Abstract
Purpose
Vitamin D is a novel potential therapeutic agent for peritoneal dialysis (PD)-related peritoneal fibrosis, but it can induce hypercalcemia and vascular calcification, which limits its applicability. In this study, we create nanotechnology-based drug delivery systems to investigate its therapeutics and side effects.
Materials and methods
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [amino-(polyethylene glycol)2000] (DSPE-PEG) and L-α-phosphatidylcholine (PC), which packages with 1α,25(OH)2D3, were used to construct vitamin D nanoliposomes. To confirm the function and safety of vitamin D nanoliposomes, peritoneal mesothelial cells were treated with TGF-β1 and the reverse was attempted using vitamin D nanoliposomes. Antibodies (Ab) against the peritoneum-glycoprotein M6A (GPM6A) Ab were conjugated with vitamin D nanoliposomes. These particles were implanted into mice by intraperitoneal injection and the animals were monitored for the distribution and side effects induced by vitamin D.
Results
Vitamin D nanoliposomes were taken up by the mesothelial cells over time without cell toxicity and it also provided the same therapeutic effect in vitro. In vivo study, fluorescent imaging showed vitamin D nanoliposomes allow specific peritoneum target effect and also ameliorate vitamin D side effect.
Conclusion
Nanoliposomes vitamin D delivery systems for the prevention of PD-related peritoneal damage may be a potential clinical strategy in the future.
Keywords: peritoneal dialysis, nanoliposome, vitamin D, fibrosis
Introduction
Peritoneal dialysis (PD) is a type of renal replacement therapy.1–4 The most important limitation of PD therapy is that patients may shift to hemodialysis (HD) involuntarily due to technique failure after several years.5–10 This technique failure is mostly attributed to peritoneal damage, and it has become an important issue in PD therapy.6,9,11–14
Conventional PD dialysate is bio-incompatible and is characterized by hypertonicity, high glucose, an acidic PH, and containing lactate and glucose degradation products (GDPs). These characteristics will induce pathological changes in the peritoneum, including the induction of the epithelial-to-mesenchymal transition (EMT) of mesothelial cells (MCs).15–18 Subsequently, the peritoneal membrane suffers from structural and functional changes, including fibrosis and neoangiogenesis. Finally, peritoneal membrane failure occurs.16,17,19,20
Our study as well as other previous studies have found that vitamin D is a potential therapy for PD-related peritoneal damage.21–24 However, the clinical application of vitamin D is limited by its side effects including hypercalcemia, hyperphosphatemia, and vascular calcification.
Recently, developments in nanotechnology have shown that nanoparticles are an ideal drug carrier. In nano drug delivery systems (nano-DDSs), the drug is transported specifically to the target location, thereby allowing drug action only on the target organ and minimizing undesirable side effects. In addition, nano-DDS protects the drug from degradation, resulting in a higher drug concentration in the target area, resulting in lower dosages of the drug being required.25 This type of therapy is particularly important if there is only a marginal difference in concentration between a therapeutic dosage and a toxic dosage.
Therefore, this study investigated the application of vitamin D nano-DDS against peritoneal fibrosis.
Materials and Methods
Synthesis of Vitamin D3-Loaded Nanoliposomes
L-α-Phosphatidylcholine (PC) (Sigma; 2.0 mg) and vitamin D (1α,25(OH)2D3) (Enzo Life Sciences; 1.0 mg) were dissolved in 5.0 mL dichloromethane (DCM) (Sigma).26 This was then stirred for 2 mins and 0.2 mg of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino-(polyethylene glycol)2000] (DSPE-PEG) (Nanocs Inc.) was added. This solution was then stirred for 5 mins to ensure thorough mixing. The solvent was then evaporated into a thin and uniform lipid-drug film with the help of a rotary evaporator.27 After thorough drying with a vacuum pump, the lipid-drug film was hydrated with 1.0 mL H2O and sonicated for 1 min in a water-bath sonicator, then transferred into a new 1.5-mL tube at 60°C for 2 hrs. Finally, the solutions were purified and filtered by using a dialysis membrane (500–1000 Daltons molecular weight cutoff (MWCO)) (Spectrum) overnight at room temperature on a stir plate. The vitamin D-loaded nanoliposomes (vit. D-NPs) were stored at 4°C for further use.
Synthesis of Rhodamine 6G (R6G)-Loaded Nanoliposomes
100 μL of R6G stock (0.1 mM) and 2.0 mg of PC were dissolved in 5.0 mL DCM and stirred for 2 mins. Next, 0.2 mg of DSPE-PEG was stirred in for 5 mins to ensure thorough mixing. The following procedures were identical to those described previously. Nanoliposomes were stored at 4°C and away from light for further use.
Nanoliposomes Conjugate with Glycoprotein M6A (GPM6A) Antibody
The amount of antibody used was the same as the amount of DSPE-PEG, and the amount of N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) (Sigma) and N-hydroxysuccinimide (NHS) (Sigma) used was 1.5 times that of the antibody used. Therefore, 1.5 nmole each of EDC and NHS were added into the solution of nanoliposomes and mixed with a gentle vortex before being incubated at 4°C. After 30 mins, 1 nmole of glycoprotein M6A (GPM6A) antibody (MBL International) was added to the reaction mixture for at least 4 hrs at 4°C.
General Procedures for the Quantification of Vitamin D Loading
High-performance liquid chromatography (HPLC) (Agilent 1260 Infinity system) was used to analyze vitamin D using a ZORBAX Eclipse PAH polymeric C18 bonded column (Agilent) with methanol (J.T.Baker) and water (92:8% v/v) as the mobile phase. The conditions were a flow rate of 2 mL/min, a column temperature of 40°C, and a variable wavelength detector (VWD) detection of 280 nm.28 A calibration curve was plotted in the concentration range of 0.01–1 mg/mL for 1α, 25(OH)2D3 by diluting 1mg/mL standard stock solution in methanol.
General Procedure for Determining the Vitamin D Release Profile
One hundred microliters of concentrated vitamin D3-loaded nanoliposomes was sealed in a dialysis membrane (MWCO 500–1000 Daltons). The dialysis bags were incubated in 50.0 mL water at 37°C with gentle shaking. A 100 μL portion of the aliquot was collected from the dialysis bags at predetermined time intervals, and the released drug was quantified using HPLC.27
Determination of the size distribution of nanoliposomes was by dynamic light scattering (DLS).
The mean particle size (hydrodynamic diameter) of the nanoliposomes was measured via the DLS method using a Zetasizer Nano 2590 (Malvern, UK).
Transmission Electron Microscopy (TEM) of Nanoliposomes
Ten microliter suspensions of nanoliposomes in H2O were measured using uranyl acetate negative staining. Ten microliters of this solution were then transferred to a TEM grid (copper grid, 3.0 mm, 200 mesh, coated with Formvar film) together with a drop of uranyl acetate (2% water solution) for 1 min, and allowed to dry. Analysis of the stained grids was performed with a JEOL JEM 2100 (Tokyo, Japan) TEM.
Human Primary Mesothelial Cells
Omentum-derived MCs were obtained from non-uremic patients undergoing abdominal surgery. The omentum samples were digested in 0.05% trypsin and 0.02% EDTA to isolate MCs.16,29 All MCs isolated from the omentum were then incubated in culture medium consisting of M199 (Gibco) medium supplemented with 10% fetal bovine serum (FBS) (Biological Industries), insulin-transferrin-selenium-sodium pyruvate (ITS-A) (Gibco), 100 μg/mL of streptomycin (MDBio, Inc.), 63.6 µg/mL (Sigma) of penicillin G, and 250 ng/mL of Fungizone (MDBio, Inc.).24
Cell Viability Assay
For the cell viability assay, 1×104 primary peritoneal MCs per well (200 μL) in M199/DMEM medium (Gibco) with 10% FBS were seeded in a 96-well plate and allowed to attach overnight in a 5% CO2 incubator at 37°C. Cells were starved for 24 hrs by replacing the medium with 1% FBS M199/serum-free Dulbecco’s modified eagle medium (DMEM), then treated with 200 μL of either free vitamin D or vit. D-NPs at different concentrations (L: 0.5, M: 1, H: 2 μM) and incubated for 24 hrs in 5% CO2 at 37°C. After 24 hrs, the medium from all wells was aspirated and 210 μL XTT (Biotium) reagents from mixing 25 μL activation reagents with 5 mL XTT solution to derive activated XTT solution in 10 mL serum-free M199/DMEM was added to each well. After incubation for 4 hrs in an incubator, the absorbance of the orange-colored product, 2,3-bis(2-methoxy-4-nitro- 5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide, was measured with a spectrophotometer at a wavelength of 450–500 nm, and the background absorbance at a wavelength between 630–690 nm.
Cellular Uptake Assay
To investigate the cellular uptake of nanoliposomes, human peritoneal mesothelial cell line (HMrSV5 cells) (ATCC) were treated with R6G-loaded nanoliposomes.
The Effect on the Epithelial–Mesenchymal Transition Process
To investigate the effects of vit. D-NPs on the MC EMT process, omentum-derived MCs were treated with 1 ng/mL human recombinant transforming growth factor-β1 (TGF-β1) (PeproTech) in culture medium to induce EMT of MC, as previous study.24,30 At the same time, we also treated omentum-derived MCs with 1α, 25(OH)2D3 only or vit. D-NPs. The epithelial marker E-cadherin (mouse anti-E-cadherin, diluted 1:500; BD Bioscience) and the mesenchymal marker Snai1 (rabbit anti-Snail; diluted 1:2000; Cell Signaling Technology Inc.) were identified to monitor the EMT process of MCs.31,32 Each of the primary antibody blots were incubated with Goat anti-Mouse IgG (H+L) Secondary Antibody and HRP conjugate alkaline phosphatase-conjugated secondary antibody (Pierce) at a dilution ratio of 1:5000, or Goat anti-Rabbit IgG (H+L) Antibody and HRP conjugate (KPL) at a dilution ratio of 1:5000 detected using enhanced chemiluminescence (ECL) (Millipore).
Nanoliposomes Conjugate with Glycoprotein M6A Antibody Observed Using Xenogen IVIS Spectrum Noninvasive Quantitative Molecular Imaging System
All in vivo mice experiments were approved by the Laboratory Animal Center of the National Cheng Kung University. Throughout the experiment, mice were handled according to the principles of animal care in experimentation “Guide for The Care and Use of Laboratory Animals” (NRC, USA 2011). The 4-6-week-old syngeneic C57BL/6 male mice were randomly separated into three groups. The diet of mice was standard diet and also free access to water. To assay the bio-distribution of nanoliposomes in mice, R6G-loaded nanoliposomes, which were conjugated with or without the GPM6A antibody (R6G-NPs/Ab-R6G-NPs), were injected into the abdominal cavity by intraperitoneal (i.p.) injection and the fluorescence of R6G was detected using an in vivo imaging system (IVIS). R6G-NPs and Ab-R6G-NPs were administered by i.p. injection once into the three groups of mice at a volume of 300 μL/mouse. The fluorescence of R6G was detected at a wavelength of 535–580 nm using an in vivo imaging system (IVIS) after 24, 48, and 72 hrs. The control group for the three mice was injected with 300 μL/mouse autoclaved PBS, which was served as the vehicle control. Mice were euthanized after 72 hrs. The parietal peritoneum and mesentery were soaked in PBS overnight away from light then investigated by IVIS to confirm the fluorescence.
Intraperitoneal Vitamin D Injection Model Experimental Protocol
The 4-6-week-old C57BL/6 male mice were separated randomly into four groups and were injected i.p. with autoclaved PBS (5 μL/g body weight (BW)), vitamin D, vit D-NPs, and Ab-vit. D-NPs (0.002 μg/g BW of vitamin D3) once daily for three consecutive weeks. Terminal blood samples (500~800 μL/mouse) were collected via cardiac puncture at the end of the three-week treatment.5,33,34 All animals were euthanized by cervical dislocation immediately after blood was collected. The aorta was then removed by dissection.
Statistical Analysis
All data were expressed as mean ± S.D. and statistical significance was analyzed with a one-way analysis of variance. A significant result was defined as P < 0.05.
Ethics Statement
This study was approved by the ethics committee/institutional review board of E-Da Hospital, and written informed consent was obtained from all patients (IRB number: EMRP18100N).
Results
Synthesis and Characterization of Vitamin D3 Nanoliposomes
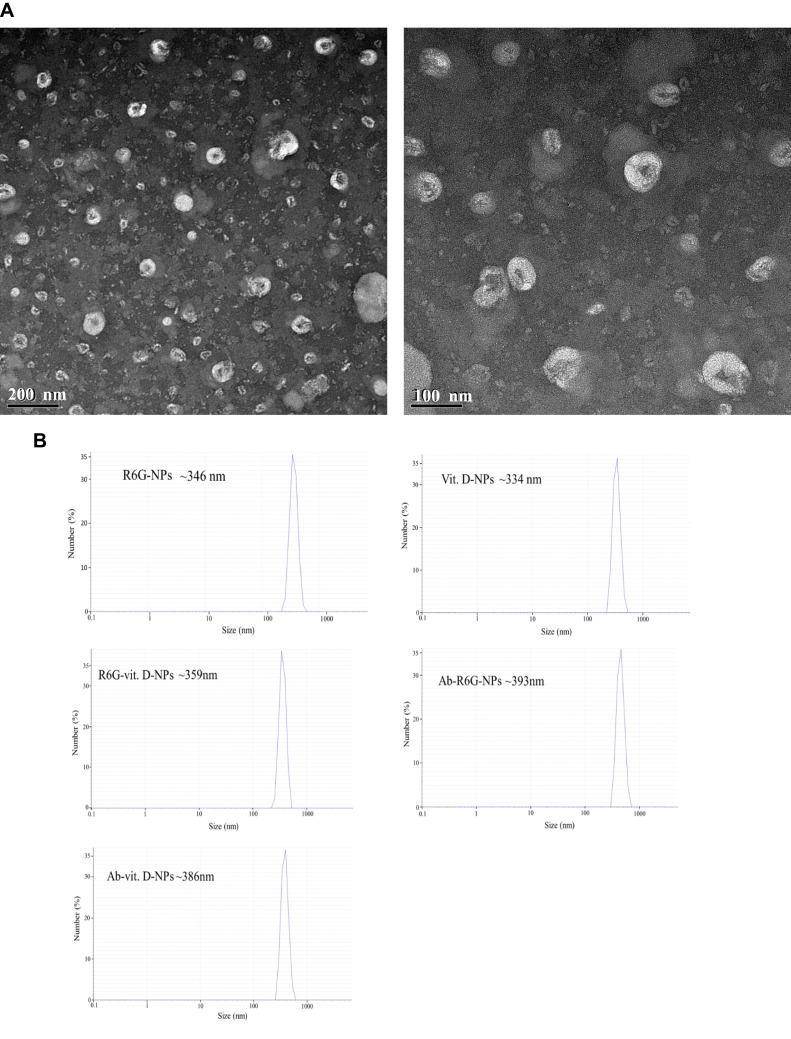
A series of material amounts of vitamin D were tested, and incubation temperatures and purified methods were analyzed to find the maximum quantity of encapsulated 1α,25(OH)2D3 in the nanoliposomes (Table 1). The most suitable incubation temperature to form nanoliposomes and develop stability and loading efficiency depended on the proportion of materials. Hence, we constructed nanoliposomes from vitamin D3 or R6G, fluorescence dye, and PC and DSPE-PEG in a 5:10:1 weight ratio using a solvent evaporation-lipid-film hydration-extrusion method (Supplementary Figure 1).35 The shape, size, and morphology of the vit. D-NPs were determined by TEM (Figure 1A). The hydrodynamic diameter of the vit. D-NPs was evaluated by DLS which is the primary technique for determining the hydrodynamic diameter of nanoliposomes in solution, which was found to be 250–300 nm (Figure 1B). From the DLS and TEM data it is clear that vit. D-NPs formed mono-dispersed, sub-200 nm spherical particles which are ideal for enhanced permeability and retention action.27
Table 1.
Construct Test Conditions of Vit D-NPs
| Vitamin D (mg) | Incubation Temperature (°C) | Loading Efficiency (%) | Purification |
|---|---|---|---|
| 0.5 | 37 | 0 | Centrifuge 60 mins |
| 1 | 37 | 25 | Centrifuge 60 mins |
| 0.5 | 60 | 16 | Centrifuge 60 mins |
| 1 | 60 | 10 | Centrifuge 60 mins |
| 1 | 60 | 4 | Centrifuge 35 mins |
| 1 | 60 | 27 | Centrifuge 60 min |
| 1 | 60 | 31 | Dialysis 3 times (2 hrs interval) |
| 1 | 60 | 32 | Dialysis 2 times (2 hrs interval) |
| 1 | 60 | 34 | Dialysis overnight |
Notes: A series of conditions were tested while constructing nanoparticles. The greatest efficiency was achieved by incubating nanoparticles at 60°C overnight using the dialysis method to purify. Loading efficiency (%) = 100 x (total drug–drug in supernatant)/(total drug).
Figure 1.
Characterization of vit. D-NPs and size distribution by transmission electron microscope (TEM) and differential light scattering (DLS). (A) TEM images show that vit. D–NPs are spherical in shape and sub-200 nm in size. (B) Hydrodynamic diameters of all liposome formulations were measured by DLS.
Release Kinetic Profile of Vitamin D from Nanoliposomes
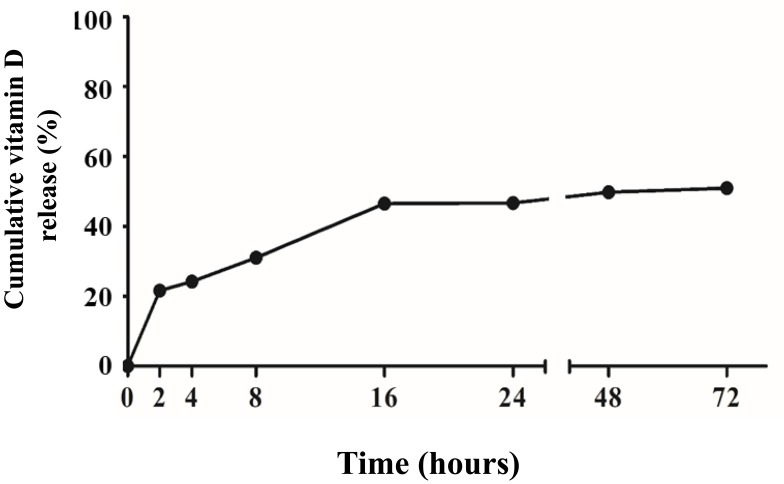
To assess the release profile of the encapsulated vitamin D, a dialysis method was used to detect the automatically diffused vit D-NPs at 37°C.35 The Vit D-NPs were incubated in a 500–1000 Daltons MWCO dialysis membrane to ensure that only free 1α, 25(OH)2D3 penetrated out of the dialysis tube. The kinetic release of vit D-NPs through nanoliposomes in the dialysis tube and sterile ultra-pure water in the outer chamber was evaluated. In our data, vit. D-NPs reveal a sustained release profile of over 72 hrs (Figure 2). These data show that the vitamin D3 released from nanoliposomes can follow both passive diffusion and slow release in a sustained manner.
Figure 2.
Release profile from vit. D-NPs over 72 hrs. From the release profile, the vit. D-NPs appear to release vitamin D in a slow and sustained manner.
In vitro Cytotoxicity and Cellular Uptake of Nanoliposomes
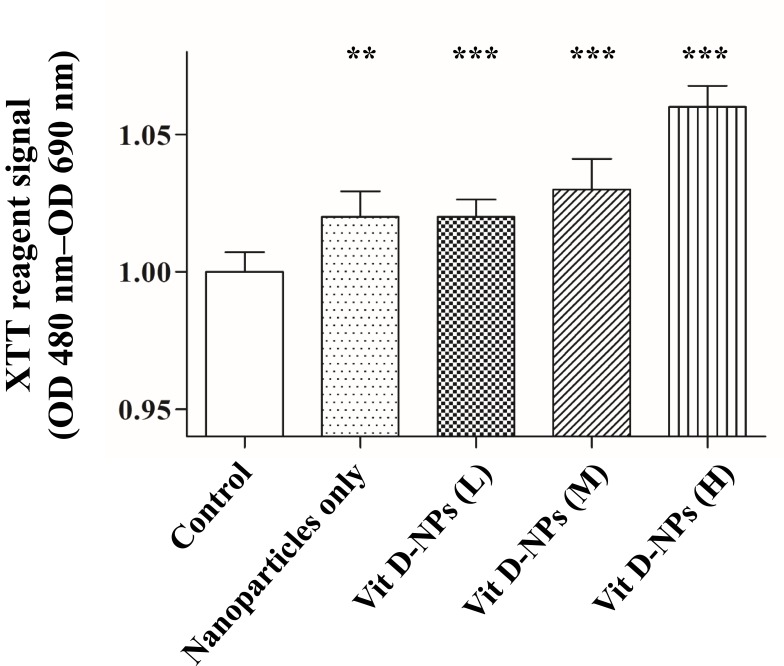
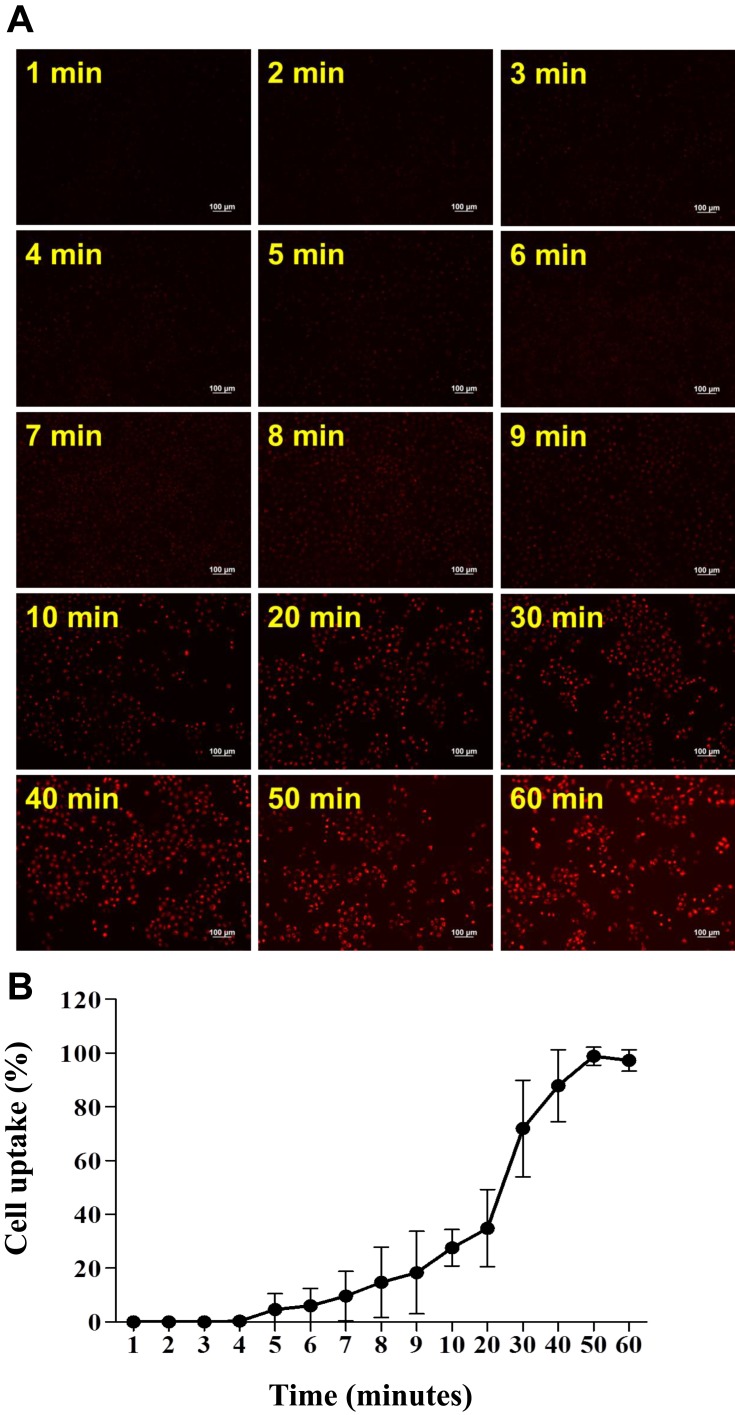
A cell viability assay was executed to determine the efficacy of vit. D-NPs in vitro. Cell viability was quantified at 24 hrs post-incubation by the XTT assay. It was expected that the in vitro study of vitamin D and the materials of nanoliposomes would be non-cytotoxic, whereas the vit D-NPs might induce cell proliferation in a time-dependent manner (Figure 3). NPs are uptaken into cells via endocytosis.36–38 To evaluate time dependency in the cellular uptake of nanoliposomes, vit. D-NPs were constructed which contain R6G (R6G-vit. D-NPs) which deliver fluorescence to display the interaction between the nanoliposomes and cells. As time progressed, the red fluorescence intensity enhanced as uptake increased in the cells (Figure 4). This result reaffirms that the materials used to construct nanoliposomes displayed non-toxicity and biocompatibility for cells and could be uptaken by cells over time.
Figure 3.
The cell viability assay of primary mesothelial cells treated with different concentrations of vit. D-NPs as analyzed via an XTT assay. Primary human peritoneal mesothelial cells were incubated with PBS, free vitamin D, or vit. D-NPs at different concentrations (L: 0.5, M: 1, H: 2μM). The XTT assay shows that the vitamin D nano-DDS were non-toxic to primary human peritoneal MCs. Data are represented as means ± SD (mean ± SD, n=6; **P<0.01, ***P<0.001, compared with the control group).
Figure 4.
Cellular uptake of R6G-vit D-NPs by mesothelial cells. (A) Cellular uptake study of R6G-vit D-NPs treated with mesothelial cells (HMrSV5). The treated cells were harvested at different time-points and cellular uptake was monitored by fluorescence microscopy (red fluorescence). (B) The percentage of mesothelial cells which uptake R6G-vit. D-NPs (mean ± SD, n=6).
Vit. D-NPs Can Debilitate the TGF-β1-Induced EMT in vitro
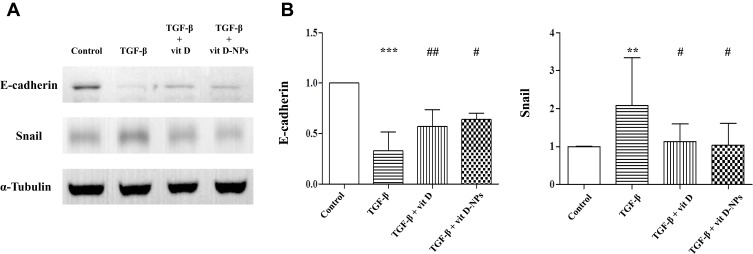
Primary human MCs were incubated with TGF-β1 in the presence or absence of vitamin D and vit. D-NPs to compare the therapeutic effects of vit. D-NPs with vitamin D. The Western blot analysis substantiates that vit. D-NPs have the same therapeutic effect as vitamin D. Vit D-NPs can significantly inhibit the TGF-β1-induced upregulation of α-SMA and the downregulation of E-cadherin (Figure 5).
Figure 5.
Vitamin D3-encapsulated nanoparticles inhibit the TGF-β1-induced epithelial-to-mesenchymal transition (EMT) of mesothelial cells (MCs) in vitro. (A) The Western blot analysis showed that 1α, 25(OH)2D3 (10−6 mol/L)-encapsulated nanoparticles had the same effect as the inhibited TGF-β1-induced EMT process of MCs. (B) Normalized data of protein levels of E-cadherin and Snail. (mean ± SD, n≥3; **P<0.01, ***P<0.001, compared with the control group; #P<0.05, ##P<0.01, compared with the TGF- β group).
Ab-R6G-NPs, Antibody Conjugated Nanoliposomes Allow Specific Tissue Fluorescent Imaging in vivo
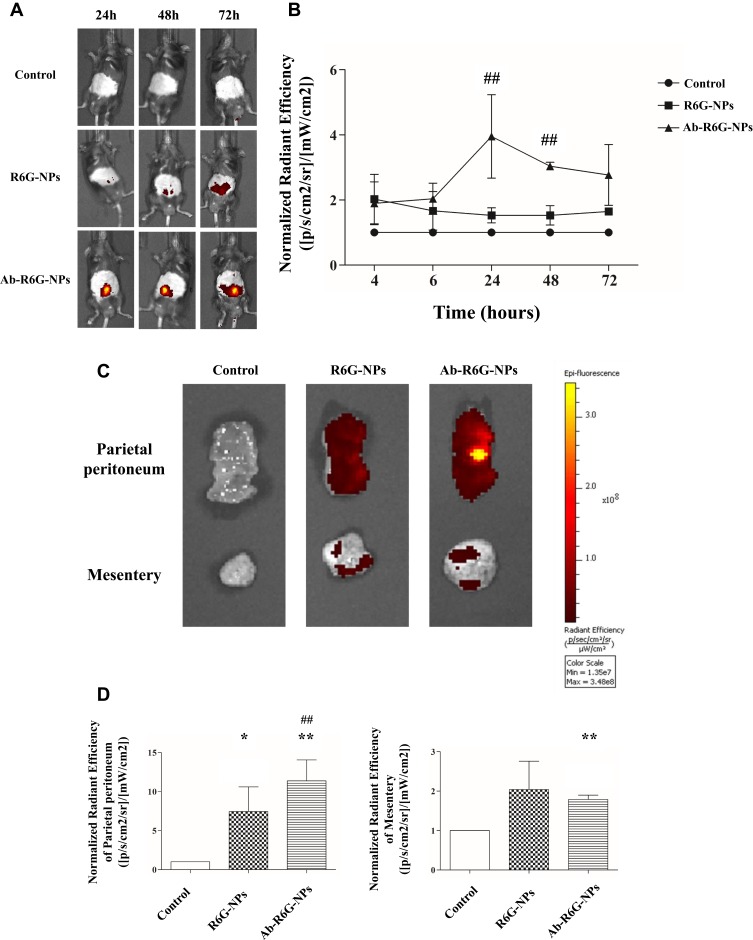
Mice were injected i.p. with nanoliposomes conjugated with or without a GPM6A antibody (300 μL, R6G, 0.1 mM/100 μL) (n=3 in each group). In the IVIS data, fluorescence was quantified within the specific abdominal cavity non-invasively at time points from 24 to 72 hrs after injection. Peritoneal fluorescence was observed in all mice but was seen significantly 72 hrs after injection in the Ab-R6G-NPs group, suggesting tissue-specificity (Figure 6).
Figure 6.
Ab-R6G-NPs displayed enhanced peritoneal targeting. C57BL/6 mice received intraperitoneal (i.p.) injection with nanoparticles conjugated with or without GPM6A antibody (R6G-NPs/Ab-R6G-NPs). Mice received i.p. instillation of PBS as a control group. Parietal peritoneum and mesentery samples were taken 72 hrs after the first i.p. injection. (A) In vivo imaging system (IVIS) show that R6G fluorescence was observed in all mice in the Ab-R6G-NPs group significantly after 72 hrs of injection. (B) Normalized radiant efficiency of R6G fluorescence of panel (A). (C) Parietal peritoneum and mesentery show R6G fluorescence after 72 hrs of injection. (D) Normalized radiant efficiency of R6G fluorescence of panel (C). (Data are represented as mean ± SD, n≥3; **P<0.01, compared with the control group; *P<0.05, **P<0.01, compared with the control group; ##P<0.01, compared with the R6G-NPs group).
Ab-Vit. D-NPs Can Ameliorate the Side Effects of Vitamin D
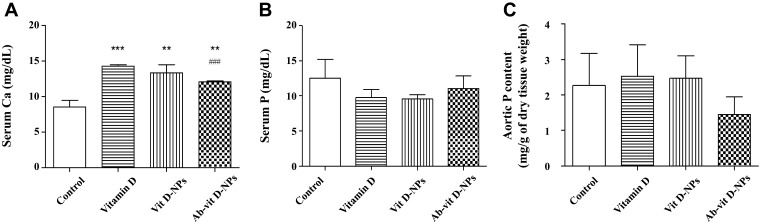
In Figure 7A, 1α, 25(OH)2D3 and vit. D-NPs induced significant hypercalcemia, but the Ab-vit. D-NPs group only displayed mild hypercalcemia. However, there is no significant difference in the serum phosphate content of the different groups (Figure 7B). Aortic calcium and phosphate deposition in the mice models were also evaluated. The values of aortic calcium were too low to be detected, and aortic phosphorus showed a trend though no significant difference of Ab-vit. D-NPs alleviating the aortic phosphate deposition (Figure 7C).
Figure 7.
Effect of vit. D-NPs and Ab-vit. D-NPs on serum calcium, phosphate, and aortic phosphate content. C57BL/6 mice received intraperitoneal (i.p.) injection daily of 1α, 25(OH)2D3, vit. D-NPs, and Ab-vit. D-NPs (0.002 μg/g BW of vitamin D3) (n=3 in each group). Mice received daily i.p. instillation of phosphate-buffered saline (PBS) in the control group. Blood samples and aorta tissue samples were taken after 21 days. (A) In the Ab-vit. D-NPs group, the hypercalcemia side effect was significantly lower than the vitamin D group. (B) No significant difference was found in the serum phosphate of the different groups. (C) The aortic phosphorus content showed a trend, though no significant difference of Ab-vit. D-NPs alleviating aortic phosphate deposition. (Data are represented as mean ± SD, n=3; **P<0.01, ***P<0.001, compared with the control group; ###P<0.001, compared with the vitamin D group).
Discussion
The anti-fibrotic effects of 1α, 25(OH)2D3 in PD-related peritoneal damage have been well reported both in vitro and in vivo.24 However, using 1α, 25(OH)2D3 to treat peritoneal damage requires supra-physiological doses which will induce some side effects such as hypercalcemia, hyperphosphatemia, and vascular calcification.24 This limits the clinical application of this type of therapy. In this study, it was found that Ab-vit. D-NPs are a potential therapy for future treatment. The results show that vit. D-NPs have the same effect in inhibiting the EMT process in vitro. In addition, the vit. D-NP was conjugated with an antibody against GPM6A, a peritoneal marker, to obtain Ab-vit. D-NPs, which possessed enhanced peritoneal targeting. Importantly, it can reduce the vitamin D side effects, including hypercalcemia in vivo.
DSPE-PEG nanoliposomes were manufactured as a promising delivery system to enhance the therapeutic potential of 1α, 25(OH)2D3. The liposome structure can encapsulate and carry compounds with poor water solubility to assemble in the layer of DSPE. The PEG shell decreases clearance via the reticuloendothelial system (RES).39,40 The DSPE-PEG-liposomal nanoliposomes were constructed which can package 1α, 25(OH)2D3 as a delivery system with a size of sub-200 nm. These nanoliposomes like stealth liposomes have a stable formulation, good biocompatibility, and good safety. Another important reason that we use liposome system to deliver vitamin D3 to treat disease is due to that liposome is a U.S. FDA-approved biomaterial for human body. Our goal is to extend our study to clinical applications in future, so the used materials are very important. Thus, using U.S. FDA-approved biomaterials is a good choice. Previously, several studies have investigated different delivery vehicles for 1α, 25(OH)2D3. Ramalho et al proved that Poly(lactic-co-glycolic acid) (PLGA) nanoliposomes as a platform for 1α, 25(OH)2D3 will enhance bioavailability by avoiding drug degradation before administration.41 Almouazen et al reported that, based on the chemical structure of 1α, 25(OH)2D3, polymeric NPs encapsulate vitamin D and have a moderate loading efficiency and rapid release profile compared to others.42 However, liposomes are able to deliver hydrophilic and hydrophobic drugs within the aqueous core and lipid bilayer membranes and have an advantage in the encapsulation of hydrophobic molecules. In addition, liposomes were the first nanomedicine to be clinically approved and marketed, and therefore are a large portion of the clinical stage nanomedicines.43 1α, 25(OH)2D3 exhibits antifibrotic activity only in supra-physiological doses associated with a high risk of hypercalcemia, hyperphosphatemia, and vascular calcification. These risks suggest that 1α, 25(OH)2D3 treatment should be focused on specific cell targeting and sustaining the effective concentration by encapsulating the nanoliposomes.
One interesting finding is that in cell viability assay, the results showed nanoliposomes alone induced in increased proliferation (Figure 3). Thereafter, future studies should be carried out to investigate the mechanism. But in our other study result, the data showed control NPs do not attenuate TGF-beta effects (Supplementary Figure 2).
Nonetheless, this study has some limitations. Firstly, the mean 1α, 25(OH)2D3 loading efficiency in nanoliposomes is only 25–30% which is not an economical method as a potential delivery system for clinical use. Secondly, although vit. D-NPs followed slow release in a sustained manner from the release profile, release was still too quick causing free vitamin D to enter systemic circulation. This result may be the reason that Ab-vit. D-NPs display a mild hypercalcemic activity. Therefore, future studies should be carried out to improve the loading efficiency and stability of the NPs, evaluate the alleviation of hypercalcemia, and further the anti-fibrotic activities of the Ab-vit. D-NPs in vivo.
Collectively, these findings show that Ab-vit. D-NPs deliver vitamin D to the peritoneum and can alleviate supra-physiological doses of vitamin D therapy-related side effects in mice. These results suggest that vitamin D nano-DDS could be a potential therapeutic option for PD-related peritoneal damage in the future.
Acknowledgments
We are indebted to Shin-Han Tseng for critical discussion and partial execution of the study. This study was supported by EDAHP-107063, EDAHP-108044 and NCKUEDA10709 from the Research Foundation of E-DA Hospital, and National Cheng Kung University, Taiwan.
Abbreviations
Ab-R6G-NPs, glycoprotein M6A antibody-rhodamine 6G nanoliposomes; Ab-vit. D-NPs, glycoprotein M6A antibody-vitamin D nanoliposomes; DCM, dichloromethane; DLS, dynamic light scattering; DMEM, Dulbecco’s modified eagle medium; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino-(polyethylene glycol)2000]; EMT, epithelial-to-mesenchymal transition; GDPs, glucose degradation products; GPM6A, glycoprotein M6A; HD, hemodialysis; HPLC, high-performance liquid chromatography; IVIS, in vivo imaging system; MCs, mesothelial cells; MWCO, molecular weight cut off; Nano-DDS, nano drug delivery system; NP, nanoliposomes; PD, peritoneal dialysis; PC,; L-α-phosphatidylcholine; PLGA, poly(lactic-co-glycolic acid); R6G, rhodamine 6G; R6G-vit D-NP, rhodamine 6G-vitamin D nanoliposomes; RES, reticuloendothelial system; Vit D-NP, vitamin D nanoliposomes.
Disclosure
Yi-Che Lee and Yuan-Yow Chiou contributed equally as corresponding authors to this work. Yi-Che Lee and Chih-Ting Huang contributed equally as first authors to this work. The authors declare that they have no conflicts of interest.
References
- 1.Fenton SS, Schaubel DE, Desmeules M, et al. Hemodialysis versus peritoneal dialysis: a comparison of adjusted mortality rates. Am J Kidney Dis. 1997;30:334–342. doi: 10.1016/S0272-6386(97)90276-6 [DOI] [PubMed] [Google Scholar]
- 2.Huang CC, Cheng KF, Wu HD. Survival analysis: comparing peritoneal dialysis and hemodialysis in Taiwan. Perit Dial Int. 2008;28(Suppl 3):S15–S20. [PubMed] [Google Scholar]
- 3.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med. 2011;171:110–118. doi: 10.1001/archinternmed.2010.352 [DOI] [PubMed] [Google Scholar]
- 4.Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006;S3–S11. doi: 10.1038/sj.ki.5001910 [DOI] [PubMed] [Google Scholar]
- 5.Han SH, Lee SC, Ahn SV, et al. Improving outcome of CAPD: twenty-five years’ experience in a single Korean center. Perit Dial Int. 2007;27:432–440. [PubMed] [Google Scholar]
- 6.Kawaguchi Y, Hasegawa T, Nakayama M, Kubo H, Shigematu T. Issues affecting the longevity of the continuous peritoneal dialysis therapy. Kidney Int Suppl. 1997;62:S105–S107. [PubMed] [Google Scholar]
- 7.Lee YC, Hung SY, Wang HH, et al. Different risk of common gastrointestinal disease between groups undergoing hemodialysis or peritoneal dialysis or with non-end stage renal disease: a nationwide population-based cohort study. Medicine (Baltimore). 2015;94:e1482. doi: 10.1097/MD.0000000000001482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamoto H, Kawaguchi Y, Suzuki H. Is technique survival on peritoneal dialysis better in Japan? Perit Dial Int. 2006;26:136–143. [PubMed] [Google Scholar]
- 9.Schaefer F, Klaus G, Muller-Wiefel DE, Mehls O. Current practice of peritoneal dialysis in children: results of a longitudinal survey. Mid European Pediatric Peritoneal Dialysis Study Group (MEPPS). Perit Dial Int. 1999;19(Suppl 2):S445–S449. [PubMed] [Google Scholar]
- 10.Woodrow G, Turney JH, Brownjohn AM. Technique failure in peritoneal dialysis and its impact on patient survival. Perit Dial Int. 1997;17:360–364. [PubMed] [Google Scholar]
- 11.Andreoli SP, Langefeld CD, Stadler S, Smith P, Sears A, West K. Risks of peritoneal membrane failure in children undergoing long-term peritoneal dialysis. Pediatr Nephrol. 1993;7:543–547. doi: 10.1007/BF00852541 [DOI] [PubMed] [Google Scholar]
- 12.Davies SJ, Phillips L, Griffiths AM, Russell LH, Naish PF, Russell GI. What really happens to people on long-term peritoneal dialysis? Kidney Int. 1998;54:2207–2217. doi: 10.1046/j.1523-1755.1998.00180.x [DOI] [PubMed] [Google Scholar]
- 13.Davies SJ, Phillips L, Naish PF, Russell GI. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol. 2001;12:1046–1051. [DOI] [PubMed] [Google Scholar]
- 14.Krediet RT. The peritoneal membrane in chronic peritoneal dialysis. Kidney Int. 1999;55:341–356. doi: 10.1046/j.1523-1755.1999.00264.x [DOI] [PubMed] [Google Scholar]
- 15.Lee YC, Tsai YS, Hung SY, et al. Shorter daily dwelling time in peritoneal dialysis attenuates the epithelial-to-mesenchymal transition of mesothelial cells. BMC Nephrol. 2014;15:35. doi: 10.1186/1471-2369-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanez-Mo M, Lara-Pezzi E, Selgas R, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348:403–413. doi: 10.1056/NEJMoa020809 [DOI] [PubMed] [Google Scholar]
- 17.Yang AH, Chen JY, Lin YP, Huang TP, Wu CW. Peritoneal dialysis solution induces apoptosis of mesothelial cells. Kidney Int. 1997;51:1280–1288. doi: 10.1038/ki.1997.175 [DOI] [PubMed] [Google Scholar]
- 18.Zheng Z, Ye R, Yu X, Bergstrom J, Lindholm B. Peritoneal dialysis solutions disturb the balance of apoptosis and proliferation of peritoneal cells in chronic dialysis model. Adv Perit Dial. 2001;17:53–57. [PubMed] [Google Scholar]
- 19.Aguilera A, Yanez-Mo M, Selgas R, Sanchez-Madrid F, Lopez-Cabrera M. Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr Opin Investig Drugs. 2005;6:262–268. [PubMed] [Google Scholar]
- 20.Selgas R, Bajo MA, Aguilera A, et al. Epithelial-mesenchymal transition in fibrosing processes. Mesothelial cells obtained ex vivo from patients treated with peritoneal dialysis as transdifferentiation model. Nefrologia. 2004;24:34–39. [PubMed] [Google Scholar]
- 21.Gonzalez-Mateo GT, Fernandez-Millara V, Bellon T, et al. Paricalcitol reduces peritoneal fibrosis in mice through the activation of regulatory T cells and reduction in IL-17 production. PLoS One. 2014;9:e108477. doi: 10.1371/journal.pone.0108477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirose M, Nishino T, Obata Y, et al. 22-Oxacalcitriol prevents progression of peritoneal fibrosis in a mouse model. Perit Dial Int. 2013;33:132–142. doi: 10.3747/pdi.2011.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CJ, Subeq YM, Lee RP, Liou HH, Hsu BG. Calcitriol decreases TGF-beta1 and angiotensin II production and protects against chlorhexide digluconate-induced liver peritoneal fibrosis in rats. Cytokine. 2014;65:105–118. doi: 10.1016/j.cyto.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 24.Lee YC, Hung SY, Liou HH, et al. Vitamin D can ameliorate chlorhexidine gluconate-induced peritoneal fibrosis and functional deterioration through the inhibition of epithelial-to-mesenchymal transition of mesothelial cells. Biomed Res Int. 2015;2015:595030. doi: 10.1155/2015/595030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nevozhay D, Kanska U, Budzynska R, Boratynski J. [Current status of research on conjugates and related drug delivery systems in the treatment of cancer and other diseases]. Postepy Hig Med Dosw. 2007;61:350–360. [PubMed] [Google Scholar]
- 26.Palvai S, Nagraj J, Mapara N, Chowdhury R, Basu S. Dual drug loaded vitamin D3 nanoparticle to target drug resistance in cancer. RSC Adv. 2014;4:57271–57281. doi: 10.1039/C4RA06475E [DOI] [Google Scholar]
- 27.Patil S, Gawali S, Patil S, Basu S. Synthesis, characterization and in vitro evaluation of novel vitamin D3 nanoparticles as a versatile platform for drug delivery in cancer therapy. J Mater Chem B. 2013;1:5742. doi: 10.1039/c3tb21176b [DOI] [PubMed] [Google Scholar]
- 28.Rongjie FU, Jianzhong LI, Wang Y. Fat-soluble vitamins analysis on an agilent ZORBAX eclipse PAH polymeric C18 bonded column. Agilent Technol. 2010;5990–5342EN. [Google Scholar]
- 29.Diaz C, Selgas R, Castro MA, Bajo MA, Fernandez de Castro M, Molina S, Jimenez C, Ortiz A, Vara F. Ex vivo proliferation of mesothelial cells directly obtained from peritoneal effluent: its relationship with peritoneal antecedents and functional parameters. Adv Perit Dial. 1998;14:19–24. [PubMed] [Google Scholar]
- 30.Loureiro J, Schilte M, Aguilera A, et al. BMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposure. Nephrol Dial Transplant. 2010;25:1098–1108. doi: 10.1093/ndt/gfp618 [DOI] [PubMed] [Google Scholar]
- 31.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025 [DOI] [PubMed] [Google Scholar]
- 32.Li C, Ren Y, Jia X, et al. Twist overexpression promoted epithelial-to-mesenchymal transition of human peritoneal mesothelial cells under high glucose. Nephrol Dial Transplant. 2012;27:4119–4124. doi: 10.1093/ndt/gfs049 [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Slominski A, Tuckey RC, et al. 20-hydroxyvitamin D(3) inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–746. [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu F, Li T, Qiu F, et al. Preventive effect of Notch signaling inhibition by a gamma-secretase inhibitor on peritoneal dialysis fluid-induced peritoneal fibrosis in rats. Am J Pathol. 2010;176:650–659. doi: 10.2353/ajpath.2010.090447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sengupta P, Basu S, Soni S, et al. Cholesterol-tethered platinum II-based supramolecular nanoparticle increases antitumor efficacy and reduces nephrotoxicity. Proc Natl Acad Sci U S A. 2012;109:11294–11299. doi: 10.1073/pnas.1203129109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614 [DOI] [PubMed] [Google Scholar]
- 37.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591 [DOI] [PubMed] [Google Scholar]
- 38.Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J Control Release. 2014;190:485–499. doi: 10.1016/j.jconrel.2014.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033 [DOI] [PubMed] [Google Scholar]
- 40.Wang R, Xiao R, Zeng Z, Xu L, Wang J. Application of poly(ethylene glycol)-distearoylphosphatidylethanolamine (PEG-DSPE) block copolymers and their derivatives as nanomaterials in drug delivery. Int J Nanomedicine. 2012;7:4185–4198. doi: 10.2147/IJN.S34489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramalho MJ, Loureiro JA, Gomes B, Frasco MF, Coelho MA, Pereira MC. PLGA nanoparticles as a platform for vitamin D-based cancer therapy. Beilstein J Nanotechnol. 2015;6:1306–1318. doi: 10.3762/bjnano.6.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almouazen E, Bourgeois S, Jordheim LP, Fessi H, Briancon S. Nano-encapsulation of vitamin D3 active metabolites for application in chemotherapy: formulation study and in vitro evaluation. Pharm Res. 2013;30:1137–1146. doi: 10.1007/s11095-012-0949-4 [DOI] [PubMed] [Google Scholar]
- 43.Kamaly N, He JC, Ausiello DA, Farokhzad OC. Nanomedicines for renal disease: current status and future applications. Nat Rev Nephrol. 2016;12:738–753. doi: 10.1038/nrneph.2016.156 [DOI] [PMC free article] [PubMed] [Google Scholar]