Abstract
Binge eating presents in the context of several eating disorders (EDs) and has been shown to be associated with negative affectivity and inhibitory control deficits. While considerable ecological momentary assessment (EMA) work in EDs has demonstrated the importance of intra-individual variability in affect in predicting binge episodes, no research has considered how fluctuations in inhibitory control and negative affect together influence binge eating, or the extent to which these relationships may differ across ED diagnoses. Therefore, the present EMA study assessed the extent to which daily inhibitory control moderated momentary associations between negative affect and binge eating, and whether the presence of regular compensatory behaviors influenced these associations. Participants were 40 women reporting regular binge eating (anorexia nervosa binge-purge type [AN-BP], bulimia nervosa [BN], binge-eating disorder [BED]/subthreshold BED) who completed a 10-day EMA protocol that included measures of affect, eating, and a daily ambulatory Go/No-go task that included palatable food and neutral stimuli. Results of generalized estimating equations indicated greater between-person food-related inhibitory control deficits were associated with greater binge likelihood, and there was a three-way interaction between momentary negative affect, daily food-related inhibitory control, and compensatory behavior group. For individuals with BN or AN-BP, the relationship between momentary negative affect and subsequent binge eating was stronger on days characterized by reduced inhibitory control, whereas no main or interactive effects of negative affect or inhibitory control were observed for those with BED/ subthreshold BED. Together these results demonstrate the importance of intra-individual variability in executive functioning and affective processes that underlie binge eating, as well as meaningful individual differences in these momentary associations.
Keywords: ecological momentary assessment, binge eating, eating disorders, inhibitory control, emotion regulation
Introduction
Binge eating is an aberrant behavior defined by consumption of a large amount of food with a sense of loss of control over eating, which commonly occurs across eating disorders (EDs; American Psychiatric Association [APA], 2013). Unfortunately, treatments for eating disorders remain modestly effective at best, which underscores the need to identify and disrupt maintenance mechanisms (Linardon, & Wade, 2018; Linardon, 2018; Murray et al., 2019). While numerous trait-level factors have demonstrated relevance to binge eating, increasing ecological momentary assessment (EMA) research has highlighted the importance of daily and momentary factors (Engel et al., 2016). EMA involves collecting multiple reports of experiences and behaviors across the day, which can serve to identify dynamic mechanisms that precipitate ED symptoms, as well as trait-level factors that influence these temporal processes. EMA is therefore a promising approach that can inform the development of more targeted, mechanistic treatments.
Affect regulation theories of binge eating have received significant empirical support using EMA, which has indicated that negative affect increases prior to and decreases following binge-eating episodes (Engel et al., 2016). Consistent with these findings, affect regulation models conceptualize binge eating as a self-regulatory failure that is maintained via negative reinforcement. Self-regulation is a multifaceted construct that broadly refers to the ongoing, adaptive modulation of one’s internal states (e.g., emotion, cognition) and behavior (Hofmann et al., 2012; Koole and Aldao, 2017). However, the self-regulatory mechanisms by which states of negative affect lead to episodes of binge eating are not well-understood. That is, despite the robust evidence demonstrating momentary relationships between affect and binge eating, it is yet unclear how, when, and for whom these relationships emerge. As such, further research is needed to examine momentary self-regulatory mechanisms that potentiate binge eating, as well as the individual differences (i.e., traits) that predispose some individuals to be more vulnerable to these mechanisms.
Executive functioning is an important factor underlying self-regulatory capacity and emotion regulation processes, and may therefore be a key variable to consider in momentary relationships between affect and binge eating (Loth et al., 2016; Ochsner and Gross, 2008; Okon-Singer et al., 2015). Specifically, inhibitory control (i.e., the ability to control attention, behavior, thoughts, and/or emotions to override internal or external prepotent responses) is a central component of executive functioning that enables individuals to exert self-control, and is therefore a neurocognitive index of self-regulation that may help to understand momentary processes contributing to binge-eating episodes (Diamond, 2013; Okon-Singer et al., 2015). Across studies, individuals with binge eating show lower trait inhibitory control and higher impulsivity (Smith et al., 2018; Wildes and Marcus, 2013); furthermore, inhibitory control deficits are larger in the context of ED-relevant stimuli (e.g., weight/shape and food-related) compared to neutral stimuli (Wu et al., 2013). High inhibitory control may therefore enable individuals to refrain from binge eating when experiencing negative affective states by increasing their ability to exert self-control, particularly to binge-related cues (e.g., palatable food). In line with this assertion, EMA studies have indicated that individuals with trait deficits in neurocognitive dimensions related to inhibitory control were more likely to engage in binge eating or loss of control eating in response to negative affect (Goldschmidt et al., 2018; Smith et al., 2019).
However, published research on inhibitory control in EDs has relied on trait-level laboratory-based assessments, which precludes consideration of the dynamic nature of inhibitory control and self-regulatory processes. Importantly, emerging research outside of EDs using ambulatory neurocognitive assessments suggests executive functions significantly vary over time within people (Sliwinski et al., 2018). Moreover, one study found that moments of lower inhibitory control predict increased snacking in the next hour (Powell et al., 2017). It is therefore possible that, in addition to trait-level inhibitory control deficits measured between persons, reductions in inhibitory control occurring within persons reflect decreased capacity for self-regulation, and in turn increase the likelihood that negative affective states will trigger binge-eating episodes. However, intra-individual variability (i.e., fluctuations relative to an individual’s own average level) in inhibitory control, and potential interactions with affective states, have not been examined in the context of binge eating.
Lastly, it is important to consider that some individuals may be more to prone than others to binge eat when experiencing high negative affect and low inhibitory control or self-regulatory capacity. Consequently, the impact of momentary affective and inhibitory control processes may be greater in some individuals with EDs than others (Pearson et al., 2015). While all EDs are associated with emotion regulation deficits, and both bulimia nervosa (BN) and binge-eating disorder (BED) show inhibitory control deficits when compared to healthy controls (Smith et al., 2018), the degree and nature of self-regulatory disturbances may differ between those with (e.g., BN) and without compensatory behaviors (e.g., BED; Lavender et al., 2015; Leehr et al., 2015). For instance, affective instability is thought to be a key factor in BN (Berner et al., 2017); research has found greater inhibitory control deficits in BN compared to BED groups (Wu et al., 2013); and some studies show BED is associated with less severe deficits in some aspects of emotion regulation compared to other EDs (Brockmeyer et al., 2014; Svaldi et al., 2012). Furthermore, emotion regulation strategies were not shown to influence momentary relationships between negative affect and binge eating in a recent EMA study of BED (Svaldi, 2019). Together, this suggests that individuals with compensatory symptoms (e.g., BN, anorexia binge-purge subtype [AN-BP]), compared to those who do not engage in compensatory behaviors (e.g., BED), evidence greater emotional lability and deficits in inhibitory control and emotion regulation. Thus, they may have more momentary difficulties in refraining from binge eating when self-regulatory resources are depleted.
Therefore, the current study aimed to provide new insights into dynamic associations of negative affect, self-regulatory capacity (indexed by inhibitory control), and binge eating by examining the extent to which daily inhibitory control is related to momentary relationships between negative affect and binge eating using EMA. In addition, we explored whether these factors, which were thought to convey momentary vulnerability for binge eating, differed based on the presence of regular compensatory behaviors. First, it was hypothesized that there would be an interaction between momentary negative affect and daily inhibitory control predicting binge episodes, in that the relationship between momentary negative affect and subsequent binge eating would be stronger on days characterized by lower self-regulatory capacity, as indexed by inhibitory control. Second, as an exploratory aim, we examined whether individuals with regular compensatory behaviors versus no regular compensatory behaviors differed in these associations, with the expectation that individuals with compensatory behavior would evidence stronger independent and interactive effects of negative affect and inhibitory control in predicting binge eating, as compared to those without compensatory behavior. Therefore, a 3-way interaction between momentary negative affect, daily inhibitory control, and group (i.e., presence/absence of regular compensatory behaviors) was assessed as a predictor of binge eating. Given evidence in binge-type EDs suggesting larger effects on inhibitory control tasks in the context of ED-relevant stimuli (Wu et al., 2013), palatable food and neutral cues were chosen as the stimuli of interest in the inhibitory control task. We expected that effects would be larger in the context of reduced inhibitory control to palatable food cues compared to neutral cues. We therefore conducted analyses separately for inhibitory control in the context of palatable food and neutral cues to compare these stimuli.
Methods
Participants
Participants were 40 adult women who were recruited from clinical and community settings (87.5% Caucasian). To be eligible for the study, participants were required to be female, between the ages of 18 and 65, and endorse ≥1 episode of objective binge eating per week over the previous three months as determined by a structured clinical interview (Structured Clinical Interview for DSM-5, Research Version [SCID-5-RV]; First and Spitzer, 2015). Based on the SCID-5-RV, the sample included 29 participants with BED, 9 participants with BN, 1 participant with AN-BP (DSM-5 mild severity category: 17.5<body mass index [BMI]<18.5; APA, 2013), and 1 participant with Other Specified Feeding or Eating Disorder (OSFED, subthreshold BED presentation). Exclusion criteria included (1) inability to read/speak English; (2) current psychosis; (3) current mania; (4) acute suicidality; (5) medical instability as determined by vital signs and blood pressure at the study visit; (6) severe cognitive impairment or intellectual disability determined by a phone screen; (7) currently pregnant or breastfeeding; (8) inpatient or partial hospitalization in the past 4 weeks; (9) changes to ED treatment in the past 4 weeks; (10) history of bariatric surgery; and (11) BMI <18.0 kg/m2. Based on a treatment history questionnaire administered at baseline that assessed treatment engagement over the past six months, 62.5% reported attending individual psychotherapy, 12.5% reported attending group therapy, 10.0% reported attending community support groups, and 40.0% reported taking medication for eating, mood, or weight-related symptoms.
Procedures
Interested participants completed a phone screen to determine initial eligibility, after which they completed the first study visit. The visit included the informed consent process, assessment of vital signs and anthropometric measures (i.e., blood pressure, pulse, height, weight), structured interviews, computerized tasks, and self-report questionnaires. Participants received training from study staff on the EMA protocol, which was administered using the Momentary Assessment Tool (MAT) system on Samsung Galaxy tablets (provided by the researchers) for the next 11 days. Participants received training by research staff on the definitions of loss of control eating and overeating, consistent with DSM-5 definitions. During each day of the EMA protocol, participants received 5 semi-random signal-contingent prompts distributed around 5 anchor points between 8:30 a.m. and 9:00 p.m. In addition, participants were asked to complete event-contingent recordings after eating episodes; if participants forgot to record an episode, they could enter this information at the next semi-random signal. Once per day at a semi-random signal in the afternoon, participants were also prompted to complete an ambulatory inhibitory control assessment (i.e., Go/No-go task). The ambulatory task was given only once per day to assess the feasibility of the approach and to minimize participant burden. The afternoon time was selected given evidence that (1) negative affect increases throughout the day, and (2) the probability of binge eating peaks in the afternoon/evening hours (Smyth et al., 2009); thus, this schedule aimed to capture inhibitory control in a time window of heightened risk for symptoms. The first day of the EMA protocol was a practice day (i.e., data were not included in analyses), after which staff contacted participants to address any questions related to the protocol. Participants then completed the 10-day EMA protocol. Following the protocol, participants completed a second study visit to return the tablet and receive payment for (Fairburn and Beglin, 2008)participation. All procedures received IRB approval.
Measures
Eating Disorder Examination Questionnaire (EDE-Q 6.0; Fairburn and Beglin, 2008).
The EDE-Q was administered prior to the EMA protocol. The EDE-Q is 28-item self-report questionnaire that assesses of a variety of ED symptoms over the past 28 days. For the present study, the global score and subscales (Weight Concern, Shape Concern, Eating Concern, and Restraint) were examined for descriptive purposes as measures of ED severity.
EMA self-report questions.
Momentary negative affect was assessed at EMA signals by Positive and Negative Affect Schedule (PANAS) items: afraid, ashamed, guilty, hostile, nervous, and upset (Thompson, 2007; Watson and Tellegan, 1998). Items were selected from the short form to minimize participant burden (Thompson, 2007), with the addition of guilt given the relevance of this affective state to EDs (Berg et al., 2013). For each item, participants endorsed the extent to which they were experiencing each affect on a Likert scale ranging from 1 (not at all) to 5 (very much). Ratings were summed to create a composite negative affect score at each EMA signal (α=.88).
Binge eating was measured at each eating episode by questions that assessed participants’ degree of loss of control eating (While you were eating, to what extent did you: feel a sense of loss of control?; feel that you could not stop eating once you started?; feel disconnected [e.g., numb, zoned out, on auto-pilot]?) and overeating (To what extent do you: feel that you overate?; think that others would consider what you ate to be an usual or excessive amount of food?), each of which was rated on a Likert scale ranging from 1 (not at all) to 5 (extremely), with a rating of 3 corresponding to moderately. For each eating episode, scores on the loss of control and overeating items were averaged to create composite scores, and the episode was categorized as a binge episode (yes/no) if the loss of control and overeating composite ratings were each ≥3 (i.e., corresponding to at least moderate levels of both loss of control and overeating). The internal consistencies of the loss of control eating and overeating composites were excellent (α=.90 and α=.94, respectively).
Ambulatory Go/No-go task.
Momentary inhibitory control was assessed by a modified version of a traditional computer-based Go/No-go task administered on the MAT platform, which included neutral (e.g., hair brush) and palatable food images (e.g., cookies). To select the stimuli for the task, we used food and non-food images from a separate study conducted to validate palatability of food images (online supplementary material). During the ambulatory Go/No-go task, an image (i.e., neutral or palatable food) was presented on the screen for 1,000ms, preceded by a 500ms fixation cross. For “go” trials, participants were instructed to respond as fast as possible to categorize the images that had either a red border or a blue border. Participants responded using a colored button (red or blue) displayed on the left or right side of the screen. During “no-go” trials, participants were asked to inhibit responses to stimuli upon seeing a green dot (the no-go signal) displayed above the image. All trials were randomized within the test block. Each task administration included 100 trials, which required approximately 3 minutes to complete. The outcome measures used for the current study were the number of food-related and neutral commission errors at each EMA signal (i.e., failure to inhibit response in the presence of food or neutral images), with a greater commission errors reflecting poorer inhibitory control. Reliability was calculated according to procedures described by Sliwinski and colleagues (2018) using intra-class correlation coefficients (ICCs). Between-person reliability was .31, indicating that 31% of the variance in performance (i.e., proportion of commission errors) was between persons (i.e., suggesting substantial within-person variability), which is consistent with other research using ambulatory neurocognitive assessments (Sliwinski et al., 2018).
Statistical Analyses
Analyses were based on available data without imputation. First, generalized estimating equations (GEEs) were used to examine the degree to which the momentary relationship between negative affect and subsequent binge eating varied based on daily inhibitory control; in order to assess the hypothesis that inhibitory control deficits in the context of food cues would have a relatively more potent effect compared to neutral cues, one GEE examined food-related commission errors while the other GEE examined commission errors in the context of neutral stimuli. The GEE models included within-and between-person effects of continuous independent variables (i.e., momentary negative affect and daily Go/No-go food-related or neutral commission errors), to predict the likelihood of binge episode occurrences on a given day, as well as the 2-way interactions between within-person negative affect and food-related/neutral commission errors. Given that the hypotheses focused on within-person relationships (i.e., interactions between momentary negative affect and daily inhibitory control), the 2-way interaction terms included only the within-subject components for these variables (i.e., within-person negative affect by within-person food-related/neutral commission errors).
Within-person effects were person mean centered, while between-person effects were grand mean centered. That is, within-person effects reflect the degree to which an individual’s momentary/daily value of a variable (i.e., negative affect or food-related/neutral commission errors) differs from that individual’s average level, whereas between-person effects reflect the degree to which an individual’s average level of a variable (i.e., aggregated across the EMA protocol) differs from the total sample mean of the variable. In order to examine temporal relationships between negative affect and binge eating, negative affect ratings were lagged from the previous EMA signal but not lagged across individuals or days. GEEs employed an AR1 serial autocorrelation given the dependence within the nested data, and a binary logistic function due to the dichotomous coding of binge episode occurrences. Age and BMI were also included as covariates in analyses (grand mean centered).
Second, we evaluated whether these associations differed based on the presence or absence of regular compensatory behavior as an exploratory aim. Two additional GEEs (one including food-related commission errors; one including neutral commission errors) were conducted, with the addition of a grouping variable as a moderator of within-person effects. Participants were grouped based on their DSM-5 ED diagnoses, such that those with AN-BP or BN were categorized in the “compensatory behavior” group, and those with BED or subthreshold BED were categorized in the “no compensatory behavior” group. Group was added as a main effect to each GEE, as well as the following 2-and 3-way interactions: (1) within-person negative affect by within-person food-related/neutral commission errors; (2) within-person negative affect by group; (3) within-person food-related/neutral commission errors by group; and (4) within-person negative affect by within-person food-related/neutral commission errors by group.
Results
Descriptive information is shown in Table 1. The compensatory and no compensatory behavior groups did not differ in the average frequency of objective binge episodes reported at the study visit during the clinical interview (t[38]=−.91, p=.371). Significant differences were observed for BMI (t[38]=2.57, p=.014) and age (t[38]=2.13, p=.040), such that participants with BED/subthreshold BED evidenced higher BMI and were older compared to those with BN or AN-BP. As shown in Table 1, those with BED/subthreshold BED reported lower EDE-Q Global (t[38]=2.94, p=.006), Restraint (t[38]=3.20, p=.003), and Eating Concern (t[37]=2.27, p=.042) scores, but did not differ significantly on levels of Shape Concern (t[38]=1.76, p=.087) or Weight Concern (t[38]=1.73, p=.092).
Table 1.
Descriptive information
| Total sample | No compensatory behavior group | Compensatory behavior group | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M | SD | Min | Max | N | M | SD | Min | Max | N | M | SD | Min | Max | |
| BMI | 40 | 34.30 | 9.84 | 18.21 | 59.16 | 30 | 36.46 | 9.63 | 21.73 | 59.16 | 10 | 27.84 | 7.63 | 18.21 | 46.45 |
| Age | 40 | 34.70 | 15.59 | 19.00 | 64.00 | 30 | 37.60 | 16.11 | 19.00 | 64.00 | 10 | 26.00 | 10.25 | 20.00 | 54.00 |
| EDEQ Restraint | 40 | 2.27 | 1.44 | 0.20 | 5.80 | 30 | 1.89 | 1.15 | 0.20 | 3.60 | 10 | 3.40 | 1.68 | 0.60 | 5.80 |
| EDEQ Eating Concern | 39 | 2.74 | 1.23 | 0.60 | 5.60 | 29 | 2.46 | 1.03 | 0.60 | 5.60 | 10 | 3.58 | 1.44 | 1.00 | 5.20 |
| EDEQ Shape Concern | 40 | 4.37 | 1.17 | 0.88 | 6.00 | 30 | 4.18 | 1.13 | 0.88 | 6.00 | 10 | 4.91 | 1.17 | 2.63 | 6.00 |
| EDEQ Weight Concern | 40 | 3.84 | 1.17 | 0.80 | 6.00 | 30 | 3.66 | 1.06 | 0.80 | 5.20 | 10 | 4.38 | 1.38 | 2.00 | 6.00 |
| EDEQ Global | 40 | 3.31 | 1.03 | 0.62 | 5.45 | 30 | 3.05 | 0.86 | 0.62 | 4.46 | 10 | 4.07 | 1.18 | 2.06 | 5.45 |
| OBEs/week | 40 | 2.43 | 1.11 | 1.00 | 5.00 | 30 | 2.33 | 1.09 | 1.00 | 5.00 | 10 | 2.70 | 1.16 | 1.00 | 4.00 |
Note. BMI=body mass index; EDEQ=Eating Disorder Examination Questionnaire; OBEs/week=average frequency of objective binge eating (OBE) episodes per week over previous 3 months (as determined by the SCID-5-RV). The compensatory group included individuals with bulimia nervosa (BN) and anorexia nervosa binge-purge subtype (AN-BP); the no compensatory behavior group included individuals with binge eating disorder (BED) and subthreshold BED.
There were a total of 2,239 EMA signals completed during the 10-day EMA protocol, with a 90.3% compliance rate for signal-contingent recordings. The mean loss of control and overeating composite scores (aggregated within each person) were 1.99±.62 and 2.09±.68, respectively. The mean number of binge episodes reported per participant during the EMA protocol was 5.82±5.56 (Md=4.00; Range: 0–22), with 77.5% of participants reporting two or more binge episodes over the 10-day protocol. Consistent with prior EMA research, the distribution of binge eating over the course of a day was considerably skewed, with 67% of binge episodes occurring after 3:00 p.m., and 51.9% of episodes occurring after 5 p.m. The associations between negative affect ratings and commission errors (aggregated within persons) were examined Spearman’s rho due to the skewed distribution of commission errors. There were small positive correlations between negative affect and food-related commission errors (ρ=.26, p=.121), and between negative affect and neutral commission errors (ρ=.26, p=.112), though these effects were not statistically significant.
GEE Results
Aim 1.
GEEs assessing the 2-way interactions involving within-person negative affect and food-related or neutral commission errors are shown in Tables 2–3. There was a main effect of between-person food-related commission errors predicting binge episodes (p=.031; Table 2), indicating that individuals who evidenced greater overall food-related commission errors (i.e., poorer inhibitory control in the context of food cues) were more likely to binge during the EMA protocol; this effect did not reach statistical significance in the context of neutral commission errors (p=.051, Table 3). No other main effects or interactions were significant.
Table 2.
Results of generalized estimating equation predicting likelihood of binge-eating episodes using GNG food-related commission errors
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| B | SE | Lower | Upper | Wald χ2 | p | |
| Intercept | −1.74 | 0.22 | −2.18 | −1.30 | 60.40 | <0.001 |
| BMI | −0.01 | 0.02 | −0.06 | 0.03 | 0.47 | 0.493 |
| Age | <0.01 | 0.02 | −0.03 | 0.03 | 0.05 | 0.822 |
| NA between | 0.07 | 0.05 | −0.04 | 0.17 | 1.46 | 0.226 |
| GNG between (food) | 4.16 | 1.92 | 0.39 | 7.92 | 4.67 | 0.031 |
| Momentary NA | 0.05 | 0.04 | −0.03 | 0.13 | 1.63 | 0.202 |
| Daily GNG (food) | −0.31 | 1.11 | −2.50 | 1.87 | 0.08 | 0.778 |
| Momentary NA X daily GNG (food) | 0.19 | 0.25 | −0.30 | 0.69 | 0.58 | 0.445 |
Note. BMI=body mass index; NA=negative affect; GNG=Go/No-go food-related commission errors. Between-person effects were grand-mean centered. Within-person effects were person-mean centered, such that within-person NA reflects momentary fluctuations, and within-person GNG reflects daily fluctuations. Within-person momentary NA was lagged from the previous signal.
Table 3.
Results of generalized estimating equation predicting likelihood of binge-eating episodes using GNG neutral commission errors
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| B | SE | Lower | Upper | Wald χ2 | p | |
| Intercept | −1.73 | 0.23 | −2.18 | −1.28 | 56.27 | <0.001 |
| BMI | −0.02 | 0.02 | −0.06 | 0.02 | 0.76 | 0.383 |
| Age | <0.01 | 0.02 | −0.03 | 0.03 | 0.05 | 0.822 |
| NA between | 0.05 | 0.06 | −0.06 | 0.16 | 0.73 | 0.391 |
| GNG between (neutral) | 3.83 | 1.96 | −0.01 | 7.67 | 3.82 | 0.051 |
| Momentary NA | 0.06 | 0.04 | −0.02 | 0.14 | 2.18 | 0.140 |
| Daily GNG (neutral) | −1.35 | 1.12 | −3.54 | 0.85 | 1.45 | 0.229 |
| Momentary NA X daily GNG (neutral) | 0.26 | 0.20 | −0.14 | 0.66 | 1.61 | 0.204 |
Note. BMI=body mass index; NA=negative affect; GNG=Go/No-go neutral commission errors. Between-person effects were grand-mean centered. Within-person effects were person-mean centered, such that within-person NA reflects momentary fluctuations, and within-person GNG reflects daily fluctuations. Within-person momentary NA was lagged from the previous signal.
Aim 2.
The GEE model assessing group as a moderator of within-person effects using food-related commission errors revealed a three-way interaction between lagged negative affect, daily food-related inhibitory control (indexed by food-related commission errors on the daily Go/No-go task), and group (compensatory behaviors vs. no compensatory behaviors) predicting the likelihood of binge eating, B=2.46, SE=1.22, p=.044 (Table 4). No other main effects or interactions were significant. As shown in Figure 1, among those with BED/subthreshold BED (no compensatory behavior group), daily levels of food-related inhibitory control were not related to the likelihood of binge eating. As predicted by the second hypothesis, for those with BN/AN-BP (compensatory behavior group), the degree of momentary negative affect and daily food-related inhibitory control were interactively related to the likelihood of binge eating. That is, higher momentary negative affect, relative to an individual’s average, was related to increased likelihood of subsequent binge eating on days characterized by higher food-related commission errors (i.e., lower inhibitory control), relative to an individual’s average level. Conversely, on days characterized by lower food-related commission errors (i.e., higher inhibitory control), lower momentary negative affect was related to a greater likelihood of binge. With respect to stimuli specificity, results of the GEE model using Go/No-go neutral commission errors did not indicate significant main or interactive effects at the between-or within-person level (Table 5).
Table 4.
Results of generalized estimating equation predicting likelihood of binge-eating episodes using GNG food-related commission errors, including the presence of compensatory behavior (group) as moderator
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| B | SE | Lower | Upper | Wald χ2 | p | |
| Intercept | −1.05 | 1.15 | −3.31 | 1.21 | 0.83 | 0.362 |
| BMI | −0.02 | 0.02 | −0.06 | 0.03 | 0.48 | 0.489 |
| Age | <0.01 | 0.02 | −0.04 | 0.03 | 0.05 | 0.827 |
| Group | −0.12 | 0.63 | −1.35 | 1.12 | 0.03 | 0.855 |
| NA between | 0.06 | 0.05 | −0.04 | 0.17 | 1.46 | 0.227 |
| GNG between (food) | 4.05 | 2.69 | −1.23 | 9.33 | 2.26 | 0.133 |
| Momentary NA | 0.05 | 0.05 | −0.03 | 0.14 | 1.44 | 0.230 |
| Daily GNG (food) | −0.24 | 1.20 | −2.60 | 2.11 | 0.04 | 0.839 |
| Group X momentary NA | 0.03 | 0.08 | −0.12 | 0.18 | 0.11 | 0.736 |
| Group X daily GNG (food) | −0.05 | 2.95 | −5.83 | 5.73 | 0.00 | 0.987 |
| Momentary NA X daily GNG (food) | −0.04 | 0.27 | −0.56 | 0.49 | 0.02 | 0.895 |
| Group X momentary NA X daily GNG (food) | 2.46 | 1.22 | 0.07 | 4.86 | 4.07 | 0.044 |
Note. BMI=body mass index; NA=negative affect; GNG=Go/No-go food-related commission errors. Between-person effects were grand-mean centered. Within-person effects were person-mean centered, such that within-person NA reflects momentary fluctuations, and within-person GNG reflects daily fluctuations. Within-person momentary NA was lagged from the previous signal. Group was coded such that the reference category is the “no compensatory behavior” group.
Figure 1.
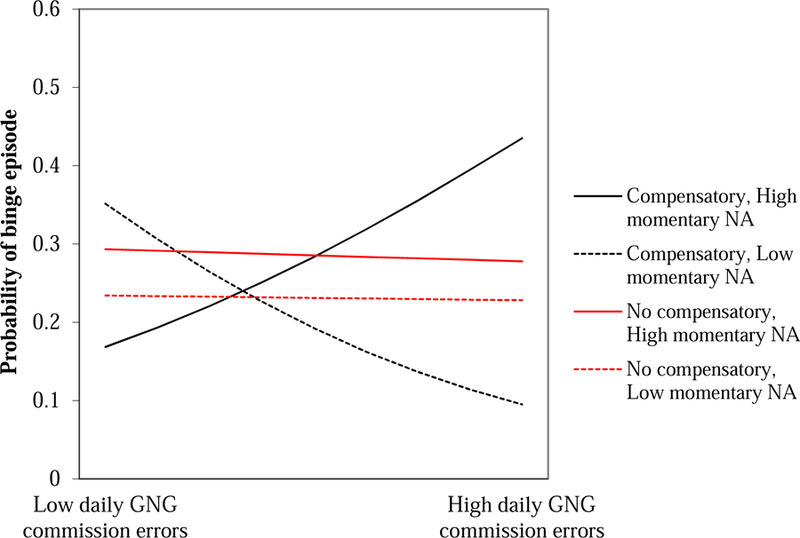
Three-way interaction between momentary negative affect, daily inhibitory control, and group predicting binge eating. (NA=momentary negative affect at the previous EMA signal; GNG=Go/No-go food-related commission errors; compensatory=compensatory behavior group; no compensatory=no compensatory behavior group).
Table 5.
Results of generalized estimating equation predicting likelihood of binge-eating episodes using GNG neutral stimuli commission errors, including the presence of compensatory behavior (group) as moderator
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| B | SE | Lower | Upper | Wald χ2 | p | |
| Intercept | −1.75 | 0.28 | −2.31 | −1.19 | 37.61 | <0.001 |
| BMI | −0.02 | 0.02 | −0.06 | 0.03 | 0.58 | 0.444 |
| Age | 0.00 | 0.02 | −0.04 | 0.03 | 0.02 | 0.888 |
| Group | 0.08 | 0.65 | −1.20 | 1.35 | 0.01 | 0.906 |
| NA between | 0.05 | 0.06 | −0.06 | 0.16 | 0.79 | 0.375 |
| GNG between (neutral) | 3.56 | 2.46 | −1.25 | 8.38 | 2.10 | 0.147 |
| Momentary NA | 0.05 | 0.05 | −0.04 | 0.14 | 1.37 | 0.241 |
| Daily GNG (neutral) | −1.71 | 1.59 | −4.82 | 1.40 | 1.16 | 0.281 |
| Group X momentary NA | 1.91 | 1.79 | −1.59 | 5.41 | 1.14 | 0.286 |
| Group X daily GNG (neutral) | 0.01 | 0.08 | −0.15 | 0.17 | 0.01 | 0.937 |
| Momentary NA X daily GNG (neutral) | 0.19 | 0.27 | −0.33 | 0.71 | 0.52 | 0.472 |
| Group X momentary NA X daily GNG (neutral) | 0.36 | 0.45 | −0.51 | 1.24 | 0.66 | 0.415 |
Note. BMI=body mass index; NA=negative affect; GNG=Go/No-go neutral commission errors. Between-person effects were grand-mean centered. Within-person effects were person-mean centered, such that within-person NA reflects momentary fluctuations, and within-person GNG reflects daily fluctuations. Within-person momentary NA was lagged from the previous signal. Group was coded such that the reference category is the “no compensatory behavior” group.
Discussion
The present study was the first to our knowledge to examine how intra-individual variability (i.e., fluctuations relative to an individual’s mean level) in self-regulatory capacity (indexed by inhibitory control) and negative affect together predict binge eating in individuals with EDs using EMA, and whether these associations differ based on the presence of regular compensatory behaviors. Results partially supported the hypotheses, in that the association between momentary negative affect and subsequent binge eating was stronger on days characterized by lower food-related inhibitory control, but only among those who reported regular compensatory behaviors (i.e., BN/AN-BP). In addition, there was a main effect of between-person food-related commission errors predicting binge eating independent of group status, which suggested individuals with poorer inhibitory control in the context of palatable food generally evidence greater binge eating. While these results should be interpreted as preliminary, findings highlight the importance of considering the interplay between intra-and inter-individual factors when addressing binge-eating symptoms, which has meaningful implications for future research and treatment. Furthermore, results were specific to inhibitory control in the context of food and not neutral cues. This supports earlier findings (Wu et al., 2013) and extends this literature by demonstrating that variability in inhibition to disorder-salient stimuli could potentiate the momentary processes leading to binge eating among individuals with bulimic symptoms.
Importantly, the results observed among those with BN or AN-BP are consistent with literature outside of EDs that suggests temporal reductions in executive functioning, in combination with other situational factors (e.g., affect), influence self-regulatory behavior (Hofmann et al., 2012). However, the micro-temporal associations between negative affect and inhibitory control could not be well-elucidated in the present study given that inhibitory control was examined only once per day. That is, it is not clear whether negative affect impairs momentary self-regulatory capacity (i.e., inhibitory control) and/or vice versa.
Prior research has suggested the relationship between inhibitory control and emotion regulation is reciprocal. For instance, training individuals to recruit inhibitory control in emotional contexts can improve adaptive emotion regulation strategies (i.e., reappraisal) and reduce maladaptive strategies (i.e., rumination), and training inhibitory control reduces amygdala reactivity to aversive stimuli (Cohen et al., 2016; Cohen and Mor, 2018; Cohen et al., 2015). In addition, it is possible other factors (e.g., dietary restraint, sleep, physical activity) impact inhibitory control fluctuations, which in turn influence the ability to exert self-control over eating in the face of negative affect. These issues regarding directionality will be important to address in future studies using multiple daily assessments of inhibitory control.
Furthermore, the present findings are consistent with the literature in EDs implicating the role of negative urgency, a facet of impulsivity defined as a trait-like tendency to act rashly when experiencing negative affect (Cyders and Smith, 2008). Negative urgency has been consistently found to be an important risk factor in EDs that predicts bulimic symptoms specifically (Culbert et al., 2015). While negative urgency is typically conceptualized as a stable, trait-like characteristic, the current results add uniquely to this literature by highlighting the confluence of temporal fluctuations in negative affect and behavioral impulsivity among individuals with bulimic symptoms. It would also be useful for future work to examine the correspondence between trait-levels of negative urgency and momentary relationships between negative affect and inhibitory control.
Interestingly, among the compensatory group, lower momentary negative affect was related to a greater likelihood of binge eating on days characterized by better food-related inhibitory control. It may be that higher food-related inhibitory control reflected greater attempts to restrain eating in the compensatory group, which in turn increased risk of binge eating. Conversely, lower momentary negative affect was related to a lower likelihood of binge eating on days characterized by poorer inhibitory control in the compensatory group. Thus, it may be that other external factors (e.g., food cues) rather than affect also interact with low levels of inhibitory control to increase the likelihood of binge eating. Additional research is necessary to explore such effects.
It is also notable that there were no significant independent or interactive effects of momentary negative affect and daily inhibitory control in predicting binge eating among individuals with BED or subthreshold BED. One possibility is that individuals with compensatory behavior may be more likely to try to exhibit restraint (Elran-Barak et al., 2015), and on days when inhibitory control is lower in this group, restraint failure may be more likely when experiencing negative affect (Mason et al., 2018; Pearson et al., 2018). Conversely, given that individuals without compensatory behavior were older and may have longer duration of illness, it could be that habit-based processes were more salient in predicting binge eating among those with BED presentations. For instance, whereas momentary negative affect and lower inhibitory control were perhaps initially predictive of binge eating, over time, conditioned cues (e.g., location) could become more powerful predictors of binge episodes; however, given that duration of illness was not assessed in the present study, further empirical examination of this possibility is warranted. Additionally, food cue responsivity and the relative reinforcing value of food are positively related to BMI (Epstein et al., 2012; Stice et al., 2010). In light of the higher BMI in this group, it is possible that external food cues and the rewarding aspects of eating (i.e., food hedonics) are more potent antecedents of binge eating in BED. Thus, going forward it will be useful for EMA studies to examine and compare the momentary relevance of restraint, habit strength, illness duration, and food-related reward across different EDs characterized by binge eating.
There are also limitations to note. The sample size was modest, and thus replication is necessary in larger groups of individuals with AN-BP, BN, and BED. Specifically, the 3-way interaction should be interpreted with caution and as preliminary given the limited sample size of each group. The Go/No-go task was also administered once per day. While this was done to establish feasibility and limit participant burden, this approach limited assessments of potentially highly dynamic associations between affect, inhibitory control, and binge eating. Nevertheless, results show utility in measuring it once in a day as well, which have clinical and practical implications. In addition, given that negative affect and the probability of binge eating has been shown to increase over the course of the day (Smyth et al., 2009), the pattern of findings may have differed if inhibitory control assessments were administered earlier in the day. It is also important to consider that commission errors were generally low, which could warrant the use of more challenging tasks in future work. Participants were adult women and primarily Caucasian, which also limits generalizability to other groups. In particular, these findings need to be replicated in male samples. Overeating was assessed via self-report questions; therefore it was not possible to determine objective intake, and the use of other dietary assessment methods (e.g., image-based) would be important to consider in future EMA work (Schembre, 2018). Finally, food stimuli in the inhibitory control task were selected based on ratings of palatability, not necessarily caloric content. Consequently, there could be different effects for stimuli assessing the relative caloric content of foods (high versus low calorie food image), as compared to the effects associated with the stimuli in the present study (i.e., food versus non-food images).
Collectively, results represent a first step in elucidating intra-individual variability in both neurocognitive and affective processes underlying binge eating in naturalistic settings. Although results need to be further replicated in light of the sample size and relatively low rate of commission errors, findings elucidated a meaningful individual difference (i.e., compensatory behaviors) that serves as a marker of vulnerability to these momentary processes. As such, it is imperative for future research and intervention efforts to consider heterogeneity among individuals who engage in binge eating. Continued EMA research along these lines could also inform the development of adaptive ecological momentary interventions (i.e., just-in-time adaptive interventions), which aim to tailor interventions based on momentary periods of risk/vulnerability (e.g., states of high negative affect and low inhibitory control) as well as individual difference factors (Nahum-Shani et al., 2015). By accounting for intra-and inter-individual factors, like those identified in the present study, these interventions may prove to be particularly useful in targeting dynamic and heterogeneous mechanisms underlying binge eating.
Supplementary Material
Acknowledgments:
The authors would like to thank Paul Rokke, Ph.D., for assistance with study recruitment, and Kayla Bjorlie, B.A., Morgan Sorby, B.A., and Marinda Kurpius-Brock, M.Ed., for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflicts of interest.
References
- American Psychiatric Association (APA), 2013. Diagnostic and statistical manual of mental disorders. Author, Washington, DC. [Google Scholar]
- Berg KC, Crosby RD, Cao L, Peterson CB, Engel SG, Mitchell JE, Wonderlich SA, 2013. Facets of negative affect prior to and following binge-only, purge-only, and binge/purge events in women with bulimia nervosa. J Abnorm Psychol 122(1), 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner LA, Crosby RD, Cao L, Engel SG, Lavender JM, Mitchell JE, Wonderlich SA, 2017. Temporal associations between affective instability and dysregulated eating behavior in bulimia nervosa. J Psychiatr Res 92, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeyer T, Skunde M, Wu M, Bresslein E, Rudofsky G, Herzog W, Friederich HC, 2014. Difficulties in emotion regulation across the spectrum of eating disorders. Compr Psychiatry 55(3), 565–571. [DOI] [PubMed] [Google Scholar]
- Cohen N, Margulies DS, Ashkenazi S, Schäfer A, Taubert M, Henik A, Villringer A, Okon-Singer H, 2016. Using executive control training to suppress amygdala reactivity to aversive information. NeuroImage 125, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Cohen N, Mor N, 2018. Enhancing reappraisal by linking cognitive control and emotion. Clinical Psychological Science 6(1), 155–163. [Google Scholar]
- Cohen N, Mor N, Henik A, 2015. Linking executive control and emotional response: A training procedure to reduce rumination. Clinical Psychological Science 3(1), 15–25. [Google Scholar]
- Culbert KM, Racine SE, Klump KL, 2015. Research Review: What we have learned about the causes of eating disorders-a synthesis of sociocultural, psychological, and biological research. J Child Psychol Psychiatry 56(11), 1141–1164. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Smith GT, 2008. Emotion-based dispositions to rash action: positive and negative urgency. Psychol Bull 134(6), 807–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, 2013. Executive functions. Annual review of psychology 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elran-Barak R, Sztainer M, Goldschmidt AB, Crow SJ, Peterson CB, Hill LL, Crosby RD, Powers P, Mitchell JE, Le Grange D, 2015. Dietary Restriction Behaviors and Binge Eating in Anorexia Nervosa, Bulimia Nervosa and Binge Eating Disorder: Trans-diagnostic Examination of the Restraint Model. Eating behaviors 18, 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SG, Crosby RD, Thomas G, Bond D, Lavender JM, Mason T, Steffen KJ, Green DD, Wonderlich SA, 2016. Ecological Momentary Assessment in Eating Disorder and Obesity Research: a Review of the Recent Literature. Current psychiatry reports 18(4), 37. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Lin H, Carr KA, Fletcher KD, 2012. Food reinforcement and obesity. Psychological moderators. Appetite 58(1), 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, Beglin SJ, 2008. Eating Disorder Examination Questionnaire (6.0), in: Fairburn CG (Ed.) Cognitive Behavior Therapy and Eating Disorders. Guilford Press, New York. [Google Scholar]
- First M, Williams JBW, Karg RS,, Spitzer R, 2015. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research vers ion; SCID-5-RV). American Psychiatric Association, Arlington, VA. [Google Scholar]
- Goldschmidt AB, Smith KE, Lavender JM, Engel SG, Haedt-Matt A, 2018. Trait-level facets of impulsivity and momentary, naturalistic eating behavior in children and adolescents with overweight/obesity. J Psychiatr Res 110, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ, Baddeley AD, 2012. Executive functions and self-regulation. Trends Cogn Sci 16(3), 174–180. [DOI] [PubMed] [Google Scholar]
- Koole SL, Aldao A, 2017. The self-regulation of emotion: Theoretical and empirical advances, in: Vohs K, Baumeister RF (Ed.) Handbook of self-regulation: Research, theory and applications. Guilford Press, New York, NY, pp. 24–41. [Google Scholar]
- Lavender JM, Wonderlich SA, Engel SG, Gordon KH, Kaye WH, Mitchell JE, 2015. Dimensions of emotion dysregulation in anorexia nervosa and bulimia nervosa: A conceptual review of the empirical literature. Clinical psychology review 40, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehr EJ, Krohmer K, Schag K, Dresler T, Zipfel S, Giel KE, 2015. Emotion regulation model in binge eating disorder and obesity-a systematic review. Neuroscience & Biobehavioral Reviews 49, 125–134. [DOI] [PubMed] [Google Scholar]
- Linardon J, 2018. Rates of abstinence following psychological or behavioral treatments for binge-eating disorder: Meta-analysis. Int J Eat Disord 51(8), 785–797. [DOI] [PubMed] [Google Scholar]
- Linardon J, Wade TD, 2018. How many individuals achieve symptom abstinence following psychological treatments for bulimia nervosa? A meta‐analytic review. International Journal of Eating Disorders 51(8), 785–797. [DOI] [PubMed] [Google Scholar]
- Loth KA, Goldschmidt AB, Wonderlich SA, Lavender JM, Neumark-Sztainer D, Vohs KD, 2016. Could the resource depletion model of self-control help the field to better understand momentary processes that lead to binge eating? Int J Eat Disord 49(11), 998–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason TB, Smith KE, Lavender JM, Lewis RJ, 2018. Independent and interactive associations of negative affect, restraint, and impulsivity in relation to binge eating among women. Appetite 121, 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SB, Quintana DS, Loeb KL, Griffiths S, Le Grange D, 2019. Treatment outcomes for anorexia nervosa: a systematic review and meta-analysis of randomized-controlled trials. Psychol Med 49(4), 701–704. [DOI] [PubMed] [Google Scholar]
- Nahum-Shani I, Hekler EB, Spruijt-Metz D, 2015. Building health behavior models to guide the development of just-in-time adaptive interventions: A pragmatic framework. Health Psychol 34S, 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ, 2008. Cognitive Emotion Regulation: Insights from Social Cognitive and Affective Neuroscience. Curr Dir Psychol Sci 17(2), 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon-Singer H, Hendler T, Pessoa L, Shackman AJ, 2015. The neurobiology of emotion– cognition interactions: fundamental questions and strategies for future research. Frontiers in Human Neuroscience 9, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CM, Mason TB, Cao L, Goldschmidt AB, Lavender JM, Crosby RD, Crow SJ, Engel SG, Wonderlich SA, Peterson CB, 2018. A test of a state-based, self-control theory of binge eating in adults with obesity. Eat Disord 26(1), 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DJ, McMinn D, Allan JL, 2017. Does real time variability in inhibitory control drive snacking behavior? An intensive longitudinal study. Health Psychol 36(4), 356–364. [DOI] [PubMed] [Google Scholar]
- Schembre SM, Liao Y, O’connor SG, Hingle MD, Shen SE, Hamoy KG, Huh J, Dunton GF, Weiss R, Thomson CA, Boushey CJ, 2018. Mobile Ecological Momentary Diet Assessment Methods for Behavioral Research: Systematic Review. JMIR mHealth and uHealth 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski MJ, Mogle JA, Hyun J, Munoz E, Smyth JM, Lipton RB, 2018. Reliability and Validity of Ambulatory Cognitive Assessments. Assessment 25(1), 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Mason TB, Crosby RD, Engel SG, Wonderlich SA, 2019. A multimodal, naturalistic investigation of relationships between behavioral impulsivity, affect, and binge eating. Appetite 136, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Mason TB, Johnson JS, Lavender JM, Wonderlich SA, 2018. A systematic review of reviews of neurocognitive functioning in eating disorders: The state-of-the-literature and future directions. Int J Eat Disord 51(8), 798–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JM, Wonderlich SA, Sliwinski MJ, Crosby RD, Engel SG, Mitchell JE, Calogero RM, 2009. Ecological momentary assessment of affect, stress, and binge-purge behaviors: day of week and time of day effects in the natural environment. Int J Eat Disord 42(5), 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A, 2010. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage 50(4), 1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaldi J, Griepenstroh J, Tuschen-Caffier B, Ehring T, 2012. Emotion regulation deficits in eating disorders: a marker of eating pathology or general psychopathology? Psychiatry Res 197(1–2), 103–111. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Werle D, Naumann E, Eichler E, & Berking M, 2019. Prospective associations of negative mood and emotion regulation in the occurrence of binge eating in binge eating disorder. Journal of Psychiatric Research. [DOI] [PubMed]
- Thompson ER, 2007. Development and validation of an internationally reliable short-form of the positive and negative affect schedule (PANAS). Journal of Cross-cultural Psychology 38(2), 227–242. [Google Scholar]
- Watson D, Clark LA,, Tellegan A, 1998. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology 54(6), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wildes JE, Marcus MD, 2013. Alternative methods of classifying eating disorders: models incorporating comorbid psychopathology and associated features. Clin Psychol Rev 33(3), 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Giel KE, Skunde M, Schag K, Rudofsky G, de Zwaan M, Zipfel S, Herzog W, Friederich HC, 2013. Inhibitory control and decision making under risk in bulimia nervosa and binge‐eating disorder. International Journal of Eating Disorders 46(7), 721–728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


