Abstract
The anthelmintic drug praziquantel (PZQ) is used to treat schistosomiasis, a neglected tropical disease that affects over 200 million people worldwide. PZQ causes Ca2+ influx and spastic paralysis of adult worms and rapid vacuolization of the worm surface. However, the mechanism of action of PZQ remains unknown even after 40 years of clinical use. Here, we demonstrate that PZQ activates a schistosome transient receptor potential (TRP) channel, christened Sm.TRPMPZQ, present in parasitic schistosomes and other PZQ-sensitive parasites. Several properties of Sm.TRPMPZQ were consistent with known effects of PZQ on schistosomes, including (i) nanomolar sensitivity to PZQ; (ii) stereoselectivity toward (R)-PZQ; (iii) mediation of sustained Ca2+ signals in response to PZQ; and (iv) a pharmacological profile that mirrors the well-known effects of PZQ on muscle contraction and tegumental disruption. We anticipate that these findings will spur development of novel therapeutic interventions to manage schistosome infections and broader interest in PZQ, which is finally unmasked as a potent flatworm TRP channel activator.
Keywords: transient receptor potential channels (TRP channels), calcium channel, calcium imaging, infectious disease, ion channel, bilharzia, flatworm, schistosomiasis, Ca2+ signaling, parasite
Introduction
Schistosomiasis (bilharzia) is a parasitic worm infection that infects millions of people worldwide (1, 2). Mature blood flukes living in the vasculature lay eggs, which become deposited in host tissues, where they trigger local inflammatory responses. Chronic infections become associated with fibrosis and obstructive disease in gastrointestinal tissues and liver (Schistosoma mansoni, Schistosoma japonicum), genitourinary disease (Schistosoma haematobium), anemia, undernutrition, and a heightened risk for other comorbidities (3). The annual disease burden has been estimated as a loss of up to 70 million disability-adjusted life years (1, 2).
In 2017, ∼100 million people (∼80 million school-aged children) received free preventive treatment for schistosomiasis. This treatment depends on a drug called praziquantel (PZQ),2 as no effective vaccine currently exists (4). The clinical formulation of PZQ is a racemate (±PZQ) composed of the enantiomers (R)-PZQ and (S)-PZQ. (R)-PZQ is the antischistosomal eutomer, known to cause Ca2+ influx and spastic paralysis of adult worms and rapid vacuolization of the worm tegumental surface (5). (S)-PZQ is regarded as the less active distomer (6). From a therapeutic perspective, it is problematic that despite decades of clinical usage, as well as demonstration of strains with lower sensitivity to PZQ in both laboratory and field, the flatworm target(s) of PZQ remains unknown (7, 8). This lack of knowledge is a longstanding roadblock for this field.
Here, we demonstrate that (R)-PZQ activates a Ca2+-permeable transient receptor potential (TRP) channel expressed in PZQ-sensitive flatworms.
Results
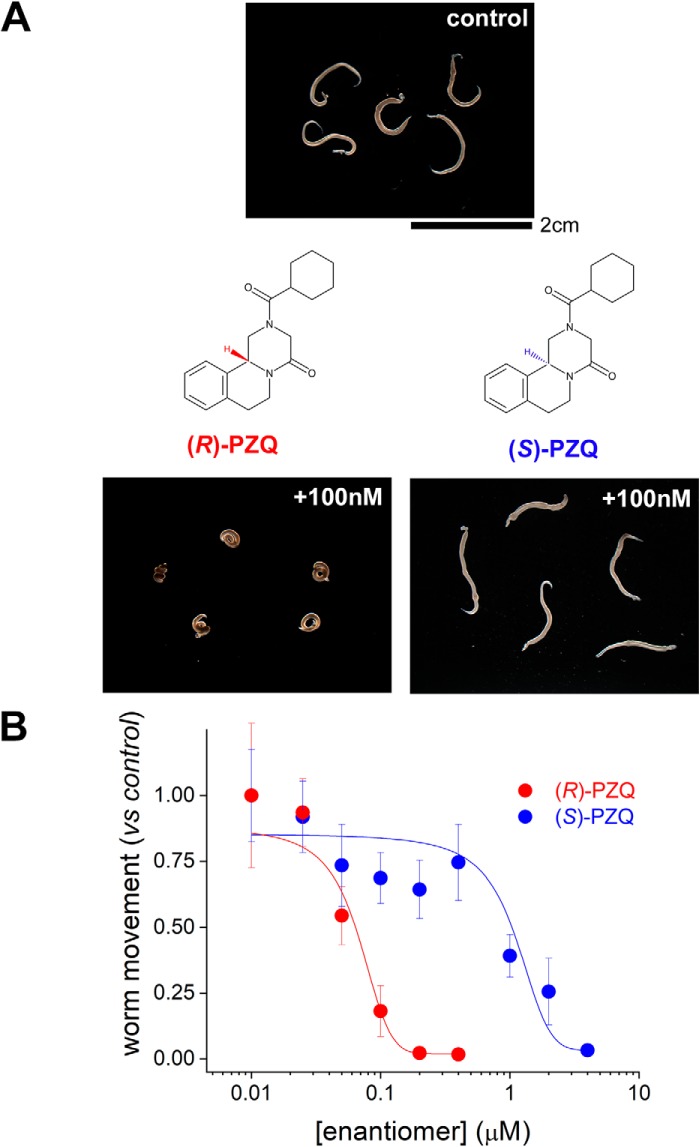
The addition of (R)-PZQ (100 nm) to adult schistosome worms ex vivo caused a rapid, spastic paralysis (Fig. 1A). The addition of the same concentration of (S)-PZQ was ineffective at causing contraction (Fig. 1A). This demonstrates the differential potency of the two PZQ enantiomers against adult schistosome worms (EC50 for (R)-PZQ = 68 ± 7 nm, EC50 for (S)-PZQ = 1.1 ± 0.4 μm; Fig. 1B) observed both ex vivo and in vivo (6).
Figure 1.
Effects of PZQ enantiomers on schistosome worms in vitro. A, images of five schistosome worms before (top) and 1 min after (bottom) the addition of a fixed concentration (100 nm) of (R)-PZQ or (S)-PZQ. Structures of (R)-PZQ and (S)-PZQ, tetracyclic tetrahydroisoquinolines, highlight chirality. B, concentration–response relationships for (R)-PZQ (red) and (S)-PZQ (blue) evoked changes in worm motility measured as described (13). Data represent mean ± S.D. (error bars) for at least three independent experiments.
Although no binding site(s) for these enantiomers has been identified in parasitic flatworms, there has been considerable recent progress in identifying targets for (R)-PZQ and (S)-PZQ in the human host (9). (R)-PZQ is a partial agonist of the human 5-hydroxytryptamine 2B receptor (5HT2BR (10)), and (S)-PZQ is a partial agonist of the human transient receptor potential melastatin-8 channel (hTRPM8 (11)). Whereas regulation of these host targets occurs over the micromolar range (10–12), molecular divergence between human and flatworm ligand-binding pockets (13, 14) makes it reasonable to anticipate different binding poises and affinities at a homologous schistosome target(s).
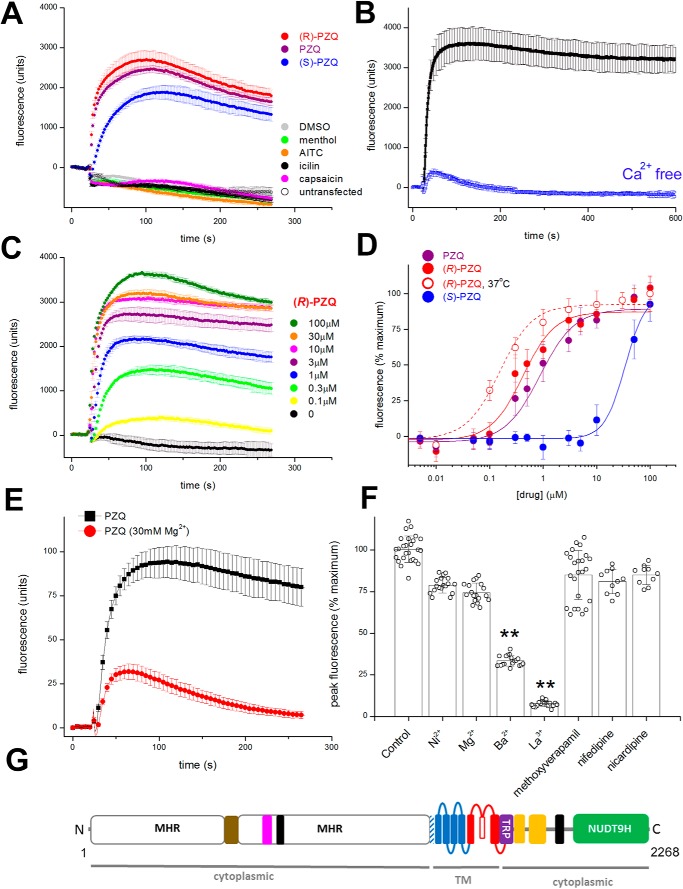
Following this logic, we searched for flatworm TRP channels exhibiting sequence homology to hTRPM8. One candidate, christened Sm.TRPMPZQ, mediated robust Ca2+ signals in response to ±PZQ and (R)-PZQ in transfected HEK293 cells that were not observed in either untransfected or vehicle-treated cells expressing Sm.TRPMPZQ (Fig. 2A). (S)-PZQ also evoked a response in Sm.TRPMPZQ-expressing cells, but with slower kinetics suggestive of a stereoselectivity toward the PZQ enantiomers that would be poorly discriminated at the high concentration of the primary screening (50 μm; Fig. 2A). Established mammalian TRP ligands (menthol, allyl isothiocyanate (AITC), icilin, and capsaicin) did not activate Sm.TRPMPZQ (Fig. 2A). The PZQ-evoked Ca2+ signal depended on Ca2+ entry across the plasma membrane, as removal of extracellular Ca2+ abolished the sustained cytoplasmic Ca2+ elevation (Fig. 2B).
Figure 2.
Properties of Sm.TRPMPZQ. A, Fluo-4 fluorescence traces in HEK293 cells in expressing Sm.TRPMPZQ monitored prior to and after the addition of ±PZQ (purple), (R)-PZQ (red), and (S)-PZQ (blue) (50 μm). Other tested TRP ligands (menthol (green), AITC (orange), icilin (black), and capsaicin (magenta) (50 μm)) did not evoke a response. For Fig. 2, all Ca2+ reporter assays were performed using a FLIPR to resolve Fluo-4 fluorescence from cells in 96-well plates. Data are presented as mean ± S.D. (error bars) of technical replicates in an individual experiment except where stated. B, PZQ-evoked Ca2+ signals depend on Ca2+ influx. Responses to ±PZQ (5 μm) in HEK293 cells expressing Sm.TRPMPZQ in normal HBSS (black) and Ca2+-free HBSS (blue, HBSS supplemented with 1 mm EGTA). C, fluorescence traces showing action of various concentrations (100 nm to 100 μm) of (R)-PZQ at Sm.TRPMPZQ. D, concentration–response relationships for ±PZQ (purple), (R)-PZQ (red), and (S)-PZQ (blue) at Sm.TRPMPZQ from experiments performed at room temperature. An (R)-PZQ concentration–response curve performed at 37 °C is shown by the dashed red line. Data are mean ± S.D. for at least three independent experiments. E, effect of Mg2+ concentration on Sm.TRPMPZQ activity. Assays were performed under the same medium conditions as described previously (15), comparing the effects of 1 μm ±PZQ at 0.4 mm Mg2+, 0.4 mm Ca2+ (1:1) and 30 mm Mg2+, 0.4 mm Ca2+ (75:1). F, pharmacological signature of Sm.TRPMPZQ. Effects of various heavy metal ions (10 mm) on ±PZQ (1 μm) evoked Sm.TRPMPZQ activity to replicate conditions reported previously (17). Effects of drugs were as follows: methoxyverapamil (D-600, 100 μm; mimicking conditions in Ref. 17) and nifedipine and nicardipine (20 μm; mimicking conditions in Ref. 18). Data show individual data points (mean ± S.D.) from a total of at least three independent transfections. One-way analysis of variance yielded significant variance between treatments. An ensuing post hoc Tukey test showed that both the Ba2+ and La3+ treatments differed significantly from other conditions (**, p < 0.01). G, domain organization of Sm.TRPMPZQ. The schematic shows distinct domains identified in recent TRPM2 structures to include the N-terminal TRPM homology region (MHR) domain containing an ankyrin-like repeat domain (brown (22)), the pre-S1 helix (shaded), the six TM-spanning helices (S1–S6) comprising the voltage sensor–like domain (VSLD; blue) and pore-forming domain (red), the TRP domain (purple), the rib and pole helices (yellow), an additional helical domain (black), and the C-terminal NUDT9H domain.
Full concentration–response curves were performed with (R)-PZQ (Fig. 2, C and D), (S)-PZQ, and ±PZQ (Fig. 2D). Sm.TRPMPZQ was activated by ±PZQ (EC50 = 1.08 ± 0.14 μm; Fig. 2D), and activation was stereoselective, with (R)-PZQ evoking Ca2+ signals over a considerably lower concentration range (EC50 = 597 ± 10 nm) than (S)-PZQ (EC50 = 27.9 ± 3.1 μm; Fig. 2D). When the incubation temperature was increased to 37 °C, (R)-PZQ activated Sm.TRPMPZQ over an even lower concentration range (EC50 = 154 ± 33 nm; Fig. 2D).
Early work on schistosomes established key pharmacological characteristics of PZQ action on parasite muscle contraction and/or 45Ca2+ uptake. These include (i) conversion of contraction from sustained to phasic in the presence of elevated Mg2+, (ii) inhibition by La3+, and (iii) insensitivity to several voltage-operated Ca2+ channel (Cav) blockers at specific doses. We therefore examined the impact of these same manipulations on Sm.TRPMPZQ activity. First, increasing the Mg2+/Ca2+ ratio to a level (75:1) that resulted in transient muscle contraction (15, 16) also resulted in a transient PZQ-evoked Ca2+ signal via Sm.TRPMPZQ (Fig. 2E). Second, preincubation of worms with La3+ (10 mm) inhibited both PZQ-evoked 45Ca2+ accumulation and PZQ-evoked contraction (17). La3+ (10 mm) also inhibited Sm.TRPMPZQ activity (Fig. 2F). Third, three Cav blockers (methoxyverapamil, nifedipine, and nicardipine) that failed to block PZQ action on worms (17, 18) also failed to inhibit PZQ-evoked Sm.TRPMPZQ activity at the same doses (Fig. 2F). Therefore, the pharmacological properties of Sm.TRPMPZQ mirror the characteristics of PZQ action on schistosome muscle.
Consistent with the homology-based search strategy, Sm.TRPMPZQ is a member of the TRP melastatin (TRPM) subfamily. Sequence analysis revealed an architecture characteristic of TRPM channels (Fig. 2G), a well-represented family within flatworm genomes (19). Features include a long N-terminal TRPM homology region (MHR) domain, followed by six predicted transmembrane (TM) domains with a pore-forming re-entry loop between TM5 and TM6, a conserved TRP helix juxtaposed to coiled-coil regions, and a cytoplasmic C-terminal enzymatic domain (Fig. 2G). This enzyme domain displayed homology with the human ADP-ribose (ADPR) pyrophosphatase NUDT9, a feature characteristic of TRPM2 channels (20–23). TRPM2 and TRPM8 are closely related “long” TRPM channels, and Sm.TRPMPZQ displays the highest sequence identity with these human TRPM variants (29.5 and 28.5% sequence identity with hTRPM2 and hTRPM8, respectively).
Analysis of flatworm genomic and transcriptomic data sets revealed the presence of Sm.TRPMPZQ homologs in other parasitic flatworms, including cestodes and flukes, known to exhibit PZQ sensitivity (Fig. S1A). To assess the broader PZQ sensitivity of schistosome TRP channels, we screened three other TRPs. First, we examined the previously characterized Sm.TRPA, which has been shown to activated by the ligands AITC and capsaicin (14). Sm.TRPA did not respond to PZQ but, as expected, did respond to the other two compounds (Fig. S1B). Next, we focused on the schistosome TRPM subfamily, which is predicted to contain seven members (Fig. S1A). The two members most closely related to Sm.TRPMPZQ (Smp_130890 and Smp_000050) did not respond to PZQ (Fig. S1, C and D). With the caveat that there is no control for functional expression, as endogenous agonists of these TRPM channels are unknown, these data suggest that schistosome TRP (and TRPM) channels are not broadly sensitive to PZQ.
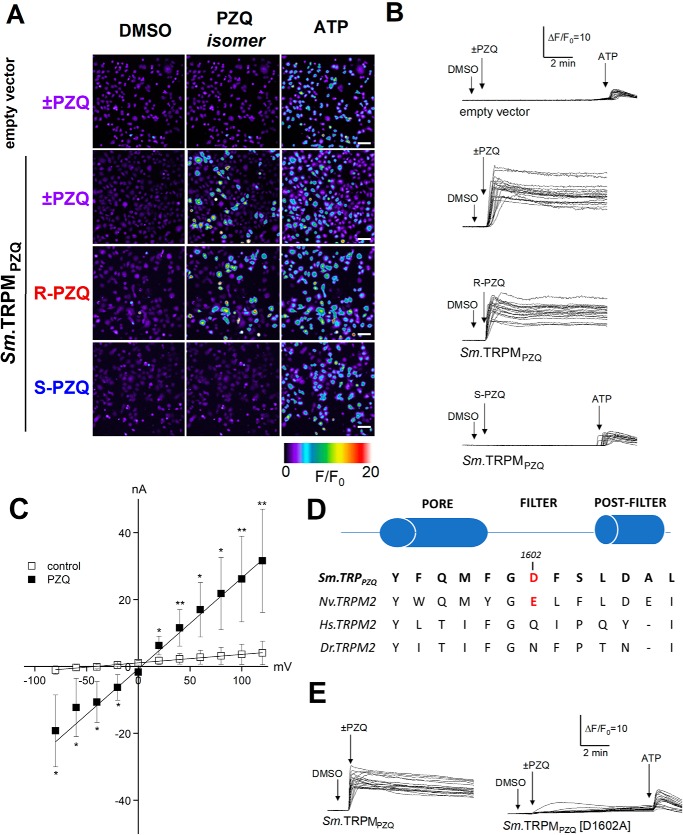
Next, to resolve the single-cell kinetics of Sm.TRPMPZQ activity, we performed confocal Ca2+ imaging. In HEK cells transfected with empty vector, the addition of ±PZQ (10 μm) failed to evoke a cytoplasmic Ca2+ signal (Fig. 3, A and B), although cells responded to ATP (100 μm), which activated endogenous purinoceptors. In contrast, in HEK cells transiently transfected with Sm.TRPMPZQ, the addition of ±PZQ (1 μm) evoked a rapid and protracted rise in cytoplasmic Ca2+ (Fig. 3, A and B). Responses were evoked by (R)-PZQ, with (S)-PZQ being ineffective at the same concentration (1 μm; Fig. 3, A and B). The large and persistent increase in fluorescence evidenced little Sm.TRPMPZQ desensitization in the presence of ±PZQ and contrasted with the smaller, transient nature of Ca2+ signals evoked by ATP. This signal was triggered by Ca2+ influx, as this response was seen only when Ca2+-containing medium was re-added to HEK cells initially exposed to ±PZQ in Ca2+-free medium (Fig. S2A). Activation of Sm.TRPMPZQ by ±PZQ was also reversible, as ±PZQ washout resulted in a decrease of signal to baseline (Fig. S2B).
Figure 3.
Characterization of Sm.TRPMPZQ-mediated Ca2+ signals by confocal imaging and electrophysiology. A, confocal Ca2+ imaging of Fluo-4 fluorescence from control (top) or Sm.TRPMPZQ-expressing HEK cells, stimulated with ±PZQ, individual PZQ enantiomers (1 μm), or ATP (100 μm). Pseudocolored images of the imaging field are captured at the indicated time points on related fluorescence traces shown in B. Scale bar, 40 μm. B, representative fluorescence traces from HEK293 cells loaded with Fluo-4 AM following the addition of DMSO vehicle, PZQ (10 μm for empty vector control transfections, 1 μm in Sm.TRPMPZQ-expressing cells), and ATP (100 μm). Time courses of fluorescence ratios are presented as ΔF/F0 (where ΔF represents change in fluorescence from baseline and F0 represents fluorescence at time 0). C, dependence of transmembrane current of Sm.TRPMPZQ-expressing HEK293 cells in whole-cell mode on the voltage in the absence (control, open squares) or presence of 2 μm PZQ (closed squares) in the bathing solution. Statistical analysis between I-V curve values in drug-treated and control conditions was performed using a Mann–Whitney test at each voltage condition (mean ± S.D. (error bars); *, p < 0.1; **, p < 0.05). Average membrane conductance and reversal potential in the absence and the presence of PZQ were 26 and 257 nS (p = 0.026) and −32 and −14 mV (p = 0.074), respectively. Data show peak current values from cells recorded from n ≥ 3 independent transfections. D, sequence alignment of the channel selectivity filter region in various TRPM2 channels to highlight the Asp-1602 residue in Sm.TRPPZQ aligned with corresponding amino acid sequence from N. vectensis (Nv.TRPM2), human TRPM2 (Hs.TRPM2), and zebrafish TRPM2 (Dr.TRPM2). E, Ca2+-imaging data showing responses of control HEK cells (top), HEK cells expressing WT Sm.TRPMPZQ (middle), and D1602A mutant (bottom) to ±PZQ (10 μm for empty vector control transfection, 1 μm in Sm.TRPMPZQ- or Sm.TRPMPZQ[D1602A]-expressing cells) from one of three representative experiments.
Electrophysiological analysis of Sm.TRPMPZQ was performed by measuring whole-cell currents in HEK cells expressing GFP alone or expressing GFP and Sm.TRPMPZQ. In cells expressing GFP alone, the addition of ±PZQ (2 μm) did not evoke currents (0 of 18 cells examined). In contrast, in HEK cells co-transfected with cDNA encoding both Sm.TRPMPZQ and GFP, the addition of ±PZQ evoked rapidly activating inward currents in all GFP-positive cells (22 of 22 cells, holding potential of −40 mV). Characterization of current magnitude after various voltage steps, in the absence and presence of PZQ (2 μm), revealed PZQ-activated Sm.TRPMPZQ-conducted large inward and outward currents with a linear I-V relationship (Fig. 3C), resembling the linear I-V relationship displayed by hTRPM2 channels (24). Based on sequence homology with another invertebrate TRPM2 channel (Nematostella vectensis TRPM2, Nv.TRPM2) that has been structurally and functionally characterized (25), we speculated that the substantial Ca2+ permeability of Sm.TRPMPZQ (Fig. 3, B and C) is supported by the presence of a negatively charged residue in the predicted pore filter of Sm.TRPMPZQ (FGD in Fig. 3D). This closely resembles the pore filter sequence of Nv.TRPM2 (YGE in Fig. 3D), which displays substantial Ca2+ permeability (25). Consistent with this idea, PZQ-evoked Ca2+ signals were strongly attenuated in HEK cells expressing the mutant Sm.TRPMPZQ[D1602A] (Fig. 3E). Sm.TRPMPZQ therefore displays several characteristics consistent with the properties of TRPM2 channels.
Discussion
These data represent the first report of a flatworm target activated by PZQ. Although further experiments would be needed to confirm Sm.TRPMPZQ as the clinically relevant target in worms, our data clearly evidence Sm.TRPMPZQ as a schistosome target of PZQ.
The properties of Sm.TRPMPZQ, a TRPM2-like channel, are, however, consistent with several key facets of PZQ action on worms. These include (i) nanomolar sensitivity to PZQ (Fig. 2, C and D); (ii) stereoselectivity toward (R)-PZQ (Figs. 2 and 3); (iii) mediation of a sustained Ca2+ entry in response to PZQ (Fig. 3B) that parallels the kinetics of worm contracture and tegumental disruption (15–17, 26); (iv) partial blockade by Mg2+ and complete inhibition by La3+, mirroring the effects of PZQ on muscle contraction and tegumental disruption (15–17, 26); (v) insensitivity to specific Cav blockers that fail to block PZQ action on worms (Fig. 2F) (16–18); and (vi) presence of homologs in other parasitic flatworms sensitive to PZQ (Fig. S1). Just as Sm.TRPMPZQ supports long-lasting cellular Ca2+ signals (Figs. 2 and 3), human TRPM2 (hTRPM2) also exhibits long channel opening times that support substantial Ca2+ influx (23, 27). hTRMP2 is a well-known effector of apoptosis being responsive to reactive oxygen species through activation by H2O2 and ADPR (23, 28). Activation of hTRMP2 at the cell surface and within intracellular organelles causes lysosomal permeabilization and cell death (28–30). Such regulation could underpin the deleterious actions of ±PZQ on worm tegument crucial for the in vivo efficacy of PZQ (31, 32). Therefore, there are many similarities between the properties of Sm.TRPMPZQ and the characteristics of PZQ action on schistosomes.
This discovery also prompts new questions. What are the endogenous agonists and/or environmental cues that regulate Sm.TRPMPZQ activity across the parasite life cycle? In what cell type(s) is Sm.TRPMPZQ expressed? How is Sm.TRPMPZQ activity regulated in juvenile worms known to be less sensitive to PZQ? Is Sm.TRPMPZQ activity altered in schistosome strains that show refractoriness to PZQ action? Mutagenesis demonstrates that single amino acid changes in Sm.TRPMPZQ can dramatically alter channel responses to ±PZQ (Fig. 3E). This discovery also prioritizes analyses of TRPMPZQ homologs in other flatworms as well as all other schistosome TRPM channels to assess broader PZQ sensitivity.
Finally, we note that (R)-PZQ is a potent activator of Sm.TRPMPZQ (Fig. 2). Known regulators of hTRPM2, including the endogenous agonist ADPR (23), act over the micromolar range. This is important as hTRPM2 is an emerging clinical target for several nervous system and inflammatory disorders (23, 27). Understanding the basis of (R)-PZQ affinity for Sm.TRPMPZQ and comparing regulation and gating of Sm.TRPMPZQ with recently solved TRPM structures (20–22, 33) may reciprocally catalyze drug design at this clinically important human target.
Experimental procedures
Reagents
Enantiomers of ±PZQ were resolved following the protocol of Woelfle et al. (34). All chemical reagents were from Sigma. Cell culture reagents were from Invitrogen. Lipofectamine 2000 was from Thermo Fisher Scientific.
Adult schistosome mobility assays
Adult schistosomes were recovered by dissection of the mesenteric vasculature in female Swiss Webster mice previously infected (∼49 days) with S. mansoni cercariae (NMRI strain) by the Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, MD). All animal experiments followed ethical regulations approved by the Medical College of Wisconsin institutional animal care and use committee. Harvested schistosomes were washed in RPMI 1640 supplemented with HEPES (25 mm), 5% heat-inactivated fetal bovine serum (FBS) (Gibco), and penicillin-streptomycin (100 units/ml) and incubated overnight (37 °C/5% CO2) in vented Petri dishes (100 × 25 mm). The following day, movement assays were performed using male worms in 6-well dishes (∼5 individual worms/3 ml of medium per well). Video recordings were captured using a Zeiss Discovery v20 stereomicroscope with a QiCAM 12-bit cooled color CCD camera controlled by Metamorph imaging software. Recordings (1 min) of worm motility (4 frames/s), during the addition of various drug concentrations were analyzed as described previously (13).
Molecular cloning
For cloning of Sm.TRPMPZQ, total RNA was isolated from adult schistosome worm pairs using TRIzol® and poly(A)-purified using a NucleoTrap® mRNA minikit. cDNA was synthesized using the SuperScriptTM III first-strand synthesis system (Invitrogen). Using the predicted sequence (Smp_246790) as a template, cDNA from transcribed sequences was amplified by PCR (LA TaqTM polymerase) and ligated into pGEM®-T Easy (Promega) for sequencing. Several splice variants of Sm.TRPMPZQ were identified within both the N-terminal TRPM homology region (MHR) and cytoplasmic C-terminal domain, which will be characterized elsewhere. The sequence used here for functional analyses represents the reference sequence (2268 amino acids, Smp_246790.5).
Cell culture and transfection
HEK293 cells (ATCC CRL-1573.3) and U2OS cells (ATCC HTB-96; Fig. S2A and B) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), and l-glutamine (290 μg/ml). For screening parasite TRP channels, codon-optimized cDNAs and mutants (Genscript) were transiently transfected into HEK293 cells using Lipofectamine-2000 at a density of 3 × 106 cells/dish (100 mm).
Ca2+-imaging assays
Ca2+-imaging assays were performed using a fluorescence imaging plate reader (FLIPRTETRA, Molecular Devices). HEK293 cells (naive or transfected) were seeded (50,000 cells/well) in a black-walled clear-bottomed poly-d-lysine–coated 96-well plate (Corning) in Dulbecco's modified Eagle's medium supplemented with 10% dialyzed FBS. After 24 h, growth medium was removed, and cells were loaded with a fluorescent Ca2+ indicator (Fluo-4 direct dye, Invitrogen) by incubation (100 μl per well, 1 h at 37 °C) in Hanks' balanced salt solution (HBSS) assay buffer containing probenecid (2.5 mm) and HEPES (20 mm). Drug dilutions were prepared in assay buffer, without probenecid and dye, in V-shaped 96-well plates (Greiner Bio-one, Frickenhausen, Germany). After loading, the Ca2+ assay was performed at room temperature. Basal fluorescence was monitored for 20 s, and then 25 μl of each drug was added, and the signal (raw fluorescence units) was monitored over an additional 250 s. For quantitative analyses, peak fluorescence in each well was normalized to maximum -fold increase over baseline.
For confocal Ca2+ imaging, HEK cells were loaded with Fluo-4-AM (4 μm) and Pluronic F127 (0.4%) for 25 min at room temperature. Cells were then washed twice with HBSS and incubated at room temperature for de-esterification (30 min). Experiments in U2OS cells (Fig. S2A and B) were done using the genetically encoded calcium indicator, GCaMP6M. Fluorescence was imaged on an Olympus IX81 microscope, and fluorescence changes (λex = 488 nm (λem = 513 ± 15-nm bandpass) were monitored using a Yogokawa spinning disk confocal (CSU-X-M1N) and an Andor iXon Ultra 888 EMCCD camera. Data were expressed as a ratio (F/F0) of fluorescence at any given time (F) relative to fluorescence prior to drug addition (F0).
Electrophysiology
For whole-cell current recordings, HEK293 cells were transfected with a plasmid encoding GFP or co-transfected with plasmids encoding GFP and Sm.TRPMPZQ. One day later, cells were replated onto round 18-mm glass coverslips. After overnight incubation, coverslips were secured in a recording chamber over a Nikon Eclipse TE200 inverted microscope. Cells were continuously superfused (6 ml/min) with an extracellular buffer consisting of 140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, and 10 mm glucose (pH 7.4, 310 ± 3 mosm at room temperature). HEK293 cells were held at a holding voltage of −40 mV, and responses were resolved after superfusion of extracellular buffer containing ±PZQ (2 μm). Recordings were made using borosilicate pipettes (Sutter Instrument Company, Novato, CA) pulled on a Sutter micropipette puller (model P-87) to resistances of 2–5 megaohms. Patch pipettes were filled with intracellular buffer containing 135 mm KCl, 10 mm NaCl, 1 mm MgCl2, 1 mm EGTA, 0.2 mm Na.GTP, 2.5 mm ATP.Na2, and 10 mm HEPES (pH 7.20, 290 ± 3 mosm at room temperature). Cell capacitance was compensated, and series resistance was kept <10 megaohms. Cells were included in analyses if the leak current stayed <200 pA. Recordings were made using an EPC10 USB amplifier (HEKA Electronics) and Patch Master software (HEKA Electronics). Patch-clamp data were analyzed using Pulse, PulseFit, or Fitmaster software (HEKA Electronics). For current-voltage measurements of HEK293 cells expressing Sm.TRPMPZQ, step potentials of 250 ms spanning the voltage range from −80 to +120 mV were delivered from a holding potential of −80 mV. For I-V curves, patch pipettes were filled with intracellular buffer containing: 140 mm CsMeSO4, 1 mm MgCl2, 1 mm EGTA, 10 mm HEPES-CsOH (pH 7.2 with CsOH, 300–310 mOsm/kg adjusted with sucrose).
Author contributions
S. K. P. and J. S. M. conceptualization; S. K. P., G. S. G., E. G. C., F. M., and P. M. investigation; P. I. D. resources; J. D. C., C. L. S., and J. S. M. supervision; J. D. C., C. L. S., and J. S. M. writing-review and editing; J. S. M. funding acquisition; J. S. M. writing-original draft; J. S. M. project administration.
Supplementary Material
Acknowledgments
Schistosome-infected mice were provided by the NIAID Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, MD) through NIAID, National Institutes of Health, Contract HHSN272201000005I for distribution via BEI Resources.
This work was supported by the Marcus Family, National Institutes of Health Grants R56AI145871 (to J. S. M.) and R01-NS040538 and RO1-NS070711 (to C. L. S.), and National Science Foundation Grant MCB1615538 (to J. S. M.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1 and S2.
- PZQ
- praziquantel
- TRP
- transient receptor potential
- AITC
- allyl isothiocyanate
- TRPM
- TRP melastatin
- TM
- transmembrane
- ADPR
- ADP-ribose
- HEK
- human embryonic kidney
- HBSS
- Hanks' balanced salt solution.
References
- 1. Hotez P. J., and Fenwick A. (2009) Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl. Trop. Dis. 3, e485 10.1371/journal.pntd.0000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. King C. H., and Dangerfield-Cha M. (2008) The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 4, 65–79 10.1177/1742395307084407 [DOI] [PubMed] [Google Scholar]
- 3. Yegorov S., Joag V., Galiwango R. M., Good S. V., Mpendo J., Tannich E., Boggild A. K., Kiwanuka N., Bagaya B. S., and Kaul R. (2019) Schistosoma mansoni treatment reduces HIV entry into cervical CD4+ T cells and induces IFN-I pathways. Nat. Commun. 10, 2296 10.1038/s41467-019-09900-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergquist R., Utzinger J., and Keiser J. (2017) Controlling schistosomiasis with praziquantel: how much longer without a viable alternative? Infect. Dis. Poverty 6, 74 10.1186/s40249-017-0286-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrews P., Thomas H., Pohlke R., and Seubert J. (1983) Praziquantel. Med. Res. Rev. 3, 147–200 10.1002/med.2610030204 [DOI] [PubMed] [Google Scholar]
- 6. Kovač J., Vargas M., and Keiser J. (2017) In vitro and in vivo activity of R- and S-praziquantel enantiomers and the main human metabolite trans-4-hydroxy-praziquantel against Schistosoma haematobium. Parasites Vectors 10, 365 10.1186/s13071-017-2293-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenberg R. M. (2013) New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology 140, 1534–1546 10.1017/S0031182013000231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas C. M., and Timson D. J. (2018) The mechanism of action of praziquantel: six hypotheses. Curr. Top. Med. Chem. 18, 1575–1584 10.2174/1568026618666181029143214 [DOI] [PubMed] [Google Scholar]
- 9. Day T. A., and Kimber M. J. (2018) Praziquantel interaction with mammalian targets in the spotlight. Trends Parasitol. 34, 263–265 10.1016/j.pt.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 10. Chan J. D., Cupit P. M., Gunaratne G. S., McCorvy J. D., Yang Y., Stoltz K., Webb T. R., Dosa P. I., Roth B. L., Abagyan R., Cunningham C., and Marchant J. S. (2017) The anthelmintic praziquantel is a human serotoninergic G-protein-coupled receptor ligand. Nat. Commun. 8, 1910 10.1038/s41467-017-02084-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gunaratne G. S., Yahya N. A., Dosa P. I., and Marchant J. S. (2018) Activation of host transient receptor potential (TRP) channels by praziquantel stereoisomers. PLoS Negl. Trop. Dis. 12, e0006420 10.1371/journal.pntd.0006420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Babes R. M., Selescu T., Domocos D., and Babes A. (2017) The anthelminthic drug praziquantel is a selective agonist of the sensory transient receptor potential melastatin type 8 channel. Toxicol. Appl. Pharmacol. 336, 55–65 10.1016/j.taap.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 13. Chan J. D., McCorvy J. D., Acharya S., Johns M. E., Day T. A., Roth B. L., and Marchant J. S. (2016) A miniaturized screen of a Schistosoma mansoni serotonergic G protein-coupled receptor identifies novel classes of parasite-selective inhibitors. PLoS Pathog. 12, e1005651 10.1371/journal.ppat.1005651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bais S., Berry C. T., Liu X., Ruthel G., Freedman B. D., and Greenberg R. M. (2018) Atypical pharmacology of schistosome TRPA1-like ion channels. PLoS Negl. Trop. Dis. 12, e0006495 10.1371/journal.pntd.0006495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blair K. L., Bennett J. L., and Pax R. A. (1992) Praziquantel: physiological evidence for its site(s) of action in magnesium-paralysed Schistosoma mansoni. Parasitology 104, 59–66 10.1017/S0031182000060807 [DOI] [PubMed] [Google Scholar]
- 16. Pax R., Bennett J. L., and Fetterer R. (1978) A benzodiazepine derivative and praziquantel: effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Naunyn Schmiedebergs Arch. Pharmacol. 304, 309–315 10.1007/BF00507974 [DOI] [PubMed] [Google Scholar]
- 17. Fetterer R. H., Pax R. A., and Bennett J. L. (1980) Praziquantel, potassium and 2,4-dinitrophenol: analysis of their action on the musculature of Schistosoma mansoni. Eur. J. Pharmacol. 64, 31–38 10.1016/0014-2999(80)90366-0 [DOI] [PubMed] [Google Scholar]
- 18. Pica-Mattoccia L., Orsini T., Basso A., Festucci A., Liberti P., Guidi A., Marcatto-Maggi A. L., Nobre-Santana S., Troiani A. R., Cioli D., and Valle C. (2008) Schistosoma mansoni: lack of correlation between praziquantel-induced intra-worm calcium influx and parasite death. Exp. Parasitol. 119, 332–335 10.1016/j.exppara.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 19. Bais S., and Greenberg R. M. (2016) TRP channels in schistosomes. Int. J. Parasitol. Drugs Drug Resist. 6, 335–342 10.1016/j.ijpddr.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang L., Fu T. M., Zhou Y., Xia S., Greka A., and Wu H. (2018) Structures and gating mechanism of human TRPM2. Science 362, eaav4809 10.1126/science.aav4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang Y., Winkler P. A., Sun W., Lü W., and Du J. (2018) Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium. Nature 562, 145–149 10.1038/s41586-018-0558-4 [DOI] [PubMed] [Google Scholar]
- 22. Kühn F. J., Kühn C., Winking M., Hoffmann D. C., and Lückhoff A. (2016) ADP-ribose activates the TRPM2 channel from the sea anemone Nematostella vectensis independently of the NUDT9H domain. PLoS One 11, e0158060 10.1371/journal.pone.0158060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perraud A. L., Fleig A., Dunn C. A., Bagley L. A., Launay P., Schmitz C., Stokes A. J., Zhu Q., Bessman M. J., Penner R., Kinet J. P., and Scharenberg A. M. (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411, 595–599 10.1038/35079100 [DOI] [PubMed] [Google Scholar]
- 24. Du J., Xie J., and Yue L. (2009) Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J. Gen. Physiol. 134, 471–488 10.1085/jgp.200910254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Z., Tóth B., Szollosi A., Chen J., and Csanády L. (2018) Structure of a TRPM2 channel in complex with Ca2+ explains unique gating regulation. eLife 7, e36409 10.7554/eLife.36409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bricker C. S., Depenbusch J. W., Bennett J. L., and Thompson D. P. (1983) The relationship between tegumental disruption and muscle-contraction in Schistosoma mansoni exposed to various compounds. Z. Parasitenkd. 69, 61–71 10.1007/bf00934011 [DOI] [PubMed] [Google Scholar]
- 27. Belrose J. C., and Jackson M. F. (2018) TRPM2: a candidate therapeutic target for treating neurological diseases. Acta Pharmacol. Sin. 39, 722–732 10.1038/aps.2018.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hara Y., Wakamori M., Ishii M., Maeno E., Nishida M., Yoshida T., Yamada H., Shimizu S., Mori E., Kudoh J., Shimizu N., Kurose H., Okada Y., Imoto K., and Mori Y. (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 9, 163–173 10.1016/S1097-2765(01)00438-5 [DOI] [PubMed] [Google Scholar]
- 29. Abuarab N., Munsey T. S., Jiang L. H., Li J., and Sivaprasadarao A. (2017) High glucose-induced ROS activates TRPM2 to trigger lysosomal membrane permeabilization and Zn2+-mediated mitochondrial fission. Sci. Signal. 10, eaal4161 10.1126/scisignal.aal4161 [DOI] [PubMed] [Google Scholar]
- 30. Lange I., Yamamoto S., Partida-Sanchez S., Mori Y., Fleig A., and Penner R. (2009) TRPM2 functions as a lysosomal Ca2+-release channel in β cells. Sci. Signal. 2, ra23 10.1126/scisignal.2000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Day T. A., Bennett J. L., and Pax R. A. (1992) Praziquantel: the enigmatic antiparasitic. Parasitol. Today 8, 342–344 10.1016/0169-4758(92)90070-I [DOI] [PubMed] [Google Scholar]
- 32. Brindley P. J., and Sher A. (1990) Immunological involvement in the efficacy of praziquantel. Exp. Parasitol. 71, 245–248 10.1016/0014-4894(90)90028-B [DOI] [PubMed] [Google Scholar]
- 33. Yin Y., Le S. C., Hsu A. L., Borgnia M. J., Yang H., and Lee S. Y. (2019) Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 363, eaav9334 10.1126/science.aav9334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woelfle M., Seerden J. P., de Gooijer J., Pouwer K., Olliaro P., and Todd M. H. (2011) Resolution of praziquantel. PLoS Negl. Trop. Dis. 5, e1260 10.1371/journal.pntd.0001260 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.