Abstract
Background:
The intestinal microbiota contributes to the pathogenesis of obesity and metabolic disorders. People living with HIV (PLWH) have a higher risk for the development of visceral adiposity with accompanying worsened cardiovascular risk.
Setting:
Convenience sample from an HIV clinic and research unit.
Methods:
To understand the relationship between adiposity and intestinal dysbiosis, we compared the gut microbiota and inflammatory markers in a cross-sectional study of viscerally obese, generally obese, and lean PLWH. Fecal intestinal microbiota was characterized by 16S ribosomal DNA sequencing. Abdominal CTs quantified subcutaneous and visceral adipose tissue (SAT; VAT). Serum hsCRP, adiponectin, leptin, IL-6, MCP-1 and sCD14 were assayed.
Results:
We studied 15, 9 and 11 participants with visceral obesity, general obesity and lean body type, respectively. The generally obese group were all women and 2/3 African-American, whereas the visceral obesity and lean groups were predominantly white and men who have sex with men. Markers of systemic inflammation and sCD14 were higher in general obesity compared with lean. sCD14 was positively correlated with VAT but not SAT. Bacterial diversity was significantly reduced in participants with visceral and general obesity and composition of intestinal microbiota was significantly different from lean body types. Bacterial alpha diversity was negatively correlated with VAT area, waist/hip ratio and sCD14, but not with SAT area.
Conclusions:
In this exploratory study, obesity in general was associated with dysbiotic intestinal microbiota. The relationships of VAT to bacterial diversity and sCD14 suggests that dysbiosis in viscerally obese PLWH could be associated with heightened inflammatory state.
Keywords: Intestinal Microbiota, Dysbiosis, Obesity, HIV, Visceral fat, Systemic inflammation
Introduction
The excess visceral adiposity present in some people living with HIV (PLWH), has been associated with systemic inflammation1–3 subclinical atherosclerosis4–6, insulin resistance, and diabetes mellitus7. Although the etiology of visceral adiposity is unknown, initiation of diverse contemporary antiretroviral regimens is associated with increases in visceral adipose tissue volume, suggesting that it is not a direct effect of specific drugs8,9. Obesity, in general, is a prevalent problem in PLWH10–13, an issue that may be exacerbated by weight gain in association with antiretroviral therapy14,15 and specifically drugs in the integrase strand transfer inhibitor class16,17.
The gut microbiota is critical for maintaining intestinal homeostasis and plays vital role in maintenance of mucosal barrier function and regulation of innate and adaptive immune responses. Dysbiosis (an imbalance in the composition of the microbiota) has been implicated in chronic inflammation associated with both visceral18–20 and general obesity as well as numerous inflammatory disorders21–23. Previous studies have reported alterations in the intestinal microbiota in PLWH3 with resultant bacterial translocation and immune activation that are now thought to be central contributors to the progression of HIV disease in the settings of both acute and chronic infection24. Microbial translocation can cause monocyte activation25, and toll-like receptor ligands have been shown to induce T-cell activation and apoptosis in vitro26. Immune activation and inflammation are thought to contribute to the pathogenesis of some HIV-related complications such as atherosclerosis6 but have not been studied extensively in relation to body fat distribution. Obesity is linked with significant alterations in the intestinal microbial communities, but the potential relationships between visceral obesity, gut microbiota, and immune activation in people living with HIV are poorly understood.
We hypothesized that the diversity and composition of the gut microbiota will differ between groups of PLWH who have visceral obesity, generalized obesity, or non-obese. We evaluated the associations of abdominal CT quantified subcutaneous and visceral adipose tissue areas with microbial diversity and composition, as well as serum markers of microbial translocation and inflammation.
Methods
Study design and subjects
We conducted a cross-sectional pilot study of a convenience sample of adults living with HIV infection recruited from the HIV clinic and HIV Clinical Trials Unit at the New York Presbyterian Hospital-Weill Cornell Medical Center. The study protocol was approved by the Weill Cornell Medical College Institutional Review Board and all participants provided written informed consent (IRB# 1007011134). Potential participants underwent an interview and physical examination to determine eligibility. Inclusion criteria were documented HIV infection, on stable combination antiretroviral therapy for >12 weeks, and meeting anthropometric criteria for inclusion into one of the following study groups (goal of n=12 per group): (1) visceral obesity (any body mass index [BMI], waist circumference >88.2 cm for men or > 75.3 cm for women, and waist:hip ratio ≥ 0.95 for men or ≥ 0.90 for women; (2) generalized obesity (BMI 30 to 39.9, any waist circumference, and waist:hip ratio < 0.95 for men or < 0.90 for women; or (3) non-obese (BMI < 25, waist circumference ≤ 88.2 cm for men or ≤ 75.3 cm for women, and waist:hip ratio ≤ 0.95 for men or ≤ 0.90 for women). Duration of antiretroviral therapy was estimated using the intention-to-treat paradigm where participants were assumed to have remained on therapy once initiated. Duration of suppressed HIV-1 viremia was defined as the duration of time prior to the study visit during which the HIV-1 viral load was documented as being continuously less than 400 copies/ml. Exclusion criteria were any antibiotic use within the preceding 90 days, history of gastrointestinal surgery, active malignancy or opportunistic infection, diarrhea lasting more than 48 hours within 90 days prior to entry, and pregnancy or breastfeeding. Participants provided fresh stool samples on the day of the study entry visit or refrigerated samples from the prior day, which were promptly stored frozen at −80°C.
Evaluation of body composition
Image acquisition: Two axial (non-helical) CT scans were obtained at the L4–L5 level, as determined by lateral scout. Images were acquired at 5 mm slice thickness, kV=120, mA=170, rotation time=1 sec, DFOV = entire body.
Image processing: Images were sent to an Advantage Workstation (GE Healthcare). Fat was identified using a pixel threshold of −190 to −30 Hounsfield units, allowing for calculation of total fat area. The abdominal wall was then segmented, leaving only subcutaneous tissue, allowing calculation of subcutaneous fat area. Visceral fat area was then calculated as (total fat area) - (subcutaneous fat area).
Measurements of bacterial translocation and systemic inflammation markers
Fasting blood samples were collected and serum was separated and frozen at −80° until analysis. Levels of high sensitivity C-reactive protein (hsCRP), adiponectin, leptin, interleukin (IL)-6, soluble CD14 (sCD14) and monocyte chemotactic protein-1 (MCP-1) were determined from pristine thawed serum samples using commercial assay kits at the Clinical and Translational Science Center Core Laboratory at Weill Cornell Medicine. Assays were run in batches upon completion of study recruitment.
DNA stool extraction and 16s rDNA sequencing
For each fecal specimen, DNA was phenol-chloroform extracted and purified, and the V1–V3 region of the 16S rRNA gene was PCR-amplified using modified universal bacterial primers. Purified PCR products were sequenced on a 454 GS FLX Titanium platform. Sequencing reads were demultiplexed and error-filtered, using maximum expected error (Emax=1). The UPARSE pipeline was used to group sequences into operational taxonomic units of 97% distance-based similarity, and to identify and remove potentially chimeric sequences (using both de novo and reference-based methods). Prior to clustering, singleton sequences were removed as part of the UPARSE protocol. Taxonomic assignment to species level was performed for representative sequences from each OTU; this was achieved through the BioPython implementation of nucleotide BLAST, with NCBI RefSeq RNA as reference training set. We used a minimum E-value threshold of 1e-10 for assignments. Sequence designations and identity scores were manually inspected for quality and consistency in terms of taxonomic structure and secondary matches. In addition to taxonomic assignment, α-diversity and β-diversity of samples were calculated at the OTU level, using the Shannon index and Sørensen dissimilarity, respectively.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism v8 (GraphPad Software, San Diego, CA) and Stata 13 (StataCorp, College Station, TX). Data were described as mean and standard deviation (SD) or standard error of mean (SEM) for quantitative variables. The Kruskal-Wallis test was used to evaluate differences in inflammatory and body composition markers between the different body types, with subsequent Wilcoxon rank-sum test for pair-wise comparisons. Pearson’s correlation evaluated associations between continuous variables. A portion of microbial community analysis was conducted using the Agile Toolkit for Incisive Microbial Analyses (ATIMA), developed by the Alkek Center for Metagenomics and Microbiome Research. Analysis of Variance (ANOVA) with Tukey’s multiple comparison’s test was used to compare the bacterial α diversity measures between the different body types. Tukey-Kramer test was then used to adjust for multiple comparisons. Sørensen similarity distance followed by permutational multivariate analysis of variance (PERMANOVA) was used to determine differences in microbial community composition. To identify differentially abundant bacterial genera between lean, obese and visceral groups, LEfSe (linear discriminant analysis effect size) method was used27. Significantly different genera were then used as input for linear discriminant analysis (LDA), and log10 effect sizes were generated. All bacterial genera abundances were compared between body composition groups (lean vs. visceral; lean vs. obese; and obese vs. visceral) using the Kruskal-Wallis test at a pre-defined α of 0.05.
Results
Baseline Clinical Characteristics of Study Participants
Table 1 summarizes the demographic and clinical characteristics of the 35 study participants. The mean ages were similar across the three groups. The majority of viscerally obese and lean individuals were men (86.7% and 90.9%, respectively), whereas all of the generally obese individuals were women. 60% of viscerally obese and 90% of lean individuals were men who have sex with men. The mean time since HIV diagnosis was similar among the three groups with a mean duration overall of 14.3 (5.8) years. Both hypertension and dyslipidemia were prevalent in all study groups. Every study participant was on antiretroviral therapy. While the duration of suppressed HIV-1 viremia was longer in obese group, the difference did not reach statistical significance. Current CD4 counts and viral RNA were similar among the three groups. Although nadir CD4 count was numerically lowest in the group with visceral obesity, the difference was not statistically different
Table 1:
Baseline Characteristics of Study Participants
| Visceral Obesity (n = 15) |
General Obesity (n = 9) |
Lean (n = 11) |
P-value | |
|---|---|---|---|---|
| Age, mean (SD) years | 51.0 (5.9) | 45.2 (6.6) | 45.7 (7.9) | 0.11a |
| Race | ||||
| African-American | 4 (26.7%) | 6 (66.7%) | 0 | 0.003b |
| White | 11 (73.4%) | 0 | 10 (90.9%) | |
| > 1 race | 0 | 3 (33.3%) | 1 (9.1%) | |
| Ethnicity | ||||
| Hispanic | 8 (53.3%) | 6 (66.7%) | 2* (20%) | 0.12c |
| Sex | ||||
| Male | 13 (86.7%) | 0 | 10 (90.9%) | < 0.001c |
| Female | 2 (13.3%) | 9 (100%) | 1 (9.1%) | |
| HIV Transmission Risk Factor | < 0.001c | |||
| MSM | 9 (60.0%) | 0 | 10 (90.9%) | |
| Heterosexual | 4 (26.7%) | 9 (100%) | 1 (9.1%) | |
| Transfusion | 1 (6.7%) | 0 | 0 | |
| MSM/IDU | 1 (6.7%) | 0 | 0 | |
| Known duration of HIV, mean (SD) years | 16.1 (6.0) | 14.6 (5.6) | 12.3 (6.0) | 0.35a |
| Height, mean (SD) cm | 171.1 (8.2) | 164.7 (9.8) | 172.1 (9.9) | 0.18a |
| Weight, mean (SD) kg | 89.6 (13.5) | 111.5 (25.3) | 70.9 (11.9) | <0.001a |
| Body mass index, mean (SD) kg/m2 | 30.8 (5.7) | 40.8 (6.9) | 23.9 (2.8) | <0.001a |
| Waist circumference, mean (SD) cm | 107.3 (9.5) | 112.2 (11.3) | 85.8 (8.3) | <0.001a |
| Hip circumference, mean (SD) cm | 101.9 (7.7) | 132.0 (19.2) | 94.9 (7.9) | <0.001a |
| Waist:hip ratio, mean (SD) | 1.06 (0.068) | 0.86 (0.076) | 0.90 (0.056) | <0.001a |
| Dorsocervical fat pad enlargement | 3 (20.0%) | 3 (33.3%) | 0 | 0.14c |
| Hypertension | 6 (40.0%) | 3 (33.3%) | 1 (9.1%) | 0.25c |
| Dyslipidemia | 3 (20.0%) | 3 (33.3%) | 4 (36.4%) | 0.64c |
| Nadir CD4, median, (Q1, Q3) cells/mm3 | 73 (47, 299) | 219 (153, 261) | 212 (108, 262) | 0.36a |
| Current CD4, median, (Q1, Q3) cells/mm3 | 657 (571, 806) | 605 (443, 816) | 630 (529, 817) | 0.84a |
| HIV RNA < 400 copies/ml | 14 (93.3%) | 6 (66.7%) | 11 (100%) | 0.050c |
| Active hepatitis B (sAg+) | 1 | 0 | 0 | |
| Active HCV | 0 | 0 | 0 | |
| Duration of antiretroviral therapy (years) | 13.0 (8.3, 14.8) N = 12 |
13.1 (11.6, 14.2) N = 4 |
3.8 (2.2, 10.1) N = 8 |
0.054a |
| Duration of suppressed HIV-1 viremia (years) | 2.6 (2.2, 8.0) N = 11 |
6.8 (4.2, 15.0) N = 3 |
2.4 (0.8, 3.3) N = 8 |
0.092a |
| Currently on PI | 9 (60.0%) | 7 (77.8%) | 4 (36.4%) | 0.20c |
| Currently on NNRTI | 6 (40.0%) | 2 (22.2%) | 7 (63.6%) | 0.20c |
| Ever on zidovudine | 10 (66.7%) | 3 (33.3%) | 4 (36.4%) | 0.22c |
| Ever on stavudine | 4 (26.7%) | 3 (33.3%) | 1 (9.0%) | 0.41c |
| Ever on didanosine | 3 (20.0%) | 3 (33.3%) | 2 (18.2%) | 0.77c |
| Ever on first generation PI | 8 (53.3%) | 3 (33.3%) | 1 (9.0%) | 0.057c |
| Currently on raltegravir | 5 (33.3%) | 1 (11.1%) | 2 (18.2%) | 0.60c |
Ethnicity was not reported on one subject
Kruskal-Wallis test
Fisher’s exact for Black vs others
Fisher’s exact test
Abbreviations: IDU, injection drug use; MSM, men who have sex with men; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; Q1, first quartile; Q3, third quartile. First generation protease inhibitors were defined as indinavir, nelfinavir, and saquinavir.
Body composition and associations with systemic markers of inflammation
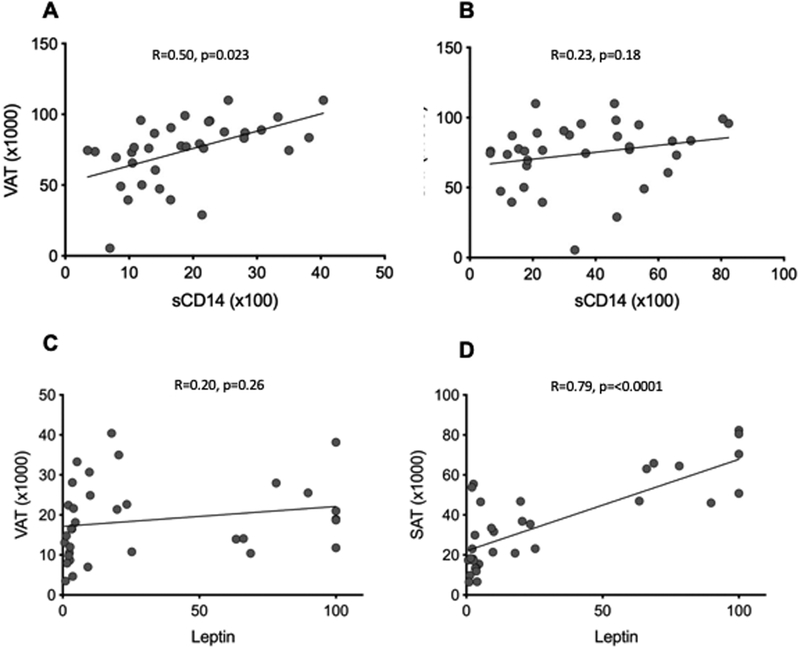
Abdominal CT imaging of study participants demonstrated significantly increased total and subcutaneous adipose tissue (TAT and SAT) in study participants with general obesity, compared with visceral obesity, and lean individuals had significantly lower TAT compared with either obese body type (Figure S1). Visceral adipose tissue (VAT) was significantly lower in participants with lean body type compared with either of the obese groups. Notably, the quantity of VAT was similar among viscerally and generally obese study participants, however the viscerally obese group had the highest VAT:SAT ratio (Figure S1D). We observed significant positive correlation between sCD14 and VAT, but not SAT (r=0.50, p=0.0023 and r=0.23, p=0.18 respectively). Levels of leptin were positively correlated with SAT area, but not with VAT (r=0.79, p=<0.0001 and r=0.20, p=0.26 respectively) (Figure 1)
Figure 1. Visceral adiposity is associated with higher levels of sCD14, but not with leptin.
Correlation of sCD14 (A, B) and leptin (C,D) with visceral and subcutaneous tissue area as determined by abdominal CT imaging.
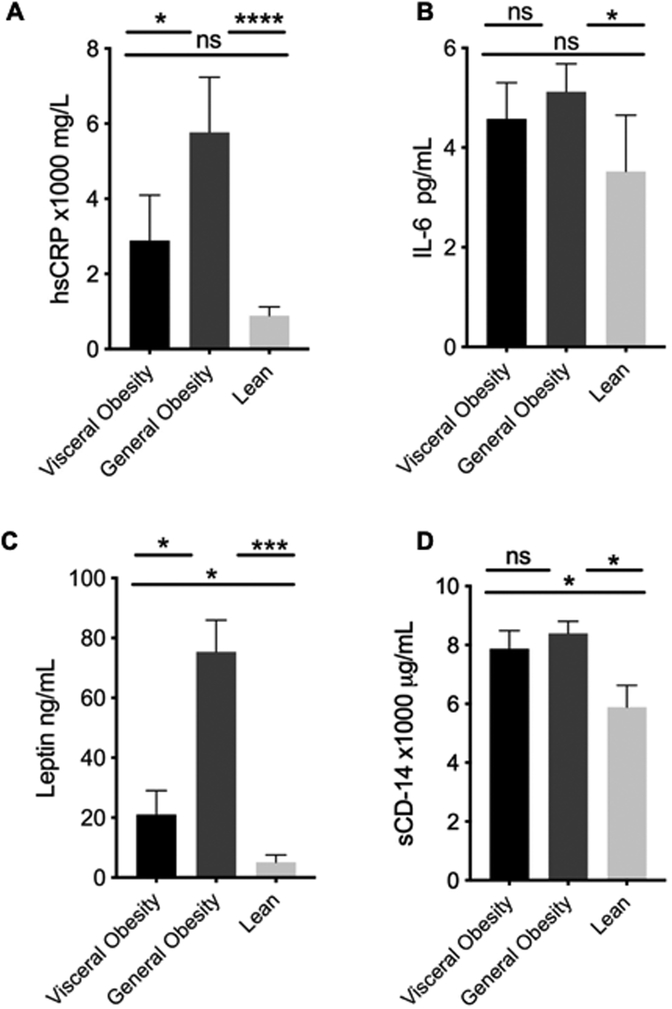
Figure 2 summarizes the adipokines and biomarkers of systemic inflammation by study group. hsCRP levels were highest in the general obesity group followed by the visceral obesity then lean group. Leptin levels were highest in the generally obese group followed by the viscerally obese then lean group. Both general and viscerally obese individuals had higher serum levels of soluble CD14 than lean individuals, but there were no significant differences between the two obese groups. Additionally, IL-6 levels were significantly elevated in participants with general obesity compared to the lean group, however there was no significant difference between lean and visceral groups. There were no statistically significant differences between adiponectin and MCP-1 levels among the groups of study participants (Figure S2).
Figure 2. Circulating adipokines and markers of systemic inflammation are altered in HIV+ participants with visceral and general obesity compared with lean body type.
Biomarkers of systemic inflammation were measured in serum and plasma of study participants by standard methods. HIV+ patients with either type of obesity had significantly elevated levels of hsCRP, IL-6, leptin and sCD14 compared with lean individuals. Additionally, hsCRP and leptin were significantly elevated in individuals with general compared with visceral obesity. p<0.05, **p<0.01, ***p<0.005, ****p<0.0001 as determined by Kruskal-Wallis test; pair-wise comparisons by Wilcoxon rank-sum test.
Intestinal Microbiota alterations in participants with visceral and general obesity
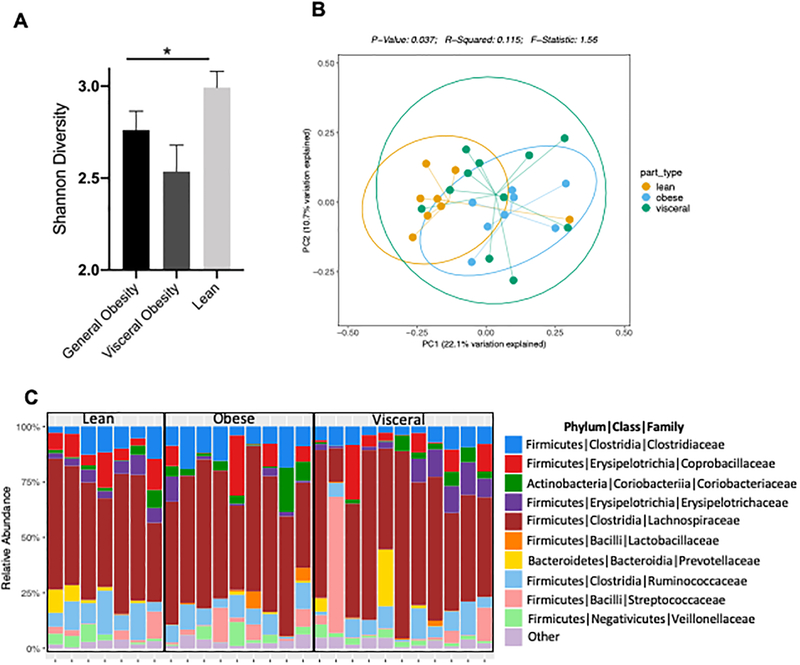
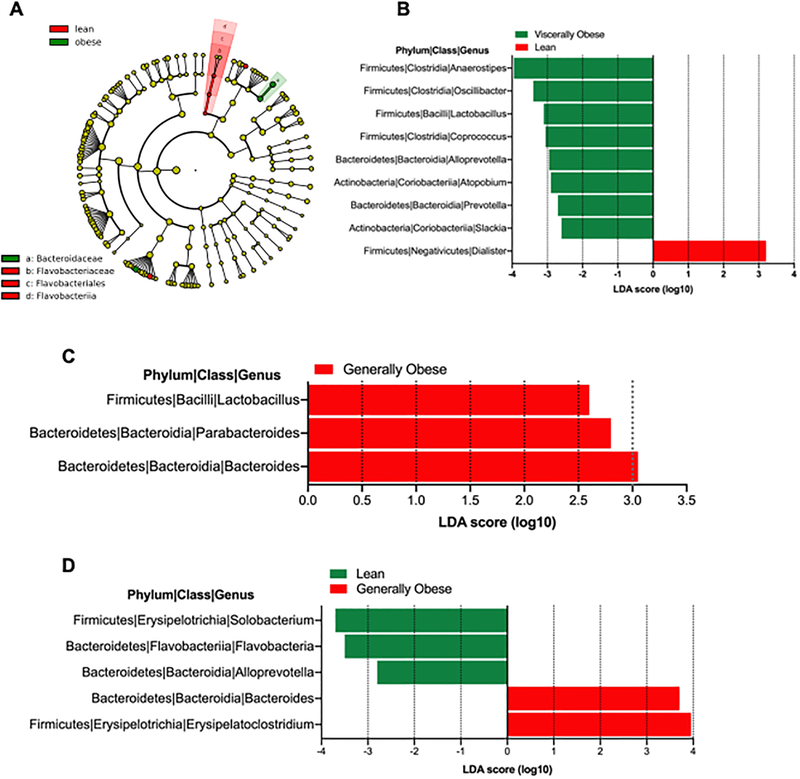
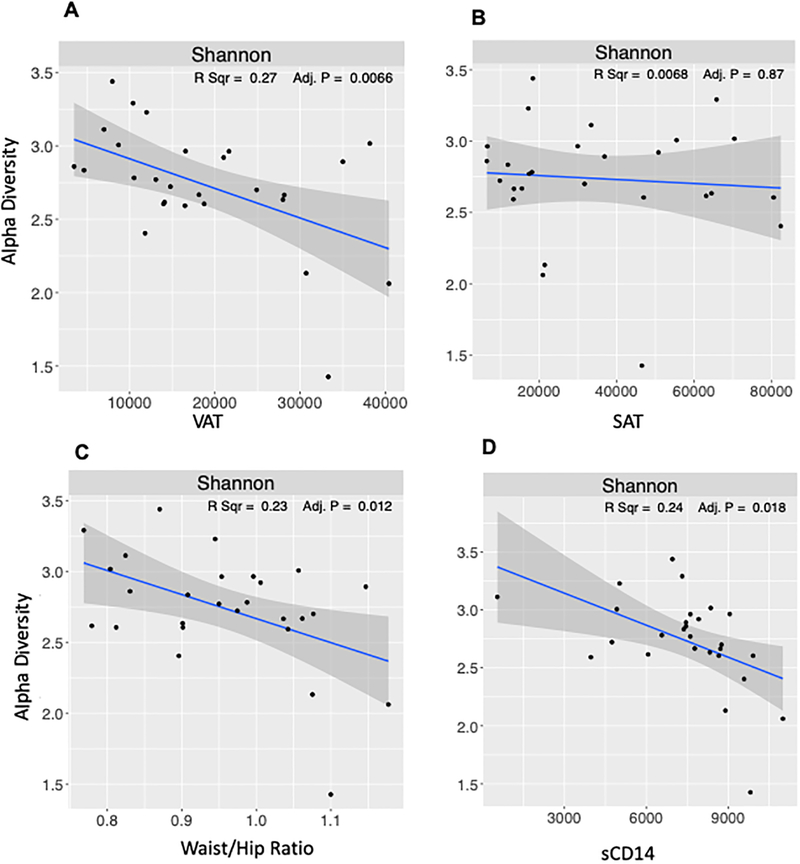
Differences in intestinal microbiota in participants with different body types were evaluated by 16S rDNA sequencing of stool samples from study participants. Study samples yielded 171,083 high-quality 16S sequences (after filtering) for analysis. Number of sequences per sample ranged between 2422 to 7806 (average 5184). Analysis of species level alpha diversity revealed significantly higher Shannon diversity index in participants with lean body type, compared with visceral and general obesity (Figure 3A). However, no difference was observed between visceral and general obesity body types. To evaluate the microbial composition, we performed principal coordinate analysis of Sørensen dissimilarity, which revealed significantly different clustering of lean and obese individuals of both types, with no differences between visceral and obese body types (Figure 3B). Notably, in microbiota diversity analysis limited to MSM, bacterial diversity was reduced in the visceral group relative to the lean group (p=0.016) (Figure S3A). Relative abundance of intestinal bacteria was different among the different body types, with an expansion of family Prevotellaceae from Bacteroidetes phylum in the lean body type and less frequently with visceral but not generally obese individuals (Figure 3C). LEfSe analysis to identify the differentially abundant bacteria among the three different study groups demonstrated two bacterial taxa belonging to Bacteroidetes phylum to be differentially represented in our study cohort. Specifically, there was a significant increase in abundance of Bacteroidaceae family in obese individuals, and significant reduction in Flavobacteriaceae (Figure 4A), both of which belong to Bacteroidetes phylum. While Bacteroidetes have been previously described to be differentially abundant in lean vs obese individuals28, they are not the most abundant members of intestinal microbiota (Figure 3C) and are underrepresented in all three groups of our study cohort. Compared with lean individuals, at a genus level viscerally obese individuals had significantly increased abundance of Dialister and reduced Anaerostipes, Coprococcus, Lactobacilli, Oscillibacter, Atopobium and Alloprevotella (Figure 4B). The difference between generally and viscerally obese individuals was minor, but generally obese individuals had significantly increased Parabacteroides and Lactobacillus compared with viscerally obese individuals (Figure 4C). Comparison of microbial composition among all three study groups showed significant increase in Erysipelatoclostridium and Bacteroides in lean individuals, compared with both obese groups and obese group had significant reductions of Solobacterium and Alloprevotella compared with lean and viscerally obese individuals (Figure 4B). Intestinal microbiota composition was not significantly different among the viscerally obese group members who were ever on zidovudine or first-generation protease inhibitors compared with general obesity and lean groups on the same medications (Figure S3B and C). In an analysis restricted to participants with HIV-1 RNA levels <400 copies/mL, bacterial diversity was reduced in the viscerally obese group relative to the other groups (p=0.039) (Figure S3D). Further analysis of microbial signatures in visceral and generally obese individuals showed a significant negative correlation between Shannon diversity index and both VAT area and waist:hip ratio, which was not observed with SAT area. Furthermore, significant negative correlation was observed between the Shannon diversity index and serum soluble CD14 (Figure 5).
Figure 3. Intestinal Microbiota is altered in HIV+ participants with visceral and general obesity.
Microbial community diversity analysis (Shannon Diversity Index) by body type (A). Measures of microbial beta-diversity based on an unweighted UniFrac distance (B). Relative abundance of a family classified fecal microbiota from patients with different body type (C).
Figure 4. Differentially represented bacteria between different body types identified by linear discriminant analysis coupled with effect size (LEfSe).
(A) Cladogram representation of differentially abundant bacteria among all three groups. Histogram of bacterial genera between (B) lean and viscerally obese individuals; (C) general vs viscerally obese individuals; (D) lean vs viscerally vs generally obese
Figure 5. Association of body fat distribution and sCD14 with bacterial diversity in people with HIV infection.
Correlation between bacterial alpha-diversity measures with adiposity (A, B, C) and sCD14 (D) in HIV+ participants. Linear regression analysis and adjusted p-values are shown.
Discussion
In this study we show that the intestinal microbiota in PLWH with general and visceral obesity exhibited greater dysbiosis compared with lean body type. We observed a distinct pattern of systemic inflammatory markers in PLWH who were generally obese when compared with viscerally obese individuals. Importantly, visceral fat area was significantly inversely correlated with the alpha diversity of intestinal bacteria, which could indicate a role of dysbiotic microbiota in the development of visceral adiposity in PLWH.
Previous studies have observed weight gain and increased BMI in individuals on combination antiretroviral therapy (cART), especially in individuals with lower baseline BMI11. Specifically, thymidine analogs and ddl-based ART are associated with increased visceral fat accumulation and increased cardiovascular risk29. However, the overall rate of obesity, irrespective of any particular cART regimen, has been steadily increasing and following the overall trend of increased incidence for obesity13. Therefore, the weight gain observed in PLWH may be a consequence of lifestyle and/or indirect effects of controlling HIV replication. For example, investigators have demonstrated a reduction of basal metabolic rate and improved appetite in association with suppressing viremia, the latter thought to be due to the effects of lower circulating cytokines on the hypothalamic structures implicated in satiety30,31. Visceral adiposity is prevalent in PLWH32 and its pathogenesis, while likely multifactorial, is not well understood33. Visceral adiposity is accompanied by increased systemic inflammation, dyslipidemia, insulin resistance and cardiovascular risk factors. Specifically, excess adipose tissue is characterized by infiltration of pro-inflammatory immune cells, causing chronic low-grade inflammation, and can provide a reservoir for latently infected immune system cells34. Obese PLWH have higher monocyte activation (sCD14, sCD163) and systemic inflammatory markers (IL-6) that are independently associated with increased body weight and associated with metabolic abnormalities and long term complications35.
The associations of intestinal microbiota with obesity and associated inflammatory responses are well established36,37. Importantly, intestinal dysbiosis and reduction of bacterial α-diversity in PLWH compared with healthy individuals has been previously described in PLWH3,38, but the link between the visceral adiposity, microbial signatures associated with different types of adiposity and their relationship with the markers of systemic inflammation has not been explored to our knowledge. In this study, we show that intestinal microbiota is altered in PLWH with specific changes in viscerally obese individuals. Previous studies have demonstrated increased abundance of Bacteroidetes and reduction of Firmicutes in lean individuals28,36. In our study cohort, Bacteroidetes was less abundant in both types of obese groups, which is consistent with previous studies. However, it was also less abundant in the lean body type group.
While use of thymidine analog drugs have been linked to lipoatrophy, associations between specific antiretroviral classes and visceral fat accumulation are less clear. We observed trends towards more frequent use of zidovudine and first-generation PIs in participants with visceral adposity. However, the analysis of microbiota diversity among the participants in all three study groups who were ever on first generation PIs or zidovudine did not show significant differences. Therefore, the intestinal dysbiosis in visceral adiposity may not be associated with the prior use of first-generation PIs or zidovudine.
It is important to note that comparisons of individuals with HIV infection, regardless of body habitus, with historical data from the general population may be confounded by lifestyle characteristics and histories of antibiotic exposure that may affect the intestinal microbiota. Importantly, our observed correlation between bacterial alpha diversity and VAT could indicate the potential contribution of intestinal microbiota in visceral fat accumulation in PLWH. Furthermore, we found higher levels of sCD14 - a possible marker of microbial translocation-- in both obesity phenotypes compared to lean individuals but overall a positive correlation between sCD14 and VAT, but not SAT. Others have reported associations between sCD14 level and additional weight gain in overweight and obese participants who initiated antiretroviral therapy39.
This exploratory study has several strengths and limitations. First, to our knowledge, this is the first study examining the intestinal dysbiosis in PLWH by body habitus. Several other studies have described the intestinal microbiota in context of HIV infection and other risk factors. However, the specific changes in microbial composition in PLWH and the correlation with visceral and body fat has been previously unexplored. Additionally, we have quantified visceral and body fat in study participants using computed tomography, which is a gold standard method of quantification. Our study also has several important limitations, including the small sample size and lack of adjustment for clinical confounders. Additionally, all of the generally obese study participants were women and 91% of lean body type individuals were men. Therefore, imbalance in sex distribution of our study population, which may be reflective of sex differences in the prevalence and patterns of obesity in PLWH40–42, could have contributed to some of the findings that we observed. Study participants were on a wide variety of antiretroviral regimens and due to the small sample size, we were unable to definitively analyze the contribution of individual drugs or classes of drugs to the change in intestinal microbiota. Additionally, previous studies have demonstrated significant differences in intestinal microbiota of MSM, which may have contributed to the observed differences43. Also, we did not assess diet or lifestyle in this study, which could be an important factor, either as a confounder of body habitus, or mediator of microbiota composition. Finally, it was difficult for us to know how exactly these findings relate to the specific context of HIV, and to what degree any of these links occur irrespective of HIV status and treatment.
Collectively, these data demonstrate that intestinal microbiota composition in PLWH with either type of obesity is dysbiotic compared with lean body type and despite the high degree of similarities, microbial signatures exist that can segregate the visceral and generally obese groups. Importantly, the dysbiotic microbiota correlates with visceral fat accumulation and markers of systemic inflammation, suggesting that microbial dysbiosis may contribute to the pathogenesis of or be a consequence of visceral adiposity and associated heightened inflammatory status.
Supplementary Material
Figure S1. Body composition of study participants by abdominal CT imaging (A-D). p<0.05, **p<0.01, ***p<0.005, ****p<0.0001 as determined by Kruskal-Wallis test; pair-wise comparisons by Wilcoxon rank-sum test.
Figure S2. Circulating adiponectin and MCP1 in HIV+ participants with visceral and general obesity compared with lean body type. p<0.05, **p<0.01, ***p<0.005, ****p<0.0001 as determined by Kruskal-Wallis test; pair-wise comparisons by Wilcoxon rank-sum test.
Figure S3. Microbial community diversity analysis (Shannon Diversity Index) by body type limited to MSM (A), participants ever on Zidovudine (B), participants ever on first-generation PIs (C) and participants with HIV-1 viral load <400 copies/mL (D) as determined by Kruskal-Wallis test
Acknowledgements:
We would like to thank Kirsis Ham, FNP for study coordination and Eric Littman for bioinformatic analysis support.
Conflict of Interest and Source of Funding: Authors declare no financial, institutional, consultant or other conflict of interest. Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000457. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Lasha Gogokhia was supported by Weill Cornell Medicine Clinical and Translational Science Center of the National Institute of Health under award number UL1TR002384.
References:
- 1.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39(1):44–54. [DOI] [PubMed] [Google Scholar]
- 2.Stanley TL, Falutz J, Mamputu J-C, Soulban G, Potvin D, Grinspoon SK. Effects of tesamorelin on inflammatory markers in HIV patients with excess abdominal fat: relationship with visceral adipose reduction. AIDS. 2011;25(10):1281–1288. doi: 10.1097/QAD.0b013e328347f3f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinh DM, Volpe GE, Duffalo C, et al. Intestinal Microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. In: Journal of Infectious Diseases.; 2015. doi: 10.1093/infdis/jiu409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glesby MJ, Hanna DB, Hoover DR, et al. Abdominal Fat Depots and Subclinical Carotid Artery Atherosclerosis in Women With and Without HIV Infection. J Acquir Immune Defic Syndr. 2018;77(3):308–316. doi: 10.1097/QAI.0000000000001606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palella FJ, Phair JP, Phair JP. Cardiovascular disease in HIV infection. Curr Opin HIV AIDS. 2011;6(4):266–271. doi: 10.1097/COH.0b013e328347876c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23(9):1059–1067. doi: 10.1097/QAD.0b013e32832b514b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glesby MJ, Hanna DB, Hoover DR, et al. Abdominal fat depots, insulin resistance, and incident diabetes mellitus in women with and without HIV infection. AIDS. 2018;32(12):1643–1650. doi: 10.1097/QAD.0000000000001873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McComsey GA, Moser C, Currier J, et al. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62(7):853–862. doi: 10.1093/cid/ciw017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McComsey GA, Kitch D, Sax PE, et al. Peripheral and Central Fat Changes in Subjects Randomized to Abacavir-Lamivudine or Tenofovir-Emtricitabine With Atazanavir-Ritonavir or Efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53(2):185–196. doi: 10.1093/cid/cir324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39(5):557–561. [PubMed] [Google Scholar]
- 11.Koethe JR, Jenkins CA, Lau B, et al. Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32(1):50–58. doi: 10.1089/aid.2015.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing Rates of Obesity among HIV-Infected Persons during the HIV Epidemic Loutfy MR, ed. PLoS One. 2010;5(4):e10106. doi: 10.1371/journal.pone.0010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor BS, Liang Y, Garduño LS, et al. High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr. 2014;65(2):e33–40. doi: 10.1097/QAI.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakey W, Yang L-Y, Yancy W, Chow S-C, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses. 2013;29(3):435–440. doi: 10.1089/aid.2012.0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taramasso L, Ricci E, Menzaghi B, et al. Weight Gain: A Possible Side Effect of All Antiretrovirals. Open forum Infect Dis. 2017;4(4):ofx239. doi: 10.1093/ofid/ofx239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norwood J, Turner M, Bofill C, et al. Brief Report: Weight Gain in Persons With HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor-Based Regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–531. doi: 10.1097/QAI.0000000000001525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menard A, Meddeb L, Tissot-Dupont H, et al. Dolutegravir and weight gain: an unexpected bothering side effect? AIDS. 2017;31(10):1499–1500. doi: 10.1097/QAD.0000000000001495 [DOI] [PubMed] [Google Scholar]
- 18.Lopes HF, Corrêa-Giannella ML, Consolim-Colombo FM, Egan BM. Visceral adiposity syndrome. Diabetol Metab Syndr. 2016;8(1):40. doi: 10.1186/s13098-016-0156-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paeschke A, Erben U, Kredel LI, Kühl AA, Siegmund B. Role of visceral fat in colonic inflammation. Curr Opin Gastroenterol. 2017;33(1):53–58. doi: 10.1097/MOG.0000000000000324 [DOI] [PubMed] [Google Scholar]
- 20.Zulian A, Cancello R, Ruocco C, et al. Differences in Visceral Fat and Fat Bacterial Colonization between Ulcerative Colitis and Crohn’s Disease. An In Vivo and In Vitro Study Heimesaat MM, ed. PLoS One. 2013;8(10):e78495. doi: 10.1371/journal.pone.0078495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 22.Flint HJ. Obesity and the Gut Microbiota. J Clin Gastroenterol. 2011;45:S128–S132. doi: 10.1097/MCG.0b013e31821f44c4 [DOI] [PubMed] [Google Scholar]
- 23.Gregor MF, Hotamisligil GS. Inflammatory Mechanisms in Obesity. Annu Rev Immunol. 2011;29(1):415–445. doi: 10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- 24.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511 [DOI] [PubMed] [Google Scholar]
- 25.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients Unutmaz D, ed. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis Unutmaz D, ed. PLoS One. 2008;3(4):e1915. doi: 10.1371/journal.pone.0001915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 29.Gelpi M, Afzal S, Fuchs A, et al. Prior exposure to thymidine analogs and didanosine is associated with long-lasting alterations in adipose tissue distribution and cardiovascular risk factors. AIDS. 2019;33(4):675–683. doi: 10.1097/QAD.0000000000002119 [DOI] [PubMed] [Google Scholar]
- 30.Sutinen J, Yki-Järvinen H. Increased resting energy expenditure, fat oxidation, and food intake in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Metab. 2007;292(3):E687–E692. doi: 10.1152/ajpendo.00219.2006 [DOI] [PubMed] [Google Scholar]
- 31.Kosmiski L. Energy expenditure in HIV infection. Am J Clin Nutr. 2011;94(6):1677S–1682S. doi: 10.3945/ajcn.111.012625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lake JE. The Fat of the Matter: Obesity and Visceral Adiposity in Treated HIV Infection. Curr HIV/AIDS Rep. 2017;14(6):211–219. doi: 10.1007/s11904-017-0368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lake JE, Stanley TL, Apovian CM, et al. Practical Review of Recognition and Management of Obesity and Lipohypertrophy in Human Immunodeficiency Virus Infection. Clin Infect Dis. 2017;64(10):1422–1429. doi: 10.1093/cid/cix178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couturier J, Lewis DE. HIV Persistence in Adipose Tissue Reservoirs. Curr HIV/AIDS Rep. 2018;15(1):60–71. doi: 10.1007/s11904-018-0378-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conley LJ, Bush TJ, Rupert AW, et al. Obesity is associated with greater inflammation and monocyte activation among HIV-infected adults receiving antiretroviral therapy. AIDS. 2015;29(16):2201–2207. doi: 10.1097/QAD.0000000000000817 [DOI] [PubMed] [Google Scholar]
- 36.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verdam FJ, Fuentes S, de Jonge C, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity. 2013;21(12):E607–E615. doi: 10.1002/oby.20466 [DOI] [PubMed] [Google Scholar]
- 38.Tuddenham SA, Koay WLA, Zhao N, et al. The Impact of Human Immunodeficiency Virus Infection on Gut Microbiota α-Diversity: An Individual-level Meta-analysis. Clin Infect Dis. March 2019. doi: 10.1093/cid/ciz258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mave V, Erlandson KM, Gupte N, et al. Inflammation and Change in Body Weight With Antiretroviral Therapy Initiation in a Multinational Cohort of HIV-Infected Adults. J Infect Dis. 2016;214(1):65–72. doi: 10.1093/infdis/jiw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson-Paul AM, Wei SC, Mattson CL, et al. Obesity Among HIV-Infected Adults Receiving Medical Care in the United States: Data From the Cross-Sectional Medical Monitoring Project and National Health and Nutrition Examination Survey. Medicine (Baltimore). 2015;94(27):e1081. doi: 10.1097/MD.0000000000001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debroy P, Sim M, Erlandson KM, et al. Progressive increases in fat mass occur in adults living with HIV on antiretroviral therapy, but patterns differ by sex and anatomic depot. J Antimicrob Chemother. 2019;74(4):1028–1034. doi: 10.1093/jac/dky551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Min Y, Ma X, Sankaran K, et al. Sex-specific association between gut microbiome and fat distribution. Nat Commun. 2019;10(1):2408. doi: 10.1038/s41467-019-10440-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noguera-Julian M, Rocafort M, Guillén Y, et al. Gut Microbiota Linked to Sexual Preference and HIV Infection . EBioMedicine. 2016;5:135–146. doi: 10.1016/j.ebiom.2016.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Body composition of study participants by abdominal CT imaging (A-D). p<0.05, **p<0.01, ***p<0.005, ****p<0.0001 as determined by Kruskal-Wallis test; pair-wise comparisons by Wilcoxon rank-sum test.
Figure S2. Circulating adiponectin and MCP1 in HIV+ participants with visceral and general obesity compared with lean body type. p<0.05, **p<0.01, ***p<0.005, ****p<0.0001 as determined by Kruskal-Wallis test; pair-wise comparisons by Wilcoxon rank-sum test.
Figure S3. Microbial community diversity analysis (Shannon Diversity Index) by body type limited to MSM (A), participants ever on Zidovudine (B), participants ever on first-generation PIs (C) and participants with HIV-1 viral load <400 copies/mL (D) as determined by Kruskal-Wallis test