Abstract
Purpose
Multigene panels (MGPs) are increasingly being used despite questions regarding their clinical utility and no standard approach to genetic counseling. How frequently genetic providers use MGP testing and how patient-reported outcomes (PROs) differ from targeted testing (eg, BRCA1/2 only) are unknown.
Methods
We evaluated use of MGP testing and PROs in participants undergoing cancer genetic testing in the multicenter Communication of Genetic Test Results by Telephone study (ClinicalTrials.gov identifier: NCT01736345), a randomized study of telephone versus in-person disclosure of genetic test results. PROs included genetic knowledge, general and state anxiety, depression, cancer-specific distress, uncertainty, and satisfaction. Genetic providers offered targeted or MGP testing based on clinical assessment.
Results
Since the inclusion of MGP testing in 2014, 395 patients (66%) were offered MGP testing. MGP testing increased over time from 57% in 2014 to 66% in 2015 (P = .02) and varied by site (46% to 78%; P < .01). Being offered MGP testing was significantly associated with not having Ashkenazi Jewish ancestry, having a history of cancer, not having a mutation in the family, not having made a treatment decision, and study site. After demographic adjustment, patients offered MGP testing had lower general anxiety (P = .04), state anxiety (P = .03), depression (P = .04), and uncertainty (P = .05) pre-disclosure compared with patients offered targeted testing. State anxiety (P = .05) and cancer-specific distress (P = .05) were lower at disclosure in the MGP group. There was a greater increase in change in uncertainty (P = .04) among patients who underwent MGP testing.
Conclusion
MGP testing was more frequently offered to patients with lower anxiety, depression, and uncertainty and was associated with favorable outcomes, with the exception of a greater increase in uncertainty compared with patients who had targeted testing. Addressing uncertainty may be important as MGP testing is increasingly adopted.
INTRODUCTION
The clinical evaluation of individuals for hereditary cancer risks has increased in complexity in recent years. The recognition of cancer risks associated with a growing number of high- and moderate-penetrance genes in common cancers (eg, breast, colorectal, and ovarian cancer) has led to the introduction of multigene panel (MGP) tests capable of simultaneously examining cancer risk across multiple loci.1,2 Although initial targeted testing of one or a few of the most likely high-risk genes remains a reasonable approach to evaluate patients with a strong family history suggestive of a particular genetic syndrome,1 the value of MGPs in efficiently evaluating patients at risk for multiple syndromes or cancer as a result of mutations in several clinically relevant genes is increasingly acknowledged.1
The growing use of clinical genetic testing and greater complexity of genetic tests offered to patients have challenged the limited genetic counseling resources available. Delivery of genetic testing results by telephone is one of several adaptations to the traditional genetic counseling practice model that have been studied in an effort to improve counseling access through the more efficient use of resources.3-5 Beginning in 2012, as part of the National Cancer Institute–funded multicenter Communication of Genetic Test Results by Telephone (COGENT) clinical trial, patients eligible for BRCA1/2 genetic testing for hereditary breast or ovarian cancer risk assessment were recruited and randomly assigned to in-person or telephone disclosure of genetic test results. In 2014, with the emergence of MGP testing and its rapid adoption in clinical practice, the study was modified to include this novel form of testing. Primary outcomes of the COGENT study6 have demonstrated that telephone disclosure is noninferior to in-person counseling across all primary and secondary short-term outcomes.
A robust literature on patient-reported outcomes (PROs) of BRCA1/2 and targeted genetic testing exists, including two randomized studies of telephone delivery of both pretesting and post-testing genetic services for patients considering genetic testing for these genes.3,5 COGENT is, to our knowledge, the first randomized study to examine uptake of MGP tests in the population of patients presenting for risk assessment to a tertiary high-risk clinic, as well as to report on PROs among patients receiving traditional targeted gene counseling and testing compared with patients receiving counseling and testing adapted to MGPs. In the current study, trends in testing choices with the advent of MGP testing and across the COGENT study time period and participating sites are reported, and PROs previously reported in aggregate are analyzed by counseling and testing modality selected for the patient.
METHODS
Study Design, Setting, and Participants
The COGENT study was a multicenter, randomized, noninferiority trial comparing the psychosocial and behavioral outcomes of phone versus in-person disclosure of genetic test results. Initially designed to include targeted BRCA1/2 testing only, the study eligibility was adapted in May 2014 to add all clinical germline genetic testing for hereditary breast, gynecologic, and/or GI cancer syndromes, allowing individuals receiving either targeted testing or MGP testing. Participants were recruited after in-person pretest counseling with a genetic counselor and completed PROs at the time of enrollment (after pretest counseling [T0]) and after results disclosure (T1).
Random Assignment
After completion of the survey given after pretest counseling, participants were assigned to either the in-person arm or telephone arm. Random assignment was stratified by study site and sex only. Participants who did not want to receive results by telephone and thus were not willing to be randomly assigned were permitted to self-select in-person disclosure and were analyzed separately.
All 22 genetic counseling professionals used a tiered and binned counseling model for pretest sessions where MGP testing was offered.7,8 All disclosures were made using standardized communication protocols and visual aids.7-9 Both a genetic counseling professional and a medical provider were present when results were provided in person, whereas individuals who received results by telephone were recommended to return to their institution to meet with a medical provider. Post-test PROs were measured immediately after in-person disclosures.
PROs
Genetic knowledge was evaluated (T0 and T1) using an adapted six-item cancer genetics knowledge scale (Cronbach’s α = .60 to .96), which is applicable to a broad range of cancer genes and focused on understanding of test results and cancer risk.10 This scale included items evaluating mechanism of inheritance (one item) and meaning of positive (two items) and negative results (three items).
State anxiety, a sensitive indicator of transient or situational changes in anxiety, was measured (T0 and T1) with the 20-item State Inventory of the State-Trait Anxiety Inventory11 (α = .96).
General anxiety was assessed (T0 and T1) with the seven-item anxiety subscale of the Hospital Anxiety and Depression Scale,12 which has been used to assess severity of general anxiety in a wide range of medical patients, including those with cancer (α = .86 to .89). Depression was assessed (T0 and T1) with the seven-item depression subscale of the Hospital Anxiety and Depression Scale (α = .82 to .84).
Cancer-specific distress was evaluated (T0 and T1) with 14 items of the Impact of Events Scale.13 We excluded one item lacking face validity in our population (α = .88 to .90).
Uncertainty was assessed (T0 and T1) using a three-item scale adapted from the Multidimensional Impact of Cancer Risk Assessment questionnaire14 (α = .80 to .84). Satisfaction with genetic services was measured (T0 and T1) with a nine-item scale evaluating participants’ perceptions of their genetic counseling and testing experience,15,16 including cognitive, affective, and time or attention items (α = .74 to .81).
Behavioral intention (T0 and T1) was included as a surrogate of early health behaviors because information on performance of behaviors is being collected in longitudinal follow-up. These behaviors included intent to perform mammography, breast magnetic resonance imaging, colonoscopy, and prophylactic surgeries (mastectomy and oophorectomy). Patients responded on a seven-item Likert scale and could mark “not applicable.”
Statistical Analysis
For baseline analyses, we characterized variables using means, proportions, standard deviations, and ranges for age. We used t tests and Fisher’s exact tests to compare characteristics between those participants offered and not offered panel testing. We used logistic regression to examine the relationship of baseline variables with being offered panel testing. We used multiple linear regressions to examine the relationship of panel testing with PROs after controlling for possible confounding baseline variables. Variables included in the tables and regressions included age, sex, race (white v nonwhite), education (college graduate v less than a college degree), Jewish ethnicity (no or missing v yes), number of first- and second-degree relatives with cancer, known cancer mutation, treatment decision, random assignment or self-selecting for in-person disclosure arm, and study site. To account for missing data in the PRO analyses, we used multiple imputation via the package IVEware (https://www.src.isr.umich.edu/software/) with 25 data sets.17 In the imputations, we included anyone who had baseline (T0) or postdisclosure (T1) response data. We did not include in the imputation analyses individuals missing both T0 and T1 response data because we felt that such individuals had too much missing data to reliably impute data. The criterion for statistical significance was P < .05.
RESULTS
Use and Predictors of MGP Testing Versus Targeted Testing
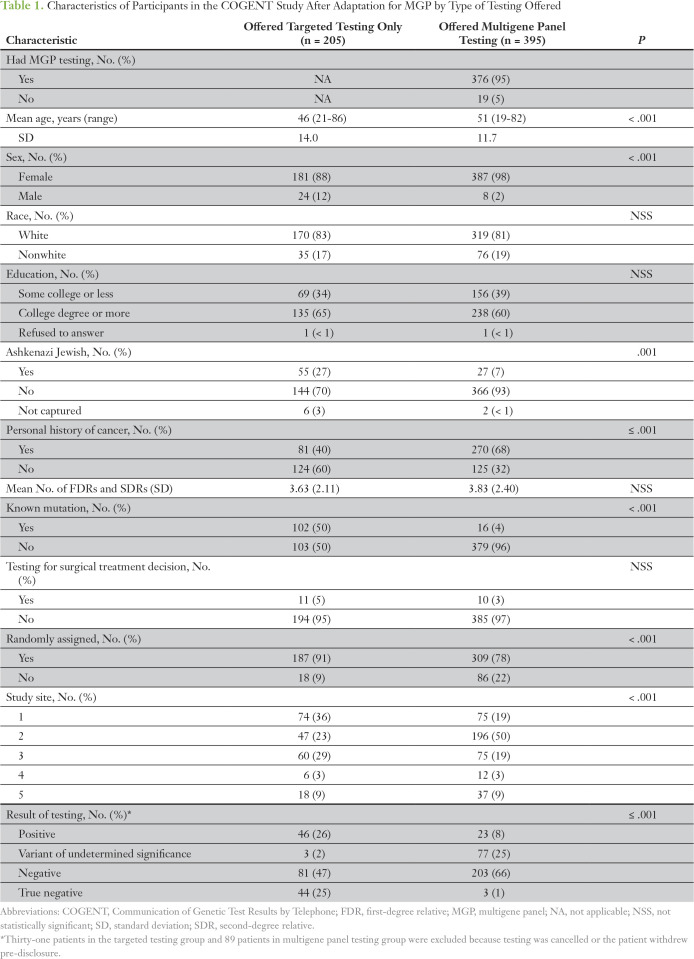
In total, 600 participants were offered genetic testing after inclusion of MGP testing; 62.7% of participants (376 of 600 participants) underwent MGP testing, whereas 37.3% of participants underwent targeted testing (Fig 1). The demographic characteristics of participants offered targeted and MGP testing are listed in Table 1. Targeted testing was most frequently offered to patients with a known familial mutation (86%) and to patients of Ashkenazi Jewish ancestry (67%), a population in which three founder mutations in BRCA1/2 are found at high frequency (prevalence of approximately 1:40). The study site accruing the largest number of participants (site 2) accounted for 50% of MGP tests (196 of 395 MGP tests) offered. Of note, of 395 patients offered MGP testing by the genetic counselor, a small fraction (19 [4.8%] of 395 patients) declined MGP testing and chose targeted testing. Characteristics associated with being more likely to be offered MGP testing were older age, female sex, non–Ashkenazi Jewish heritage, personal history of cancer, no known familial mutation, nonrandomization status (ie, preference for in-person disclosure), and study site.
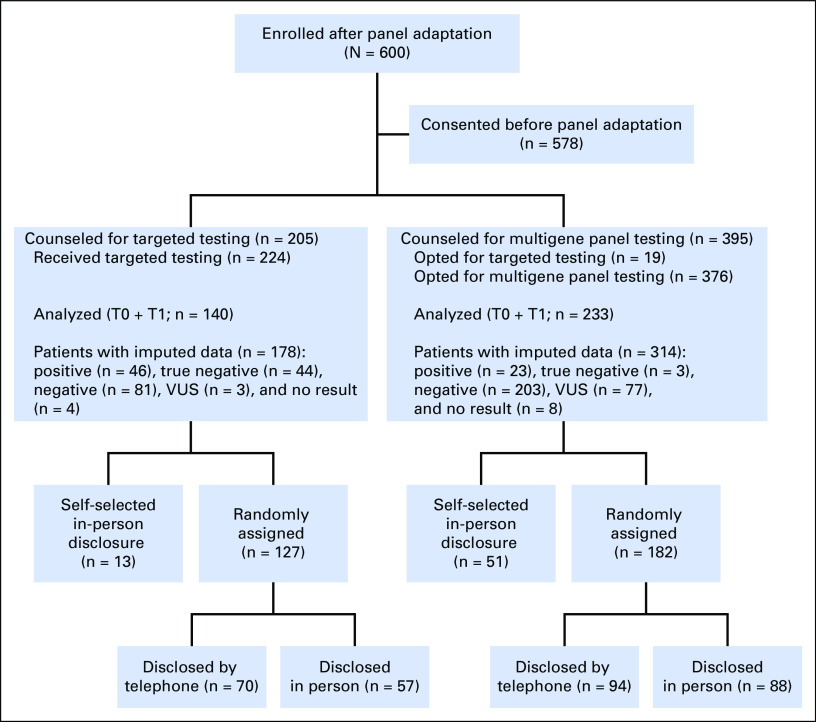
Fig 1.
CONSORT diagram. T0, after pretest counseling; T1, after results disclosure; VUS, variant of undetermined significance.
Table 1.
Characteristics of Participants in the COGENT Study After Adaptation for MGP by Type of Testing Offered
The percentage of patients offered MGP testing increased over time (from 57% in 2014 to 66% in 2015; P = .02) and was found to vary significantly by study site (range, 46% to 81% of all tests offered; Fig 2). By the end of 2015, at least 50% of testing offered was MGP testing. In a full multivariable logistic regression analysis, being offered MGP testing was associated with not being of Ashkenazi descent (odds ratio [OR], 5.28; P < .001), having a history of cancer (OR, 2.03; P = .012), not having a familial mutation (OR, 24.5; P < .001), not making a treatment decision (OR, 4.02; P = .011), requesting in-person disclosure (OR, 2.34; P = .018), and study site (OR, 6.06 for highest offer site v lowest offer site; P < .001). Identification of carriers and true-negative results were higher in the targeted testing group, which is likely secondary to a higher number of patients with a known familial mutation (Table 1). The frequency of uncertain variants was higher in the MGP group (25% v 2% with targeted testing).
Fig 2.
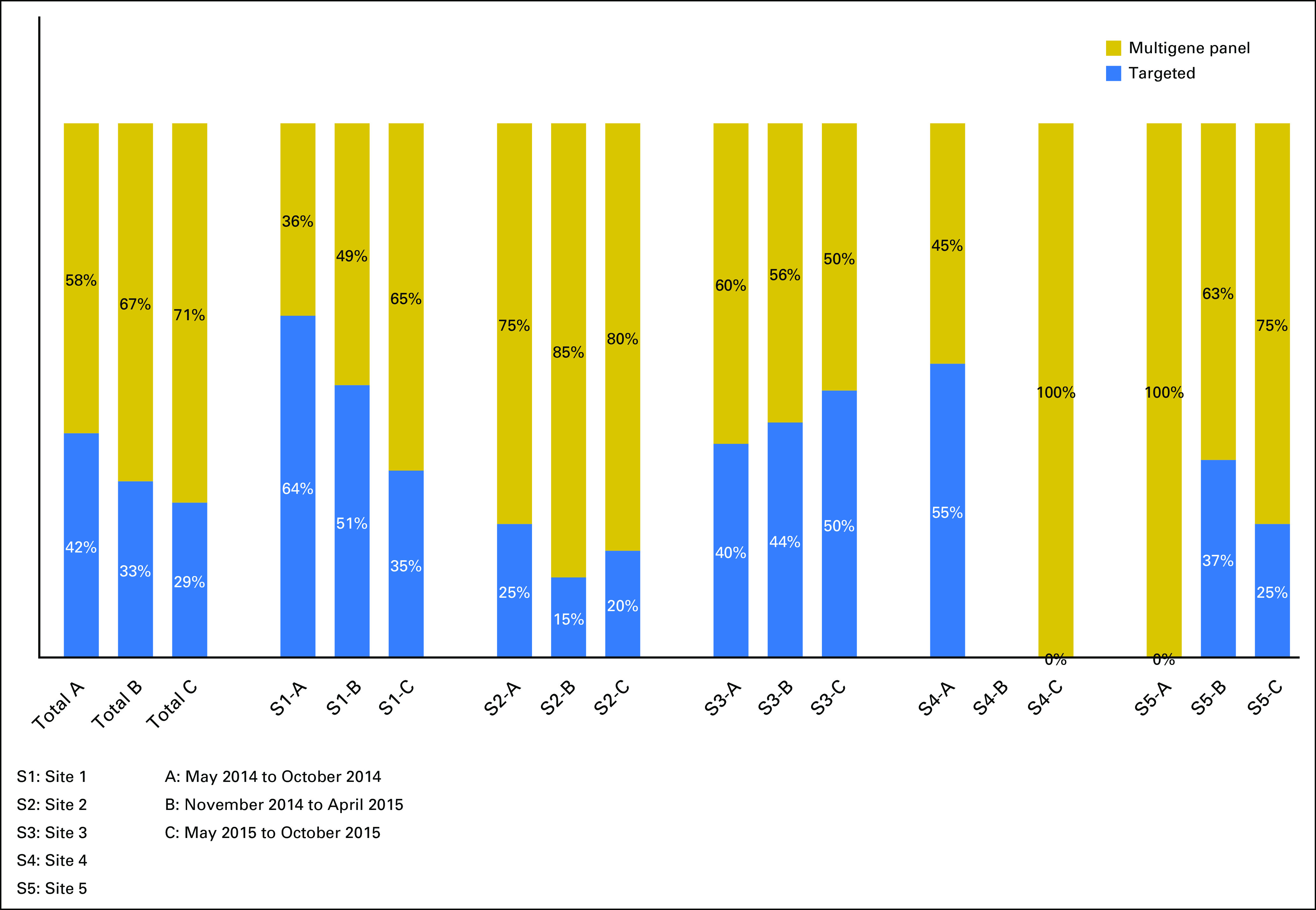
Type of testing offered by site.
PROs Assessed by Offer of Targeted Testing Versus MGP Testing
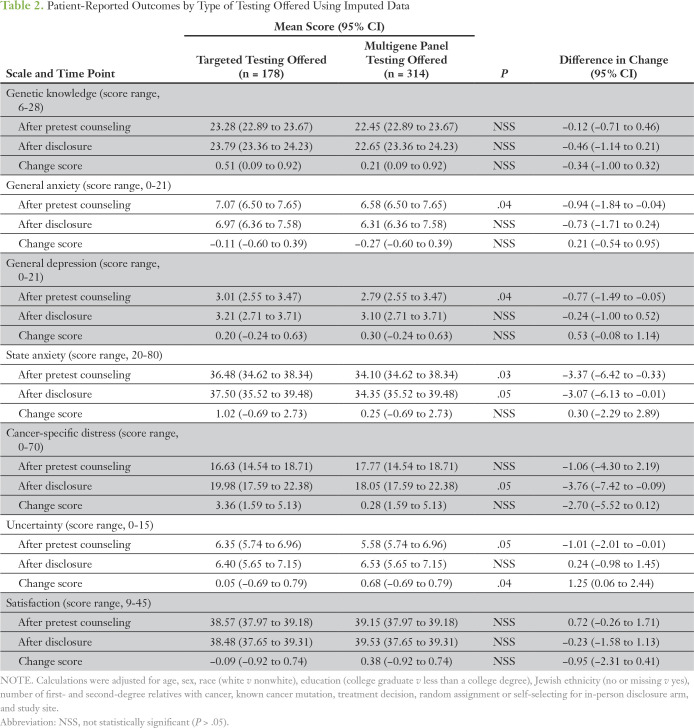
In full models, adjusting for baseline variables, PROs were assessed and compared by whether patients were offered MGP testing or targeted testing. In the final model, patients offered MGP testing had lower general anxiety (regression coefficient [β] = −0.94, P = .04), state anxiety (β = −3.37, P = .03), depression (β = −0.77, P = 0.04), and uncertainty (β = −1.01, P = .05) pre-disclosure compared with patients offered targeted testing (Table 2). Patients offered MGP testing did not differ in genetic knowledge, cancer-specific distress, or satisfaction pre-disclosure compared with patients offered targeted testing. State anxiety (β = −3.07, P = .05) and cancer-specific distress (β = −3.76, P = .05) were lower after disclosure in the MGP group compared with the targeted group. Even among participants with no familial mutation who underwent targeted testing, both baseline and follow-up anxiety levels were significantly higher than those in participants who were tested with an MGP when examined in unadjusted analyses (P = .025 and P = .029, respectively). There were no significant differences between groups in knowledge, general anxiety, depression, uncertainty, or satisfaction after disclosure, although there was a greater increase in change in uncertainty (β = 1.25, P = .04) among participants who underwent MGP testing. To understand this change, we additionally controlled for result type (positive v true negative v uncertain variant/indeterminate) in the regressions of uncertainty but found that significance inferences did not change. This suggests that the nature of MGP testing, and not the result, leads to increased uncertainty. Finally, there were no significant differences in knowledge at any time point.
Table 2.
Patient-Reported Outcomes by Type of Testing Offered Using Imputed Data
DISCUSSION
In the largest study to date of PROs after MGP testing, we found that patients eligible for hereditary cancer risk assessment and offered MGP, compared with targeted testing, had similar predisclosure and postdisclosure knowledge levels but lower predisclosure anxiety, depression, and uncertainty. In addition, postdisclosure state anxiety and cancer-specific distress were also lower among patients offered MGP, but uncertainty increased in this group compared with patients offered targeted testing. These results demonstrate that genetic counselors can incorporate MGP testing into their practice without compromising patient understanding, even when results are delivered by telephone.6 One of the concerns commonly raised with MGP testing is the possibility of knowledge deficits as a result of the complexity of information communicated. The potential negative impact of information overload from MGP counseling or testing on psychological outcomes is not supported by our findings, because we found that postdisclosure anxiety and depression were similar between the MGP and targeted testing groups. It is notable, however, that despite lower predisclosure uncertainty among those offered MGP testing, uncertainty increased more in the MGP testing group after disclosure, potentially indicating greater resilience among those offered MGP testing to the negative psychological implications of uncertainty. When the lower levels of predisclosure anxiety and depression among participants offered MGP are considered, our results also suggest that counselors may be more likely to offer complex MGP testing to patients they feel are at lower risk of negative psychological outcomes. In this way, these findings suggest that counselors are more than just gatekeepers to genetic testing and that comprehensive counseling includes both knowledge exchange and shared decision making with patients about concerns and preferences for genetic evaluation. In the higher rates of selection of in-person disclosure among patients offered MGP testing, we observe counselors and patients counterbalancing a patient’s ability to manage the complexity and uncertainty of MGP testing with the standard approach of in-person results disclosure.
These findings suggest that telephone disclosure is readily scalable to MGP testing and thus has an important role in the future of genomic medicine. Our study is particularly timely because a growing number of complex molecular diagnostic results will need to be communicated to patients. Although other studies have previously supported the efficacy of alternative telephone-based modalities of genetics services delivery,3,5 these studies have not included MGP testing and have, notably, not reported detailed PROs. One study recruited women at increased risk of hereditary breast and/or ovarian cancer and randomly assigned participants to in-person or telephone counseling, whereas the second study randomly assigned women with at least a 10% risk of having a BRCA1/2 mutation but without cancer to telephone counseling versus usual care. In both studies, telephone counseling was noninferior to in-person counseling for the selected outcomes (which included anxiety, cancer-specific distress, perceived control, and decisional conflict in one study5 and satisfaction, distress, physical functioning, decisional conflict, and knowledge at 3 months and 1 year in the other study3), although these results are specific to BRCA1/2 testing. A recent population-based study18 examined targeted testing (BRCA1/2) versus MGP testing among women with incident breast cancer. Similar to COGENT, a substantial shift toward MGP testing was seen over 2 years, although overall testing rates remained stable. Although PROs were not ascertained, the rate of preference for and receipt of mastectomy was similar regardless of whether a BRCA1/2 pathogenic variant or a pathogenic variant in a gene other than BRCA1/2 was identified by MGP, arguing that MGP may lead to more mastectomies over time. PROs have not been explored in other areas of medicine where MGP testing has entered clinical care.
Lower postdisclosure anxiety and distress among patients offered MGP testing is consistent with differences seen pre-disclosure and reflect counselor- and patient-driven selection of less anxious patients toward MGP testing. Patients offered targeted testing had a higher likelihood of a pathogenic mutation, explaining in part their higher anxiety. For some, this was a result of Ashkenazi Jewish ancestry, which is a group with a high prevalence of BRCA1/2 mutations. Ashkenazi patients were nearly four times more likely to be offered targeted testing compared with MGP testing. For others, high pretest risk was secondary to a known familial mutation; 50% of the targeted testing population had a known familial mutation compared with 4% of the MGP testing population. Similarly, patients tested by MGP had lower pretest likelihood of a pathogenic mutation, although the risk of identifying uncertain variants was higher as a result of the larger number of genes sequenced.
Although no other randomized studies have been conducted involving MGP, Lumish et al19 performed a post-test survey measuring PROs in patients undergoing testing for hereditary breast or ovarian cancer and in whom MGP testing was performed. They found older age, nonwhite race or non-Hispanic ethnicity, lower educational attainment, and lower knowledge to be associated with higher anxiety and adverse psychological effects from counseling. Analyses by cancer status (affected or unaffected) and stratified by result also found differential impact on psychological outcomes.19 Nonetheless, it remains uncertain how the differences would have changed had MGP testing been replaced by targeted testing. Finally, it is interesting that patients offered MGP testing in our study had lower baseline levels of anxiety and uncertainty (P = .05) but experienced greater increases in uncertainty that were not accompanied by increases in anxiety (P > .05). This may reflect greater tolerance toward genomic uncertainty in this group.20 Studies of uncertainty intolerance related to genomic testing21 have shown that uncertainty intolerance is associated with impaired decision making, feelings of vulnerability, and other adverse psychological outcomes.22
Overall, we found that use of MGP testing increased over the study period.23 Several factors are likely driving this change, including the need for more efficient delivery of genetic services. The declining costs and the competitive marketplace for commercial testing established by the US Supreme Court’s decision against Myriad Genetic Laboratories and gene patenting have improved access to genetic testing. The growing number of clinically relevant tests in diverse medical disciplines (eg, oncology, cardiology) has also fueled demand. Regional and community hospitals may be particularly disadvantaged when it comes to access to genetic counseling services and thus could be the earliest beneficiaries of models that incorporate remote counseling and testing options. In practice, commercial telephone counseling and telegenetics programs are already operational; thus, our research validates that newer models can effectively be adopted when properly designed. Finally, increased use of MGP testing may also be a result of patient preferences for larger and more comprehensive testing via MGP.24
This study had several limitations. The groups compared here (targeted testing v MGP testing) were not a result of random assignment. However, after accounting for nonrandom differences between the targeted and MGP testing groups, short-term postdisclosure PROs were generally similar, suggesting that the benefits of counseling rest in the live conversation between the counselor and patient and not in their proximity. Whether the results seen here will be sustained over time remains uncertain, and longitudinal data are needed. Importantly, the impact of MGP tests on PROs conducted without professional counseling and using different models of counseling could differ from our findings. Time and resource limitations have undermined the traditional rigorous pre- and post-test counseling model used in COGENT; for example, Katz et al25 found that 47% of patients did not receive counseling. In addition, few COGENT participants were tested to guide a breast cancer treatment decision, and a recent population-based study found that MGP testing was associated with lower rates of testing among patients with breast cancer who had preoperative testing.18 Finally, it also possible that patients choosing an MGP test versus targeted testing do so with family and family cancer risks in mind, so there may be additional information on testing selection gained from studying patterns of communication of results with relatives.
In summary, the world of clinical genetic testing is changing rapidly, and use of MGP testing has increased over time. Post-test knowledge, general anxiety, and depression were no worse and state anxiety was lower among patients who received MGP testing compared with those who received targeted testing. Longitudinal data, including uptake of screening and risk reducing behaviors, will further inform the clinical risks and benefits of clinical use of MGP testing.
Footnotes
Supported by National Cancer Institute Grant No. R01 CA160847.
AUTHOR CONTRIBUTIONS
Conception and design: Michael J. Hall, Linda J. Patrick-Miller, Brian L. Egleston, Angela R. Bradbury
Provision of study material or patients: Michael J. Hall, Susan M. Domchek, Mary B. Daly, Olufunmilayo I. Olopade, Pamela Ganschow, Generosa Grana, Angela Bradbury
Collection and assembly of data: All authors
Data analysis and interpretation: Michael J. Hall, Brian L. Egleston, Susan M. Domchek, Mary B. Daly, Pamela Ganschow, Generosa Grana, Dominique Fetzer, Angela R. Bradbury
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Michael J. Hall
Patents, Royalties, Other Intellectual Property: I share a patent with several Fox Chase investigators for a novel method to investigate hereditary colorectal cancer genes (Inst)
Travel, Accommodations, Expenses: Foundation Medicine
Other Relationship: Myriad Genetics, Foundation Medicine, Invitae, Caris Life Sciences
Linda J. Patrick-Miller
No relationship to disclose
Brian L. Egleston
No relationship to disclose
Susan M. Domchek
Honoraria: AstraZeneca, Clovis Oncology, Bristol-Myers Squibb
Research Funding: AstraZeneca (Inst), Clovis Oncology (Inst), PharmaMar (Inst)
Mary B. Daly
No relationship to disclose
Pamela Ganschow
No relationship to disclose
Generosa Grana
Honoraria: Novartis, Pfizer
Olufunmilayo I. Olopade
Employment: CancerIQ (I)
Leadership: CancerIQ
Stock and Other Ownership Interests: CancerIQ, Tempus
Research Funding: Novartis (Inst)
Other Relationship: Tempus, Color Genomics, Genentech, Myriad Genetics, BIO Ventures for Global Health
Dominique Fetzer
No relationship to disclose
Amanda Brandt
No relationship to disclose
Rachelle Chambers
No relationship to disclose
Dana F. Clark
No relationship to disclose
Andrea Forman
Consulting or Advisory Role: Invitae, AstraZeneca, Ambry
Speakers' Bureau: AstraZeneca, Ambry Genetics, Invitae
Rikki Gaber
Honoraria: CancerIQ
Cassandra Gulden
No relationship to disclose
Janice Horte
No relationship to disclose
Jessica M. Long
Employment: DePuy Companies (I)
Stock and Other Ownership Interests: DePuy Companies (I)
Travel, Accommodations, Expenses: DePuy Companies (I)
Terra Lucas
No relationship to disclose
Shreshtha Madaan
Stock and Other Ownership Interests: Counsyl
Kristin Mattie
No relationship to disclose
Danielle McKenna
No relationship to disclose
Susan Montgomery
No relationship to disclose
Sarah Nielsen
No relationship to disclose
Jacquelyn Powers
Employment: Carevive Systems
Honoraria: CureConnect
Consulting or Advisory Role: Carevive Systems
Travel, Accommodations, Expenses: Hospital of the University of Pennsylvania
Kim Rainey
No relationship to disclose
Christina Rybak
No relationship to disclose
Michelle Savage
No relationship to disclose
Christina Seelaus
No relationship to disclose
Jessica Stoll
No relationship to disclose
Jill E. Stopfer
No relationship to disclose
Xinxin (Shirley) Yao
Employment: BioReference Laboratories/GeneDx
Angela R. Bradbury
No relationship to disclose
REFERENCES
- 1.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines for Breast Cancer Screening and Diagnosis. . https://www.nccn.org/professionals/physician_gls/recently_updated.asp
- 2.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines for Colorectal Cancer Screening and Diagnosis. . https://www.nccn.org/professionals/physician_gls/recently_updated.asp
- 3.Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 2014;32:618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinney AY, Butler KM, Schwartz MD, et al. Expanding access to BRCA1/2 genetic counseling with telephone delivery: A cluster randomized trial. J Natl Cancer Inst. 2014;106:dju328. doi: 10.1093/jnci/dju328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinney AY, Steffen LE, Brumbach BH, et al. Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counseling: 1-Year follow-up. J Clin Oncol. 2016;34:2914–2924. doi: 10.1200/JCO.2015.65.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradbury AR, Patrick-Miller LJ, Egleston BL, et al. Randomized noninferiority trial of telephone vs in-person disclosure of germline cancer genetic test results. J Natl Cancer Inst. 2018;110:985–993. doi: 10.1093/jnci/djy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradbury AR, Patrick-Miller LJ, Egleston BL, et al. Patient feedback and early outcome data with a novel tiered-binned model for multiplex breast cancer susceptibility testing. Genet Med. 2016;18:25–33. doi: 10.1038/gim.2015.19. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury AR, Patrick-Miller L, Long J, et al. Development of a tiered and binned genetic counseling model for informed consent in the era of multiplex testing for cancer susceptibility. Genet Med. 2015;17:485–492. doi: 10.1038/gim.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrick-Miller LJ, Egleston BL, Fetzer D, et al. Development of a communication protocol for telephone disclosure of genetic test results for cancer predisposition. JMIR Res Protoc. 2014;3:e49. doi: 10.2196/resprot.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly K, Leventhal H, Marvin M, et al. Cancer genetics knowledge and beliefs and receipt of results in Ashkenazi Jewish individuals receiving counseling for BRCA1/2 mutations. Cancer Contr. 2004;11:236–244. doi: 10.1177/107327480401100405. [DOI] [PubMed] [Google Scholar]
- 11.Speilberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 12.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: A measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Cella D, Hughes C, Peterman A, et al. A brief assessment of concerns associated with genetic testing for cancer: The Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002;21:564–572. [PubMed] [Google Scholar]
- 15.Pieterse AH, van Dulmen AM, Beemer FA, et al. Cancer genetic counseling: Communication and counselees’ post-visit satisfaction, cognitions, anxiety, and needs fulfillment. J Genet Couns. 2007;16:85–96. doi: 10.1007/s10897-006-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrick-Miller L, Egleston BL, Daly M, et al. Implementation and outcomes of telephone disclosure of clinical BRCA1/2 test results. Patient Educ Couns. 2013;93:413–419. doi: 10.1016/j.pec.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepkowski JM, Raghunathan TE, Solenberger P, et al. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 18.Kurian AW, Ward KC, Hamilton AS, et al. Uptake, results, and outcomes of germline multiple-gene sequencing after diagnosis of breast cancer. JAMA Oncol. 2018;4:1066–1072. doi: 10.1001/jamaoncol.2018.0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumish HS, Steinfeld H, Koval C, et al. Impact of panel gene testing for hereditary breast and ovarian cancer on patients. J Genet Couns. 2017;26:1116–1129. doi: 10.1007/s10897-017-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newson AJ, Leonard SJ, Hall A, et al. Known unknowns: Building an ethics of uncertainty into genomic medicine. BMC Med Genomics. 2016;9:57. doi: 10.1186/s12920-016-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biesecker BB, Klein W, Lewis KL, et al. How do research participants perceive “uncertainty” in genome sequencing? Genet Med. 2014;16:977–980. doi: 10.1038/gim.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han PK, Klein WM, Arora NK. Varieties of uncertainty in health care: A conceptual taxonomy. Med Decis Making. 2011;31:828–838. doi: 10.1177/0272989X11393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooker GW, Clemens KR, Quillin J, et al. Cancer genetic counseling and testing in an era of rapid change. J Genet Couns. 2017;26:1244–1253. doi: 10.1007/s10897-017-0099-2. [DOI] [PubMed] [Google Scholar]
- 24.Courtney E, Li ST, Shaw T, et al. Predictors of next-generation sequencing panel selection using a shared decision-making approach. NPJ Genom Med. 2018;3:11. doi: 10.1038/s41525-018-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36:1218–1224. doi: 10.1200/JCO.2017.76.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]