Abstract
Introduction
Targeted therapies in cancer aim to inhibit specific molecular targets responsible for enhanced tumor growth. AKT/PKB (protein kinase B) is a serine threonine kinase involved in several critical cellular pathways including survival, proliferation, invasion, apoptosis, and angiogenesis. Although phosphatidylinositol-3 kinase (PI3K) is the key regulator of AKT activation, numerous stimuli and kinases initiate pro-proliferative AKT signaling which results in the activation of AKT pathway to drive cellular growth and survival. Activating mutations and amplification of components of the AKT pathway are implicated in the pathogenesis of many cancers including breast and ovarian. Given its importance, AKT, it has been validated as a promising therapeutic target.
Areas covered
This article summarizes AKT’s biological function and different classes of AKT inhibitors as anticancer agents. We also explore the efficacy of AKT inhibitors as monotherapies and in combination with cytotoxic and other targeted therapies.
Expert opinion
The complex mechanism following AKT inhibition, requires the addition of other therapies to prevent resistance and improve clinical response Further studies are necessary to determine additional rational combinations that can enhance efficacy of AKT inhibitors, potentially by targeting compensatory mechanisms, and/or enhancing apoptosis. The identification of biomarkers of response is essential for the development of successful therapeutics.
Keywords: AKT activation, tumorigenicity, breast and ovarian cancer, AKT inhibitors, targeted therapy, combinational therapy
1. Introduction
Breast and ovarian cancers are two of the most frequent malignancies in women, with breast cancer being the second and ovarian cancer being the fifth leading causes of cancer-related death in women[1, 2]. Although endocrine therapy and chemotherapy are effective, many tumors have intrinsic or acquired resistance to standard therapies[3, 4]. Extensive research has revealed many potential cellular processes such as epigenetic, genomic change, DNA repair and molecular cross talk that underlie therapeutic resistance[5, 6]. Determining targetable molecular biomarkers as alternative or combinational treatments can add to the clinical efficacy of the current therapies and overcome potential resistance.
Over the past few decades, molecular characterization of differentially expressed genes in breast and ovarian cancer patients has demonstrated that novel molecular targeted therapies were successful approaches[7, 8]. Genomic alterations in the phosphatidylinositol 3-kinase (PI3K)/AKT pathway have been reported in several types of cancer, including breast and ovarian[9, 10]. AKT, also known as protein kinase B (PKB), is a critical growth regulator in PI3K signaling, that phosphorylates over 9,000 proteins[11, 12] and is an attractive therapeutic target in cancer. Targeting AKT with the available small molecule inhibitors may enhance approved or investigational anticancer treatments[13]. In this review, the biological function and activation of AKT and its contribution to tumor development and progression are explained. Furthermore, the efficacy of clinically relevant AKT inhibitors as monotherapy and in combination with cytotoxic and other targeted therapies in the treatment of breast and ovarian cancers are discussed.
2. AKT function and structure
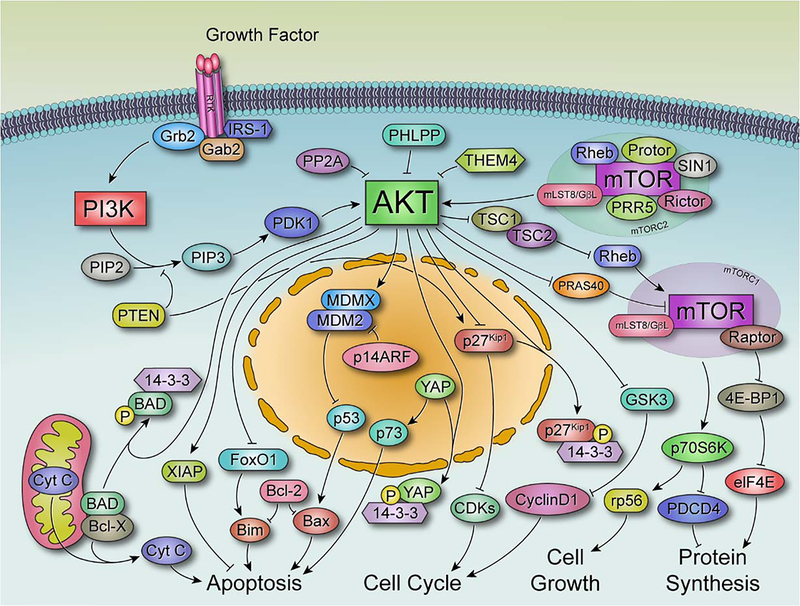
AKT is a serine threonine kinase that mediates various biological functions (Figure 1) such as cell proliferation, survival, glucose metabolism, protein synthesis, genome stability, and inhibition of apoptosis in response to different growth factors and extracellular stimuli[14, 15]. Besides its pivotal role in normal cellular physiology, many studies have demonstrated the activation of AKT cascade in various types of human cancer that often results in tumor aggressiveness and drug resistance[16, 17]. Several pathways regulated by AKT are implicated in cancer-associated phenotypes. AKT inactivates pro-apoptotic proteins, such as BCL-2-antagonist of cell death (BAD) and procaspase-9, to block apoptosis. During cell cycle progression, AKT phosphorylates and inhibits glycogen synthase kinase 3β (GSK3β) to prevent cyclin D1 degradation[18, 19, 20].
Figure 1.
Representation of AKT pathway and downstream effectors. Shown are the key molecular targets involved in AKT signaling. Upon growth factor binding to receptor tyrosine kinase (RTK), phosphoinositol bisphosphate (PIP2) is converted to phosphoinositol trisphosphate (PIP3), which recruits AKT and phosphoinositide-dependent kinase-1 (PDK1) to the plasma membrane by binding the PH domain to membrane lipids. PDK1 and mammalian target of rapamycin complex 2 (mTORC2) phosphorylate AKT, resulting in full activation of AKT. Phosphatase and tensin homolog (PTEN) and PH domain leucine-rich-protein phosphatase (PHLPP) negatively regulate AKT through dephosphorylation of PIP3 and AKT (serine-473 residue) respectively. Once activated AKT phosphorylates and inhibits the proline-rich AKT substrate of 40 kDa (PRAS40) and tuberous sclerosis complex 1 and 2 (TSC1 and TSC2) to promote protein synthesis. AKT blocks apoptosis through inhibition of BCL-2-antagonist of cell death (BAD), BCL-2-like protein 11 (BIM), and forkhead box protein O1 (FoxO1). It also phosphorylates MDM2, resulting in p53 degradation. AKT prevents cell cycle arrest and cyclin D1 degradation by phosphorylating and inhibiting cyclin dependent kinase inhibitor p27, and glycogen synthase kinase 3β (GSK3β).
There are three AKT isoforms in humans: AKT1 (PKB-α), AKT2 (PKB-β) and AKT3 (PKB-γ) that share a common structure and a similar activation mechanism[21]. The pleckstrin homology (PH) domain at the N-terminus of AKT interacts with membrane lipids to facilitate AKT recognition and membrane translocation by upstream kinases[22]. The center catalytic region of the protein is the kinase domain, which contains a threonine residue that needs to be phosphorylated for AKT activation[23]. The C-terminal regulatory hydrophobic region of AKT contains a conserved serine residue required for the kinase phosphorylation and activation[24]. All three AKT isoforms share 80% homology in their amino acid sequences with isoform-specific functions[25]. While AKT1 is mainly involved in regulating cell growth and division, AKT2 plays an important role in cellular energy and metabolism[26]. AKT3, the least studied AKT isoform, has been proposed as critical for brain development and the viability of malignant glioma cells[27].
3. AKT activation mechanism and signaling pathway
The main event that triggers AKT activation is the binding of ligands to cell membrane receptors. These ligands include growth factors such as IGF-1 (insulin-like growth factor 1) and PDGF (platelet-derived growth factor), cytokines, hormones, and mitogens[28, 29]. Identical mechanisms downstream of PI3K pathway, as the key element of the AKT signaling cascade, activate all three AKT isoforms [14]. Upon growth factor binding to receptor tyrosine kinase (RTK), the regulatory subunit of PI3K (p85) is targeted to phosphotyrosine-containing cytoplasmic domains of activated RTK. This leads to the activation of the catalytic domain of PI3K (p110), which then recruits AKT to the plasma membrane by binding the PH domain to membrane lipids[30]. The AKT undergoes a conformational change, resulting in the phosphorylation of Thr308 residue by phosphoinositide-dependent kinase-1 (PDK1) and phosphorylation of Ser473 by target of rapamycin complex 2 (mTORC2) of the mammalian target of rapamycin (mTOR) kinase[31, 32]. In addition to mTORC2, multiple different kinases, including DNA-PK (DNA-dependent protein kinase) which is a PI3K-like kinase (PIKK), are responsible for phosphorylation and activation of AKT at Ser473[33].
Once phosphorylated, activated AKT translocate to various intracellular locations, where it phosphorylates and modulates the function of numerous substrates. Many of these are involved in cancer initiation and progression[34]. Tumor suppressors phosphatase and tensin homolog (PTEN) and PH domain leucine-rich-protein phosphatase (PHLPP) negatively regulate AKT through dephosphorylation[35]. PTEN dephosphorylates PIP3 component of PI3K back to PIP2 and PHLPP removes phosphate group from AKT at S473 residue[36].
Activated AKT phosphorylates a variety of protein substrates involved in survival and cellular growth. mTOR, the main downstream target of PI3K/AKT signaling, is a key cell metabolism regulator that once activated phosphorylates ribosomal protein S6 kinase (p70S6K) and eIF4E binding-protein-1 (4E-BP1) to promote protein synthesis. Tuberous sclerosis complex 1 and 2 (TSC1 and TSC2) tumor suppressors, which are negative regulators of mTOR/S6K pathway, are phosphorylated by AKT, resulting in their inhibition[37, 38]. In addition, AKT phosphorylates the proline-rich AKT substrate of 40 kDa (PRAS40) at Thr246 and the yes-associated protein (YAP) at Ser127 to induce their interactions with 14–3-3. These interactions correlate with their inactivations[39, 40, 41]. AKT exerts its effect on cell cycle progression by phosphorylating and inhibiting cyclin dependent kinase inhibitors, p21 and p27, which function as G1 checkpoints to arrest cell cycle[38]. AKT regulates apoptosis through inhibition of BAD, BCL-2-like protein 11 (BIM), caspase 9, and forkhead box protein O1 (FoxO1)[42, 43, 44, 45]. It also phosphorylates MDM2, allowing its entry into the nucleus. This results in p53 degradation[46, 47, 48].
4. AKT activation in breast and ovarian cancers
Aberrant expression of AKT has been observed in a variety of human cancers such as breast, lung, ovarian, pancreatic, and gastric carcinomas[49]. AKT1 amplification, unlike other AKT isoforms, is commonly reported in cancer. However, an activating somatic mutation in the PH domain of AKT1 has been identified in 8.2% of breast, 2% of ovarian and 5.9% of colorectal cancers[50]. The AKT1-E17K mutation plays a crucial role in cancer development as it has been shown to express as mutually exclusive to the PIK3CA mutation and PTEN loss. AKT1-E17K stimulates AKT membrane localization, induction of cellular transformation, and leukemia in mice[50]. The first genomic alteration in the AKT family was observed in AKT2. A large-scale multicenter study identified AKT2 overexpression in 12% (16 of 132) of ovarian and 3% (3 of 106) of breast carcinomas[7]. Interestingly, AKT2 amplification was more frequent in high grade ovarian tumors and correlated with a poor prognosis for patients[51]. Furthermore, overexpression studies of AKT2 showed increased invasion and metastasis of human breast and ovarian cancer cells[52].
The function of AKT3, the least studied isoform in the AKT family, in cancer initiation and progression is unknown. TCGA analysis has reported the upregulation of AKT3 mRNA in 28% of triple negative breast cancer (TNBC)[53]. In a recent study, targeted exome-sequencing has identified a novel AKT3 mutation in a human epidermal growth factor receptor 2 (HER2) amplified breast cancer patient with acquired resistance to trastuzumab[54]. Additionally, 20% of ovarian tumor subtypes and 40% of primary melanomas exhibited increased AKT3 expression[27, 55]. AKT3 induces G2/M transition during cell cycle progression in ovarian cancer cell lines[55].
5. AKT inhibitors as anticancer treatments
Inhibiting AKT as the central component of frequently disrupted PI3K/AKT signaling has long been an attractive therapeutic approach in cancer. Several compounds for targeting AKT are in clinical development (Table. 1). There are two classes of AKT inhibitors: ATP-competitive and allosteric inhibitors. The ATP-competitive inhibitors bind to the active site of AKT, blocking ATP binding; whereas, allosteric inhibitors bind to the PH domain, preventing AKT phosphorylation and activation[56]. Although the three AKT isoforms have structural similarity, there are differences in their functions, tissue distribution and substrate specificity[57]. Consequently, the impact of novel therapeutics on different isoforms in vivo needs further study.
Table 1.
Drugs targeting AKT in clinical trials.
| Inhibitor | Class | Target | Status |
|---|---|---|---|
| Ipatasertib (GDC-0068) | ATP-competitive | AKT1, 2 and 3 | Phase I/Phase II/ Phase III clinical trials |
| Capivasertib (AZD5363) | ATP-competitive | AKT1, 2 and 3 | Phase I/Phase II clinical trials |
| ARQ751 and ARQ092 | Allosteric | AKT1, 2 and 3 | Phase I clinical trials |
| Afuresertib (GSK2110183) and Uprosertib (GSK2141795) | ATP-competitive | AKT1, 2 and 3 | Phase I/Phase II clinical trials |
| MK-2206 | Allosteric | AKT1 and 2 | Phase I/Phase II clinical trials |
| Perifosine | Allosteric | AKT1, 2 and 3 | Phase I clinical trials |
| BAY1125976 | Allosteric | AKT1 and 2 | Phase I clinical trials |
| Triciribine | Allosteric | AKT1, 2 and 3 | Phase I clinical trials |
| TAS-117 | Allosteric | AKT1, 2 and 3 | Phase II clinical trials |
| LY2780301 | ATP-competitive | AKT1, 2, 3 and p70S6K | Phase I clinical trials |
| MSC2363318A | ATP-competitive | AKT1, 3 and p70S6K | Phase I clinical trials |
| Cenisertib (R763/AS703569) | ATP-competitive | AKT, FLT3, VEGFR2, LYN, BTK and KIT | Phase I clinical trials |
5.1. Ipatasertib (GDC-0068)
Ipatasertib (GDC-0068), a potent and selective ATP-competitive AKT1, 2 and 3 inhibitor, was discovered during a structural based optimization design[58]. Ipatasertib treatment inhibits AKT signaling and cell cycle in human cancer cell lines and induces a robust antitumor response in PIK3CA/AKT driven tumor xenograft models[59].
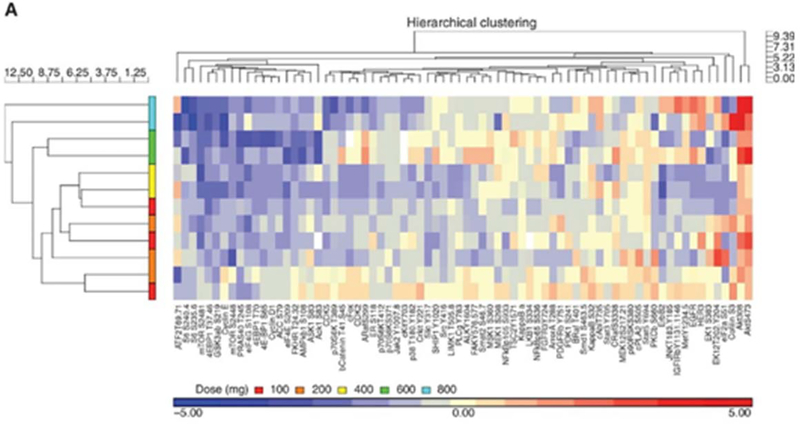
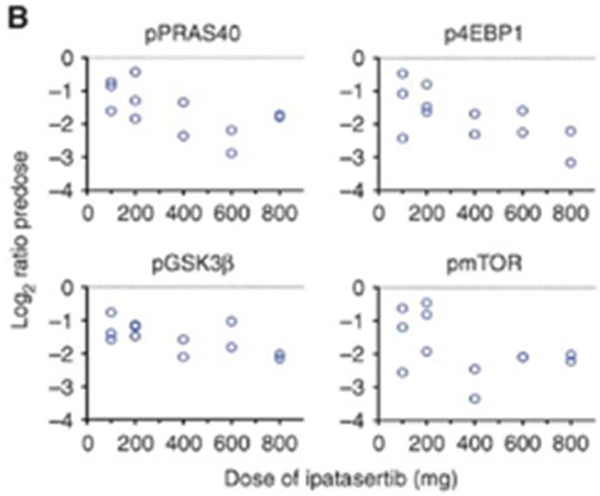
A first monotherapy of ipatasertib in human demonstrated robust and safe targeting of AKT with downregulation of AKT pathway targets in patients with diverse solid tumors (Figure 2A)[60]. Ipatasertib was well tolerated in this phase I, with most common adverse events being gastrointestinal and grade 1–2. The study also demonstrated that ipatasertib ≥200 mg daily in patients resulted in exposure corresponding to concentrations that resulted in over 90% tumor growth inhibition (TGI90) in preclinical xenograft models with PTEN-null status. Pharmacodynamic studies in platelet rich plasma (pGSK3β, not shown) and paired pre-treatment and on-treatment biopsies (pPRAS40, pGSK3β, p4E-BP1 and pmTOR) confirmed target inhibition (Figure 2B).
Figure 2.
Pharmacodynamic effects of AKT inhibition. Patients undergoing treatment with ipatasertib underwent pre-treatment and on-treatment tumor biopsies. Pharmacodynamics effects were evaluated with reverse phase protein array for multiple AKT pathway targets. A) Ipatasertib caused accumulation of AKT phosphorylation (Threonine-308 and Serine-473) and downregulation of multiple AKT targets such as B) proline rich AKT substrate of 40 kDa (PRAS40), eIF4E binding-protein-1 (4E-BP1), glycogen synthase kinase 3β (GSK3β), and mammalian target of rapamycin (mTOR) in a dose-dependent manner. Figure was reprinted with permission from “A First-in-Human Phase I Study of the ATP-Competitive AKT Inhibitor Ipatasertib Demonstrates Robust and Safe Targeting of AKT in Patients with Solid Tumors” by Cristina Saura, 2016. Cancer Discovery, Volume 7, Pages 102–113.
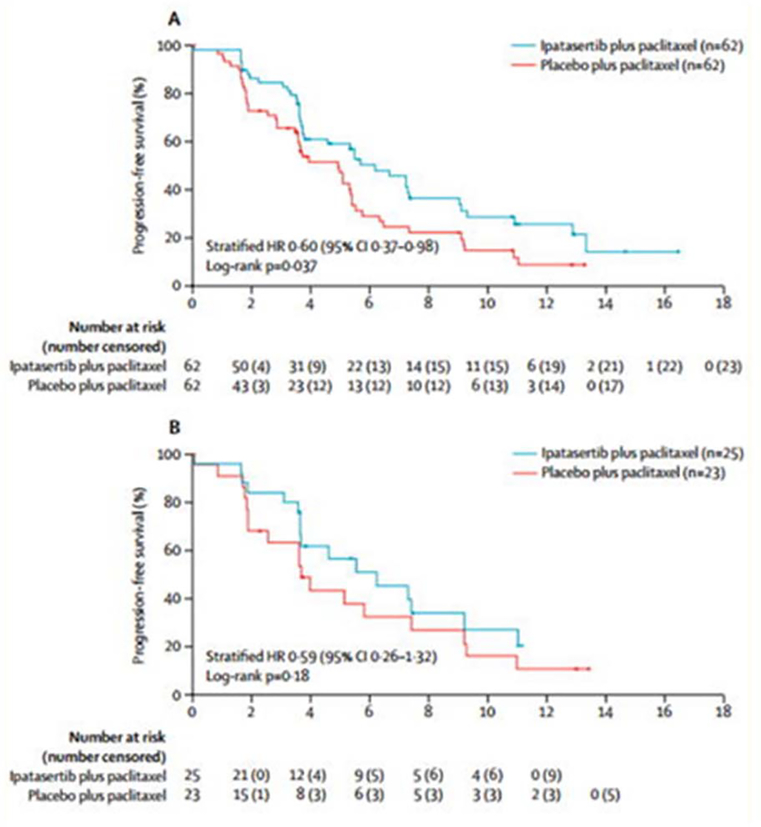
A phase Ib trial of ipatasertib and paclitaxel combination showed a good safety profile with antitumor activity seen in TNBC and HER2-negative breast patients, harboring PIK3K/AKT pathway activation[61]. Furthermore, the addition of ipatasertib to paclitaxel in a phase II trial was associated with improved progression-free survival in metastatic TNBC patients[62]. In this randomized placebo-controlled, double-blind phase II trial, patients with measurable and inoperable locally advanced or metastatic TNBC previously untreated with systemic therapy were treated with paclitaxel plus ipatasertib and paclitaxel plus placebo. Median progression-free survival in the intention-to-treat population was 6.2 months (95% CI 3.8—9.0) with ipatasertib plus paclitaxel versus 4.9 months (3.6–5.4) with placebo plus paclitaxel (hazard ratio 0.60, 95% CI 0.37–0.98, p= 0.037) (Figure 3A). Notably, the effect of ipatasertib in patients with PTEN-low tumors by immunohistochemistry (IHC) was not greater than those with non-PTEN-low tumors. However, efficacy in the patients with PIK3CA/AKT1/PTEN-altered tumors on next generation sequencing (NGS) showed an encouraging progression-free survival (HR of 0.44 and an increase of 4.1 months in the median progression-free survival) with a median of 9.0 months in the ipatasertib group, versus 4.9 months in the placebo group (Figure 3B). These results are encouraging for the utility of NGS for patient selection to benefit AKT inhibitors. The lack of enhanced efficacy when using only PTEN loss by IHC as a stratifier may be due to other mechanisms of pathway alteration diluting the effect. However, PTEN IHC may also have additional challenges as a predictive marker as previous studies have reported a high rate of discordance between primary and metastasis[63], suggesting tumor heterogeneity and/or PTEN status evolution.
Figure 3.
Progression-free survival (PFS) in randomized clinical trial of ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple negative breast cancer. A) PFS in the intention-to-treat population unselected by biomarker, and B) PFS in patients with tumors bearing PIK3CA/AKT1/ phosphatase and tensin homolog (PTEN) alterations. Figure was reprinted with permission from “Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial” by Sung-Bae Kim, 2017. The Lancet Oncology, Volume 18, Pages 1360–1372.
5.2. Capivasertib (AZD5363; AstraZeneca)
Capivasertib (AZD5363) is an ATP-competitive inhibitor that inhibits all three AKT isoforms with a potency of ≤10 nM in cell-free assays and good preclinical drug metabolism and pharmacokinetics properties[64]. In preclinical studies, capivasertib inhibits phosphorylation of PRAS40 and GSK3β (AKT substrates) as well as S6 and 4E-BP1; however, it increases the phosphorylation of AKT at Ser473 and Thr308[65]. In a standard growth assay using a panel of 182 tumor cell lines, capivasertib inhibits the proliferation of 41 cell lines with most sensitivity observed in breast cancer cells (63%)[64]. Additionally, activating mutations in PIK3CA and AKT1 or loss of the PTEN tumor suppressor gene significantly enhanced capivasertib sensitivity.
Capivasertib monotherapy shows encouraging clinical efficacy in various AKT1-E17K mutant solid tumors, including ER-positive breast, cervical, and ovarian cancer. In a phase I study, capivasertib as a single drug resulted in partial response in two patients, one with breast and the other one with ovarian cancer, both with AKT1-E17K mutation[66]. In a multihistology basket study of capivasertib in patients with advanced solid tumors with AKT1-E17K mutation, median progression-free survival was 5.5 months, 6.6 months, and 4.2 months in patients with ER-positive breast cancer, gynecologic, and other solid tumors respectively[67]. In an exploratory biomarker analysis, the presence of coincident PI3K pathway hotspot mutations enhanced efficacy (HR 0.21, p=0.045). Recently in the NCI-MATCH trial, capivasertib caused partial responses or stable disease in nearly 70% of the patients with metastatic AKT1-mutant tumor[68]. Notably in this study, ER-positive patients were allowed to continue endocrine therapy. Overall, studies to date suggest that capivasertib is an active agent especially in tumors with AKT1-E17K mutation.
There are several ongoing phase I and II clinical studies determining capivasertib therapeutic effect as a single agent or in combination with other drugs in breast and ovarian cancers. The combination of capivasertib with estrogen receptor antagonist fulvestrant in AKT1 or PTEN-mutant ER-positive metastatic breast cancer is currently in clinical development[69]. Recently, in a phase II trial of postmenopausal women with ER-positive and HER2-negative endocrine resistant advanced breast cancer(), the addition of capivasertib to fulvestrant resulted in significantly longer progression-free survival compared to placebo with fulvestrant (PFS 10.3 months vs 4.8 months; Hazard Ratio (HR) 0.57; 95% CI: 0.39 to 0.84; one-sided p = 0.0017; two-sided 0.0035), with a trend toward improved survival (26 vs 20 months)[70].
Moreover, the combination of capivasertib with paclitaxel in a phase II trial of metastatic TNBC demonstrated longer progression-free survival and a significant increase in overall-survival[71]. This combination is being further explored as a first line therapy for TNBC. Combination with targeted therapy is also being explored. Phase I expansion of PARP inhibitor olaparib and capivasertib indicated evidence of antitumor activity in ovarian, endometrial, and TNBC with 50% overall response rate observed in endometrial cancer cohort[72].
5.3. ARQ751 and ARQ092
ARQ092 and its analog ARQ751 are highly potent allosteric inhibitors of all three AKT isoforms and AKT-E17K. ARQ092 and ARQ751 dephosphorylate active AKT and prevent its plasma membrane localization[73, 74]. Both compounds exhibited strong antitumor activity across several tumor types, being most potent in breast, endometrial, leukemia, and colorectal cancer cell lines [74].
In a phase I study of ARQ092 in subjects with advanced solid tumors, two patients with activating mutation in PIK3CA gene experienced durable partial responses[75] and antitumor activity was observed in patients harboring AKT1-E17K and PIK3CA-H1047R mutations in a phase Ib trial[76]. Results from another phase Ib study of ARQ092 in combination with carboplatin plus paclitaxel demonstrated encouraging clinical activity in ovarian patients. Two ovarian cancer patients, pretreated with carboplatin and paclitaxel, achieved complete response (AKT-E17K mutant) and partial response (unknown AKT mutant)[77]. Ongoing ARQ751 phase I dose escalation study in patients with advanced solid tumors with AKT-1, 2, and 3 genomic alterations, PI3K activating mutations, PTEN-null, and other PTEN actionable mutations demonstrated a manageable safety profile[78]. Interestingly, a non-oncological phase I trial of ARQ092 is also being conducted for the treatment of Proteus syndrome in children and adults patients[79].
5.4. Afuresertib (GSK2110183) and Uprosertib (GSK2141795)
Afuresertib (GSK2110183) and uprosertib (GSK2141795) are potent ATP-competitive inhibitors of AKT1, 2, and 3 that demonstrate more sensitivity in hematological cell lines[80]. Afuresertib, which has more potency against AKT1, is shown to be safe and well-tolerated with manageable adverse events, including hyperglycemia in a clinical study[81]. Single agent activity was observed with afuresertib in patients with multiple myeloma[81]. A phase I/II clinical study on afuresertib, in combination with paclitaxel and carboplatin, showed favorable activity in patients with platinum-resistant ovarian cancer[82]. Continuous daily dosing of afuresertib was poorly tolerated in combination with MEK inhibitor trametinib in a phase I study of patients with solid tumors. However, an intermittent dose schedule of afuresertib with trametinib provided a more tolerable safety profile[83]. Uprosertib, a close analog of afuresertib, has more inhibition potency with greater off-target effects. In a phase I study, uprosertib was safe and well-tolerated, with preliminary clinical activity seen as monotherapy in patients with PIK3CA mutation or PTEN loss[84].
5.5. MK-2206
Allosteric AKT inhibitor, MK-2206 (Merck), predominantly inhibits AKT1 and 2 with preclinical single agent activity that prevents phosphorylation of downstream AKT signaling in a range of cancer cell lines[85]. The anti-proliferative effect of MK-2206 was greater in tumor cell lines having AKT2 amplification, PIK3CA mutation, PTEN loss/mutation, or RTK (such as HER2) constitutive activation[85, 86].
In spite of promising preclinical antitumor activity in the diseases, MK-2206 has shown limited efficacy in monotherapy in phase II trials[87]. MK-2206 has been investigated in a phase II trial for the treatment of platinum-resistant ovarian, primary peritoneal or fallopian tube cancers, as well as breast cancer with PIK3CA/AKT mutations or PTEN loss. MK-2206 was also tested in a Phase II trial in advanced breast cancer patients selected for PIK3CA/AKT1 mutations or PTEN loss/PTEN mutation[88]. In spite of the fact that preclinical models with PIK3CA/AKT and PTEN alterations showed antitumor efficacy of MK-2206, MK-2206 monotherapy has limited clinical activity in these biomarker-selected, metastatic breast cancer patients. Notably, MK-2206 treatment was associated with a significant decline in pAKT-S473 and pAKT-T308 and PI3K activation score in peripheral blood mononuclear cells (PBMC) and platelet-rich plasma (PRP), but pathway inhibition was not observed upon comparison of pre-treatment and on-treatment tumor biopsies. By IHC, there was no significant decrease in median pAKT-S473 or Ki-67 staining in the overall cohort, but a drop was observed in the two patients with clinical benefit (one with partial response, one with prolonged stable disease). This suggests that there may be inadequate target inhibition at tolerable doses in heavily pre-treated patients with pathway activation.
In a preclinical breast cancer model, upregulation of AKT3 due to epigenetic reprogramming conferred resistance to MK-2206, which was reversible upon drug removal[89]. Therefore, attempts were focused on combinational therapies. The evaluation of MK-2206 treatment alone or in combination with trastuzumab or lapatinib (HER inhibitors) in several phase I studies of HER2-positive breast cancer showed antitumor activity[90]. Phase I trial of MK-2206 combined with trastuzumab or lapatinib was tolerated with clinical efficacy in HER2-positive breast cancer.
The combination of MK-2206 and paclitaxel in breast cancer and solid tumors was well-tolerated with evidence of antitumor activity[91]. In the adaptive neoadjuvant I-SPY2 trial, the combination of MK-2206 with paclitaxel improved the predicted pathologic response rates in several HR-negative and HER2-positive breast cancers compared to standard chemotherapy[92].
MK-2206 was also assessed in ER-positive breast cancer patients in a window-of-opportunity trial and a neoadjuvant trial in combination with endocrine therapy, both with concerns raised about tolerability[93, 94]. Both of these trials performed pharmacodynamics analysis of pretreatment and on-treatment tumor samples and also suggested that there may be suboptimal target inhibition at clinically achievable doses[95].
5.6. Perifosine
Perifosine is a relatively non-selective AKT allosteric inhibitor that disrupts the interaction between AKT and phospholipids in the plasma membrane and triggers apoptosis in vitro and in vivo of human tumor models[96]. Although perifosine displayed significant anti-proliferative activity in preclinical studies, no objective response was observed in a phase II trial of perifosine in pretreated metastatic breast cancer patients[97]. Perifosine plus docetaxel was also assessed in a phase I study in platinum-resistant epithelial ovarian cancer patients[98]. The combination demonstrated evidence of efficacy with acceptable safety profile, but progression-free survival and overall-survival was 1.9 and 4.5 months respectively. One patient with a PTEN mutation achieved partial remission (PR) for 7.5 months, suggesting that targeting AKT with next generation inhibitors in ovarian cancer having PI3K pathway alterations may be warranted.
5.7. BAY1125976
BAY1125976 is a potent and selective allosteric AKT1 and 2 inhibitor with anti-proliferative activity in a panel of human cancer cell lines––particularly breast and prostate cancer cells[99]. It was also well tolerated in vivo with antitumor efficacy observed in tumor models having PI3K/AKT pathway alterations, including AKT1-E17K and PTEN-loss. BAY1125976 is currently in early phase clinical trials (ClinicalTrials.gov Identifier: ).
5.8. Triciribine (PTX-200)
Triciribine phosphate monohydrate (TCN-PM) is an allostericinhibitor of all AKT isoforms which binds to the PH domain and prevents AKT phosphorylation and subsequent activation[100]. In preclinical testing, it has been shown to inhibit AKT phosphorylation, cause cell cycle arrest, and induce apoptosis[101, 102]. The combination treatment of triciribine with gemcitabine in pancreatic cancer cells augmented the antitumor activity and overcame the resistance mediated by AKT activation[103].
Preliminary evidences from phase I studies in patients with solid tumors containing activated AKT and advanced hematologic malignancies indicated that triciribine reduced AKT phosphorylation levels, but its efficacy as a single agent was limited due to toxicity[104, 105]. The clinical activity of carboplatin plus triciribine in patients with platinum-resistance recurrent ovarian cancer is being explored in ongoing studies (ClinicalTrials.gov Identifier: ).
5.9. TAS-117
TAS-117 is a highly potent and selective allosteric AKT inhibitor with a high affinity for AKT1, 2, and 3. TAS-117 induced a significant growth inhibition in multiple myeloma cell lines with high level of baseline AKT phosphorylation[106]. In vivo, TAS-117 was effective in combination with chemotherapeutic agents and greatly improved the sensitivity of carboplatin or irinotecan in a human ovarian cancer cell line-derived (A2780A) xenograft model[107]. Phase II trial of TAS-117 in subjects with advanced solid tumors having PI3K/AKT pathway aberrations is under investigation (ClinicalTrials.gov Identifier: ).
5.10. LY2780301
LY2780301 is a highly selective and potent ATP-competitive dual inhibitor of p70S6K and AKT that exhibited anti-proliferative activity in a broad range of cancer cell lines with effective tumor growth inhibition in xenograft models of A2780 (ovarian), HCT116 (colon), H460 (lung), and PC3 (prostate). (Data on File from Eli Lilly Company).
A phase I trial of LY2780301 in patients with advanced or metastatic cancers was conducted to determine the recommended phase II dose as a single agent[108]. Among patients receiving 500 mg daily, more than 50% exhibited reduced S6 phosphorylation in skin biopsies on Day 8 of treatment. However, this effect was not maintained with any correlation of pharmacokinetics and S6 phosphorylation levels the in skin. The pharmacokinetic and drug interaction of LY2780301 in combination with paclitaxel is being examined in women with HER2-negative metastatic breast cancer[109]. Combining LY2780301 and paclitaxel in a recent phase Ib/II trial of HER2-negative, locally advanced or metastatic breast cancer patients, showed preliminary evidences of efficacy, independently of PI3K/AKT activation (ClinicalTrials.gov Identifier: ). The addition of LY2780301 to gemcitabine in another phase Ib dose escalation study of patients with PI3K/AKT pathway alterations demonstrated acceptable toxicity with 74% disease control at cycle 2, and a 5% partial response rate (responses in breast and cervical cancer)[110].
5.11. MSC2363318A
MSC2363318A is a selective and potent ATP-competitive inhibitor of p70S6K, AKT1 and 3 with strong growth inhibition activity in many solid tumor cell lines especially those with molecular alterations in PI3K/AKT pathway[111]. Evaluation of MSC2363318A in patient-derived xenograft models of breast cancer suggested that dual p70S6K/AKT inhibitor may improve AKT pathway inhibition by blocking the negative AKT activation emanating through the negative feedback loop[112]. The unique capacity of MSC2363318A to pass the blood-brain barrier[111] allows clinical investigations of cancers with PI3K/AKT pathway dysregulation that involved central nervous system malignancies. MSC2363318A alone and in combination with trastuzumab and tamoxifen has been investigated in a phase I dose escalation study of patients with advanced malignancies[113].
5.12. Cenisertib (R763/AS703569)
Cenisertib is a potent inhibitor of Aurora kinases that blocks the kinase activity of AKT as well as FLT3, VEGFR2, LYN, BTK, and KIT and induces growth inhibition, apoptosis and cell cycle arrest in various cancer cell lines both in vitro and in vivo [114, 115]. Data from two phase I trials in advanced solid tumors (two arm trial) and hematological malignancies indicated that cenisertib was well tolerated with early evidence of activity observed in patients with leukemia[116, 117]. The combination of cenisertib with gemcitabine in subjects with advanced solid tumors is in phase I clinical study[118].
6. Conclusions
Over the past few decades, a growing body of literature has emphasized the key role of AKT during tumor development, survival, and progression. In fact, AKT hyperactivation contributes to many hallmarks of cancer, such as evading apoptosis, self-sufficiency in growth, invasion, and metastasis[119]. Given the role of AKT as an attractive target, several small molecule inhibitors have been discovered and progressed in trial studies. However, none of the AKT inhibitors have been approved for clinical use to date. The structural similarity between AKT isoforms and the off-target inhibition of AGC kinase family hindered the discovery of selective and efficacious compounds inhibiting AKT. First generation AKT inhibitors exhibited limited clinical efficacy as single agent and have limitations due to toxicity. Clinical trials that are designed based on mechanistic insights of AKT pathway and combinational treatments will provide a better clinical perspective of AKT inhibitors and use the full potential of these compounds.
7. Expert opinion
One of the main goals of translational research in the era of precision medicine is the identification of targeted agents and their predictive biomarkers for the development of successful therapeutic strategies. Identification and validation of potential biomarkers in response to AKT inhibitors treatment is increasingly essential to design trials for novel targeting agents and pave the way for clinical practice of these compounds. Several studies have indicated that genomic aberrations in PI3K/AKT pathway are associated with sensitivity to AKT inhibitors[120]. These observations suggest that genomic alterations in this pathway (activating AKT and PIK3CA mutations, and inactivating PTEN mutations) are candidates for biomarkers of response to AKT inhibitors. In contrast, PTEN loss by IHC may be less robust as a biomarker. The expression of PTEN may be lost due to non-genomic mechanisms; therefore, DNA sequencing in addition to IHC quantification will provide more extensive insight.
In the clinic, the AKT1-E17K mutation, which occurs at a relatively low rate in breast and ovarian tumors, is shown to be a strong predictive biomarker of response to AZD5363 monotherapy[67]. The fact that AKT1-E17K presents at a low frequency should not minimize its potent biological effect during tumorigenicity and potential impact on outcomes in patients with tumors bearing this mutation. Although accumulation of sequencing data has identified more AKT mutations, most of these alterations do not activate pathway signaling or affect growth compared to a wild type. In a functional characterization study of more than twenty AKT mutations, E17K (AKT1 and AKT2), L52R, Q79K, and D323H (AKT1) were the only mutants that showed pathway activations. Therefore, the majority of AKT variants identified by clinical tumor sequencing are passenger mutations with few functional consequences[121]. In addition to analysis of somatic alterations in PI3K/AKT pathway, transcriptomic and proteomic signatures of AKT inhibitors sensitivity also need to be explored to better select patients that may benefit from AKT inhibitors.
Despite the development of a large number of compounds with promising results for inhibiting AKT, there is a gap between preclinical data and the lack of a clinically effective AKT inhibitor. Although AKT inhibitors have compelling antitumor activity in tumors having specific genetic alterations, the complex cellular function of AKT and activation of potential feedback signaling limit the efficacy of AKT as a single agent. AKT inhibitors have been shown to improve the therapeutic effect of standard anticancer agents in several preclinical studies. Therefore, combinational therapy approaches appeared to be a major research direction for the clinical utilization of AKT inhibitors.
The antitumor activity of ipatasertib and paclitaxel combination seen in patients with TNBC harboring PIK3K/AKT pathway activation supports the hypothesis that PI3K/AKT activation predicts sensitivity to AKT inhibitors[62]. The efficacy seen with capivasertib and paclitaxel also support the hypothesis that targeting AKT in tumors with PI3K pathway alterations can enhance chemosensitivity and improve clinical outcomes. Further studies are necessary to determine additional rational combinations that can enhance efficacy of AKT inhibitors, potentially by targeting compensatory mechanisms, and/or enhancing apoptosis.
Article highlights.
Aberrant activation of AKT plays an important role in the pathogenesis of many human tumors, including breast and ovarian cancers.
Many compounds have been developed to inhibit AKT as a validated therapeutic target.
Tumors with PI3K/AKT pathway genomic alterations demonstrated more sensitivity to AKT inhibitions.
Combining AKT inhibitors with other therapeutic agents can greatly enhance the efficacy of these inhibitors.
the identification of targeted agents and their predictive biomarkers is necessary for the development of successful therapeutic strategies.
Acknowledgments
We thank Visual Art at the University of Texas, MD Anderson Cancer Center for generating the illustration in Figure 1.
Funding
The work of the authors was supported in part by the Nellie B. Connally Breast Cancer Research Endowment, The Cancer Prevention and Research Institute of Texas RP150535, Sheikh Khalifa Al Nahyan Ben Zayed Institute for Personalized Cancer Therapy, MD Anderson Cancer Center Support grant (P30 CA016672).
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.DeSantis C, Siegel R, Bandi P, et al. Breast cancer statistics, 2011. Ca-Cancer J Clin. 2011. November-December;61(6):409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Stewart SL, Harewood R, Matz M, et al. Disparities in ovarian cancer survival in the United States (2001–2009): Findings from the CONCORD-2 study. Cancer-Am Cancer Soc. 2017. December 15;123:5138–5159. doi: 10.1002/cncr.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan WM, Chang JJ, Fu PF. Endocrine therapy resistance in breast cancer: current status, possible mechanisms and overcoming strategies. Future Med Chem. 2015;7(12):1511–1519. doi: 10.4155/fmc.15.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monk BJ, Coleman RL. Changing the Paradigm in the Treatment of Platinum-Sensitive Recurrent Ovarian Cancer From Platinum Doublets to Nonplatinum Doublets and Adding Antiangiogenesis Compounds. Int J Gynecol Cancer. 2009. December;19:S63–S67. doi: 10.1111/IGC.0b013e3181c104fa. [DOI] [PubMed] [Google Scholar]
- 5.Osborne CK, Schiff R. Mechanisms of Endocrine Resistance in Breast Cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Zyl B, Tang D, Bowden NA. Biomarkers of platinum resistance in ovarian cancer: what can we use to improve treatment. Endocr-Relat Cancer. 2018. May;25(5):R303–R318. doi: 10.1530/Erc-17-0336. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa A, de Feo D, Godwin AK, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995. August 22;64(4):280–5. [DOI] [PubMed] [Google Scholar]
- 8.Dahl E, Sadr-Nabavi A, Klopocki E, et al. Systematic identification and molecular characterization of genes differentially expressed in breast and ovarian cancer. J Pathol. 2005. January;205(1):21–28. doi: 10.1002/path.1687. [DOI] [PubMed] [Google Scholar]
- 9.Ma CX. The PI3K Pathway as a Therapeutic Target in Breast Cancer. Am J Hematol-Oncol. 2015. March;11(3):23–29. [Google Scholar]
- 10.Mabuchi S, Kuroda H, Takahashi R, et al. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015. April;137(1):173–179. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Hay N The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005. September;8(3):179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001. August;114(16):2903–2910. [DOI] [PubMed] [Google Scholar]
- 13.Steelman LS, Stadelman KM, Chappell WH, et al. Akt as a therapeutic target in cancer. Expert Opin Ther Tar. 2008. September;12(9):1139–1165. doi: 10.1517/14728220802351707. [DOI] [PubMed] [Google Scholar]
- 14.Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017. April 20;169(3):381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mundi PS, Sachdev J, McCourt C, et al. AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol. 2016. October;82(4):943–56. doi: 10.1111/bcp.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheon DJ, Orsulic S. Mouse Models of Cancer. Annu Rev Pathol-Mech. 2011;6:95–119. doi: 10.1146/annurev.pathol.3.121806.154244. [DOI] [PubMed] [Google Scholar]
- 17.Singh M, Johnson L. Using genetically engineered mouse models of cancer to aid drug development: An industry perspective. Clin Cancer Res. 2006. September 15;12(18):5312–5328. doi: 10.1158/1078-0432.Ccr-06-0437. [DOI] [PubMed] [Google Scholar]
- 18.Arcaro A, Guerreiro AS. The phosphoinositide 3-kinase pathway in human cancer: Genetic alterations and therapeutic implications. Curr Genomics. 2007. August;8(5):271–306. doi: Doi 10.2174/138920207782446160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downward J PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004. April;15(2):177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Testa JR, Bellacosa A. Commentary - AKT plays a central role in tumorigenesis. P Natl Acad Sci USA. 2001. September 25;98(20):10983–10985. doi: DOI 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Zhao W, Guo H, et al. AKT isoform-specific expression and activation across cancer lineages. BMC Cancer. 2018. July 16;18(1):742. doi: 10.1186/s12885-018-4654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanada M, Feng JH, Hemmings BA. Structure, regulation and function of PKB/AKT - a major therapeutic target. Bba-Proteins Proteom. 2004. March 11;1697(1–2):3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Hemmings BA. Structure, regulation and function of PKB/Akt - A major therapeutic target. Cell Mol Biol Lett. 2003;8(2a):527–527. [Google Scholar]
- 24.Barnett SF, Defeo-Jones D, Fu S, et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem J. 2005. January 15;385(Pt 2):399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007. April;35(Pt 2):231–5. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 26.Zhou GL, Tucker DF, Bae SS, et al. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J Biol Chem. 2006. November 24;281(47):36443–53. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]
- 27.Easton RM, Cho H, Roovers K, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005. March;25(5):1869–78. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2(1):19–42. [PMC free article] [PubMed] [Google Scholar]
- 29.Scheid MP, Woodgett JR. Unravelling the activation mechanisms of protein kinase B/Akt. Febs Lett. 2003. July 3;546(1):108–112. doi: 10.1016/S0014-5793(03)00562-3. [DOI] [PubMed] [Google Scholar]
- 30.Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997. April 1;7(4):261–269. doi: Doi 10.1016/S0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 31.Conus NM, Hannan KM, Cristiano BE, et al. Direct identification of tyrosine 474 as a regulatory phosphorylation site for the Akt protein kinase. Journal of Biological Chemistry. 2002. October 11;277(41):38021–38028. doi: 10.1074/jbc.M203387200. [DOI] [PubMed] [Google Scholar]
- 32.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005. February 18;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 33.Feng J, Park J, Cron P, et al. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004. September 24;279(39):41189–96. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 34.Gray CW, Coster ACF. Crosstalk in transition: the translocation of Akt. J Math Biol. 2018. October 9. doi: 10.1007/s00285-018-1297-8. [DOI] [PubMed] [Google Scholar]
- 35.O’Neill AK, Niederst MJ, Newton AC. Suppression of survival signalling pathways by the phosphatase PHLPP. FEBS J. 2013. January;280(2):572–83. doi: 10.1111/j.1742-4658.2012.08537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005. April 1;18(1):13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Altomare DA, Khaled AR. Homeostasis and the Importance for a Balance Between AKT/mTOR Activity and Intracellular Signaling. Curr Med Chem. 2012. August;19(22):3748–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006. October 6;127(1):125–137. doi: DOI 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Li XJ, Leem SH, Park MH, et al. Regulation of YAP through an Akt-dependent process by 3, 3 ‘-diindolylmethane in human colon cancer cells. Int J Oncol. 2013. December;43(6):1992–1998. doi: 10.3892/ijo.2013.2121. [DOI] [PubMed] [Google Scholar]
- 40.Xu Q, Liu LZ, Qian X, et al. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012. January;40(2):761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison DK. The 14–3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009. January;19(1):16–23. doi: 10.1016/j.tcb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997. October 17;91(2):231–241. doi: Doi 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 43.Qi XJ, Wildey GM, Howe PH. Evidence that Ser(87) of Bim(EL) is phosphorylated by Akt and regulates BimEL apoptotic function. Journal of Biological Chemistry. 2006. January 13;281(2):813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 44.Sangawa A, Shintani M, Yamao N, et al. Phosphorylation status of Akt and caspase-9 in gastric and colorectal carcinomas. Int J Clin Exp Patho. 2014;7(6):3312–3317. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang XB, Tang NM, Hadden TJ, et al. Akt, FoxO and regulation of apoptosis. Bba-Mol Cell Res. 2011. November;1813(11):1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Ogawara Y, Kishishita S, Obata T, et al. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. Journal of Biological Chemistry. 2002. June 14;277(24):21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 47.Park S, Kim D, Dan HC, et al. Identification of Akt Interaction Protein PHF20/TZP That Transcriptionally Regulates p53. Journal of Biological Chemistry. 2012. March 30;287(14):11151–11163. doi: 10.1074/jbc.M111.333922. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Park S, Kim D, Dan HC, et al. Identification of Akt interaction protein PHF20/TZP that transcriptionally regulates p53 (vol 287, pg 11151, 2012). Journal of Biological Chemistry. 2016. October 21;291(43): 22852–22852. doi: 10.1074/jbc.A111.333922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi Y, Liu X, Han EK, et al. Optimal classes of chemotherapeutic agents sensitized by specific small-molecule inhibitors of akt in vitro and in vivo. Neoplasia. 2005. November;7(11):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007. July 26;448(7152):439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 51.Kroeger PT, Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Curr Opin Obstet Gyn. 2017. February;29(1):26–34. doi: 10.1097/Gco.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arboleda MJ, Lyons JF, Kabbinavar FF, et al. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003. January 1;63(1):196–206. [PubMed] [Google Scholar]
- 53.Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012. October 4;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carmona FJ, Montemurro F, Kannan S, et al. AKT signaling in ERBB2-amplified breast cancer. Pharmacol Ther. 2016. February;158:63–70. doi: 10.1016/j.pharmthera.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cristiano BE, Chan JC, Hannan KM, et al. A specific role for AKT3 in the genesis of ovarian cancer through modulation of G(2)-M phase transition. Cancer Research. 2006. December 15;66(24):11718–11725. doi: 10.1158/0008-5472.Can-06-1968. [DOI] [PubMed] [Google Scholar]
- 56.Bhutani J, Sheikh A, Niazi AK. Akt inhibitors: mechanism of action and implications for anticancer therapeutics. Infect Agents Cancer. 2013. December 13;8. doi: Artn 49, 10.1186/1750-9378-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fortier AM, Asselin E, Cadrin M. Functional specificity of Akt isoforms in cancer progression. Biomol Concepts. 2011. April 1;2(1–2):1–11. doi: 10.1515/bmc.2011.003. [DOI] [PubMed] [Google Scholar]
- 58.Blake JF, Xu R, Bencsik JR, et al. Discovery and Preclinical Pharmacology of a Selective ATP-Competitive Akt Inhibitor (GDC-0068) for the Treatment of Human Tumors. J Med Chem. 2012. September 27;55(18):8110–8127. doi: 10.1021/jm301024w. [DOI] [PubMed] [Google Scholar]
- 59.Lin J, Sampath D, Nannini MA, et al. Targeting Activated Akt with GDC-0068, a Novel Selective Akt Inhibitor That Is Efficacious in Multiple Tumor Models. Clin Cancer Res. 2013. April 1;19(7):1760–1772. doi: 10.1158/1078-0432.Ccr-12-3072. [DOI] [PubMed] [Google Scholar]
- 60.Saura C, Roda D, Rosello S, et al. A First-in-Human Phase I Study of the ATP-Competitive AKT Inhibitor Ipatasertib Demonstrates Robust and Safe Targeting of AKT in Patients with Solid Tumors. Cancer Discov. 2017. January;7(1):102–113. doi: 10.1158/2159-8290.Cd-16-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isakoff SJ, Infante JR, Juric D, et al. Phase Ib Dose-Escalation Study of the Akt Inhibitor Ipatasertib (Ipat) with Paclitaxel (P) in Patients (Pts) with Advanced Solid Tumors. Ann Oncol. 2014. September;25. [Google Scholar]
- 62.Kim SB, Dent R, Im SA, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017. October;18(10):1360–1372. doi: 10.1016/S1470-2045(17)30450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011. June;10(6):1093–101. doi: 10.1158/1535-7163.MCT-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Addie M, Ballard P, Buttar D, et al. Discovery of 4-Amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide (AZD5363), an Orally Bioavailable, Potent Inhibitor of Akt Kinases. J Med Chem. 2013. March 14;56(5):2059–2073. doi: 10.1021/jm301762v. [DOI] [PubMed] [Google Scholar]
- 65.Davies BR, Greenwood H, Dudley P, et al. Preclinical Pharmacology of AZD5363, an Inhibitor of AKT: Pharmacodynamics, Antitumor Activity, and Correlation of Monotherapy Activity with Genetic Background. Mol Cancer Ther. 2012. April;11(4):873–887. doi: 10.1158/1535-7163.Mct-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 66.Davies BR, Guan N, Logie A, et al. Tumors with AKT1(E17K) Mutations Are Rational Targets for Single Agent or Combination Therapy with AKT Inhibitors. Mol Cancer Ther. 2015. November;14(11):2441–2451. doi: 10.1158/1535-7163.Mct-15-0230. [DOI] [PubMed] [Google Scholar]
- 67.Hyman DM, Smyth LM, Donoghue MTA, et al. AKT Inhibition in Solid Tumors With AKT1 Mutations. J Clin Oncol. 2017. July 10;35(20):2251-+. doi: 10.1200/Jco.2017.73.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capivasertib Active against AKT1-Mutated Cancers. Cancer Discov. 2019;9(1):OF7–OF7. doi: 10.1158/2159-8290.cd-nb2018-153. [DOI] [PubMed] [Google Scholar]
- 69.Smyth LM, Oliveira M, Ciruelos E, et al. AZD5363 in combination with fulvestrant in AKT1-mutant ER-positive metastatic breast cancer. Cancer Research. 2018. February;78(4). [Google Scholar]
- 70.Vicier C, Isambert N, Dalenc F, et al. TAKTIC: A prospective, multicenter, uncontrolled, phase IB/II study of LY2780301 (LY) in combination with weekly paclitaxel (wP) in HER2-negative locally advanced (LA) or metastatic breast cancer (MBC) patients. J Clin Oncol. 2019;37(15_suppl):1091–1091. doi: 10.1200/JCO.2019.37.15_suppl.1091. [DOI] [Google Scholar]
- 71.Schmid P, Abraham J, Chan S, et al. AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): A randomised, double-blind, placebo-controlled, phase II trial. J Clin Oncol. 2018. May 20;36(15). doi: DOI 10.1200/JCO.2018.36.15_suppl.1007. [DOI] [PubMed] [Google Scholar]
- 72.Westin S, Litton J, Williams R, et al. Phase I expansion of olaparib (PARP inhibitor) and AZD5363 (AKT inhibitor) in recurrent ovarian, endometrial and triple negative breast cancer. Ann Oncol. 2017. September;28. [Google Scholar]
- 73.Lapierre JM, Eathiraj S, Vensel D, et al. Discovery of 3-(3-(4-(1-Aminocyclobutyl)phenyl)-5-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine (ARQ 092): An Orally Bioavailable, Selective, and Potent Allosteric AKT Inhibitor. J Med Chem. 2016. July 14;59(13):6455–6469. doi: 10.1021/acs.jmedchem.6b00619. [DOI] [PubMed] [Google Scholar]
- 74.Yu Y, Savage RE, Eathiraj S, et al. Targeting AKT1-E17K and the PI3K/AKT Pathway with an Allosteric AKT Inhibitor, ARQ 092. Plos One. 2015. October 15;10(10). doi: ARTN e0140479, 10.1371/journal.pone.0140479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tolcher A, Harb W, Sachdev J, et al. Results from a phase 1 study of ARQ 092, a novel pan AKT-inhibitor, in subjects with advanced solid tumors or recurrent malignant lymphoma. Eur J Cancer. 2015. September;51:S66–S66. doi: Doi 10.1016/S0959-8049(16)30201-5. [DOI] [Google Scholar]
- 76.Abbadessa G, Yu Y, Eathiraj S, et al. Abstract B181: Association of AKT1E17K and PIK3CAH1047R mutations with efficacy of ARQ 092 in vitro, in vivo and in patients. Molecular Cancer Therapeutics. 2015;14(12 Supplement 2):B181–B181. doi: 10.1158/1535-7163.targ-15-b181. [DOI] [Google Scholar]
- 77.Lakhani N, Tolcher AW, Rasco DW, et al. Results of a phase Ib study of ARQ 092 in combination with carboplatin (C) plus paclitaxel (P), or with P in patients (pts) with solid tumors. J Clin Oncol. 2017. May 20;35. doi: 10.1200/JCO.2017.35.15_suppl.2524. [DOI] [Google Scholar]
- 78.Pant S, Subbiah V, Rodon J, et al. Abstract CT024: Results of a phase I dose escalation study of ARQ 751 in adult subjects with advanced solid tumors with AKT1, 2, 3 genetic alterations, activating PI3K mutations, PTEN-null, or other known actionable PTEN mutations. Cancer Research. 2018;78(13 Supplement):CT024–CT024. doi: 10.1158/1538-7445.am2018-ct024. [DOI] [Google Scholar]
- 79.Lindhurst MJ, Yourick MR, Yu Y, et al. Repression of AKT signaling by ARQ 092 in cells and tissues from patients with Proteus syndrome. Sci Rep-Uk. 2015. December 11;5. doi: ARTN 17162, 10.1038/srep17162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dumble M, Crouthamel MC, Zhang SY, et al. Discovery of novel AKT inhibitors with enhanced anti-tumor effects in combination with the MEK inhibitor. PLoS One. 2014;9(6):e100880. doi: 10.1371/journal.pone.0100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spencer A, Yoon SS, Harrison SJ, et al. The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood. 2014. October 2;124(14):2190–2195. doi: 10.1182/blood-2014-03-559963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blagden SP, Gabra H, Hamilton AL, et al. Phase I/II dose-escalation and expansion study of afuresertib plus carboplatin and paclitaxel in recurrent ovarian cancer. J Clin Oncol. 2016. May 20;34(15). doi: 10.1200/JCO.2016.34.15_suppl.2551. [DOI] [Google Scholar]
- 83.Tolcher AW, Patnaik A, Papadopoulos KP, et al. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemoth Pharm. 2015. January;75(1):183–189. doi: 10.1007/s00280-014-2615-5. [DOI] [PubMed] [Google Scholar]
- 84.Aghajanian C, Bell-McGuinn KM, Burris HA 3rd, et al. A phase I, open-label, two-stage study to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral AKT inhibitor GSK2141795 in patients with solid tumors. Invest New Drugs. 2018. December;36(6):1016–1025. doi: 10.1007/s10637-018-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sangai T, Akcakanat A, Chen HQ, et al. Biomarkers of Response to Akt Inhibitor MK-2206 in Breast Cancer. Clin Cancer Res. 2012. October 15;18(20):5816–5828. doi: 10.1158/1078-0432.Ccr-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu W, Defeo-Jones D, Davis L, et al. In vitro and in vivo antitumor activities of MK-2206, a new allosteric Akt inhibitor. Cancer Research. 2009. May 1;69. [Google Scholar]
- 87.Myers AP, Broaddus R, Makker V, et al. Phase II, two-stage, two-arm, PIK3CA mutation stratified trial of MK-2206 in recurrent endometrial cancer (EC). J Clin Oncol. 2013;31(15_suppl):5524–5524. doi: 10.1200/jco.2013.31.15_suppl.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xing Y, Lin NU, Maurer MA, et al. Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Research. 2019. 2019/07/05;21(1):78. doi: 10.1186/s13058-019-1154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stottrup C, Tsang T, Chin YR. Upregulation of AKT3 Confers Resistance to the AKT Inhibitor MK2206 in Breast Cancer. Mol Cancer Ther. 2016. August;15(8):1964–74. doi: 10.1158/1535-7163.MCT-15-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumler I, Tuxen MK, Nielsen DL. A systematic review of dual targeting in HER2-positive breast cancer. Cancer Treat Rev. 2014. March;40(2):259–270. doi: 10.1016/j.ctrv.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez-Angulo AM, Krop I, Akcakanat A, et al. SU2C Phase Ib Study of Paclitaxel and MK-2206 in Advanced Solid Tumors and Metastatic Breast Cancer. Jnci-J Natl Cancer I. 2015. March;107(3). doi: ARTN dju493, 10.1093/jnci/dju493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tripathy D, Chien AJ, Hylton N, et al. Adaptively randomized trial of neoadjuvant chemotherapy with or without the Akt inhibitor MK-2206: Graduation results from the I-SPY 2 Trial. J Clin Oncol. 2015. May 20;33(15). [Google Scholar]
- 93.Kalinsky K, Sparano JA, Zhong X, et al. Pre-surgical trial of the AKT inhibitor MK-2206 in patients with operable invasive breast cancer: a New York Cancer Consortium trial. Clin Transl Oncol. 2018. November;20(11):1474–1483. doi: 10.1007/s12094-018-1888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ma CX, Suman V, Goetz MP, et al. A Phase II Trial of Neoadjuvant MK-2206, an AKT Inhibitor, with Anastrozole in Clinical Stage II or III PIK3CA-Mutant ER-Positive and HER2-Negative Breast Cancer. Clin Cancer Res. 2017. November 15;23(22):6823–6832. doi: 10.1158/1078-0432.CCR-17-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akcakanat A, Meric-Bernstam F. MK-2206 window of opportunity study in breast cancer. Ann Transl Med. 2018. November;6(Suppl 1):S57. doi: 10.21037/atm.2018.10.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006. May 15;107(10):4053–4062. doi: DOI 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leighl NB, Dent S, Clemons M, et al. A Phase 2 study of perifosine in advanced or metastatic breast cancer. Breast Cancer Res Tr. 2008. March;108(1):87–92. doi: 10.1007/s10549-007-9584-x. [DOI] [PubMed] [Google Scholar]
- 98.Fu SQ, Hennessy BT, Ng CS, et al. Perifosine plus docetaxel in patients with platinum and taxane resistant or refractory high-grade epithelial ovarian cancer. Gynecol Oncol. 2012. July;126(1):47–53. doi: 10.1016/j.ygyno.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Politz O, Siegel F, Barfacker L, et al. BAY 1125976, a selective allosteric AKT1/2 inhibitor, exhibits high efficacy on AKT signaling-dependent tumor growth in mouse models. International Journal of Cancer. 2017. January 15;140(2):449–459. doi: 10.1002/ijc.30457. [DOI] [PubMed] [Google Scholar]
- 100.Berndt N, Yang H, Trinczek B, et al. The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane. Cell Death Differ. 2010. November;17(11):1795–1804. doi: 10.1038/cdd.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dieterle A, Orth R, Daubrawa M, et al. The Akt inhibitor triciribine sensitizes prostate carcinoma cells to TRAIL-induced apoptosis. International Journal of Cancer. 2009. August 15;125(4):932–941. doi: 10.1002/ijc.24374. [DOI] [PubMed] [Google Scholar]
- 102.Evangelisti C, Ricci F, Tazzari P, et al. Preclinical Testing of the Akt Inhibitor Triciribine in T-Cell Acute Lymphoblastic Leukemia. J Cell Physiol. 2011. March;226(3):822–831. doi: 10.1002/jcp.22407. [DOI] [PubMed] [Google Scholar]
- 103.Kim R, Yamauchi T, Husain K, et al. Triciribine Phosphate Monohydrate, an AKT Inhibitor, Enhances Gemcitabine Activity in Pancreatic Cancer Cells. Anticancer Res. 2015. September;35(9):4599–4604. [PubMed] [Google Scholar]
- 104.Garrett CR, Coppola D, Wenham RM, et al. Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Invest New Drug. 2011. December;29(6):1381–1389. doi: 10.1007/s10637-010-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sampath D, Malik A, Plunkett W, et al. Phase I clinical, pharmacokinetic, and pharmacodynamic study of the Akt-inhibitor triciribine phosphate monohydrate in patients with advanced hematologic malignancies. Leukemia Res. 2013. November;37(11):1461–1467. doi: 10.1016/j.leukres.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mimura N, Ohguchi H, Cirstea D, et al. TAS-117, a Novel Selective Akt Inhibitor Demonstrates Significant Growth Inhibition in Multiple Myeloma Cells in Vitro and in Vivo. Blood. 2012. November 16;120(21). [Google Scholar]
- 107.Ichikawa K, Abe T, Nagase H, et al. TAS-117, a highly selective non-ATP competitive inhibitor of AKT demonstrated antitumor activity in combination with chemotherapeutic agents and molecular targeted drugs. Mol Cancer Ther. 2013. November;12(11). doi: 10.1158/1535-7163.Targ-13-C177. [DOI] [Google Scholar]
- 108.Azaro A, Rodon J, Calles A, et al. A first-in-human phase I trial of LY2780301, a dual p70 S6 kinase and Akt Inhibitor, in patients with advanced or metastatic cancer. Invest New Drug. 2015. June;33(3):710–719. doi: 10.1007/s10637-015-0241-7. [DOI] [PubMed] [Google Scholar]
- 109.Rezai K, Huguet S, Madar O, et al. Abstract 2049: Pharmacokinetic drug-drug interaction: a phase Ib dose escalation study of LY2780301 in combination with weekly paclitaxel. Cancer Research. 2016;76(14 Supplement):2049–2049. doi: 10.1158/1538-7445.am2016-2049. [DOI] [Google Scholar]
- 110.Angevin E, Cassier PA, Italiano A, et al. Safety, tolerability and antitumour activity of LY2780301 (p70S6K/AKT inhibitor) in combination with gemcitabine in molecularly selected patients with advanced or metastatic cancer: a phase IB dose escalation study. Eur J Cancer. 2017. September;83:194–202. doi: 10.1016/j.ejca.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 111.Machl A, Wilker EW, Tian H, et al. M2698 is a potent dual-inhibitor of p70S6K and Akt that affects tumor growth in mouse models of cancer and crosses the blood-brain barrier. Am J Cancer Res. 2016;6(4):806–818. [PMC free article] [PubMed] [Google Scholar]
- 112.Huck BR, Tian H, Syed S, et al. Evaluation of p70S6K/Akt inhibitor MSC2363318A in patient derived xenograft (PDX) models of breast cancer. Cancer Research. 2014. October 1;74(19). doi: 10.1158/1538-7445.Am2014-4516. [DOI] [Google Scholar]
- 113.Tsimberidou AM, Verschraegen C, Heestand G, et al. A First in Human, Dose Escalation Trial of Msc2363318a-a Dual P70s6k/Akt Inhibitor, for Patients with Advanced Malignancies. Ann Oncol. 2015. Mar;26:25–25. doi: 10.1093/annonc/mdv094.1. [DOI] [Google Scholar]
- 114.McLaughlin J, Markovtsov V, Li H, et al. Preclinical characterization of Aurora kinase inhibitor R763/AS703569 identified through an image-based phenotypic screen. J Cancer Res Clin. 2010. January;136(1):99–113. doi: 10.1007/s00432-009-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peter B, Herrmann H, Gleixner KV, et al. The Aurora-Kinase Inhibitor R763/AS703569 Exerts Major Growth-Inhibitory and Apoptosis-Inducing Effects on Neoplastic Mast Cells. Blood. 2010. November 19;116(21):1620–1620. [Google Scholar]
- 116.Sonet A, Graux C, Maertens J, et al. Phase I, Dose-Escalation Study of 2 Dosing Regimens of AS703569, An Inhibitor of Aurora and Other Kinases, Administered Orally in Patients with Advanced Hematological Malignancies. Blood. 2008;112(11):2963–2963. [Google Scholar]
- 117.Renshaw JS, Patnaik A, Gordon M, et al. A phase I two arm trial of AS703569 (R763), an orally available aurora kinase inhibitor, in subjects with solid tumors: preliminary results. J Clin Oncol. 2007. 2007/06/20;25(18_suppl):14130–14130. doi: 10.1200/jco.2007.25.18_suppl.14130. [DOI] [Google Scholar]
- 118.Awada A, Alexandre J, Gianella-Borradori A, et al. Phase I and pharmacokinetic (PK) study of two regimens combining the aurora kinase inhibitor AS703569 and gemcitabine in patients with advanced solid tumors. Cancer Research. 2010. April;70. doi: 10.1158/1538-7445.Am10-2754. [DOI] [Google Scholar]
- 119.Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005. November 14;24(50):7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- 120.She QB, Chandarlapaty S, Ye Q, et al. Breast Tumor Cells with PI3K Mutation or HER2 Amplification Are Selectively Addicted to Akt Signaling. Plos One. 2008. August 26;3(8). doi: ARTN e306510.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Yi KH, Lauring J. Recurrent AKT mutations in human cancers: functional consequences and effects on drug sensitivity. Oncotarget. 2016. January 26;7(4):4241–51. doi: 10.18632/oncotarget.6648. [DOI] [PMC free article] [PubMed] [Google Scholar]