Abstract
PURPOSE
Despite concerns that power morcellation may adversely affect prognosis of patients with occult uterine cancer, empirical evidence has been limited and inconclusive. In this study, we aimed to determine whether uncontained power morcellation at the time of hysterectomy or myomectomy is associated with increased mortality risk in women with occult uterine cancer.
METHODS
By linking statewide hospital discharge records with cancer registry data in New York, we identified 843 women with occult endometrial carcinoma and 334 women with occult uterine sarcoma who underwent a hysterectomy or myomectomy for presumed benign indications during the period October 1, 2003, through December 31, 2013. Within this cohort, we compared disease-specific and all-cause mortality of women who underwent laparoscopic supracervical hysterectomy/laparoscopic myomectomy (LSH/LM), a surrogate indicator for uncontained power morcellation, with women who underwent supracervical abdominal hysterectomy and total abdominal hysterectomy (TAH), which did not involve power morcellation. Multivariable Cox regressions and propensity score method were used to adjust for patient characteristics.
RESULTS
Among women with occult uterine sarcoma, LSH/LM was associated with a higher risk for disease-specific mortality than TAH (adjusted hazard ratio [aHR], 2.66, 95% CI, 1.11 to 6.37; adjusted difference in 5-year disease-specific survival, −19.4%, 95% CI, −35.8% to −3.1%). In the subset of women with leiomyosarcoma, LSH/LM was associated with an increased risk for disease-specific mortality compared with supracervical abdominal hysterectomy (aHR, 3.64, 95% CI, 1.50 to 8.86; adjusted difference in 5-year disease-specific survival, −31.2%, 95% CI, −50.0% to −12.3%) and TAH (aHR, 4.66, 95% CI, 1.97 to 11.00; adjusted difference in 5-year disease-specific survival, −37.3%, 95% CI, −54.2% to −20.3%). Among women with occult endometrial carcinoma, there was no significant association between surgical approach and disease-specific mortality.
CONCLUSION
Uncontained power morcellation was associated with higher mortality risk in women with occult uterine sarcoma, especially in those with occult leiomyosarcoma.
INTRODUCTION
Patient safety when undergoing hysterectomy (ie, surgical removal of the uterus) and myomectomy (ie, surgical removal of uterine fibroids with retention of the uterus) is of vital importance because nearly 700,000 women undergo these procedures annually in the United States.1 With advancing medical technology, many of these procedures are performed laparoscopically, providing patients the benefit of minimally invasive surgery (eg, lower complication rate, shorter recovery time).2,3 Power morcellation has played an important role by facilitating efficient fragmentation and removal of uterine or fibroid tissue via small incisions. However, the safety of this technique has been debated since the US Food and Drug Administration cautioned against its use in 2014.4
One major concern is that the rapidly rotating cylindrical blade during morcellation may inadvertently spread cancer cells to the peritoneal cavity if there is unrecognized uterine malignancy.4 However, empirical research on the impact of uncontained power morcellation on prognosis of patients with occult uterine cancer has been sparse and provided mixed evidence.5,6 Moreover, existing studies often relied on small samples from selected referral centers or had limited risk adjustment,6-8 limiting generalizability and robustness of the findings. The current literature has also focused on occult leiomyosarcoma, a subtype of uterine cancer that has a particularly poor prognosis and is difficult to detect preoperatively,4 leading to scant data on how power morcellation may affect prognosis of patients with other forms of uterine cancer. In particular, endometrial carcinoma accounts for more than 90% of uterine cancers and affects considerably more women than does leiomyosarcoma.9
To address this important knowledge gap, we examined whether uncontained power morcellation was associated with increased mortality risk in women with occult uterine cancer who underwent hysterectomy or myomectomy for presumed benign indications. We used a large population-based sample with rigorous adjustment for patient characteristics and comprehensive assessment of all major subtypes of uterine cancer.
METHODS
Data and Sample Selection
The New York Statewide Planning and Research Cooperative System10 collects detailed information on diagnosis and procedure codes and procedure dates of all inpatient and outpatient encounters (regardless of payer) at civilian hospitals and ambulatory surgery centers in the state. Using data from this system, we identified adult women who underwent a hysterectomy or myomectomy during the period October 1, 2003, through December 31, 2013, on the basis of the International Classification of Diseases, Ninth Revision (ICD-9), diagnosis and procedure codes and current procedural terminology (CPT) codes. These women were then linked to the New York State Cancer Registry data via a unique patient identifier and their date of birth.11 The cancer registry data allowed for ascertainment of uterine cancer and vital status through December 31, 2015.
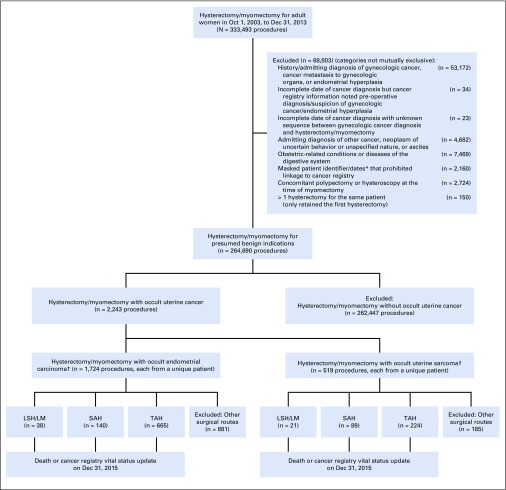
We identified women with a cancer registry–based diagnosis of corpus uteri cancer within 28 days after the index hysterectomy or myomectomy, and defined them as having occult uterine cancer if they did not have any of the following: (1) admitting diagnosis of any malignancy, neoplasm of uncertain behavior or unspecified nature, endometrial hyperplasia, or ascites; (2) discharge diagnosis of index hysterectomy or myomectomy indicating personal history of gynecologic cancer; (3) discharge diagnosis or CPT codes in the 9 months before index hysterectomy or myomectomy indicating gynecologic cancer, cancer metastasis to gynecologic organs, or endometrial hyperplasia; or (4) prior diagnosis of malignancy in corpus uteri, cervix uteri, or fallopian tubes or supporting ligaments in cancer registry (Fig 1).12,13 Corpus uteri cancer was determined using International Classification of Diseases for Oncology, 3rd edition, site code C54.x or C55.x in conjunction with behavior code 3 (for malignant neoplasm), excluding histology codes 9050 to 9055, 9140, and 9590 to 9992.14 We limited our sample to women diagnosed with corpus uteri cancer within 28 days after the index admission, because few patients were diagnosed after 28 days and prolonged time adds ambiguity regarding presence of cancer at the index hysterectomy or myomectomy. This study was approved by the Yale Human Investigation Committee.
FIG 1.
Patient flow diagram. (*) The New York Statewide Planning and Research Cooperative System (SPARCS) redacted information on patient identifiers and dates to protect the confidentiality for abortion- or HIV-related encounters. (†) Four patients had both occult endometrial carcinoma and occult uterine sarcoma and were analyzed as uterine sarcoma patients since uterine sarcoma had worse prognosis. LSH/LM, laparoscopic supracervical hysterectomy/laparoscopic myomectomy; SAH, supracervical abdominal hysterectomy; TAH, total abdominal hysterectomy.
Comparison Groups
We compared patients with occult uterine cancer who underwent a laparoscopic supracervical hysterectomy (LSH)/laparoscopic myomectomy (LM) with those who underwent supracervical abdominal hysterectomy (SAH) or total abdominal hysterectomy (TAH). These surgical routes were determined using ICD-9 and CPT procedure codes.
Women who underwent LSH/LM encompassed the group of primary interest. LSH involved laparoscopic removal of the uterine corpus while leaving the cervix intact (ie, subtotal hysterectomy). LM involved laparoscopic excision of fibroids while retaining both the uterus and the cervix. The ICD-9 procedure code for LSH (68.31) first became available on October 1, 2003, and uncontained power morcellation of the uterine or fibroid tissue was the standard technique used in LSH/LM before the 2014 US Food and Drug Administration safety warning.15-17 Therefore, by using data from October 1, 2003, through December 31, 2013, we were able to use LSH/LM as a surrogate indicator for uncontained power morcellation and, consistent with prior research,16,17 we classified all patients in the LSH/LM group as having undergone uncontained power morcellation.
Women undergoing SAH also had subtotal hysterectomy (similar to LSH), but their uterine tissue was removed via the open abdominal incision created during the procedure; hence, power morcellation was not used. Comparison between the LSH/LM and SAH groups would reveal the effect of power morcellation. We also considered abdominal myomectomy as part of this comparison group but did not identify any patients with occult uterine cancer undergoing abdominal myomectomy.
Women in the TAH group also did not undergo power morcellation of uterine tissue and they constituted another comparison group. Although TAH removes both the uterine corpus and the cervix (ie, total hysterectomy), our focus on disease-specific survival should alleviate the potential confounding effect of the additional removal of the cervix.
Measures
Our primary outcome measure was disease-specific survival, defined as the time in months from date of diagnosis to date of death if the patient died of corpus uteri cancer. Date and cause of death were extracted from the cancer registry and confirmed via New York State death certificates, US National Death Index, and/or Social Security Death Index. If no record of death was identified from these sources, the patient was presumed alive. For patients who died of other causes or were presumed alive, we used their date of death or last vital status update in the cancer registry as the date of censoring.
As a secondary outcome, we also examined patients’ all-cause survival. It was measured as the time in months from date of diagnosis to date of death (regardless of cause of death) if the patient died during the study period or to date of last vital status update if the patient was alive.
We measured patients’ age, race/ethnicity, tumor characteristics, cancer treatment, and cancer history, using data from the cancer registry. Cancer stage was categorized as localized, regional, distant, or unknown (Appendix, online only), and grade was categorized as low, high, or unknown. Patients were classified as having endometrial carcinoma versus uterine sarcoma and then more refined subtypes based on histology codes. For each patient, we ascertained whether she received radiation therapy and chemotherapy for uterine cancer and whether she had a history of other cancer, based on diagnosis before or within 28 days of the index hysterectomy or myomectomy.
Using diagnosis codes in New York Statewide Planning and Research Cooperative System data from the index admission and other inpatient and outpatient encounters in the past 9 months,18 we measured 25 benign comorbidities on the basis of the Elixhauser index.19,20 The summed number of conditions reflected a patient’s disease burden.
Statistical Analysis
We analyzed endometrial carcinoma and uterine sarcoma separately, given their distinct prognoses. Four patients had both endometrial carcinoma and uterine sarcoma and were analyzed as patients with uterine sarcoma, because of its worse prognosis. We also performed a separate analysis for leiomyosarcoma to facilitate comparison with prior studies.
We compared patient characteristics across surgical groups (ie, LSH/LM, SAH, and TAH) using Kruskal-Wallis test for continuous variables and χ2 or Fisher’s exact test for categorical variables. Disease-specific and all-cause survival were compared across groups using Kaplan-Meier curves and log-rank tests, as well as multivariable Cox proportional hazards regressions. The regressions adjusted for patient tumor characteristics (ie, stage, grade, cancer subtype, and receipt of chemotherapy and radiation therapy) and other potential confounding factors (ie, age, race/ethnicity, history of other cancer, and number of benign comorbidities).21-23 We verified the proportional hazards assumption for the effect of surgical group and reported results of the Cox regressions using hazard ratios (HRs). An alternative approach using subdistribution hazards models accounting for other deaths as a competing risk showed similar findings.
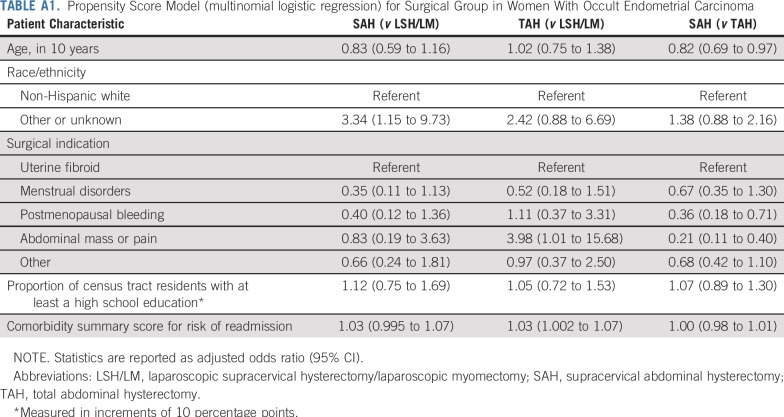
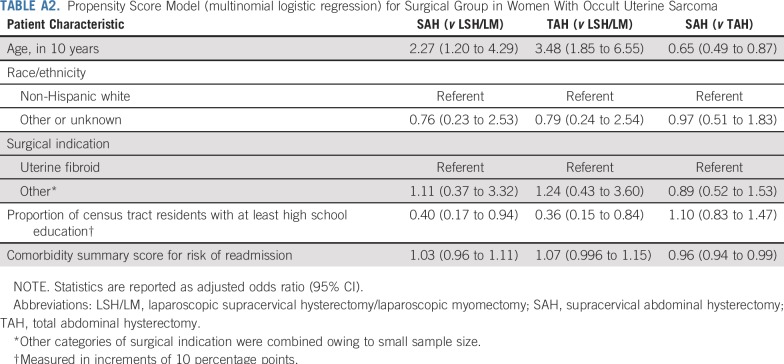
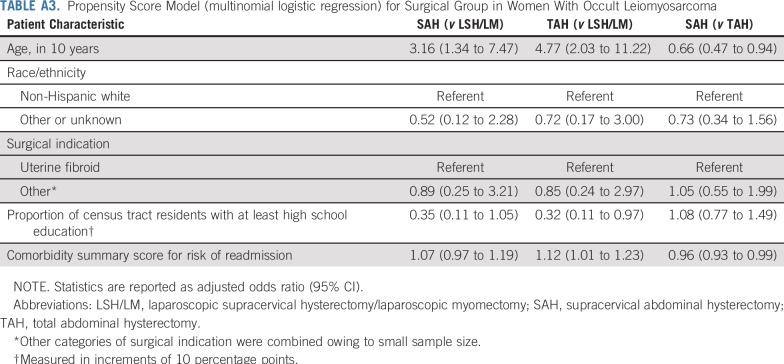
To account for other patient characteristics that might differ across groups, we also applied a propensity score method (Appendix; Appendix Tables A1-A3).24 A multinomial logistic regression was estimated to derive each patient’s propensity for undergoing LSH/LM, SAH, or TAH. This propensity score model adjusted for patient age, race/ethnicity, surgical indication, proportion of census tract residents age 25 years or older with at least high school education (indicating local socioeconomic status that could affect access to laparoscopic procedures), and a risk score for 30-day readmission based on comorbidities25 (indicating acuity of patients’ health condition, which could influence surgical route selection). We then included the propensity scores as additional covariates in the Cox regressions.
For each type of uterine cancer, we calculated the mean adjusted survival probability at 1, 3, and 5 years on the basis of characteristics of patients in the sample and coefficient estimates from the Cox regression, while assuming all patients had undergone LSH/LM, SAH, or TAH, respectively (Appendix). We also calculated 95% CIs for the difference in these adjusted probabilities between surgical groups.
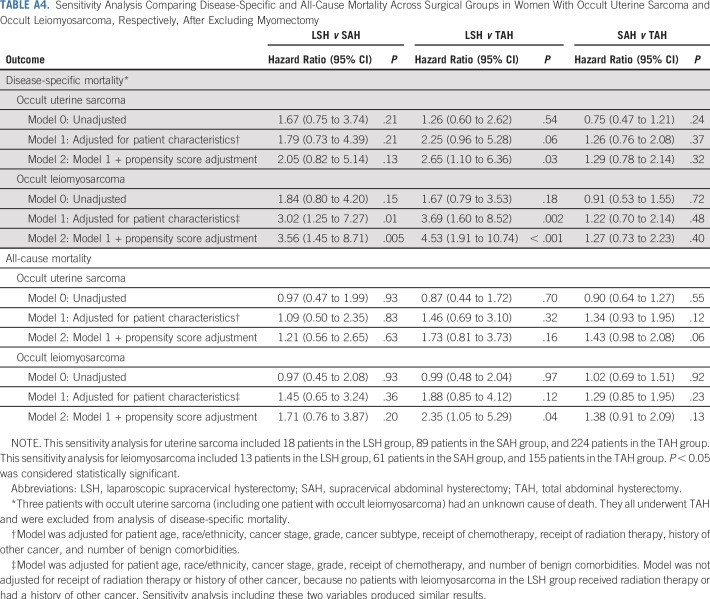
We performed two sensitivity analyses. The first excluded myomectomies to address potential differences in patient population and confounding effect of uterine disruption,26 and the second added total laparoscopic hysterectomy for uteri weighing no more than 250 g (which typically would not involve power morcellation) as another comparison group to inform potential confounding effect of laparoscopic versus abdominal surgery27 (Appendix). P < 0.05 was considered statistically significant.
RESULTS
Sample Characteristics
The final sample included 843 women with occult endometrial carcinoma and 334 with occult uterine sarcoma (a subset of 231 women had leiomyosarcoma). Median (interquartile range) follow-up was 53 (29-79) months, 41 (18-75) months, and 38 (18-67) months for women with endometrial carcinoma, uterine sarcoma, and leiomyosarcoma, respectively.
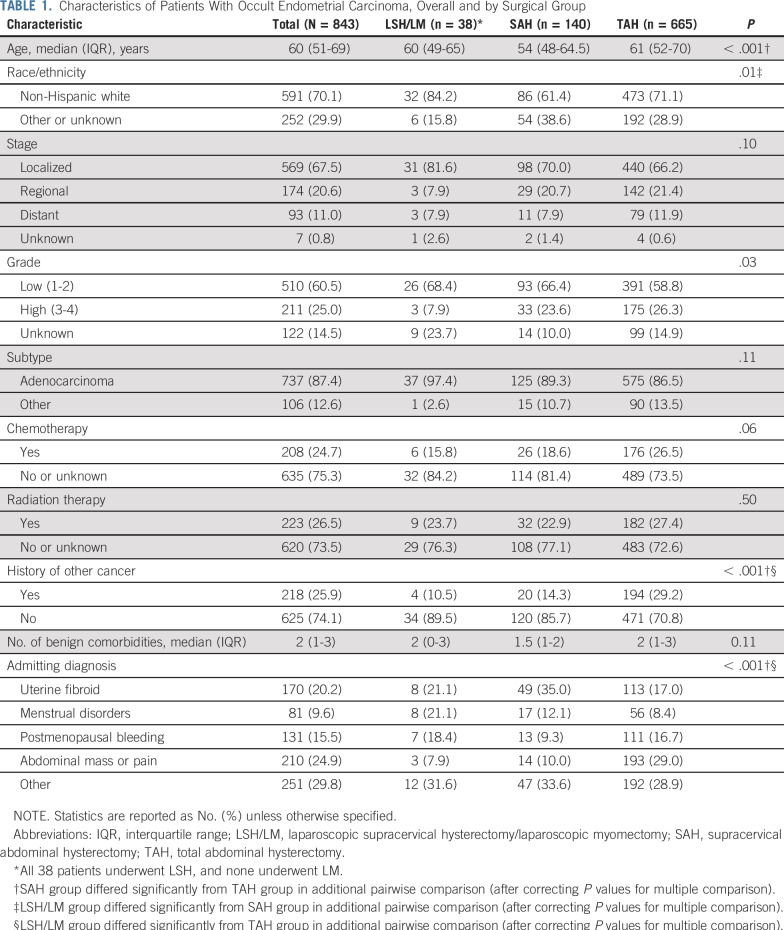
Among women with occult endometrial carcinoma, 38 were in the LSH/LM group (all underwent LSH), 140 underwent SAH, and 665 underwent TAH. Patients differed significantly by age, race/ethnicity, cancer grade, and history of other malignancy across surgical groups (Table 1). Women in the TAH group tended to have worse health attributes than women in the LSH/LM and SAH groups.
TABLE 1.
Characteristics of Patients With Occult Endometrial Carcinoma, Overall and by Surgical Group
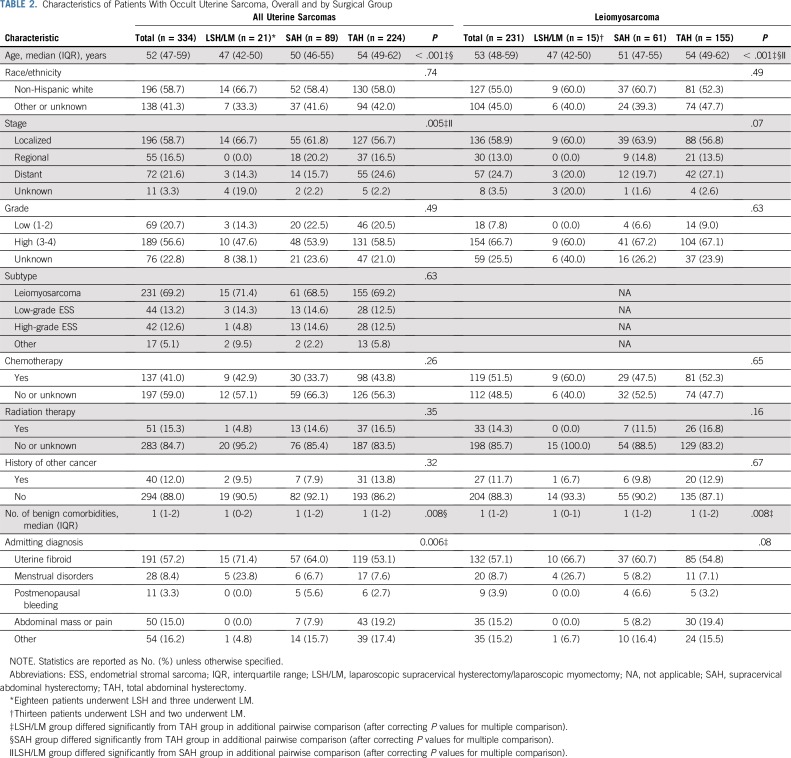
Among women with occult uterine sarcoma, 21 were in the LSH/LM group (18 underwent LSH and three underwent LM), 89 underwent SAH, and 224 underwent TAH. Patients in the three surgical groups differed significantly by age, stage, and comorbidities, generally favoring the LSH/LM group (Table 2). The subset of women having occult leiomyosarcoma exhibited a similar pattern of patient characteristics.
TABLE 2.
Characteristics of Patients With Occult Uterine Sarcoma, Overall and by Surgical Group
Disease-Specific Mortality
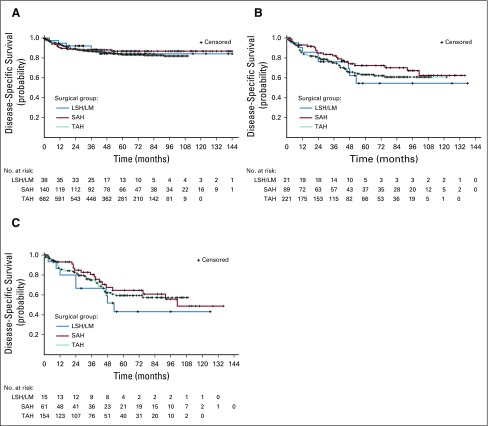
On the basis of log-rank tests, unadjusted survival curves showed no significant difference in disease-specific mortality across surgical groups for women with occult endometrial carcinoma, uterine sarcoma, or leiomyosarcoma (Fig 2). For women with occult endometrial carcinoma, surgical group was not associated with disease-specific survival even after adjusting for patient characteristics and propensity scores in multivariable analysis (Table 3).
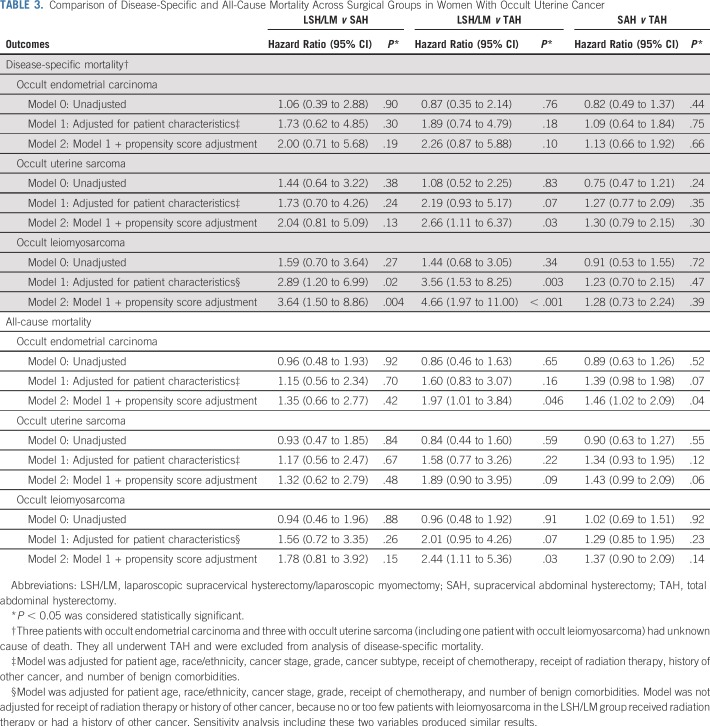
FIG 2.
(A) Disease-specific survival for occult endometrial carcinoma (unadjusted). Log-rank test for difference across surgical groups has P = .72. (B) Disease-specific survival for occult uterine carcinoma (unadjusted). Log-rank test for difference across surgical groups has P = .46. (C) Disease-specific survival for occult leiomyosarcoma (unadjusted). Log-rank test for difference across surgical groups has P = .53. LSH/LM, laparoscopic supracervical hysterectomy/laparoscopic myomectomy; SAH, supracervical abdominal hysterectomy; TAH, total abdominal hysterectomy.
TABLE 3.
Comparison of Disease-Specific and All-Cause Mortality Across Surgical Groups in Women With Occult Uterine Cancer
However, for women with occult uterine sarcoma, after adjusting for patient characteristics and propensity scores, LSH/LM was associated with a higher risk for disease-specific mortality than TAH (HR, 2.66; 95% CI, 1.11 to 6.37), but difference between the LSH/LM and SAH groups did not reach statistical significance (Table 3). In the subset of women with occult leiomyosarcoma, LSH/LM was associated with an increased risk for disease-specific mortality than SAH (HR, 3.64; 95% CI, 1.50 to 8.86) and TAH (HR, 4.66; 95% CI, 1.97 to 11.00). Meanwhile, disease-specific mortality did not differ between the SAH and TAH groups, suggesting no confounding effect of total (v subtotal) hysterectomy on disease-specific survival.
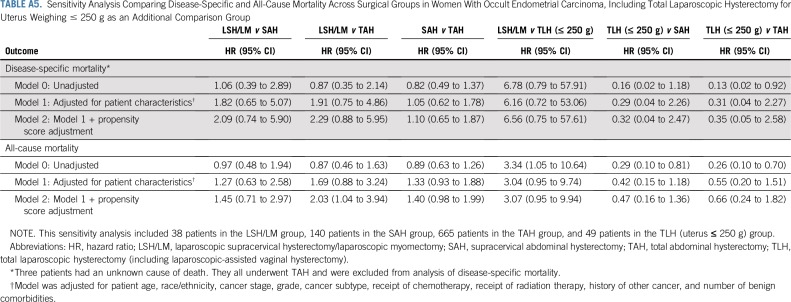
Sensitivity analysis excluding myomectomies showed similar results (Appendix Table A4). There was also no difference in adjusted mortality risk between total laparoscopic hysterectomy (uterus ≤ 250 g) and TAH, suggesting no evidence for confounding effect of laparoscopy (Appendix Table A5).
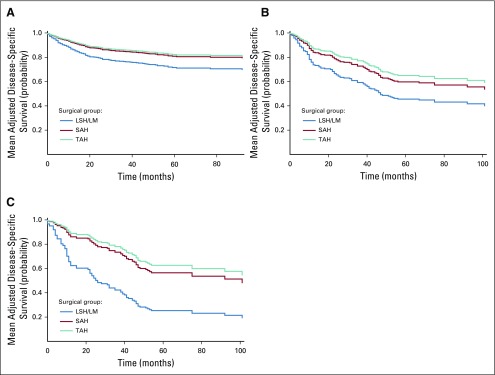
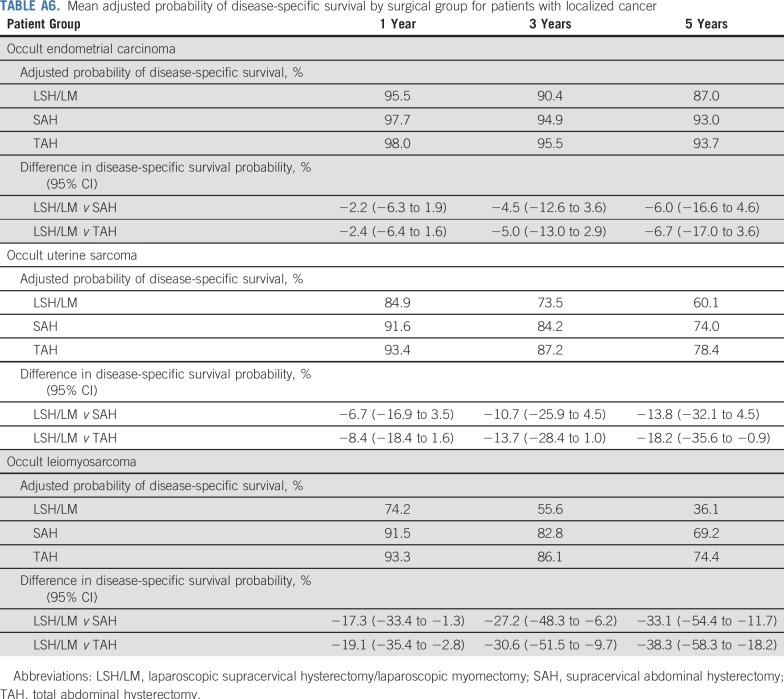
Table 4 and Fig 3 report mean adjusted probability of disease-specific survival by surgical group. For instance, for women with occult leiomyosarcoma, 5-year adjusted probability of disease-specific survival was 25.3% if they underwent LSH/LM, in contrast to 56.5% if they underwent SAH and 62.6% if they underwent TAH, resulting in a significant difference in survival probability of −31.2% (95% CI, −50.0% to −12.3%) and −37.3% (95% CI, −54.2% to −20.3%), respectively. Mean adjusted survival probability of patients with localized cancer is presented in Appendix Table A6 and Appendix Figure A1.
TABLE 4.
Mean Adjusted Probability of Disease-Specific Survival by Surgical Group
FIG 3.
(A) Mean adjusted disease-specific survival for occult endometrial carcinoma. (B) Mean adjusted disease-specific survival for occult uterine sarcoma. (C) Mean adjusted disease-specific survival for occult leimyosarcoma. The survival curve in this figure for each surgical group reflects mean adjusted survival probability at each time point among patients in the sample. Each patient’s survival probability at a given time point was estimated based on her characteristics and regression Model 2 for disease-specific survival in Table 3, while assuming that she was in the LSH/LM, SAH, or TAH group, respectively. The sample average survival probability at each time point was then derived for each group. LSH/LM, laparoscopic supracervical hysterectomy/laparoscopic myomectomy; SAH, supracervical abdominal hysterectomy; TAH, total abdominal hysterectomy.
All-Cause Mortality
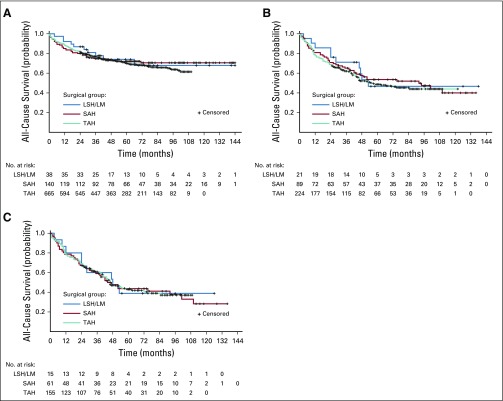
All-cause mortality did not differ across surgical groups in unadjusted analysis (Appendix Fig A2). After adjusting for patient characteristics and propensity scores, both the LSH/LM group (HR, 1.97; 95% CI, 1.01 to 3.84) and the SAH group (HR, 1.46; 95% CI, 1.02 to 2.09) had a higher risk for all-cause mortality than TAH in women with endometrial carcinoma, likely reflecting the effect of total (v subtotal) hysterectomy on overall survival (Table 3). However, in women with occult leiomyosarcoma, the risk for all-cause mortality only differed between LSH/LM and TAH (HR, 2.44; 95% CI, 1.11 to 5.36), not between SAH and TAH.
DISCUSSION
Empirical evidence for how power morcellation may affect prognosis of patients with occult uterine cancer has been sparse. Our study extends this literature by demonstrating that compared with SAH or TAH, which did not involve power morcellation, LSH/LM with presumed uncontained power morcellation was associated with higher mortality risk in women with occult uterine sarcoma, especially leiomyosarcoma.
Our findings suggest an adverse survival impact of uncontained power morcellation. This is consistent with recent studies of patients with leiomyosarcoma from Kaiser Permanente reporting a fivefold higher risk of 1-year mortality associated with morcellation28 and from a clinical trial network in Italy showing a threefold increase in mortality risk associated with power morcellation.29 A meta-analysis of 24 studies published up to 2015 also found a marked difference in 5-year survival for patients with leiomyosarcoma who underwent power morcellation versus those who did not (30% v 60%), although the difference was not statistically significant.6 The unique strengths of our study (ie, population-based data, large sample size, inclusion of all uterine cancer subtypes, and careful risk adjustment) substantively add to this evidence base in terms of generalizability, methodological rigor, and clinical breadth.
Uncontained power morcellation may affect mortality risk for several reasons. First, it may cause intraperitoneal dissemination of cancer cells, accelerating disease progression. Research has shown malignant peritoneal implants after power morcellation and higher risk for upstaged cancer after morcellated versus nonmorcellated tissue removal.30-34 Because survival decreases progressively with advancing stage,22 upstaging due to morcellation could substantially increase mortality risk. Second, morcellated specimens make it difficult to appropriately stage and assess tumor histopathology, complicating subsequent treatment.35 Moreover, compromised pathologic evaluation of morcellated specimens may lead to missed diagnosis, which also warrants attention in future research.
Our findings underscore the importance of evaluation for leiomyosarcoma and other uterine sarcomas in women undergoing hysterectomy or myomectomy. For preoperative evaluation, the American College of Obstetricians and Gynecologists and the American Association of Gynecologic Laparoscopists recommend imaging and endometrial tissue sampling.36,37 Intraoperative evaluation via biopsy and frozen sections offers another opportunity to identify underlying malignancy, but low accuracy may limit its utility.38-41 For patients at higher risk for malignancy (eg, postmenopausal women26,36), avoiding uncontained power morcellation may be beneficial.37 On the other hand, morcellation facilitates minimally invasive surgery, which helps reduce complications of hysterectomy. Hence, the risks and benefits of power morcellation should be carefully considered. For instance, simulation studies accounting for both surgery- and occult cancer-related outcomes suggest that laparoscopic hysterectomy with morcellation may be beneficial for younger women, given the associated reduced risk of surgical complications and their lower risk of unexpected cancer.42,43 In addition, cautions about power morcellation do not necessarily preclude patients from undergoing laparoscopic hysterectomy (eg, uterus can often be removed intact vaginally in total laparoscopic hysterectomy).
We recognize limitations of several measures used in our study. First, lacking direct information regarding method of specimen removal, we assumed that all patients in the LSH/LM group underwent power morcellation. Likewise, we could not determine use of manual morcellation, which might also affect prognosis, although to a lesser degree than power morcellation.6 It is possible that not all LSH/LM procedures used power morcellation or some abdominal hysterectomies involved manual morcellation. However, if this were the case, differences in mortality risk between the LSH/LM and abdominal groups observed in our study would underestimate the true effect of power morcellation. Yet, we still found a significant adverse effect of LSH/LM. Second, we cannot rule out the possibility that some malignancies were suspected before surgery, especially in the abdominal groups (because no patient should undergo LSH/LM if cancer was suspected). However, because patients with suspected cancer tend to have more advanced disease with worse prognosis, this would bias our findings in favor of LSH/LM, leading to conservative estimates for the effect of morcellation. Third, our risk adjustment used cancer stage documented in the cancer registry, which reflected the final stage assigned using all information available up to 4 months after initial diagnosis or through the completion of staging surgery. Hence, it might mask potential upstaging caused by morcellation and underestimate the impact of morcellation on mortality risk. In addition, morcellated specimens may complicate pathologic examination. Hence, a larger proportion of patients in the LSH/LM group had unknown cancer stage. This might lead to less effective risk adjustment. Fourth, ambulatory cancer treatment (eg, chemotherapy and radiation therapy) tend to be under reported to cancer registries.44 However, because this affected all surgical groups similarly, its impact on our evaluation of between-group differences is likely small.
Other limitations should be acknowledged as well. Like all observational studies, there may be unmeasured differences in patient or tumor characteristics between surgical groups. Moreover, our data came from a single state and may not reflect patients’ experience elsewhere in the country. We might lack statistical power for detecting the smaller effect of morcellation in endometrial carcinoma, given its better prognosis.9 In addition, we evaluated different subtypes of uterine cancer and surgical groups where multiple comparison might arise. However, this risk should be low, given the coherent nature of our findings. Finally, uterine sarcomas have high rates of recurrence, which also warrant close investigation in future research.45
In sum, we found evidence for increased mortality risk associated with uncontained power morcellation. Rigorous patient evaluation is needed to minimize the impact of unexpected cancers. For women at higher risk for occult uterine cancer, judicious selection of surgical approaches that avoid uncontained power morcellation may help optimize outcomes.
ACKNOWLEDGMENT
We thank colleagues at the New York Statewide Planning and Research Cooperative System for their assistance with data acquisition.
APPENDIX
Additional Technical Details of Research Methodology
Measurement of uterine cancer stage.
We categorized stage of uterine cancer as localized, regional, distant, or unknown by integrating information from the American Joint Committee on Cancer (AJCC) stage and SEER stage (using the more advanced stage if there was a discrepancy). AJCC stage I was considered localized, AJCC stages II and III were considered regional, and AJCC stage IV was considered distant.
Propensity score method.
To account for other patient characteristics that might differ across the three surgical groups, we additionally applied a propensity score method. Specifically, for each type of uterine cancer, we first performed a multinomial logistic regression to derive each patient’s propensity for undergoing laparoscopic supracervical hysterectomy/laparoscopic myomectomy (LSH/LM), supracervical abdominal hysterectomy (SAH), or total abdominal hysterectomy (TAH). The propensity score model adjusted for each patient’s age, race/ethnicity, surgical indication, proportion of residents age 25 years or older in patient’s census tract or zip code (if census tract was not available) with at least a high school education (an indicator for local socioeconomic status that could affect access to laparoscopic procedures), and a risk score for 30-day readmission based on comorbidities,25 which should reasonably reflect acuity of patients’ health condition influencing surgical route selection. The propensity score of a patient for each of the surgical groups (ie, LSH/LM, SAH, or TAH) was defined as the estimated probability that the patient would be assigned to the group, given her characteristics. Complete results of the propensity score models are reported in Appendix Tables A1-A3. Each patient would have three propensity scores, corresponding to the probabilities of belonging to the three respective surgical groups (LSH/LM, SAH, and TAH). We then included two of the propensity scores as additional covariates in the Cox regression models. We used this propensity score adjustment approach because the small sample size in the LSH/LM group was not amenable to propensity score stratification or matching and might be unduly influenced by extreme weights if inverse propensity score weighting approach was used. For similar reasons, we used a composite risk score for comorbidities (instead of a series of binary indicators for individual comorbidities) in the propensity score model to minimize potential over-parameterization given the small number of patients undergoing LSH/LM.
Sensitivity analysis.
We recognize that patients undergoing myomectomy tend to differ from patients undergoing hysterectomy (eg, younger, intend to preserve fertility). There are also concerns that disruption of the uterus itself in the process of myomectomy (regardless of laparoscopic or abdominal approach) might affect cancer dissemination.26 Moreover, because surgical route for myomectomy was only distinguishable via current procedural terminology (CPT) codes in outpatient data where abdominal myomectomy was rare, we did not identify any patients with occult uterine cancer who underwent abdominal myomectomy. As a result, the abdominal surgery groups in our analysis did not encompass a direct comparison for laparoscopic myomectomy. Therefore, we performed a sensitivity analysis excluding patients who underwent laparoscopic myomectomy. Because no women with occult endometrial carcinoma underwent laparoscopic myomectomy, this sensitivity analysis was only implemented in women with occult uterine sarcoma and women with occult leiomyosarcoma. Results from this sensitivity analysis are reported in Appendix Table A4.
Given recent evidence suggesting worse prognosis of patients with gynecologic cancer undergoing laparoscopic versus abdominal hysterectomy,27 we performed another sensitivity analysis by including women with occult uterine cancer who underwent a total laparoscopic hysterectomy (TLH; including laparoscopic-assisted vaginal hysterectomy) for uteri weighing no more than 250 g as an additional comparison group. That is, for women with occult uterine cancer, we compared their mortality risk across four groups: LSH/LM, SAH, TAH, and TLH (≤ 250 g). Because patients in the TLH (≤ 250 g) group typically would not undergo power morcellation, comparing these patients with the TAH group helped inform the potential confounding effect of laparoscopic versus abdominal surgery. These patients were identified using CPT codes (which were only available in outpatient data). However, only CPT codes for laparoscopic hysterectomy distinguish uterine weight, whereas CPT codes for abdominal hysterectomy do not differentiate uterine weight. Therefore, only the TLH group was restricted to patients whose uterus weighed no more than 250 g. This sensitivity analysis was limited to women with occult endometrial carcinoma, because too few patients with occult uterine sarcoma had a CPT code for TLH with a uterus weighing no more than 250 g. Results from this sensitivity analysis are reported in Appendix Table A5.
Mean adjusted survival curve and survival probability.
For each type of uterine cancer, using coefficient estimates from the Cox regression (model 2 in Table 3), we calculated the predicted survival curve for each patient in the sample on the basis of her characteristics and assuming all patients underwent LSH/LM (https://support.sas.com/documentation/onlinedoc/stat/141/phreg.pdf). This survival curve was averaged across all patients in the sample to obtain the mean adjusted survival curve for LSH/LM. The mean adjusted survival curves for SAH and TAH were calculated similarly. On the basis of these calculations, we derived the mean adjusted survival probability at 1, 3, and 5 years for patients in the sample had they undergone LSH/LM, SAH, and TAH, respectively. We also calculated 95% CIs for the difference in these adjusted probabilities between surgical groups. The mean adjusted survival curves are presented in Figure 3 and mean adjusted survival probabilities are reported in Table 4. In addition, we calculated mean adjusted survival curves and mean adjusted survival probabilities for the subset of patients with localized cancer. To do so, we used coefficient estimates of the Cox regressions from model 2 in Table 3 and applied them to the subset of women in our sample with localized cancer. The corresponding results are reported in Appendix Figure A1 and Appendix Table A6.
FIG A1.
Mean adjusted disease-specific survival curves for patients with localized cancer. (A) Occult endometrial carcinoma. (B) Occult uterine sarcoma. (C) Occult leiomyosarcoma. LSH/LM, laparoscopic supracervical hysterectomy/laparoscopic myomectomy; SAH, supracervical abdominal hysterectomy; TAH, total abdominal hysterectomy.
FIG A2.
Unadjusted Kaplan-Meier curves for all-cause survival. (A) Occult endometrial carcinoma (log-rank test for difference across surgical groups, P = .75). (B) Occult uterine sarcoma (log-rank test for difference across surgical groups, P = .75). (C) Occult leiomyosarcoma (log-rank test for difference across surgical groups, P = .99). LSH/LM, laparoscopic supracervical hysterectomy/laparoscopic myomectomy; SAH, supracervical abdominal hysterectomy; TAH, total abdominal hysterectomy.
TABLE A1.
Propensity Score Model (multinomial logistic regression) for Surgical Group in Women With Occult Endometrial Carcinoma
TABLE A2.
Propensity Score Model (multinomial logistic regression) for Surgical Group in Women With Occult Uterine Sarcoma
TABLE A3.
Propensity Score Model (multinomial logistic regression) for Surgical Group in Women With Occult Leiomyosarcoma
TABLE A4.
Sensitivity Analysis Comparing Disease-Specific and All-Cause Mortality Across Surgical Groups in Women With Occult Uterine Sarcoma and Occult Leiomyosarcoma, Respectively, After Excluding Myomectomy
TABLE A5.
Sensitivity Analysis Comparing Disease-Specific and All-Cause Mortality Across Surgical Groups in Women With Occult Endometrial Carcinoma, Including Total Laparoscopic Hysterectomy for Uterus Weighing ≤ 250 g as an Additional Comparison Group
TABLE A6.
Mean adjusted probability of disease-specific survival by surgical group for patients with localized cancer
Footnotes
Presented at the Society of Gynecologic Oncology annual meeting, Honolulu, HI, March 16-19, 2019.
Supported by Grant No. R01HS024702 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The New York State Cancer Registry was supported in part by the Centers for Disease Control and Prevention’s National Program of Cancer Registries through cooperative agreement 5NU58DP006309 awarded to the New York State Department of Health.
AUTHOR CONTRIBUTIONS
Conception and design: Xiao Xu, Cary P. Gross, Peter E. Schwartz, Vrunda B. Desai
Administrative support: Xiao Xu
Collection and assembly of data: Xiao Xu, Francis P. Boscoe, Lindsey M. Hutchison
Data analysis and interpretation: Xiao Xu, Haiqun Lin, Jason D. Wright, Gary P. Gross, Peter E. Schwartz, Vrunda B. Desai
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Association Between Power Morcellation and Mortality in Women With Unexpected Uterine Cancer Undergoing Hysterectomy or Myomectomy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jason D. Wright
Consulting or Advisory Role: Clovis Oncology
Research Funding: Merck (Inst)
Cary P. Gross
Research Funding: Johnson & Johnson, Pfizer
Travel, Accommodations, Expenses: Flatiron Health
Vrunda B. Desai
Employment: CooperSurgical
Stock and Other Ownership Interests: CooperSugical
Consulting or Advisory Role: CooperSurgical, Amgen (I), Boehringer Ingelheim (I), Relypsa (I), Cytokinetics (I), Novartis (I)
Research Funding: Amgen (I)
Travel, Accommodations, Expenses: Amgen (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Tsui C, Klein R, Garabrant M. Minimally invasive surgery: National trends in adoption and future directions for hospital strategy. Surg Endosc. 2013;27:2253–2257. doi: 10.1007/s00464-013-2973-9. [DOI] [PubMed] [Google Scholar]
- 2.Turner LC, Shepherd JP, Wang L, et al. Hysterectomy surgery trends: A more accurate depiction of the last decade? Am J Obstet Gynecol. 2013;208:277.e1–277.e7. doi: 10.1016/j.ajog.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitkin RM, Parker WH. Operative laparoscopy: A second look after 18 years. Obstet Gynecol. 2010;115:890–891. doi: 10.1097/AOG.0b013e3181daf60a. [DOI] [PubMed] [Google Scholar]
- 4. https://www.fda.gov/downloads/medicaldevices/safety/alertsandnotices/ucm393589.pdf US Food and Drug Administration: Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids. Summary and key findings. April 17, 2014. Silver Spring, MD, US Food and Drug Administration.
- 5. US Food and Drug Administration: FDA updated assessment of the use of laparoscopic power morcellators to treat uterine fibroids. December 2017. Silver Spring, MD, US Food and Drug Administration. https://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/SurgeryandLifeSupport/UCM584539.pdf.
- 6. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids/research-2017 Hartmann KE, Fonnesbeck C, Surawicz T, et al: Management of uterine fibroids. Comparative Effectiveness Review No. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD, Agency for Healthcare Research and Quality, December 2017.
- 7.Zhao WC, Bi FF, Li D, et al. Incidence and clinical characteristics of unexpected uterine sarcoma after hysterectomy and myomectomy for uterine fibroids: A retrospective study of 10,248 cases. OncoTargets Ther. 2015;8:2943–2948. doi: 10.2147/OTT.S92978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JY, Kim HS, Nam EJ, et al. Outcomes of uterine sarcoma found incidentally after uterus-preserving surgery for presumed benign disease. BMC Cancer. 2016;16:675. doi: 10.1186/s12885-016-2727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kosary CL: Cancer of the corpus uteri, in Ries LAG, Young JL, Keel GE, et al (eds): SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. NIH Pub. No. 07-6215. Bethesda, MD, National Cancer Institute, SEER Program, 2007. [Google Scholar]
- 10.New York State Department of Health Statewide Planning and Research Cooperative System (SPARCS)https://www.health.ny.gov/statistics/sparcs/
- 11.New York State Department of Health NYS Cancer Registry and Cancer Statisticshttps://www.health.ny.gov/statistics/cancer/registry/
- 12. Desai VB, Wright JD, Gross CP, et al: Prevalence, characteristics, and risk factors of occult uterine cancer in presumed benign hysterectomy. Am J Obstet Gynecol. 221:39.e1-39.e14, 2019. [DOI] [PMC free article] [PubMed]
- 13. Desai VB, Wright JD, Gross CP, et al: Risk of unexpected uterine cancer in women undergoing myomectomy: A population-based study. Eur J Obstet Gynecol Reprod Biol. 238:188-190, 2019. [DOI] [PubMed]
- 14.National Cancer Institute Site Recode ICD-O-3/WHO 2008 Definitionhttps://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html
- 15.Arkenbout EA, van den Haak L, Driessen SR, et al. Assessing basic “physiology” of the morcellation process and tissue spread: A time-action analysis. J Minim Invasive Gynecol. 2015;22:255–260. doi: 10.1016/j.jmig.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH) Arch Gynecol Obstet. 2015;292:665–672. doi: 10.1007/s00404-015-3696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez AM, Zeybek B, Asoglu MR, et al: Incidence of occult leiomyosarcoma in presumed morcellation cases: A database study. Eur J Obstet Gynecol Reprod Biol 197:31-35, 2016 [Erratum: Eur J Obstet Gynecol Reprod Biol 197:31-35, 2016]. [DOI] [PMC free article] [PubMed]
- 18.Baldwin LM, Klabunde CN, Green P, et al. In search of the perfect comorbidity measure for use with administrative claims data: Does it exist? Med Care. 2006;44:745–753. doi: 10.1097/01.mlr.0000223475.70440.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Healthcare Cost and Utilization Project: Elixhauser Comorbidity Software, Version 3.7 www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp.
- 20.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Glaser LM, Friedman J, Tsai S, et al. Laparoscopic myomectomy and morcellation: A review of techniques, outcomes, and practice guidelines. Best Pract Res Clin Obstet Gynaecol. 2018;46:99–112. doi: 10.1016/j.bpobgyn.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 22.American Cancer Society Survival rates for uterine sarcomahttps://www.cancer.org/cancer/uterine-sarcoma/detection-diagnosis-staging/survival-rates.html
- 23.Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: A National Cancer Database study. Gynecol Oncol. 2017;145:61–70. doi: 10.1016/j.ygyno.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Pirracchio R, Resche-Rigon M, Chevret S. Evaluation of the propensity score methods for estimating marginal odds ratios in case of small sample size. BMC Med Res Methodol. 2012;12:70. doi: 10.1186/1471-2288-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore BJ, White S, Washington R, et al. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: The AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55:698–705. doi: 10.1097/MLR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 26.Sizzi O, Manganaro L, Rossetti A, et al. Assessing the risk of laparoscopic morcellation of occult uterine sarcomas during hysterectomy and myomectomy: Literature review and the ISGE recommendations. Eur J Obstet Gynecol Reprod Biol. 2018;220:30–38. doi: 10.1016/j.ejogrb.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 28.Raine-Bennett T, Tucker LY, Zaritsky E, et al. Occult uterine sarcoma and leiomyosarcoma: Incidence of and survival associated with morcellation. Obstet Gynecol. 2016;127:29–39. doi: 10.1097/AOG.0000000000001187. [DOI] [PubMed] [Google Scholar]
- 29.Raspagliesi F, Maltese G, Bogani G, et al. Morcellation worsens survival outcomes in patients with undiagnosed uterine leiomyosarcomas: A retrospective MITO group study. Gynecol Oncol. 2017;144:90–95. doi: 10.1016/j.ygyno.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Brölmann H, Tanos V, Grimbizis G, et al. Options on fibroid morcellation: A literature review. Gynecol Surg. 2015;12:3–15. doi: 10.1007/s10397-015-0878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Einstein MH, Barakat RR, Chi DS, et al. Management of uterine malignancy found incidentally after supracervical hysterectomy or uterine morcellation for presumed benign disease. Int J Gynecol Cancer. 2008;18:1065–1070. doi: 10.1111/j.1525-1438.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 32.Mowers EL, Skinner B, McLean K, et al. Effects of morcellation of uterine smooth muscle tumor of uncertain malignant potential and endometrial stromal sarcoma: Case series and recommendations for clinical practice. J Minim Invasive Gynecol. 2015;22:601–606. doi: 10.1016/j.jmig.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Oduyebo T, Rauh-Hain AJ, Meserve EE, et al. The value of re-exploration in patients with inadvertently morcellated uterine sarcoma. Gynecol Oncol. 2014;132:360–365. doi: 10.1016/j.ygyno.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Tantitamit T, Huang KG, Manopunya M, et al. Outcome and management of uterine leiomyosarcoma treated following surgery for presumed benign disease: Review of literature. Gynecol Minim Invasive Ther. 2018;7:47–55. doi: 10.4103/GMIT.GMIT_10_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai VB, Wright JD, Schwartz PE, et al. In reply. Obstet Gynecol. 2018;132:519–520. doi: 10.1097/AOG.0000000000002770. [DOI] [PubMed] [Google Scholar]
- 36.American College of Obstetricians and Gynecologists ACOG Committee Opinion No. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238–e248. doi: 10.1097/AOG.0000000000003126. [DOI] [PubMed] [Google Scholar]
- 37.American Association of Gynecologic Laparoscopists Tissue Extraction Task Force Members. Morcellation during uterine tissue extraction: An update. J Minim Invasive Gynecol. 2018;25:543–550. doi: 10.1016/j.jmig.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 38.AAGL Advancing Minimally Invasive Gynecology Worldwide AAGL practice report: Morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517–530. doi: 10.1016/j.jmig.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 39. Lynam S, Young L, Morozov V, et al: Risk, risk reduction and management of occult malignancy diagnosed after uterine morcellation: A commentary. Womens Health (Lond) 11:929-944, 2015. [DOI] [PubMed]
- 40.Stewart EA.Differentiating uterine leiomyomas (fibroids) from uterine sarcomashttps://www.uptodate.com/contents/differentiating-uterine-leiomyomas-fibroids-from-uterine-sarcomas
- 41.Tulandi T, Ferenczy A. Biopsy of uterine leiomyomata and frozen sections before laparoscopic morcellation. J Minim Invasive Gynecol. 2014;21:963–966. doi: 10.1016/j.jmig.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Siedhoff MT, Doll KM, Clarke-Pearson DL, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroids: An updated decision analysis following the 2014 Food and Drug Administration safety communications. Am J Obstet Gynecol. 2017;216:259.e1–259.e6. doi: 10.1016/j.ajog.2016.11.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251. doi: 10.1093/jnci/djv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malin JL, Kahn KL, Adams J, et al. Validity of cancer registry data for measuring the quality of breast cancer care. J Natl Cancer Inst. 2002;94:835–844. doi: 10.1093/jnci/94.11.835. [DOI] [PubMed] [Google Scholar]
- 45.Hensley ML.Treatment and prognosis of uterine leiomyosarcomahttps://www.uptodate.com/contents/treatment-and-prognosis-of-uterine-leiomyosarcoma