Abstract
Hypoglycemia in the first hours to days after birth remains one of the most common conditions facing practitioners across Canada who care for newborns. Many cases represent normal physiologic transition to extrauterine life, but another group experiences hypoglycemia of longer duration. This statement addresses key issues for providers of neonatal care, including the definition of hypoglycemia, risk factors, screening protocols, blood glucose levels requiring intervention, and managing care for this condition. Screening, monitoring, and intervention protocols have been revised to better identify, manage, and treat infants who are at risk for persistent, recurrent, or severe hypoglycemia. The role of dextrose gels in raising glucose levels or preventing more persistent hypoglycemia, and precautions to reduce risk for recurrence after leaving hospital, are also addressed. This statement differentiates between approaches to care for hypoglycemia during the ‘transitional’ phase—the first 72 hours post-birth—and persistent hypoglycemia, which occurs or presents for the first time past that point.
Keywords: Dextrose gel, Hypoglycemia, Newborn, Point-of-care
This statement updates a previous Canadian Paediatric Society document from 2004. Hypoglycemia in the first hours to days after birth remains one of the most common conditions facing practitioners across Canada who care for newborns, and despite the passage of many years since the last statement was published, essential questions about neonatal hypoglycemia remain largely the same (1). The main evolution in guidance is that unlike previous versions of this statement, transitional hypoglycemia within the first 72 hours post-birth is now explicitly defined as a blood glucose level <2.6 mmol/L. This change helps to differentiate ‘normal’, transition-related hypoglycemia from persistent hypoglycemia, which is defined by blood glucose levels <3.3 mmol/L beyond the first 72 hours post-birth.
An algorithm is included to direct the management and care of infants at risk for neonatal hypoglycemia in the first 72 hours post-delivery (Supplementary Figure 1).
SEARCH STRATEGY
A MEDLINE search was performed for studies up to March 2017, using the keywords ‘Hypoglycemia’, ‘Blood Glucose’, and ‘All Infant: birth to 23 months’, limited to ‘Human’, ‘English’, and ‘French’, and including all trials, reviews, clinical practice guidelines, follow-up studies and meta-analyses. The Cochrane Database was searched for reviews and articles relating to glucose and infant feeding. No randomized clinical trials related to strategies for screening for neonatal hypoglycemia in at-risk infants were identified. All case-control and cohort studies were reviewed. Levels of evidence and grades of recommendations were assigned in accordance with the Oxford Centre for Evidence-Based Medicine guideline (2).
DEFINING HYPOGLYCEMIA
It remains difficult to define neonatal hypoglycemia using a single glucose value (3). In the first 48 to 72 hours post-birth, infants may develop signs of hypoglycemia, with blood glucose at levels that are substantially lower than normal adult levels. In adults or older children, Whipple’s triad (signs and symptoms of hypoglycemia, low serum glucose level, and the resolution of signs and symptoms with the provision of glucose) can be used, but this is often impractical in the neonate yet the principles should be adhered to if possible. Clinical signs of newborn hypoglycemia in approximate order of frequency are: jitteriness or tremors, cyanotic episodes, convulsions, intermittent apneic spells or tachypnea, weak or high-pitched crying, limpness or lethargy, difficulties feeding, and eye-rolling. Sweating, sudden pallor, hypothermia, and cardiac arrest and failure may also occur (Table 1). Because other conditions (e.g., meningitis or seizures unrelated to hypoglycemia) can share these clinical manifestations, it is critical to document hypoglycemia and confirm whether signs disappear with the administration of sufficient glucose to raise the blood sugar to normal levels (4).
Table 1.
Infants at risk for hypoglycemia
| Weight <10th percentile (SGA) |
| Intrauterine growth restriction (IUGR) |
| Weight >90th percentile (LGA) |
| Infants of diabetic mothers (IDMs) |
| Preterm infants <37 weeks GA |
| Maternal labetalol use |
| Late preterm exposure to antenatal steroids |
| Perinatal asphyxia |
| Metabolic conditions (e.g., CPT-1 deficiency, particularly in Inuit infants) |
| Syndromes associated with hypoglycemia (e.g., Beckwith- Wiedemann) |
CPT-1 Carnitine palmitoyltransferase 1; GA Gestational age; LGA Large for gestational age; SGA Small for gestational age.
So-called ‘normal ranges’, whether they are being used for diagnostic or therapeutic purposes, also depend on an infant’s size, gestational age, previous history of hypoglycemia, and current clinical condition, as well as on the availability of energy sources and ongoing energy demands. Definitions of hypoglycemia should be flexible enough to encompass all of these factors.
There are four approaches to defining a safe range for blood glucose, all with limitations (5,6).
Using clinical manifestations
Neonates may develop clinical signs suggestive of hypoglycemia but, as outlined above, these can be difficult to recognize or differentiate from other conditions.
Using normative ranges
Studies of exclusively breastfed, appropriate-for-gestational-age (AGA), term infants have shown that blood glucose levels fall immediately after birth, from two-thirds of maternal levels to as low as 1.8 mmol/L at 1 hour of age (Level 2b) (7,8). They subsequently rise to levels >2.0 mmol/L, which are generally maintained for 72 hours (8). Some 12% to 14% of well, AGA, breastfed newborns have a blood glucose level of <2.6 mmol/L in the first 72 hours after birth (9). Past this point, they generally maintain a glucose level >3.3 mmol/L (6). Preterm infants may take longer to reach this threshold.
Using the presence or absence of acute normal physiological, metabolic, and endocrine changes
There are normal physiological responses to hypoglycemia, such as a rise in ketones, growth hormone, cortisol, and catecholamines, and the suppression of insulin (10,11).
Using the presence or absence of sequelae
A number of studies of at-risk term, preterm, and small-for-gestational-age (SGA) infants (weight <10th percentile) have associated blood glucose levels of <2.6 mmol/L with abnormal short- (Level 4) and long-term (Level 2a) neurological or neuroimaging changes (Level 4) (12–16). Other studies have linked long-term sequelae with even lower glucose level within the first 72 hours post-birth (17). Still others have shown no harm from transient hypoglycemia (18,19) but rather, an increased risk for long-term sequelae with recurrent episodes of hypoglycemia (16).
Cohort and case-control studies are not able to demonstrate causation between low blood glucose and adverse outcomes in newborns for three reasons: ‘normal’ blood glucose levels have a wide range, the presence of comorbidities with hypoglycemia can be significant, and low blood glucose can have multiple causes. However, even with these definitional limits and a scarcity of studies beyond the transitional period, current evidence suggests that the therapeutic goal for glucose levels in infants with persistent hypoglycemia should be ≥3.3 mmol/L after 72 hours post-birth (6,20,21).
HOW SHOULD SCREENING FOR NEONATAL HYPOGLYCEMIA BE PERFORMED?
Conventionally, and for reasons of convenience, blood glucose is usually measured using chemical strips or portable, bedside glucose meters rather than formal laboratory analysis. However, many ‘point-of-care’ methods are unreliable at lower glucose levels and prone to sample or user error (22,23) (Level 3b). Also, variations between capillary and venous blood (24), blood and plasma, and immediate and stored samples, may confound results (Level 3b). In particular, delays in processing may result in lower measurable glucose levels. Capillary and venous whole blood and plasma glucose levels (Level 3b) can vary within a 10% range, with whole blood readings being lower than plasma readings (25). While acute management can be initiated based on point-of-care samples to prevent delay, a diagnosis of persistent hypoglycemia should be confirmed by laboratory assays. More accurate bedside technologies are being developed and will likely improve the quality and ease of screening in future.
Continuous glucose monitors (CGMs) have looked promising and may prove to be beneficial for monitoring neonates. However, current barriers to this technology include inaccuracy at lower glucose levels, delay in obtaining results, need for frequent recalibration, a limited surface area for sensor placement on small neonates, and a lack of treatment protocols (26,27). The accuracy of CGMs when measuring hypoglycemia in neonates remains in question (26).
RISK FACTORS FOR HYPOGLYCEMIA
Impairment of gluconeogenesis (27) is the most common cause of hypoglycemia in infants (28). Specific etiologies include excess insulin production, altered counter-regulatory hormone production, or inadequate substrate supply. Risk factors are listed in Table 1. Classically, these states can occur transiently in SGA infants (Table 2) (29), large-for-gestational-age (LGA) infants (weight >90th percentile), infants of diabetic mothers (IDMs), and preterm infants (Level 3/4) (30–33).
Table 2.
10th and 90th %ile cut-offs for birthweight at term in Canadian infants
| Gestation (completed weeks) | Birth weight (g) | |||
|---|---|---|---|---|
| 10th %ile | 90th %ile | |||
| Male | Female | Male | Female | |
| 37 | 2,552 | 2,452 | 3,665 | 3,543 |
| 38 | 2,766 | 2,658 | 3,877 | 3,738 |
| 39 | 2,942 | 2,825 | 4,049 | 3,895 |
| 40 | 3,079 | 2,955 | 4,200 | 4,034 |
| 41 | 3,179 | 3,051 | 4,328 | 4,154 |
| 42 | 3,233 | 3,114 | 4,433 | 4,251 |
Adapted with permission from reference (29).
Questions exist as to whether nonsyndromic LGA non-IDM infants are truly at risk for hypoglycemia, but infants with conditions such as Beckwith-Wiedemann syndrome need to be followed closely (34). A number of additional maternal and fetal factors, including maternal labetalol use or late preterm administration of antenatal steroids (35,36) as well as intrauterine growth restriction (IUGR) and perinatal asphyxia, can also cause hypoglycemia. More rarely, metabolic and endocrine disorders may lead to persistent neonatal hypoglycemia (28), but their disease-specific investigations and management (37) are beyond the scope of this statement. Thresholds for treating hypoglycemia may differ depending on etiology. While <2.6 mmol/L has been generally adopted as the threshold for treatment in otherwise healthy infants in the transitional period, those with documented hyperinsulinism may require a target threshold of ≥3.3 mmol/L, due to low energy resources levels in the hypoketotic state (38).
SCREENING AT-RISK INFANTS
No study evaluating optimal timing and intervals for glucose screening has been identified. Furthermore, there is insufficient evidence to support routine screening for asymptomatic infants with no risk factors for hypoglycemia.
Lack of evidence for adverse effects of glucose levels between 1.8 mmol/L and 2.5 mmol/L in asymptomatic infants at several hours of age suggests that a staged approach to screening and intervention is reasonable. Because feeding raises blood glucose (39) and stimulates ketosis (40), it is also reasonable to continue feeding at-risk infants at regular intervals, while screening before feeds.
One early study (33) showed that IDM (and, by inference, LGA) infants were the most likely to develop hypoglycemia in the first few hours post-birth. Therefore, screening is not required for this population after 12 hours of age if blood glucose levels remain ≥2.6 mmol/L. SGA and preterm infants may become hypoglycemic as late as the second day, although a decline in blood glucose levels may be prevented by establishing peroral intake. If there are no feeding concerns and the infant is well, screening may be discontinued at 24 hours of age (Level 2b). It is reasonable to screen once or twice on day 2 when there has been more than one glucose reading <2.6 mmol/L in the first 24 hours, to ensure levels remain at or above this level. An approach to testing frequency in at-risk infants is shown in Supplementary Figure 1.
Symptomatic infants should have a blood glucose assessment without delay. Parents should be aware of the reasons for regular blood testing and the symptoms that health care providers may be watching for, so that they can help with monitoring.
BLOOD GLUCOSE LEVELS REQUIRING INTERVENTION
In the first 72 hours post-birth
Symptomatic hypoglycemia
Because symptomatic hypoglycemia causes neuronal injury (41), urgent intervention to maintain glucose levels ≥2.6 mmol/L in unwell at-risk infants is recommended.
Asymptomatic hypoglycemia
Population data have suggested that blood glucose levels as low as 2.0 mmol/L (or even 1.8 mmol/L at 1 hour of age) are not uncommon in healthy newborns. For at-risk infants, however, outcome data support raising the intervention threshold. Lucas et al. (42) suggested that glucose levels of <2.6 mmol/L found from 3 to 30 days of life in preterm infants may have adverse long-term effects (Level 2b). Another study (43) assessed neurodevelopmental outcomes in a cohort of 85 SGA preterm infants in relation to episodes of hypoglycemia (defined as <2.6 mmol/L) (Level 2b). Compared with non-hypoglycemic control subjects, six or more episodes of hypoglycemia were associated with lower head circumference at 12 and 18 months as well as at 5 years of age. Furthermore, psychometric testing using the McCarthy’s test at 3.5 years of age demonstrated impairments compared with controls who did not experience neonatal hypoglycemia. It must be noted that 58% of this cohort had severe hypoglycemia, with blood glucose levels of 0.6 mmol/L to 1.6 mmol/L. It is uncertain whether these results would be similar for a cohort of more aggressively managed infants. Stenninger et al. (44) followed up 28 IDMs (13 with hypoglycemia <1.5 mmol/L and 15 without) at 8 years of age and matched, healthy control subjects. Using the Griffiths developmental and Movement-ABC tests, the authors discovered evidence of minimal neurological dysfunction whether the IDMs had been hypoglycemic or not (Level 2b). It is worth noting that most of the hypoglycemic infants were asymptomatic.
Williams (45) supports the cut-off of <2.6 mmol/L in at-risk infants at 4 to 6 hours of age. Cornblath et al. (6) proposed the concept of operational thresholds, the range of blood glucose concentrations at which clinicians should consider intervention. They distinguished between the threshold glucose value that requires action (2.0 mmol/L) and the target glucose level that interventions aim for (≥2.6 mmol/L) (Level 5). Recently, the 4.5-year follow-up study of a large cohort (15) indicated increased risk in some measures of neurological impairment, such as executive functioning and visual motor function, with recurrent (three or more episodes <2.6 mmol/L) or severe (<2.0 mmol/L) hypoglycemia.
In at-risk infants, blood glucose levels <2.6 mmol/L, particularly when persistent, are associated with adverse outcomes. More randomized clinical trials to compare interventions, intervention thresholds, and long-term outcomes are needed.
Recommendations for the management of hypoglycemia are outlined in Supplementary Figure 1.
Beyond 72 hours post-birth
Previous guidelines suggested raising threshold glucose levels and treatment goal levels for infants after the transitional period, defined as the first 48 hours and, more recently, as 3 days post-birth (19,20). Much of the evidence to recommend higher target levels is based on follow-up studies of children with hyperinsulinism, a major cause of persistent hypoglycemia. They are known to be at increased risk for neurological sequelae due to depleted alternate energy stores providing ketones to the brain. Another rationale has been the development of neuroglycopenic and neuroendocrine responses in adults and older children at glucose levels as low as 2.7 mmol/L or 2.8 mmol/L (46,47).
While glucose values may increase slightly with age, normal glucose ranges do not differ much between children and adults. For example, one study showed that the mean glucose in children after a prolonged 24-hour fast was 3.6 mmol/L (range: 2.7 to 4.5 mmol/L) in children 1 to 12 months old, 3.3 mmol/L (range: 2.8 to 3.8 mmol/L) in children 1 to 7 years old, and 3.8 mmol/L (range: 3.0 to 4.3) in children 7 to 15 years old (48,49).
While no single glucose value defining hypoglycemia can fully ensure patient safety or limit morbidity, or completely prevent over-investigation or over-treatment past the transitional period, a laboratory-confirmed value of <3.3 mmol/L effectively indicates persistent hypoglycemia that warrants further assessment (Level 2b). Infants requiring ongoing treatment for persistent hypoglycemia should have therapeutic targets of ≥3.3 mmol/L, which is well above the cut-off for neurogenic and neuroglycopenic symptoms. A critical sample to aid diagnosis for persistent hypoglycemia at ≥72 hours should be collected whenever a point-of-care glucose is <2.8 mmol/L. The distinction between the two values, one for investigation and the other for therapeutic targeting, reflects the need to distinguish normal or expected biochemical physiological responses from underlying etiology. At glucose levels <2.8 mmol/L, care providers would expect to see suppressed insulin, elevated ketones, and increased counter-regulatory hormones, such as growth hormone and cortisol. A further consideration is the discrepancy between point-of-care and laboratory-tested glucose values, which can vary as much as 20% (50–52). Due to the blood volume required for critical sampling, it is prudent to restrict collection to situations in which a point-of-care reading of <2.8 mmol/L has been obtained. This precaution aids interpretation of the critical sample results, particularly if the laboratory glucose is ≥3.3 mmol/L. Thresholds and timing for defining hypoglycemia using a critical sample are provided in Table 3.
Table 3.
Thresholds for investigation and treatment of hypoglycaemia
| Birth to 72 hours of age | ≥72 hours of age | |
|---|---|---|
| Therapeutic goal | 2.6 | 3.3 |
| Investigation threshold | 2.6 | 2.8* |
*A critical sample should be sent to help determine etiology and confirm lab glucose level. It may be drawn earlier than the time frame suggested if an endocrine or metabolic condition is suspected.
Neonates being monitored for persistent hypoglycemia should have a 5 to 6 hour fast before being discharged from hospital to ensure their safety at home if time between feeds is prolonged. Maintenance of glucose levels ≥3.3 mmol/L at 4 and 5 hours postfeed should be documented before discharge is considered. Also before discharge, an underlying diagnosis for neonatal hypoglycemia should be ascertained and specific medical management (e.g., with diazoxide in hyperinsulinism) initiated. In preparation for care at home, parents should be counselled regarding frequency of feeding, home blood glucose monitoring, medication delivery (if needed), or other treatment measures for hypoglycemia.
Current CPS guidance aligns with recent Pediatric Endocrine Society (PES) recommendations (20). While the PES statement used a cut-off of 48 hours rather than 72 hours, the transition point likely lies somewhere between these periods. Concerns of practitioners over increasing admission rates for hypoglycemia and uncertainty around the 48-hour cut-off informed consensus that a 72-hour cut-off balanced safety considerations with impacts on families and care providers. Most importantly, distinguishing between care during the transition period and beyond allows a higher, more practical index of suspicion for initiating the investigations and interventions required for hypoglycemia that persists below target levels.
INTERVENTIONS FOR NEONATAL HYPOGLYCEMIA
There are two essential approaches to treating hypoglycemia in the newborn. The first supports increasing energy intake, orally or intravenously, while the second supports the mobilization of energy stores using counter-regulatory hormones, such as glucagon or corticosteroids (53,54). The urgency and process of intervention depend on clinical signs and severity. Conjunctive treatments are specific to etiology, such as administering diazoxide or octreotide for hyperinsulinemia or recombinant growth hormone for growth hormone deficiency.
Asymptomatic hypoglycemia
Common clinical practices to prevent or treat asymptomatic hypoglycemia include increasing breastfeeding frequency, supplementing feeds with breast milk or a breast milk substitute, applying intrabuccal dextrose gel, or administering IV glucose (55,56). Clinical trials have not yet demonstrated greater benefit of one form of supplementation over another (or indeed, over breastfeeding on demand (57) for long-term outcome. Frequent (on demand) breastfeeding should be encouraged for at-risk infants, and, if they are being formula-fed or supplemented, the volume of enteral intake should be adjusted based on an infant’s size, chronological age, and gestational age (58). To raise blood glucose levels, slow feeding with breast milk or formula using a pump rather than bolus feeding may be considered, particularly when IV access is difficult. Also, delaying the first bath has been found to reduce incidence of hypoglycemia and may be considered for at-risk infants (59).
There is some evidence that increasing carbohydrate intake prevents low blood glucose levels from developing in healthy term breastfed infants. Randomized clinical trials in SGA (60) and AGA (61) infants found that augmented glucose formulas raise blood glucose and prevent hypoglycemia (Level 1b).
When feeding interventions are administered, blood glucose levels should always be rechecked 30 minutes later to ensure response.
If increasing enteral carbohydrate intake is not effective, the next intervention would traditionally have been to administer IV glucose. More recently, however, dextrose gels have been gaining traction in the management of hypoglycemia (see below). However, in keeping with studies that have measured glucose flux in newborns, when the IV route is chosen the initial glucose infusion should be 80 mL/kg/day of 10% dextrose, providing 5.5 mg/kg/min of glucose (62–65) (Level 3b). Infants with very low glucose levels, particularly those with levels <1.8 mmol/L, should be treated expediently with IV therapy, although provision of glucose gel while trying to establish access is a reasonable approach. A response to IV glucose should occur within 30 minutes, and should be confirmed in a timely manner (66). A single bolus of 2 mL/kg of 10% dextrose at the start of an infusion can achieve steady-state levels more rapidly, but the benefits of this practice for asymptomatic infants are unclear (Level 4). The short duration of action for glucose suggests that after one or two boluses of dextrose, the rate or concentration of dextrose infusion should be increased.
Symptomatic hypoglycemia
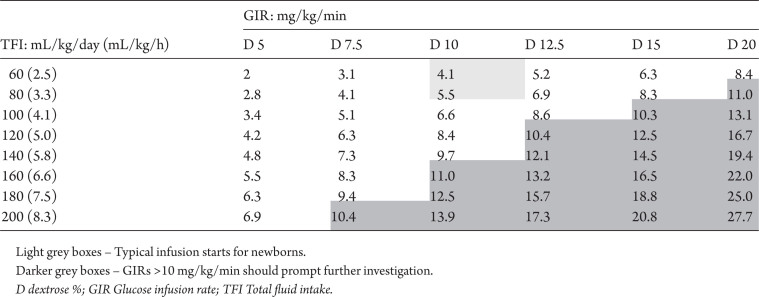
There is both observational evidence and clinical consensus that unwell hypoglycemic infants, particularly those with neurological signs, should be treated immediately with an IV infusion of glucose. Response to IV glucose should be rechecked after 30 minutes. Failure to respond to this first intervention requires a stepwise increase in glucose supply, with a review of levels 30 minutes after each increment. The effect of changes in the glucose infusion rate, either from increasing the total fluid intake and/or the concentration of dextrose, are shown in Table 4. Online calculators can help the practitioner to determine the effects of dextrose concentration or infusion rates (67).
Table 4.
Effect of fluid adjustments and dextrose concentration on glucose infusion rates
When infusions fail to maintain blood glucose at appropriate levels or an especially high rate (>10 mg/kg/min) of infusion is required, further investigation, specialist referral, and/or pharmacological intervention (e.g., IV glucagon) should be considered (68–72) (Level 4). In most cases, referral to endocrinology and investigation for cause of persistent hypoglycemia should wait until the infant is 72 hours old unless the presence of an endocrine or metabolic condition is also strongly suspected. Administering glucagon by IV bolus (0.1 mg/kg to 0.3 mg/kg) or infusion (10 µg/kg/hour to 30 µg/kg/hour) can raise blood glucose levels and prevent recurrent hypoglycemia in both term and preterm infants. Alternative therapies include hydrocortisone, diazoxide, and octreotide, but data in support of their use for initial management of hypoglycemia are limited. Previous CPS guidance regarding the need to access a central vein for concentrations ≥15% is probably obsolete. Recent evidence supports the integrity of peripheral veins with dextrose concentrations as high as 20%. No difference in rate of IV loss was noted for infants randomized to receive dextrose in more highly concentrated solutions (73).
In the transitional period, breastfeeding can be continued without risk of over-hydration because the volume of colostrum is small. To avoid over-hydration and dilutional hyponatremia in supplemented infants, oral and IV intake should not exceed 100 mL/kg/day. When these levels are utilized, careful monitoring of serum electrolytes is required. Blood glucose levels should be checked frequently until they stabilize, and failure to achieve a target level of >2.6 mmol/L requires re-evaluation and consultation. IV dextrose can be weaned when levels have been stable for 12 hours.
Dextrose gel
Intrabuccal 0.5 mL/kg of 40% dextrose gel is now available for managing neonatal hypoglycemia (Level 1b) (56). This dose provides 200 mg/kg of glucose, equivalent to an IV bolus of 2 mL/kg of D10W solution. Glucose gel should be provided with a breastfeed or measured quantity of expressed breast milk (or donor milk from an approved milk bank) or, if neither of these options is possible, formula. The Sugar Babies trial (74) compared administration of dextrose gel with placebo for treating hypoglycemia. The trial’s primary outcome was to achieve blood glucose levels >2.6 mmol/L after one or two doses. Dextrose gel also reduced frequency of treatment failure compared with placebo (14% versus 24%; relative risk [RR] 0.57, 95% confidence interval [CI] 0.33 to 0.98; P=0.04). Further benefits included reduced admissions to neonatal intensive care units (NICUs) for hypoglycemia (14% versus 25%; RR 0.54, 95% CI 0.31 to 0.93; P=0.03) and reduced need for supplementation with formula (7% versus 10%; P=0.04).
OUTCOMES
The cohort from the Sugar Babies trial and a second trial of 102 infants who received dextrose gel upon admission to NICU for monitoring with amplitude-integrated EEG were evaluated together at 2 years of age (18). For both studies, the goal was to maintain blood glucose ≥2.6 mmol/L. For the Sugar Babies cohort alone, no difference in developmental outcomes was noted between control and dextrose gel groups. A finding of concern, however, was that 66 children (36% of all patients enrolled) were experiencing neurosensory impairment (1 severe, 6 moderate, 59 mild), with similar rates for both groups (dextrose 38% versus placebo 34%; RR 1.11, 95% CI 0.75 to 1.63). Combining the patients from the two trials found no difference in neurosensory impairment (RR 0.95; 95% CI 0.75 to 1.20; P=0.67).
Reasons for the high rate of neurosensory impairment overall are unclear. Whether such outcomes were caused by conditions that often underlie hypoglycemia or by detrimental effects of hypoglycemia itself is not yet known. Supporting the former theory is research showing that both late preterm and IUGR infants have independent risk for neurosensory impairment, even with normoglycemia (75). One interesting finding was that infants whose blood glucose levels tended to be >4.0 mmol/L in the first 48 hours post-birth experienced more serious neurodevelopmental outcomes. While this result was not strong enough to have statistical significance, it raises the question of whether overcorrecting low blood glucose may cause harm.
INVESTIGATING CAUSES OF PERSISTENT HYPOGLYCEMIA
As noted above, infants experiencing hypoglycemia in the first 72 hours post-birth generally do not require investigation unless there is a clinical suspicion of an underlying condition with risk for persistent, recurrent, or severe hypoglycemia. Infants with hypoglycemia that persists beyond 72 hours should be evaluated further. When a blood glucose reading <2.8 mmol/L is obtained beyond the transitional period, a critical sample should be drawn as soon as possible. The workup should include a confirmatory plasma glucose, beta-hydroxybutyrate, bicarbonate, lactate, free fatty acids, insulin, growth hormone, cortisol, carnitine, and acylcarnitine profiling. Further workup should be conducted in collaboration with specialists in endocrinology and inborn errors of metabolism.
SUMMARY
While transient blood glucose levels as low as 1.8 mmol/L may be considered normal in healthy infants in the first few hours post-birth, adverse short- and long-term outcomes may result from levels lower than 2.6 mmol/L in at-risk infants, particularly when hypoglycemia is persistent or symptomatic. A higher threshold for investigation of 2.8 mmol/L, and 3.3 mmol/L as the therapeutic target, are recommended after the transitional period. Screening, monitoring, and intervention protocols have been revised to better identify, manage, and treat infants who are at risk for persistent, recurrent, or severe hypoglycemia.
RECOMMENDATIONS
Routine screening of appropriate-for-gestational-age infants at term is not recommended (Grade C recommendation).
Infants of diabetic (gestational or type 1 or 2) mothers (IDMs), asphyxiated infants, preterm infants (<37 weeks gestational age [GA]) and small-for-gestational-age (SGA) infants (weight <10th %ile) should be routinely screened for neonatal hypoglycemia (Grade C recommendation). Until further data are available, large-for-gestational-age (LGA) infants (weight >90th %ile) should also be considered at risk for neonatal hypoglycemia (Grade D recommendation).
Blood glucose screening of asymptomatic, at-risk infants should be performed at 2 hours of age and every 3 to 6 hours after that, in conjunction with breastfeeding. Testing can be discontinued after 12 hours in LGA infants and IDMs when blood glucose levels remain ≥2.6 mmol/L, and after 24 hours in SGA and preterm infants when feeding has been established and blood glucose levels remain at ≥2.6 mmol/L. Symptomatic and unwell infants require immediate glucose testing (Grade C recommendation).
Blood glucose measurement methods that are quality-controlled, accurate, and reliable within the range of 1 mmol/L to 3 mmol/L should be implemented in all birthing and newborn care centres (Grade D recommendation).
In the first 72 hours post-birth, at-risk infants with one measured glucose reading <2.6 mmol/L (assuming one effective feed) require intervention (Grade C recommendation). Symptomatic infants with a blood glucose reading <2.6 mmol/L should be treated immediately, with concurrent investigation for underlying cause and appropriate management.
To augment caloric intake and before starting intravenous (IV) dextrose, enteral supplementation should be provided for asymptomatic infants with blood glucose levels of 1.8 mmol/L to 2.5 mmol/L. Levels should be rechecked 30 minutes later to identify persistent hypoglycemia (Grade D recommendation).
Both symptomatic, hypoglycemic infants and asymptomatic infants who have failed to respond to enteral supplementation should be treated with IV dextrose solution.
Dextrose gel may be an alternative to IV therapy for asymptomatic infants with a blood glucose <2.6 mmol/L and should be administered in conjunction with enteral supplementation. In symptomatic infants, dextrose gel may be used as a temporizing measure to raise blood glucose while waiting to establish an IV dextrose bolus and infusion (Grade B recommendation).
Infants with hypoglycemia persisting beyond the first 72 hours post-birth should be investigated further when glucose levels remain ≤2.8 mmol/L. A critical sample should be collected.
Infants with persistent hypoglycemia (beyond 72 hours post-birth) should have a therapeutic glucose target of ≥3.3 mmol/L.
Before discharge from hospital, infants with persistent hypoglycemia should have a 5 to 6 hour fast, while maintaining blood glucose levels ≥3.3 mmol/L, to ensure safety at home.
RECOMMENDED RESOURCE FOR PARENTS
CaringforKids. Checking blood glucose in newborn babies. https://www.caringforkids.cps.ca/handouts/blood_glucose_ in_newborn_babies
Supplementary Material
All Canadian Paediatric Society position statements and practice points are reviewed regularly and revised as needed. Consult the Position Statements section of the CPS website www.cps.ca/en/documents for the most current version. Retired statements are removed from the website.
Acknowledgements
The authors wish to acknowledge and thank Drs. Jill Boulton, Kevin Coughlin, and Alfonso Solimano, from the Acute Care of At-Risk Newborns (ACoRN) program, for their collaboration and for sharing the hypoglycemia algorithm. This position statement was reviewed by the Drug Therapy and Hazardous Substances, Community Paediatrics, and Nutrition and Gastroenterology Committees of the Canadian Paediatric Society. The authors also wish to thank these members of the Canadian Pediatric Endocrine Group for their review: Drs. Rose Girgis (University of Alberta), Daniel Metzger (University of British Columbia), John Mitchell (McGill University), and Isabelle Rousseau-Nepton (Université Laval).
CANADIAN PAEDIATRIC SOCIETY FETUS AND NEWBORN COMMITTEE
Members: Heidi Budden MD (Board Representative), Mireille Guillot MD (Resident member), Leonora Hendson MD, Thierry Lacaze-Masmonteil MD, PhD (past Chair), Brigitte Lemyre MD, Michael R. Narvey MD (Chair), Vibhuti Shah MD
Liaisons: Radha Chari MD, The Society of Obstetricians and Gynaecologists of Canada; James Cummings MD, Committee on Fetus and Newborn, American Academy of Pediatrics; William Ehman MD, College of Family Physicians of Canada; Roxanne Laforge RN, Canadian Perinatal Programs Coalition; Chantal Nelson PhD, Public Health Agency of Canada; Eugene H. Ng MD, CPS Neonatal-Perinatal Medicine Section; Doris Sawatzky-Dickson RN, Canadian Association of Neonatal Nurses
Principal authors: Michael R. Narvey MD, Seth D. Marks MD
References
- 1. Aziz K, Dancey P; Canadian Paediatric Society, Fetus and Newborn Committee Screening guidelines for newborns at risk for low blood glucose. Paediatr Child Health 2004;9(10):723–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oxford Centre for Evidence-Based Medicine. Levels of evidence and grades of recommendation https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (Accessed August 19, 2019).
- 3. Cornblath M, Reisner SH. Blood glucose in the neonate and its clinical significance. N Engl J Med 1965;273(7):378–81. [DOI] [PubMed] [Google Scholar]
- 4. Behrman RE, Kliegman R, Jenson HB.. Nelson Textbook of Pediatrics, 16th edn. Philadelphia, PA: WB Saunders, 2000:533–4. [Google Scholar]
- 5. Sinclair JC. Approaches to the definition of neonatal hypoglycemia. Acta Paediatr Jpn 1997;39 (Suppl 1):S17–20. [PubMed] [Google Scholar]
- 6. Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: Suggested operational thresholds. Pediatrics 2000;105(5):1141–5. [DOI] [PubMed] [Google Scholar]
- 7. Hoseth E, Joergensen A, Ebbesen F, Moeller M. Blood glucose levels in a population of healthy, breast fed, term infants of appropriate size for gestational age. Arch Dis Child Fetal Neonatal Ed 2000;83(2):F117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diwakar KK, Sasidhar MV. Plasma glucose levels in term infants who are appropriate size for gestation and exclusively breast fed. Arch Dis Child Fetal Neonatal Ed 2002;87(1):F46–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nicholl R. What is the normal range of blood glucose concentrations in healthy term newborns? Arch Dis Child 2003;88(3):238–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bougneres PF, Lemmel C, Ferré P, Bier DM. Ketone body transport in the human neonate and infant. J Clin Invest 1986;77(1):42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaussain JL, Georges P, Calzada L, Job JC. Glycemic response to 24-hour fast in normal children: III. Influence of age. J Pediatr 1977;91(5):711–4. [DOI] [PubMed] [Google Scholar]
- 12. Koivisto M, Blanco-Sequeiros M, Krause U. Neonatal symptomatic and asymptomatic hypoglycemia: A follow-up study of 151 children. Dev Med Child Neurol 1972;14(5):603–14. [DOI] [PubMed] [Google Scholar]
- 13. Kinnala A, Rikalainen H, Lapinleimu H, Parkkola R, Kormano M, Kero P. Cerebral magnetic resonance imaging and ultrasonography findings after neonatal hypoglycemia. Pediatrics 1999;103(4 Pt 1):724–9. [DOI] [PubMed] [Google Scholar]
- 14. Cornblath M, Schwartz R. Outcome of neonatal hypoglycaemia. Complete data are needed. BMJ 1999;318(7177):194–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McKinlay CJD, Alsweiler JM, Anstice NS, et al. ; Children with Hypoglycemia and their Later Development (CHYLD) Study Team. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr 2017;171(10):972–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kerstjens JM, Bocca-Tjeertes IF, de Winter AF, Reijneveld SA, Bos AF. Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics 2012;130(2):e265–72. [DOI] [PubMed] [Google Scholar]
- 17. Brand PL, Molenaar NL, Kaaijk C, Wierenga WS. Neurodevelopmental outcome of hypoglycaemia in healthy, large for gestational age, term newborns. Arch Dis Child 2005;90(1):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKinlay CJ, Alsweiler JM, Ansell JM, et al. ; CHYLD Study Group. Neonatal glycemia and neurodevelopmental outcomes at 2 years. N Engl J Med 2015;373(16):1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stanley CA, Baker L. The causes of neonatal hypoglycemia. N Engl J Med 1999;340(15):1200–1. [DOI] [PubMed] [Google Scholar]
- 20. Thornton PS, Stanley CA, De Leon DD, et al. ; Pediatric Endocrine Society. Recommendations from the Pediatric Endocrine Society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J Pediatr. 2015;167(2):238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hume R, McGeechan A, Burchell A. Failure to detect preterm infants at risk of hypoglycemia before discharge. J Pediatr 1999;134(4):499–502. [DOI] [PubMed] [Google Scholar]
- 22. Marcus C. How to measure and interpret glucose in neonates. Acta Paediatr 2001;90(9):963–4. [DOI] [PubMed] [Google Scholar]
- 23. Hussain K, Sharief N. The inaccuracy of venous and capillary blood glucose measurement using reagent strips in the newborn period and the effect of haematocrit. Early Hum Dev 2000;57(2):111–21. [DOI] [PubMed] [Google Scholar]
- 24. Kuwa K, Nakayama T, Hoshino T, Tominaga M. Relationships of glucose concentrations in capillary whole blood, venous whole blood and venous plasma. Clin Chim Acta 2001;307(1–2):187–92. [DOI] [PubMed] [Google Scholar]
- 25. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. Validation of the continuous glucose monitoring sensor in preterm infants. Arch Dis Child Fetal Neonatal Ed 2013;98(2):F136–40. [DOI] [PubMed] [Google Scholar]
- 26. McKinlay CJD, Chase JG, Dickson J, Harris DL, Alsweiler JM, Harding JE. Continuous glucose monitoring in neonates: A review. Matern Health Neonatol Perinatol 2017;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalhan S, Parimi P. Gluconeogenesis in the fetus and neonate. Semin Perinatol 2000;24(2):94–106. [DOI] [PubMed] [Google Scholar]
- 28. Cornblath M, Ichord R. Hypoglycemia in the neonate. Semin Perinatol 2000;24(2):136–49. [DOI] [PubMed] [Google Scholar]
- 29. Kramer MS, Platt RW, Wen SW, et al. ; Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 2001;108(2):E35. [DOI] [PubMed] [Google Scholar]
- 30. Lubchenco LO, Bard H. Incidence of hypoglycemia in newborn infants classified by birth weight and gestational age. Pediatrics 1971;47(5):831–8. [PubMed] [Google Scholar]
- 31. Hawdon JM, Ward Platt MP. Metabolic adaptation in small for gestational age infants. Arch Dis Child 1993;68(3 Spec No):262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stenninger E, Schollin J, Aman J. Early postnatal hypoglycemia in newborn infants of diabetic mothers. Acta Paediatr 1997;86(12):1374–6. [DOI] [PubMed] [Google Scholar]
- 33. Holtrop PC. The frequency of hypoglycemia in full-term large and small for gestational age newborns. Am J Perinatol 1993;10(2):150–4. [DOI] [PubMed] [Google Scholar]
- 34. de Rooy L, Hawdon J. Nutritional factors that affect the postnatal metabolic adaptation of full-term small- and large-for-gestational-age infants. Pediatrics 2002;109(3):E42. [DOI] [PubMed] [Google Scholar]
- 35. Bateman BT, Patorno E, Desai RJ, et al. Late pregnancy ß blocker exposure and risks of neonatal hypoglycemia and bradycardia. Pediatrics 2016;138(3).pii:e20160731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. ; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med 2016;374(14):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burton BK. Inborn errors of metabolism in infancy: A guide to diagnosis. Pediatrics 1998;102(6):E69. [DOI] [PubMed] [Google Scholar]
- 38. Hussain K, Blankenstein O, De Lonlay P, Christesen HT. Hyperinsulinaemic hypoglycaemia: Biochemical basis and the importance of maintaining normoglycaemia during management. Arch Dis Child 2007;92(7):568–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beard AG, Panos TC, Marasigan BV, Eminians J, Kennedy HF, Lamb J. Perinatal stress and the premature neonate. II. Effect of fluid and calorie deprivation on blood glucose. J Pediatr 1966;68(3):329–43. [DOI] [PubMed] [Google Scholar]
- 40. Hawdon JM, Ward Platt MP, Aynsley-Green A. Patterns of metabolic adaptation for preterm and term infants in the first neonatal week. Arch Dis Child 1992;67(4 Spec No):357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Auer RN, Siesjö BK. Hypoglycemia: Brain neurochemistry and neuropathology. Baillieres Clin Endocrinol Metab 1993;7(3):611–25. [DOI] [PubMed] [Google Scholar]
- 42. Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ 1988;297(6659):1304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duvanel CB, Fawer CL, Cotting J, Hohlfeld P, Matthieu JM. Long-term effects of neonatal hypoglycemia on brain growth and psychomotor development in small-for-gestational-age preterm infants. J Pediatr 1999;134(4):492–8. [DOI] [PubMed] [Google Scholar]
- 44. Stenninger E, Flink R, Eriksson B, Sahlèn C. Long-term neurological dysfunction and neonatal hypoglycaemia after diabetic pregnancy. Arch Dis Child Fetal Neonatal Ed 1998;79(3):F174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Williams AF. Hypoglycaemia of the newborn: A review. Bull World Health Organ 1997;75(3):261–90. [PMC free article] [PubMed] [Google Scholar]
- 46. Steinkrauss L, Lipman TH, Hendell CD, Gerdes M, Thornton PS, Stanley CA. Effects of hypoglycemia on developmental outcome in children with congenital hyperinsulinism. J Pediatr Nurs 2005;20(2):109–18. [DOI] [PubMed] [Google Scholar]
- 47. Mitrakou A, Ryan C, Veneman T, et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol 1991;260(1 Pt 1):E67-74. [DOI] [PubMed] [Google Scholar]
- 48. Bonnefont JP, Specola NB, Vassault A, et al. The fasting test in paediatrics: Application to the diagnosis of pathological hypo and hyperketotic states. Eur J Pediatr. 1990;150(2):80–5. [DOI] [PubMed] [Google Scholar]
- 49. van Veen MR, van Hasselt PM, de Sain-van der Velden MG, et al. Metabolic profiles in children during fasting. Pediatrics 2011;127(4):e1021–7. [DOI] [PubMed] [Google Scholar]
- 50. Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol 2017;11(3):558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reynolds GJ, Davies S. A clinical audit of cotside blood glucose measurement in the detection of neonatal hypoglycaemia. J Pediatr Child Health 1993;29(4): 289–91. [DOI] [PubMed] [Google Scholar]
- 52. Ho HT, Yeung WK, Young BW. Evaluation of ‘point of care’ devices in the measurement of low blood glucose in neonatal practice. Arch Dis Child Fetal Neonatal Ed 2004;89(4):F356–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mehta A. Prevention and management of neonatal hyopoglycaemia. Arch Dis Child Fetal Neonatal Ed 1994;70(1):F54–9; discussion F59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hawdon JM, Ward Platt MP, Aynsley-Green A. Prevention and management of neonatal hypoglycaemia. Arch Dis Child Fetal Neonatal Ed 1994;70(1):F60–4; discussion F65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cornblath M. Neonatal hypoglycemia. In: Donn SM, Fisher CW, eds. Risk Management Techniques in Perinatal and Neonatal Practice. Armonk, NY: Futura Publishing Co., 1996:437–48. [Google Scholar]
- 56. Weston PJ, Harris DL, Battin M, Brown J, Hegarty JE, Harding JE. Oral dextrose gel for the treatment of hypoglycaemia in newborn infants. Cochrane Database Syst Rev 2016;(5):CD011027. [DOI] [PubMed] [Google Scholar]
- 57. Marchini G, Persson B, Berggren V, Hagenäs L. Hunger behaviour contributes to early nutritional homeostasis. Acta Paediatr 1998;87(6):671–5. [DOI] [PubMed] [Google Scholar]
- 58. Nechyba C, Gunn VL, eds. Harriet Lane Handbook: A Manual for Pediatric House Officers, 16th edn. Philadelphia, PA: Mosby, 2002:385–6. [Google Scholar]
- 59. McInerney CM, Gupta A. Delaying the first bath decreases the incidence of neonatal hypoglycemia. J Obstet Gynecol Neonatal Nurs 2015;44(1):S73–4. [Google Scholar]
- 60. Singhal PK, Singh M, Paul VK, et al. Prevention of hypoglycemia: A controlled evaluation of sugar fortified milk feeding in small-for-gestational age infants. Indian Pediatr 1992;29(11):1365–9. [PubMed] [Google Scholar]
- 61. Singhal PK, Singh M, Paul VK, Malhotra AK, Deorari AK, Ghorpade MD. A controlled study of sugar-fortified milk feeding for prevention of neonatal hypoglycemia. Indian J Med Res 1991;94:342–5. [PubMed] [Google Scholar]
- 62. Kalhan SC, Savin SM, Adam PA. Measurement of glucose turnover in the human newborn with glucose-1-13C. J Clin Endocrinol Metab 1976;43(3):704–7. [DOI] [PubMed] [Google Scholar]
- 63. Bier DM, Leake RD, Haymond MW, et al. Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes 1977;26(11):1016–23. [DOI] [PubMed] [Google Scholar]
- 64. Sunehag A, Ewald U, Larsson A, Gustafsson J. Glucose production rate in extremely immature neonates (< 28 weeks) studied by use of deuterated glucose. Pediatr Res 1993;33(2):97–100. [DOI] [PubMed] [Google Scholar]
- 65. King KC, Tserng KY, Kalhan SC. Regulation of glucose production in newborn infants of diabetic mothers. Pediatr Res 1982;16(8):608–12. [DOI] [PubMed] [Google Scholar]
- 66. Lilien LD, Pildes RS, Srinivasan G, Voora S, Yeh TF. Treatment of neonatal hypoglycemia with minibolus and intravenous glucose infusion. J Pediatr 1980;97(2):295–8. [DOI] [PubMed] [Google Scholar]
- 67. NICU Tools. Glucose Delivery Calculator http://nicutools.org/ (Accessed August 19, 2019).
- 68. Wu PY, Modanlou H, Karelitz M. Effect of glucagon on blood glucose homeostasis in infants of diabetic mothers. Acta Paediatr Scand 1975;64(3):441–5. [DOI] [PubMed] [Google Scholar]
- 69. Carter PE, Lloyd DJ, Duffty P. Glucagon for hypoglycaemia in infants small for gestational age. Arch Dis Child 1988;63(10):1264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hawdon JM, Aynsley-Green A, Ward Platt MP. Neonatal blood glucose concentrations: Metabolic effects of intravenous glucagon and intragastric medium chain triglyceride. Arch Dis Child 1993;68(3 Spec No):255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Charsha DS, McKinley PS, Whitfield JM. Glucagon infusion for treatment of hypoglycemia: Efficacy and safety in sick, preterm infants. Pediatrics 2003;111(1):220–1. [DOI] [PubMed] [Google Scholar]
- 72. Miralles RE, Lodha A, Perlman M, Moore AM. Experience with intravenous glucagon infusions as a treatment for resistant neonatal hypoglycemia. Arch Pediatr Adolesc Med 2002;156(10):999–1004. [DOI] [PubMed] [Google Scholar]
- 73. Vanhatalo T, Tammela O. Glucose infusions into peripheral veins in the management of neonatal hypoglycemia—20% instead of 15%? Acta Paediatr 2010;99(3):350–3. [DOI] [PubMed] [Google Scholar]
- 74. Harris DL, Weston PJ, Signal M, Chase JG, Harding JE. Dextrose gel for neonatal hypoglycaemia (the Sugar Babies Study): A randomised, double-blind, placebo-controlled trial. Lancet 201321;382(9910):2077–83. [DOI] [PubMed] [Google Scholar]
- 75. Arcangeli T, Thilaganathan B, Hooper R, Khan KS, Bhide A. Neurodevelopmental delay in small babies at term: A systematic review. Ultrasound Obstet Gynecol 2012;40(3):267–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.