Abstract
Background:
A growing body of research has examined relationships between neighborhood characteristics and exposure to air toxics in the United States. However, a limited number of studies have addressed neighborhood isolation, a measure of spatial segregation. We investigated the spatial distribution of carcinogenic air toxics in the St. Louis metropolitan area and tested the hypothesis that neighborhood isolation and sociodemographic characteristics are associated with exposure to carcinogenic air toxics.
Methods:
We obtained lifetime air toxics cancer risk data from the United States Environmental Protection Agency’s National Air Toxic Assessment and sociodemographic data from the American Community Survey. We used geographic information systems to identify statistically significant clusters of census tracts with elevated all-site cancer risk due to air toxics in the St. Louis metropolitan area. Relative Risks (RR) were estimated for the association between neighborhood characteristics and air toxic hot spots. Using a local spatial isolation index to evaluate residential segregation, we also evaluated the association between neighborhood racial and economic isolation and air toxic hot spots.
Results:
Approximately 14% (85 of the 615) of census tracts had elevated cancer risk due to air toxics (p < 0.01). These air toxic hot spots were independently associated with neighborhoods with high levels of poverty and unemployment and low levels of education. Census tracts with the highest levels of both racial isolation of Blacks and economic isolation of poverty were more likely to be located in air toxic hotspots than those with low combined racial and economic isolation (RR = 5.34; 95% CI = 3.10–9.22).
Conclusions:
These findings provide strong evidence of unequal distribution of carcinogenic air toxics in the St. Louis metropolitan area. Study results may be used to inform public health efforts to eliminate sociodemographic inequalities in exposure to air pollutants.
Keywords: Cancer, Air pollution, Sociodemographic, Disparity, Geographic Information System
BACKGROUND
Hazardous air pollutants, also referred to as “air toxics,” are chemical compounds that are associated with adverse health outcomes [1, 2]. In the United States (US), there have been well-documented disparities in exposure to air toxics, such as benzene, particulate matter, and other pollutants known to, or suspected to, cause cancer or other non-cancer adverse health effects, such as respiratory, cardiovascular, and neurological effects [3–6]. These disparities have been a longstanding focus of the environmental justice movement, with socially disadvantaged communities experiencing disproportionately higher exposures [7].
Previous US studies have examined associations between neighborhood characteristics, such as poverty, educational attainment, race and ethnicity, and exposure to carcinogenic air toxics [8–15]. For example, state-wide studies of cancer risk in Maryland and South Carolina and region-wide studies of cancer risk in Houston, TX Cancer Alley, LA, and Memphis, TN have reported that census tracts with higher percentages of minority residents experience greater cancer risk (from all air toxics present in a tract) than census tracts with lower percentages of minorities [8, 10, 11, 13, 14]. The current literature on associations between racial and ethnic composition and exposure to hazardous air pollutants in the United States, however, has been limited by crude measures of residential segregation. In general, these studies have utilized less precise measures (e.g. percent African Americans in the neighborhood) that capture segregation in a particular area without regard for the levels of segregation in the surrounding neighborhoods. As a result, these measures fail to account for contextual factors throughout the entire study area, leading to measurement errors that may influence observed associations with exposure to ambient air pollution.
US studies of segregation and health have employed either a global or local approach to evaluating residential segregation [16]. Based on an extensive and in-depth literature review on various segregation measures, Massey and Denton classified segregation measures into five dimensions: (1) evenness (i.e., the differential distribution of population groups); (2) exposure or, its counterpart, isolation (i.e., the potential interaction of population groups); (3) concentration, similar to the concept of density (i.e., the distributional intensity of population groups); (4) centralization (i.e., the dispersion of population groups with respect to the city center); and (5) clustering (i.e., the degree of spatial separation or proximity of population groups) [17]. Reardon and O’Sullivan [18] and Johnston et. al [19], however, later concluded that evenness and isolation were the two distinct conceptual dimensions of segregation. In US studies of segregation and exposure to ambient air pollution, for example, evenness may be used to indicate how segregation modifies an association between an exposure and health outcome across metropolitan areas, while isolation may be used to indicate how minority residents within an area experiencing residential segregation [9, 20]. In a literature review examining the relationships between segregation and health, Kramer and Hogue suggested that, compared with the evenness dimension, the isolation dimension may be the stronger measure for evaluating unhealthy environments and exposures [16]. When capturing the isolation dimension, the P* index of isolation [21] has been widely used in studies of residential racial segregation [17, 18]. While the P* index has been widely used for studying the inter-city or inter-MSA (Metropolitan Statistical Area) analysis, Oka and Wong recently modified the P* index and introduced the local spatial isolation index (SI[i]) for studying intra-MSA variations in segregation at the neighborhood level [22].
In this study, we conducted a spatial analysis of cancer risk in the St. Louis, MO–IL MSA (hereafter referred to as the St. Louis metropolitan area) because few studies, to date, have examined local spatial isolation as a dimension of segregation in relation to exposure to air toxics. Most of the previous work on inequalities in exposure to air toxics in the US has focused on locations in the Southern US, a region with the highest percentages of poverty, rural residents, and African American residents in the country. Few studies of air toxic cancer risks have focused exclusively on the American Midwest as an area of study.
The St. Louis metropolitan area, with an estimated population of 2.8 million residents, is one of the largest metropolitan areas in the American Midwest, and the 21st largest metropolitan area in the United States [23]. The area includes the independent city of St. Louis and 14 surrounding counties in the states of Missouri (Franklin, Jefferson, Lincoln, St. Charles, St. Louis, Warren, and Washington) and Illinois (Bond, Calhoun, Clinton, Jersey, Macoupin, Madison, Monroe, and St. Clair) [24]. The Mississippi River separates the two states, and approximately 75% of metropolitan area residents reside in Missouri [23]. In 2010, approximately 76% of St. Louis metropolitan area residents identified as white and 18% identified as African American, with the remaining identifying as Asian (2%), American Indian or Pacific Islander (1%), or some other race. Approximately 3% of St. Louis area residents were Hispanic or Latino of any race. St. Louis is one of the most racially segregated metropolitan area in the United States [25]. African American residents, for example, are mostly concentrated in neighborhoods in northern St. Louis City, northern St. Louis County, and in the city of East St. Louis in Illinois.
The St. Louis metropolitan area has well documented racial and socioeconomic disparities in health outcomes and quality of life [26]. However, the distribution of potential environmental health hazards, such as air toxics, are less well studied. Using local spatial isolation to evaluate residential segregation, we investigated relationships between the isolation and sociodemographic characteristics of St. Louis area neighborhoods and cancer risk from air toxics. Understanding the relationships between neighborhood characteristics and exposure to air toxics is critical to informing the design and evaluation of effective public health interventions.
METHODS
Cancer Risk from Air Toxics
We obtained lifetime air toxics cancer risk data from the United States Environmental Protection Agency’s National Air Toxic Assessment (NATA). NATA is EPA’s nationwide assessment of air toxics known to, or suspected to, cause cancer or non-cancer adverse health effects[1]. The assessment includes four steps: (1) compiling a national emissions inventory of air toxics emissions from outdoor sources; (2) estimating ambient concentrations of air toxics across the United States; (3) estimating population exposures across the United States; and (4) characterizing potential public health risk due to inhalation of air toxics including both cancer and non-cancer effects. NATA determines air toxics concentrations and cancer risk estimates at the census tract level [1]. Cancer risk estimates assume a person breathes these emissions each year over a lifetime (or approximately 70 years). Cancer risk is therefore defined as the probability of contracting cancer over the course of a lifetime [1].
NATA has provided an estimation of lifetime all-site cancer risk from inhalation of air toxics since 1996 [1]. Cancer risks from eight different emission sources were included in the assessment, including (a) pollutants directly emitted into the atmosphere (on-road mobile sources (e.g. passenger and commercial trucks, vehicles, and buses), non-road mobile sources (e.g. aircraft, heavy equipment, locomotives), point sources (major stationary sources), non-point sources (area and other smaller stationary sources), biogenic sources (from trees, plants and soil microbes), and fire sources), (b) pollutants that form in the air as a result of atmosphere transformation of precursor chemicals (secondary sources), and (c) background emissions (pollutant concentrations without a specific source) [27, 28]. Total cancer risk from air toxics was determined as the sum of the risks of the eight aforementioned emissions sources. The current study utilized NATA data from 2011 to evaluate cancer risk data for each census tract in the St. Louis metropolitan area. The 2011 NATA included emissions, ambient concentrations, and exposure estimates for 180 Clean Air Act air toxics [1].
Sociodemographic and Isolation Characteristics
Census tract-level sociodemographic data were obtained from the US Census Bureau’s American Community Survey (ACS) 2010–2014 Five-Year Estimates.[29] Sociodemographic variables about race, ethnicity, and socioeconomic status were selected based on previous research.[6, 8–11, 14, 30–34] These variables included: (a) percent of the population that is non-white, (b) percent of the population that is African American, (c) percent of the population that is Hispanic, d) percent of the population that is without a high school education, (e) per capita income, (f) median household income, (g) percent of the population in poverty, (h) percent of the population that is unemployed.
We also included measures of the racial and socioeconomic isolation to capture spatial patterns of residential segregation within the St. Louis metropolitan area. These measures of residential segregation, developed by Oka and Wong, [22] account for the spatial relationships between census tracts to reflect residential segregation at the neighborhood scale. Instead of using the percentages of populations belonging to a particular group (e.g. percentage black and percentage poverty) as an indicator of residential segregation, Oka and Wong proposed that such measures should take into account how populations are spread across surrounding neighborhoods [35]. The isolation measures detail local patterns of residential segregation across a study area using the concept of “composite population counts” [36]. The composite population count includes the population of neighboring units in evaluating the population of a reference unit. For example, the formula for calculating a local black isolation index is as follows:
where cbi is the composite population count of black population in census tract i, B is the population count of black population for the entire study area (i.e., St. Louis metropolitan area), and cti is the composite population of the total population in census tract i. This probabilistic approach to evaluating segregation is useful for considering the degree of isolation for a specific group. Isolation measures may be interpreted as the probability of a particular population group member meeting another member of the same group within a neighborhood (i.e., census tract). Therefore, the higher the probability, the greater the proportion of that neighborhood’s (or census tract’s) population who are members of the same group. In the current study, we used Oka and Wong’s black isolation and poverty isolation measures as the indicator of spatial separation from other racial/ethnic populations and from non-poverty populations, respectively. These measures were selected because of St. Louis metropolitan area’s well-documented racial (Black/White) and economic health inequalities [26].
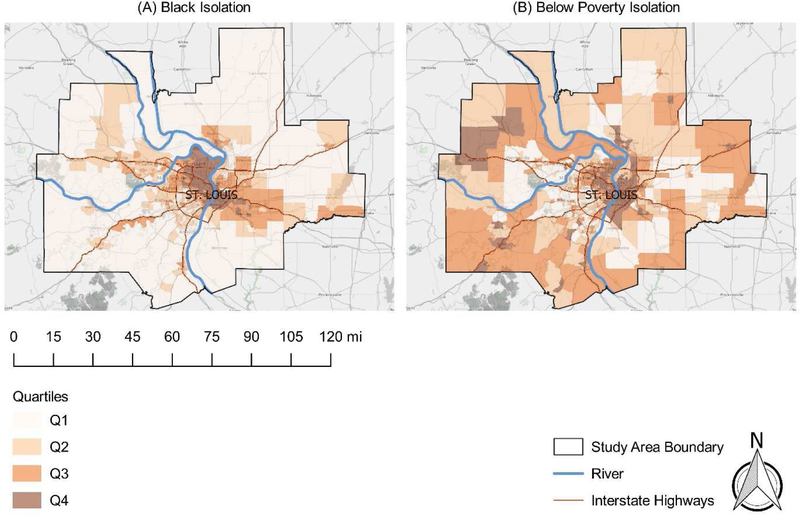
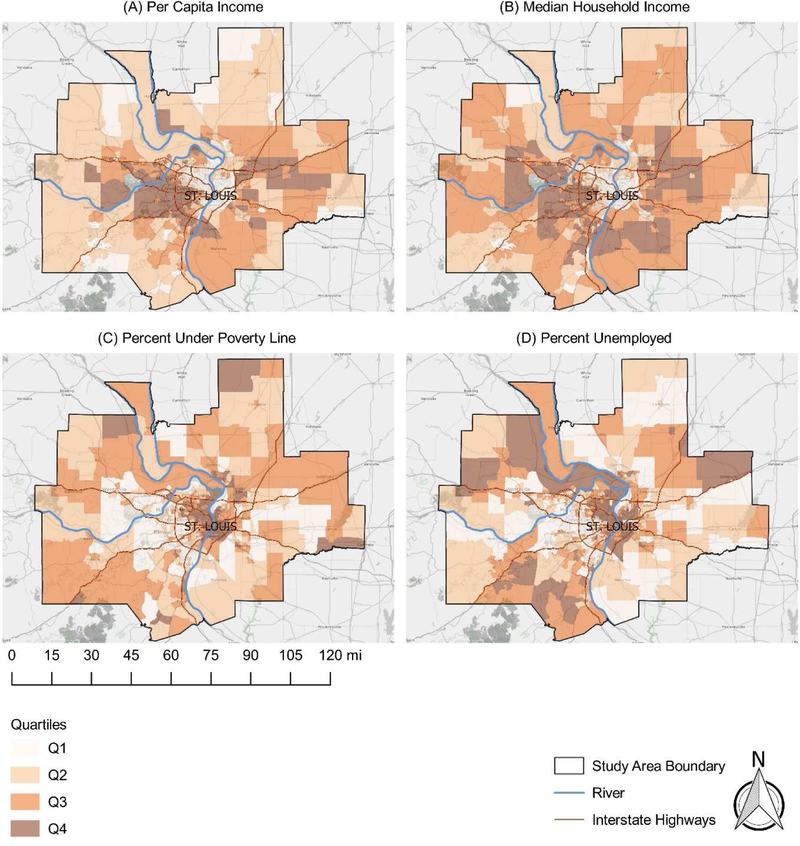
Cancer risk estimates were linked to census tract-level sociodemographic and isolation measures using the US Federal Information Processing Standards (FIPS) code. Our final analytical dataset included cancer risk estimates, sociodemographic data, and isolation data for a total of 615 St. Louis metropolitan area census tracts. Figures 1–3 display the spatial distributions of cancer risk and sociodemographic and isolation characteristics across all St. Louis census tracts by quartile.
Figure 1.
Map of percent non-white (a), percent African American (b), and percent Hispanic (c) in the St. Louis metropolitan area
Figure 3.
Map of racial (a) and economic (b) segregation in the St. Louis metropolitan area
Spatial Clustering
Moran’s I test confirmed the presence of spatial autocorrelation of cancer risk [37, 38]. The Getis–Ord Gi* statistic was then used to identify cancer risk clustering patterns across all census tracts in St. Louis metropolitan area by generating a z-score for each census tract. Briefly, each census tract is examined within the context of its neighboring census tracts. The cancer risk for a census tract and its neighboring census tracts is compared proportionally to the cancer risk values within the entire study area. To be considered a statistically significant hot spot, a census tract with a high cancer risk must be surrounded by other census tracts with high cancer risk as well. The Gi* statistic returned for each census tract is a z-score (standardized value) to which a p-value is associated. The null hypothesis for this statistic is that cancer risks in the St. Louis metropolitan area exhibit a random spatial pattern. When the p-values are statistically significant, the assumption is that the spatial distribution of cancer risks is not random. We used the Getis-Ord Gi* statistic within ArcGIS with alphas of 0.05 (95% confidence) and 0.01 (99% confidence) significance to identify cancer risk hotspots. We used a threshold of z-score ≥ 2.58 to define a 99% hotspot and a threshold of z-score ≥ 1.96 to define a 95% hotspot. Coldspots were defined using thresholds of z-score < −2.58 (99% coldspot) and z-score < −1.96 (95% coldspots).
Data Analyses
Relative Risk
Association between neighborhood characteristics and cancer risk were estimated as relative risks with 95% confidence intervals from log-binomial regression models. For the purposes of these analyses, census tracts were dichotomized into air toxic hotspots and non-hotspots. For each sociodemographic and isolation characteristic, census tracts were categorized into four sequential quartile groups, with the first group (Q1) representing census tracts with values up to the 25th percentile, the second group (Q2) representing census tracts with values between the 25th percentile and the median, the third group (Q3) representing census tracts with values between the median and the 75th percentile, and the fourth group (Q4) representing census tracts with values above the 75th percentile. To compare cancer risk, relative risk (RR) and 95% confidence interval (CI) were estimated as the ratio of the proportion of high cancer risk tracts in each quartile group, with each of the three highest groups compared with the first quartile (reference group).
We examined hotspot risks associated with combined racial and economic isolation by creating four groups of census tracts based on their racial and economic composition: (High racial and high poverty isolation, high racial and low poverty isolation, low racial and high poverty isolation, low racial and low poverty isolation). “High racial isolation” census tracts were defined as census tracts with racial isolation values above the 75th percentile, while “low racial isolation” census tracts were defined as census tracts with racial isolation values at or below the 75th percentile. Similarly, “high economic isolation” census tracts were defined as census tracts with economic isolation values above the 75th percentile and “low economic isolation” census tracts were defined as census tracts with economic isolation values at or below the 75th percentile.
All spatial analyses were conducted in ArcGIS version 10.5 (ESRI, Redlands, CA, USA), and statistical analyses were conducted in SAS version 9.4 (Cary, NC, USA).
RESULTS
Lifetime Cancer Risk from Air Toxics
The distribution of cancer risk estimates in the St. Louis metropolitan area, Missouri, Illinois, and the US are displayed in Table 1. The median lifetime total air toxics cancer risk for the St. Louis metropolitan area was higher than the median cancer risks for Missouri, Illinois, and the United States as a whole.
Table 1.
Lifetime Cancer Risk (persons per million) from Air Toxics by Region
| Region | Mean | 5th Percentile | 50th Percentile | 95th Percentile |
|---|---|---|---|---|
| St. Louis Metropolitan Area (n = 615 tracts) | 48.15 | 34.14 | 49.70 | 58.55 |
| Missouri (n= 1391 tracts) | 43.35 | 30.73 | 43.00 | 56.03 |
| Illinois (n = 3115 tracts) | 35.88 | 26.07 | 34.87 | 47.92 |
| United States (n = 73450 tracts) | 40.03 | 21.52 | 39.52 | 58.95 |
Table 2 presents the distribution of cancer risks by emission sources in the St. Louis metropolitan area. Secondary sources (pollutants formed in the atmosphere from precursor chemicals) were the largest contributors to cancer risk. Of the direct emissions sources (on-road, non-road, point, non-point, biogenics, and fire), on-road sources (vehicles used on roads and highways) contributed the most cancer risk (Table 2). Descriptive statistics for neighborhood isolation and sociodemographic characteristics are summarized in Table 3.
Table 2.
Distribution of Cancer Risk by Emission Source in the St. Louis Metropolitan Area
| Source | Mean | 5th Percentile | 50th Percentile | 95th Percentile |
|---|---|---|---|---|
| On-road | 6.79 | 1.74 | 7.12 | 11.41 |
| Non-road | 2.39 | 0.71 | 2.35 | 4.33 |
| Point | 2.89 | 0.47 | 2.14 | 7.38 |
| Non-point | 2.24 | 0.91 | 2.12 | 4.12 |
| Biogenics | 3.26 | 2.37 | 3.11 | 4.46 |
| Fire | 0.83 | 0.63 | 0.84 | 1.07 |
| Secondary | 26.02 | 20.30 | 26.94 | 29.26 |
| Background | 3.37 | 3.37 | 3.37 | 3.38 |
| Total | 48.15 | 34.14 | 49.70 | 58.55 |
Table 3.
Neighborhood characteristics of the St. Louis Metropolitan Area (n = 615 census tracts)
| Mean | 5th Percentile | 25th Percentile | 50th Percentile | 75th Percentile | 95th Percentile | |
|---|---|---|---|---|---|---|
| % Non-white | 26.72 | 1.10 | 3.90 | 10.70 | 36.90 | 98.50 |
| % African American | 22.30 | 0.00 | 0.80 | 5.00 | 29.80 | 97.10 |
| % Hispanic | 2.39 | 0.00 | 0.60 | 1.70 | 3.30 | 6.40 |
| % Without high school education | 12.88 | 2.40 | 6.50 | 10.90 | 16.80 | 29.80 |
| Per capita income per $10,000 | 27.40 | 12.34 | 20.68 | 25.77 | 31.61 | 48.19 |
| Median household income per $10,000 | 55.33 | 21.48 | 39.11 | 52.16 | 67.63 | 101.60 |
| % Poverty | 13.60 | 1.20 | 4.60 | 9.10 | 19.30 | 40.90 |
| % Unemployment | 5.69 | 1.80 | 3.20 | 4.70 | 7.10 | 13.10 |
| Isolation measures (range 0–1)* | ||||||
| Racial (Black) Isolation | 0.08 | 0.00 | 0.00 | 0.01 | 0.08 | 0.43 |
| Economic (below poverty) Isolation | 0.15 | 0.01 | 0.03 | 0.07 | 0.20 | 0.54 |
Isolation measures range from 0 to 1, with higher values indicating a greater proportion of that neighborhood’s (or census tract’s) population who are members of the same group.
Air Toxic Hotspots
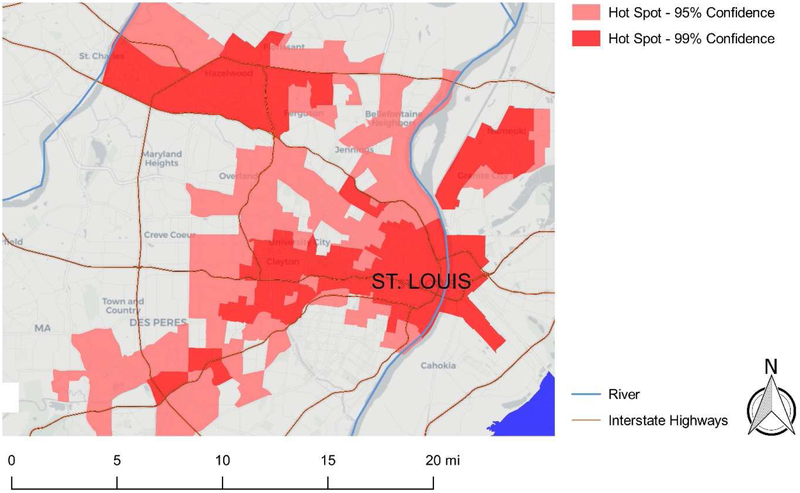
As shown in Figure 4, spatial autocorrelation analyses identified carcinogenic air toxic hotspots and coldspots in the St. Louis metropolitan area. Hotspot census tracts with 95% (light red) and 99% (dark red) significance are presented in Figure 5. Approximately 25% (154 of the 615) of census tracts were hotspots at the 95% confidence level, and 14% (85 of the 615) of census tracts were hotspots at the 99% confidence level. There were six distinct hotspots at the 99% confidence level; four of these hotspot area included intersections of major interstate highways (Figure 5).
Figure 4.
Hotspot and coldspot cluster map for cancer risk in the St. Louis metropolitan area. Census tracts with elevated cancer risk (red) represent hotspots. Census tracts with low cancer risk (blue) represent coldspots.
Figure 5.
Zoomed-in view showing clusters for cancer risk in the St. Louis metropolitan area. Census tracts with elevated cancer risk at 0.01 (dark red) significance represent hotspot clusters.
Relative Risk
Table 4 displays the percentage of census tracts in hotspot areas and the Relative Risk (RR) by quartile for each sociodemographic measure. There was a significant association between air toxic hotspots and each sociodemographic measure except Hispanic race. For example, census tracts with the highest proportion of non-white residents (Q4) were 18.5 times more likely to be identified as air toxic hot spots (at the 99% significance level) than census tract with the lowest proportion of non-whites (Q1). Relative Risks were of similar magnitude for the African American (Hotspot-99% Q4 versus Q1 RR = 17.4) and Poverty (Hotspot-99% Q4 versus Q1 RR = 12.0) variables. RRs for Q4 versus Q1 were also significant for the racial and economic isolation variables. We observed significant inverse associations between both per capita income and median household income and air toxic hotspots (Table 4).
Table 4.
Relative Risk for Cancer Risk Air Toxic Hotspots in the St. Louis MSA
| Air Toxic Hotspot-95% %* | Air Toxic Hotspot-99% %* | Air Toxic Hotspot-95% RR | Air Toxic Hotspot-99% RR | Air Toxic Hotspot-95% %* | Air Toxic Hotspot-99% %* | Air Toxic Hotspot-95% RR | Air Toxic Hotspot-99% RR | ||
|---|---|---|---|---|---|---|---|---|---|
| % Non-white | % African American | ||||||||
| Q4 | 49.8 | 28.6 | 12.13(7.64–19.25) | 18.49(7.79–43.90) | Q4 | 64.3 | 27.9 | 10.92 (6.89–17.29) | 17.40(7.32–41.35) |
| Q3 | 28.6 | 18.7 | 6.50(4.60–9.19) | 8.92(4.66–17.05) | Q3 | 37.8 | 18.0 | 6.01 (4.25–8.48) | 8.52(4.45–16.31) |
| Q2 | 15.3 | 3.9 | 3.48 (2.76–4.39) | 4.30 (2.79–6.63) | Q2 | 17.6 | 6.1 | 3.30(2.63–4.16) | 4.17(2.71–6.43) |
| Q1 | 6.4 | 4.0 | Reference | Reference | Q1 | 11.4 | 2.9 | Reference | Reference |
| % Hispanic | % without High School education | ||||||||
| Q4 | 34.2 | 15.5 | 0.84(0.55–1.28) | 0.79(0.38–1.65) | Q4 | 37.0 | 21.6 | 2.49(1.63–3.80) | 2.75(1.32–5.72) |
| Q3 | 29.4 | 10.6 | 0.88(0.36–1.21) | 0.84(0.49–1.45) | Q3 | 23.7 | 11.3 | 1.98(1.44–2.72) | 2.13(1.23–3.70) |
| Q2 | 30.4 | 11.8 | 0.92(0.50–1.13) | 0.89(0.62–1.28) | Q2 | 19.2 | 12.6 | 1.58(1.28–1.95) | 1.66(1.15–2.39) |
| Q1 | 38.9 | 18.0 | Reference | Reference | Q1 | 20.2 | 9.9 | Reference | Reference |
| Per capita income | Median household income | ||||||||
| Q4 | 28.1 | 14.9 | 0.39(0.25–0.61) | 0.39(0.25–0.61) | Q4 | 15.3 | 5.2 | 0.18(0.11–0.27) | 0.08(0.04–0.19) |
| Q3 | 11.8 | 5.9 | 0.50 (0.36–0.69) | 0.46 (0.26–0.80) | Q3 | 13.3 | 7.2 | 0.27 (0.20–0.37) | 0.15(0.08–0.28) |
| Q2 | 17.7 | 9.7 | 0.63 (0.50–0.78) | 0.60(0.41–0.86) | Q2 | 27.6 | 14.3 | 0.42 (0.34–0.52) | 0.29(0.19–0.43) |
| Q1 | 42.4 | 24.8 | Reference | Reference | Q1 | 43.8 | 28.8 | Reference | Reference |
| % Poverty | % Unemployment | ||||||||
| Q4 | 45.3 | 29.2 | 5.76(3.71–8.95) | 12.04 (5.25–27.6) | Q4 | 43.4 | 24.0 | 2.96(1.90–4.61) | 3.37(1.59–7.14) |
| Q3 | 22.7 | 12.1 | 3.72(2.68–5.17) | 6.47(3.47–12.04) | Q3 | 16.8 | 10.7 | 2.26(1.62–3.15) | 2.49(1.42–4.37) |
| Q2 | 19.2 | 9.2 | 2.40(1.92–2.99) | 3.47 (2.29–5.25) | Q2 | 16.8 | 9.2 | 1.72(1.38–2.15) | 1.84(1.26–2.67) |
| Q1 | 12.8 | 4.6 | Reference | Reference | Q1 | 23.2 | 11.4 | Reference | Reference |
| Racial (black) Isolation | Economic (below poverty) Isolation | ||||||||
| Q4 | 52.7 | 32.5 | 14.14(10.60–27.72) | 41.16(15.63–108.37) | Q4 | 50.3 | 31.2 | 7.58(4.83–11.88) | 14.63 (6.29–34.02) |
| Q3 | 29.1 | 16.2 | 8.43 (5.88–12.08) | 16.25 (7.86–33.59) | Q3 | 19.2 | 11.7 | 4.57 (3.26–6.40) | 7.48 (3.97–14.09) |
| Q2 | 13.8 | 5.8 | 4.14(3.26–5.26) | 6.42(3.95–10.41) | Q2 | 15.3 | 6.5 | 2.75 (2.20–3.45) | 3.82(2.51–5.83) |
| Q1 | 4.4 | 0.7 | Reference | Reference | Q1 | 15.3 | 5.9 | Reference | Reference |
Percentage of census tracts for the analytic subgroup that were hotspot census tracts (% = hotspot census tractssubgroup/total census tractssubgroup)
Relative risks for the combined racial and economic isolation measure are presented in Table 5. Census tracts with highest proportion of both racial and economic isolation were five times more likely to be located in air toxic hotspots than those with lowest proportions of racial and economic isolation.
Table 5.
Racial and Economic Isolation and Cancer Risk Air Toxic Hotspots
| Cancer Risk Hotspot-95% % | Cancer Risk Hotspot-99% % | Hotspot-95% RR | Hotspot-99% RR | |
|---|---|---|---|---|
| Isolation category | ||||
| High racial and high economic | 67.3 | 27.3 | 4.52 (2.40–6.03) | 5.34(3.10–9.22) |
| High racial and low economic | 52.3 | 25.0 | 3.10(2.50–3.85) | 3.51 (2.33–5.29) |
| Low racial and high economic | 40.9 | 34.1 | 2.13(1.84–2.45) | 2.31(1.75–3.04) |
| Low racial and low economic | 21.2 | 7.0 | 1.0 | 1.0 |
DISCUSSION
This study examined associations between neighborhood isolation and sociodemographic characteristics and cancer risk from air toxics in the St. Louis metropolitan area. We found that census tract–level measures of poverty, undereducation, and unemployment were associated with air toxic hotspots, while factors such as per capita income and median household income were inversely associated with air toxic hotspots. In the present study, we used local spatial isolation measures to examine relationships between neighborhood segregation and cancer risk from air toxics. We observed that air toxic hotspots were associated with census tracts with high levels of combined racial and economic isolation.
Multiple studies have documented sociodemographic disparities in exposure to air pollution [8–15, 31]. Given the growing evidence for associations between environmental exposures and cancer risk,[39–42] these disparities in exposure to air toxics may confer a greater risk for cancer in low socioeconomic status populations. Researchers have long theorized that disparities in exposure to ambient environmental hazards may result in racial disparities in health outcomes,[43–46] including the hypothesis that residential segregation leads to differential exposure to air pollutants. In the US, a history of discriminatory housing policies and unequal access to affordable loans and mortgages has made it difficult for Blacks and other minorities to move into predominantly white communities.[47] Although overall segregation has decreased, low-income Black and minorities are more likely to be spatially isolated.[48] Kramer and Hogue proposed four hypothesized pathways between segregation and health: (1) segregation begetting individual socioeconomic status; (2) segregation perpetuating unhealthy neighborhood environments; (3) segregation modifying social capital; and (4) segregation modifying individual risk behaviors or exposure to stress.[16] With regard to the relationship between segregation and exposure to air toxics, it is unclear how spatial forms of segregation disproportionately expose certain population groups. Segregation may lead to the unequal distribution of socioeconomic resources in a metropolitan area, which may lead to the economic and environmental disenfranchisement of predominantly low-income, minority comminutes, and limited community involvement in land use planning. The results of segregation are neighborhoods with low social capital, few community resources, and low property values which may, in turn, attract more low-income and minority residents, thus perpetuating the cycle of segregation and exposure to unhealthy air toxics.[31, 49, 50] In our study, cancer hotspot risk was associated with high levels of combined racial and economic isolation, a result that was consistent with previous studies on segregation.[6, 9, 20, 33, 51–53] However, much remains to be elucidated about the mechanisms by which segregation influences exposure to air toxics. Examining associations by type of emissions sources (e.g. on-road sources versus point sources), for example, will lead to a better understanding of the relationship between segregation and exposure to carcinogenic air toxics.[20]
The geographical focus of this study was St. Louis, MO–IL MSA, a major metropolitan area in the Midwestern United States. The results of our study are consistent with several studies of cancer risk stemming from southern US regions.[10, 14, 31, 32, 54] In Houston, Texas, cancer risk from air toxics was disproportionately concentrated in census tracts with the highest percentages of Hispanic residents and social disadvantage (e.g. percent of residents living below poverty, percent over 25 with less than high school education, and percent households on public assistance).[10] There were significant racial disparities in cancer risks in the Memphis, Tennessee metropolitan area where census tracts with higher percentages of African American residents displaying higher cancer risk burden than census tracts with low percentages of African American residents.[32] In a study of cancer risks in South Carolina, measures of economic deprivation and white/non-white isolation segregation were associated with air toxics cancer risk at the census tract level.[31] Similar associations were observed in a follow-up study of South Carolina with observed associations between air toxic cancer risk and census tract-level percentages of non-white persons, percentage of Hispanic persons, and percentage of residents living below poverty.[14]
Limitations
Our analyses were cross-sectional in nature, and although we observed associations between sociodemographic factors and cancer risks from air toxic risk, we cannot infer causality from this data. Cancers are multifactorial in nature, with risk factors that include poor diet, lack of physical activity, obesity, and tobacco and alcohol use. The study was limited by census track-level analyses as we could not account for these individual-level factors and other exposures that may contribute to cancer risk. We evaluated associations at the census tract-level, and spatial clustering analyses may have failed to account for a scenario in which a census tract with a high cancer risk level is next to census tracts with low cancer risk levels. The limitations associated with the EPA’s NATA data set should also be noted. NATA cancer risks were estimated using EPA risk assessment models and thus, were not based on human cancer data. Cancer risks were modeled for only a selected number (n=180) of pollutants, which does not allow for a comprehensive estimates of cancer risk from all potential air toxics, including indoor pollutants. Finally, cancer risks were determined for inhalation exposures and did not take into account exposures from other routes of entry into the body, such as dermal absorption and ingestion.
Implications for policy and future research
Our study is an important contribution as it allowed for a better understanding of air toxic cancer risks throughout the St. Louis metropolitan area. Our findings have implications for the development of policies and interventions to reduce and eliminate sociodemographic disparities in exposure to carcinogenic air pollutants. In addition to identifying local areas of concern within the region, our results support the promotion and evaluation of (1) initiatives aimed at emission reductions and (2) housing policies to reduce racial and economic segregation. Reducing segregation may lead to a reduction in social disparities and an improvement in neighborhood environments, including higher property values and better-resourced public services. These improved neighborhood environments may attract more affluent residents, which could lead to more public and political engagement over land-use policy decisions and result in the reduction the both the disparities in exposure to air toxics and the disparities in air toxics-related cancer risk. In our study, secondary pollutants and emissions on-road mobile sources were the top two contributors to cancer risk. Sample policies to reduce these emissions could include raising vehicle emission standards and promoting public transportation. Furthermore, any effort to address air toxic exposure disparities in this region must confront residential segregation. Mixed-income housing and rental vouchers are examples of policy approaches that could challenge racial and economic segregation in the region. Ongoing monitoring and evaluation will enable policymakers to make informed decisions with regard to these issues.
Hotspot analysis can be applied to subsequent investigations of ambient air quality in the St. Louis metropolitan area, and mixed models, accounting for spatial correlation,[55] should also be employed in future studies. Research priorities should include local field monitoring for air toxics. Local monitoring would allow for more focused source apportionment studies to identify and prioritize source compounds. In addition to quantifying disproportionate exposures, these methods could be applied to exposure assessments for future epidemiologic studies of air toxics and health outcomes. Future research efforts should also prioritize community-engaged strategies. Key community engagement components include a mutual understanding of community needs and goals, community involvement at each step of the research process, community understanding of the strengths and limitations of air monitoring, and effective dissemination of research results.[56, 57] Research partnerships with communities can be utilized to identify and address environmental exposure disparities in a manner that will be meaningful to the populations who live, work, and play within air toxic hotspots.
CONCLUSIONS
There is an unequal distribution of cancer risk from air toxics in the St. Louis metropolitan area, with neighborhood characteristics such as poverty, unemployment, low educational attainment, and racial and economic isolation associated with increased risk. Future investigations are warranted to identify priority sources of hazardous air pollutants and inform public health efforts to eliminate sociodemographic disparities in exposure to air toxics.
Figure 2.
Map of per capita income (a), median household income (b), percent below poverty (c), and percent unemployment (d) in the St. Louis metropolitan area
Highlights.
We examined lifetime cancer risk from air toxics using a spatial analysis
Neighborhood isolation was measured using a local spatial isolation index
Relationships between neighborhood isolation and lifetime cancer risk were examined
Neighborhood racial and poverty isolation were associated with lifetime cancer risk
Acknowledgements
Funding
This study was supported in part by the Centers for Disease Control and Prevention [U19 OH008868] and the National Cancer Institute Cancer Center Support Grant [P30 CA091842] to the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
LIST OF ABBREVIATIONS
- ACS
American Community Survey
- EPA
Environmental Protection Agency
- GIS
Geographic Information System
- MSA
Metropolitan Statistical Area
- NATA
National Air Toxic Assessment
- RR
Relative Risk
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Availability of supporting data
Not applicable
Competing Interests
The authors declare no conflict of interest.
References
- 1.U.S. Environmental Protection Agency. National Air Toxics Assessment. 2018. [cited 2019 September 5, 2019]; Available from: https://www.epa.gov/national-air-toxics-assessment/nata-overview.
- 2.Curtis L, et al. , Adverse health effects of outdoor air pollutants. Environment international, 2006. 32(6): p. 815–830. [DOI] [PubMed] [Google Scholar]
- 3.Association AL, Urban air pollution and health inequities: a workshop report. Environmental Health Perspectives, 2001. 109(suppl 3): p. 357–374. [PMC free article] [PubMed] [Google Scholar]
- 4.Ringquist EJ, Assessing evidence of environmental inequities: A meta-analysis. Journal of Policy Analysis and Management: The Journal of the Association for Public Policy Analysis and Management, 2005. 24(2): p. 223–247. [Google Scholar]
- 5.Brooks N and Sethi R, The distribution of pollution: community characteristics and exposure to air toxics. Journal of environmental economics and management, 1997. 32(2): p. 233–250. [Google Scholar]
- 6.Lopez R, Segregation and black/white differences in exposure to air toxics in 1990. Environmental Health Perspectives, 2002. 110(Suppl 2): p. 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker G, Environmental justice: concepts, evidence and politics. 2012: Routledge. [Google Scholar]
- 8.Apelberg BJ, Buckley TJ, and White RH, Socioeconomic and racial disparities in cancer risk from air toxics in Maryland. Environmental health perspectives, 2005. 113(6): p. 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morello-Frosch R and Jesdale BM, Separate and unequal: residential segregation and estimated cancer risks associated with ambient air toxics in US metropolitan areas. Environmental health perspectives, 2006. 114(3): p. 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linder SH, Marko D, and Sexton K, Cumulative cancer risk from air pollution in Houston: disparities in risk burden and social disadvantage. 2008, ACS Publications. [DOI] [PubMed] [Google Scholar]
- 11.James W, Jia C, and Kedia S, Uneven magnitude of disparities in cancer risks from air toxics. International journal of environmental research and public health, 2012. 9(12): p. 4365–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young GS, et al. , Differential exposure to hazardous air pollution in the United States: a multilevel analysis of urbanization and neighborhood socioeconomic deprivation. International journal of environmental research and public health, 2012. 9(6): p. 2204–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia C and Foran J, Air toxics concentrations, source identification, and health risks: An air pollution hot spot in southwest Memphis, TN. Atmospheric environment, 2013. 81: p. 112–116. [Google Scholar]
- 14.Wilson S, et al. , Assessment of sociodemographic and geographic disparities in cancer risk from air toxics in South Carolina. Environmental research, 2015. 140: p. 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grineski SE, Collins TW, and Morales DX, Asian Americans and disproportionate exposure to carcinogenic hazardous air pollutants: A national study. Social Science & Medicine, 2017. 185: p. 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer MR and Hogue CR, Is segregation bad for your health? Epidemiologic reviews, 2009. 31(1): p. 178–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massey DS and Denton NA, The dimensions of residential segregation. Social forces, 1988. 67(2): p. 281–315. [Google Scholar]
- 18.Reardon SF and O’Sullivan D, Measures of spatial segregation. Sociological methodology, 2004. 34(1): p. 121–162. [Google Scholar]
- 19.Johnston R, Poulsen M, and Forrest J, Ethnic and racial segregation in US Metropolitan areas, 1980–2000: the dimensions of segregation revisited. Urban Affairs Review, 2007. 42(4): p. 479–504. [Google Scholar]
- 20.Morello-Frosch R and Lopez R, The riskscape and the color line: examining the role of segregation in environmental health disparities. Environmental research, 2006. 102(2): p. 181–196. [DOI] [PubMed] [Google Scholar]
- 21.Lieberson S, An asymmetrical approach to segregation. Ethnic segregation in cities., 1981: p. 61–82. [Google Scholar]
- 22.Oka M and Wong DW, Segregation: a multi-contextual and multi-faceted phenomenon in stratified societies, in Handbook of Urban Geography, Schwanen T and Van Kempen R, Editors. 2019, Edward Elgar: Cheltenham. p. 255–280. [Google Scholar]
- 23.U.S. Census Bureau. Annual Estimates of the Resident Population: April 1, 2010 to July 1, 2017. 2018 December 1, 2018]; Available from: http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk.
- 24.U.S. Office of Management and Budget. OMB Bulletin No. 10–02: Update of Statistical Area Definitions and Guidance on Their Uses. 2009 December 1, 2018]; Available from: https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/bulletins/2010/b10-02.pdf.
- 25.Massey DS and Tannen J, A Research Note on Trends in Black Hypersegregation. Demography, 2015. 52(3): p. 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.For the Sake of All. Segregation in St. Louis: Dismantling the divide. 2018 February 26, 2019]; Available from: https://forthesakeofall.org/wp-content/uploads/2018/08/Segregation-in-St.-Louis-Dismantling-the-Divide.pdf.
- 27.U.S. Environmental Protection Agency. 2011 National Air Toxics Assessment. 2019. [cited 2019 September 5, 2019]; Available from: https://www.epa.gov/national-air-toxics-assessment/2011-national-air-toxics-assessment.
- 28.Weinhold B, Pollution portrait: the fourth National-Scale Air Toxics Assessment. Environmental health perspectives, 2011. 119(6): p. A255–A257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Census Bureau, 2010–2014 American Community Survey 5-year estimates. 2015.
- 30.Gilbert A and Chakraborty J, Using geographically weighted regression for environmental justice analysis: Cumulative cancer risks from air toxics in Florida. Social Science Research, 2011. 40(1): p. 273–286. [Google Scholar]
- 31.Rice LJ, et al. , Use of segregation indices, Townsend Index, and air toxics data to assess lifetime cancer risk disparities in metropolitan Charleston, South Carolina, USA. International journal of environmental research and public health, 2014. 11(5): p. 5510–5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia C, James W, and Kedia S, Relationship of racial composition and cancer risks from air toxics exposure in Memphis, Tennessee, U.S.A. Int J Environ Res Public Health, 2014. 11(8): p. 7713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ard K, By all measures: An examination of the relationship between segregation and health risk from air pollution. Population and Environment, 2016. 38(1): p. 1–20. [Google Scholar]
- 34.Mikati I, et al. , Disparities in Distribution of Particulate Matter Emission Sources by Race and Poverty Status. Am J Public Health, 2018. 108(4): p. 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oka M and Wong DW, Capturing the two dimensions of residential segregation at the neighborhood level for health research. Frontiers in public health, 2014. 2: p. 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong DW, Measuring multiethnic spatial segregation. Urban Geography, 1998. 19(1): p. 77–87. [Google Scholar]
- 37.Ord JK and Getis A, Local spatial autocorrelation statistics: distributional issues and an application. Geographical analysis, 1995. 27(4): p. 286–306. [Google Scholar]
- 38.Wong DW-S and Lee J, Statistical analysis of geographic information with ArcView GIS and ArcGIS. 2005: John Wiley & Sons Hoboken, NJ. [Google Scholar]
- 39.Clapp RW, Jacobs MM, and Loechler EL, Environmental and occupational causes of cancer: new evidence 2005–2007. Reviews on environmental health, 2008. 23(1): p. 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S, et al. , Substantial contribution of extrinsic risk factors to cancer development. Nature, 2016. 529(7584): p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jagai JS, et al. , County-level cumulative environmental quality associated with cancer incidence. Cancer, 2017. 123(15): p. 2901–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray JM, et al. , State of the evidence 2017: An update on the connection between breast cancer and the environment. Environmental Health, 2017. 16(1): p. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans GW and Kantrowitz E, Socioeconomic status and health: the potential role of environmental risk exposure. Annual review of public health, 2002. 23(1): p. 303–331. [DOI] [PubMed] [Google Scholar]
- 44.Brulle RJ and Pellow DN, Environmental justice: human health and environmental inequalities. Annu. Rev. Public Health, 2006. 27: p. 103–124. [DOI] [PubMed] [Google Scholar]
- 45.Richardson EA, et al. , Particulate air pollution and health inequalities: a Europe-wide ecological analysis. International journal of health geographics, 2013. 12(1): p. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajat A, Hsia C, and O’Neill MS, Socioeconomic disparities and air pollution exposure: a global review. Current environmental health reports, 2015. 2(4): p. 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massey DS and Denton NA, American apartheid: Segregation and the making of the underclass. 1993: Harvard University Press. [Google Scholar]
- 48.Massey DS and Fischer MJ, How segregation concentrates poverty. Ethnic and racial studies, 2000. 23(4): p. 670–691. [Google Scholar]
- 49.Williams DR and Collins C, Racial residential segregation: a fundamental cause of racial disparities in health. Public health reports, 2001. 116(5): p. 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson SM, An ecologic framework to study and address environmental justice and community health issues. Environmental Justice, 2009. 2(1): p. 15–24. [Google Scholar]
- 51.Gee GC and Payne-Sturges DC, Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environmental health perspectives, 2004. 112(17): p. 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Downey L, et al. , Environmental inequality in metropolitan America. Organization & environment, 2008. 21(3): p. 270–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones MR, et al. , Race/ethnicity, residential segregation, and exposure to ambient air pollution: the Multi-Ethnic Study of Atherosclerosis (MESA). American journal of public health, 2014. 104(11): p. 2130–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins TW, et al. , Understanding environmental health inequalities through comparative intracategorical analysis: Racial/ethnic disparities in cancer risks from air toxics in El Paso County, Texas. Health & Place, 2011. 17(1): p. 335–344. [DOI] [PubMed] [Google Scholar]
- 55.Samoli E, et al. , Spatial variability in air pollution exposure in relation to socioeconomic indicators in nine European metropolitan areas: A study on environmental inequality. Environ Pollut, 2019. 249: p. 345–353. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed SM and Palermo A-GS, Community Engagement in Research: Frameworks for Education and Peer Review. American Journal of Public Health, 2010. 100(8): p. 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Commodore A, et al. , Community-based participatory research for the study of air pollution: a review of motivations, approaches, and outcomes. Environmental Monitoring and Assessment, 2017. 189(8): p. 378. [DOI] [PubMed] [Google Scholar]