Abstract
Introduction
Few studies have simultaneously examined the role of sanitation conditions at the home, school, and community on soil-transmitted helminth (STH) infection. We examined the contribution of each domain that children inhabit (home, village, and school) to STH infection and estimated the association of STH infection with sanitation in each domain.
Methods
Using data from 4,104 children from Kwale County, Kenya, who reported attending school, we used logistic regression models with cross-classified random effects to calculate measures of general contextual effects and estimate associations of village sanitation coverage (percentage of households with reported access to sanitation), school sanitation coverage (number of usable toilets per enrolled pupil), and sanitation access at home with STH infection.
Findings
We found reported use of a sanitation facility by households was associated with reduced prevalence of hookworm infection but not with reduced prevalence of T. trichiura infection. School sanitation coverage > 3 toilets per 100 pupils was associated with lower prevalence of hookworm infection. School sanitation was not associated with T. trichiura infection. Village sanitation coverage > 81% was associated with reduced prevalence of T. trichiura infection, but no protective association was detected for hookworm infection. General contextual effects represented by residual heterogeneity between village and school domains had comparable impact upon likelihood of hookworm and T. trichiura infection as sanitation coverage in either of these domains.
Conclusion
Findings support the importance of providing good sanitation facilities to support mass drug administration in reducing the burden of STH infection in children.
Author summary
Infection by the soil-transmitted helminths (STH) whipworm and hookworm results from either ingestion of eggs or larvae or through skin exposure to larvae. These eggs and larvae develop in suitable soils contaminated with openly-deposited human faeces. Safe disposal of faeces should reduce transmission of STH, yet evidence of the impact of sanitation on STH transmission remains limited. A large, community-wide survey was conducted in 2015 to measure prevalence of STH infections in Kwale County, Kenya. Here, we used this data and observations made in schools to examine the relationship between sanitation conditions at home, school, and village and the presence of STH infection among 4,104 children who attend school. We found that sanitation access at home and school sanitation coverage (number of usable toilets per enrolled pupil), but not the overall level of village sanitation coverage (percentage of households with reported access to sanitation), was protective against hookworm infection. In contrast, only high village sanitation coverage, but not home or school sanitation, was protective against whipworm infection. Current STH control strategies emphasise periodic deworming of at-risk populations, including school-age children. Our findings highlight the need for continued efforts, alongside deworming, to extend access to good sanitation facilities at homes, schools, and across communities.
Introduction
In 2016, it was estimated that world-wide more than 1.5 billion people were infected with at least one species of soil-transmitted helminth (STH)[1]. Infection by roundworm, Ascaris lumbricoides, and whipworm, Trichuris trichiura, results from ingestion of embryonated eggs. The hookworms, Necator americanus and Ancylostoma duodenale, infect humans through skin exposure to or ingestion of larvae (A. duodenale) that develop in warm, moist soils from eggs in openly-deposited human faeces [2, 3]. Preventing human contact with excreta through consistent safe disposal of faeces should reduce STH transmission, yet evidence of the impact of sanitation on STH remains limited [4–6]. The concept of private and public domains of transmission for STH has been described previously [7, 8], but to our knowledge few studies have simultaneously examined the role of sanitation conditions at the home, school, and community on STH infection [9–11].
Multilevel statistical models provide a means to estimate effects of individual factors, using measures of association. They can simultaneously assess general contextual effects upon individual health outcomes, using measures of within-unit clustering and between-unit heterogeneity [12, 13]. Such models provide a useful complement to mathematical modelling of transmission dynamics for examining specific effects and possible areas for intervention [14]. Identifying effects of sanitation within each of the domains that a child inhabits will contribute to evidence for the prioritization of sanitation promotion activities alongside the regular deworming of at-risk populations, including school-aged children, recommended by the World Health Organization [15, 16].
Employing multilevel modelling, we investigate the relative importance of household, village, and school domains and estimate the association of sanitation in each domain on STH infection among Kenyan children who attend school.
Methods
Study population
The study took place in Kwale County on the south Kenyan coast. Data were collected during a cross-sectional parasitological survey conducted between March-May 2015 as the baseline for TUMIKIA, a randomised, controlled trial to evaluate the impact and cost-effectiveness of school-based versus community-wide deworming on STH transmission (NCT02397772, www.clinicaltrials.gov).
Study design, baseline findings, and impact have been described previously [17–19]. The TUMIKIA trial was implemented via community units (CUs)—government health-service delivery structures of approximately 1,000 households, comprising 2 to 7 villages. For the baseline survey, 225 households were randomly selected within 120 CUs. Among consenting households, a structured questionnaire was conducted with the head of household or primary caregiver to collect information on demographics, ownership of key assets, and sanitation, hygiene, and water conditions [20]. One household member (aged ≥ 2 years) was randomly selected to provide a stool sample. A questionnaire was then conducted with individuals who provided samples or their caregiver (for those under 5 years old) to collect information on deworming within the last year and observe their footwear. School facility surveys were conducted across Kwale County in June 2015 and July 2016. During visits, student enrolment was recorded from school registers, and sanitation conditions were assessed using structured observations. One school surveyed in 2015 was missing the total number of children enrolled, so the 2016 enrolment number was used. For 51 schools attended by children for which we had no 2015 data, enrolment and sanitation conditions from the 2016 survey were used. All data were collected on smartphones running the Android operating system (Google, Mountain View, CA, USA) using SurveyCTO (Dobility, Inc., Cambridge, MA, USA). Records from school and household surveys were linked based on the school each child reported attending. Geographic coordinates (based on WGS84 datum) were systematically collected at each household and school using the smartphones’ global positioning systems. Missing coordinates for 4 schools were obtained from Google Maps (Google, Mountain View, CA, USA). Children were excluded a priori if they lived in villages or attended schools in semi-arid areas unsuitable for STH transmission [19]. Children were eligible if they were sampled in the 2015 TUMIKIA baseline parasitological survey, aged 5 to 14 years, and reported attending school.
STH infection
Kato-Katz microscopy was used to enumerate STH eggs (A. lumbricoides, T. trichiura, and hookworm) per gram of stool. Duplicate slides were prepared from a single stool sample and read by independent microscopists. STH infection was classified based on categories of infection intensity [21], and frequencies were tabulated across categories of household, community, and school sanitation as detailed below. For both hookworm and T. trichiura, our outcome was a dichotomous indicator for the presence of > 0 eggs in stool samples (i.e. prevalence). A. lumbricoides was not examined in detail since few cases were detected.
Sanitation measures
The measure for household sanitation access was combined from reported use of a toilet facility on or off the household’s compound. Using all households sampled per village for the TUMIKIA baseline survey, this measure of household sanitation was aggregated for village sanitation coverage, as the percentage of households with reported access to sanitation. During structured observations at schools, the number of latrines considered usable (not assigned to teachers, locked, or with full pits) was quantified, excluding urinals, for both girls and boys. School sanitation coverage was calculated as the number of usable toilets per enrolled pupil, in contrast to the indicator of students per toilet [22]. Village and school sanitation coverage were categorised based on estimated quartiles to explore possible non-linear relationships during modelling.
Covariates
Candidate individual covariates included median-centred age in years, sex, a dichotomous indicator for observed shoe wearing, an indicator for whether household floor was covered (relative to an earth/sand floor), and an indicator for reported deworming with albendazole in past year. Relative wealth was determined using a factor analysis, separately for rural and urban households, of dichotomous indicators for ownership of household assets, household wall and roof materials, and household access to electricity. The indices were then divided into quintiles within urban and rural settings, and then second, third, and fourth quintiles were combined prior to analysis [19]. For villages and schools, environmental and sociodemographic conditions were hypothesized to influence both STH transmission and sanitation coverage. Areas were classified as urban, peri-urban, or rural using 2015 estimates of population density [23]. Percentage soil sand content was extracted from soilgrids.org at a resolution of 250 m [24]. Sand content was first categorised into tertiles and then a dichotomous indicator for highest sand content. A measure of aridity was obtained from the Consortium for Spatial Information (CGIAR-CSI) [25]. These measures were assembled in a geographic information system using ArcGIS 10.3 (ESRI, Redlands, CA, USA), and values were extracted for each school and household based on GPS coordinates [19]. We then aggregated mean continuous and mode categorical household values per village, using all households sampled per village.
Ethical approval
The TUMIKIA trial protocol was approved by the Kenya Medical Research Institute and National Ethics Review Committee (SSC Number 2826) and the London School of Hygiene & Tropical Medicine (LSHTM) Ethics Committee (7177). Written informed consent was sought from the household head or adult answering the household-level questionnaire. Written informed consent was also sought from adults (≥18 years) selected to provide the stool sample and complete the individual-level questionnaire. Parental consent was sought for individuals aged 2 to 17 years, and written assent was additionally obtained from children aged 13 to 17 years. All information and consent procedures were conducted in Kiswahili.
Statistical analyses
We estimated associations between sanitation conditions in the domains of interest (household, school, village) and presence of hookworm and T. trichiura infection, separately, using logistic regression models with cross-classified (non-nested) random effects to account for membership of children within village of residence and school attended.
We fit a series of generalised mixed models (with logit link), excluding observations with missing outcome or covariate information, which assumes the probability of having complete data is independent of the outcome after adjusting for included covariates. First, we fit an intercept-only model containing school- and village-specific random effects to quantify between school and between village variation in STH infection. We then fit a series of models to explore changes in general contextual effects for village and school domains with the inclusion of fixed sanitation effects and confounders at the different levels. Next, we fit models with fixed effects for sanitation conditions in each domain separately and then together in a combined crude model. Finally, we fit a model containing all sanitation effects, adjusting for potential confounders. Confounders were selected from candidate individual, village, and school covariates based on existing knowledge and encoding possible causal relationships in directed acyclic graphs. We then implemented d-separation in DAGitty to identify minimal sufficient sets of available covariates to control to estimate effects of sanitation on both hookworm (S1 Fig and S1 Text) and T. trichiura infections (S2 Fig and S2 Text) [26, 27]. Estimation used Hamiltonian Monte Carlo sampling to improve mixing and reduce auto-correlation [28]. We conducted a sensitivity analysis excluding outliers in and missingness of distance from child’s home to reported school attended.
We report prevalence odds ratios (PORs) with 95% credible intervals (CIs) as fixed effects for sanitation in each domain. In addition to the specific contextual (fixed) effects for village and school sanitation, we also calculated measures to quantify general contextual effects on individual infection [13, 29]. Proportion of total individual variation in the outcome attributable to between-school and between-village variation, or Variance Partition Coefficient (VPC), was calculated using the latent variable method. This method converts individual variance to the logistic scale from the probability scale and assumes an underlying continuous propensity for infection following a logistic distribution with individual variance of π2/3 [30]. The VPC is interpreted in the same way as the Intraclass Correlation Coefficient (ICC) only when the structure of the information is hierarchical. We calculated median odds ratios (MORs), as measures of residual heterogeneity on the odd ratio scale. MORs are always greater than or equal to 1 (MOR = 1 if there is no variation between areas) and interpreted as the median value of the odds ratios between comparable individuals drawn randomly from high and low risk areas and always having the individual from the higher risk area in the numerator. MORs are useful as they measure how much individual infection is determined by domain membership and are directly comparable to fixed effects [30]. The VPC is recommended, however, for estimating general contextual effects as a measure of clustering that incorporates both between- and within-area variance [12]. We calculated 80% interval odds ratios (80% IORs) for village and school sanitation fixed effects. This measure does not reflect the estimate’s precision but instead is recommended to consider residual variation in the interpretation of fixed effects [31]. A wider interval indicates greater unexplained between-area variation, and the inclusion of 1 indicates between-area variance is large compared to the specific fixed effect. Analyses were conducted in Stata 15 (StataCorp LP, College Station, TX, USA) and in R 3.5 (r-project.org) using the ‘brms’ package.
Results
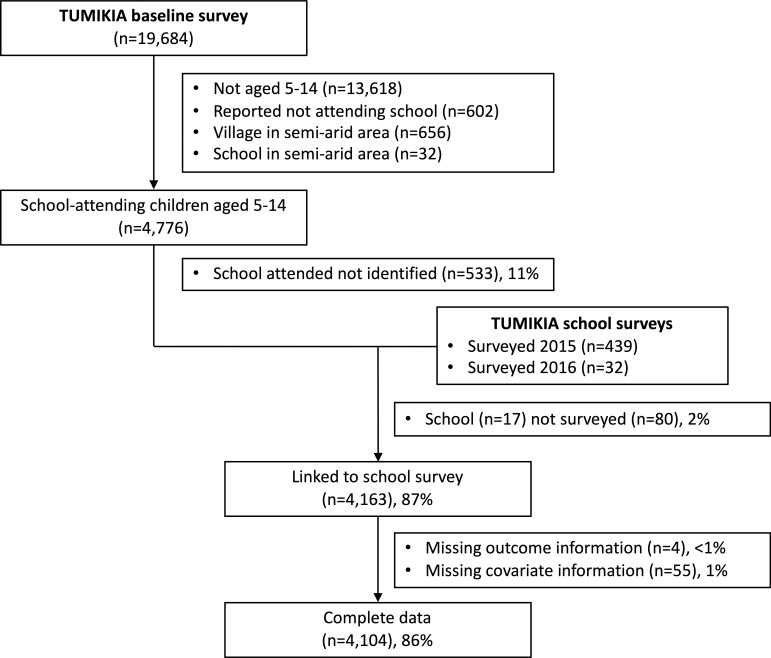
Of 6,066 school-aged children with matched samples in the survey, 602 (10%) reported not attending school, and 688 (11%) reported attending school but resided in a village or were linked to a school in a semi-arid area (Fig 1). Among these excluded children, prevalence of infection was 3% (22/688), < 1% (4/688), and 0% (0/688) for hookworm, T. trichiura and A. lumbricoides, respectively. Of 4,776 eligible children, the school reported to be attended was identified for 4,243 children (89%) and school survey data was available for 4,163 children (87%). Eligible children without school information were younger, more often being boys, less likely to have been dewormed, and less poor (S2 Table). Data were available for 4,104 eligible children (86%).
Fig 1. Flow chart of participants in the TUMIKIA baseline survey who were included in the current analysis.
Eligible sample included children aged 5–14 years who reported attending school and not residing in villages or attending schools in semi-arid areas. The proportion of eligible subjects with complete data is 86% (4,104/4,776).
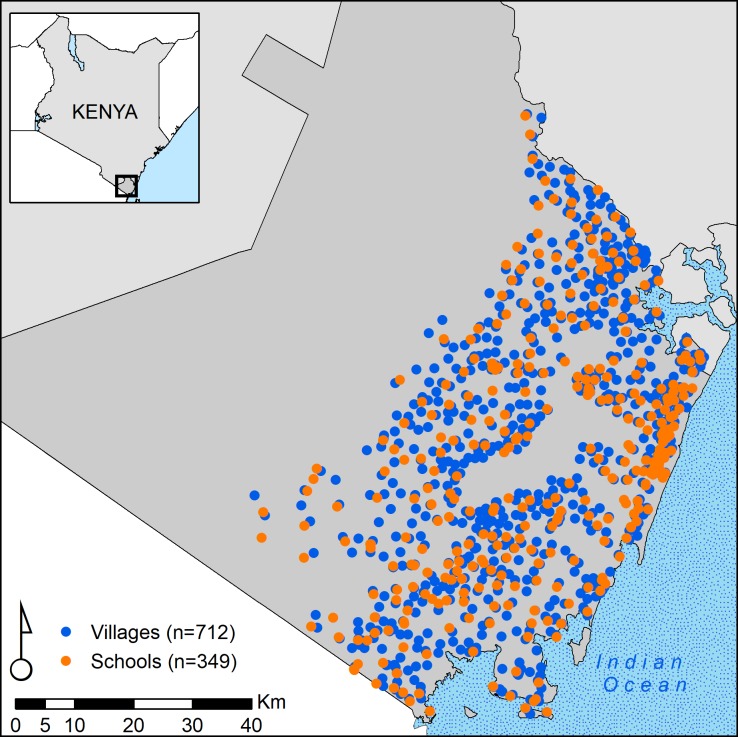
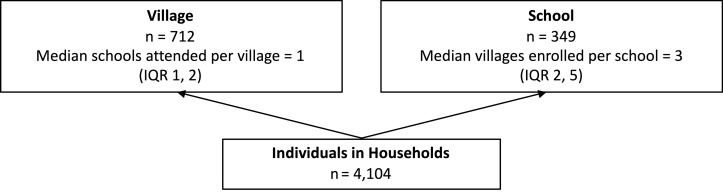
Participants resided in 712 villages (median sampled per village 4, range 1, 38) and reported attending 349 schools (median sampled per school 9, Range 1, 49) (Fig 2). Fig 3 describes the structure of the data. In half of all villages, resident children reported attending the same school (range 1 to 8 schools per village). Included schools enrolled children from 1 up to 13 villages. Table 1 describes individual, household, and village characteristics of included children.
Fig 2. Village of residence and attended schools among 4,104 school-attending children aged 5–14 years in Kwale County, Kenya, 2015.
Fig 3. Diagram for the classification model of individuals, villages, and schools.
Table 1. Summary characteristics for 4,104 school-attending children in coastal Kenya, 2015.
| Characteristics | No. or Mean | % or SD | |
|---|---|---|---|
| Individual level (n = 4,104) | |||
| Age (years) | 9.46 | 2.65 | |
| Being girls | 2,129 | 51.88 | |
| Observed wearing shoes | 1,443 | 35.16 | |
| Reported deworming in past year | 2,219 | 54.07 | |
| Reported household access to toilet | 2,255 | 54.95 | |
| Reported improved water source | 2,216 | 54.00 | |
| Missing | 13 | 0.32 | |
| Time to fetch water, < 30 min | 3,344 | 81.48 | |
| Missing | 14 | 0.34 | |
| Covered floor | 833 | 20.30 | |
| Household wealth quintile | |||
| Most poor, quintile 1 | 1,190 | 29.00 | |
| Quintiles 2–4 | 2,158 | 52.58 | |
| Least poor, quintile 5 | 756 | 18.42 | |
| Distance to school (km) (n = 3,906) | 2.25 | 5.82 | |
| Village level (n = 712) | |||
| Proportion reporting toilet access | 0.53 | 0.31 | |
| Urbanization | |||
| Rural | 545 | 76.54 | |
| Periurban | 141 | 19.80 | |
| Urban | 26 | 3.65 | |
| Aridity index | |||
| Dry sub-humid (> 0.5–0.65) | 284 | 39.89 | |
| Humid (> 0.65) | 428 | 60.11 | |
| Soil sand content ≥ 62% | 256 | 35.96 | |
| School level (n = 349) | |||
| Usable toilets per pupil | 0.03 | 0.02 | |
| Pupils per usable toilet | 57.79 | 47.35 | |
| Total enrolment | 479.47 | 270.67 | |
| Urbanization | |||
| Rural | 234 | 67.05 | |
| Periurban | 84 | 24.07 | |
| Urban | 31 | 8.88 | |
| Aridity index | |||
| Dry sub-humid (> 0.5–0.65) | 130 | 37.25 | |
| Humid (> 0.65) | 219 | 62.75 | |
| Soil sand content ≥ 62% | 126 | 36.10 | |
Overall prevalence of any detected infection was 17.8% and 6.0% for hookworm and T. trichiura, respectively, and most infections were classified as light (Table 2). Comparing MOR in Tables 3 and 4, school attended had greater impact than village of residence upon hookworm infection (School MOR 2.73, 95% CI 2.50, 2.98; Village MOR 2.09, 95% CI 1.74, 2.38). School and village membership had comparable impacts on T. trichiura infection (School MOR 2.92, 95% CI 2.40, 3.43; Village MOR 2.78, 95% CI 2.28, 3.29). Calculated VPC showed similar results.
Table 2. Intensity and any presence of STH infections by household, school, and village sanitation conditions among 4,104 school-attending children in coastal Kenya, 2015.
| Intensity | |||||||
|---|---|---|---|---|---|---|---|
| Infection | N | None % |
Light % |
Moderate % |
Heavy % |
Any % |
|
| Hookworm | Overall | 4,104 | 82.21 | 16.91 | 0.56 | 0.32 | 17.79 |
| Household sanitation access |
|||||||
| Yes | 2,255 | 84.12 | 15.25 | 0.40 | 0.22 | 15.88 | |
| No | 1,849 | 79.88 | 18.93 | 0.76 | 0.43 | 20.12 | |
| School sanitation coverage (per 100) | |||||||
| < 1.49 | 1,182 | 81.30 | 17.77 | 0.68 | 0.25 | 18.70 | |
| 1.49–2.17 | 1,181 | 80.10 | 19.14 | 0.68 | 0.08 | 19.90 | |
| 2.18–3.13 | 1,077 | 83.84 | 15.51 | 0.46 | 0.19 | 16.16 | |
| > 3.13 | 664 | 84.94 | 13.70 | 0.30 | 1.05 | 15.06 | |
| Village sanitation coverage | |||||||
| ≤ 0.25 | 792 | 83.59 | 15.53 | 0.63 | 0.25 | 16.41 | |
| 0.26–0.54 | 1,123 | 82.37 | 16.92 | 0.53 | 0.18 | 17.63 | |
| 0.54–0.81 | 1,130 | 77.88 | 20.71 | 0.88 | 0.53 | 22.12 | |
| > 0.81 | 1,059 | 85.65 | 13.88 | 0.19 | 0.28 | 14.35 | |
| T. trichiura | Overall | 4,104 | 94.05 | 5.43 | 0.49 | 0.02 | 5.95 |
| Household sanitation access | |||||||
| Yes | 2,255 | 94.10 | 5.54 | 0.35 | 0.00 | 5.90 | |
| No | 1,849 | 94.00 | 5.30 | 0.65 | 0.05 | 6.00 | |
| School sanitation coverage (per 100) | |||||||
| < 1.49 | 1,182 | 92.89 | 6.35 | 0.68 | 0.08 | 7.11 | |
| 1.49–2.17 | 1,181 | 95.17 | 4.40 | 0.42 | 0.00 | 4.83 | |
| 2.18–3.13 | 1,077 | 94.15 | 5.48 | 0.37 | 0.00 | 5.85 | |
| > 3.13 | 664 | 93.98 | 5.57 | 0.45 | 0.00 | 6.02 | |
| Village sanitation coverage | |||||||
| ≤ 0.25 | 792 | 94.19 | 4.80 | 1.01 | 0.00 | 5.81 | |
| 0.26–0.54 | 1,123 | 94.57 | 5.08 | 0.36 | 0.00 | 5.43 | |
| 0.54–0.81 | 1,130 | 93.27 | 6.28 | 0.35 | 0.09 | 6.73 | |
| > 0.81 | 1,059 | 94.24 | 5.38 | 0.38 | 0.00 | 5.76 | |
| A. lumbricoides | Overall | 4,104 | 99.32 | 0.41 | 0.24 | 0.02 | 0.68 |
Hookworm intensity categories (epg = eggs per gram): none 0 epg; light <2,000 epg; moderate 2,000-<4,000 epg; heavy ≥4,000 epg
T. trichiura intensity categories: none 0 epg; light <1,000 epg; moderate 1,000-<10,000 epg; heavy ≥10,000 epg
A. lumbricoides intensity categories: none 0 epg; light <5,000 epg; moderate 5,000-<50,000 epg; heavy ≥50,000 epg
Table 3. Sanitation and contextual effects on presence of hookworm infection among 4,104 school-attending children in coastal Kenya.
| Intercept-Only | Crude | Adjusted1 | |||||
|---|---|---|---|---|---|---|---|
| Fixed Effects | POR | (95% CI) | POR | (95% CI) | POR | (95% CI) | 80% IOR |
| Household sanitation access |
-- |
-- |
0.63 |
(0.51, 0.79) |
0.76 |
(0.61, 0.95) |
-- |
| School sanitation coverage (per 100) | |||||||
| 1.49–2.17 | -- | -- | 0.89 | (0.55, 1.42) | 0.79 | (0.51, 1.21) | (0.14, 4.52) |
| 2.18–3.13 | -- | -- | 0.86 | (0.54, 1.36) | 0.64 | (0.40, 0.98) | (0.11, 3.66) |
| > 3.13 | -- | -- | 0.58 | (0.35, 0.98) | 0.51 | (0.31, 0.83) | (0.09, 2.94) |
| Village sanitation coverage | |||||||
| 0.26–0.54 | -- | -- | 1.28 | (0.89, 1.86) | 1.40 | (0.96, 2.08) | (0.33, 6.06) |
| 0.54–0.81 | -- | -- | 1.52 | (1.01, 2.29) | 1.86 | (1.22, 2.86) | (0.43, 8.01) |
| > 0.81 | -- | -- | 1.21 | (0.75, 1.92) | 1.40 | (0.86, 2.34) | (0.33, 6.06) |
| Contextual Effects | |||||||
| School | |||||||
| Variance | 1.11 | (0.92, 1.31) | 1.12 | (0.93, 1.32) | 0.93 | (0.74, 1.14) | -- |
| MOR | 2.73 | (2.50, 2.98) | 2.74 | (2.51, 2.99) | 2.51 | (2.27, 2.77) | -- |
| VPC | 0.22 | (0.20, 0.24) | 0.22 | (0.20, 0.24) | 0.19 | (0.17, 0.21) | -- |
| Village | |||||||
| Variance | 0.60 | (0.34, 0.83) | 0.61 | (0.34, 0.85) | 0.65 | (0.41, 0.89) | -- |
| MOR | 2.09 | (1.74, 2.38) | 2.11 | (1.74, 2.41) | 2.16 | (1.84, 2.46) | -- |
| VPC | 0.12 | (0.07, 0.15) | 0.12 | (0.07, 0.16) | 0.13 | (0.09, 0.17) | -- |
POR = Prevalence Odds Ratio; CI = Credible Interval; MOR = Median Odds Ratio; IOR = Interval Odds Ratio; VPC = Variance Partition Coefficient
1Adjusted for age (centred at 9), being female, reported deworming in past year, observed shoe-wearing, household floor covered, village high soil sand content, village aridity index (scaled 100x), village urban/periurban/rural, school high soil sand content, school aridity index (scaled 100x), school urban/periurban/rural
Table 4. Sanitation and contextual effects on presence of T. trichiura infection among 4,104 school-attending children in coastal Kenya.
| Intercept-Only | Crude | Adjusted1 | |||||
|---|---|---|---|---|---|---|---|
| Fixed Effects | POR | (95% CI) | POR | (95% CI) | POR | (95% CI) | 80% IOR |
| Household sanitation access |
-- |
-- |
0.95 |
(0.66, 1.38) |
1.00 |
(0.68, 1.46) |
-- |
| School sanitation coverage (per 100) | |||||||
| 1.49–2.17 | -- | -- | 0.75 | (0.36, 1.51) | 1.23 | (0.70, 2.23) | (0.29, 5.26) |
| 2.18–3.13 | -- | -- | 0.85 | (0.42, 1.70) | 1.07 | (0.60, 1.93) | (0.25, 4.57) |
| > 3.13 | -- | -- | 0.81 | (0.36, 1.73) | 1.06 | (0.56, 1.95) | (0.25, 4.53) |
| Village sanitation coverage | |||||||
| 0.26–0.54 | -- | -- | 1.00 | (0.51, 1.92) | 0.59 | (0.30, 1.15) | (0.09, 4.05) |
| 0.54–0.81 | -- | -- | 1.57 | (0.79, 3.16) | 0.82 | (0.41, 1.62) | (0.12, 5.57) |
| > 0.81 | -- | -- | 0.84 | (0.37, 1.90) | 0.30 | (0.14, 0.68) | (0.04, 2.07) |
| Contextual Effects | |||||||
| School | |||||||
| Variance | 1.26 | (0.84, 1.67) | 1.34 | (0.92, 1.78) | 0.64 | (0.11, 1.10) | -- |
| MOR | 2.92 | (2.40, 3.43) | 3.02 | (2.50, 3.57) | 2.14 | (1.37, 2.72) | -- |
| VPC | 0.22 | (0.17, 0.26) | 0.23 | (0.18, 0.27) | 0.13 | (0.03, 0.19) | -- |
| Village | |||||||
| Variance | 1.15 | (0.75, 1.56) | 1.17 | (0.77, 1.59) | 1.12 | (0.75, 1.51) | -- |
| MOR | 2.78 | (2.28, 3.29) | 2.81 | (2.31, 3.33) | 2.74 | (2.28, 3.23) | -- |
| VPC | 0.20 | (0.15, 0.24) | 0.20 | (0.15, 0.24) | 0.22 | (0.18, 0.26) | -- |
POR = Prevalence Odds Ratio; CI = Credible Interval; MOR = Median Odds Ratio; IOR = Interval Odds Ratio; VPC = Variance Partition Coefficient
1Adjusted for household SES category, village high soil sand content, village aridity index (scaled 100x), village urban/periurban/rural, school high soil sand content, school aridity index (scaled 100x), school urban/periurban/rural
In order to explore changes in these general contextual effects, inclusion of either a crude or a household sanitation measure adjusted for individual confounders in the model for hookworm infection did not change estimated MORs or VPCs (S3 Table), though school MOR and VPC were slightly reduced adjusting for village and school confounders. Further including school and village sanitation effects without adjustment for confounders at these levels did not change general contextual effects. Including a crude household sanitation measure, or with adjustment for household SES, in the model for T. trichiura did not change estimated school and village general contextual effects (S4 Table). School MOR and VPC were reduced with further adjustment for confounders in these domains. Including crude fixed effects for sanitation at the village and school level in separate models for hookworm and T. trichiura did not meaningfully change measures of variance and heterogeneity (S3 Table and S4 Table). Including all domain sanitation measures in the crude model did not change calculated MOR or VPC values for hookworm or T. trichiura relative to the intercept-only model (Tables 3 and 4). Adjusting for all potential confounders, residual heterogeneity between schools decreased for T. trichiura and, to a lesser extent, hookworm infection, but residual heterogeneity between villages was unchanged.
Reported presence of household sanitation access reduced odds of hookworm infection by 37%, compared to no household sanitation access (POR 0.63, 95% CI 0.51, 0.79), among children in villages and schools with similar sanitation conditions (Table 3). Adjusting for potential confounders, this association was attenuated towards null (POR 0.76, 95% CI 0.61, 0.95). School sanitation coverage in the two highest quartiles (> 2.17 toilets per 100 students) was associated with lower hookworm prevalence, compared to the lowest coverage quartile (< 1.49 toilets per 100 students), adjusting for potential confounders and household and village sanitation (POR 0.64, 95% CI 0.40, 0.98; POR 0.51, 95% CI 0.31, 0.83). No evidence of association of household or school sanitation with T. trichiura infection was detected (Table 4). Children in villages where 54 to 81% of households had sanitation access, compared to children in villages with ≤ 25% sanitation coverage, had 1.86 times the odds of hookworm infection (POR 1.86, 95% CI 1.22, 2.86). Children in villages where > 81% of households had sanitation access, compared to children in villages with ≤ 25% sanitation coverage, had 70% lower odds of T. trichiura infection (POR 0.30, 95% CI 0.14, 0.68). Results were robust to the exclusion of 199 children without household coordinates and 197 reporting attending a school > 6.5 km from their house.
Calculated 80% IORs from adjusted models for hookworm and T. trichiura were wide and included one for both village and school sanitation measures, indicating sanitation was less important for explaining individual infection, compared to residual variation between these domain levels. For example, comparing children with identical characteristics but drawn from either a village with sanitation coverage ≤ 25% or a village with sanitation coverage > 81%, odds of T. trichiura infection will be between 0.04 and 2.07 in 80% of such comparisons.
Discussion
In this analysis of baseline data from the TUMIKIA trial, we found notable differences between two species of STH in the relationship between sanitation availability and prevalence of infection within different domains among school-attending children in coastal Kenya. Reported use of a sanitation facility by households was associated with reduced prevalence of hookworm infection but was not associated with reduced prevalence of T. trichiura infection. Meanwhile, village sanitation coverage > 81% was associated with reduced prevalence of T. trichiura infection, but no protective association was detected for hookworm infection. School sanitation coverage > 3.13 toilets per 100 pupils was associated with lower prevalence of hookworm infection. This coverage level, corresponding to a pupil:toilet ratio of 32:1, supports the minimum ratios (25:1 for girls and 35:1 for boys) currently recommended by the Kenyan government [32]. School sanitation was not associated with T. trichiura infection, however. We found that general contextual effects, represented by residual heterogeneity between village and school domains, had comparable impact upon likelihood of hookworm and T. trichiura infection as sanitation coverage in either of these domains.
Three published meta-analyses record considerable heterogeneity in estimates of the effect of sanitation access on hookworm and T. trichiura infection. Ziegelbauer et al. found that sanitation was protective against both hookworm and T. trichiura infection [5]. Strunz et al. found no association of sanitation access with hookworm infection, but it was protective against T. trichiura [4]. Freeman et al., in the most recent review, found no association of sanitation access with T. trichiura infection but sanitation access was protective against hookworm [6]. Our finding of association of household and school sanitation with hookworm infection is consistent with this latter result, while adjusting for village sanitation coverage plus potential confounders and conditional on village and school membership. Albendazole, the medication used for Kenya’s National School-Based Deworming Programme (NSBDP), is less effective against T. trichiura, compared to hookworm and A. lumbricoides [33]. The observed associations of household and school sanitation with lower hookworm prevalence in this population of school-attending children could reflect impacts of sanitation in these domains on reinfection, following school-based deworming. In contrast, due to albendazole’s lower effectiveness against T. trichiura infection, Freeman et al. concluded that only a long-term impact of sanitation access might be observable following deworming, which our finding would also support [6].
The three meta-analyses described above did not distinguish between sanitation access at home or at school. We estimated the independent effects of sanitation access at both the household and school on STH infection. We also expanded upon previous work to estimate the effect of village sanitation coverage [9, 10]. The protective association of village sanitation coverage against T. trichiura may reflect longer-term impacts of sanitation and a possible community-wide (herd) effect at access levels > 80%, independent of household sanitation access and other factors. In contrast, we found no consistent pattern between village sanitation coverage and hookworm infection. We may not have observed an association because many of the included villages with the lowest sanitation coverage were also in the most arid environments, limiting their suitability for transmission.
Our results clearly show the large contextual effects of village and school domains relative to the estimated fixed effects. The 80% IOR does not indicate precision but provides an interval around our estimated village and school sanitation effects that incorporates unexplained variability between these domains. This result, coupled with the calculated VPCs and MORs, indicates that sanitation coverage in these domains is not a strong predictor of hookworm or T. trichiura infection in this setting, though some protective associations were observed. Others have also reported that village membership alone has a large impact on the likelihood of hookworm or T. trichiura infection [34], and that heterogeneity of prevalence is associated with multiple environmental and socioeconomic factors [35]. Adjusting for potential individual, village and school level confounders in our models did not meaningfully explain further heterogeneity in hookworm infection between villages or schools but did explain some heterogeneity in T. trichiura infection between schools.
Though our general contextual effects indicate that village is a relevant context for analysis, the representativeness of village measures is a limitation of the current study. We aggregated village measures from all households sampled for the baseline survey. Because sampling for TUMIKIA was based on CUs, the number of units sampled per village varied. We assumed that included units were representative, but our sample may not have adequately characterised village conditions. Village membership was based on an administrative rather than geographic grouping, so it may also not represent sanitation conditions in the area surrounding study households. While useful for implementation purposes, village may not be the most suitable scale at which to assess community-wide sanitation effects. Future studies could use varying spatial buffers with complete household samples to examine community-wide effects of sanitation coverage on STH infection and identify target thresholds [36]. Our household sanitation measure was based on a reported measure and may not reflect actual consistent usage and faeces disposal by household members or faecal contamination levels in the area. Our outcome measure was based on a single stool sample, which may also have underestimated prevalence [37]. Our assumption that the probability of complete data is independent of the outcome after adjusting for included covariates may have been incorrect. Only a small proportion of subjects were excluded due to missing data on covariates and outcomes, so we would expect the magnitude of bias from this source to be minimal. We attempted to control for imbalance in characteristics and conditions between individuals in different exposure groups, but, as with any observational research study, potential residual confounding and our cross-sectional design remain important limitations.
In the current study, sanitation conditions, as measured, explained little of the heterogeneity in transmission between villages and schools. Further studies should examine the role of sanitation in different domains against STH infections within the context of school- and community-based mass drug administration (MDA). We found evidence of a protective effect of sanitation access at the household against hookworm infection and a sanitation coverage threshold at which a community-wide effect against T. trichiura was observed. We also found evidence in support of current school sanitation coverage guidelines towards the control of hookworm infection. In summary, our findings highlight the need for continued efforts, alongside MDA, to extend access to good sanitation facilities at homes, schools, and across communities.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank the study participants for their time and patience answering our many questions. We also thank the members of our study team, including supervisors, field officers, laboratory technicians, drivers, and data entry personnel. We thank Claudio Fronterre for advice with modelling and Emma Beaumont for assistance during database assembly and Jessie Hamon for administrative support. We thank our reviewers for helpful feedback and suggestions.
Data Availability
De-identified data that underlie the results reported in this paper are available on LSHTM Data Compass: https://doi.org/10.17037/DATA.00001306. Researchers can request access through the Data Compass portal. Requests for release of the data will be reviewed by the relevant institutional review boards.
Funding Statement
The study was supported by the Bill & Melinda Gates Foundation (#OPP1033751) [https://www.gatesfoundation.org/], the Joint Global Health Trials Scheme (MR/N00597X/1) of the Medical Research Council, the UK Department for International Development, and the Wellcome Trust [https://wellcome.ac.uk/funding/joint-global- health-trials-scheme]; and the Children’s Investment Fund Foundation (#PRG0180EDU) [https://ciff.org/]. At the time survey was conducted, SJB was supported by a Wellcome Trust Senior Fellowship in Basic Biomedical Science (098045), which also supported RLP and KEH. The funders played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.GBD Compare Data Visualization [Internet]. Institute for Health Metrics and Evaluation, University of Washington. 2016 [cited July 19, 2018]. Available from: http://vizhub.healthdata.org/gbd-compare.
- 2.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–61. Epub 2006/05/02. 10.1016/S0065-308X(05)62007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feachem RG, Bradley DJ, Garelick H, Mara DD. Sanitation and disease: Health aspects of excreta and wastewater management. Bank W, editor. New York, NY: John Wiley & Sons; 1983. [Google Scholar]
- 4.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11(3):e1001620 Epub 2014/03/29. 10.1371/journal.pmed.1001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegelbauer K, Speich B, Mausezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9(1):e1001162 Epub 2012/02/01. 10.1371/journal.pmed.1001162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman MC, Garn JV, Sclar GD, Boisson S, Medlicott K, Alexander KT, et al. The impact of sanitation on infectious disease and nutritional status: A systematic review and meta-analysis. Int J Hyg Environ Health. 2017;220(6):928–49. Epub 2017/06/13. 10.1016/j.ijheh.2017.05.007 . [DOI] [PubMed] [Google Scholar]
- 7.Brooker S, Alexander N, Geiger S, Moyeed RA, Stander J, Fleming F, et al. Contrasting patterns in the small-scale heterogeneity of human helminth infections in urban and rural environments in Brazil. Int J Parasitol. 2006;36(10–11):1143–51. Epub 2006/07/04. 10.1016/j.ijpara.2006.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairncross S, Blumenthal U, Kolsky P, Moraes L, Tayeh A. The public and domestic domains in the transmission of disease. Trop Med Int Health. 1996;1(1):27–34. Epub 1996/02/01. 10.1046/j.1365-3156.1996.d01-9.x . [DOI] [PubMed] [Google Scholar]
- 9.Freeman MC, Chard AN, Nikolay B, Garn JV, Okoyo C, Kihara J, et al. Associations between school- and household-level water, sanitation and hygiene conditions and soil-transmitted helminth infection among Kenyan school children. Parasites & vectors. 2015;8:412 Epub 2015/08/08. 10.1186/s13071-015-1024-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garn JV, Mwandawiro CS, Nikolay B, Drews-Botsch CD, Kihara JH, Brooker SJ, et al. Ascaris lumbricoides Infection Following School-Based Deworming in Western Kenya: Assessing the Role of Pupils' School and Home Water, Sanitation, and Hygiene Exposures. Am J Trop Med Hyg. 2016;94(5):1045–54. Epub 2016/02/24. 10.4269/ajtmh.15-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gass K, Addiss DG, Freeman MC. Exploring the relationship between access to water, sanitation and hygiene and soil-transmitted helminth infection: a demonstration of two recursive partitioning tools. PLoS Negl Trop Dis. 2014;8(6):e2945 Epub 2014/06/13. 10.1371/journal.pntd.0002945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin PC, Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat Med. 2017;36(20):3257–77. Epub 2017/05/26. 10.1002/sim.7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merlo J, Wagner P, Austin PC, Subramanian SV, Leckie G. General and specific contextual effects in multilevel regression analyses and their paradoxical relationship: A conceptual tutorial. SSM Popul Health. 2018;5:33–7. Epub 2018/06/13. 10.1016/j.ssmph.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diez Roux AV, Aiello AE. Multilevel analysis of infectious diseases. J Infect Dis. 2005;191 Suppl 1:S25–33. Epub 2005/01/01. 10.1086/425288 . [DOI] [PubMed] [Google Scholar]
- 15.Freeman MC, Ogden S, Jacobson J, Abbott D, Addiss DG, Amnie AG, et al. Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: a rationale for inter-sectoral collaboration. PLoS Negl Trop Dis. 2013;7(9):e2439 Epub 2013/10/03. 10.1371/journal.pntd.0002439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Guideline: preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups Geneva: World Health Organization, 2017. [PubMed] [Google Scholar]
- 17.Brooker SJ, Mwandawiro CS, Halliday KE, Njenga SM, McHaro C, Gichuki PM, et al. Interrupting transmission of soil-transmitted helminths: a study protocol for cluster randomised trials evaluating alternative treatment strategies and delivery systems in Kenya. BMJ Open. 2015;5(10):e008950 Epub 2015/10/21. 10.1136/bmjopen-2015-008950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pullan RL, Halliday KE, Oswald WE, McHaro C, Beaumont E, Kepha S, et al. Effects, equity, and cost of school-based and community-wide treatment strategies for soil-transmitted helminths in Kenya: a cluster-randomised controlled trial. Lancet. 2019;393(10185):2039–50. Epub 2019/04/23. 10.1016/S0140-6736(18)32591-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halliday KE, Oswald WE, McHaro C, Beaumont E, Gichuki PM, Kepha S, et al. Community-level epidemiology of soil-transmitted helminths in the context of school-based deworming: Baseline results of a cluster randomised trial on the coast of Kenya. PLoS Negl Trop Dis. 2019;13(8):e0007427 Epub 2019/08/10. 10.1371/journal.pntd.0007427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oswald WE, Okiya S. TUMIKIA Project Household Questionnaire & Stool Sample Collection Form London, U.K: London School of Hygiene & Tropical Medicine; 2017. Available from: https://datacompass.lshtm.ac.uk/1311/. [Google Scholar]
- 21.Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level Geneva, Switzerland: World Health Organization, 1998. WHO/CTD/SIP/98.1. [Google Scholar]
- 22.WHO/UNICEF. Core questions and indicators for monitoring WASH in Schools in the Sustainable Development Goals Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 23.WorldPop. The WorldPop demography project 2015. Available from: http://www.worldpop.org.uk/.
- 24.Hengl T, Mendes de Jesus J, Heuvelink GB, Ruiperez Gonzalez M, Kilibarda M, Blagotic A, et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS One. 2017;12(2):e0169748 Epub 2017/02/17. 10.1371/journal.pone.0169748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CGIAR-CSI. Consortium for Spatial Information 2019. Available from: http://www.cgiar-csi.org/.
- 26.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd Ed Philadelphia, PA: Lippincott, Williams, & Wilkins; 2008. [Google Scholar]
- 27.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745 Epub 2011/08/04. 10.1097/EDE.0b013e318225c2be . [DOI] [PubMed] [Google Scholar]
- 28.Burkner P. Advanced Bayesian Multilevel Modeling with the R Package brms: r-project.org; 2018. Available from: https://cran.r-project.org/web/packages/brms/vignettes/brms_multilevel.pdf.
- 29.Merlo J, Wagner P, Ghith N, Leckie G. An Original Stepwise Multilevel Logistic Regression Analysis of Discriminatory Accuracy: The Case of Neighbourhoods and Health. PLoS One. 2016;11(4):e0153778 Epub 2016/04/28. 10.1371/journal.pone.0153778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290–7. Epub 2006/03/16. 10.1136/jech.2004.029454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–8. Epub 2004/12/24. 10.1093/aje/kwi017 . [DOI] [PubMed] [Google Scholar]
- 32.KMOH. Kenya Environmental Sanitation and Hygiene Policy 2016–2030 Nairobi, Kenya: Republic of Kenya Ministry of Health, 2016. [Google Scholar]
- 33.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299(16):1937–48. Epub 2008/04/24. 10.1001/jama.299.16.1937 . [DOI] [PubMed] [Google Scholar]
- 34.Benjamin-Chung J, Nazneen A, Halder AK, Haque R, Siddique A, Uddin MS, et al. The Interaction of Deworming, Improved Sanitation, and Household Flooring with Soil-Transmitted Helminth Infection in Rural Bangladesh. PLoS Negl Trop Dis. 2015;9(12):e0004256 Epub 2015/12/02. 10.1371/journal.pntd.0004256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisanzio D, Mutuku F, Bustinduy AL, Mungai PL, Muchiri EM, King CH, et al. Cross-sectional study of the burden of vector-borne and soil-transmitted polyparasitism in rural communities of Coast Province, Kenya. PLoS Negl Trop Dis. 2014;8(7):e2992 Epub 2014/07/25. 10.1371/journal.pntd.0002992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuller JA, Villamor E, Cevallos W, Trostle J, Eisenberg JN. I get height with a little help from my friends: herd protection from sanitation on child growth in rural Ecuador. Int J Epidemiol. 2016;45(2):460–9. Epub 2016/03/05. 10.1093/ije/dyv368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2(11):e331 Epub 2008/11/05. 10.1371/journal.pntd.0000331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
De-identified data that underlie the results reported in this paper are available on LSHTM Data Compass: https://doi.org/10.17037/DATA.00001306. Researchers can request access through the Data Compass portal. Requests for release of the data will be reviewed by the relevant institutional review boards.