austin, Tex). Archived biopsies of tumor specimens, optional skin biopsies, and fresh tumor biopsies obtained before cycle 1 and after cycle 2 were collected to be evaluated by immunohistochemical tissue staining and laser-scanning cytometry. Eight tissue biomarkers were selected for evaluation: ALK1, BMP9, BMP10, CD105 (endoglin), CD31 (platelet endothelial cell adhesion molecule), helix-loophelix protein Id2, phosphorylated SMAD5, and growth/differentiation factor 5 (GDF-5).
Summary statistics as well as percentage changes from baseline were used to evaluate baseline levels of each biomarker and postbaseline levels during dalantercept treatment. Archived tumor specimens and fresh tissue samples, donated voluntarily during the study by patients with telangiectasias, were individually evaluated.
RESULTS
Patient Characteristics
In total, 46 patients were enrolled between October 2011 and July 2013. Summary demographics and baseline characteristics for these patients are provided in Table 1. Two patients were enrolled at the fixed dose of 80mg, 13 patients were enrolled at the 0.6-mg/kg dose level, and 31 were enrolled at the 1.2-mg/kg dose level. Patients had received a median of 2 prior therapies, and the primary site of disease was oropharynx or oral cavity in 78.3% of patients. The rates of HPV-positive and HPV-negative patients were 45.7% and 41.3%, respectively, and 13% (n =6) had unknown HPV status.
TABLE 1.
Patient Characteristics
| No. of Patients (%) | ||||
|---|---|---|---|---|
| Dalantercept Dose | ||||
| Characteristic | 80 mg, N = 2 | 0.6 mg/kg, N = 13 | 1.2 mg/kg, N = 31 | Overall, N = 46 |
| Age: Median [range], y | 56.5 [47–66] | 60 [50–68] | 61 [45–78] | 60.5 [45–78] |
| Sex | ||||
| Men | 0 (0) | 13 (100) | 26 (83.9) | 39 (84.8) |
| Women | 2 (100) | 0 (0) | 5 (16.1) | 7 (15.2) |
| ECOG PS | ||||
| 0 | 0 (0) | 6 (46.2) | 9 (29) | 15 (32.6) |
| 1 | 2 (100) | 7 (53.8) | 22 (71) | 31 (67.4) |
| Site of primary tumor | ||||
| Oropharynx | 0 (0) | 11 (84.6) | 10 (32.3) | 21 (45.7) |
| Oral cavity | 2 (100) | 2 (15.4) | 11 (35.5) | 15 (32.6) |
| Larynx | 0 (0) | 0 (0) | 6 (19.4) | 6 (13) |
| Unknown | 0 (0) | 0 (0) | 4 (12.9) | 4 (8.7) |
| HPV status | ||||
| Positive | 0 (0) | 9 (69.2) | 12 (38.7) | 21 (45.7) |
| Negative | 2 (100) | 4 (30.8) | 13 (41.9) | 19 (41.3) |
| Unknown | 0 (0) | 0 (0) | 6 (19.4) | 6 (13) |
| Smoking status | ||||
| Current | 0 (0) | 1 (7.7) | 1 (3.2) | 2 (4.3) |
| Former | 1 (50) | 6 (46.2) | 23 (74.2) | 30 (65.2) |
| Never | 1 (50) | 6 (46.2) | 7 (22.6) | 14 (30.4) |
| No. of prior regimens Median [range] | 3 [3–3] | 2 [1–8] | 2 [1–6] | 2 [1–8] |
| Prior radiotherapy to metastatic disease | 0 (0) | 3 (23.1) | 11 (35.5) | 14 (30.4) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; HPV, human papillomavirus.
Efficacy
Antitumor activity was assessed in 40 evaluable patients at the 0.6-mg/kg and 1.2-mg/kg dose levels (Table 2). The 2 patients at the fixed 80-mg dose level were excluded from the primary efficacy analysis, because the protocol had been amended to incorporate weight-based dosing for the remainder of the study population. The ORR was 5%. There were no partial responses at the 0.6-mg/kg dose level of dalantercept. Two heavily pretreated patients—1HPV-positive (who received 2 lines of cisplatin-based therapy) and the other HPV-negative (who received 3 lines of carboplatin-based therapy)—at the 1.2-mg/kg dose level experienced partial responses with −50% and −56% tumor shrinkage, respectively. Both patients continued to receive treatment and maintained at least a stable disease response beyond 1 year. Another 14 patients (35%) experienced stable disease, which lasted at least 3 months in 5 patients. The disease control rate was numerically greater (but the difference was not significant) at the 1.2-mg/kg dose level compared with the 0.6-mg/kg dose level (44.4% vs 30.8%; 1-sided Fisher exact test; P=.3177). The HPV-positive patients, as a group (N=20), experienced a greater disease control rate compared with the HPV-negative patients (N=15; 55% vs 20%; 1-sided Fisher exact test; P=.0392) (Supporting Table 1; see online supporting information).
TABLE 2.
Antitumor Activity of Dalantercept by Dose Levela
| No. of Patients (%) | |||
|---|---|---|---|
| Dalantercept Dose | |||
| Endpoint | 0.6 mg/kg, N = 13 |
1.2 mg/kg, N = 27 |
Overall, N = 40 |
| Partial response | 0 (0) | 2(7.4) | 2 (5) |
| Stable disease | 4 (30.8) | 10 (37) | 14 (35) |
| Disease control rate | 4 (30.8) | 12 (44.4) | 16 (40) |
| Disease control rate ≥ 3 mo | 2 (15.4) | 5 (18.5) | 7(17.5) |
| Progressive disease | 9 (69.2) | 15 (55.6) | 24 (60) |
All patients received at least 1 dose of dalantercept and had 1 postbaseline tumor imaging study assessed by an investigator according to Response Evaluation Criteria in Solid Tumors (RECIST).
The overall median PFS was 1.4 months and was similar across both dose levels. The median OS was 7.1 months in the 0.6-mg/kg group, 9.5 months in the 1.2mg/kg group, and 7.1 months overall. The OS rate at 1 year was 22.7%. There were no statistical differences in PFS or OS between the dosing groups. Details on the maximum reduction in target lesion size according to the best overall response and OS Kaplan-Meier curves by dose group are provided in Supporting Figures 1 and 2, respectively (see online supporting information).
Safety
Treatment-emergent adverse events that occurred in >10% of patients are listed in Table 3. Adverse events that were reported as related to dalantercept (>10%) included anemia, fatigue, peripheral edema, headache, hyponatremia, and pleural effusion (Table 4). No worsening in cardiac function was observed clinically or on the echocardiograms obtained at baseline and after therapy. Telangiectasias, clinical pharmacodynamic manifestations of ALK1 pathway inhibition that have a known association with genetic ALK1 deficiency syndrome (hereditary hemorrhagic telangiectasia 2), were reported in 4 patients (8.7%). Anemia, peripheral edema, hyponatremia, and pleural effusions occurred more frequently at the 1.2-mg/ kg dose level compared with the 0.6-mg/kg dose level. Grade ≥3 treatment-related adverse events occurred in 15.2% of patients (N=7) and occurred most frequently at the 1.2-mg/kg dose level (N=5). Hyponatremia was the most frequent treatment-related grade ≥3 adverse event (N=3). Adverse events leading to treatment discontinuation included amylase/lipase elevations without pancreatitis (n =1) and pleural effusion (n=1). Thirteen patients (28.3%) experienced a serious adverse event. Three of those patients (6.5%) experienced a treatment-related serious adverse event(s) of pleural effusion (n=2), pulmonary edema (n =1), and tracheal obstruction (n =1). There were no treatment-related deaths. No antidrug antibodies were detected.
TABLE 3.
Treatment-Emergent Adverse Events (>10%): All Grades
| No. of Patients (%) | ||||
|---|---|---|---|---|
| Dalantercept Dose | ||||
| Preferred AE Term | 80 mg, N = 2 | 0.6 mg/kg, N = 13 | 1.2 mg/kg, N = 31 | Overall, N = 46 |
| Fatigue | 1 (50) | 8 (61.5) | 12 (38.7) | 21 (45.7) |
| Anemia | 0 (0) | 2 (15.4) | 16 (51.6) | 18 (39.1) |
| Headache | 1 (50) | 4 (30.8) | 11 (35.5) | 16 (34.8) |
| Peripheral edema | 0 (0) | 2 (15.4) | 11 (35.5) | 13 (28.3) |
| Hyponatremia | 1 (50) | 1 (7.7) | 9 (29) | 11 (23.9) |
| Decreased appetite | 0 (0) | 3 (23.1) | 5 (16.1) | 8 (17.4) |
| Dyspnea | 0 (0) | 2 (15.4) | 5 (16.1) | 7 (15.2) |
| Arthralgia | 0 (0) | 0 (0) | 6 (19.4) | 6 (13) |
| Constipation | 1 (50) | 3 (23.1) | 2 (6.5) | 6 (13) |
| Cough | 0 (0) | 2 (15.4) | 4 (12.9) | 6 (13) |
| Hyperglycemia | 0 (0) | 0 (0) | 6 (19.4) | 6 (13) |
| Nausea | 1 (50) | 1 (7.7) | 4 (12.9) | 6 (13) |
| Pleural effusion | 0 (0) | 1 (7.7) | 5 (16.1) | 6 (13) |
| Vomiting | 1 (50) | 2 (15.4) | 3 (9.7) | 6(13) |
| Face edema | 0 (0) | 0 (0) | 5 (16.1) | 5 (10.9) |
| Hypoalbuminemia | 0 (0) | 2 (15.4) | 3 (9.7) | 5 (10.9) |
Abbreviation: AE: adverse event.
TABLE 4.
Treatment-Emergent Adverse Events Considered Related to Study Drug (>10%): All Grades
| No. of Patients (%) | ||||
|---|---|---|---|---|
| Dalantercept Dose | ||||
| Preferred AE Term | 80 mg, N = 2 | 0.6 mg/kg, N = 13 | 1.2 mg/kg, N = 31 | Overall, N = 46 |
| Patients with ≥ 1 AE | 1 (50) | 13 (100) | 27 (87.1) | 41 (89.1) |
| Anemia | 0 (0) | 2 (15.4) | 10 (32.3) | 12 (26.1) |
| Fatigue | 1 (50) | 3 (23.1) | 7 (22.6) | 11 (23.9) |
| Peripheral edema | 0 (0) | 2 (15.4) | 9 (29) | 11 (23.9) |
| Headache | 0 (0) | 3 (23.1) | 5 (16.1) | 8 (17.4) |
| Hyponatremia | 1 (50) | 0 (0) | 6 (19.4) | 7 (15.2) |
| Pleural effusion | 0 (0) | 1 (7.7) | 4 (12.9) | 5 (10.9) |
Abbreviation: AE: adverse event.
Pharmacokinetics
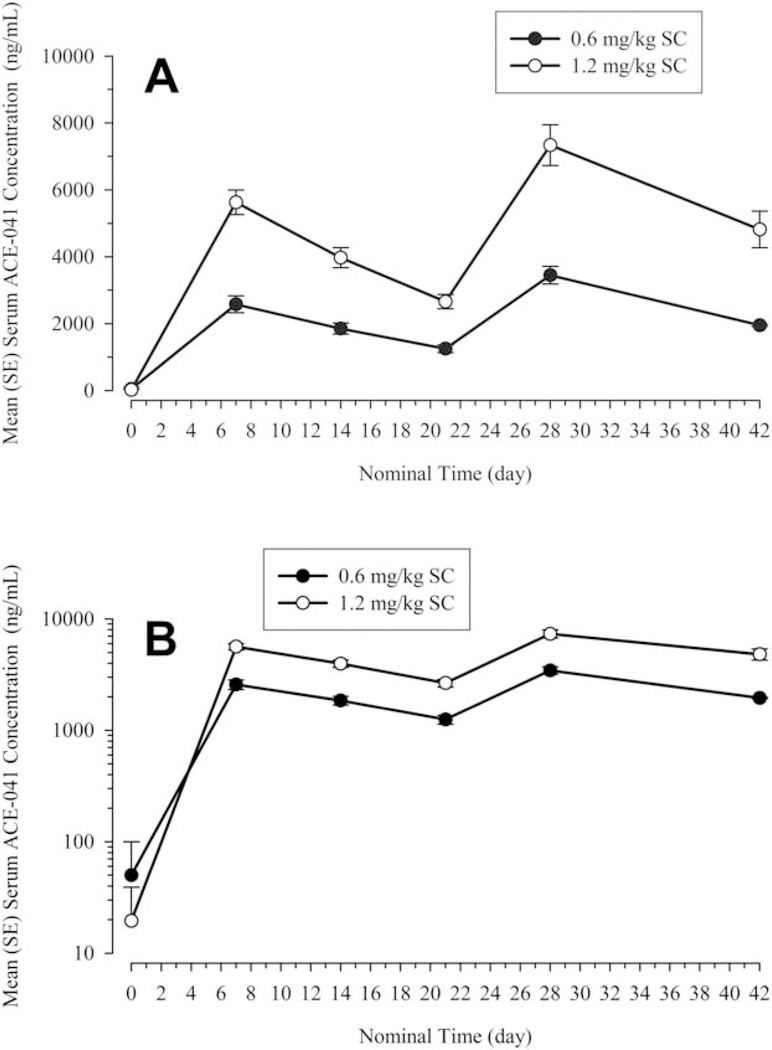
Peak serum concentrations of dalantercept were reached around 7 days for both dose levels, whereas concentrations declined in a monoexponential fashion (Fig. 1). Exposure was dose proportional for cycle 1 and was slightly more than dose proportional for cycle 2, whereas the time to peak concentration (approximately 7 days) was consistent across dose levels and cycles (Table 5).
Figure 1.
(A,B) Mean±standard error (SE) serum concentrations versus time profiles of dalantercept (ACE-041) are illustrated after single or multiple subcutaneous (SC) doses of dalantercept (semi-log and linear scale).
TABLE 5.
Summary Pharmacokinetics Parameters for Dalantercept After Single or Multiple Doses of Dalantercept
| Serum ACE-041: Geometric Mean (CV% Geometric Mean) | ||||
|---|---|---|---|---|
| Cycle 1 | Cycle 2 | |||
| Parameter | 0.6 mg/kg SC | 1.2 mg/kg SC | 0.6 mg/kg SC | 1.2 mg/kg SC |
| No. of patients | 12 | 26 | 11 | 19 |
| AUC0-t, ng●d/mL | 32,992 (35.1) | 69,572 (44.7) | 29,674 (59.4) | 72,725 (91.4) |
| Cmax, ng/mL | 2446 (35.1) | 5336 (37.8) | 3326 (30.3) | 7067 (44.9) |
| Tmax, da | 6.85 [5.96, 8.92] | 6.88 [5.98, 20.71] | 6.92 [6.84, 8.81] | 6.90 [6.73, 11.86] |
| Tlast, da | 20.75 [19.94, 21.99] | 20.78 [7.00, 27.73] | 20.80 [6.84, 22.91] | 20.84 [6.87, 27.87] |
Abbreviations: ACE-041, dalantercept; AUC0-t, area under the plasma concentration-time curve; Cmax, maximum (peak) serum drug concentration; CV%, percentage coefficient of variation; SC, subcutaneously; Tlast, last quantified plasma concentration; Tmax, maximum observed plasma concentration.
a Values indicate the median [minimum, maximum].
Exploratory Biomarkers
In total, 46 patients provided serum samples for biomarker analysis; however only patients who received dalantercept doses of 0.6 and 1.2 mg/kg (n=44) were included in the analysis. Summary statistics for each biomarker at baseline, before the second and third doses, and at the last study visit are presented in Supporting Table 2. Serum samples were summarized using the entire study population, because subgroup analyses by HPV status or dose level led to very small sample sizes. Serum concentrations of 2 biomarkers, BMP9 and VEGF, generally increased from baseline; whereas concentrations of placental growth factor and VEGFR3 consistently decreased at each post-baseline visit. Serum concentrations of soluble endoglin and VEGFR2 were similar at each study visit.
Because of the limited distribution of staining scores in archived tissue samples and the suboptimal quality of the fresh tissue samples collected before and during treatment, no additional observations could be made regarding biomarker expression levels. Two patients underwent fresh skin biopsies of telangiectasias; and, in both samples, ALK1 and BMP9 staining was low, with staining scores of 1 or 2 on a scale from 1 to 9.
DISCUSSION
Antiangiogenic strategies targeting the VEGF pathway have demonstrated limited clinical activity and, in some cases, significant toxicities, hindering further development in SCCHN. RM-SCCHN is a highly angiogenic disease; therefore, further exploration of novel angiogenic modulators is justified. It is known that the ALK1 pathway is activated in a variety of cancers, including SCCHN. Here, we report the first clinical study investigating dalantercept, an ALK1 pathway inhibitor, in patients with RM-SCCHN. Dalantercept monotherapy demonstrated modest clinical activity (2 patients achieved a partial response, and 7 patients experienced disease control that lasted ≥3 months) and did not meet the primary efficacy objective of the study. The median OS was 7 months.
Overall, dalantercept was well tolerated, the main side effects (fatigue, anemia, and peripheral edema) were dose-dependent, and the safety and pharmacokinetic profiles were consistent with those reported in the phase 1 study. Hyponatremia (24%) emerged as a unique adverse event in this study compared with the phase 1 experience. Hyponatremia has been described as a paraneoplastic Syndrome in squamous cancers typically involving the lung but also recognized to occur in SCCHN.20,21 In addition, cisplatin induces salt wasting, and prior neck dissections and radiotherapy as part of the treatment for SCCHN can also contribute to the development of hyponatremia.22,23 Serum and urine laboratory studies in some of these patients were consistent with serum of inappropriate antidiuretic hormone (SIADH) release. However, hypervolemic hyponatremia also contributed in some patients to edema-related events associated with dalantercept. Finally, the management of edema with diuretics also may have contributed to hyponatremia in some patients. Currently, the mechanism of dalantercept-associated edema is not fully understood. ALK1 is expressed on lymphatic endothelial cells and, thus, disruption of lymphatic vessel integrity may lead to vascular leak resulting in fluid-related adverse events, such as peripheral edema, pleural effusions, and pulmonary edema.23 It is noteworthy that the safety profile of dalantercept remains unique among the class of antiangiogenic agents, because the anti-VEGF–based therapies are more commonly associated with bleeding and other dose-limiting adverse events, such as diarrhea, hypertension, proteinuria, and hand-foot syndrome.
Given the limited antitumor activity of dalantercept and the limited distribution of patient outcomes, no definitive conclusions can be drawn from the exploratory biomarker data collected in the serum and tumor specimens. Because dalantercept binds to the angiogenic ligand BMP9, postbaseline changes in BMP9 levels were the primary focus of the biomarker analysis. It is noteworthy that 1 patient who had a baseline BMP9 serum level of 64.0 pg/mL (well above the population baseline mean of 7.1 pg/mL) experienced a 95% decrease in BMP9 serum concentrations after the first dose of dalantercept and was 1 of the 2 partial responders in the study. No significant dose-dependent biomarker changes were observed. Of the patients who developed telangiectasias, all had stable disease as their best response, and 3 were in the highest dose level tested. Immunohistochemical studies on 2 of these patients’ skin biopsies revealed low levels of ALK1 and BMP9 expression, consistent with an on-target effect of dalantercept.
The recently reported results from a trial of the programmed death receptor 1 (PD-1) nivolumab in SCCHN, indicating improved OS compared with a single-agent investigator’s choice of chemotherapy in patients with platinum-refractory RM-SCCHN, highlight the value of targeting the tumor microenvironment in SCCHN.24 The reasons behind the lack of activity or intrinsic nonresponsiveness of antiangiogenic therapy in patients with SCCHN is currently unknown but may include challenges with drug delivery to the locoregional head and neck area, in which the vasculature has been compromised by surgery and radiation.25
This study had several important limitations. First, the patient population was heterogeneous, with both HPV-positive and HPV-negative patients and numerous prior therapies. Second, the exploration of 2 dose levels, although it yielded valuable information on drug tolerability, also introduced heterogeneity. Together with a limited total number of patients treated, these elements of heterogeneity led to an inability to detect statistically different treatment effects in subgroups. Other factors, including a predominantly HPV-positive population and subsequent therapies, likely contributed to the favorable survival results.
In conclusion, subcutaneous administration of dalantercept in this unselected population of patients with RM-SCCHN demonstrated modest dose-dependent anticancer activity and a favorable safety profile. Although the ORR was 5%, OS at 1 year was 22.7%. Patients with HPV-positive disease and who received dalantercept at the higher dose level of 1.2mg/kg appeared to derive the greatest clinical benefit. Post-treatment serum BMP9 levels generally increased as tumors progressed, an observation that will be further evaluated in ongoing studies exploring the combination of dalantercept with VEGF pathway inhibitors. Given the overall favorable safety profile of dalantercept, combinations with chemotherapy and/or radiation therapy are additional approaches that could be considered in patients with RM-SCCHN.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This study was supported by Acceleron Pharma.
CONFLICT OF INTEREST DISCLOSURES
The following authors were primary investigators for this clinical trial and thus received research support from Acceleron Pharma (Cambridge, Mass): Antonio Jimeno, Marshall R. Posner, Lori J. Wirth, Nabil F. Saba, Roger B. Cohen, Elizabeta C. Popa, Athanassios Argiris, Kenneth Grossmann, Ammar Sukari, and Jared Weiss. The following authors were employees of Acceleron Pharma: Dawn Wilson, Xiaosha Zhang, Jade Sun, Chad Glasser, Kenneth M. Attie, Matthew L. Sherman, and Susan S. Pandya. Antonio Jimeno has received honoria from Astrazeneca for work performed outside of this study. Lori J. Wirth reports personal fees from Merck and Novartis outside the submitted work. Kenneth F. Grossmann reports support from Roche/Genentech outside the submitted work. Kenneth M. Attie reports personal fees from Acceleron Pharma outside the submitted work. Susan S. Pandya is a former employee of Acceleron Pharma and owns stock in the company. Jared Weiss reports grants from AstraZeneca, Celgene, Novartis, Merck, Pfizer, and EMD Serono and personal fees from AstraZeneca, Biodesix, Eli Lilly, Pfizer, and Clovis, all outside the submitted work.
Footnotes
We are indebted to the patients and their families and to the clinical teams who facilitated patient coordination as well as sample and data acquisition.
Additional supporting information may be found in the online version of this article
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, et al. Cancer Statistics, 2005. CA Cancer J Clin. 2005;55:10–30. [DOI] [PubMed] [Google Scholar]
- 3.Chung CH, Parker JS, Karaca G, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489–500. [DOI] [PubMed] [Google Scholar]
- 4.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Canc Netw. 2011;9:665–673. [DOI] [PubMed] [Google Scholar]
- 5.Vermorken JB, Herbst RS, Leon X, Amellal N, Baselga J. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008;112:2710–2719. [DOI] [PubMed] [Google Scholar]
- 6.Denaro N, Russi EG, Colantonio I, et al. The role of antiangiogenic agents in the treatment of head and neck cancer. Oncology. 2012;83: 108–116. [DOI] [PubMed] [Google Scholar]
- 7.Gold KA, Lee HY, Kim ES. Targeted therapies in squamous cell carcinoma of the head and neck. Cancer. 2009;115:922–935. [DOI] [PubMed] [Google Scholar]
- 8.Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25: 3766–3773. [DOI] [PubMed] [Google Scholar]
- 9.Machiels JP, Henry S, Zanetta S, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006–01. J Clin Oncol. 2010;28:21–28. [DOI] [PubMed] [Google Scholar]
- 10.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93:682–689. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez LA, Sanz-Rodriguez F, Blanco FJ, Bernabeu C, Botella LM. Hereditary hemorrhagic telangiectasia, a vascular dysplasia affecting the TGF-beta signaling pathway. Clin Med Res. 2006;4:6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niessen K, Zhang G, Ridgway JB, Chen H, Yan M. ALK1 signaling regulates early postnatal lymphatic vessel development. Blood. 2010; 115:1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunha SI, Pardali E, Thorikay M, et al. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med. 2010;207:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunha SI, Pietras K. ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood. 2011;117:6999–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alimzhanov M, Lee M, Solban N, et al. Dalantercept, an ALK1 inhibitor of angiogenesis, in combination with cisplatin inhibits tumor growth in a xenograft model of squamous cell carcinoma of the head and neck. Abstract 1692. Paper presented at: American Association for Cancer Res. (AACR) Annual Meeting 2014; April 5–9 2014; San Diego, California. [Google Scholar]
- 16.Mitchell D, Pobre EG, Mulivor AW, et al. ALK1-Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Mol Cancer Ther. 2010;9:379–388. [DOI] [PubMed] [Google Scholar]
- 17.Hu-Lowe DD, Chen E, Zhang L, et al. Targeting activin receptor like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti-VEGF therapies. Cancer Res. 2011;71:1362–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendell JC, Gordon MS, Hurwitz HI, et al. Safety, #pharmacokinetics, |pharmacodynamics, and antitumor activity of dalantercept, an activin receptor-like kinase-1 ligand trap, in patients with advanced cancer. Clin Cancer Res. 2014;20:480–489. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 20.Ferlito A1, Rinaldo A, Devaney KO Syndrome of inappropriate antidiuretic hormone secretion associated with head neck cancers: review of the literature. Ann Otol Rhinol Laryngol. 1997;106:878–883. [DOI] [PubMed] [Google Scholar]
- 21.Diagnosis Raftopoulos H. and management of hyponatremia in cancer patients. Support Care Cancer. 2007;15:1341–1347. [DOI] [PubMed] [Google Scholar]
- 22.Feinstein AJ, Davis J, Gonzalez BS, et al. Hyponatremia and perioperative complications in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38:E1370–E1374. [DOI] [PubMed] [Google Scholar]
- 23.Zacay G, Bedrin L, Horowitz Z, et al. Syndrome of inappropriate antidiuretic hormone or arginine vasopressin secretion in patients following neck dissection. Laryngoscope. 2002;112:2020–2024. [DOI] [PubMed] [Google Scholar]
- 24.Gillison M, Blumenschein G, Fayette J, et al. Nivolumab (nivo) vs investigator’s choice (IC) for recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): CheckMate-141. Paper presented at: American Association for Cancer Res. (AACR) Annual Meeting 2016; April 16–20, 2016; New Orleans, Louisiana. [Google Scholar]
- 25.Vassilakopoulou M, Psyrri A, Argiris A. Targeting angiogenesis in head and neck cancer. Oral Oncol. 2015;51:409–415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.