Abstract
Filtration of high-energy short-wave visible light (blue light) to improve vision and protect against damage has evolved both in aquatic animals and terrestrial species. In humans, pigments in the inner layer of the macula absorb wavelengths between 400 and 520 nm and function to improve visual performance. In patients who undergo cataract surgery, replacing cataractous lenses with artificial intraocular lenses (IOLs) that do not mimic normal healthy adult lenses could result in preventable negative visual effects, including glare disability. Blue light–filtering (BLF) IOLs were designed to filter short-wave light in addition to ultraviolet light and mimic the natural crystalline lens. Current studies indicate that BLF IOLs may provide protection from blue light–induced retinal damage and slow the development and progression of age-related macular degeneration. Additionally, BLF IOLs have been shown to improve chromatic contrast, reduce photostress recovery time, reduce glare disability and discomfort, and generally improve visual performance under glare conditions. Although a number of concerns have been raised about the relative risks versus the benefits of BLF IOLs, recent studies reported no adverse effects on visual function or contrast under photopic conditions, no long-term effects on color vision, and no detrimental effects on circadian rhythms with BLF IOLs. Based on the current understanding of the field, evidence suggests that BLF IOLs would be returning the eye to a more natural state compared with non-BLF lenses.
Keywords: blue light filtration, intraocular lens, cataract surgery
Introduction
High-energy, short-wave visible light (400–500 nm) is often referred to as “blue light” based on our perceptual response to that segment of the spectrum. It has been addressed extensively in the scientific literature because of its disproportionate effects on human visual function, as well as actinic effects on ocular tissues.1,2 Humans are not the only species that appear to be strongly influenced by blue light. There are aquatic animals and terrestrial species, such as prairie dogs and squirrels, that have evolved yellow intraocular lenses that act as blue light filters, as described by Walls and Judd and reviewed by Hammond.3,4 These filters are adaptations that developed in ecological niches where visual function could be impaired owing to bright sunlight and where damage could occur as a result of continuous exposure to highly actinic short-wave light.4
Humans have evolved specific mechanisms for producing intraocular blue light filters. The macular pigments (meso-zeaxanthin and dietary lutein and zeaxanthin) are naturally occurring intraocular filters found in the inner layers of the macula that absorb wavelengths between ~400 and 520 nm, with an absorption peak at 460 nm.5–7 These pigments can be found in very high proportion (over a log unit optical density) in and around the fovea (central 10°–12°), effectively screening central cones and rods of the retina.8 Empirical studies have found that this screening yields numerous beneficial effects to vision. For example, there are multiple double-blinded randomized controlled trials (reviewed by Mares) showing that supplementing lutein and zeaxanthin leads to improved visual function under glare conditions when compared with placebo.9
The greatest source of both high-energy ultraviolet (UV) radiation and blue light is natural sunlight; artificial sources of UV radiation include welding and UV lamps, whereas artificial sources of blue light include electronic devices (eg, computers) and some indoor lights.2,10 Blue light–filtering (BLF) intraocular lenses (IOL) were designed to filter short-wave light and, like many IOLs, to selectively reduce the transmission of UV radiation. Most of UV radiation is blocked by the native lens (UV-B, 280–315 nm and UV-A, 315–400 nm) or absorbed by the cornea (<295 nm).2 However, recent data indicate that clinically significant amounts of UV-B radiation reach the retina of individuals until about the age of 30 years;11 IOLs without significant UV absorbance could quickly lead to significant retinal damage.12 BLF IOLs were different than typical UV-filtering IOLs because of the addition of short-wave light filtering to their absorbance profile. Therefore, the resulting light transmission of a BLF lens resembled that of a healthy natural crystalline lens.13–15 The basic premise of the BLF lens was that replacing an opacified natural lens with a non-BLF UV-filtering IOL (which would have been more similar to the lens of a human infant than a healthy older adult) created an unnatural condition. The large increase in blue light transmission with a non-BLF UV-filtering IOL in an older eye (eg, an eye with short-wave photosensitizers like the lipofuscin fluorophore diretinoid–pyridinium–ethanolamine [A2E], a byproduct of light-induced release of all-trans-retinal16) may place older patients at unnecessary risk. The basic hypothesis behind the BLF lens was that returning the eye to a more natural state would represent better clinical practice.
This approach was supported by evidence from several categories of empirical data. First, a variety of data ranging from animal to human and laboratory to clinic showed retinal risk from the so-called “blue light hazard.”2 The second category of data was derived from the effects of blue light on visual function. The objective of this paper is to review the literature on BLF lenses, focusing on three key areas: the protection/oxidative stress hypothesis; the glare hypothesis, and the visibility hypothesis and to explore empirical data on visual function in more depth.
Oxidative Photodegradation of Ocular Tissues
There is a scientific consensus that excessive light exposure can damage surface tissues, such as the eye and skin. Ocular damage mediated by light is the primary etiologic factor in a number of eye diseases, such as pinguecula/pterygium and photokeratitis (for an extensive review of ocular phototoxicity and its prevention, see Hammond et al1). Damage initiated by excessive light exposure is also implicated in numerous other ocular conditions, such as age-related cataract and macular degeneration (AMD).17 Blue light appears to be especially damaging to the retina owing to the existence of photosensitizers in that wave-band (eg, A2E); hence, its ability to cause photochemical (not just thermal) damage. In healthy adults, visible light passes through the crystalline lens and macular pigments of the inner retina, both of which have the capability to absorb blue light.1 The crystalline lens accumulates oxidative damage with age, causing it to become yellow and absorb higher amounts of UV and visible short-wave light, thus providing increased protection from blue light.18 In an in vitro study, blue light has been linked to oxidative damage mediated by photoexcitation of the visual chromophore retinal.19 Another in vitro study reported that A2E-laden retinal pigment epithelial cells had significantly less damage when exposed to light through a BLF lens compared with a UV-blocking lens.20 Additionally, Rezai et al showed that BLF IOL significantly reduced blue light-induced apoptosis in retinal pigment epithelial cells compared with UV-only IOL.21 An in vitro study by Kernt et al provided additional evidence that BLF IOLs may help reduce apoptotic cell death from extensive light exposure.22 Because of the common use of blue light in workspaces (eg, blue argon lasers), light safety standards are often aimed at reducing exposure to this region of the visible spectrum, recognized as the “blue light hazard.”23
If blue light does pose a special risk to the retina, then it follows that filtering such light would have a protective effect, analogous to sunscreen protecting the skin. For example, it is the standard of care to use eye protection on babies with jaundice when blue or white light is used to break up the dermal accumulation of bilirubin.24
There is good evidence from both animal and cell culture studies that a BLF IOL can protect against damage from short-wave light.25 For example, when exposing human uveal melanoma cell lines to blue light stress, BLF IOLs reduced cellular proliferation rates compared with UV-only absorbing lenses.26 Additionally, two small studies examined the influence of BLF IOLs on the development and progression of AMD. One study measured changes in fundus autofluorescence, which can predict the development of AMD, after implantation of BLF and UV-filtering IOLs.27 Two years after implantation, the incidence of AMD was significantly higher in the UV-filtering IOL group (11%; n=79) compared with the BLF IOL group (2%; n=52; P=0.042), suggesting that BLF IOLs may help prevent AMD at this late stage.27 Another study evaluated the effect of BLF IOLs on disease progression in 66 eyes with geographic atrophy and reported a slower progression of the disease in patients with BLF IOLs versus non-BLF IOLs.14
Although BLF crystalline lenses in nature may reduce degenerative changes in the retina, it is unlikely they evolved to serve that purpose. Similar to other naturally selected traits, mechanisms for accumulating BLF filters likely evolved based on some reproductive pressure (hence, earlier in life). This implies that the filters were influencing some aspect of function that affected fecundity. Work on the human macular pigments, for instance, has looked at various likely candidates, many of them connected to improving visual function under bright light conditions.4
Glare Disability and Discomfort Hypothesis
Glare generally refers to the loss of visual performance that accompanies exposure to light that is in excess of an individual’s adaptive state, as originally described by Holladay in 1926 and Stiles in 1929.28,29 Glare is generally separated into two broad categories. The first is glare disability (GD), which focuses specifically on the loss of visual function arising mostly from intraocular scattered light (this scatter originates from inhomogeneities in the anterior media, primarily the lens). Reflection glare is a specialized case of GD where the loss of visual performance is specific to bright light reflected from surfaces such as windshields, monitors, or water. The second category is glare discomfort (GDC), which refers to the slight pain or discomfort from exposure to bright light.
Glare disability is assessed using intense light sources often based on simulated sunlight in a laboratory or clinical setting. For instance, contrast sensitivity can be measured under glare conditions with clinical devices (eg, the CVS1000HGT chart-based test [VectorVision, Greenville, OH] that can produce reliable measurements of the contrast sensitivity function).30,31 Alternatively, contrast sensitivity can be measured using a 1-degree grating target surrounded by an annular source of broadband white light,32 where the intensity of the annulus is increased until the glare disability threshold is reached (in this case, glare disability threshold is defined as the magnitude of glare that causes the central grating to be occluded owing to light scattering in the media of the eye). By changing the spatial frequency of the central grating, it is possible to evaluate the entire contrast sensitivity function under glare conditions. These two main approaches differ in how they manipulate the glare source. The former measures contrast sensitivity function with a light source of constant intensity (eg, two halogen lights that bracket a series of gratings or variable spatial frequencies reducing in contrast). The latter approach typically uses a single grating (one point on the contrast sensitivity function) with an adjustable light source: the glare source is increased in a continuous manner until light scatter in the eye is sufficient to veil the grating. An important variable with either technique is the light spectrum used for the glare source. For example, measuring glare disability using a light source that matches the broadband spectrum of the sun can provide results that better predict visual function outdoors.33
Glare discomfort is, by definition, subjective, and it does not always accompany the loss of visual function. Methods for measuring GDC are aimed at quantifying aversion. These include questionnaire assessments, such as the De Boer scale; squinting (assessed by measuring the diameter of the palpebral fissure or the reaction of the orbicularis muscle); as well as head aversion and blinking rates.34–37
Many studies on glare disability and discomfort use centrally fixated stimuli assessing light scatter that obscures foveal targets. However, light entering the anterior segment can also spread well outside the macula in what is often described as halos and starbursts (Figure 1). Because of this, filtering by macular pigment would not influence this type of glare. Clinically, this is referred to as positive dysphotopsia38 and tends to affect patients who have undergone surgical procedures that affect the anterior ocular media, such as corneal ablations and implantations of IOLs after cataract surgery. Furthermore, the contact between the implant and capsule is a critical area where scatter can originate, depending on the smoothness of the junction.
Figure 1.
Illustration of visual disturbances: halos and spokes.
Humans are visually sensitive to a relatively small portion of the overall electromagnetic spectrum. Sensitivity peaks in the center (around 570 nm) and trails off at the shorter and longer ends of the range (the spectral sensitivity curve). Even at equal energy (wavelength is inversely related to photon energy), visible light is not equally damaging. Ham et al were among the first to show in 1976 that the potential for retinal damage is inversely and monotonically related to wavelength: shorter wavelengths of light are more damaging than longer wavelengths, even at equal energy.39 Stringham et al tested whether discomfort, quantified by the squint response, followed the same pattern. The squint response was elicited by exposure to equiluminant stimuli presented every 10 nm from 440 to 640 nm. The study reported that the GDC function closely correlated to the threshold-energy retinal damage reported in direct-light damage studies by Ham et al.34,39
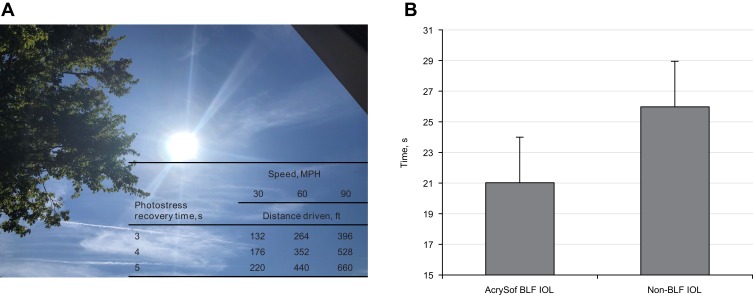
Blue light–filtering IOLs have been shown to improve visual performance in the presence of glare by reducing GD and photostress recovery time (the recovery of normal visual function after temporary blindness induced by exposure to a very bright light source, which plays a critical role during driving; Figure 2A). In a study in which experimenters were blinded to treatment design, patients with the BLF lenses (n=17) had shorter photostress recovery times and significantly reduced GD (P<0.02) compared with patients who received conventional UV-filtering IOLs (n=20).40 These findings were confirmed in a later study using a contralateral (within-subject) design. In a study with 52 patients who elected to have a BLF IOL implanted in one eye and a conventional UV-filtering IOL in the other eye, both GD and photostress recovery (Figure 2B) were significantly improved in the eye with the BLF IOL (P=0.04 and P=0.02, respectively).41 Another study assessed the effects of BLF achieved by clip-on glasses with characteristics similar to BLF IOLs. Patients wearing clip-on BLF glasses had a significantly higher GD threshold (P=0.00014) and a significantly lower photostress recovery time (P=0.0001) compared with patients wearing clear glasses.42
Figure 2.
Photostress recovery times in patients experiencing glare while driving (A) and contralateral comparison of photostress recovery times in patients (n=50) with AcrySof® (Alcon Vision LLC, Fort Worth, TX) BLF IOL and non-BLF IOL. (B) Data from Panel B was derived from Hammond et al.41
Abbreviations: BLF, blue light–filtering; IOL, intraocular lens.
Even small increments of time gained by drivers may improve driving performance and safety, as has been suggested by the addition of the center high mounted stop lamps (CHMSL) to cars and trucks. Drivers following cars and trucks with CHMSL had reaction times that were 0.11 and 0.09 seconds shorter, respectively, than drivers following cars and trucks without CHMSL. Addition of CHMSL was associated with a 5% reduction in rear-impact crashes between 1986 and 1991.43
To address the effects of BLF IOLs on real-world tasks, a cross-sectional prospective study evaluated the performance of 33 patients (18 with BLF IOLs and 15 with UV-filtering IOLs) in a driving simulator. Patients with BLF IOLs had a significantly lower glare susceptibility and greater safety margins than those with UV-filtering IOLs,44 suggesting that BLF benefits different functional aspects of vision, including static detection and identification in the presence of veiling glare, as well as dynamic motion-in-depth effects in the presence of glare. On the other hand, patients without BLF IOLs overestimated the time to collision of the approaching cars because of the glare-induced reduction in contrast of the incrementally expanding retinal image (ie, motion-in-depth signal).45
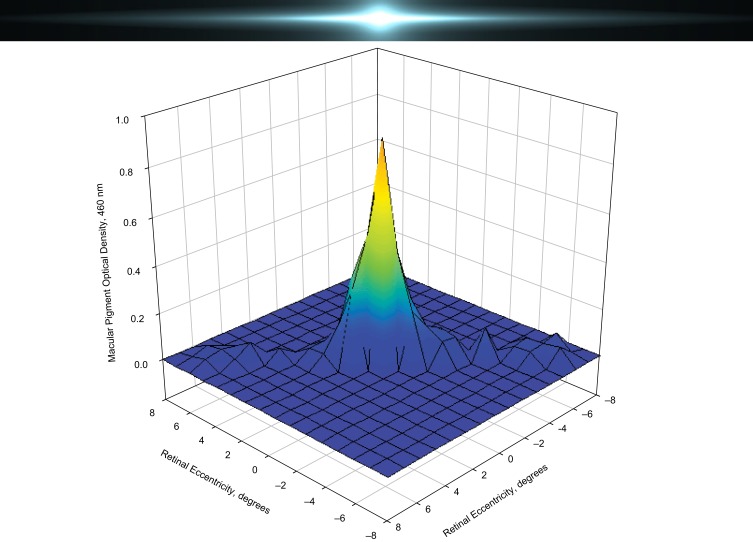
Although macular pigment affects GD and photostress recovery time, it does not influence other aspects of glare; specifically, macular pigment does not offer protection from peripheral glare (Figure 3). Filtering of the light by macular pigment is confined to approximately the central 5 degrees of the macula, whereas light from glare can spread past the fovea and parafovea (ie, positive dysphotopsia), which often follows cataract surgery or laser correction for myopia.38 Therefore, for filtering to be effective at reducing this type of peripheral glare, the source of the filter should be anterior to the retina and screen the entire retina, which can be achieved by colored spectacles, contact lenses, or IOLs, but not macular pigment.46 Future studies will need to address the effects of BLF IOLs on visual disturbances such as halos and spokes. Based on currently available studies, it is not clear whether BLF has any effect on the incidence of posterior capsule opacification, a common side effect in patients after cataract surgery.
Figure 3.
Macular pigment absorbance distribution. Macular pigment affects central but not peripheral glare.8
Visibility Hypothesis
Blue haze that formed over fields and forests was initially described by Went, who largely attributed it to light scatter arising from plant emissions.47 Haze particles tend to be bigger than air molecules but smaller than fog droplets causing haze to appear gray or bluish (the Tyndall effect).47 Ferman et al noted that sulfate aerosols were the most significant particles reducing visibility in the Shenandoah Valley/Blue Ridge mountain area.48 Although haze is most common over heavily forested areas because of monoterpenes from plants reacting with atmospheric oxidants to form aerosols, haze arising from human activity (ie, emission from power plants) is becoming increasingly common, mostly because of the emission of sulfur dioxide.49 For example, Wu et al quantified the number of haze events in the Beijing region of China between 1980 and 2013 and noted an increase in event frequency. Based on their criteria, it was determined that 49 haze events occurred during that period, each event lasting at least 3 days and significantly limiting visibility: 7 of these events occurred in the 1980s (23 days), 9 events occurred in the 1990s (33 days), 17 events occurred from 2000 to 2009 (70 days), and 16 events occurred from 2010 to 2013 (77 days spent in haze).50
Visibility generally refers to how far we can see (ie, the range) and how well we can see (ie, discrimination of targets) through the earth’s atmosphere, and it has long been argued that haze would limit visibility.51 The specifics of visual range depend on many factors within a given scene, such as target size and reflectance, illumination conditions, state of the observer, angle of regard, and the size and distribution of scattering particles. Age-related increases in crystalline lens transmittance can result in decreased transmission of shorter wavelengths (about 420),52 suggesting that variation in the density of the crystalline lens could contribute to individual differences in how far a given individual can see. Wooten and Hammond were the first to consider such factors and to carefully model how the macular pigments might increase visual range.53 They argued that, all things being equal, macular pigment would increase visual range as a linear function of the amount.
This modeling was empirically tested by measuring contrast thresholds under blue haze conditions, created using optical filtering in conjunction with xenon broadband light and by simulating changes in macular pigment using a variable path-length xanthophyllic filter that matched the absorbance characteristics of macular pigment. The results of the study demonstrated that an artificial macular pigment simulated by variable path-length filter improved a given subject’s contrast thresholds by an average of 25%.54 A second study measured macular pigment density directly in a sample of young healthy individuals with a range of macular pigment densities. The study reported that visual range measured under blue haze conditions was strongly correlated with macular pigment density.55 The effects of BLF IOLs on visual range have not been assessed.
Blue Light Filtration and Chromatic Contrast
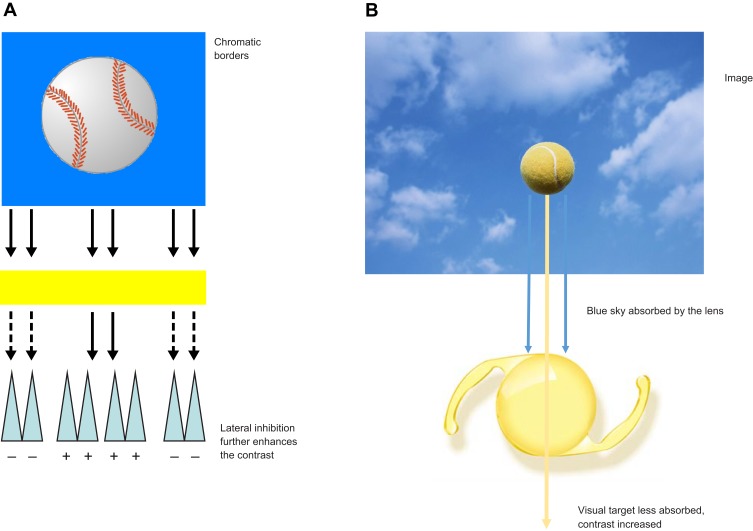
Walls and Judd originally argued that blue light filters enhance chromatic contrast.3 Enhancing contrast is an important aspect of spatial vision, particularly as it applies to defining the edges of objects in the real world. Edges drive vision (as previously discussed by Hammond in a 2012 review, the retina has been described as a “contrast engine”4) and the visual system is organized to accentuate edges (eg, lateral inhibition in receptive fields). Edges define object boundaries and are necessary to segment, register, and ultimately identify objects in a scene. Retinex algorithms (a major theory of color vision), for example, emphasize the importance of color borders.56 Simple cells within the cortex are maximally sensitive to edges of a given orientation, and lateral inhibition within the retina accentuates discontinuities within our visual field. Anything that accentuates edges would be expected to improve spatial vision and the detection of objects against a background. Luminance differences constitute one way an edge can be defined.4 However, in the real world, things are rarely achromatic. Consequently, other differences, such as wavelength composition, are used to define edges. This is the reason colored filters can make objects appear more “crisp.” Yellow filters, for instance, will make a yellow target with a blue surround more visible by selectively reducing the surround relative to the center (Figure 4A and B). This simple optical effect enhances the contrast between a mid- or long-wave target and a background with short-wave energy. Both Luria and Wolffsohn et al demonstrated that the visibility of stimuli like these is improved when viewed through yellow lenses.57,58
Figure 4.
Effects of blue light filtration on chromatic contrast. Colored filters can create luminance edges (A) and enhance chromatic contrast (B).
Renzi and Hammond originally showed that the human macular pigments improved contrast when absorbing a short-wave background more strongly than a mid-wave target: supplementing lutein and zeaxanthin, which increases macular pigment density, has been shown to also improve chromatic contrast relative to placebo.59,60 Not surprisingly, BLF IOLs improve chromatic contrast by selective reduction of blue backgrounds.40,41
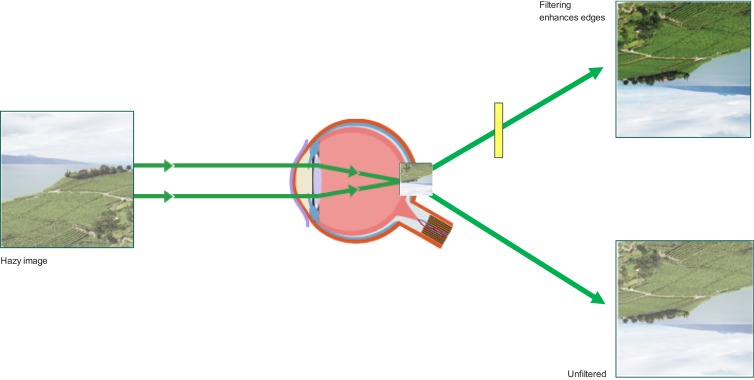
Although the stimuli in such experiments are highly specific (ie, yellow targets on a blue background) and do not appear to be applicable to many examples of everyday vision, this simple stimulus arrangement is, in fact, highly generalizable. Rayleigh scatter makes blue backgrounds quite common; additionally, objects in our line of sight are rarely short-wave dominant. The edge-enhancing properties of BLF are illustrated in Figure 5.
Figure 5.
Blue light filtration can enhance visibility under hazy conditions.
Note that this discussion focuses on chromatic stimuli. Contrast sensitivity in the clinic is most often measured using achromatic gratings. Concerns have been raised about the relative risks and benefits of BLF IOL implantation in patients following cataract surgery based on contrast sensitivity function measured using these methods.61 Multiple studies have been performed that addressed the effects of BLF IOLs on visual performance in achromatic conditions, including visual acuity and contrast sensitivity; a meta-analysis of the results found that there were no significant differences in best-corrected visual acuity or postoperative contrast sensitivity in patients with BLF IOLs compared with patients with UV-filtering IOLs.62–69 Other concerns, discussed below, have also been raised about the effects of BLF IOLs on scotopic vision, color perception, and circadian rhythms.61
Effects of Blue Light Filtration on Scotopic Vision
Blue light filtration has no effect on visual function under photopic conditions.62 This is not unexpected, given that the sensitivity of the photopic system is off-peak relative to blue light filters (555 nm). It is reasonable to speculate that a more sensitive scotopic system operating at a luminance level below −3.8 log cd/m2, with its blue-shifted peak (507 nm), might be more sensitive to interference from blue light filters.70–73 However, recent findings have not supported this view. Evidently, implantation of a BLF IOL, on average, results in a significant increase in blue light transmission compared with a natural crystalline lens in a typical older individual.74 A review of recent studies concluded that BLF IOLs did not decrease scotopic sensitivity compared with either conventional UV-filtering IOLs or natural lenses.25 As originally noted by Werner, sensitivity adjustment by the scotopic system is far larger than filtering by a BLF IOL.70 The eye is not like a static camera; neural sensitivity can be adjusted across variations in lighting levels.
Effects of Blue Light Filtering on Color Vision
The ability of the visual system to compensate for changes in the filtering characteristics of the eye and variations in the light source is a well-understood phenomenon in visual science (ie, color constancy).75 As a consequence, aging and extreme changes in the eye, such as cataract extraction and implantation of an IOL, are accompanied with only relatively minor changes in color vision, as described by studies such as Delahunt et al.76 A change from a very opaque cataract to a UV-filtering IOL has minimal long-term effect on color vision, although until the system normalizes, patients with UV-filtering IOLs can experience cyanopsia, or transient blue tints surrounding objects.76,77 Therefore, it is unlikely that implantation of a BLF IOL, which matches the absorbance spectrum of a young adult’s natural lens, would result in long-term changes in color perception.
Several tests are available to assess different aspects of color vision. Pseudoisochromatic plate tests are common and easy to use. Newer versions, such as the standard pseudoisochromatic plates part 2 and Hardy Rand and Rittler, are also available and were designed specifically to assess acquired color vision deficiencies; about 4.5% of the population have some form of anomalous color vision.78 Other color vision assessments include chromatic discrimination tests (ie, Farnsworth–Munsell 100-hue test and Farnsworth panel D-15), spectral sensitivity and saturation discrimination evaluations, and anomaloscope color matching tests.73
In a prospective study, Cionni and Tsai assessed color perception in age-matched patients with BLF IOLs, UV-only filtering IOLs, and a phakic control group. Based on the results of the Farnsworth–Munsell 100-hue test, no significant difference in color discrimination was observed in patients with BLF compared with phakic controls or non-BLF IOLs under photopic conditions.79
Khokhar et al tested whether implanting a BLF IOL would influence color discrimination using a variety of clinical-based tests of color vision, including the Ishihara pseudoisochromatic test, Edridge–Green lantern test, Heidelberg anomaloscope, and Farnsworth–Munsell 100-hue test. These tests showed no effects of BLF IOLs on color discrimination.80 Henderson and Grimes reported that of the studies that had been conducted up until that time (10 independent assessments), none showed long-term effects on color tasks assessed using a variety of techniques.81 A more recent review of the existent data reached a similar conclusion.25
Effects of Blue Light Filtration on Circadian Rhythm
As a general rule, cataract surgery tends to improve sleep-wake cycles because of the increased amounts of light that can reach the retina.82 However, it has been hypothesized that BLF could disrupt circadian rhythms by blocking photosensitive retinal ganglion cells that have maximal light sensitivity of approximately 480 nm (although it is not clear why a BLF IOL would be expected to have this effect more than a natural lens or macular pigment).83 This speculation has not been confirmed by empirical studies. For example, a recent large study (n=5070) found that even nuclear cataracts, which absorb more blue light than an IOL, had no effect on sleep quality.84 Similar results were observed when BLF IOLs were studied directly.85 A prospective study of 961 patients found similar overall Pittsburgh Sleep Quality Index scores at 12 months in patients who received BLF IOLs and UV-blocking IOLs.86 Considered together, the empirical data to date suggest that IOLs have no negative influence on circadian rhythms.25
Conclusions
The need for surgical correction of cataracts is expected to increase dramatically with an aging population, and choosing the best approach to cataract surgery is critical. In a population with high levels of macular pigment, minimal light exposure, and healthy crystalline lenses, this matter would be purely academic. However, dietary intake of lutein and zeaxanthin are generally low,87 and, consequently, many individuals are at increased risk of photo-oxidative ocular damage. It is common to replace cataractous lenses with UV-filtering IOLs that do not mimic normal healthy adult lenses. Consequently, it is also likely that, following cataract surgery, many patients will not achieve their full potential for vision and could experience preventable negative visual effects, such as disability and discomfort from glare. Because BLF IOLs mimic natural properties of the native lens in ageing adults, patients receiving these lenses may be protected from ocular damage generated by short-wave blue light. Additionally, BLF IOLs can improve visual performance under glare conditions and reduce GD. Although concerns have been raised about a possible negative impact of BLF IOL on some aspects of visual function, current evidence suggests that BLF does not have a significant effect on visual function under photopic or scotopic conditions, chromatic contrast, or color discrimination. The potential advantages of BLF IOLs should be taken into consideration by ophthalmologists; however, additional studies are needed to demonstrate whether BLF IOLs provide significant long-term improvements compared with non-BLF IOLs.
Acknowledgments
Medical writing assistance was provided by Maria Hovenden, PhD, and Natalia Zhukovskaya, PhD, of Complete Healthcare Communications, LLC (North Wales, PA), an ICON plc company, and was funded by Alcon.
Funding
This study was sponsored by Alcon Research LLC, Fort Worth, TX, USA. The sponsor participated in manuscript preparation, review, and approval.
Disclosure
B. R. Hammond received research funding from and was a consultant for Alcon. V. Sreenivasan and R. Suryakumar are Alcon employees. The authors report no other conflicts of interest in this work.
References
- 1.Hammond BR, Johnson BA, George ER. Oxidative photodegradation of ocular tissues: beneficial effects of filtering and exogenous antioxidants. Exp Eye Res. 2014;129:135–150. doi: 10.1016/j.exer.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006;84(1):4–15. doi: 10.1111/j.1600-0420.2005.00627.x [DOI] [PubMed] [Google Scholar]
- 3.Walls GL, Judd HD. The intra-ocular colour-filters of vertebrates. Br J Ophthalmol. 1933;17(11):641–675. doi: 10.1136/bjo.17.11.641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond BR Jr. The visual effects of intraocular colored filters. Scientifica (Cairo). 2012;2012:424965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatty S, Boulton M, Henson D, Koh HH, Murray IJ. Macular pigment and age related macular degeneration. Br J Ophthalmol. 1999;83(7):867–877. doi: 10.1136/bjo.83.7.867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993;34(6):2033–2040. [PubMed] [Google Scholar]
- 7.Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985;25(11):1531–1535. doi: 10.1016/0042-6989(85)90123-3 [DOI] [PubMed] [Google Scholar]
- 8.Hammond BR Jr., Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am a Opt Image Sci Vis. 1997;14(6):1187–1196. doi: 10.1364/JOSAA.14.001187 [DOI] [PubMed] [Google Scholar]
- 9.Mares J. Lutein and zeaxanthin isomers in eye health and disease. Annu Rev Nutr. 2016;36:571–602. doi: 10.1146/annurev-nutr-071715-051110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behar-Cohen F, Baillet G, de Ayguavives T, et al. Ultraviolet damage to the eye revisited: eye-sun protection factor (E-SPF(R)), a new ultraviolet protection label for eyewear. Clin Ophthalmol. 2014;8:87–104. doi: 10.2147/OPTH.S46189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond BR Jr., Renzi-Hammond L. Individual variation in the transmission of UVB radiation in the young adult eye. PLoS One. 2018;13(7):e0199940. doi: 10.1371/journal.pone.0199940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Domene MC, Perez-Vives C, Peris-Martinez C, Artigas JM. Comparison of the ultraviolet light filtering across different intraocular lenses. Optom Vis Sci. 2018;95(12):1129–1134. doi: 10.1097/OPX.0000000000001309 [DOI] [PubMed] [Google Scholar]
- 13.Sparrow JR, Miller AS, Zhou J. Blue light-absorbing intraocular lens and retinal pigment epithelium protection in vitro. J Cataract Refract Surg. 2004;30(4):873–878. doi: 10.1016/j.jcrs.2004.01.031 [DOI] [PubMed] [Google Scholar]
- 14.Pipis A, Touliou E, Pillunat LE, Augustin AJ. Effect of the blue filter intraocular lens on the progression of geographic atrophy. Eur J Ophthalmol. 2015;25(2):128–133. doi: 10.5301/ejo.5000520 [DOI] [PubMed] [Google Scholar]
- 15.Marshall J, Cionni RJ, Davison J, et al. Clinical results of the blue-light filtering AcrySof Natural foldable acrylic intraocular lens. J Cataract Refract Surg. 2005;31(12):2319–2323. doi: 10.1016/j.jcrs.2004.11.061 [DOI] [PubMed] [Google Scholar]
- 16.Sparrow JR, Fishkin N, Zhou J, et al. A2E, a byproduct of the visual cycle. Vision Res. 2003;43(28):2983–2990. doi: 10.1016/S0042-6989(03)00475-9 [DOI] [PubMed] [Google Scholar]
- 17.Schick T, Ersoy L, Lechanteur YT, et al. History of sunlight exposure is a risk factor for age-related macular degeneration. Retina. 2016;36(4):787–790. doi: 10.1097/IAE.0000000000000756 [DOI] [PubMed] [Google Scholar]
- 18.Werner JS. Development of scotopic sensitivity and the absorption spectrum of the human ocular media. J Opt Soc Am. 1982;72(2):247–258. doi: 10.1364/JOSA.72.000247 [DOI] [PubMed] [Google Scholar]
- 19.Ratnayake K, Payton JL, Lakmal OH, Karunarathne A. Blue light excited retinal intercepts cellular signaling. Sci Rep. 2018;8(1):10207. doi: 10.1038/s41598-018-28254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanagi Y, Inoue Y, Jang WD, Kadonosono K. A2e mediated phototoxic effects of endoilluminators. Br J Ophthalmol. 2006;90(2):229–232. doi: 10.1136/bjo.2005.076711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezai KA, Gasyna E, Seagle BL, Norris JR Jr., Rezaei KA. AcrySof Natural filter decreases blue light-induced apoptosis in human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 2008;246(5):671–676. doi: 10.1007/s00417-006-0484-2 [DOI] [PubMed] [Google Scholar]
- 22.Kernt M, Neubauer AS, Liegl R, et al. Cytoprotective effects of a blue light-filtering intraocular lens on human retinal pigment epithelium by reducing phototoxic effects on vascular endothelial growth factor-alpha, Bax, and Bcl-2 expression. J Cataract Refract Surg. 2009;35(2):354–362. doi: 10.1016/j.jcrs.2008.10.052 [DOI] [PubMed] [Google Scholar]
- 23.Bullough JD. The blue-light hazard: a review. J Illum Eng Soc. 2000;29(2):6–14. doi: 10.1080/00994480.2000.10748312 [DOI] [Google Scholar]
- 24.Pratesi S, Di Fabio S, Bresci C, et al. Broad-spectrum light versus blue light for phototherapy in neonatal hyperbilirubinemia: a randomized controlled trial. Am J Perinatol. 2015;32(8):779–784. doi: 10.1055/s-0034-1396685 [DOI] [PubMed] [Google Scholar]
- 25.Downes SM. Ultraviolet or blue-filtering intraocular lenses: what is the evidence? Eye (Lond). 2016;30(2):215–221. doi: 10.1038/eye.2015.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall JC, Gordon KD, McCauley CS, de Souza Filho JP, Burnier MN. The effect of blue light exposure and use of intraocular lenses on human uveal melanoma cell lines. Melanoma Res. 2006;16(6):537–541. doi: 10.1097/CMR.0b013e3280112b86 [DOI] [PubMed] [Google Scholar]
- 27.Nagai H, Hirano Y, Yasukawa T, et al. Prevention of increased abnormal fundus autofluorescence with blue light-filtering intraocular lenses. J Cataract Refract Surg. 2015;41(9):1855–1859. doi: 10.1016/j.jcrs.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 28.Holladay LL. The fundamentals of glare and visibility. J Opt Soc Am Rev Sci Instrum. 1926;12(4):271–319. doi: 10.1364/JOSA.12.000271 [DOI] [Google Scholar]
- 29.Stiles WS. The effect of glare on the brightness difference threshold. Proc R Soc Lond Ser B Containing Pap Biol Charact. 1929;104(731):322–351. doi: 10.1098/rspb.1929.0012 [DOI] [Google Scholar]
- 30.Thurman SM, Davey PG, McCray KL, Paronian V, Seitz AR. Predicting individual contrast sensitivity functions from acuity and letter contrast sensitivity measurements. J Vis. 2016;16(15):15. doi: 10.1167/16.15.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pomerance GN, Evans DW. Test-retest reliability of the CSV-1000 contrast test and its relationship to glaucoma therapy. Invest Ophthalmol Vis Sci. 1994;35(9):3357–3361. [PubMed] [Google Scholar]
- 32.Stringham JM, Hammond BR. Macular pigment and visual performance under glare conditions. Optom Vis Sci. 2008;85(2):82–88. doi: 10.1097/OPX.0b013e318162266e [DOI] [PubMed] [Google Scholar]
- 33.Renzi-Hammond LM, Hammond BR Jr. The effects of photochromic lenses on visual performance. Clin Exp Optom. 2016;99(6):568–574. doi: 10.1111/cxo.12394 [DOI] [PubMed] [Google Scholar]
- 34.Stringham JM, Fuld K, Wenzel AJ. Action spectrum for photophobia. J Opt Soc Am a Opt Image Sci Vis. 2003;20(10):1852–1858. doi: 10.1364/JOSAA.20.001852 [DOI] [PubMed] [Google Scholar]
- 35.Bullough JD. A method for estimating discomfort glare from exterior lighting systems. ASSIST. 2011;9(1):1–7. [Google Scholar]
- 36.De Boer JB. Visual perception in road traffic and the field of vision of the motorist In: De Boer JB, editor. Public Lighting. Netherlands; Eindhoven: Philips Technical Library; 1967:11–96. [Google Scholar]
- 37.Bargary G, Furlan M, Raynham PJ, Barbur JL, Smith AT. Cortical hyperexcitability and sensitivity to discomfort glare. Neuropsychologia. 2015;69:194–200. doi: 10.1016/j.neuropsychologia.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 38.Hu J, Sella R, Afshari NA. Dysphotopsia: a multifaceted optic phenomenon. Curr Opin Ophthalmol. 2018;29(1):61–68. doi: 10.1097/ICU.0000000000000447 [DOI] [PubMed] [Google Scholar]
- 39.Ham WT Jr., Mueller HA, Sliney DH. Retinal sensitivity to damage from short wavelength light. Nature. 1976;260(5547):153–155. doi: 10.1038/260153a0 [DOI] [PubMed] [Google Scholar]
- 40.Hammond BR, Bernstein B, Dong J. The effect of the AcrySof natural lens on glare disability and photostress. Am J Ophthalmol. 2009;148(2):272–276 e272. doi: 10.1016/j.ajo.2009.03.014 [DOI] [PubMed] [Google Scholar]
- 41.Hammond BR Jr., Renzi LM, Sachak S, Brint SF. Contralateral comparison of blue-filtering and non–blue-filtering intraocular lenses: glare disability, heterochromatic contrast, and photostress recovery. Clin Ophthalmol. 2010;4:1465–1473. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammond BR. Attenuating photostress and glare disability in pseudophakic patients through the addition of a short-wave absorbing filter. J Ophthalmol. 2015;2015:607635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahane CJ, Hertz E. The long-term effectiveness of center high mounted stop lamps in passenger cars and light trucks. NHTSA technical report number DOT HS 808 696; 1998.
- 44.Gray R, Hill W, Neuman B, Houtman D, Potvin R. Effects of a blue light-filtering intraocular lens on driving safety in glare conditions. J Cataract Refract Surg. 2012;38(5):816–822. doi: 10.1016/j.jcrs.2011.11.047 [DOI] [PubMed] [Google Scholar]
- 45.Gray R, Perkins SA, Suryakumar R, Neuman B, Maxwell WA. Reduced effect of glare disability on driving performance in patients with blue light–filtering intraocular lenses. J Cataract Refract Surg. 2011;37(1):38–44. doi: 10.1016/j.jcrs.2010.07.034 [DOI] [PubMed] [Google Scholar]
- 46.Hammond B, Renzi-Hammond L. Macular pigment and glare geometry. Presented at: BON Conference 11–13 July 2018; 2018; Downing College, Cambridge University, UK. [Google Scholar]
- 47.Went FW. Blue hazes in the atmosphere. Nature. 1960;187:641–643. doi: 10.1038/187641a0 [DOI] [Google Scholar]
- 48.Ferman MA, Wolff GT, Kelly NA. The nature and sources of haze in the Shenandoah Valley/Blue Ridge Mountains area. J Air Pollut Control Assoc. 1981;31(10):1074–1082. doi: 10.1080/00022470.1981.10465329 [DOI] [Google Scholar]
- 49.Zhang R, Wang L, Khalizov AF, et al. Formation of nanoparticles of blue haze enhanced by anthropogenic pollution. Proc Natl Acad Sci U S A. 2009;106(42):17650–17654. doi: 10.1073/pnas.0910125106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu P, Ding Y, Liu Y. Atmospheric circulation and dynamic mechanism for persistent haze events in the Beijing-Tianjin-Hebei region. Adv Atmos Sci. 2017;34:429–440. doi: 10.1007/s00376-016-6158-z [DOI] [Google Scholar]
- 51.Walls GL. The Vertebrate Eye and Its Adaptive Radiation. Bloomfield Hills, Michigan: Cranbrook Institute of Science; 1942. [Google Scholar]
- 52.Artigas JM, Felipe A, Navea A, Fandino A, Artigas C. Spectral transmission of the human crystalline lens in adult and elderly persons: color and total transmission of visible light. Invest Ophthalmol Vis Sci. 2012;53(7):4076–4084. doi: 10.1167/iovs.12-9471 [DOI] [PubMed] [Google Scholar]
- 53.Wooten BR, Hammond BR. Macular pigment: influences on visual acuity and visibility. Prog Retin Eye Res. 2002;21(2):225–240. doi: 10.1016/S1350-9462(02)00003-4 [DOI] [PubMed] [Google Scholar]
- 54.Hammond BR Jr., Wooten BR, Engles M, Wong JC. The influence of filtering by the macular carotenoids on contrast sensitivity measured under simulated blue haze conditions. Vision Res. 2012;63:58–62. doi: 10.1016/j.visres.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 55.Fletcher LM, Engles M, Hammond BR Jr. Visibility through atmospheric haze and its relation to macular pigment. Optom Vis Sci. 2014;91(9):1089–1096. doi: 10.1097/OPX.0000000000000355 [DOI] [PubMed] [Google Scholar]
- 56.Tang S, Dong M, Ma J, Zhou Z, Li C. Color image enhancement based on retinex theory with guided filter. Presented at: 2017 29th Chinese Control And Decision Conference (CCDC), 28-30 May 2017, 2017; 57. Luria SM. Vision with chromatic filters. Am J Optom Arch Am Acad Optom. 1972;49(10):818–829. doi: 10.1097/00006324-197210000-00002 [DOI] [PubMed] [Google Scholar]
- 57.Luria SM. Vision with chromatic filters. Am J Optom Arch Am Acad Optom. 1972;49(10):818–829. doi: 10.1097/00006324-197210000-00002 [DOI] [PubMed] [Google Scholar]
- 58.Wolffsohn JS, Dinardo C, Vingrys AJ. Benefit of coloured lenses for age-related macular degeneration. Ophthalmic Physiol Opt. 2002;22(4):300–311. doi: 10.1046/j.1475-1313.2002.00036.x [DOI] [PubMed] [Google Scholar]
- 59.Hammond BR, Fletcher LM, Roos F, Wittwer J, Schalch W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Invest Ophthalmol Vis Sci. 2014;55(12):8583–8589. doi: 10.1167/iovs.14-15573 [DOI] [PubMed] [Google Scholar]
- 60.Renzi LM, Hammond BR. The effect of macular pigment on heterochromatic luminance contrast. Exp Eye Res. 2010;91(6):896–900. doi: 10.1016/j.exer.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 61.Li X, Kelly D, Nolan JM, Dennison JL, Beatty S. The evidence informing the surgeon’s selection of intraocular lens on the basis of light transmittance properties. Eye (Lond). 2017;31(2):258–272. doi: 10.1038/eye.2016.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu XF, Zou HD, Yu YF, Sun Q, Zhao NQ. Comparison of blue light-filtering IOLs and UV light-filtering IOLs for cataract surgery: a meta-analysis. PLoS One. 2012;7(3):e33013. doi: 10.1371/journal.pone.0033013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leibovitch I, Lai T, Porter N, et al. Visual outcomes with the yellow intraocular lens. Acta Ophthalmol Scand. 2006;84(1):95–99. doi: 10.1111/j.1600-0420.2005.00607.x [DOI] [PubMed] [Google Scholar]
- 64.Mester U, Holz F, Kohnen T, Lohmann C, Tetz M. Intraindividual comparison of a blue-light filter on visual function: AF-1 (UY) versus AF-1 (UV) intraocular lens. J Cataract Refract Surg. 2008;34(4):608–615. doi: 10.1016/j.jcrs.2007.11.049 [DOI] [PubMed] [Google Scholar]
- 65.Neumaier-Ammerer B, Felke S, Hagen S, et al. Comparison of visual performance with blue light-filtering and ultraviolet light-filtering intraocular lenses. J Cataract Refract Surg. 2010;36(12):2073–2079. doi: 10.1016/j.jcrs.2010.06.069 [DOI] [PubMed] [Google Scholar]
- 66.Vuori ML, Mantyjarvi M. Colour vision and retinal nerve fibre layer photography in patients with an Acrysof Natural intraocular lens. Acta Ophthalmol Scand. 2006;84(1):92–94. doi: 10.1111/j.1600-0420.2005.00579.x [DOI] [PubMed] [Google Scholar]
- 67.Wang H, Wang J, Fan W, Wang W. Comparison of photochromic, yellow, and clear intraocular lenses in human eyes under photopic and mesopic lighting conditions. J Cataract Refract Surg. 2010;36(12):2080–2086. doi: 10.1016/j.jcrs.2010.07.024 [DOI] [PubMed] [Google Scholar]
- 68.Hayashi K, Hayashi H. Visual function in patients with yellow tinted intraocular lenses compared with vision in patients with non-tinted intraocular lenses. Br J Ophthalmol. 2006;90(8):1019–1023. doi: 10.1136/bjo.2006.090712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pandita D, Raj SM, Vasavada VA, et al. Contrast sensitivity and glare disability after implantation of AcrySof IQ Natural aspherical intraocular lens: prospective randomized masked clinical trial. J Cataract Refract Surg. 2007;33(4):603–610. doi: 10.1016/j.jcrs.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 70.Werner JS. Night vision in the elderly: consequences for seeing through a “blue filtering” intraocular lens. Br J Ophthalmol. 2005;89(11):1518–1521. doi: 10.1136/bjo.2005.073734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mainster MA, Sparrow JR. How much blue light should an IOL transmit? Br J Ophthalmol. 2003;87(12):1523–1529. doi: 10.1136/bjo.87.12.1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mainster MA, Turner PL. Blue-blocking IOLs decrease photoreception without providing significant photoprotection. Surv Ophthalmol. 2010;55(3):272–289. doi: 10.1016/j.survophthal.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 73.Cao D. Chapter 10 - Color vision and night vision Ryan SJ, editor. Retina. 5th ed Vol. 1 Elsevier Inc.; 2012:285–299. [Google Scholar]
- 74.Schwiegerling J. Blue-light-absorbing lenses and their effect on scotopic vision. J Cataract Refract Surg. 2006;32(1):141–144. doi: 10.1016/j.jcrs.2005.11.021 [DOI] [PubMed] [Google Scholar]
- 75.Werner JS. Visual problems of the retina during ageing: compensation mechanisms and colour constancy across the life span. Prog Retin Eye Res. 1996;15(2):621–645. doi: 10.1016/1350-9462(96)00001-8 [DOI] [Google Scholar]
- 76.Delahunt PB, Webster MA, Ma L, Werner JS. Long-term renormalization of chromatic mechanisms following cataract surgery. Vis Neurosci. 2004;21(3):301–307. doi: 10.1017/S0952523804213025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan Z, Reinach P, Yuan J. Contrast sensitivity and color vision with a yellow intraocular lens. Am J Ophthalmol. 2004;138(1):138–140. doi: 10.1016/j.ajo.2004.02.024 [DOI] [PubMed] [Google Scholar]
- 78.Patel JR, Trivedi H, Patel A, Patil S, Jethva J. Assessment of colour vision deficiency in medical students. Int J Community Med Public Health. 2017;3(1):230–235. [Google Scholar]
- 79.Cionni RJ, Tsai JH. Color perception with AcrySof natural and AcrySof single-piece intraocular lenses under photopic and mesopic conditions. J Cataract Refract Surg. 2006;32(2):236–242. doi: 10.1016/j.jcrs.2005.12.129 [DOI] [PubMed] [Google Scholar]
- 80.Khokhar SK, Jindal A, Agarwal T, Panda A. Comparison of color perception after tinted blue light-filtering and clear ultraviolet-filtering intraocular lens implantation. J Cataract Refract Surg. 2011;37(9):1598–1604. doi: 10.1016/j.jcrs.2011.03.044 [DOI] [PubMed] [Google Scholar]
- 81.Henderson BA, Grimes KJ. Blue-blocking IOLs: a complete review of the literature. Surv Ophthalmol. 2010;55(3):284–289. doi: 10.1016/j.survophthal.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 82.Zheng L, Wu XH, Lin HT. The effect of cataract surgery on sleep quality: a systematic review and meta-analysis. Int J Ophthalmol. 2017;10(11):1734–1741. doi: 10.18240/ijo.2017.11.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Foster RG, Hankins MW. Circadian vision. Curr Biol. 2007;17(17):R746–R751. doi: 10.1016/j.cub.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 84.Chen Y, Nondahl DM, Schubert CR, et al. The relation between sleep disruption and cataract in a large population study. Ophthalmic Epidemiol. 2017;24(2):111–115. doi: 10.1080/09286586.2016.1259640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Landers JA, Tamblyn D, Perriam D. Effect of a blue-light-blocking intraocular lens on the quality of sleep. J Cataract Refract Surg. 2009;35(1):83–88. doi: 10.1016/j.jcrs.2008.10.015 [DOI] [PubMed] [Google Scholar]
- 86.Alexander I, Cuthbertson FM, Ratnarajan G, et al. Impact of cataract surgery on sleep in patients receiving either ultraviolet-blocking or blue-filtering intraocular lens implants. Invest Ophthalmol Vis Sci. 2014;55(8):4999–5004. doi: 10.1167/iovs.14-14054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eisenhauer B, Natoli S, Liew G, Flood VM. Lutein and zeaxanthin-food sources, bioavailability and dietary variety in age-related macular degeneration protection. Nutrients. 2017;9:2. doi: 10.3390/nu9020120 [DOI] [Google Scholar]