Abstract
Exercise has emerged as a powerful variable that can improve cognitive function and delay age-associated cognitive decline and Alzheimer’s disease (AD), however, the underlying mechanisms are poorly understood. To determine if protective mechanisms may occur at the transcriptional level, we used microarrays to investigate the relationship between physical activity levels and gene expression patterns in the cognitively-intact aged human hippocampus. In parallel, hippocampal gene expression patterns associated with Aging and AD were assessed using publicly available microarray data profiling hippocampus from young (20–59 yrs), cognitively-intact aging (73–95 yrs) and age-matched AD cases. To identify “anti-Aging/AD” transcription patterns associated with physical activity, probesets significantly associated with both physical activity and Aging/AD were identified and their directions of expression change in each condition were compared. Remarkably, of the 2210 probesets significant in both datasets, nearly 95% showed opposite transcription patterns with physical activity compared to Aging/AD. The majority (>70%) of these anti-Aging/AD genes showed increased expression with physical activity and decreased expression in Aging/AD. Enrichment analysis of the anti-Aging/AD genes showing increased expression in association with physical activity revealed strong overrepresentation of mitochondrial energy production and synaptic function, along with axonal function and myelin integrity. Synaptic genes were notably enriched for synaptic vesicle priming, release and recycling, glutamate and GABA signaling and spine plasticity. Anti-Aging/AD genes showing decreased expression in association with physical activity were enriched for transcription-related function. These data reveal that physical activity is associated with a more youthful profile in the hippocampus across multiple biological processes, providing a potential molecular foundation for how physical activity can delay age- and AD-related decline of hippocampal function.
Keywords: exercise, microarray, plasticity, synaptic vesicle trafficking, mitochondria, white matter, myelin, axon
1. INTRODUCTION
Lifestyle factors have a striking ability to empower the adult brain - strengthening plasticity, building cognitive reserve, and preventing functional decline. Physical activity in particular has emerged as a powerful life-style factor that can improve cognitive function (Buchman et al., 2012; Buchman et al., 2008; Colcombe and Kramer, 2003; Colcombe et al., 2003; Erickson et al., 2010; Erickson et al., 2011; Kramer and Erickson, 2007a, b; Smith et al., 2010; Stubbs et al., 2017; Suwabe et al., 2018), reduce the risk of age-associated cognitive decline and AD (Buchman et al., 2012; Erickson et al., 2012; Hillman et al., 2008; Kirk-Sanchez and McGough, 2014; Korol et al., 2013; Prakash et al., 2015; Weuve et al., 2004; Yaffe et al., 2001), counteract age- and AD-related losses of grey and white matter (Baker et al., 2010; Best et al., 2017; Bherer et al., 2013; Colcombe et al., 2003; Erickson et al., 2014; Lautenschlager et al., 2008; Okonkwo et al., 2014; Suzuki et al., 2012; Voss et al., 2013; Zlatar et al., 2015) and counteract age- and AD-related declines in cognitive neural network function (Colcombe et al., 2004; Huang et al., 2016; Voss et al., 2010). While the benefits of physical activity are evident, the underlying mechanisms by which physical activity supports brain function and health and slows Aging- and AD-related declines in the human brain are poorly understood.
Gene expression studies to address this question have been precluded by a paucity of human autopsy cases that are well-defined for late-life lifestyle factors, and in particular, where physical activity has been objectively documented in a manner that avoids recall bias. Participants in the Rush Memory and Aging Project (MAP) meet these criteria. MAP participants have been followed longitudinally for up to 18 years, until death, with detailed annual assessments of cognitive function and multiple lifestyle variables including quantitative assessment of physical activity (Bennett et al., 2012). Importantly, physical activity was continuously monitored over multiple days with actigraphy, capturing both exercise and non-exercise activity (e.g., light physical activity) to provide a highly accurate account of the total daily activity levels (Buchman et al., 2012).
In this study, we use post-mortem hippocampal tissue from cognitively-healthy, aged (76–100 years) MAP cases that captured a broad spectrum of physical activity level in combination with a microarray-based genome-wide approach to investigate mechanisms by which physical activity may promote cognitive health and hippocampal function, and counteract Aging- and AD-related transcription patterns in the human brain. The data suggest that gene expression patterns associated with physical activity have the potential to extensively counteract transcriptional changes that occur in the human hippocampus with Aging or AD. In particular, physical activity may slow Aging/AD-related declines in hippocampal health and function by enhancing mitochondrial energy production and synaptic function, improving axonal function and myelin integrity, and maintaining appropriate transcriptional control mechanisms.
2. MATERIALS AND METHODS
Samples
To investigate the relationship between physical activity and gene expression patterns in the cognitively intact aged human hippocampus, frozen post-mortem hippocampal brain tissue was obtained from 47 clinically well-characterized cognitively intact cases (aged 76–100 years) from MAP, an ongoing longitudinal, study of older individuals without known dementia recruited from northeastern Illinois. All tissue was obtained from the mid-hippocampus region containing all hippocampal subfields. Participants were diagnosed as cognitively-intact based on annual assessment of cognitive status using a battery of 19 cognitive tests, at which time they were additionally assessed for other lifestyle factors including cognitive frequency, social frequency and depression, among other variables (Bennett et al., 2012). Participants underwent annual assessment of physical activity using actigraphs worn continuously over multiple days. Post-mortem tissue was assessed for pathology as previously reported (Bennett et al., 2012), and cases with Lewy Bodies, infarcts, or hippocampal sclerosis were excluded from the study. Hippocampal RNA from 35 participants (76–100 yrs, average 87.62 ± 6.36 yrs) was of high quality sufficient to process on microarrays and an additional 12 samples were included in the Nanostring analysis.
Quantification of Physical activity
Physical activity was measured with actigraphy (Actical: Mini Mitter, Bend OR). Total daily exercise and non exercise physical activity was measured 24 hours per day for up to 10 days, with actigraphs worn on the non-dominant wrist. Average daily physical activity was calculated as the average across days of the total daily activity counts recorded. Average physical activity ranged from 0.13 to 5.6 × 105 activity counts/day (Supplemental Figure 1). Data were converted to approximate metabolic equivalents (METs) using the distance conversion metrics reported by Hall et al. (Hall et al., 2013) and standard MET equivalent values (Ainsworth et al., 1993). Using these metrics, 1.0 × 105 counts/day corresponded to 20 min/day moderate-high activity (walking, 3.5 mph) (equivalent to 1.5 MET-hrs of activity/day), and 5.0 × 105 counts/day was the equivalent of 100 min/day of moderate-high activity (7.5 MET-hrs/day). Previously, Weuve et al. (Weuve et al., 2004), described a dose-dependent effect of physical activity on cognitive performance, with highest cognitive performance in individuals with activity estimates above 26 MET-hrs/week.
RNA extraction and Microarray quality control
RNA from approximately 25 mg tissue (mid-level hippocampus containing all hippocampal subregions) was extracted using Trizol, treated with DNAse, and purified using Qiagen spin columns as described previously (Berchtold et al., 2008). RNA quality was assessed using the Agilent Bioanalyzer and RNA integrity number (RIN), and samples with RIN ≥ 6.5 were further processed on microarrays. CEL file quality control was assessed using the affyQC automated R workflow available through ArrayAnalysis (http://www.arrayanalysis.org). Quality control parameters were: beta-actin 3’/5’ ratio < 3, GAPDH 3’/5’ ratio < 1.25, internal hybridization control signal (BioB=Present), Percent Present ≥ 49%, Percent Present spread ≤ 10%, Background spread ≤ 20%) and log scale factor spread ≤ 3.
Actical samples:
Of the 47 hippocampal tissues, high quality RNA was obtained from 35 samples (RIN: 7.36 ± 0.58, range 6.5–8.7) which were processed individually on Affymetrix HgU133plus 2.0 microarrays by the Genomic high-Throughput Facility at UC Irvine, following manufacturer’s recommendations. CEL file quality control was assessed using the affyQC workflow in ArrayAnalysis (http://www.arrayanalysis.org). One microarray did not meet all quality control parameters in ArrayAnalysis and was excluded. Sample characteristics and quality control measures are provided in Supplemental Tables 1A and 1D. The remaining 34 CEL files were submitted to GeneSpring software GS version 14.5 (GS14.5) (Agilent Technologies) for preprocessing by MAS and GC-RMA. MAS and GC-RMA values were quantile normalized and underwent baseline transformation to the median of all samples across all genes as implemented in GS14.5. The CEL files and microarray data are available through the public gene expression omnibus (GEO)(http://www.ncbi.nlm.nih.gov/geo), dataset accession number GSE110298.
Young, Aged, and AD samples:
To characterize gene expression changes associated with Aging and AD, 58 CEL files profiling gene expression patterns in hippocampus from young (20–59 yrs), cognitively intact aging cases (69–99 yrs), and AD cases (73–99 yrs) were downloaded from the public gene expression omnibus (http://www.ncbi.nlm.nih.gov/geo) dataset, accession numbers GSE11882 and GSE48350. CEL file quality control was assessed using the affyQC workflow in ArrayAnalysis (http://www.arrayanalysis.org) and identified 48 microarrays that met all quality control and study parameters (young (n=16), cognitively intact aging cases (n=18), and AD cases (n=14)). Sample characteristics and quality control measures are provided in Supplemental Tables 1B and 1E.
Microarray data analysis – identifying genes associated with physical activity
Poorly performing probesets were eliminated by filtering the array data based on flags (Present, Marginal, Absent) using MAS preprocessing, retaining only probesets flagged Present on all samples of at least one physical activity group (low, moderate, high activity: Supplemental Figure 1) (23,712 probesets) and that overlapped with the probeset list used for the analysis of Aging and AD (21,002 probesets). Using the programming environment R (http://www.R-project.org/), multiple linear regression was applied to GC-RMA normalized values using lm( ) to adjust for technical factors that were potential confounding variables (batch, RIN, and PMI) and to evaluate the relationship between gene expression and late-life physical activity levels across the 34 samples (Miller et al., 2017). For each probeset, the estimated regression coefficient for physical activity, its standard error, and the t-ratio and P-value for a two-sided test were extracted using infer( ) with robust standard errors. Significance was set at p ≤ 0.05 and q values were calculated with Graph Pad Prism 7 statistical software using the two-stage step-up method of Benjamini, Krieger and Yekutiessli. To estimate fold changes across low, moderate and high physical activity tiers, multiple linear regression was applied to each 2 group comparison, and p and q values were calculated. Log values were transformed to linear scale using Excel software. All calculated values are provided in Supplemental Table 2.
Microarray analysis – Identifying genes associated with Aging and AD
The 54,675 probesets on the array were filtered using MAS preprocessing derived flags to retain only probesets flagged Present on all samples of at least one treatment group (21,002 probesets) and that overlapped with the probeset list used for the analysis of gene expression associated with physical activity (21,002). Using R, multiple linear regression was applied to GC-RMA normalized values to adjust for batch, RIN, and PMI and to evaluate gene expression changes in Aging (Aged vs. young) and AD (AD vs. Aged). For each probeset, the estimated regression coefficient for physical activity, its standard error, and the t-ratio and P-value for a two-sided test were extracted using infer() with robust standard errors. Significance was set at p ≤ 0.05 and q values were calculated using Graph Pad Prism 7 statistical software. Log values were transformed to linear scale using Excel software. All calculated values are provided in Supplemental Table 2
Anti-Aging/AD patterns associated with exercise
To investigate mechanisms by which physical activity may protect the brain from Aging and AD-related change, probesets significantly associated with both physical activity and Aging or AD were selected, patterns of expression change were compared for each probeset, and probesets showing opposite patterns of change with physical activity versus Aging or AD were identified (henceforth referred to as “anti-Aging/AD probesets”) (See Methods in Figure 1). Significance of the overlap patterns between physical activity and Aging-AD (eg, opposite direction of gene expression change, or similar direction) was assessed using the Fisher exact test. 2 approaches were used to estimate the number of probesets expected to occur by chance in the comparisons (a) physical activity and aging or (b) physical activity and AD. The first approach assumes normality of the p value distribution and independence of the comparisons, and was calculated as (2*(0.052) – (0.05)3) * 21,002 probesets tested. This approach estimated the number of putative false positives to be 102 probesets (e.g., p ≤ 0.004875 × 21,002), corresponding to a false positive rate (FDR) estimate of 4.6% (e.g., 102 false discoveries/2210 true discoveries). Permutation analysis was used as a second approach where normality of the p value distribution is not assumed. Using the R function “transform( )” activity labels were randomized across samples in the physical activity dataset, and across the Young, Aged, and AD samples for the Aging-AD analysis, and the number of overlapping significant probes was calculated for the permuted data. 100 permutation analyses were performed, resulting in estimations ranging from 10 – 1,057 false positives, with an average ± standard deviation estimate of 176 ± 192 and a median estimate of 109 false positives. The computed average false positives (176) corresponds to a false positive rate of 7.9% and the median false positive estimate (109) corresponds to a false positive rate of 4.9%, similar to the FDR estimate (4.6%) calculated under the assumption of normal distribution of p values.
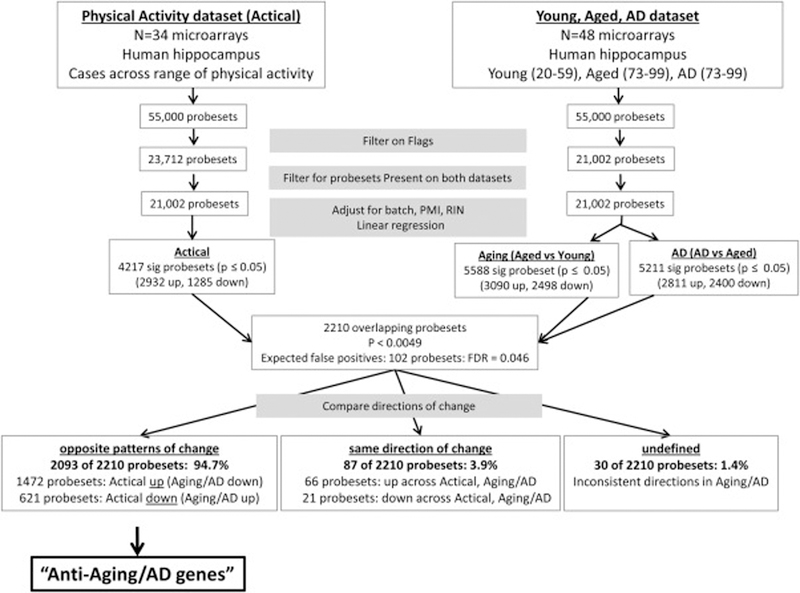
Figure 1.
Flowchart of the Methods to determine if physical activity-related gene expression patterns counter aging and AD-related expression change. 34 microarrays profiling gene expression in the aged human hippocampus were used to determine the relationship between gene expression and physical activity. Poorly performing probesets were eliminated by filtering the array data based on flags (Present, Marginal, Absent) using MAS preprocessing, retaining only probesets flagged Present on all samples of at least one group (low, moderate, high activity) (23,034 probesets). Using the programming environment R (http://www.R-project.org/), multiple linear regression was applied to GC-RMA normalized values using lm( ) to adjust for technical factors that were potential confounding variables (batch, RIN, and PMI) and to evaluate the relationship between gene expression and late-life physical activity levels across the 34 samples. In parallel, Aging and AD-related gene expression change was assessed using 51 CEL files from the public gene expression omnibus (http://www.ncbi.nlm.nih.gov/geo, dataset accession number GSE:11882) profiling expression patterns of 54,675 probesets in hippocampal samples from young cases (20–59 yrs, n=17), cognitively intact aged cases (73–99 yrs, n=20), and AD cases (73–99 yrs, n=14). The ~55,000 probesets on the array were filtered using MAS preprocessing derived flags to retain only probesets flagged Present on all samples of at least one treatment group (21,002 probesets). Using R, multiple linear regression was applied to GC-RMA normalized values to adjust for batch, RIN, and PMI and to evaluate gene expression changes in Aging (aging vs young) and AD (AD vs aged). Probesets significantly associated with both physical activity (p<0.05 and Aging or AD (p<0.05) were selected, patterns of expression change were compared for each probeset, and probesets showing opposite patterns of change with physical activity versus aging or AD were identified. The number of probesets expected to occur by chance in the overlap of the 2 studies is 52 probesets, calculated by multiplying the product of the p-values (0.0025) by the number of probesets that were tested in both datasets (21,002). The analysis identified 2138 probesets significantly associated with both physical activity and Aging or AD, 94.9% (2029 probesets) of which showed the opposite pattern of change with physical activity compared to Aging/AD (“Anti-Aging/AD probesets”). These probesets were analyzed for functional enrichment using DAVID.
DAVID Functional enrichment analysis
Functional enrichment analysis of the Anti-Aging/AD genes was used to identify biological modules of Aging/AD that show the opposite pattern of change with physical activity. Significant gene lists were submitted to the high-throughput data mining bioinformatics resources DAVID (the Database for Annotation, Visualization and Integrated Discovery, https://david.ncifcrf.gov) (Dennis et al., 2003; Huang da et al., 2009a, b), to identify over-represented biological functions and pathways. Significant probesets were translated to DAVID ids followed by mapping of unambiguous DAVID ids to terms in the Gene Ontology (GO) databases (Cell Component (GOTERM-CC), Biological Process (GOTERM-BP), Molecular Function (GOTERM-MF), KEGG and BioCarta pathways, or the UniProt keywords database. Enrichment was assessed against a background gene set of the 21,002 probesets with reliable expression on both datasets, EASE score threshold p ≤ 0.01, and Benjamini multiple testing correction p ≤ 0.10. Benjamini p-values are indicated in the results.
Nanostring nCounter™ gene expression system
Microarray expression changes associated with physical activity were validated using the NanoString nCounter™ gene expression system to assess gene expression on an expanded set of human hippocampal tissues, for experimental and independent replication of the microarray data. Experimental replication included 8 additional low activity cases and 4 additional high physical activity cases, in addition to low (n=10) and high activity (n=12) cases included in the microarray analysis (Supplemental Tables 1C, 1D). One low activity sample (sample 1_1-E) included in the microarray analysis was not included in the nanostring run due to sample degradation. NanoString provides similar sensitivity to TaqMan-based RQ-PCR and SYBR Green I fluorescent dye-based RQ-PCR but does not involved reverse transcription of mRNA and subsequent cDNA amplification, thus eliminating amplification bias (Geiss et al., 2008; Veldman-Jones et al., 2015). Gene expression was assessed in low activity (n= 18) and high activity (n=16) cases for 13 synaptic genes that showed decreased expression with Aging/AD and increased expression with physical activity (GABRG2, GLRB, GRIN2a, SLC1a6, SYN2, VAMP2, SNAP25, USP14, NRXN1,NRXN3, SYT4, SSTR1, Munc13–3), and for 10 genes where microarray analysis demonstrated decreased expression with Aging/AD and trends for increased expression with physical activity (GABBR1, GABRA2, GABRA4, GABRB3, GABRG1, GRIA2, DOC2a, SCN2b, DLG3/SAP102, AKAP5). 7 putative internal control genes (ATF6, DDX52, METTL20, GAPDH, SLC38a7, ZNF780b, ZNF791) were first identified based on microarray data demonstrating expression homogeneity across physical activity tier and low variance, and were evaluated using Nanostring for suitability. Putative control genes were excluded if Nanostring gene expression showed a trend (p ≤ 0.1) for a significant correlation with physical activity across cases or a trend for a significant t-test comparing low vs. high physical activity groups. ZNF780b and DDX52 were identified as suitable control genes. Nanostring expression values underwent sample normalization to the geometric mean of the control genes and Graph Pad Prism 7 statistical software was used to calculate means, standard error of the mean (sem) and 2 group comparisons of the low activity (n=18) and high activity (n=16) groups across the genes.
For independent validation of the microarray data, only the new Nanostring cases were included in the analysis (n=8 low activity, n=4 high activity). The Mann-Whitney test was used to determine whether the nanostring-measured genes, as a group, tended to move in the same direction as those genes did on the array. For each gene, the average nanostring value for the low and high activity groups were calculated, and the Mann-Whitney test was applied using Graph pad Prism 7 statistical software.
3. RESULTS
3.1. Physical activity is associated with anti-Aging/AD gene expression patterns in the hippocampus
Hippocampal tissue from 34 cognitively-intact aged cases well-characterized for physical activity levels were run on microarrays to investigate gene expression patterns significantly associated with physical activity levels. In parallel, microarray data profiling hippocampal gene signatures from young (20–59 yrs), cognitively intact aging (69–99 yrs) and AD (73–99) cases were analyzed to identify gene expression patterns associated with Aging and AD (gene expression omnibus (GEO) dataset accession number GSE11882, http://www.ncbi.nlm.nih.gov/geo). To investigate mechanisms by which physical activity may protect the brain from Aging and AD-related change, probesets significantly associated with both physical activity and Aging or AD were selected patterns of expression change were compared for each probeset, and probesets showing opposite patterns of change with physical activity versus Aging or AD were identified (henceforth referred to as “anti-Aging/AD probesets”) (Figure 1).
Of the 21,002 probesets with reliable expression on both datasets, the analysis identified 2210 probesets that were significantly associated with both physical activity and Aging/AD, a number far greater than expected to occur by chance (e.g. ~102 expected false positives/2210 significant probesets, FDR = 4.6%), with the significance of the overlap calculated to be p <0.0001 (Fisher exact test). Remarkably, of these 2210 probesets, 94.7% (2,093 probesets) showed the opposite patterns of change in association with physical activity compared with Aging/AD (henceforth called “anti-Aging/AD probesets”). The majority (>70%) of these anti-Aging/AD probesets showed increased expression with physical activity (1,472 probesets), while ~30% (621 probesets) showed decreased expression in association with physical activity. These data suggest that gene expression patterns associated with physical activity extensively counteract gene expression changes that occur in the human hippocampus with Aging or AD, particularly with respect to those genes undergoing transcriptional decline.
3.2. Anti-Aging/AD genes are enriched for several core biological functions
To investigate potential repercussions of physical activity for hippocampal health and function, we investigated if the anti-Aging/AD probesets significantly associated with physical activity were enriched for biological functions using the integrated data-mining bioinformatics resource DAVID version 6.8 (Dennis et al., 2003; Huang da et al., 2009a, b). The anti-Aging/AD probeset lists (1472 with increased expression and 621 with decreased expression in association with physical activity) were uploaded and translated to DAVID ids followed by mapping of unambiguous DAVID ids to terms in the GO databases, KEGG pathways or UniProt keywords database. Benjamini p-values are indicated below.
For anti-Aging/AD probesets showing increased expression in association with physical activity, the probeset list translated into 1176 unique and unambiguous DAVID ids of which 1117 genes could be mapped to database terms. DAVID analysis revealed robust enrichment for several functional categories key to cellular health and synaptic function (Table 1). Highly significant enrichment was found for mitochondrial-related genes (e.g., UniProt keyword “Mitochondrion”, p =2 × 10−5) especially for electron transport chain function, as well as for genes associated with the synapse (e.g., UniProt keyword “Synapse”, p = 5 ×10−7), myelin sheath (GOTERM “myelin sheath”, p =1 × 10−4), and axon (GOTERM “axon”, p =3 × 10−3). These data suggest that Aging/AD is accompanied by diminishing gene expression for these core biological functions, and that physical activity may slow Aging/AD-related declines in hippocampal health and function by enhancing mitochondrial energy production, synaptic health and axon function.
Table 1.
DAVID enrichment categories for unambiguous probesets with hippocampal expression significantly increased in association with physical activity and significantly decreased with Aging or AD. Note that significant genes may be contained with in multiple enriched terms within a category.
| Mitochondria-related categories | Database | Term | Count | % | EASE PValue | List Total | Pop Hits | Pop Total | Fold Enrichment |
Benjamini |
|---|---|---|---|---|---|---|---|---|---|---|
| Mitochondrion | UP_KEYWORDS | 143 | 12.16 | 1.98E-07 | 1138 | 881 | 10612 | 1.51 | 2.04E-05 | |
| Mitochondrion | GOTERM_CC_DIRECT | GO:0005739 | 162 | 13.78 | 3.57E-08 | 1047 | 1026 | 9961 | 1.50 | 7.04E-06 |
| Mitochondrion inner membrane | UP_KEYWORDS | 55 | 4.68 | 2.12E-09 | 1138 | 218 | 10612 | 2.35 | 4.39E-07 | |
| Mitochondrion inner membrane | GOTERM_CC_DIRECT | GO:0005743 | 74 | 6.29 | 2.77E-08 | 1047 | 364 | 9961 | 1.93 | 8.20E-06 |
| Mitochondrion outer membrane | UP_KEYWORDS | 19 | 1.62 | 3.41E-03 | 1138 | 85 | 10612 | 2.08 | 9.60E-02 | |
| Respiratory chain | UP_KEYWORDS | 17 | 1.45 | 1.41E-04 | 1138 | 55 | 10612 | 2.88 | 8.26E-03 | |
| oxidative phosphorylation | KEGG_PATHWAY | hsa00190 | 33 | 2.81 | 2.19E-09 | 380 | 107 | 3909 | 3.17 | 5.54E-07 |
| mitochondrial respiratory chain complex I | GOTERM_CC_DIRECT | GO:0005747 | 15 | 1.28 | 5.70E-05 | 1047 | 42 | 9961 | 3.40 | 4.81E-03 |
| ubiquinone | UP_KEYWORDS | 10 | 0.85 | 1.88E-03 | 1138 | 28 | 10612 | 3.33 | 6.83E-02 | |
| mitochondrial electron transport, NADH to ubiquinone | GOTERM_BP_DIRECT | GO:0006120 | 15 | 1.28 | 6.51E-05 | 972 | 43 | 9380 | 3.37 | 9.24E-02 |
| mitochondrial proton-transporting ATP synthase complex | GOTERM_CC_DIRECT | GO:0005753 | 8 | 0.68 | 2.23E-03 | 1047 | 19 | 9961 | 4.01 | 9.67E-02 |
| Metabolic pathways | KEGG_PATHWAY | hsa01100 | 103 | 8.76 | 6.18E-04 | 380 | 792 | 3909 | 1.34 | 2.21E-02 |
| Synapse, plasticity -related categories | Database | Term | Count | % | EASE PValue | List Total | Pop Hits | Pop Total | Fold Enrichment |
Benjamini |
|---|---|---|---|---|---|---|---|---|---|---|
| Synapse | UP_KEYWORDS | 60 | 5.10 | 1.23E-09 | 1138 | 245 | 10612 | 2.28 | 5.06E-07 | |
| synaptic vesicle membrane | GOTERM_CC_DIRECT | GO:0030672 | 16 | 1.36 | 4.16E-06 | 1047 | 39 | 9961 | 3.90 | 4.92E-04 |
| chemical synaptic transmission | GOTERM_BP_DIRECT | GO:0007268 | 31 | 2.64 | 3.31E-05 | 972 | 134 | 9380 | 2.23 | 9.38E-02 |
| Postsynaptic cell membrane | UP_KEYWORDS | 26 | 2.21 | 1.46E-04 | 1138 | 108 | 10612 | 2.24 | 7.50E-03 | |
| Postsynaptic membrane | GOTERM_CC_DIRECT | GO:0045211 | 29 | 2.47 | 2.08E-04 | 1047 | 132 | 9961 | 2.09 | 1.36E-02 |
| crebPathway:Transcription factor CREB and its extracellular signals | BIOCARTA | 9 | 0.77 | 1.89E-03 | 102 | 26 | 1060 | 3.60 | 6.89E-02 | |
| axon | GOTERM_CC_DIRECT | GO:0030424 | 38 | 3.23 | 3.40E-05 | 1047 | 178 | 9961 | 2.03 | 3.35E-03 |
| myelin sheath | GOTERM_CC_DIRECT | GO:0043209 | 36 | 3.06 | 7.04E-07 | 1047 | 140 | 9961 | 2.45 | 1.04E-04 |
| growth cone | GOTERM_CC_DIRECT | GO:0030426 | 23 | 1.96 | 1.62E-04 | 1047 | 92 | 9961 | 2.38 | 1.19E-02 |
| neuron projection | GOTERM_CC_DIRECT | GO:0043005 | 31 | 2.64 | 1.87E-03 | 1047 | 165 | 9961 | 1.79 | 9.56E-02 |
| Cell junction | UP_KEYWORDS | 78 | 6.63 | 4.37E-06 | 1138 | 433 | 10612 | 1.68 | 3.61E-04 | |
| Cell junction | GOTERM_CC_DIRECT | 66 | 5.61 | 2.16E-08 | 1047 | 308 | 9961 | 2.04 | 1.28E-05 |
| Other categories | Database | Term | Count | % | EASE PValue | List Total | Pop Hits | Pop Total | Fold Enrichment |
Benjamini |
|---|---|---|---|---|---|---|---|---|---|---|
| gap junction | KEGG_PATHWAY | hsa04540 | 16 | 1.36 | 1.25E-03 | 380 | 66 | 3909 | 2.49 | 3.12E-02 |
| Circadian entrainment | KEGG_PATHWAY | hsa04713 | 17 | 1.45 | 6.86E-04 | 380 | 69 | 3909 | 2.53 | 2.15E-02 |
| Transport | UP_KEYWORDS | 172 | 14.63 | 4.71E-04 | 1138 | 1265 | 10612 | 1.27 | 2.14E-02 | |
| Transit peptide | UP_KEYWORDS | 77 | 6.55 | 1.07E-05 | 1138 | 436 | 10612 | 1.65 | 7.33E-04 | |
| chrebpPathway:ChREBP regulation by carbohydrates and cAMP | BIOCARTA | 9 | 0.77 | 3.51E-05 | 102 | 16 | 1060 | 5.85 | 6.62E-03 | |
| agpcrPathway:Attenuation of GPCR Signaling | BIOCARTA | 7 | 0.60 | 2.02E-04 | 102 | 11 | 1060 | 6.61 | 1.89E-02 | |
| cskPathway:Activation of Csk by cAMP-dependent
Protein Kinase Inhibits Signaling through the T Cell Receptor |
BIOCARTA | 7 | 0.60 | 1.03E-03 | 102 | 14 | 1060 | 5.20 | 6.30E-02 | |
| plcePathway:Phospholipase C-epsilon pathway | BIOCARTA | 6 | 0.51 | 1.22E-03 | 102 | 10 | 1060 | 6.24 | 5.60E-02 | |
| raccPathway:Ion Channels and Their Functional Role in Vascular Endothelium | BIOCARTA | 6 | 0.51 | 2.07E-03 | 102 | 11 | 1060 | 5.67 | 6.31E-02 | |
| nos1Pathway:Nitric Oxide Signaling Pathway | BIOCARTA | 7 | 0.60 | 2.35E-03 | 102 | 16 | 1060 | 4.55 | 6.15E-02 | |
| gpcrPathway:Signaling Pathway from G-Protein Families | BIOCARTA | 9 | 0.77 | 3.19E-03 | 102 | 28 | 1060 | 3.34 | 7.28E-02 |
A different enrichment pattern was observed for anti-Aging/AD probesets showing decreased expression in association with physical activity. The 621 anti-Aging/AD translated into 408 unique and unambiguous DAVID ids of which 378 genes could be mapped to an enrichment term. Anti-Aging/AD genes showing decreased expression with physical activity were significantly enriched primarily for transcription-related function (e.g., UniProt keyword “Transcription”, p = 7 × 10−3), notably for negative regulation of transcription (e.g., GOTERM “negative regulation of transcription from RNA polymerase II promoter”, p = 8.8 × 10−2) (Table 2). These data suggest that Aging/AD is accompanied by increased expression of genes that suppress transcription, and that physical activity may restrain this transcriptional dysregulation to maintain appropriate transcriptional control of gene expression in the Aging and AD hippocampus.
Table 2.
DAVID enrichment results for unambiuous probesests with hippocampal expression significantly decreased in association with physical activity and significantly increased with Aging or AD. Note that significant genes may be contained with in multiple enriched terms within a category.
| Category | Database | Term | Count | % | EASE PValue | List Total | Pop Hits | Pop Total | Fold Enrichment |
Benjamini |
|---|---|---|---|---|---|---|---|---|---|---|
| Nucleus | UP_KEYWORDS | 155 | 37.99 | 3.08E-04 | 383 | 3398 | 10612 | 1.26 | 1.93E-02 | |
| Transcription | UP_KEYWORDS | 81 | 19.85 | 9.04E-05 | 383 | 1480 | 10612 | 1.52 | 7.14E-03 | |
| Transcription regulation | UP_KEYWORDS | 79 | 19.36 | 8.03E-05 | 383 | 1428 | 10612 | 1.53 | 8.45E-03 | |
| negative regulation of transcription from RNA polymerase II promoter | GOTERM_BP | GO:0000122 | 35 | 8.58 | 4.93E-05 | 357 | 436 | 9380 | 2.11 | 8.80E-02 |
| Repressor | UP_KEYWORDS | 31 | 7.60 | 3.64E-04 | 383 | 428 | 10612 | 2.01 | 1.91E-02 | |
| Activator | UP_KEYWORDS | 30 | 7.35 | 1.13E-03 | 383 | 438 | 10612 | 1.90 | 4.37E-02 | |
| Polymorphism | UP_KEYWORDS | 274 | 67.16 | 4.03E-08 | 383 | 6188 | 10612 | 1.23 | 1.28E-05 | |
| Alternative splicing | UP_KEYWORDS | 269 | 65.93 | 9.26E-04 | 383 | 6639 | 10612 | 1.12 | 4.11E-02 | |
| Isopeptide bond | UP_KEYWORDS | 48 | 11.76 | 1.94E-03 | 383 | 849 | 10612 | 1.57 | 6.60E-02 |
| Category | Term | Count | % | PValue | List Total | Pop Hits | Pop Total | Fold Enrichment | Benjamini |
|---|---|---|---|---|---|---|---|---|---|
| UP_KEYWC | Polyymorphism | 274 | 67.15686 | 4.03E-08 | 383 | 6188 | 10612 | 1.226871 | 1.28E-05 |
| UP_KEYWC | Phosphoprotein | 259 | 63.48039 | 3.12E-07 | 383 | 5849 | 10612 | 1.226921 | 4.94E-05 |
| UP_KEYWC | Transcription | 81 | 19.85294 | 9.04E-05 | 383 | 1480 | 10612 | 1.516428 | 0.007139 |
| UP_KEYWC | Transcription | 79 | 19.36275 | 8.03E-05 | 383 | 1428 | 10612 | 1.532842 | 0.008448 |
| UP_KEYWC | Repressor | 31 | 7.598039 | 3.64E-04 | 383 | 428 | 10612 | 2.006857 | 0.019076 |
| UP_KEYWC | Nucleus | 155 | 37.9902 | 3.08E-04 | 383 | 3398 | 10612 | 1.263883 | 0.019342 |
| UP_KEYWC | Alternative | 269 | 65.93137 | 9.26E-04 | 383 | 6639 | 10612 | 1.12266 | 0.041106 |
| UP_KEYWC | Activator | 30 | 7.352941 | 0.001128 | 383 | 438 | 10612 | 1.897779 | 0.043743 |
| UP_KEYWC | Isopeptide | 48 | 11.76471 | 0.001936 | 383 | 849 | 10612 | 1.566506 | 0.065984 |
| GOTERM_E | GO:000012 | 35 | 8.578431 | 4.93E-05 | 357 | 436 | 9380 | 2.109192 | 0.088027 |
| UP_KEYWC | DNA-bindin | 56 | 13.72549 | 0.003426 | 383 | 1061 | 10612 | 1.462417 | 0.103095 |
3.2.1. Physical activity-associated gene expression patterns oppose Aging/AD-related declines in mitochondrial function and energy production
DAVID analysis revealed that amongst anti-Aging/AD genes increased with physical activity, mitochondria-related genes were the most highly enriched class and represented 12–13% of the gene list, a 1.5-fold enrichment (GOTERM-CC, p = 7.0 × 10−6). These included pronounced enrichment for oxidative phosphorylation (KEGG pathway, 3.17-fold enrichment, p = 5.5 × 10−7) along with components of the mitochondrial inner membrane (GOTERM-CC: 1.93-fold enrichment, p = 8.2 × 10−6) and mitochondrial outer membrane (GOTERM-CC: 2.08-fold enrichment, p = 9.6 × 10−2). These mitochondrial-associated genes were next investigated in greater detail to determine which aspects of mitochondrial function that decline with Aging/AD may be particularly amenable to improvement with physical activity. Genes in these enriched mitochondria-related categories have been compiled in Supplemental Table 3.
Approximately 30% of the mitochondrial anti-Aging/AD genes were associated with energy production via oxidative phosphorylation and the tricarboxylic acid cycle (TCA). Anti-Aging/AD genes were 3.4-fold enriched for cellular components of the mitochondrial respiratory chain complex I (GOTERM CC, p = 4.8 × 10−3) and 4.0-fold enriched for mitochondrial respiratory chain complex V (ATP synthase complex) (GOTERM CC, p = 9.67 × 10−2). Overlay of all genes significantly associated with physical activity onto the KEGG pathway “oxidative phosphorylation” (3.17-fold enriched, p = 5.5 × 10−7) reveals the extensive engagement of genes critical for energy production. Increased expression was found for approximately 30% of the genes encoding mitochondrial complex I - the entry point to the electron transport pathway - along with 35% of the genes encoding Complex IV (cytochrome C oxidase) and Complex V (ATP synthase), as well as 2 components (SLC25A27, SLC25A14) of the uncoupling protein (Figure 2). The broadly increased expression of oxidative phosphorylation genes was accompanied by increased expression of key genes driving pyruvate metabolism and the TCA cycle, which generates energy from pyruvate metabolism and provides NADH and FADH2 reducing equivalents for the electron transport chain. These included dihydrolipoamide dehydrogenase (DLD), malate dehydrogenase 1 (MDH1), malate dehydrogenase 2 (MDH2), malic enzyme 1 (ME1), malic enzyme 3 (ME3), pyruvate dehydrogenase (PDHA1) and succinate CoA ligase (beta subunit, SUCLA2). In addition to the numerous genes involved in energy production, other notably mitochondrial anti-Aging/AD genes included increased expression in association with physical activity of 16 mitochondrial ribosomal structural genes and 8 genes that negatively regulate neuronal apoptosis (B-Raf proto-oncogene serine/threonine kinase (BRAF), DnaJ heat shock protein family Hsp40 member C5 (DNAJC5), PTEN induced putative kinase 1 (PINK1), oxidation resistance 1 (OXR1), peptidylprolyl cis/trans isomeras (PIN1), syntaxin binding protein 1 (STXBP1), synucleins alpha and beta (SNCA, SNCB), among other genes (Supplemental Table 3).
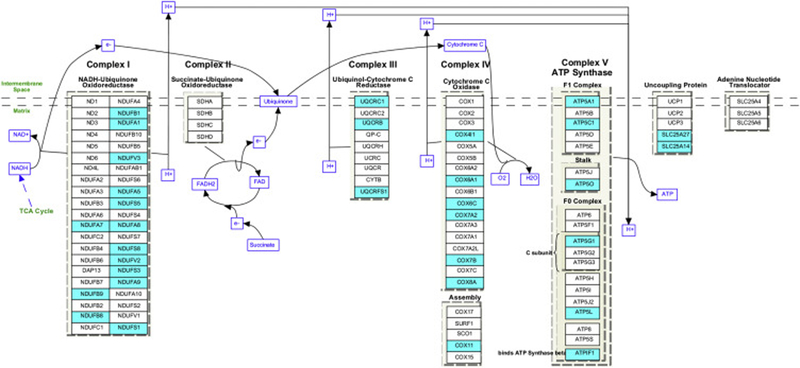
Figure 2.
The KEGG pathway “oxidative phosphorylation” (3.17-fold enriched, p = 5.5 × 10−7) overlaid with microarray data - blue highlight identifies genes showing significantly increased expression in association with physical activity and decreased expression in Aging or AD. Significant genes included approximately 30% of the genes encoding mitochondrial complex I (NADH:ubiquinone oxidoreductase) (NDUFA1, NDUFA7, NDUFA8, NDUFA9, NDUFAF5, NDUFB1,NDUFB8, NDUFB9, NDUFS1, NDUFS3, NDUFS5, NDUFV2 and NDUFV3) approximately 35% of the genes encoding Complex IV (cytochrome C oxidase)(subunits COX4l1, COX6A1, COX6C, COX7B, COX7A2, COX8A and COX11) and Complex V (ATP synthase)(ATP5A1, ATP5C1, ATP5O, ATP5G1, ATP5L, ATP51), as well as 2 components (SLC25A27, SLC25A14) of the uncoupling protein. No genes in the pathway showed decreased expression in association with physical activity.
Taken together, these data suggest that multiple components necessary for mitochondrial health and energy production undergo declining gene expression with Aging/AD, and that physical activity may slow Aging/AD-related declines in hippocampal function by counteracting these gene expression patterns to enhance mitochondrial health and capacity for energy production.
3.2.2. Physical activity-associated gene expression patterns oppose Aging/AD-related declines in synaptic gene expression
Genes related to synaptic and neuronal function constituted the second most significantly enriched class of genes amongst anti-Aging/AD genes increased with physical activity. For example, genes in the Uniprot Keyword category “Synapse” represented ~5% of the gene list (60 genes), a 2.28-fold enrichment (p = 5.0 × 10−7) (Table 1). Significant genes-related to synaptic function could be grouped into the following main functional categories: (1) synaptic vesicle priming, release and recycling; (2) Glutamate and GABA signaling and receptor trafficking; and (3) synapse formation, plasticity, and axon health. Significant genes in enriched terms related to synapse (Uniprot Keyword, p = 5.0 × 10−7), synaptic vesicle membrane (GOTERM-CC, 3.9-fold enriched, p = 4.9 × 10−4), chemical synaptic transmission (GOTERM-BP 2.2-fold enriched, p=9.4 × 10−2) and post-synaptic membrane (GOTERM-CC, 2.1-fold enriched, p = 1.4 × 10−2) are listed in Supplemental Table 4.
3.2.2.1. Synaptic vesicle priming, release, and recycling
Our data revealed that numerous anti-Aging/AD genes showing increased activity with physical activity were associated with synaptic vesicle priming, regulating the readily releasable and reserve pools of synaptic vesicles, and synaptic vesicle recycling and recovering (Table 1). Key enriched terms associated with these functions included synaptic vesicle membrane (GOTERM-CC, 3.9-fold enriched, p = 4.9 × 10−4) and chemical synaptic transmission (GOTERM-BP, p = 9.4 × 10−2).
Several key genes that regulate synaptic vesicle priming and docking at the presynaptic active zone were present in the anti-Aging/AD genes showing increased expression with physical activity. Synaptic vesicle priming and docking require the assembly of 3 essential SNARE proteins into a complex, followed by binding of the SNARE complex to Sec1/Munc18-like (SM) proteins and other chaperones to catalyze vesicle fusion to the presynaptic membrane. Our data revealed that anti-Aging/AD genes included 2 of the 3 core SNARE proteins (synaptosome associated protein 25 (SNAP25), synaptobrevin/VAMP2) that are essential for active zone priming and docking, as well as several SNARE chaperones that included syntaxin-binding proteins (STXBP1/Munc18–1, STXBP5), amyloid precursor protein (APP), the RIM-binding protein ERC2, alpha and beta synuclein (SNCA, SNCB) and rab-3 interacting molecules (RIMS2, RIMS3).
In parallel, many of the anti-Aging/AD genes increased with physical activity are key regulators of presynaptic vesicle availability. For example, among the anti-Aging/AD genes were several active zone scaffolding proteins that regulate the balance between the reserve and the readily releasable pools of neurotransmitter, including synapsin II (SYN2), cyclin-dependent kinase 5 (CDK5) and STXBP5. In addition, there were numerous genes that regulate endocytosis and recovery of synaptic vesicles following neurotransmitter release, highly important steps for maintaining presynaptic synaptic vesicle availability, particularly in the hippocampus. These genes included increased expression of dynamins (DNM1L, DNM3), which are fundamental to synaptic vesicle endocytosis and recycling, along with synaptojanin1 (SYNJ1) and endophilin1 (SH3GL2), which work in concert and are required for ultrafast endocytosis and clathrin uncoating of endocytosed synaptic vesicles. Several additional anti-Aging/AD genes associated with synaptic vesicle endocytosis and recovery were the clathrin adaptor protein complex 2 (AP2M1), the Bloc-1 subunit pallidin (BLOC1S6), Syndapin/PACSIN1, amphiphysin (AMPH), DNAJC5, secretory carrier membrane protein 5 (SCAMP5), syntaxin 12, TorsinA (TOR1A) and multiple V-ATPase subunits for APT6V1 (subunits A, C1, D, E1, G2) (Figure 3).
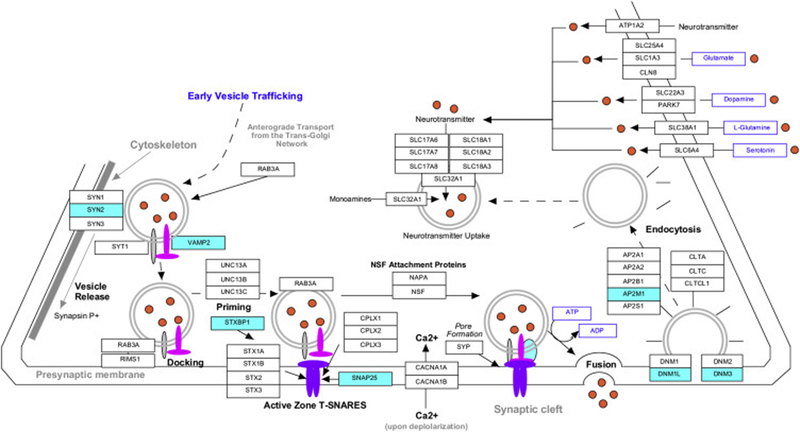
Figure 3.
The synaptic vesicle KEGG pathway overlaid with microarray analysis with blue highlight identifying genes showing significantly increased expression in association with physical activity and decreased expression in Aging or AD. Significant genes are involved in synaptic vesicle trafficking, priming and docking at the active zone: SNAP25, synaptobrevin/VAMP2, synapsin 2 (SYN2), syntaxin binding protein 1 (STXBP1)) as well as synaptic vesicle endocytosis and recycling (dynamin-like 1 (DNML1), DNML3, adaptor protein complex 2 (AP2M1). No genes in the pathway showed decreased expression in association with physical activity.
Taken together, these data suggest that Aging/AD are accompanied by declining expression of multiple components of the synaptic vesicle release, trafficking, and recycling machinery, and that physical activity may counteract these gene expression patterns and maintain a more effective presynaptic machinery.
3.2.2.2. Glutamate and GABA signaling: neurotransmitter levels, receptors, and receptor trafficking
Further analysis of the anti-Aging/AD genes showing increased expression with physical activity revealed numerous genes that regulate postsynaptic signaling efficiency of glutamatergic and GABAergic neurotransmission. These included several genes that regulate glutamate availability (glutaminase (GLS), high affinity glutamate transporter SLC1A6), glutamate receptor levels (NMDA receptor subunit 2a (GRIN2a), kainate receptor 2 (GRIK2), AMPA receptor subunit 4 (GRIA4)) and auxiliary components that modulate glutamate receptor signaling kinetics (ferric chelate reductase 1 like (FRRS1L), neuropilin and tolloid like 1 and 2 (NETO1, NETO2), homer scaffolding protein 1 (HOMER1)). In addition, there were several genes that regulate glutamate receptor availability in the post-synaptic density (PSD), primarily genes that promote removal of AMPA GluR1 from the PSD (glutamate receptor interacting protein 1 (GRIP1), ATPase family AAA domain containing 1 (ATAD1), SYNJ1, VAMP2), but also genes that increase AMPA GluR1 anchoring (sortilin related VPS10 domain containing receptor 3 (SORCS3), leucine rich repeat transmembrane neuronal 4 (LRRTM4)), GRIN2A clustering in the PSD (rabphilin 3A: RPH3A) and GRIN2B localization in the PSD (lin-7 homologs A and B: LIN7A, LIN7B). At the same time, anti-Aging/AD genes included genes regulating GABAergic neurotransmission, including increased expression of GABAA receptors (GABRA1, GABRG2) and gephyrin (GPHN) - the neuronal assembly protein that anchors GABA receptors to the inhibitory PSD – along with several neuromodulators that augment GABA tone (glycine receptor beta (GLRB), somatostatin receptor 1 (SSTR1) and cholecystokinin (CCK)).
These data indicate that physical activity increases expression of many genes that regulate postsynaptic signaling efficiency of glutamatergic and GABAergic neurotransmission. The constellation of gene expression changes with increased physical activity may function to enhance excitatory signaling and at the same time to dampen neuronal responsiveness within neural circuits.
3.2.2.3. synapse formation, plasticity, and axon health
In parallel, genes showing increased expression in association with physical activity and decreased expression with Age/AD included central organizing molecules which promote new synapse formation and maturation, molecular pathways key for activity-dependent plasticity, and genes supporting axonal function and white matter integrity.
Notable categories associated with synapse formation, maturation and plasticity showing enrichment included a 2.24-fold enrichment for genes associated with the ‘post-synaptic cell membrane’ (p = 7.5 × 10−3) (Supplemental Table 5) and 3.6 fold enrichment for the BioCarta pathway “transcription factor CREB and its extracellular signals” (p = 6.89 × 10−2). Genes regulating spine genesis and synapse maturation included presynaptic neurexins (NRXN1, NRX3) and calsyntenin 3 (CLSTN3) a synaptogenic adhesion molecule which works in concert with neurexins, along with leucine-rich repeat molecules (LRRTM4, LRRC4C, LRRC7), cell adhesion molecule 2 and 3 (CADM2, CADM3) and other genes: Rho guanine nucleotide exchange factor 7 (ARHGEF7), myocyte enhancer factor 2C (MEF2C), inositol-trisphosphate 3-kinase A (ITPKA), caytaxin (ATCAY), ATPase plasma membrane Ca2+ transporting 2 (ATPB2), phosphatase and actin regulator 1 (PHACTR1), teneurin transmembrane protein 2 (TENM2), diacylgycerol kinase (DGK), glycoprotein M6A (GPM6A), cell division cycle 42 (CDC42), neuralized E3 ubiquitin protein ligase 1 (NEURL1). In addition, several of the anti-Aging/AD genes increased in association with physical activity are key molecules that enable dynamic plasticity critical for memory formation. These included kinases that drive the signaling in the nucleus required for sustained long-term potentiation (LTP) such as the catalytic and regulatory subunits of protein kinase A (PRKACB, PRKAR1A, PRKAR1B, PRKAR2B) and protein kinase C (beta subunit; PRKCB), genes regulating signaling through the CREB transcription factor (e.g., Adenylate cyclase 1 (ADCY1), calcium/calmodulin-dependent protein kinase delta (CAMK2D), GNAS complex (GNAS), growth factor receptor bound (GRB2)), and genes that facilitate trafficking and signaling of brain-derived neurotrophic factor (BDNF), a key plasticity molecule that facilitates LTP induction and stability and that is essential for hippocampus-dependent learning (e.g., endophilin (SH3GL2), synaptotagmin 4 (SYT4), ubiquitin specific peptidase 14 (USP14)).
Interestingly, genes showing increased expression in association with physical activity and decreased expression with Age/AD were also enriched for genes supporting axonal connectivity and function and white matter integrity. There was strong enrichment for the term ‘growth cone’ (2.38-fold enriched, p=1.19 ×10−2) (Supplemental Table 6), an actin-supported extension of a developing or regenerating neurite seeking its synaptic target, and a 1.79 fold enrichment for “Neuron projection” (p = 9.56 × 10−2). It is possible that this gene expression pattern reflects axonal outgrowth from newly generated neurons in the neurogenic subgranular zone of the hippocampus, as increased expression was seen for several genes that regulate axonal outgrowth and targeting (e.g., RAC1-activated kinases (PAK1, PAK3, PAK5, PAK6), ephrins (EFNB3, EFNA5 and its receptor EPHA3), slit guidance ligand 2 (SLIT2), roundabout guidance receptor 2 (ROBO2), SLIT-ROBO Rho GTPase activating protein (SRGAP3)), along with a number of important genes that enhance proliferation, survival and migration of immature neurons (Supplemental Table 7) (e.g., doublecortin (DCX), cyclin-dependent kinase 5 (CDK5), glycogen synthase kinase 3 beta (GSK3b), platelet activating factor acetylhydrolase (PAFAH1B1), TMF1-regulated nuclear protein 1 (TRNP1), ephexin 1 (NGEF), and ROBO2). However, while stimulation of neurogenesis with physical activity is one of the most consistently documented effects of exercise in the animal hippocampus, there is controversy concerning the capacity for neurogenesis in the adult human hippocampus. It is possible that these expression patterns are not indicative of active neurogenesis but rather reflect promotion of axonal health and function. Indeed, anti-Aging/AD genes undergoing decreased expression in the Aging/AD hippocampus but increased expression in association with physical activity showed strong enrichment for genes associated with the axon (GOTERM-CC, 2.03-fold enrichment, p=3.53 ×10−3) (Supplemental Table 8) as well as the myelin sheath (GOTERM-CC, 2.45-fold enriched, p = 1.0 ×10−4) (Supplemental Table 9).
Taken together, these data suggest that late-life physical activity may counteract Age/AD-associated declines in synaptic plasticity by enhancing the molecular machinery for spine formation and activity-dependent plasticity, and promoting white matter integrity along with axonal connectivity and function.
3.2.3. Anti-Aging/AD genes decreased in association with physical activity are enriched for transcriptional control
DAVID analysis of the 621 anti-Aging/AD probesets showing decreased expression with physical activity identified 408 unique and unambiguous DAVID ids of which 378 genes could be mapped to an enrichment term. Anti-Aging/AD genes showing decreased expression with physical activity were significantly enriched primarily for transcription-related function (Table 2). Significantly enriched terms notably included “Transcription regulation” (UniProt keyword, 1.5-fold enriched, p = 8.45 × 10−3), “Repressor” (UniProt keyword, 2-fold enriched, p = 1.9 × 10−2), “Activator” (UniProt keyword, 1.9-fold enriched, p = 4.37 × 10−2) and “negative regulation of transcription from RNA polymerase II promoter (GOTERM, 2.11-fold enriched, p=8.8 ×10−2) (Supplemental Table 10). Transcriptional control genes that were increased with Aging/AD but decreased in association with physical activity included, among others, several epigenetic regulators of histones (histone deacetylase 1 (HDAC1), lysine-specific histone demethylase 1B (KDM1B), lysine 9 histone 3 demethylase (KDM3A), histone lysine methyltransferase 2C (KMT2C)), components of the HDAC complex (Sin3A associated proteins 18 and 30 (SAP18, SAP30), MDS1 And EVI1 Complex Locus Protein MDS1 (MECOM), bHLH Transcription Factor 1 (TAL1), Transformer-2 Alpha (TRA2A), numerous zinc finger proteins (ZFHX3, ZNF24, ZNF37A, ZNF160, ZNF274, ZNF397, ZNF532, ZNF652, ZN721, ZBTB20, ZBTB47), along with the transcription factor SP3, which recruits HDAC2 to suppress transcription of synaptic-plasticity associated genes. Importantly, these genes are ones that show increased expression with Aging and/or AD but decreased in association with physical activity, suggesting that physical activity may offset transcriptional dysregulation and help maintain appropriate transcriptional control of gene expression in the Aging and AD hippocampus.
3.3. Replication: Nanostring technology
NanoString nCounter™ gene expression system was used to assess expression levels of an expanded set of human hippocampal tissues that included 12 additional cases to those used in the microarray analysis. Gene expression was compared in low (n=18) vs high activity (n=16) cases for 13 synaptic genes that showed decreased expression with Aging/AD and significantly increased expression with physical activity (GABRG2, GLRB, GRIN2a, SLC1a6, SYN2, VAMP2, SNAP25, USP14, NRXN1, NRXN3, SYT4, SSTR1, Munc13–3), and for 10 genes where microarray analysis demonstrated decreased expression with Aging/AD and trends that did not reach statistical significance for increased expression with physical activity (GABBR1, GABRA2, GABRA4, GABRB3, GABRG1, GRIA2, DOC2a, SCN2b, DLG3/SAP102, AKAP5).
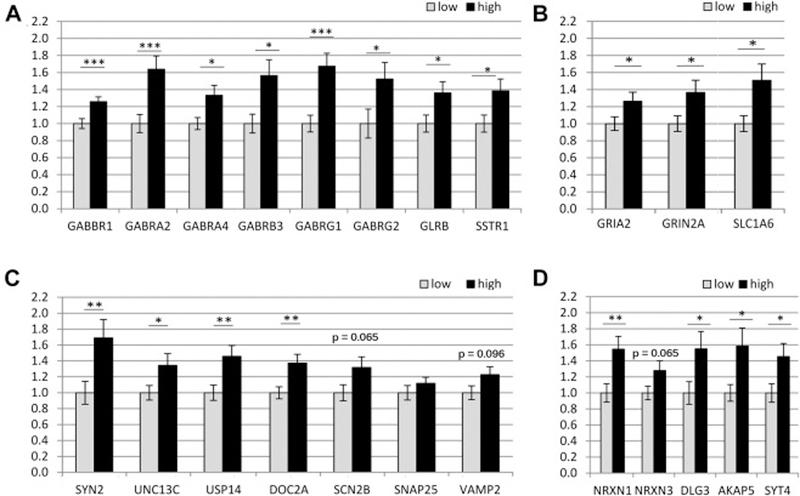
Nanostring analysis using an expanded set of samples demonstrated increased gene expression with high physical activity for GABRG2 (p=0.038), GRIN2a (p=0.03), GLRB (p<0.03), SLC1a6 (p=0.013), SYN2 (p=0.01), USP14 (p=0.008), NRXN1 (p=0.007), NRXN3 (p=0.05) and SYT4 (p=0.022) SSTR1 (p=0.026), and UNC13c/Munc13–3 (p=0.039), confirming the microarray data, while expression for VAMP2 (p=0.08), and SNAP25 (p>0.10) failed to reach statistical significance. The nanostring analysis additionally demonstrated increased gene expression with high physical activity for GABBR1 (p=0.002), GABRA2 (p=0.001), GABRA4 (p=0.013), GABRB3 (p=0.009), GABRG1 (p=0.001), GRIA2 (p=0.04), DOC2a (p=0.03), SCN2b (p=0.05), DLG3/SAP102 (p=0.03), AKAP5 (p=0.019) (Figure 4).
Figure 4.
The NanoString nCounter™ gene expression system was used to assess gene expression changes with physical activity, using an expanded set of human hippocampal samples (low activity, n=18; high activity, n=16) (A) 8 genes associated with GABA signaling: the G-protein coupled metabotropic GABAB receptor 1 (GABBR1), ionotropic GABAA receptor alpha subunits (GABRA2, GABRA4), GABAA receptor beta subunit 3 (GABRB3), GABAA receptor gamma subunits (GABRG1, GABRG2), glycine receptor beta (GLRB) and somatostatin receptor 1 (SSTR1). (B) 3 genes regulating glutamate signaling: Glutamate ionotropic Receptor AMPA Type Subunit 2 (GRIA2), Glutamate ionotropic Receptor NMDA Type Subunit 2A (GRIN2a), and the neuron specific Sodium-Dependent Glutamate/Aspartate Transporter (Solute Carrier Family 1 Member 6: SLC1a6). (C) 7 genes involved in synaptic vesicle trafficking and release: Synapsin II (SYN2), Unc-13 Homolog C (UNC13c/Munc13–3), Ubiquitin Specific Peptidase 14 (USP14), Double C2 Domain Alpha (DOC2a), voltage-gated sodium channel (type II, subunit B: SCN2B), Synaptosomal-associated protein 25 (SNAP25) and Vesicle Associated Membrane Protein 2 (VAMP2). (D) 5 genes involved in building new synapses and the maintenance and plasticity of the post-synaptic density: Neurexin 1 (NRXN1), Neurexin 3 (NRXN3), Discs Large Homolog 3 (DLG3, also known as synapse associated protein SAP102), A-Kinase Anchoring Protein 5 (AKAP5), Synaptotagmin 4 (SYT4), Mean ± SEM. * p ≤ 0.05, ** p ≤ 0.01, ***p ≤0.005.
Finally, we analyzed the Nanostring data using only the subset of Nanostring cases that represented the 12 new cases not used in the microarray (n=8 low activity, n=4 high activity) as an independent confirmation of the microarray data. 100% of the genes showed directional agreement between the Nanostring and microarray data. Mann-Whitney comparison of average values across the genes revealed significantly higher expression in the high activity group vs. low activity group (medians: 0.894 (low), 1.12 (high); p<0.0001). The data thus confirm in an independent set of experimental samples that expression levels in the nanostring-measured genes, as a group, moved in the same direction as in the microarray analysis.
4.0. DISCUSSION
Multiple studies have established that physical activity is associated with delayed age-related decline and onset of AD in humans (Best et al., 2017; Buchman et al., 2012; Erickson et al., 2012; Hillman et al., 2008; Kirk-Sanchez and McGough, 2014; Korol et al., 2013; Prakash et al., 2015; Suwabe et al., 2018; Weuve et al., 2004; Yaffe et al., 2001). This study sought to identify underlying mechanisms by which physical activity supports brain health and slows Aging/AD-related declines in hippocampal function. Using microarrays, hippocampal transcription patterns associated with late-life physical activity levels were compared to hippocampal gene expression changes associated with Aging and AD. The data demonstrate that late-life physical activity is associated with major reprogramming of hippocampal gene expression, with a striking number of these genes showing significant but opposite patterns of expression change in Aging/AD. The majority (>70%) of these anti-Aging/AD genes show increased expression in association with physical activity but decreased expression in Aging/AD, with an extensive representation of genes regulating mitochondrial energy production, synaptic plasticity at the structural and signaling levels, and axon function.
With age a decline in energy production has been reported across organs and multiple species (Lane et al., 2015; Lin and Beal, 2006). Our data show that physical activity is linked to increased gene expression that included multiple components of the electron transport chain along with key genes driving the TCA cycle, which operates in the mitochondrial matrix and provides key intermediaries (NADH and FADH2) to the electron transport chain and enhances energy production. Importantly, the genes showing increased expression with physical activity are the same genes that undergo reduced expression in Aging and AD. Enhancement of mitochondrial function may serve as a core mechanism by which physical activity delays Age/AD-related cognitive decline, considering that a ready supply of energy is essential for the maintenance of synaptic fidelity (Harris et al., 2012; Hebert-Chatelain et al., 2016; Smith et al., 2016).
One of the major causes of cognitive decline in Aging and AD is synaptic dysfunction and ultimately failure that progressively disrupts connectivity patterns critical to ongoing functions of the brain (Koffie et al., 2011; Prieto et al., 2017; Selkoe, 2002). Our data suggest that gene expression patterns associated with physical activity may promote synaptic fidelity in the aged hippocampus not only by targeting energy production but also by counteracting Age/AD-related declines in synaptic components and processes central to neurotransmission. Notably, anti-Aging/AD genes showing increased expression in association with physical activity included many presynaptic components that regulate synaptic vesicle priming, release and recycling, post-synaptic GABA and glutamate neurotransmitter receptors that control the excitatory/inhibitory balance, and PSD scaffolding molecules that tune NMDA and AMPA glutamate signaling, all of which are major mechanisms to regulate synaptic efficacy. Further, many of the anti-Aging/AD genes showing increased expression with physical activity target myelin and axon health, suggesting that physical activity may promote white matter integrity and axonal connectivity and function. The strong relationship between physical activity and expression of genes targeting axon functioning are important, given that white matter degeneration and reduced axonal efficacy are notable components of cognitive decline in both normal aging and in AD pathogenesis (Bartzokis et al., 2003; Fletcher et al., 2018; Kruggel et al., 2017).
Approximately 30% of the anti-Aging/AD genes showed decreased expression with physical activity, but increased expression in Aging/AD. Interestingly, these genes were primarily enriched for transcriptional regulation, including many genes driving negative regulation of transcription. Our data suggest that Aging/AD is accompanied by augmented transcriptional suppression, and that physical activity may offset transcriptional dysregulation and help maintain appropriate transcriptional control of gene expression in the Aging and AD hippocampus. The transcriptional control genes that were increased with Aging/AD but decreased in association with physical activity included several epigenetic regulators of histones including lysine-specific histone demethylases (KDM1B, KDM3A), histone lysine methyltransferase (KMT2C), histone deacetylases (HDAC1) and components of the HDAC complex (SAP18, SAP30, MECOM, TAL1), along with multiple transcriptional repressors and activators. The possibility that physical activity may reduce Aging/AD-associated transcriptional dysregulation would represent a powerful mechanism that could have far-reaching consequences for hippocampal function. For example, one gene of interest is the transcription factor SP3, which forms a chromatin modifying complex with HDAC2 that suppresses transcription of synaptic-plasticity associated genes, by removing acetylation from the promoter region (Yamakawa et al., 2017). Previous studies demonstrate that SP3 is elevated in brains of AD patients and in mouse models of AD, and that reducing SP3, or the SP3-HDAC2 interaction, improves synaptic plasticity and cognitive function without affecting HDAC2 function in other processes (Yamakawa et al., 2017). Our data reveal that SP3 is significantly elevated in the hippocampus already with Aging, shows a further increase with AD, and is reduced in association with physical activity, which may be one mechanism by which physical activity can facilitate synaptic gene expression in the hippocampus. Consistent with this idea, our data reveal that several synaptic target genes that are negatively co-regulated by HDAC2 and SP3 (Yamakawa et al., 2017) show increased expression in association with physical activity, including GRIK2, LIN7A, SYNGR3, and DLGAP1. Taken together, these data suggest that physical activity may offset Age/AD-associated transcriptional dysregulation and help maintain appropriate gene expression in the hippocampus.
While many aspects of human brain aging appear to be counteracted by transcriptional patterns associated with physical activity, an unexpected finding was that physical activity was not associated with extensive involvement of genes regulating immune function and inflammation. We had hypothesized that physical activity would be associated with decreased expression of this gene class based on the literature that the hippocampus undergoes extensive immune gene activation in aging and AD (Barrientos et al., 2015; Berchtold et al., 2008; Cribbs et al., 2012) and the literature that physical activity can constrain activation of immune/inflammatory processes in the hippocampus of animal models (Barrientos et al., 2011; Kohman et al., 2013; Littlefield et al., 2015). While analysis of the anti-Aging/AD genes showing decreased expression in association with physical activity revealed some over-representation of immune/inflammation related genes, the enrichment did not meet statistical significance. Nonetheless, we observed decreased expression in association with physical activity of a few notable genes that drive immune/inflammatory responses, including several activators of the NF-kB pathway, such as adipocyte enhancer-binding protein 1 (AEBP1), CD40 and filamin A (FLNA), all of which have been implicated in the progression of AD pathology (Giunta et al., 2010; Shijo et al., 2018; Wang et al., 2012). Overall however, our data did not reveal a strong relationship between physical activity levels and expression levels of immune/inflammation-related genes, particularly for immune/inflammation-related genes that show increased expression with Aging and AD.
The transcriptional signatures identified in our study are suported by the human and animal literature reporting widespread benefits of lifestyle activity to brain structure and function. Imaging studies in humans have demonstrated that exercise increases hippocampal volume and density along with cortical gray and white matter volumes and integrity, notably in brain regions that undergo aging-associated atrophy and functional decline (Colcombe et al., 2006; Erickson et al., 2011; Kleemeyer et al., 2016; Ruscheweyh et al., 2011; Voss et al., 2013). Such changes are consistent with our transcriptional data suggesting that physical activity promotes synaptic and cellular health from augmented energy availability, synaptic function, and axon integrity. The findings in human studies are paralleled by the animal literature demonstrating structural and functional changes in the hippocampus and cortex with physical activity including synaptogenesis and increased dendritic complexity (Eadie et al., 2005; Stranahan et al., 2007), hippocampal neurogenesis (Erickson et al., 2013; Fabel et al., 2009; Gould et al., 1999; van Praag et al., 1999b) enhanced synaptic plasticity (Farmer et al., 2004; van Praag et al., 1999a), improved myelin integrity (Zhang et al., 2017; Zhou et al., 2018) and improved spatial learning and memory (Berchtold et al., 2010; Creer et al., 2010; Fordyce and Wehner, 1993; Intlekofer et al., 2013; van Praag et al., 2005). In addition, animal studies have demonstrated that exercise targets multiple aspects mitochondrial function (Cechella et al., 2017; Kim et al., 2010; Lee et al., 2014; Marques-Aleixo et al., 2012; Rampon et al., 2000; Stranahan et al., 2008; Tong et al., 2001). Finally, a handful of animal studies have investigated anti-Aging/AD benefits of physical activity, demonstrated that exercise reverses age or AD-related changes in hippocampal gene expression, most notably for genes associated with mitochondrial function, synaptic plasticity, and axon/myelin integrity (Boveris and Navarro, 2008; Choi et al., 2018; Kohman et al., 2011). We note that some of the findings of animal studies were not recapitulated in the human brain (e.g., immune gene responses). The discrepancy between our data and the animal literature may be a function of differences in the extent or intensity of exercise participation between humans in this study vs. animals, with the exercise undertaken in animal studies generally being of higher frequency (daily), intensity, and distance (>2 km), but usually shorter duration (<4 weeks).
Taken together, our results suggest that physical activity may broadly shift multiple biological processes towards improved functioning; stimulating core functions that undergo Age/AD-related decline but that are critical for maintaining brain health and synaptic function. We note that most of these genes followed a pattern of only modest expression change in the course of cognitively normal aging followed by progressively greater expression change in AD, and conversely, small expression change with moderate physical activity followed by a relatively large expression change in the highest tier of physical activity. While a relatively modest expression change of any individual gene on its own may not significantly impact hippocampal plasticity and cognitive function, the cumulative effect of reduced expression of multiple genes in a particular functional category, and the gestalt effect of compromised function across multiple categories (e.g. energy production, synaptic efficacy, axon function, transcriptional control), may well be a root cause of age-related decline in hippocampal plasticity and cognitive function. Similarly, the well-established benefit of physical activity to delay age-related cognitive decline and onset of AD may well arise from the cumulative expression change of multiple genes across multiple categories of function. By preserving a more youthful profile across multiple biological processes, these transcriptional effects could empower the brain to strengthen plasticity and build cognitive reserve, providing several potential mechanisms for the cognitive-preserving effects of lifestyle. While the data presented here do not provide direct evidence of a causal relationship, these gene expression patterns may guide the combinatorial use of pharmaceutical agents that target biological domains untouched or minimally associated with physical activity in the human hippocampus transcription pattern.
Supplementary Material
Total daily physical activity (exercise and non-exercise) was measured 24 hours/day with actigraphy (Actical: Mini Mitter, Bend OR). The relationship between probeset expression and physical activity levels across 34 cases was used in the analysis to identify probesets significantly associated with physical activity. For visualization of potential dose-response relationships, relative expression levels (Supplemental Table 2) were calculated across cases grouped into low, moderate, and high physical activity, using a heuristic approach and natural break points across the data to categorize the cases into 3 tiers of physical activity (low activity: < 1.05 × 105 activity counts/day, moderate activity: 1.06 × 105 × 2.5 × 105 activity counts/day, high activity: > 2.5 × 105 activity counts/day). These thresholds correspond closely to the activity quintiles defined by Weuve et al. (Weuve et al., 2004), who found a dose-dependent effect of physical activity on cognitive performance, with highest cognitive performance in individuals with activity estimates above 26 MET-hrs/week
HIGHLIGHTS.
Physical activity is extensively associated with gene expression in the aged human hippocampus.
Gene expression patterns associated with physical activity restore a more youthful state in the hippocampus, and opposed transcription patterns associated with Aging and Alzheimer’s disease.
Gene expression is primarily increased, with genes targeting multiple core cellular functions.
Genes showed robust enrichment for mitochondrial energy production, synaptic signaling mechanisms, axon and white matter health, and control of transcription.
5.0 ACKNOWLEDGEMENTS
This work was made possible by National Institute on Aging (RO1AG34667, RO1AG051807, PPG AG000538, R01AG17917, R01AG15819, RO1AG057558), NIH-NINDS (R01NS78009), the Illinois Department of Public Health, the Robert C. Borwell Endowment Fund, and through access to the Genomic High Throughput Facility Shared Resource of the Cancer Center Support Grant (CA-62203) at the University of California, Irvine. The authors declare no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. LITERATURE CITED
- Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr., Montoye HJ, Sallis JF, Paffenbarger RS Jr., 1993. Compendium of physical activities: classification of energy costs of human physical activities. Medicine and science in sports and exercise 25(1), 71–80. [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S, 2010. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of neurology 67(1), 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF, 2011. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci 31(32), 11578–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR, Maier SF, 2015. Neuroinflammation in the normal aging hippocampus. Neuroscience 309, 84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J, 2003. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Archives of neurology 60(3), 393–398. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS, 2012. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res 9(6), 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Castello N, Cotman CW, 2010. Exercise and time-dependent benefits to learning and memory. Neuroscience 167(3), 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Cribbs DH, Coleman PD, Rogers J, Head E, Kim R, Beach T, Miller C, Troncoso J, Trojanowski JQ, Zielke HR, Cotman CW, 2008. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proceedings of the National Academy of Sciences of the United States of America 105(40), 15605–15610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Rosano C, Aizenstein HJ, Tian Q, Boudreau RM, Ayonayon HN, Satterfield S, Simonsick EM, Studenski S, Yaffe K, Liu-Ambrose T, 2017. Long-term changes in time spent walking and subsequent cognitive and structural brain changes in older adults. Neurobiology of aging 57, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bherer L, Erickson KI, Liu-Ambrose T, 2013. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res 2013, 657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Navarro A, 2008. Brain mitochondrial dysfunction in aging. IUBMB life 60(5), 308–314. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA, 2012. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78(17), 1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Bennett DA, 2008. Total daily activity is associated with cognition in older persons. Am J Geriatr Psychiatry 16(8), 697–701. [DOI] [PubMed] [Google Scholar]
- Cechella JL, Leite MR, Pinton S, Zeni G, Nogueira CW, 2017. Neuroprotective Benefits of Aerobic Exercise and Organoselenium Dietary Supplementation in Hippocampus of Old Rats. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, Aronson J, Zhang C, Miller SJ, Lesinski A, Chen JW, Kim DY, van Praag H, Spiegelman BM, Gage FH, Tanzi RE, 2018. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science (New York, N.Y 361(6406). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF, 2003. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 14(2), 125–130. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF, 2003. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 58(2), 176–180. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF, 2006. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61(11), 1166–1170. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P, 2004. Neurocognitive aging and cardiovascular fitness: recent findings and future directions. J Mol Neurosci. 24(1), 9–14. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ, 2010. Running enhances spatial pattern separation in mice. Proceedings of the National Academy of Sciences of the United States of America 107(5), 2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW, 2012. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. Journal of neuroinflammation 9, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA, 2003. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4(5), P3. [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR, 2005. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. The Journal of comparative neurology 486(1), 39–47. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Gildengers AG, Butters MA, 2013. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci 15(1), 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, Weinstein AM, 2014. Physical activity, fitness, and gray matter volume. Neurobiology of aging 35 Suppl 2, S20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH, 2010. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology 75(16), 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF, 2011. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America 108(7), 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Weinstein AM, Lopez OL, 2012. Physical activity, brain plasticity, and Alzheimer’s disease. Arch Med Res 43(8), 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G, 2009. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci 3, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR, 2004. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124(1), 71–79. [DOI] [PubMed] [Google Scholar]
- Fletcher E, Gavett B, Harvey D, Farias ST, Olichney J, Beckett L, DeCarli C, Mungas D, 2018. Brain volume change and cognitive trajectories in aging. Neuropsychology 32(4), 436–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM, 1993. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain research 619(1–2), 111–119. [DOI] [PubMed] [Google Scholar]
- Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K, 2008. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26(3), 317–325. [DOI] [PubMed] [Google Scholar]
- Giunta B, Rezai-Zadeh K, Tan J, 2010. Impact of the CD40-CD40L dyad in Alzheimer’s disease. CNS Neurol Disord Drug Targets 9(2), 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ, 1999. Learning enhances adult neurogenesis in the hippocampal formation [see comments]. Nature neuroscience 2(3), 260–265. [DOI] [PubMed] [Google Scholar]
- Hall KS, Howe CA, Rana SR, Martin CL, Morey MC, 2013. METs and accelerometry of walking in older adults: standard versus measured energy cost. Medicine and science in sports and exercise 45(3), 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D, 2012. Synaptic energy use and supply. Neuron 75(5), 762–777. [DOI] [PubMed] [Google Scholar]
- Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, Busquets-Garcia A, Pagano Zottola AC, Delamarre A, Cannich A, Vincent P, Varilh M, Robin LM, Terral G, Garcia-Fernandez MD, Colavita M, Mazier W, Drago F, Puente N, Reguero L, Elezgarai I, Dupuy JW, Cota D, Lopez-Rodriguez ML, Barreda-Gomez G, Massa F, Grandes P, Benard G, Marsicano G, 2016. A cannabinoid link between mitochondria and memory. Nature 539(7630), 555–559. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF, 2008. Be smart, exercise your heart: exercise effects on brain and cognition. Nature reviews 9(1), 58–65. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA, 2009a. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA, 2009b. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols 4(1), 44–57. [DOI] [PubMed] [Google Scholar]
- Huang P, Fang R, Li BY, Chen SD, 2016. Exercise-Related Changes of Networks in Aging and Mild Cognitive Impairment Brain. Front Aging Neurosci 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, Wood MA, Cotman CW, 2013. Exercise and Sodium Butyrate Transform a Subthreshold Learning Event into Long-Term Memory via a Brain-Derived Neurotrophic factor-Dependent Mechanism. Neuropsychopharmacology 38(10), 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS, 2010. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Experimental gerontology 45(5), 357–365. [DOI] [PubMed] [Google Scholar]
- Kirk-Sanchez NJ, McGough EL, 2014. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging 9, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemeyer MM, Kuhn S, Prindle J, Bodammer NC, Brechtel L, Garthe A, Kempermann G, Schaefer S, Lindenberger U, 2016. Changes in fitness are associated with changes in hippocampal microstructure and hippocampal volume among older adults. NeuroImage 131, 155–161. [DOI] [PubMed] [Google Scholar]
- Koffie RM, Hyman BT, Spires-Jones TL, 2011. Alzheimer’s disease: synapses gone cold. Mol Neurodegener 6(1), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS, 2013. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. Journal of neuroinflammation 10, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Rodriguez-Zas SL, Southey BR, Kelley KW, Dantzer R, Rhodes JS, 2011. Voluntary wheel running reverses age-induced changes in hippocampal gene expression. PLoS ONE 6(8), e22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Gold PE, Scavuzzo CJ, 2013. Use it and boost it with physical and mental activity. Hippocampus 23(11), 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, 2007a. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci 11(8), 342–348. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, 2007b. Effects of physical activity on cognition, well-being, and brain: human interventions. Alzheimers Dement 3(2 Suppl), S45–51. [DOI] [PubMed] [Google Scholar]
- Kruggel F, Masaki F, Solodkin A, 2017. Analysis of longitudinal diffusion-weighted images in healthy and pathological aging: An ADNI study. J Neurosci Methods 278, 101–115. [DOI] [PubMed] [Google Scholar]
- Lane RK, Hilsabeck T, Rea SL, 2015. The role of mitochondrial dysfunction in age-related diseases. Biochim Biophys Acta 1847(11), 1387–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP, 2008. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. Jama 300(9), 1027–1037. [DOI] [PubMed] [Google Scholar]
- Lee MC, Rakwal R, Shibato J, Inoue K, Chang H, Soya H, 2014. DNA microarray-based analysis of voluntary resistance wheel running reveals novel transcriptome leading robust hippocampal plasticity. Physiol Rep 2(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF, 2006. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113), 787–795. [DOI] [PubMed] [Google Scholar]
- Littlefield AM, Setti SE, Priester C, Kohman RA, 2015. Voluntary exercise attenuates LPS-induced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. Journal of neuroinflammation 12, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Aleixo I, Oliveira PJ, Moreira PI, Magalhaes J, Ascensao A, 2012. Physical exercise as a possible strategy for brain protection: evidence from mitochondrial-mediated mechanisms. Prog Neurobiol 99(2), 149–162. [DOI] [PubMed] [Google Scholar]
- Miller JA, Guillozet-Bongaarts A, Gibbons LE, Postupna N, Renz A, Beller AE, Sunkin SM, Ng L, Rose SE, Smith KA, Szafer A, Barber C, Bertagnolli D, Bickley K, Brouner K, Caldejon S, Chapin M, Chua ML, Coleman NM, Cudaback E, Cuhaciyan C, Dalley RA, Dee N, Desta T, Dolbeare TA, Dotson NI, Fisher M, Gaudreault N, Gee G, Gilbert TL, Goldy J, Griffin F, Habel C, Haradon Z, Hejazinia N, Hellstern LL, Horvath S, Howard K, Howard R, Johal J, Jorstad NL, Josephsen SR, Kuan CL, Lai F, Lee E, Lee F, Lemon T, Li X, Marshall DA, Melchor J, Mukherjee S, Nyhus J, Pendergraft J, Potekhina L, Rha EY, Rice S, Rosen D, Sapru A, Schantz A, Shen E, Sherfield E, Shi S, Sodt AJ, Thatra N, Tieu M, Wilson AM, Montine TJ, Larson EB, Bernard A, Crane PK, Ellenbogen RG, Keene CD, Lein E, 2017. Neuropathological and transcriptomic characteristics of the aged brain. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik R, Gallagher CL, Dowling NM, Carlsson CM, Bendlin BB, LaRue A, Rowley HA, Christian BT, Asthana S, Hermann BP, Johnson SC, Sager MA, 2014. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology 83(19), 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash RS, Voss MW, Erickson KI, Kramer AF, 2015. Physical activity and cognitive vitality. Annu Rev Psychol 66, 769–797. [DOI] [PubMed] [Google Scholar]
- Prieto GA, Trieu BH, Dang CT, Bilousova T, Gylys KH, Berchtold NC, Lynch G, Cotman CW, 2017. Pharmacological Rescue of Long-Term Potentiation in Alzheimer Diseased Synapses. J Neurosci 37(5), 1197–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, Tsien JZ, Hu Y, 2000. Effects of environmental enrichment on gene expression in the brain. Proceedings of the National Academy of Sciences of the United States of America 97(23), 12880–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, Volker K, Ho HV, Mooren F, Knecht S, Floel A, 2011. Physical activity and memory functions: an interventional study. Neurobiology of aging 32(7), 1304–1319. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, 2002. Alzheimer’s disease is a synaptic failure. Science (New York, N.Y 298(5594), 789–791. [DOI] [PubMed] [Google Scholar]
- Shijo M, Honda H, Suzuki SO, Hamasaki H, Hokama M, Abolhassani N, Nakabeppu Y, Ninomiya T, Kitazono T, Iwaki T, 2018. Association of adipocyte enhancer-binding protein 1 with Alzheimer’s disease pathology in human hippocampi. Brain Pathol 28(1), 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HL, Bourne JN, Cao G, Chirillo MA, Ostroff LE, Watson DJ, Harris KM, 2016. Mitochondrial support of persistent presynaptic vesicle mobilization with age-dependent synaptic growth after LTP. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A, 2010. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic medicine 72(3), 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E, 2007. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus 17(11), 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP, 2008. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiology of aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs B, Chen LJ, Chang CY, Sun WJ, Ku PW, 2017. Accelerometer-assessed light physical activity is protective of future cognitive ability: A longitudinal study among community dwelling older adults. Experimental gerontology 91, 104–109. [DOI] [PubMed] [Google Scholar]
- Suwabe K, Byun K, Hyodo K, Reagh ZM, Roberts JM, Matsushita A, Saotome K, Ochi G, Fukuie T, Suzuki K, Sankai Y, Yassa MA, Soya H, 2018. Rapid stimulation of human dentate gyrus function with acute mild exercise. Proceedings of the National Academy of Sciences of the United States of America 115(41), 10487–10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, Uemura K, Lee S, Park H, 2012. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol 12, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau V, Balazs R, Cotman CW, 2001. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiology of disease 8(6), 1046–1056. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH, 1999a. Running enhances neurogenesis, learning, and long-term potentiation in mice. PNAS 96(23), 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 1999b. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci 2(3), 266–270. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH, 2005. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 25(38), 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman-Jones MH, Brant R, Rooney C, Geh C, Emery H, Harbron CG, Wappett M, Sharpe A, Dymond M, Barrett JC, Harrington EA, Marshall G, 2015. Evaluating Robustness and Sensitivity of the NanoString Technologies nCounter Platform to Enable Multiplexed Gene Expression Analysis of Clinical Samples. Cancer Res 75(13), 2587–2593. [DOI] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wojcicki TR, White SM, Gothe N, McAuley E, Sutton BP, Kramer AF, 2013. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp 34(11), 2972–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wojcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF, 2010. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Bakshi K, Frankfurt M, Stucky A, Goberdhan M, Shah SM, Burns LH, 2012. Reducing amyloid-related Alzheimer’s disease pathogenesis by a small molecule targeting filamin A. J Neurosci 32(29), 9773–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F, 2004. Physical activity, including walking, and cognitive function in older women. Jama. 292(12), 1454–1461. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K, 2001. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Archives of internal medicine 161(14), 1703–1708. [DOI] [PubMed] [Google Scholar]
- Yamakawa H, Cheng J, Penney J, Gao F, Rueda R, Wang J, Yamakawa S, Kritskiy O, Gjoneska E, Tsai LH, 2017. The Transcription Factor Sp3 Cooperates with HDAC2 to Regulate Synaptic Function and Plasticity in Neurons. Cell Rep 20(6), 1319–1334. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chao FL, Luo YM, Xiao Q, Jiang L, Zhou CN, Zhang Y, Lv FL, He Q, Ma J, Tang Y, 2017. Exercise Prevents Cognitive Function Decline and Demyelination in the White Matter of APP/PS1 Transgenic AD Mice. Curr Alzheimer Res 14(6), 645–655. [DOI] [PubMed] [Google Scholar]
- Zhou CN, Chao FL, Zhang Y, Jiang L, Zhang L, Luo YM, Xiao Q, Chen LM, Tang Y, 2018. Sex Differences in the White Matter and Myelinated Fibers of APP/PS1 Mice and the Effects of Running Exercise on the Sex Differences of AD Mice. Front Aging Neurosci 10, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatar ZZ, McGregor KM, Towler S, Nocera JR, Dzierzewski JM, Crosson B, 2015. Self-reported physical activity and objective aerobic fitness: differential associations with gray matter density in healthy aging. Front Aging Neurosci 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total daily physical activity (exercise and non-exercise) was measured 24 hours/day with actigraphy (Actical: Mini Mitter, Bend OR). The relationship between probeset expression and physical activity levels across 34 cases was used in the analysis to identify probesets significantly associated with physical activity. For visualization of potential dose-response relationships, relative expression levels (Supplemental Table 2) were calculated across cases grouped into low, moderate, and high physical activity, using a heuristic approach and natural break points across the data to categorize the cases into 3 tiers of physical activity (low activity: < 1.05 × 105 activity counts/day, moderate activity: 1.06 × 105 × 2.5 × 105 activity counts/day, high activity: > 2.5 × 105 activity counts/day). These thresholds correspond closely to the activity quintiles defined by Weuve et al. (Weuve et al., 2004), who found a dose-dependent effect of physical activity on cognitive performance, with highest cognitive performance in individuals with activity estimates above 26 MET-hrs/week