Abstract
Introduction:
Injuries to the peripheral auditory system are among the most common results of high intensity impulsive acoustic exposure. Prior studies of high intensity sound transmission by the ossicular chain have relied upon measurements in animal models, measurements at more moderate sound levels (i.e. < 130 dB SPL), and/or measured responses to steady-state noise. Here, we directly measure intracochlear pressure in human cadaveric temporal bones, with fiber optic pressure sensors placed in scala vestibuli (SV) and tympani (ST), during exposure to shock waves with peak positive pressures between ~7–83 kPa.
Methods:
Eight full-cephalic human cadaver heads were exposed, face-on, to acoustic shock waves in a 45 cm diameter shock tube. Specimens were exposed to impulses with nominal peak overpressures of 7, 28, 55, & 83 kPa (171, 183, 189, & 192 dB pSPL), measured in the free field adjacent to the forehead. Specimens were prepared bilaterally by mastoidectomy and extended facial recess to expose the ossicular chain. Ear canal (EAC), middle ear, and intracochlear sound pressure levels were measured with fiber-optic pressure sensors. Surface-mounted sensors measured SPL and skull strain near the opening of each EAC and at the forehead.
Results:
Measurements on the forehead showed incident peak pressures approximately twice that measured by adjacent free-field and EAC entrance sensors, as expected based on the sensor orientation (normal vs tangential to the shock wave propagation). At 7 kPa, EAC pressure showed gain, calculated from the frequency spectra, consistent with the ear canal resonance, and gain in the intracochlear pressures (normalized to the EAC pressure) were consistent with (though somewhat lower than) previously reported middle ear transfer functions. Responses to higher intensity impulses tended to show lower intracochlear gain relative to EAC, suggesting sound transmission efficiency along the ossicular chain is reduced at high intensities. Tympanic membrane (TM) rupture was observed following nearly every exposure 55 kPa or higher.
Conclusions:
Intracochlear pressures reveal lower middle-ear transfer function magnitudes (i.e. reduced gain relative to the ear canal) for high sound pressure levels, thus revealing lower than expected cochlear exposure based on extrapolation from cochlear pressures measured at more moderate sound levels. These results are consistent with lowered transmissivity of the ossicular chain at high intensities, and are consistent with our prior report measuring middle ear transfer functions in human cadaveric temporal bones with high intensity tone pips.
Keywords: Laser Doppler vibrometry, intracochlear pressure, blast overpressure, acoustic shock wave, stapedial annular ligament
1. INTRODUCTION
Noise induced hearing loss and related diseases are a major concern for the modern military: tinnitus and hearing loss are the two most common disabilities for which the U.S. Department of Veterans Affairs provides compensation (VBA, 2017). A majority of all injuries in veterans returning from Iraq and Afghanistan are a result of exposure to blast from improvised explosive devices (Owens et al., 2008), thus auditory dysfunction, defined in this paper as hearing loss and tinnitus, is more likely to be correlated with a blast-related traumatic brain injury (TBI) than any other type of TBI, affecting upwards of 60% of these patients (Fausti et al., 2005; Lew et al., 2007). Understanding the mechanisms underlying these auditory injuries thus may enable better predictions of injury, and enhanced protection and recovery from blast exposure.
The literature contains many case reports describing human exposure to blast waves (e.g. Bruins et al., 1991; Chandler et al., 1997; Remenschneider et al., 2014; Yetiser et al., 1993), and the effects of blast exposure on hearing outcomes has been evaluated systematically in animals (Cho et al., 2013; Price et al., 1989) and humans (Patterson Jr et al., 1994a; Patterson Jr et al., 1994b). However, fewer studies have investigated the propagation of energy into the inner ear during an exposure directly. Prior studies of high intensity sound transmission by the ossicular chain have relied upon either measurements in animal models (e.g. Guinan et al., 1967; Price, 1974b) or measurements at more moderate sound levels (e.g. < 130 dB SPL: Hato et al., 2003; Nakajima et al., 2009). In particular, the effects of nonlinearities in ossicular chain motion on sound transmission (Greene et al., 2017; Guinan et al., 1967; Price, 1974a) are not well understood, complicating predictions of injury resulting from high level impulse noise (Henderson et al., 1986).
Several tools have been developed to estimate injury and determine safe exposure limits to impulse noise. The recently adopted MIL-STD-1474E (DoD, 2015) military acquisition standard includes two such tools, LIAeq100ms, an “equal energy” model that computes the noise dose equivalent to a 100 ms impulse, and that may be used by the Air Force and Navy, and the Auditory Hazard Assessment Algorithm for Humans (Price et al., 1991) which must be used for Army Materiel acquisition. The AHAAH model is an electroacoustic analog model of the human ear designed to predict injury from impulse noise exposure (Price et al., 1991). The AHAAH model represents an interesting approach to injury prediction, and significant insights have been derived from the model; however, the model was designed using data from studies in animals (particularly cat; Kalb et al., 1987), and several components need to be updated and the results validated (Wightman et al., 2010). In particular, ossicular chain nonlinearities have a substantial effect on AHAAH model results, but the extent and degree of nonlinear effects have not been well characterized in the human ear.
Frequency and level dependent nonlinearities in ossicular chain motion, particularly due to the limiting effect of the stapedial annular ligament (SAL), have been shown to reduce sound transmission into the cochlea during continuous sound exposure in the cat (Price, 1974a). When adapted to the human, this transmission reduction leads to the counterintuitive and contentious prediction of a non-monotonic dose-response function for large caliber weapons in the output of the AHAAH model, above which higher intensity exposures are predicted to cause reduced injury (Price, 2007). In support of such a result, reports have provided evidence in support of a compressive nonlinearity in the ossicular chain motion (Price et al., 2017). Others, however, have suggested that while the displacement limitation is present, the implementation in the AHAAH model is overly-compressive, and that reduction (but not elimination) of the SAL compliance eliminates this behavior (Smoorenburg, 2003; Zagadou et al., 2016). The controversy surrounding the dose-response inversion (in addition to other controversial aspects) of the AHAAH model have been discussed previously (Wightman et al., 2010), and have been the subject of a recent exchange in the literature (Price et al., 2017; Zagadou et al., 2016; Zagadou et al., 2017).
We have previously shown that stapes motion is indeed limited at high sound exposure levels in a cadaveric human preparation, but that this limitation occurs at a higher sound level (by at least 10 dB) than predicted by the AHAAH model, thus supporting the assertion that the model is overly compressive (Greene et al., 2017). That report established, for pure tones, that ossicular chain displacement and the sound pressure level generated in the cochlea may be limited at high levels; however, results indicated substantial frequency dependence in the relationships between incident sound pressure level, stapes displacement, and the generated intracochlear sound pressure level. The AHAAH model is explicitly designed to predict injury in response to impulse noise exposure, which is by definition a broad-band stimulus, thus cross-frequency effects may lead to further unanticipated effects in the sound transmission to the cochlea. Empirical measurements are necessary to fully characterize the relationship between impulse noise exposure and the risk to hearing, and the accuracy of the AHAAH model in predicting this injury. To begin to fill this gap, here we report results of intracochlear pressure measurements in the scala vestibuli (SV) and scala tympani (ST), in human cadaveric temporal bones, during exposure to shock waves with peak positive pressures between ~7 to > 83 kPa.
2. MATERIALS AND METHODS
The use of temporal bone tissue was in compliance with the University of Colorado Anschutz Medical Campus Institutional Biosafety Committee. Eight fresh-frozen whole-head specimens with no history of middle ear disease (except presbycusis) were evaluated. Tissue was obtained from cadavers undergoing autopsy with permission to use tissues and organs for research (Lone Tree Medical Donation, Littleton, CO).
2.1. Temporal bone preparation
Temporal bone preparation procedures were similar to several recent reports from our laboratory (e.g. Deveze et al., 2010; Greene et al., 2017; Greene et al., 2015; Mattingly et al., 2015; Maxwell et al., 2017). Briefly, the temporal bones were prepared bilaterally: The pinna and surrounding skin were reflected during preparation but left intact. Temporal bones were prepared with a canal-wall-up mastoidectomy with an extended facial recess. The facial canal was opened and the facial nerve removed to maximize access and visibility of the middle-ear structures, which were inspected for damage and abnormalities. The ossicular chain was not disturbed (including the stapedius muscle/tendon). The cochlear promontory was thinned near the oval and round windows in preparation for pressure probe insertion.
2.2. Instrumentation
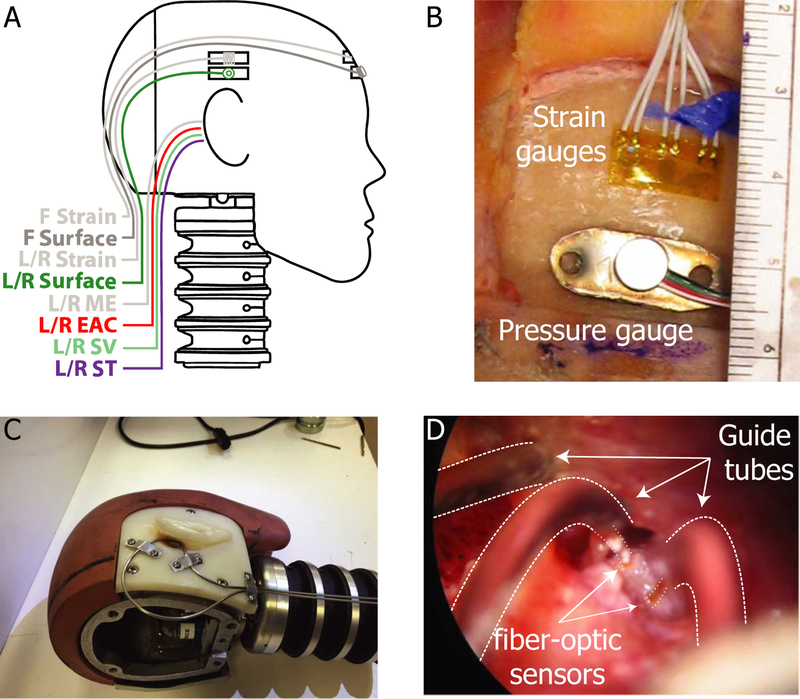
Sensor placement is illustrated in Figure 1A: specimens were outfitted with an array of sensors in the ears bilaterally, as well as on the surface of the skull, and in the air adjacent to the front surface of the head. The exposure level was measured in the free field adjacent to the specimen’s forehead with a “pancake”, or “splitter” style pressure probe (Endevco 8530C), oriented with the sensor surface 90° to the long-axis of the shock tube. Surface sensors were attached ~2–3 cm above the pinna (in line, vertically, with the entrance of the ear canal) outside the area of coverage by a set of ear muffs (note: results of hearing protection use are not discussed in this report, see section 2.4 for additional details), as well as centered on the forehead (on the horizontal circumference of the head determined by the left and right surface sensors, ~2 cm above the brow line). The skin overlying the skull in these locations was reflected. Strain gauges (Omega KFH-3–350-D17–11LM2S) were secured to the skull with cyanoacrylate adhesive, and were covered with the reflected flap of skin which was sutured into place. Pressure gauges (Endevco 8515C-15) were similarly affixed to stainless steel plates, which were likewise secured to the skull with cyanoacrylate adhesive, and the overlying skin removed (Figure 1B). Wires were tightly sutured to the surface of the skin along the circumference of the head. Note, this report presents the results of measurements by the pressure probes only, thus strain gauge responses are outside the scope of this report and are not discussed.
Fig. 1:
Locations and fixation methods for strain gauges and pressure sensors. A) Strain gauges and surface-mount pressure gauges were placed in three locations on the skull (Front/Left/Right). Fiber-optic pressure sensors (FOP-M) were placed into both ear canals (L/R EAC) and both middle ear (L/R ME) cavities. Fiber-optic pressure sensors (FOP-M260) were inserted bilaterally into the scala vestibuli (L/R SV) and scala tympani (L/R ST). B) Strain gauges and surface-mount pressure gauges were fixed to the surface of the skull in three locations with cyanoacrylate adhesive. C) Stainless steel tubing was securely mounted to the skull to guide and protect the fiber-optic pressure sensors (shown on a modified Hybrid III head). D) Fiber optic pressure sensors (bracketed by dotted lines) were inserted into the cochlea via small cochleostomies, sealed into the cochlea with alginate dental impression material, and fixed in place with cyanoacrylate adhesive along the length of the guide tube. Note, strain gauge responses are not presented in this report.
Prior to sensor placement in the ear, pre-test velocity measurements (see next section) were made on both ears. Fiber-optic pressure sensors were then inserted into the ear (into the external auditory canal, PEAC, scala vestibuli, PSV, scala tympani, PST, and middle ear, PME), run through custom-fitted stainless steel guide tubes (demonstrated on a modified Hybrid III male head in Figure 1C). The guide tubes were run under the skin from the base of the skull into the mastoidectomy (through a channel cut in the bone), and fitted such that no deformation was introduced around the pinna (which could interfere with ear muff placement). The end of the guide tube terminated in a hole cut in the bony wall of the ear canal (for PEAC) and < 1 cm from the intended target (PSV & PST; Figure 1D). Fiber-optic sensors (FISO FOP-M-NS-1037A for PEAC & PME, and FOP-M260-NS-1036A for PSV and PST; FISO Inc., Quebec, Quebec, Canada) were then inserted through the guide tubes, inserted underwater (to avoid introduction of air bubbles) into the cochlea via small cochleostomies made with a sharp pick (for PSV and PST), or until the sensor tip could just be seen (~100 μm) extending from the probe tube into the middle ear (PME) or the ear canal (PEAC). The tip of the PEAC probe was fixed in place within ~2 mm of the tympanic membrane (TM), PME was placed ~1 cm posterior to the incus short process in the middle ear cavity. PSV and PST probes were sealed in place in the cochlea with alginate dental impression material (Jeltrate). Once placed, stapes and round window velocity measurements were repeated and compared to the pre-test responses. The reflected skin and pinna were then fixed back into place over the mastoidectomy with heavy suture, and the skin margin re-sealed with cyanoacrylate adhesive. Optic fibers and sensor wires were then bundled together at the back of the head, and affixed to the specimen support structure (shielded by the head and rubber/plastic covers), outside of the shock tube. In this manner, the sensors and the optic fibers were securely fixed in place and protected from damage.
All pressure sensors (both FISO and Endevco) were new (i.e. were not re-used from one specimen to the next), and came calibrated from their respective suppliers. Sensors were not re-calibrated on site, and if a sensor failed or provided faulty readings, was replaced (if possible) before subsequent measurements were collected. All sensors were zeroed to ambient pressure prior to each recording.
Motion of the sensor tips, as well as motion of the fiber optic cable, can introduce noise and artifact to the recorded signal. While it was not possible to eliminate all motion of sensor leads due to the high intensity nature of the stimulus, care was taken to minimize motion of sensor tips and leads. Surface mount gauges were firmly affixed to the skull via stainless steel mounting plates and cyanoacrylate adhesive, and wire leads were tightly sutured to the scalp surface, leading to the nape of the neck. Fiber optic pressure sensors were likewise firmly held in place within the guide tubes by applying cyanoacrylate adhesive to the far end of the guide tubes such that the liquid adhesive wicked into the guide tube via capillary action, while the guide tubes that were rigidly mounted to the skull in at least two places with stainless steel mounting plates and screws. Intracochlear pressure sensors were sealed inside the cochlea with alginate dental impression material. Each specimen was inspected following all recordings, and no evidence of probe motion (cracked or missing alginate material) was observed. Guide tubes from both ears were routed to the nape of the neck, where they were sutured together. All sensor leads ran through a section of flexible, clear PVC tubing, and zip-tied together to form a single cable bundle that ran up the back of the neck (e.g. Figure 1C) to the aluminum mounting bracket, and zip tied along the leeward edge of the mounting bracket (Figure 3C). The bundle was routed to the steel support struts, to which it was firmly attached by covering the length of the exposed cable with black adhesive vinyl tape. The cable bundle then passed into the shock tube suppressor and out to the data acquisition system via a cable pass-through. The cable bundle was allowed to flex behind the neck to allow relatively natural movement of the head in response to the exposure, but was rigidly supported along all other segments to minimize motion and artifact.
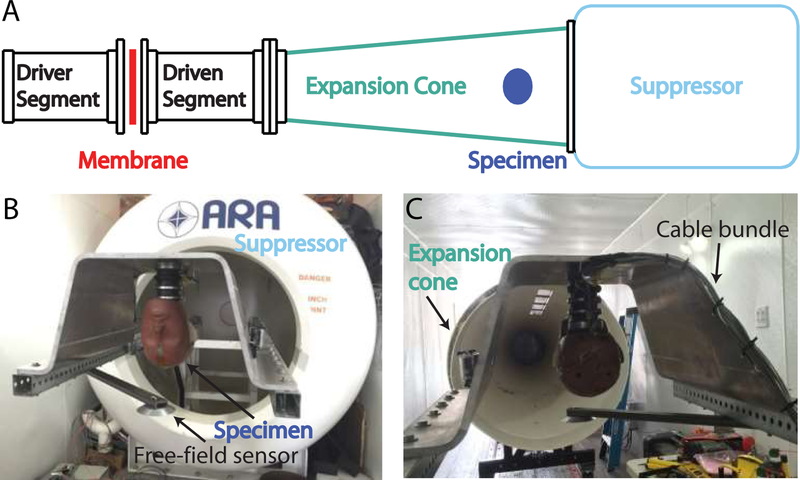
Fig. 3:
Shock waves were generated with a 45 cm diameter shock tube mounted inside a semi-trailer (A). The specimen (a Hybrid III dummy head shown here) was mounted (B) inside the expansion cone (C) of the shock tube, held in place up-side down inside an aluminum mount, affixed to a steel frame. A pancake-style free-field pressure gauge was mounted adjacent to the specimen’s head (slightly forward of the forehead). The bundle of wires and fiber-optic cabling were run up the neck, along the back of the aluminum mount, and attached to the steel supports, to protect the bundle from the shock wave.
2.3. Pre-test velocity measurements
At the beginning of each experiment a number of velocity measurements were made in each ear in order to probe specimen condition. Velocity measurements were conducted as described previously (Greene et al., 2017; Greene et al., 2015; Mattingly et al., 2015): measured using a single-axis laser-Doppler vibrometer (OFV-534 and OFV-5000; Polytec Inc., Irvine, CA, U.S.A.), acoustic stimuli delivered to the ear canal via PVC tubing from a closed-field magnetic loudspeaker (TDT CF1; Tucker-Davis Technologies Inc., Alachua, FL, U.S.A.), and the sound pressure level near the TM recorded with a probe-tube microphone (type 4182; Brüel & Kjær, Nærum, Denmark). Stimulus presentation and data recordings were performed at a sampling rate of 96 kHz, using an external sound card (Hammerfall Multiface II; RME, Haimhausen, Germany) modified to eliminate high-pass filtering on the analog output, and controlled using a custom program developed in MATLAB (MathWorks Inc., Natick, MA, U.S.A.). Stimuli were three repetitions of 1s duration tone pips logarithmically spaced at 4 frequencies/octave between 100 Hz to 12 kHz, presented attenuated by 30 dB from full scale (approximately 90 – 110 dB SPL).
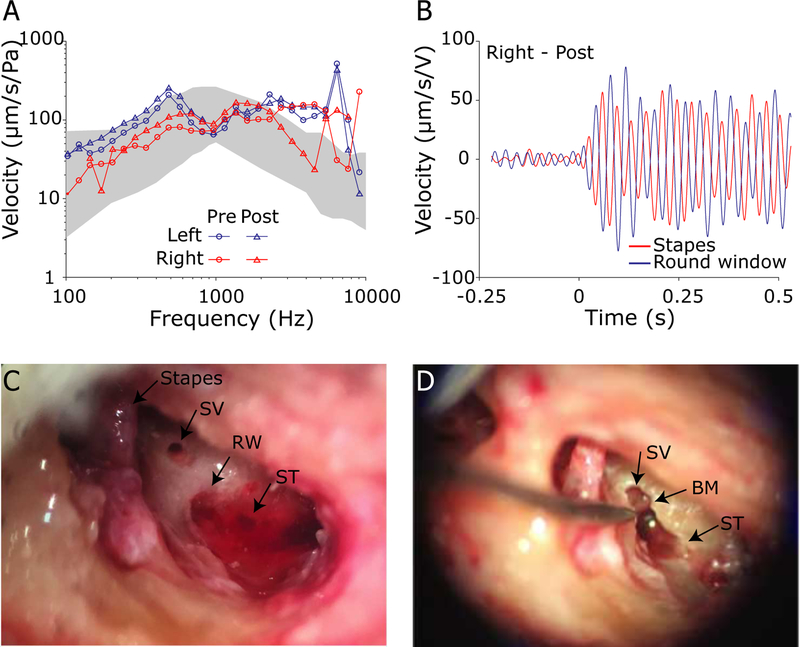
Figure 2 shows a representative set of validation measurements in one ear (359L). Following the mastoidectomy and facial recess, but prior to making cochleostomies (opening the cochlea) and sensor placement, the average stapes velocity transfer function across the three repetitions (Figure 2A) was recorded (labeled PRE, circles) for comparison to prior reports in the literature (grey band: Mattingly et al., 2015; Rosowski et al., 2007). Similarly, the round window velocity (Figure 2B) was measured at a low frequency (400 Hz, 110 dB SPL) to verify opposite (180°) motion relative to the stapes to rule out the presence of air in the cochlea (Nakajima et al., 2009). Responses were then compared to velocities measured after pressure probe insertion and fixation (Figure 2A, labeled POST) to quantify any changes in these responses. Note, the orientation of the LDV laser could not be held constant between Pre and Post measurements, due to the guide tube placement procedure, thus some changes in transfer function magnitudes were expected; however, the shape of the magnitude curve did not change substantially across frequency after the pressure sensors were inserted and sealed into the cochlea. Response phases (not shown) matched prior reports, and were essentially identical in Pre and Post measurements in all ears included for analysis. Finally, after the completion of all experimental procedures, the fiber optic probes and guide tubes were removed from the specimen to inspect for gross anatomical damage (Figure 2C; none was noted), and the bone between the SV and ST cochleostomies was removed to verify the position of the cochlear partition between the two (Figure 2D).
Fig. 2:
Validation procedures. A) Stapes velocity transfer functions (HStap = VStap/PEAC; mean across three repetitions in specimen 359L) were measured before (○) and after (△) intracochlear pressure sensor insertion to verify comparable magnitudes (note that the angle of the LDV with respect to the stapes motion differed between measurements, thus absolute magnitudes could vary somewhat). Gray band represents the 95% confidence interval (CI) from (Rosowski et al., 2007). B) Round window velocity (blue) was compared to Stapes velocity (red) at 400 Hz to verify a 180° phase difference. The stimulus was a 1s duration tone pip. C) Cochleostomies were created near the base of the stapes and the round window (RW) to enter into the scala vestibuli (SV) and scala tympani (ST) respectively. D) After all experimental conditions were completed, intracochlear pressure probe placement was verified by dissecting away the bone between the two cochleostomies, and verifying that the basilar membrane (BM) was between the two fenestrations.
2.4. Shock wave exposure
When preparations and pre-test velocity measurements were complete, specimens were transported to the test apparatus. Acoustic shock waves were generated with a ~45 cm diameter shock tube with a ~2.4 m long expansion cone and spheroidal suppressor, mounted inside a ~16 m semi-trailer (Figure 3 A; ARA Inc., Littleton, CO, U.S.A.). Specimens were suspended upside-down from a steel and aluminum support structure inside the expansion cone of the shock tube, and fixed in place facing the shock tube driver with the interaural axis horizontal to the ground (Figure 3 B–C; demonstrated here with a Hybrid III dummy head). The shock tube was driven with compressed air, and used mylar (for 7 kPa peak exposures) or aluminum (for higher pressures) membranes that were allowed to rupture spontaneously. A wire mesh screen near the membrane prevented membrane material from contacting the specimen. Pressure was monitored in both the driver and driven segments, near the membrane. A high intensity light source provided illumination for a Phantom v7.3 (Vision Research, Wayne, NJ) high speed camera, which filmed at 2000 frames/sec. through a transparent polycarbonate window in the expansion cone, time locked to the shock wave exposure. Data acquisition was performed by a Hi-Techniques, Inc. (Madison, WI) meDAQ system operating at 1 MS/s. Recordings were triggered by the pressure sensor adjacent to the specimen’s forehead.
Peak SPL (pSPL) values are calculated as: pSPL = 20*log10(Pp / 20 μPa), where Pp is the instantaneous peak pressure in Pascals (ANSI, 1973). Specimens were exposed to impulses with nominal peak overpressures of 7, 28, 55, & 83 kPa nominal peak pressures (171, 183, 189 & 192 dB pSPL). In general, exposure levels resulting from the 55 & 83 kPa nominal peak pressure exposures were more variable, and the sensor responses less consistent than the results collected at the lower levels, thus discussion of the responses to the higher two exposure levels is limited.
Specimens were exposed to these pressure levels in both an open-ear condition, and with one or more hearing protective devices (HPDs) fitted (3M EAR Classic, 3M Combat Arms, ARA Shot Shield, and 3M Peltor over-the-head ear muffs with gel ear cup seals). In a subset of specimens measurements were additionally made with an Advanced Combat Helmet (ACH) fitted to the specimen’s head. Note, the results from measurements with HPD and helmet use are outside the scope of this report, and only results from open-ear, no helmet conditions are presented in the results below. Preliminary results from HPD measurements have been presented elsewhere (Walilko et al., 2017).
No specimen was tested under all exposure conditions, instead specimens were exposed to a pseudorandom set of conditions, starting with an open-ear 7 kPa nominal peak pressure exposure and ending with an open-ear exposure at the highest condition tested for the day. Open-ear, 7 kPa nominal peak pressure exposures were repeated throughout the duration of testing in order to assess changes in the specimen’s response after repeated exposures.
3. RESULTS
3.1. Shock wave characterization
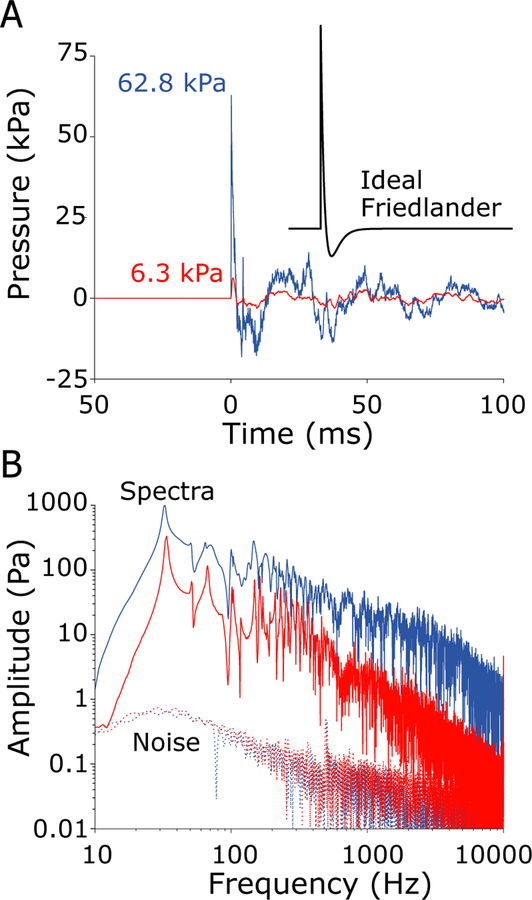
Figure 4 demonstrates waveforms recorded during ~ 7 and ~55 kPa nominal peak pressure exposures (6.3 and 62.8 kPa actual peaks, red and blue lines respectively) recorded by the free-field pressure sensor during testing. The shock waves (Figure 4A) approximated a Friedlander waveform (i.e. an “ideal” shock waveform consisting of a fast onset and short positive pressure component, followed by a shorter, lower magnitude negative component, given by the equation P = PS(exp(−t/τ))(1-(t/τ)), PS = 62.8 kPa, τ = 2 ms; see inset) at the waveform onset (Chandra et al., 2012). Specifically, the shock waves generated reproduced the fast, positive pressure onset as well as the slower negative-going phase; however the waveform showed ringing with a period of ~ 35 ms that was related to the length of the shock tube system and persisted for some time after the initial onset.
Fig. 4:
Example shock waves generated by the shock tube measured by the free-field pressure gauge during testing of specimen 359. A) Time-domain recordings show a 7 kPa nominal peak pressure (red; 6.3 kPa actual), and a 55 kPa nominal peak pressure (blue; 62.8 kPa actual) shock wave exposures. An ideal Friedlander waveform with a 2 ms A-duration is shown in the inset. B) The frequency spectra (solid) of the shock waves shown in A, along with the noise floors (dashed) estimated from the period preceding the exposure, reveal a high signal-to-noise ratio from ~10 Hz - 10 kHz that extends to a wider range of frequencies with increasing SPL. Spectra are computed over a 1 sec. window beginning at the onset in the free-field sensor.
Shock waves are characterized using two characteristic durations: the A-duration (the time interval between impulse arrival at the free-field sensor and the first negative-going zero crossing) is ~2 ms for both levels, and the B-duration (the duration to the last point on the waveform envelope 20 dB down from the peak) is ~250 ms for the 7 kPa, and ~80 ms for the 55 kPa peak impulses (following: Coles et al., 1968; Hynson et al., 1976). The onset time, the time of arrival at each sensor, is defined as the point at which the pressure exceeds a value 20 dB below the peak, or 4 standard deviations of the response prior to the free field onset, whichever is lower. Median and first and third quartiles (25th and 75th percentiles) of the onset time, as well as A- and B- durations, are provided for each sensor, for each of the four intensities in Table 1. Onset times generally decrease above 6.3 kPa, and are relatively consistent at higher exposure levels. Notably, onset times are shortest in the front surface (FSurf) sensor, and are comparable in the lateral surface (Surf), ear canal (EAC), and intracochlear pressure sensors (SV & ST) at 6.3 kPa; however, the intracochlear pressure sensors show somewhat shorter onset times than Surf or EAC sensors at higher exposure levels. However, the interquartile range is relatively large, with substantial overlap across levels. A-durations are relatively consistent at ~2 ms across all four levels, and across all seven sensors, whereas B-durations decreased substantially from ~200 ms to 50–100 ms with increasing level. The decrease in B-duration is evidence of a relative reduction of the ongoing low frequency oscillations relative to the peak pressure at the onset of the exposure, suggesting that the shock wave intensity increases nonlinearly relative to the level of the ongoing reverberations within the shock tube. Note, only responses showing no evidence of sensor failure are included, thus the N varies from one condition to the next.
Table 1:
Medians, first (Q1) and third (Q3) quartiles, and number (N) of Onset times, A-, and B-durations calculated for each pressure sensor, at each exposure level. All times are shown (in ms) relative to the free-field (Field) sensor. FSurf: front surface; Surf: surface sensor adjacent to the ear; EAC: external auditory canal, adjacent to the tympanic membrane; SV: scala vestibuli; ST: scala tympani; ME: middle ear; std dev: standard deviation; N: number of included measurements.
| Onset (ms) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 kPa | 28 kPa | 55 kPa | 83 kPa | |||||||||
| Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | |||||
| Field | 0.00 | (-,-) | 8 | 0.00 | (-,-) | 7 | 0.00 | (-,-) | 3 | 0.00 | (-,-) | 3 |
| Fsurf | 0.41 | (0.37, 0.45) | 8 | 0.31 | (0.24, 0.34) | 6 | 0.30 | (0.28, 0.31) | 3 | 0.21 | (0.18, 0.31) | 3 |
| Surf | 0.74 | (0.64, 0.79) | 16 | 0.51 | (0.46, 0.58) | 14 | 0.51 | (0.49, 0.51) | 6 | 0.44 | (0.40, 0.64) | 6 |
| EAC | 0.66 | (0.59, 0.71) | 14 | 0.55 | (0.50, 0.61) | 11 | 0.52 | (0.51, 0.55) | 3 | 0.44 | (0.44, 0.47) | 5 |
| SV | 0.66 | (0.55, 0.73) | 12 | 0.42 | (0.34, 0.46) | 11 | 0.36 | (0.17, 0.39) | 4 | 0.39 | (0.25, 0.51) | 5 |
| ST | 0.74 | (0.64, 0.94) | 13 | 0.45 | (0.38, 0.52) | 11 | 0.41 | (0.31, 0.54) | 4 | 0.35 | (0.23, 0.40) | 4 |
| ME | 0.86 | (0.83, 1.12) | 10 | 0.61 | (0.52, 0.67) | 8 | 0.55 | (0.52, 0.57) | 3 | 0.48 | (0.45, 0.52) | 3 |
| A-Duration (ms) | ||||||||||||
| 7 kPa | 28 kPa | 55 kPa | 83 kPa | |||||||||
| Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | |||||
| Field | 2.03 | (1.88, 2.14) | 8 | 2.00 | (0.68, 2.14) | 7 | 3.05 | (0.73, 3.20) | 3 | 2.00 | (1.94, 6.36) | 3 |
| Fsurf | 2.26 | (2.19, 2.80) | 8 | 3.05 | (2.56, 3.33) | 6 | 2.93 | (2.87, 3.27) | 3 | 2.26 | (2.07, 6.38) | 3 |
| Surf | 2.64 | (2.55, 2.69) | 16 | 2.43 | (2.27, 2.76) | 14 | 2.34 | (2.28, 2.68) | 6 | 4.02 | (2.32, 5.97) | 6 |
| EAC | 2.46 | (2.37, 2.59) | 14 | 2.13 | (1.62, 2.46) | 11 | 3.20 | (3.05, 4.77) | 3 | 2.62 | (0.70, 5.89) | 5 |
| SV | 3.23 | (2.31, 3.70) | 12 | 2.94 | (1.98, 3.25) | 11 | 2.92 | (1.96, 2.97) | 4 | 3.37 | (3.01, 5.81) | 5 |
| ST | 2.95 | (2.47, 3.66) | 13 | 3.20 | (2.87, 3.27) | 11 | 2.86 | (2.18, 2.92) | 4 | 3.06 | (1.72, 5.43) | 4 |
| ME | 2.95 | (2.68, 3.53) | 10 | 3.03 | (2.73, 3.22) | 8 | 3.01 | (2.78, 4.21) | 3 | 3.24 | (2.80, 6.50) | 3 |
| B-Duration (ms) | ||||||||||||
| 7 kPa | 28 kPa | 55 kPa | 83 kPa | |||||||||
| Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | |||||
| Field | 258 | (256, 259) | 8 | 49 | (48, 115) | 7 | 61 | (56, 120) | 3 | 54 | (54, 54) | 3 |
| Fsurf | 256 | (205, 259) | 8 | 266 | (183, 425) | 6 | 103 | (63, 294) | 3 | 27 | (26, 29) | 3 |
| Surf | 291 | (256, 316) | 16 | 116 | (81, 136) | 14 | 55 | (53, 66) | 6 | 86 | (42, 90) | 6 |
| EAC | 196 | (181, 257) | 14 | 81 | (49, 141) | 11 | 62 | (60, 182) | 3 | 116 | (89, 258) | 5 |
| SV | 364 | (324, 405) | 12 | 235 | (177, 294) | 11 | 117 | (88, 137) | 4 | 90 | (38, 210) | 5 |
| ST | 349 | (266, 452) | 12 | 189 | (135, 323) | 10 | 103 | (82, 139) | 4 | 149 | (117, 225) | 4 |
| ME | 289 | (258, 324) | 10 | 197 | (195, 198) | 8 | 117 | (60, 119) | 3 | 118 | (117, 118) | 3 |
The frequency spectra (calculated from the first 1 s after the shock wave arrival, t = 0 to 1 s, and smoothed with a 1/8 octave wide moving average filter for visualization) of the shock waves (Figure 4B) reveal an overall trend towards low frequencies (i.e. < 200 Hz), in addition to a substantial peak at ~30 Hz corresponding to the resonant period of the shock tube system (solid red and blue lines show the spectra for the corresponding shock waves presented in Figure 4A). The signal was well above the noise floor (dotted lines), which was estimated from the recording immediately prior to the impulse, for frequencies between ~ 10 Hz - 10 kHz for 7 kPa nominal peak pressure impulses, and over a broader bandwidth for higher-level impulses. Note, measurements were collected in both ears in all specimens; here we treat each ear independently, and do not directly compare the two ears within each specimen, as small variations in head orientation or tissue condition/preparation in the two ears may cause slight differences in results between ears.
3.2. Otoscopic examination
The external and in-ear condition of each specimen was inspected prior to the first, and after each shock wave exposure. Two ears had TM perforations prior to testing and were excluded from further analysis (2/16). No visible damage was noted after any 7 kPa exposure (14/16 ears), and only one ear showed a perforation after a 28 kPa exposure (1/14 ears), which was likely caused by an experimenter rather than the shock wave. In contrast, TM rupture was observed after nearly every 55 kPa (4/5 ears) or 83 kPa (6/6 ears) peak exposure. Note, most specimens were exposed to both 7 and 28 kPa, but no specimen was exposed to both 55 and 83 kPa. Testing ceased once both TMs were perforated.
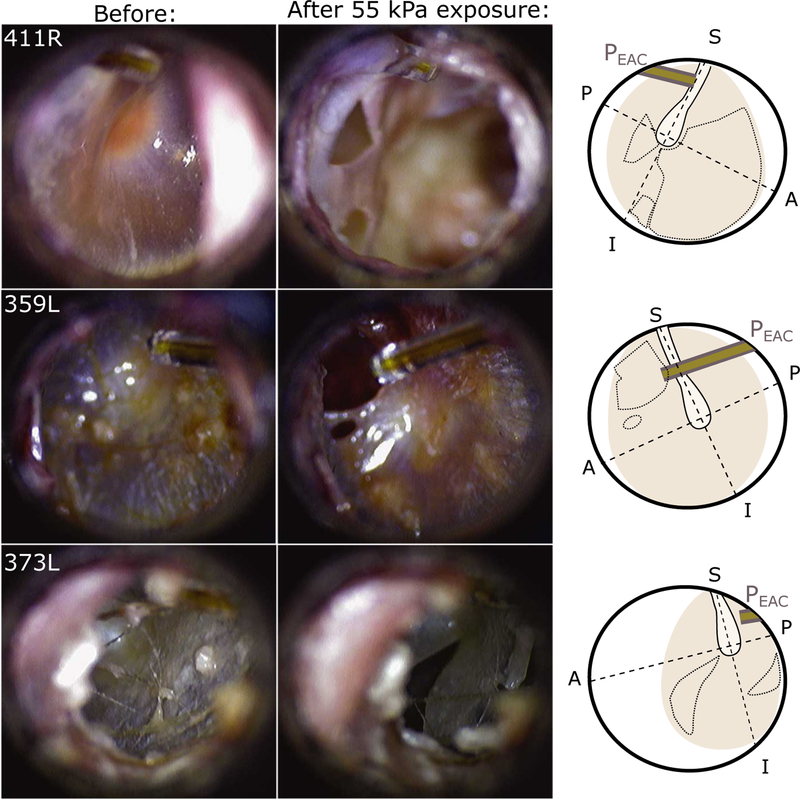
Figure 5 shows examples of the TM ruptures observed. The left column shows intact TM from three ears (411R, 359L, and 373L) shortly prior to the peak exposure causing each TM rupture. Note: the location and orientation of the fiber-optic pressure probe is visible at the top left (top) or right (center, bottom) of each image. The center column shows the same ears after subsequent unprotected 55 kPa nominal peak exposure, and the right column shows an illustration demonstrating the orientation and location of each TM (colored area), the location of the fiber-optic sensor (grey bars), the location of the manubrium of the malleus (lighter area with a solid border), and the region in which the TM rupture(s) were visible (dotted lines). Several perforations are visible, showing the range of TM perforations observed in this study: top: a large perforation encompassing both anterior quadrants through which the cochlear promontory is visible (though out of focus), and two smaller perforations in each of the posterior quadrants; center: a circular perforation in the anterior-superior quadrant; bottom: a “slit-like” perforation in the anterior-inferior quadrant, that extends outward radially from the center (Kuroda, 1993). In some cases perforations broadened when subjected to additional exposures (not shown). In the results below, responses are only assessed if the TM was intact prior to the exposure (i.e. there was no pre-existing perforation).
Fig. 5:
Photomicrographs of tympanic membranes taken before (left) and after (center) high level shock wave exposures from three specimens/ears (specimen number and ear (L or R) noted in the upper left of each panel). The external auditory canal fiber-optic pressure sensor (EAC) is visible in the upper left or right corners. The approximate position of the umbo, orientation of the manubrium (solid outline), and perforated area (dotted lines) is highlighted in an illustration (right) of each TM.
3.3. Acoustic shock wave exposures
3.3.1. 7 kPa nominal peak pressure
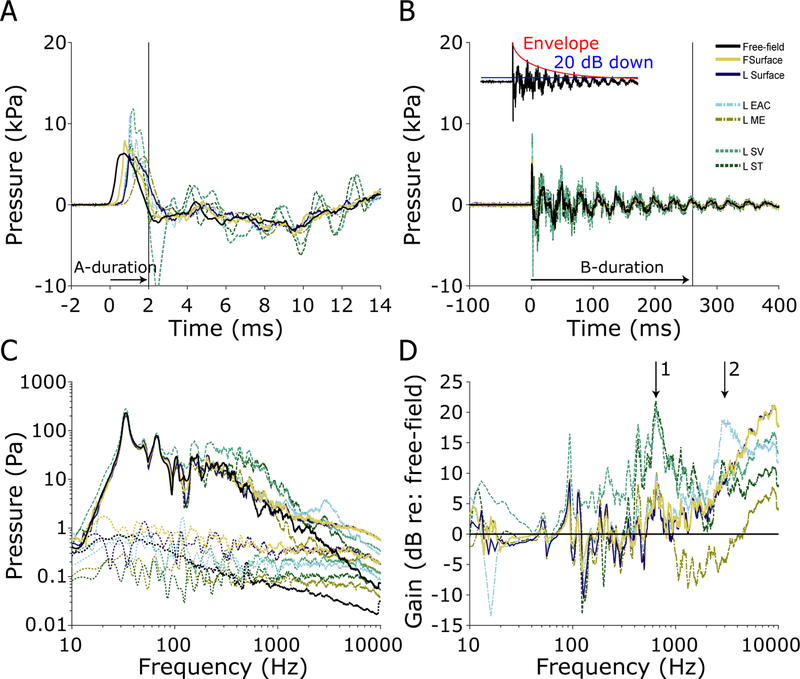
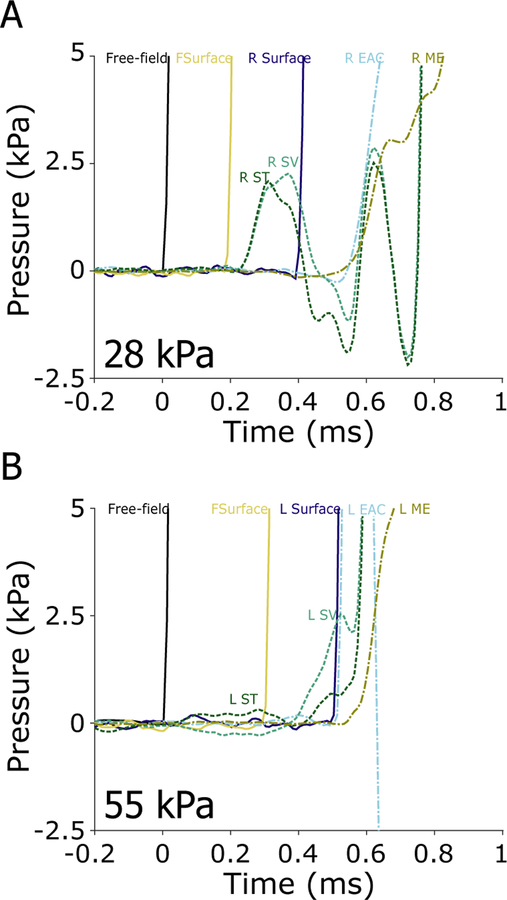
All eight specimens (16 ears) were exposed to 7 kPa nominal peak pressure shock waves at the beginning of each set of exposures, and again after certain larger exposures to verify the condition of the ears and probes throughout the duration of the experiment. If a response to the 7 kPa exposure changed substantially in one or more sensor channels, the sensor was repaired or replaced, if possible, or noted as a failure and excluded from subsequent analysis otherwise. Figure 6 shows the first 14 ms (Figure 6A) and first 400 ms (Figure 6B) of the responses of the pressure probes to a single 7 kPa nominal peak pressure exposure (ear 359L, the same as in Figure 2). The peak exposure level (computed from the free field pressure sensor) for this exposure was ~6.3 kPa, or ~170 dB pSPL. The pressure wave arrived at the free-field pressure sensor first (at time = 0 ms), followed by the front surface (forehead) sensor (‘FSurface’) at ~0.4 ms, and the remaining sensors at ~0.7 ms (see Table 1). The forehead surface sensor showed a somewhat higher pressure peak than the free-field sensor (~1.9 dB), presumably due to the sensor orientation (the shock wave angle of incidence is normal to the forehead). Two main differences between the in-ear sensors and the free-field/surface sensors are visible. First, the in-ear sensors showed substantially higher pressure peaks resulting from the gain of the pinna and ear canal (Wiener et al., 1946). In particular, the EAC sensors showed up to ~6 dB, and the intracochlear pressure sensors ~ 5.5 dB higher peak pressures than the free-field pressure sensor. Second, an oscillation with a ~1.5 ms period is visible (particularly between 8–14 ms) in Figure 6A (in addition to the ~30 ms fluctuation observed in Figure 6B), in the responses of the SV and ST sensors, that is not present in the other curves. Note, a large negative peak is observed in the SV sensor at ~2.5 ms that appears to be a component of the 1.5 ms period oscillation, but was not observed in all specimens thus may be either an artifact or a unique feature of this specimen.
Fig. 6:
Pressures measured during a 7 kPa nominal peak pressure exposure in ear 359L. The first 14 ms (A), and 400 ms (B) of the recording are shown, illustrating the A- and B- durations (horizontal arrows) of the free-field pressure gauge measurement of the shock wave. For all sensors, A-duration (2.0 ms) is defined as the first zero crossing, while the B-duration (259.1 ms) is defined as the time to the last point exceeding 20 dB down from the peak (see inset). The frequency spectra (solid) and noise floors (dotted) of each sensor (C). The gain of each sensor relative to the free-field pressure gauge (D).
The frequency spectra, calculated over a 1 s period beginning at shock wave arrival at the free-field sensor, and the difference in the spectra relative to that of the free-field sensor (in dB), are shown in Figures 6C–D (smoothed with a 1/8 octave wide moving average filter for visualization). The spectra are largely similar to the responses observed in the free-field sensor for low frequencies (i.e. < 200 Hz); however, the spectra deviate substantially from the free-field at higher frequencies. In particular, two distinct peaks in the gain plots, centered at 600–800 Hz (arrow 1) and at 2–3 kHz (arrow 2), are visible in all signals. First, a substantial (~10 dB) peak at 2–3 kHz, representing the ear canal resonance, appears in the EAC probes. This gain propagates through the ear and is visible in the downstream pressure sensors. Second, a substantial peak (~20 dB) at 600–800 Hz, corresponding with the ~1.5 ms period oscillations visible in the time-domain plots, appears in the intracochlear pressures, indicative of the middle ear gain. Together, these effects increase the amplitude of signals entering the cochlea by 10–20 dB, relative to the incident sound pressure level, for frequencies above ~300 Hz. Lower frequency sounds show little or no difference relative to the incident exposure. Note: all signals are well above the noise floor for frequencies between ~ 10 Hz – 10 kHz.
The relative peak pressure amplitudes (computed from waveforms) are compared across sensor location, and across exposure level, in Table 2. Mean and standard deviation of the peak pressure measured in the free field is shown at left, and reveals peak pressures comparable to the nominal peak pressure exposures by which responses are grouped (i.e. mean exposure was 7.6 ± 1.9 kPa for the 7 kPa nominal peak pressure exposure condition). Responses of the other sensors are reported in dB gain relative to the free field sensor, as reported above. In general, peak pressure responses are higher in the front surface sensor, comparable in the lateral surface sensors, higher in the ear canals, and lower in the intracochlear pressures (SV & ST) and middle ear sensors than the free field sensor, but responses are variable, with standard deviations exceeding means for most measures.
Table 2:
Mean and standard deviation (SD) of peak pressure (in kPa) measured in the free field for each exposure level, and the gain in peak pressure level for each sensor relative to the free field pressure gauge (in dB re: free field). Abbreviations defined in Table 1.
| Peak Pressure (kPa) | Gain (dB re: free field) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Field | FSurf | Surf | EAC | SV | ST | ME | |||
| 7 kPa | Mean | 7.59 | +2.7 | +0.3 | +3.9 | 2.7 | −2.5 | −3.1 | |
| SD | 1.89 | 1.7 | 1.4 | 5.3 | 5.3 | 5.3 | 6.9 | ||
| N | 16 | 16 | 16 | 14 | 13 | 13 | 11 | ||
| 28 kPa | Mean | 31.19 | +4.8 | +1.3 | +4.8 | −4.6 | −5.1 | −8.0 | |
| SD | 3.10 | 1.4 | 1.5 | 6.9 | 6.2 | 6.5 | 9.3 | ||
| N | 14 | 14 | 14 | 12 | 11 | 11 | 9 | ||
| 55 kPa | Mean | 57.50 | +4.3 | +1.4 | −0.6 | −0.5 | −0.6 | −11.5 | |
| SD | 4.46 | 0.6 | 1.7 | 5.8 | 2.1 | 4.2 | 17.5 | ||
| N | 6 | 6 | 6 | 4 | 4 | 4 | 4 | ||
| 83 kPa | Mean | 92.63 | +4.6 | −9.5 | −6.4 | −6.5 | −6.0 | −7.3 | |
| SD | 1.82 | 2.5 | 13.3 | 3.0 | 5.0 | 6.6 | 1.2 | ||
| N | 6 | 6 | 6 | 6 | 5 | 5 | 3 | ||
3.3.2. 28 kPa nominal peak pressure
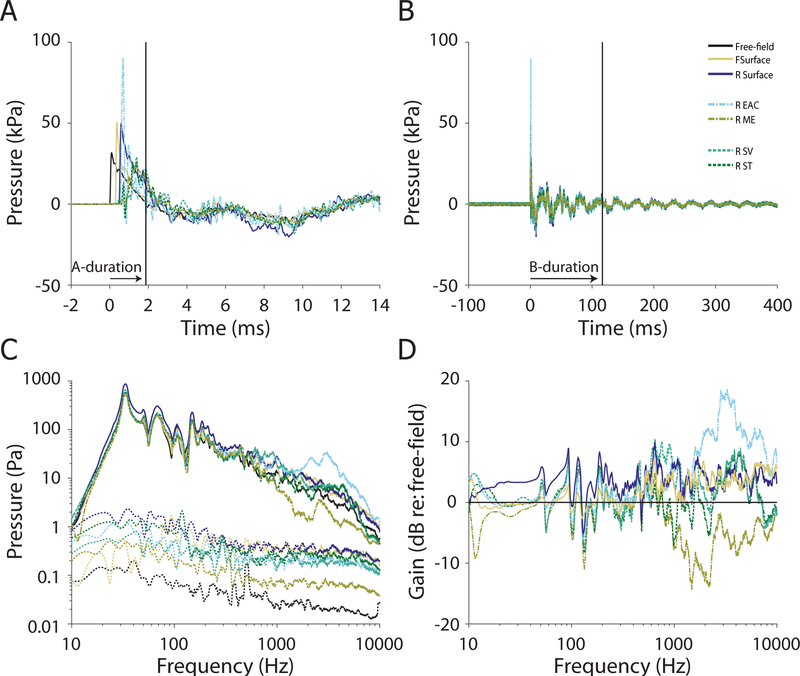
Seven of the eight specimens (14 of the 16 ears) were exposed to the 28 kPa nominal peak pressure exposure condition. Figure 7 shows the first 14 ms (A) and first 400 ms (B) of the responses of the pressure probes to a single 28 kPa nominal peak pressure exposure (ear 411R). The peak pressure level (calculated from the free field sensor) for this exposure was close to ~37.6 kPa, or ~185 dB pSPL. Similar to the 7 kPa exposure, the pressure wave arrived at the free-field pressure sensor first (t = 0). However, the intensity of the shock wave was higher, thus the speed of the wave front was higher and the delay to each of the sensors shorter relative to the 7 kPa stimulus: the front surface (forehead) sensor was delayed by ~0.3 ms, and the remaining sensors ~ 0.5 ms relative to free-field. Once again, the front surface (forehead) sensor (~4.5 dB), and the EAC pressure sensors (~2.6 dB), showed higher peak pressures than the free-field sensor. However, compared to the lower intensity exposure the shock wave rise-time was shorter, the peaks sharper, and the B-duration shorter (as described in section 3.1 and Table 1). The intracochlear pressure sensors showed somewhat lower peak pressures than the free-field sensor (~−1 dB). This decrease is prominent for higher frequency components of the response, as revealed in the frequency spectra (Figures 7C–D; smoothed with a 1/8 octave wide moving average filter for visualization). Peak pressures showed a similar trend as the 7 kPa exposure: peak pressures were higher in the front surface and ear canal sensors, and intracochlear and middle ear pressures were lower than the free field sensor (Table 2).
Fig. 7:
Pressures measured during a 28 kPa nominal peak pressure exposure in ear 411R. The first 14 ms (A), and 400 ms (B) of the recording are shown, illustrating the A- and B- durations (horizontal arrows; 2.1 & 48.4 ms respectively) of the free-field pressure gauge measurement of the shock wave. The frequency spectra (solid) and noise floors (dotted) of each sensor (C). The gain of each sensor relative to the free-field pressure gauge (D).
3.3.3. 55 & 83 kPa nominal peak pressures
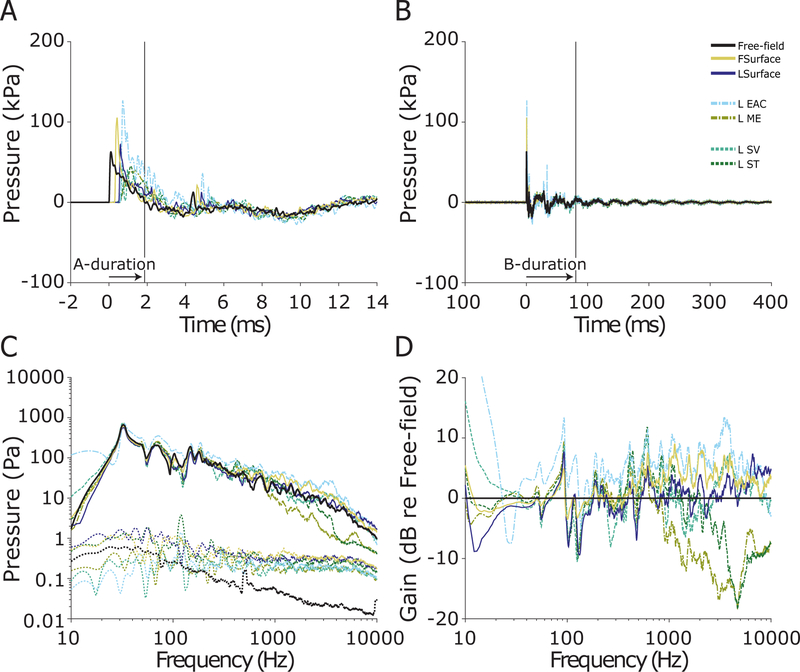
Three of the eight specimens were exposed to the 55 kPa, and three to the 83 kPa (6 of the 16 ears each), nominal peak pressure shock waves (two specimens were not exposed to either due to previous damage). Figure 8 shows the first 400 ms (A) and first 14 ms (B) of the responses of the pressure probes to a single 55 kPa nominal peak pressure exposure (ear 359L, the same as Figures 6 & 2). The 55 kPa nominal peak pressures were somewhat more variable than the lower levels (e.g. Table 2), and the peak exposure level in this specimen was ~62.8 kPa, or ~190 dB pSPL. Similar to the 28 kPa exposure, the pressure wave arrived at the free-field pressure sensor first, at the forehead sensor delayed by ~0.3 ms, and at the remaining sensors delayed by ~ 0.5 ms (Table 1).
Fig. 8:
Pressures measured during a 55 kPa nominal peak pressure exposure in ear 359L (same as Figure 6). The first 14 ms (A), and 400 ms (B) of the recording are shown, illustrating the A- and B- durations (horizontal arrows; 3.1 & 62.4 ms respectively) of the free-field pressure gauge measurement of the shock wave. The frequency spectra (solid) and noise floors (dotted) of each sensor (C). The gain of each sensor relative to the free-field pressure gauge (D).
Peak pressures across sensor locations showed a less consistent trend compared to either lower level exposure (Figures 6 & 7), as revealed by the difference in spectra re: free field (Figure 8D): the front surface (forehead) sensor gain remained relatively similar, however, intracochlear pressures (SV & ST) showed pressures comparable in peak magnitude relative to the free field (compared to reduced peak pressures at lower levels), and the lateral surface and EAC probes both showed significantly lower peak pressures than the free field sensor in the 83 kPa exposure (significantly reduced gain compared to those observed at lower levels). One cause of the relatively increased peaks in intracochlear pressures could be the relative increase in sound transmission through a non-ossicular pathway, such as though bone conduction.
3.4. Evidence for a bone-conducted component of shock wave propagation
In the 28- and 55 kPa nominal peak exposures shown in Figures 7 and 8, a small disturbance is visible in both intracochlear pressure sensors at ~ 0.3–0.4 ms. These responses are shown expanded in figures 9A and B respectively. This pressure arrives in the cochlea 0.1–0.15 ms prior to the air-conducted shock wave arrival at the entrance or within the ear canal (~0.5 ms), thus must be the result of an alternate sound conduction pathway such as through the skull bone (which has a speed of sound of ~3 km/s for the longitudinal mode (White et al., 2006), nearly an order of magnitude faster than the ~343 m/s speed of sound in air at 20° C), stimulating the cochlea directly. This pressure typically arrives in both SV and ST sensors simultaneously, and generally revealed an increase in sound pressure level, as illustrated in figure 9A. Note, the speed of sound increases with increasing shock wave peak overpressure, thus the 343 m/s estimate provided here represents a lower bound on the speed of sound in air. This bone-conducted sound pressure is quickly over-ridden by the air-conducted pressure wave arrival at 0.5–0.6 ms, thus we cannot directly differentiate their contributions to the overall sound pressure observed. Nevertheless, these early sound pressures observed are substantial, although L SV showed a somewhat higher peak than L ST, rising to ~ 2.2 kPa & ~2.5 kPa (in Figure 9A and B respectively) by the time the shock wave is visible in the EAC sensor at ~ 0.5 ms. Similar pressure increases preceding shock wave arrival in the EAC are observed in all measurements during exposures 28 kPa or higher, although absolute pressures and time courses vary somewhat across specimens/ears.
Fig. 9:
Pressures measured during the 28 (A) and 55 kPa (B) nominal peak pressure exposures, from Figures 7 (ear 411R) & 8 (ear 359L), respectively, expanded to show the first 1 ms after the shock wave arrival at the free field sensor. Note the pressure change in SV and ST appears after the increase in FSurface, but prior to that in the EAC in both examples indicating a non-ossicular (likely bone-conducted) component to the sound transmission. Note, results in A and B are from two different specimens, and absolute arrival times vary between A and B.
3.5. Intracochlear pressure transfer functions
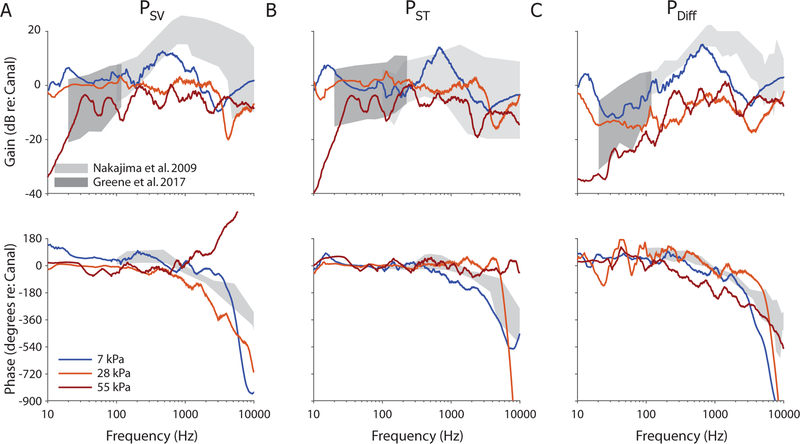
In order to more directly compare results to prior reports (e.g., Greene et al., 2017), intracochlear pressure transfer functions (HSV/ST/Diff) were calculated from the frequency spectra of PSV (Figure 10A; HSV), PST (Figure 10B; HST) and PDiff (Figure 10C; HDiff, the complex difference between SV and ST), by normalizing to PEAC, the sound pressure level in the corresponding ear canal. Figure 10 shows transfer function magnitudes (top) and phases (bottom) for the three examples (from two ears) shown in figures 6–8 (measured during the 7, 28, and 55 kPa nominal peak pressure exposures). Transfer function phase was calculated from the complex component of the frequency spectrum (calculated from a 1 sec. window after response onset in the free-field sensor), represents the delay between the EAC and each sensor, and was unwrapped using the Matlab function laplacianUnwrap.m, available on the Mathworks file exchange website (Bouwman, 2016). Both magnitude and phase responses are smoothed with a 1/5 octave wide moving average filter for visualization. Responses are superimposed onto the range of normal responses observed previously in the literature at moderate sound pressure levels (light grey, Nakajima et al., 2009), as well as the responses recorded at somewhat higher levels and lower frequencies (dark grey, Greene et al., 2017), measured with short tone pips. The responses shown in grey represent the range of responses expected for normal, healthy temporal bones (that is, stimuli were presented at levels that elicit responses that vary linearly with sound pressure level), thus deviations from these responses represent nonlinearities in the sound transmission pathway.
Fig. 10:
Example intracochlear pressure transfer function magnitudes and phases for the specimens and exposures shown in figures 6–8 (i.e. responses in ears 359L, 411R, and 359L, during 7, 28, and 55 kPa nominal peak pressure exposures respectively). Responses are shown superimposed onto the range of responses observed to moderate sound pressure level tone pips (gray shading) reported previously (dark: Greene et al., 2017; light: Nakajima et al., 2009).
As we have discussed previously, nonlinearities in middle ear motion are dependent upon both stimulus level and frequency (Greene et al., 2017), thus complicating comparisons between the current results and prior results based on tone-pips. Nevertheless, transfer function magnitudes for the 7 kPa exposure (blue) were generally consistent with the prior reports, although magnitudes were somewhat lower for high frequencies (> ~600 Hz) during the impulse noise pressures in the HSV and HDiff. Responses to the 28 kPa nominal peak pressure exposures revealed reduced transfer function magnitudes in all three measures, particularly showing a decrease in the frequency range showing maximum gain (~600 Hz – 2 kHz) such that the gain was relatively constant (at or below zero dB re: EAC) across frequencies in all three transfer functions. The 55 kPa peak exposure level showed an additional (small) decrease in gain across frequency relative to the 28 kPa exposure. A notable deviation from the prior reports is visible in HSV for the 55 kPa exposure, which showed an increasing rather than decreasing phase angle for frequencies greater than 1 kHz. This phase disparity generally increased with level, and may be an artifact of the phase unwrapping algorithm, or evidence of the bone conducted sound transmission altering the response at the exposure onset.
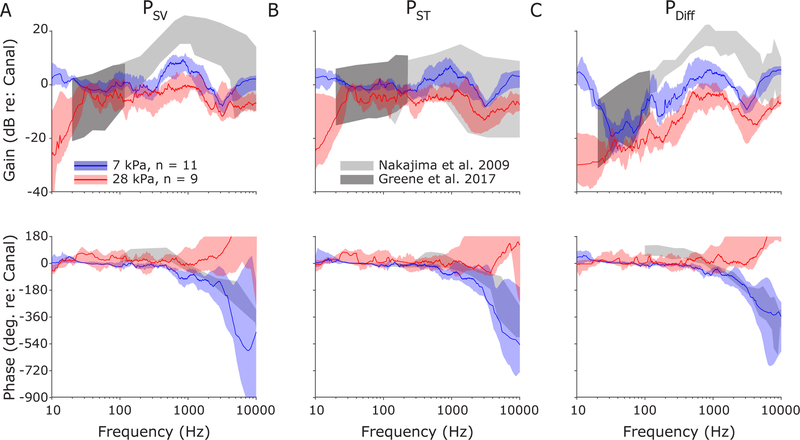
Responses are summarized across the population of specimens/ears in Figure 11. As in Figure 10, transfer function magnitude (top) and phase (bottom) are shown for PSV (Figure 11A; HSV), PST (Figure 11B; HST) and PDiff (Figure 11C; HDiff). The median ± interquartile range of responses for each of the intracochlear pressure transfer function magnitudes (top), and phases (bottom) are show for the 7 kPa (blue) and 28 kPa (red) peak pressure exposures. Responses from 55 kPa and 83 kPa exposures were comparable to those of the 28 kPa, but are excluded here for clarity (and due to the small number of exposures; n = 4 and n = 3 respectively). Transfer function magnitudes are consistently lower during the higher exposures than during 7 kPa exposures in all three measures. PSV and PST show a substantial decrease between approximately 400 Hz – 1 kHz, decreasing by a mean (± standard deviation) of −8.8 ± 1.1 dB and −9.2 ± 2.0 dB, respectively. This decrease is substantially larger than at lower frequencies (30–400 Hz), where the decrease was only −3.0 ± 1.9 dB and −4.4 ± 2.2 dB, respectively. PDiff, on the other hand, shows a more consistent decrease across frequencies, showing declines of −9.6 ± 2.2 dB and −7.8 ± 1.3 dB in the 30–400 Hz and 400–1000Hz bands respectively. PSV and PDiff were lower in magnitude than previously reported, even for the 7 kPa exposure, which may indicate the nonlinearity may begin at a lower intensity than we tested here, or may reflect the difference in methodology between measurements (e.g. steady-state vs impulse noise exposure). Overall, the 7 kPa and 28 kPa peak pressure exposure transfer function magnitudes are comparable to the ~140 dB SPL and the ~160 dB SPL steady-state tone exposures described previously (Greene et al., 2017).
Fig. 11:
Median and interquartile ranges for intracochlear pressure transfer function magnitudes for all specimens tested, shown for 7 and 28 kPa nominal peak pressure exposures. Responses are shown superimposed onto the range of responses observed to moderate sound pressure level tone pips (gray shading) reported previously (Greene et al., 2017; Nakajima et al., 2009).
Transfer function phases were generally consistent with prior reports (Greene et al., 2017; Nakajima et al., 2009) to tone stimuli, particularly for low frequencies (< ~1 kHz); transfer function phases for the 28 kPa (and 55 & 83 kPa) exposure appear to deviate from the prior reports at higher frequencies (> 1 kHz), showing an increasing phase with frequency suggesting a phase lead in the intracochlear pressures compared to EAC. This deviation (and the large variability across subjects) may be due to noise in the signal, errors in the phase unwrapping algorithm, or evidence of a substantial contribution from a non-ossicular sound conduction pathway (e.g. via bone conduction). Evidence for this final possibility is visible in the shorter intracochlear pressure onset times relative to EAC (Table 1).
4. DISCUSSION
Results of intracochlear pressure measurements during impulse noise exposure reveal that, similarly to continuous stimuli (Greene et al., 2017), middle ear gain is diminished with increasing sound pressure level for high-level sound stimulation. Intracochlear sound pressure transfer functions were comparable between moderate-level continuous stimuli and the impulse stimulus at 7 kPa nominal peak pressures (i.e. ~170 dB pSPL); these were diminished in magnitude for higher level impulse stimuli in much the same way as for continuous stimuli. These results are likely frequency dependent (e.g. figures 10–11), thus results may be expected to vary according to the frequency spectrum of the impulse noise source. These results inform strategies for improving injury prediction methods, as well as the limitations of current injury prediction models, which we discuss in the following sections.
4.1. Responses as a function of SPL
The shock tube utilized to generate shock waves in the current study relies upon spontaneous membrane rupture (i.e. there is no puncture mechanism), thus free-field pSPLs are dependent upon driver section fill rate (controlled with a manual air valve) and membrane material properties. Despite these sources of variability, the shock tube was well characterized, and run by experienced operators, and exposure levels were generally within ~ 1 dB of the target pressure. Surface and EAC SPLs generally increased linearly with free-field SPLs (e.g. EAC gain is comparable in Figure 6D and 7D), although measurements were substantially more variable at higher levels (table 2) potentially due to sensor variability or nonlinearities in sound transmission through the outer ear. In contrast, intracochlear pressures increased linearly for low-level exposures, but deviated from proportionality and increased at a lower rate for higher SPLs (e.g. the substantial gain observed in Figure 6D is not visible in Figure 7D). This effect leads to a decrease in transfer function magnitude during exposures above 7 kPa nominal peak pressure (particularly at frequencies around 1 kHz), as evident in Figures 10–11. This nonlinearity is consistent with our previous reports for tonal stimuli (Greene et al., 2017), and is likely an indication of the limiting effect of the SAL on cochlear input (Guinan et al., 1967; Price, 1974a), as well as possible contributions of nonlinear material properties of the TM and other middle ear ligaments.
Importantly, while the rate of increase in intracochlear pressure declined with increasing exposure level, the absolute sound pressure levels recorded continued to increase. In other words, no dose-response inversion was observed with increasing sound pressure level, despite the sound levels increasing above levels at which middle-ear components (particularly the SAL) limit ossicular chain motion. The responses observed here thus support a correction to the SAL compliance similar to that proposed by Zagadou et al. (2016) that eliminates this anomalous behavior in the AHAAH model.
4.2. Bone-conducted sound transmission
The initial pressure fluctuation recorded in each sensor represents the shortest propagation distance (i.e. a straight path from the ruptured shock tube membrane to the specimen), as any other path (e.g. reflections off of the shock tube walls) will necessarily involve longer transmission times. In 28 kPa and higher peak exposures, a prominent pressure fluctuation is visible in the intracochlear pressures that followed the EAC and front surface pressure sensors but preceded the pressure wave in the lateral surface sensors, ear canal or middle ear. The only transmission pathway that could lead to a result in which the shock wave arrives in the cochlea (embedded in the skull) prior to the lateral surface or ear canal pressure probes, is through coupling of the air to the front surface of the skull (i.e. the forehead), and transmission of the shockwave through the skull directly. Vibration induced in the support structure supporting the specimen is an unlikely source of this vibration for a number of reasons. First, the support structure is attached to the trailer floor inside the shock suppressor, several meters behind the specimen, thus vibrations induced in the shock tube/floor must travel several fold further than the direct transmission path. Second, the surface area of the support structure facing the shock wave source is minimal, thus coupling of the support structure with the shock wave should be minimal, and induced vibrations should be minimal. Finally, the specimen is mounted to the support structure via the cervical spine, and no rigid conduction pathway is created from the support structure to the skull. Any vibrations transmitted through this pathway must travel through the soft tissue, which would slow and low-pass filter any induced vibrations (Mattingly et al., 2015). Likewise, while the surgical preparation includes a wide mastoidectomy, thus potentially introducing a direct air-conducted signal pathway into the middle ear, this is an unlikely source of the intracochlear pressures observed due to the tight closure of the mastoidectomy (see methods), as well as the long delays and substantial low-pass filtering observed in the recorded responses. Overall, the presence of the pressures in the cochlea preceding any other pressure in the skull is strong evidence for a bone-conducted signal.
These results suggest that, at high levels, the effects of bone-conducted sound transmission become prominent. These effects may be substantial at lower levels also, but were not visible in the measurements presented here. In these conditions the impulse is transmitted from the air to the skull directly (e.g. through the forehead), and is conducted through the skull to the inner ear. Since the speed of sound in bone is considerably faster than in air, this initial pressure peak can be observed in the inner ear pressures prior to the onset of the air-conducted component. Superposition of the air and bone conducted components of the intracochlear pressure limit our ability to quantify the effects of the bone conduction pathway. Nevertheless, these results suggest that this pathway must be taken into account when investigating high level noise exposure.
Previous reports (summarized by (Berger et al., 2003)) using well-fit hearing protection devices to block the air conduction pathway, have estimated that bone conduction thresholds are 40–60 dB higher than air conduction thresholds (depending upon the frequency). A preliminary report by Clavier et al. (2012) suggested that intracranial sound pressure levels and skull vibrations induced by nearby small arms fire scales linearly with exposure level in a head simulant. In contrast, previous reports from our laboratory and others suggest that air conducted cochlear stimulation increases less than linearly with increasing level (Greene et al., 2017; Price, 1974a). Our results are generally consistent with these prior observations (although we cannot test them directly here), where the putative bone-conducted component is clearly visible in the 28 and higher, but not the 7 kPa exposure levels (Figure 9), and surface, and ear canal peak pressures increase (from 2.7 and 0.3 dB to 4.8 and 1.3 dB re free field respectively), but SV and ST pressures decrease (−2.7 and −2.5 dB to −4.6 and −5.1 dB re free field respectively) relative to the free field with increasing exposure level from 7 to 28 kPa peak (see Table 2). Note this relationship does not appear as consistent in the higher level exposures, potentially due to sensor unreliability. These results demonstrate that bone-conducted stimulation can be substantial during high level noise exposure, and is likely sufficient to directly contribute to hearing loss. At very least, these results suggest that the bone conduction pathway represents an upper bound on the protection provided by a HPD.
4.3. Acoustic hazard prediction and protection
Predicting injury to the auditory system due to impulse noise exposure is difficult due to level and frequency dependent sound transmission through the middle ear for high level sounds; several tools have been developed to assist in such predictions (reviewed recently by (De Paolis et al., 2017)). The AHAAH model (Price et al., 1991) has recently been implemented in the DOD acquisition standard MIL-STD-1474E to assess the risk associated with impulse noise exposure; however, several components (particularly the nonlinear transmission elements) of the model have not been validated in humans, or at levels known to cause permanent hearing loss.
A review of hearing damage risk criteria (DRC) concluded that the AHAAH model is “not fully developed and verified”, and that “a more quantitative understanding of middle ear function at high levels (linearities, non-linearities)” was a prominent point requiring more research (Wightman et al., 2010). Many of the points raised by this review, and related controversies surrounding the AHAAH model, stem from the fact that many model parameters have been estimated from results derived in small animals since comparable results in humans are not available or readily obtainable. Two main nonlinearities are included in AHAAH: the first involves contraction of middle ear muscles either in response, or in anticipation of a noise exposure, and is the subject of two recent studies exploring this phenomena in humans (Flamme et al., 2017; Jones et al., 2017); the second, which we have explored recently (Greene et al., 2017), and was the focus of this study, involves the frequency and level dependence of middle ear motion in response to high level noise exposure. One recent report attempted to address several of the concerns, including updating several parameters with more recent parameters derived from human cadavers (particularly an alteration to SAL compliance that eliminates the dose-response inversion), and introduction of a new damage risk correlate (integrated cochlear energy, ICE) (Zagadou et al., 2016); however, this attempt to modify the model met with controversy, and both the model’s authors (Price et al., 2017), and the article’s authors (Zagadou et al., 2017) have published commentary.
The sound pressure levels of the exposures presented here are known to be hazardous to human hearing (Patterson Jr et al., 1994a; Patterson Jr et al., 1994b), and likely cause permanent hearing loss. The intracochlear pressures measured, therefore, are consistent with those that cause permanent loss, thus these results can be used to validate and test the predictions of the AHAAH model at these levels. Subsequent results can therefore be used to update parameters in the AHAAH model to improve injury predictions, especially nonlinearities associated with the joints of the ossicular chain and the SAL. Additionally, the current results reveal the presence of a substantial bone-conducted component of the sound transmission to the cochlea, thus inclusion of this secondary transmission pathway may substantially improve the predictive power in a future cochlear model for use with auditory damage risk assessment.
Intracochlear pressure represents a direct measurement of the input force that drives the cochlear partition (Dancer et al., 1980; Lynch et al., 1982; Nakajima et al., 2009). These measurements therefore represent an ideal platform to measure the attenuation provided by hearing protective devices, as all nonlinearities in the signal transduction pathway are included (in contrast to use of an acoustical manikin). Additionally, the differential pressure (i.e. the pressure difference across the basilar membrane), along with the volume velocity of the basilar membrane, make up the inputs to the newly proposed damage risk correlate, ICE (Zagadou et al., 2016). Furthermore, this method allows for testing of hearing protective devices at much higher levels than methods involving human performance (e.g. Real Ear Attenuation at Threshold, REAT testing (Berger, 1986)), due to the reduced risks involved, thus allowing for more accurate assessment of high-level performance of these devices. In addition to the open-ear data reported here, responses were collected at each exposure level with use of several HPDs. The results of this testing is outside the scope of this report, but preliminary results have been reported recently (Walilko et al., 2017).
The data presented here show for the first time that middle ear gain decreases with increasing sound pressure level for impulse noise exposure. These results are consistent with our prior report suggesting middle ear gain decreases, while cochlear input impedance remains constant, with increasing SPL of short tone pip presentation (Greene et al., 2017). These results thus support the AHAAH assumption that middle ear motion saturates at high levels (Price et al., 1991), derived from earlier measurements in cat (Price, 1974a), but suggest that this saturation occurs at a higher sound pressure level than predicted by the AHAAH model (Greene et al., 2017). This result is consistent with the approach pursued by a recent attempt at an update to the AHAAH model (Zagadou et al., 2016), which decreased both annular ligament resistance and cochlear input resistance, thereby eliminating the anomalous dose-response inversion observed in AHAAH responses. Note, however, that while cochlear input impedance does not appear to vary with exposure level, it does vary (by an order of magnitude) across frequency (Greene et al., 2017; Nakajima et al., 2009). Neither version of the AHAAH model accounts for this variation, likely degrading injury prediction performance.
4.4. Tympanic membrane rupture and auditory injury assessment
TM rupture occurred in every exposure with a peak pressure of 55 kPa or higher. This is somewhat more common than would be predicted from previous estimates of TM burst threshold, although it is not always clear whether previous measurements report pressures in the free-field or ear-canal (which can show significantly higher pressures due to the effects of the pinna and ear canal). A recent review of TM rupture differentiated between the lowest levels at which TM rupture are observed, and levels that are widely used as a threshold for rupture (Staab, 2012). Specifically, tympanic membrane rupture is unlikely to occur at ~7 kPa (Cameron et al., 1992), but can be observed as low as 35 kPa (Stewart et al., 2006). TM rupture thresholds are generally defined as the peak pressure at 50% probability of rupture, which varies between 43 kPa (Beveridge, 2011) to ~ 100 kPa (Richmond et al., 1989; Stewart et al., 2006). Investigations of large animals (pigs and sheep) exposed to blast waves inside armored vehicles revealed ~36% of ears were damaged in response to the lowest (41 ± 21 kPa) peak overpressure blasts, whereas 71% were injured in response to the highest (154 ± 66 kPa) blast waves, and higher peak pressures were associated with greater rupture incidence (Phillips et al., 1989). Studies in human cadavers (summarized by (Richmond et al., 1989)) have generally observed similar responses, showing TM ruptures: varying between 37–298 kPa with a mean of 158 kPa in normal ears (Zalewski, 1906), with a 50% incidence rate at 100 kPa (Blake et al.), and incidence rising from 1% at 21 kPa, to 50% at 110 kPa (Hirsch, 1968). These studies further suggest rise-time, duration, and frequency content of the blast exposure, as well as health and condition of the tissue affect TM rupture threshold. Overall, while occurring at somewhat lower levels than observed in some studies, the TM ruptures observed here (at ~55 kPa or higher peak overpressure levels) is consistent with the range of rupture thresholds observed previously.
4.5. Limitations to this study
In this report we developed equipment and procedures intended to minimize artifacts in the recorded measurements; however, some methodological issues are unavoidable when making sensitive measurements in such a high intensity noise environment. In particular, while we went to great lengths to fix the sensor tips, and shield the sensor leads along their entire length, motion artifacts are unavoidable, especially at the highest levels tested. Additionally, the signal conditioner used to drive the fiber optic interferometry system, and which provides an analog output of the ongoing sound pressure level detected, includes a digital stage, which allows optional filtering and sample averaging (which we disabled during these measurements), limits the conditions under which results may be collected. The digital stage operates at 200 kHz sampling rate, which is sufficient for most sound stimuli, but is too slow to capture the microsecond duration rise time observed with high intensity shock wave exposure (Chandra et al., 2012). Likewise, another potential source of error is the related to the maximum rate-of-change of the fiber optic sensors and signal conditioning, and failures of the system’s fringe-tracking system observed in some measurements (not shown). Note, responses of sensors exhibiting these failures are not included in the analysis presented above. Finally, the fiber-optic pressure sensors operate linearly within a limited pressure range, and while the sound pressure levels used here should be within the calibrated range of these sensors, it is possible that the pressures reported are underestimates of the actual pressures encountered, especially for the highest level exposures.
The nature of the stimuli presented, in addition to the level at which they were presented, also limit the comparability of the current results with prior characterizations of middle ear transfer functions. In particular, the shock waves presented here show a frequency response with substantial energy at low, and less energy at high frequencies. The motion and transmission of the middle ear is highly frequency dependent (Guinan et al., 1967), as are nonlinearities in the ossicular chain (Kalb et al., 1987; Price, 1974a), thus direct comparisons to transfer functions collected with steady state tones (Greene et al., 2017), and/or at more moderate sound pressure levels (Nakajima et al., 2009), may not be appropriate. Overall, since the middle ear is expected to respond nonlinearly, transfer functions provide limited utility in describing the generalized response of the system to these high level stimuli. For this reason, it is important to thoroughly characterize the responses of the middle ear to high intensity, impulse noise stimuli in order to develop accurate injury prediction criteria.
5. CONCLUSIONS
These results represent the first measurements of SPL in the cadaveric human cochleae during shock wave exposure. The results agree with prior studies concluding that reductions in ossicular chain motion reduces cochlear stimulation at high exposure levels (acoustic impulses at or above 170 dB Peak), but did not reveal any evidence of a dose-response inversion with increasing sound level. These results may be used to test HPDs, and models of auditory risk and injury may be validated and extended with these results.
6. ACKNOWLEDGEMENTS
The authors gratefully acknowledge the technical assistance of Luke Aurich, Michael Hall, PhD, Ryan Lowe, PhD, Evan Sandstrom, Greg Weiss, and Dan Welsh. Funding was provided by the U.S. Army Medical Research and Material Command under Contract No. W81XWH-15-2-0002.
REFERENCES
- ANSI. 1973. Terminology, ANSI Psychoacoustical S3. 20. New York: American National Standards Institute. [Google Scholar]
- Berger EH 1986. Methods of measuring the attenuation of hearing protection devices. J Acoust Soc Am 79, 1655–87. [DOI] [PubMed] [Google Scholar]
- Berger EH, Kieper RW, Gauger D 2003. Hearing protection: surpassing the limits to attenuation imposed by the bone-conduction pathways. J Acoust Soc Am 114, 1955–67. [DOI] [PubMed] [Google Scholar]
- Beveridge A 2011. Forensic investigation of explosions CRC press. [Google Scholar]
- Blake P, Douglas J, Krohn P, Zuckerman S Rupture of the Eardrum by Blast Ministry of Home Security Report BPC 43/179/WS 21, Military Personnel Research Committee (Medical Research Council), Department of Human Anatomy. Oxford University, Oxford, England. [Google Scholar]
- Bouwman JG 2016. Quantitative Susceptibility Mapping for MRI part 1 [Online] https://www.mathworks.com/matlabcentral/fileexchange/48557-quantitative-susceptibility-mapping-for-mri-part-1?focused=6248682&tab=function (verified 3 Nov 2017). [Google Scholar]
- Bruins WR, Cawood RH 1991. Blast injuries of the ear as a result of the Peterborough lorry explosion: 22 March 1989. J Laryngol Otol 105, 890–5. [DOI] [PubMed] [Google Scholar]
- Cameron JR, Skofronick JG, Grant RM 1992. Medical Physics: physics of the body Medical Physics Publishing Corporation. [Google Scholar]
- Chandler DW, Edmond CV 1997. Effects of blast overpressure on the ear: case reports. J Am Acad Audiol 8, 81–8. [PubMed] [Google Scholar]
- Chandra N, Ganpule S, Kleinschmit N, Feng R, Holmberg A, Sundaramurthy A, Selvan V, Alai A 2012. Evolution of blast wave profiles in simulated air blasts: experiment and computational modeling. Shock Waves, 1–13. [Google Scholar]
- Cho SI, Gao SS, Xia A, Wang R, Salles FT, Raphael PD, Abaya H, Wachtel J, Baek J, Jacobs D, Rasband MN, Oghalai JS 2013. Mechanisms of hearing loss after blast injury to the ear. PLoS One 8, e67618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavier OH, Dietz AJ, Wilbur JC, Zechmann EL, Murphy WJ 2012. Measurements of bone-conducted impulse noise from weapons using a head simulator. The Journal of the Acoustical Society of America 132, 2014–2014. [Google Scholar]
- Coles RR, Garinther GR, Hodge DC, Rice CG 1968. Hazardous exposure to impulse noise. J Acoust Soc Am 43, 336–43. [DOI] [PubMed] [Google Scholar]
- Dancer A, Franke R 1980. Intracochlear sound pressure measurements in guinea pigs. Hear Res 2, 191–205. [DOI] [PubMed] [Google Scholar]
- De Paolis A, Bikson M, Nelson JT, de Ru JA, Packer M, Cardoso L 2017. Analytical and numerical modeling of the hearing system: Advances towards the assessment of hearing damage. Hearing Research. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Deveze A, Koka K, Tringali S, Jenkins HA, Tollin DJ 2010. Active middle ear implant application in case of stapes fixation: a temporal bone study. Otol Neurotol 31, 1027–34. [DOI] [PubMed] [Google Scholar]
- DoD. 2015. MIL-STD-1474E, Department of Defense Design Criteria Standard: Noise Limits. [Google Scholar]
- Fausti SA, Wilmington DJ, Helt PV, Helt WJ, Konrad-Martin D 2005. Hearing health and care: the need for improved hearing loss prevention and hearing conservation practices. J Rehabil Res Dev 42, 45–62. [DOI] [PubMed] [Google Scholar]
- Flamme GA, Deiters KK, Tasko SM, Ahroon WA 2017. Acoustic reflexes are common but not pervasive: evidence from the National Health and Nutrition Examination Survey, 1999–2012. Int J Audiol 56, 52–62. [DOI] [PubMed] [Google Scholar]
- Greene NT, Jenkins HA, Tollin DJ, Easter JR 2017. Stapes displacement and intracochlear pressure in response to very high level, low frequency sounds. Hear Res 348, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene NT, Mattingly JK, Jenkins HA, Tollin DJ, Easter JR, Cass SP 2015. Cochlear Implant Electrode Effect on Sound Energy Transfer Within the Cochlea During Acoustic Stimulation. Otol Neurotol 36, 1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ Jr., Peake WT 1967. Middle-ear characteristics of anesthetized cats. J Acoust Soc Am 41, 1237–61. [DOI] [PubMed] [Google Scholar]
- Hato N, Stenfelt S, Goode RL 2003. Three-dimensional stapes footplate motion in human temporal bones. Audiol Neurootol 8, 140–52. [DOI] [PubMed] [Google Scholar]
- Henderson D, Hamernik RP 1986. Impulse noise: critical review. J Acoust Soc Am 80, 569–84. [DOI] [PubMed] [Google Scholar]
- Hirsch FG 1968. Effects of overpressure on the ear--a review. Ann N Y Acad Sci 152, 147–62. [DOI] [PubMed] [Google Scholar]
- Hynson K, Hamernik R, Henderson D 1976. B‐duration impulse definition: Some interesting results. The Journal of the Acoustical Society of America 59, S30–S30. [Google Scholar]
- Jones HG, Greene NT, Ahroon WA 2017. Assessment of Middle Ear Function During the Acoustic Reflex Using Laser-Doppler Vibrometry. U.S. Army Aeromedical Research Laboratory, Ft. Rucker, AL. [Google Scholar]
- Kalb JT, Price GR 1987. Mathematical model of the ear’s response to weapons impulses, Proceedings of the Third Conference on Weapon Launch Noise Blast Overpressure, U.S.Army Ballistics Research Lab, Aberdeen Proving Ground, MD. [Google Scholar]
- Kuroda R 1993. [Clinical study on perforation of the tympanic membrane and discussion based on experimentally induced tympanic rupture]. Nihon Jibiinkoka Gakkai Kaiho 96, 1490–500. [DOI] [PubMed] [Google Scholar]
- Lew HL, Jerger JF, Guillory SB, Henry JA 2007. Auditory dysfunction in traumatic brain injury. J Rehabil Res Dev 44, 921–8. [DOI] [PubMed] [Google Scholar]
- Lynch TJ 3rd, Nedzelnitsky V, Peake WT 1982. Input impedance of the cochlea in cat. J Acoust Soc Am 72, 108–30. [DOI] [PubMed] [Google Scholar]
- Mattingly JK, Greene NT, Jenkins HA, Tollin DJ, Easter JR, Cass SP 2015. Effects of Skin Thickness on Cochlear Input Signal Using Transcutaneous Bone Conduction Implants. Otol Neurotol 36, 1403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AK, Banakis Hartl RM, Greene NT, Benichoux V, Mattingly JK, Cass SP, Tollin DJ 2017. Semicircular Canal Pressure Changes During High-intensity Acoustic Stimulation. Otol Neurotol 38, 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima HH, Dong W, Olson ES, Merchant SN, Ravicz ME, Rosowski JJ 2009. Differential intracochlear sound pressure measurements in normal human temporal bones. J Assoc Res Otolaryngol 10, 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens BD, Kragh JF, Wenke JC, Macaitis J, Wade CE, Holcomb JB 2008. Combat Wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J. Trauma Inj. Infect. Crit. Care 64, 295–299. [DOI] [PubMed] [Google Scholar]
- Patterson JH Jr, Johnson DL 1994a. Temporary threshold shifts produced by high intensity freefield impulse noise in humans wearing hearing protection. US Army Aeromedical Research Laboratory, Ft. Rucker, AL. [Google Scholar]
- Patterson JH Jr, Hamernik RP, Hargett CE, Ahroon WA 1994b. An isohazard function for impulse noise. J. Acoust. Soc. Am 93, 2860–2869. [Google Scholar]
- Phillips YY, Mundie TG, Hoyt R, Dodd KT 1989. Middle ear injury in animals exposed to complex blast waves inside an armored vehicle. Ann Otol Rhinol Laryngol Suppl 140, 17–22. [DOI] [PubMed] [Google Scholar]
- Price GR 1974a. Upper limit to stapes displacement: implications for hearing loss. J Acoust Soc Am 56, 195–9. [DOI] [PubMed] [Google Scholar]
- Price GR 1974b. Transformation function of the external ear in response to impulsive stimulation. J Acoust Soc Am 56, 190–4. [DOI] [PubMed] [Google Scholar]
- Price GR 2007. Validation of the auditory hazard assessment algorithm for the human with impulse noise data. J Acoust Soc Am 122, 2786–802. [DOI] [PubMed] [Google Scholar]
- Price GR, Wansack S 1989. Hazard from an intense midrange impulse. J Acoust Soc Am 86, 2185–91. [DOI] [PubMed] [Google Scholar]
- Price GR, Kalb JT 1991. Insights into hazard from intense impulses from a mathematical model of the ear. The Journal of the Acoustical Society of America 90, 219–227. [DOI] [PubMed] [Google Scholar]
- Price GR, Kalb JT, Jokel CR 2017. Brief critical examination of the article:“Impulse noise injury prediction based on the cochlear energy” by Zagadou, Chan, Ho and Shelly . Hearing Research 350, 43–44. [DOI] [PubMed] [Google Scholar]
- Remenschneider AK, Lookabaugh S, Aliphas A, Brodsky JR, Devaiah AK, Dagher W, Grundfast KM, Heman-Ackah SE, Rubin S, Sillman J, Tsai AC, Vecchiotti M, Kujawa SG, Lee DJ, Quesnel AM 2014. Otologic outcomes after blast injury: the Boston Marathon experience. Otol Neurotol 35, 1825–34. [DOI] [PubMed] [Google Scholar]
- Richmond DR, Fletcher ER, Yelverton JT, Phillips YY 1989. Physical correlates of eardrum rupture. Annals of Otology, Rhinology & Laryngology 98, 35–41. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ, Chien W, Ravicz ME, Merchant SN 2007. Testing a method for quantifying the output of implantable middle ear hearing devices. Audiol Neurootol 12, 265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoorenburg G 2003. Risk of Hearing Loss from Exposure to Impulse Sounds. Brussels: North Atlantic Treaty Organization; 2003; Report No. RTO-TR-017. [Google Scholar]
- Staab W 2012. Eardrum Rupture – At What Pressure? [Online]. Available by Hearing Health and Technology Matters, LLC http://hearinghealthmatters.org/waynesworld/2012/eardrum-rupture-at-what-pressure/ (verified 11/9/2017). [Google Scholar]
- Stewart C, Jagoda A, Howell JM 2006. Blast injuries: preparing for the inevitable. Emergency Medicine Practice+ Em Practice Guidelines Update 8, 1–26. [Google Scholar]
- VBA. 2017. VBA Annual Benefits Report Fiscal Year 2016. [Online]. Available by VBA (Veterans Benefits Administration; ) (verified 06/19/2017). [Google Scholar]
- Walilko TJ, Lowe RD, Argo TF, Meegan GD, Greene NT, Tollin DJ 2017. Experimental Evaluation of Blast Loadings on the Ear and Head with and Without Hearing Protection Devices, Mechanics of Biological Systems and Materials, Volume 6 Springer; pp. 101–109. [Google Scholar]
- White PJ, Clement GT, Hynynen K 2006. Longitudinal and shear mode ultrasound propagation in human skull bone. Ultrasound Med Biol 32, 1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener FM, Ross DA 1946. The pressure distribution in the auditory canal in a progressive sound field. The Journal of the Acoustical Society of America 18, 401–408. [Google Scholar]
- Wightman F, Flamme G, Campanella A, Luz G, Rosentahal T, Gallo S 2010. AIBS peer review of injury prevention and reduction research task area impulse noise models. American Institute of Biological Sciences. [Google Scholar]
- Yetiser S, Ustun T 1993. Concussive blast-type aural trauma, eardrum perforations, and their effects on hearing levels: an update on military experience in Izmir, Turkey. Mil Med 158, 803–6. [PubMed] [Google Scholar]
- Zagadou B, Chan P, Ho K, Shelley D 2016. Impulse noise injury prediction based on the cochlear energy. Hear Res 342, 23–38. [DOI] [PubMed] [Google Scholar]
- Zagadou B, Chan P, Ho K, Shelley D 2017. Reply to “Critical examination of the article: Impulse noise injury prediction based on the cochlear energy”. Hearing Research 350, 217–221. [DOI] [PubMed] [Google Scholar]
- Zalewski T 1906. Experimentelle untersuchungen uber die resistenzfahigkeit des trommelfells. Z Ohrenheilkd 52, 109. [Google Scholar]