Abstract
Sustained viremia following acute HIV infection is associated with profound CD4+ T cell loss and exhaustion of HIV-specific CD8+ T cell responses. To determine the impact of combination antiretroviral therapy (cART) on these processes, we examined the evolution of immune responses in acutely infected individuals initiating treatment prior to peak viremia. Immediate treatment of Fiebig stage I-II infection led to a rapid decline in viral load and diminished magnitude of HIV-specific (tetramer+) CD8+ T cell responses compared to untreated donors. There was a strong positive correlation between cumulative viral antigen exposure prior to full cART-induced suppression and immune responses measured by MHC class I tetramers, IFN-γ ELISPOT, and CD8+ T cell activation (CD38+HLA-DR+ among CD8+T cells). HIV-specific CD8+ T responses of early treated subjects were characterized by increased CD127 and BCL-2 expression, greater in vitro IFN-γ secretion, and enhanced differentiation into effector memory (Tem) cells. Transcriptional analysis of tetramer-positive CD8+ T cells from treated persons revealed reduced expression of genes associated with activation and apoptosis, with concurrent up-regulation of pro-survival genes including BCL-2, AXL, and SRC. Early treatment also resulted in robust HIV-specific CD4+ T cell responses compared to untreated HIV-infected individuals. Our data show that limiting acute viremia results in enhanced functionality of HIV-specific CD4+ and CD8+ T cells, preserving key antiviral properties of these cells.
One sentence summary:
Immediate initiation of antiviral therapy in acute HIV infection results in functional and persistent T cell responses.
Introduction
Despite considerable prevention efforts, continuing global HIV transmissions result in increasing numbers of life-long infections; moreover, substantial scientific challenges remain in the quest for an effective vaccine or cure (1, 2). Following transmission, there is considerable heterogeneity in the rate of disease progression, which is impacted by the magnitude of the set point viral load and the initial CD4+ T cell loss (3–5). Therefore, investigating factors that influence the quality of antiviral immune responses during the earliest stages of HIV infection may reveal specific responses that affect the clinical course of disease and inform vaccine development.
The emergence of HIV-specific CD8+ T cell responses has consistently been associated with reduction in peak virus replication during primary infection (6–10). Rapid escape from HIV-specific CD8+ T cell responses has been observed in acute infection, indicating antiviral function for at least a subset of these cells (11, 12). By twice-weekly screening of uninfected women at high risk of HIV infection in South Africa from the FRESH (Females Rising Through Education Support and Health) Cohort (13, 14)), we identified persons with hyperacute infection (defined as the period from onset of plasma viremia to peak viral load) and previously showed that untreated infection is associated with massive HIV-specific CD8+ T cell activation, and that the magnitude and kinetics of the initial response impact viral load (8); similar results have been reported in untreated cohorts in Thailand and East Africa (15, 16). These studies identified defects in early responses that could contribute to lack of complete viral suppression, including increased T cell apoptosis and the inability to up-regulate pro-survival molecules, such as CD127 required for establishment of long-term immunologic memory (8). However, how these defects relate to overall antigen exposure, and whether such defects could be abrogated by limiting antigen exposure in the earliest stages of infection, remain unknown.
In contrast to untreated acute infection, cART initiation during and following peak viremia in primary HIV infection is associated with improved T cell functionality and reduction in the size of the viral reservoir, and in some cases has been associated with prolonged remission following treatment discontinuation (17–23), suggesting that cART treatment very early in infection has a positive effect on antiviral immune responses. Moreover, numerous studies have shown that early therapy augments HIV-specific CD4+ T cell responses (reviewed in (24)). More recent studies demonstrate that initiation of cART prior to peak viremia limits antigen exposure and results in maintenance of CD4+ T cell numbers (13, 25), but abrogates antibody responses and can result in non-reactive HIV serology (13, 26). However, the impact of immediate cART on the induction, evolution, and function of HIV-specific CD4+ and CD8+ T cells has not been fully elucidated.
In this study, we conducted a detailed analysis of immune responses generated when cART is initiated in Fiebig stage I-II infection, prior to peak viremia, compared to treatment initiation at Fiebig stage III and later. Our findings have implications for the HIV vaccine and cure research and provide insights into the relationship between acute antigen exposure and T cell functionality.
Results
Description of the study design and sample collection
We studied a total of 46 HIV-infected FRESH cohort participants classified into 3 groups (table S1). Group 1 consisted of 26 participants identified in Fiebig I-II who were initiated on cART within 24 to 48 hours of detection of plasma HIV RNA (Tx Fiebig I-II). Group 2 consisted of 8 individuals identified in Fiebig III-V who were similarly immediately initiated on cART (Tx Fiebig III-V), and group 3 consisted of 12 participants identified in Fiebig I-V who did not initiate therapy during acute HIV infection (UnTx). A subset of the untreated individuals was also sampled after subsequent ART initiation, which was based on the then-current South African guidelines of a CD4 count below 350 cells/μL. Group 1 samples were collected at a median of 28 days after enrollment and treatment initiation (IQR 19.2–30 days), Group 2 samples at a median of 25 days (IQR 16.5–29.5) after enrollment and treatment initiation, and untreated samples at a median of 28 days (IQR 24–30.5) after enrollment. For groups 1 and 2, most samples were at a time when complete plasma viral suppression had already been achieved. Linear regression analysis showed that differences in treatment duration prior to sample collection among early treated individuals did not significantly affect the measured immunological parameters reported in subsequent figures (Supplementary Table S2).
Comparative analysis of initial HIV-specific CD8+ T cell responses during hyperacute HIV infection
Very early cART initiation in hyperacute infection leads to lack of antibody seroconversion in the majority of infected individuals (Supplementary Table S3) (13, 26), but the impact of early therapy on the induction and magnitude of emerging CD8+ T cell responses is not well characterized. To address this, we assessed HIV-specific CD8+ T cell responses in individuals initiating therapy during Fiebig Stage I-II or Stage III-V and compared these responses to analogous time points during untreated infection.
First, we employed IFN-γ ELISPOT using overlapping consensus clade C peptides (OLPs) spanning HIV Gag, Nef and Env proteins to measure the breadth of HIV-specific T cell responses at the time of peak CD8+ T cell activation in untreated infection (8). Eighteen of 26 (69%) treated (Tx) Fiebig I-II individuals had detectable ELISPOT responses, whereas 5 of 6 (83%) Tx Fiebig III-V and 12 of 12 UnTx persons had detectable responses, differences that did not reach statistical significance (Chi-square X2=4.84, df=2, p=0.09) (Fig. 1A). However, untreated subjects targeted significantly more peptides compared to Tx Fiebig I-II (Mann Whitney p=0.0001) or Tx Fiebig III-V (p=0.02) (Fig. 1b).
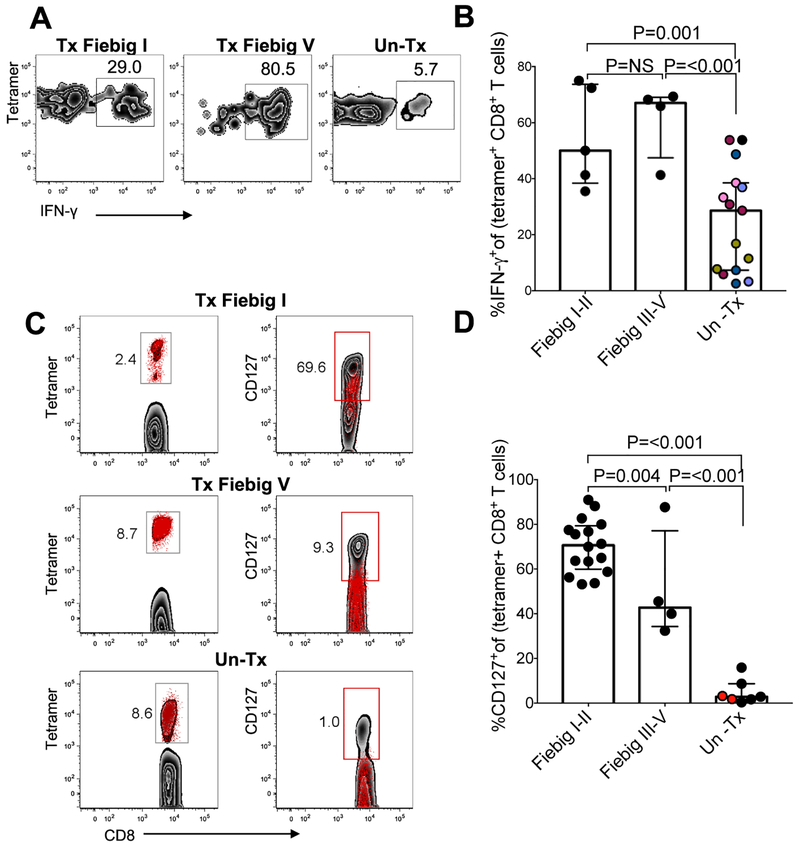
Figure 1: Very early cART initiation is associated with the induction of HIV-specific CD8+ T cell responses that are lower in magnitude and breadth compared to untreated acute HIV infection.
(a) Number of study participants tested in each of the three donor groups using ELISPOT assay. PBMCs were stimulated with individual overlapping peptides (OLP) spanning Gag, Nef, and Env proteins derived from HIV-1 clade C. (b) ELISPOT data denoting cumulative number of responses to clade C HIV Gag, Nef and Env peptides in 24 Fiebig I-II, 6 Fiebig III-V and 12 UnTx (c) Number of study participants tested in each group using the CFSE proliferation assays. PBMCs were stimulated with Gag, Nef, and Env peptide pools derived from HIV-1 clade C. One peptide pool was used for each protein (d) Cumulative percent of proliferating CD8+ T cells in response to Gag, Nef and Env HIV-1 clade C peptide pools in 20 Fiebig I-II, 6 Fiebig III-V and 6 UnTx. (e) Left column shows longitudinal plasma HIV RNA (red, RNA copies/ml plasma) and absolute CD4+ T cell counts (blue, CD4+ T lymphocytes/ul) before HIV infection and following onset of detectable plasma viremia in three representative individuals. The right column shows representative immunodominant tetramer positive responses for each subject measured at the peak of the response (D14 to D42 after diagnosis). The data are arranged according to treatment initiation status. (f) Number of responding and non-responding participants tested in each group using the MHC class I tetramers. (g) Frequencies of immunodominant tetramer+CD8+ T cells in Fiebig I-II, Fiebig III-V and untreated subjects in 21 Fiebig I-II, 8 Fiebig III-V and 11 UnTx. Statistical significance was calculated using multilevel mixed-effects linear regression analyses when comparing between groups to account for multiple measurements within some individuals. Black dots denote a single tetramer measurement per donor. Same coloured dots denote sum of multiple tetramer measurements from a single donor. Horizontal lines represent median with interquartile range.
Next, we performed HIV-specific CD8+ T cell proliferation assays following stimulation with Gag, Nef, and Env peptide pools. These were performed at similar time points to those used in the ELISPOT assays, but because of sample limitations not all individuals could be tested with both assays. 18 of 20 Tx Fiebig I-II subjects tested (90%) had detectable proliferative responses, as did 6 of 7 (86%) Tx Fiebig III-V, and 5 of 6 (83%) UnTx donors. There was no significant difference between groups in the number of donors with detectable responses (Chi-square X2=0.23, df=2, p=0.9 Fig. 1c), nor in the overall breadth of responses (Mann Whitney p=ns) (Fig. 1d).
Next, we performed tetramer staining in samples from Tx Fiebig I-II individuals as a quantitative measure of individual HIV-specific T cell responses, independent of function, at similar time points as the ELISPOT and proliferation analyses. These studies were limited to those who expressed HLA class I alleles for whom tetramers were available, with representative examples shown in Fig. 1e. All untreated (n=12) and Tx Fiebig III-V (n=8) participants had detectable tetramer responses (Fig 1f), and 80% of Tx Fiebig I-II participants had detectable responses, a difference that was not statistically significant (Chi-square X2=4.92 df=2 p=0.08). Early treated individuals had significantly lower magnitude of tetramer+ cells compared to untreated individuals (mixed-effects linear regression analysis: Tx Fiebig I-II vs UnTx, p=<0.001; Tx Fiebig III-V vs UnTx p=0.02, Fig. 1g).
Overall, 25 of 26 (96%) Tx Fiebig I-II individuals had detectable HIV-specific CD8+ T cells responses by at least one of the assays employed. Of note, 7 of the Tx Fiebig I-II individuals with detectable CD8+ T cell responses had no detectable HIV antibodies responses by either western blot or by enzyme immunoassay (Supplementary Table S3). Thus, counter to what is observed for HIV-specific antibody responses, the majority of early treated individuals develop detectable HIV-specific CD8+ T cell responses, but with reduced magnitude and breadth compared to untreated individuals.
Cumulative HIV exposure impacts induction and persistence of HIV-specific CD8+ T cell responses
We next investigated the impact of cumulative viral exposure on the generation of CD8+ T cell responses by calculating the area under the plasma viral load curve over time. We termed this parameter “viremia copy days” (VCD), similar to how cumulative HIV burden has been estimated in chronic HIV infection (27). VCD for Tx Fiebig I-II donors ranged over 2000-fold, from as little as 3.2 log10 VCD to as much as 6.6 log10 VCD (IQR 4.1–5.7 log10 VCD). We observed a positive correlation between VCD and the magnitude of tetramer positive responses at peak time point in the 21 Tx Fiebig I-II participants for whom class I tetramer responses were detected (Spearman r=0.6, p=0.007) (Fig 2a). Similarly, there was a positive correlation between the breadth of responses (number of positive responses) measured by IFN-γ ELISPOT and VCD in the 26 Tx Fiebig I-II participants with positive ELISPOT responses (Spearman r=0.6, p=0.003) (Fig. 2b).
Figure 2: Cumulative HIV antigen load correlates with the magnitude of HIV-specific CD8+ T cell responses.
Correlation between HIV antigen burden prior to cART-induced complete plasma viral suppression defined as viremic copy days (VCD) and the frequency of (a) tetramer+CD8+ T cells in 21 Fiebig I-II, (b) breadth of CD8+ T cells in 21 Fiebig I-II and (c) frequency of activated (CD8+,CD38+, HLA-DR+) cells in 20 Fiebig I-II individuals respectively at 14 to 42 days after detection of plasma viremia. Spearman’s rank correlation test was used. Two tailed p values are reported.
We and others have previously shown that during hyperacute HIV infection most activated (CD38+HLA-DR+) CD8+ T cells are directed towards HIV antigens (8, 16). Therefore, we next investigated the relationship between the frequency of antigen-specific CD8+ T cells measured by co-expression of activation markers (CD38+HLA-DR+) and VCD. Similarly, we found a strong positive correlation between the two parameters in the 20 Fiebig I-II participants tested (Spearman r=0.8, p=0.001) (Fig 2c). We then used the Youden Index, typically used to determine the optimal cut-off for diagnostic tests (28), to identify the minimal VCD that would predict the development of a positive HIV-specific T-cell ELISPOT response. We found the cutoff to be 3.8 log10 VCD (Youden Index = 0.541, sensitivity=0.94, specificity=0.60, area under receiver-operator curve (ROC) at cut off point=0.77). Taken together, these data indicate that total antigen exposure in persons intiating ART during Fiebig stage I-II infection influences the detection, magnitude and breadth of HIV-specific CD8+ T cell responses.
Treated hyperacute infection leads to greater HIV-specific CD8+ T cell IFN-γ and CD127 expression.
We previously demonstrated that untreated hyperacute HIV infection is associated with high magnitude HIV-specific CD8+ T cells responses exhibiting functional defects including decreased IFN-γ secretion, down-regulation of the anti-apoptotic marker BCL-2, and lower expression of the memory marker CD127, which is required for long-term survival (8). We next investigated if prompt cART-mediated reduction in HIV replication alters the expression of these proteins. Tetramer staining together with intracellular cytokine staining (ICS) (29) demonstrated that early treatment improved IFN-γ secretion capacity of HIV-specific responses following stimulation with Gag peptides compared to untreated controls (Fig. 3a), and that this was significant for both Tx Fiebig I-II and Tx Fiebig III-V donors (mixed-effects linear regression analysis: Tx Fiebig I-II vs. UnTx p=0.001, Tx Fiebig III-V vs. UnTx p<0.001) (Fig. 3b).
Figure 3: HIV-specific CD8+ T cells in early treated individuals produce more IFN-γ and are more likely to express CD127 compared to untreated hyperacute HIV infection.
HLA class I-tetramer binding cells were tested by ICS for IFN-γ production in response to HIV peptide stimulation. (a) Representative data for one Fiebig stage I-II treated, one Fiebig stage III-V treated subject and one untreated donor are shown. Flow panels are gated on IFN-γ-secreting cells. (b) Aggregate data depicting IFN-γ-secreting tetramer+ CD8+ T cells. Black dots denote a single measurement per donor in 5 Fiebig I-II, 4 Fiebig III-V and 6 UnTx. Same coloured dots denote multiple measurements from a single donor. (c) All flow plots gated on CD8+ T cells. The left column shows flow plots gated on tetramer+ cells (red dots). The right column shows tetramer+ cells (red dots) overlaid over total CD8+ T cells (gray background), (d) Aggregate for frequencies of CD127+ tetramer+ cells in 16 Fiebig I-II, 4 Fiebig III-V and 6 UnTx. Black dots denote a single measurement per donor, same coloured dots denote multiple measurements from a single donor. Samples were tested between 21–28 days after diagnosis, as indicated in the figures. Statistical significance for aggregate data was calculated using multilevel mixed-effects linear regression analyses when comparing between groups to account for multiple measurements within some individuals. Horizontal lines represent median with interquartile range.
Another profound defect observed in HIV-specific CD8+ T cell differentiation in untreated infection is reduced ability to express the IL-7 receptor-α (CD127) (8, 16, 30, 31), which is required for long-term cell survival (32) and has been shown to inversely correlate with viral load set point (33). We next evaluated the impact of early treatment on expression of this marker for long-lived memory cells. Representative results (Fig 3c) and aggregate data (Fig. 3d) show that HIV-specific CD8+T cells associated with early treatment, whether in Fiebig I-II or III-V, resulted in higher frequencies of tetramer+CD127+ cells among tetramer+ cells compared to untreated infection (Mann Whitney p=<0.001), indicating that cumulative antigen exposure impacts long term survival of antigen-specific cells.
cART-mediated reduction in antigen burden limits transcriptional activation of HIV-specific CD8+ T cells in acute infection.
The observed impact of early treatment on cytokine secretion and CD127 expression by HIV-specific CD8+ T cells led us to investigate the broader impact of treatment by performing longitudinal transcriptional analysis on this cell population. Tetramer+ CD8+ T cells were sorted at multiple time points following infection, using PBMC from 4 early treated (Fiebig III-V) and 4 untreated individuals, and profiled by RNA-seq (Fig. 4a). Additionally, we also sorted CMV-specific tetramer+ CD8+ T cells in 1 early treated and 2 untreated subjects and CD8+ T cell populations depleted of tetramer+ cells for all subjects. Strikingly, across all time points sampled, the HIV-specific tetramer+ CD8+ T cells, in both early treated and untreated individuals demonstrated the largest number of differentially expressed genes (False Discovery Ratio adjusted p-value, q<0.05) during the first year of infection (Supplementary Fig. S1A, Supplementary Tables S4 & S5). We observed very few transcriptional changes in the CMV-specific CD8+ T cells or the remaining bulk CD8+ T cells at all the time points measured compared to pre-HIV infection time point, consistent with our earlier report of a lack of bystander CD8+ T cell activation in acute HIV infection (8). Furthermore, direct comparison of CMV-specific and HIV-specific transcriptome data at the available time points revealed no significant differentially expressed genes between these infection-specific responses at late and long-term time points (Supplementary Table S6).
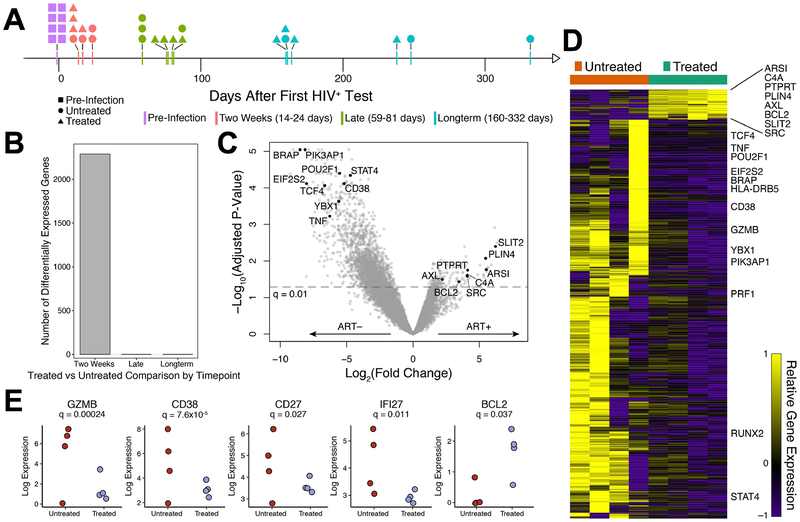
Figure 4: HIV-specific CD8+ T cell responses in treated hyperacute and untreated hyperacute HIV infection have distinct transcriptional signatures.
Transcriptional responses of CD8+ T cells in untreated (n=4) and early treated (n=4) individuals along the course of acute infection. (a) Timeline of collected RNA samples with samples binned into time-frames denoted by color. (b) Number of differentially expressed genes between HIV-specific CD8+ T cells from untreated and early treated individuals at various time-points. (c) Volcano plot depicting differentially expressed genes (False Discovery Ratio (FDR), q < 0.01, edgeR Likelihood Ratio Test) in HIV-specific CD8+ T cells at the two week time-frame frame comparing treated and untreated individuals. Genes of interest are annotated by name. (d) Heatmap depicting differentially expressed genes from (c), row normalized expression. (e) Scaled log normalized expression values of genes of interest. The significances reported were calculated as in (c).
Comparing the transcriptomes of the HIV-specific tetramer+ CD8+ T cells between early treated and untreated individuals as a function of time since HIV detection, we observed substantial differential expression only at the time point closest to peak viremia (“Two Weeks”, Fig. 4b, Supplementary Table S7). Notably, early treatment mitigated the transcriptional response in HIV-specific CD8+ T cells (Fig. 4c and 4d). Specifically, genes associated not only with immune activation (e.g. CD38, TNF, STAT4), but also cellular translation (e.g. BRAP, POU2F1, EIF2S2), were down-regulated in tetramer+ cells from treated individuals compared to untreated persons. Interestingly, fewer genes were up-regulated in cells from treated individuals compared to untreated, suggesting a much tighter transcriptional response in the presence of cART and an antigen-limited environment. Some of the genes up-regulated in cells from treated individuals (e.g. BCL-2, AXL, SRC) have known roles in cell survival with the potential to give rise to memory CD8+ T cells (34–36), whereas many of the genes up-regulated in cells from untreated individuals were significantly enriched for pathways associated with over-activation and cellular dysfunction (Supplementary Fig. S1B). In particular, the Eukaryotic Initiation Factor 2 (EIF2) signaling pathway, involved in protein synthesis, and the integrated stress response, were strongly enriched in untreated individuals (p = 7.9×10−23), as well as several genes associated with apoptosis (Supplementary Fig. S1C).
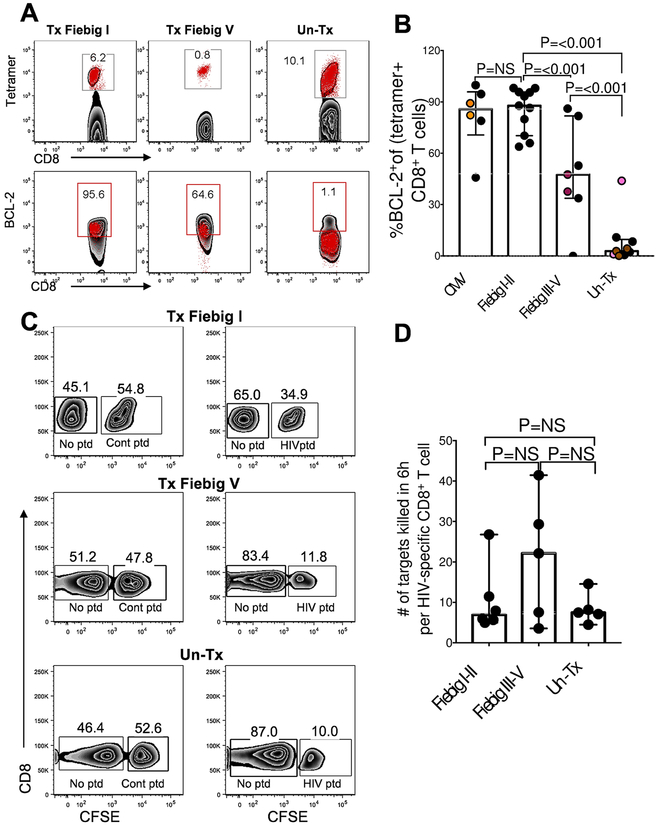
One of the few genes that was differentially upregulated in cells from treated donors compared to untreated donors was BCL-2 (q=0.04), an anti-apoptotic molecule implicated in memory generation (35). We verified the transcriptional data (Fig 4e) by measuring the BCL-2 protein expression in tetramer-sorted HIV-specific CD8+ T cells at peak viremia and found highest expression in Fiebig stage I-II treated compared to untreated participants (Fig 5a, b, mixed-effects linear regression analysis: test p<0.001). Interestingly, early treatment led to BCL-2 expression comparable to CMV-specific CD8+ T cells (Fig 5b).
Figure 5: The effect of transient antigen exposure on the functional quality of HIV-specific CD4+ and CD8+ T cell responses.
(a) PBMCs isolated within 28 days of ART initiation were stained with a panel of MHC class I peptide-tetramers specific for HIV epitopes and antibodies against BCL-2. All flow plots are gated on CD8+ T cells. Upper panels show flow plots gated on tetramer+ CD8+ T cells for each HIV tetramer tested. The lower panel shows tetramer+ cells (red dots) overlaid on total CD8+ T cells (black background), (b) Aggregate BCL-2 expression on tetramer+ cells specific for CMV or HIV measured in 5 persons with CMV responses and 11 Fiebig I-II, 6 Fiebig III-V, 6 UnTx with HIV-specific responses. Black dots denote single measurement per donor, same coloured dots denote multiple measurements within a donor. (c) Representative results of direct killing activity of HIV-specific CD8+ T cells measured in a four-hour killing assay. Peptide-pulsed CFSEhi CD8-depleted cells designated as targets were mixed with CFSElo unpulsed control cells in a 1:1 ratio and co-incubated with autologous CD8+ T cells. Reduction in the CFSEhi population was compared to target cells pulsed with an irrelevant peptide. (d) The killing capacity was calculated as percent reduction in CFSEhi HIV peptide-pulsed targets relative to control ovalbumin (SIINFEKL) peptide-pulsed condition. 6 Fiebig I-II, 5 Fiebig III-V treated subjects and 5 UnTx were used for these experiments. Statistical significance for aggregated data (b and d) was determined using linear mixed-effects linear regression analyses when comparing between groups to account for multiple measurements within some individuals. Horizontal lines represent median with interquartile range.
Transcriptional analysis also revealed that HIV-specific CD8+ T cells from untreated donors expressed significantly more granzyme B (FDR, q=0.00024) compared to early treated donors (11). Thus, we investigated whether higher mRNA expression of cytolytic genes translated into superior killing of HIV infected targets by measuring the intrinsic killing capacity of HIV-specific CD8+ T cells using a 4 hour direct killing assay. Representative plots (Fig. 5c) and summary data (Fig. 5d) show CD8+ T cells killing peptide-pulsed targets incubated at a 1:1 effector target ratio. To account for differences in the frequencies of effector cells among the individuals studied, we measured frequency of tetramer+ cells specific for the optimal peptide (loaded on target cells) to calculate per-cell CD8+ T cell killing capacity. Despite the higher granzyme B expression in cells from untreated infection, this analysis found no significant difference in the per-cell CD8+ T cell killing activity between the groups. Together, these data demonstrate that at peak viremia the HIV-specific CD8+ T cell response is dominated by highly activated, short-lived effector cells, whereas early treatment skews CD8+ T cells toward enhanced survival capacity and memory generation without impairing cytolytic function.
Limited cART-induced antigen exposure affects the differentiation status and durability of HIV-specific CD8+ T cell responses
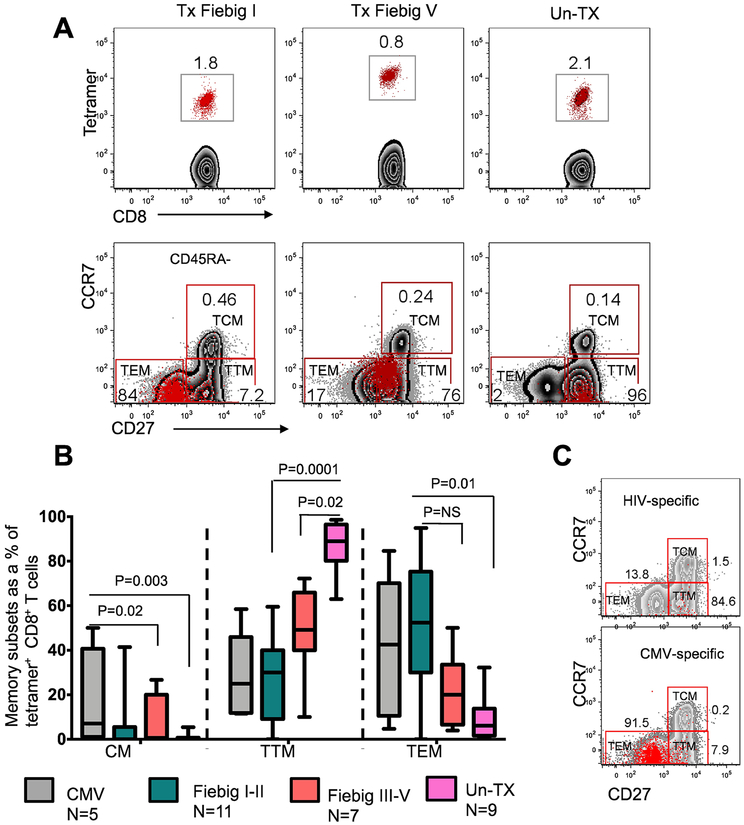
The functional attributes of antigen-specific T cell responses are influenced by the differentiation state of the cell (37). Chronic HIV infection has been shown to skew the maturation of HIV-specific CD8+ T cells towards a transitional memory phenotype, with suboptimal effector functions (38, 39). However, the effect of very early treatment on memory differentiation has not been explored. Transcriptional analysis of HIV-specific CD8+ T cells at time points near peak viral load (2–4 weeks after diagnosis) revealed that early treatment had a profound effect on the transcriptional landscape of HIV-specific CD8+ T cell responses. To determine the effect of distinct transcriptional signatures on HIV-specific CD8+ T cell phenotypes, we assessed the differentiation state of tetramer+ cells at 2 to 5 weeks after detection of plasma viremia using the well-defined phenotypic makers CD45RA, CCR7, and CD27 that are typically used to define central memory Tcm (CD45RA−CD27+CCR7+), transitional memory Ttm (CD45RA−CD27+CCR7−), and effector memory Tem (CD45RA−CD27−CCR7−). As shown in the representative flow cytometry plot (Fig. 6a), the phenotype of untreated responses was dominated by pre-differentiated transitional memory (Ttm) cells (UnTx vs Tx Fiebig I-II Mann Whitney p= 0.0001, UnTx vs Tx Fiebig III-V, p=0.02), analogous to what has been reported in chronic HIV infection (38). In contrast, responses in early treated donors were predominantly effector memory phenotype (Tem, Tx Fiebig I-II vs UnTx, Mann Whitney p= 0.01), comparable to CMV-specific responses in the same individuals (Fig 6b). Moreover, representative comparative analysis of antigen-specific cells in a late treated (Fiebig V) individual showed that the vast majority of HIV-specific CD8+ T cells were skewed toward Ttm whereas the majority of CMV-specific responses in the same donor were predominantly Tem (Fig. 6c). These data suggest that very early cART-mediated viral suppression affects phenotypic differentiation leading to an increased frequency of more differentiated HIV-specific CD8+ T cells.
Figure 6: Phenotypic characterization of HIV-specific CD8+ T cell responses in treated and untreated hyperacute HIV infection.
HIV-specific (tetramer+) CD8+ T cell memory subpopulations defined using CD45RA, CD27 and CCR7 at 14 to 36 days after viremia using flow cytometry. These markers were used to discriminate three distinct memory populations: central memory Tcm (CD45RA−CD27+CCR7+), transitional memory Ttm (CD45RA−CD27+CCR7−) and effector memory Tem (CD45RA−CD27−CCR7−). (a) Representative flow plots for early treated subjects and untreated subjects are shown. (b) Aggregate data for the frequencies of the three memory subsets in 11 Tx Fiebig I-II, 7 Tx Fiebig III-V, and 9 UnTx subjects, as well as 6 CMV responses in early treated subjects are shown. Statistical significance was calculated using two sided Mann Whitney test. Horizontal lines represent median with interquartile range. (c) Representative flow plot showing intra-donor differences in the phenotype of CMV and HIV specific CD8+ T cells in a Fiebig V treated donor is shown.
To understand the dynamics of the virus-specific CD8+ T cell response in more detail, we next examined the effect of early treatment on durability of T cell clonotypes. For 2 early treated and 2 untreated individuals, we sorted tetramer+ populations reactive to the same epitope at an acute (28 or 35 days post diagnosis) and a chronic (336 days post-diagnosis) time point. Repertoire diversity was assessed by sequencing TCR ß genes in each population. Consistent with the transcriptional profiles and differentiation status observed for CD8+ T cells in early treated individuals, we found that the dominant TCR ß clones present at the acute time point in both individuals were maintained at the chronic time point (Supplementary Fig. S2 and Supplementary Table S8). In contrast, in both untreated individuals, we observed that the dominant TCR ß clone present at the acute time point diminished in frequency and was replaced by a new dominant clone at the chronic time point. This was illustrated for an identical public B58-TW10-reactive TCR clone present in one early treated and one untreated participant, suggesting that the difference was due to variation in the CD8+ T cell response rather than intrinsic characteristics of individual TCR. Overall, the changes in TCR ß clonotype frequency (for all clones observed more than once) was greater in the untreated individuals (Mann Whitney, p=0.002). These results indicate that early treatment results in better maintenance of dominant TCR clonotypes elicited during acute infection.
Prolonged viral suppression skews HIV-specific CD8+ T cell differentiation towards to central memory phenotype
Next, we investigated the effect of prolonged HIV suppression on the phenotypic differentiation trajectories of HIV-specific CD8+ T cells. Tetramer+ CD8+ T cells were phenotypically analyzed at 2 to 5 weeks and at a median of 336 days (IQR 252–378) after onset of plasma viremia. Representative flow plots for a Tx Fiebig I donor and aggregate data for 5 Tx Fiebig I-II donors show a significant shift in the phenotype of HIV-specific CD8+ T cells from a predominantly Tem to a Tcm phenotype (change in TCM frequencies between Day 14–42 and Day >256 days: Mann Whitney p=0.01) (Figure 7a). Similarly, 5 Fiebig III-V donors showed a significant increase in the Tcm phenotype with a concurrent reduction in the Tem phenotype (Day 14–42 vs Day: TCM >256 days: Mann Whitney p=0.001: Tem >256 days: p=0.04) (Figure 7b). There was no notable difference in the memory differentiation at the acute and late time points in 4 untreated individuals in spite of plasma viral suppression by cART in some, at the chronic time point (Figure 7c). These data show that the timing of treatment initiation has a profound effect on HIV-specific CD8+ T cell differentiation.
Figure 7: Longitudinal characterization of HIV-specific CD8+ T cell responses in treated and untreated hyperacute HIV infection.
HIV-specific (tetramer+) CD8+ T cell memory subpopulations defined using CD45RA, CD27, and CCR7 during acute (14 to 36 days after diagnosis) and chronic (more that 250 days after diagnosis) infection. (a) Representative flow plots for a Fiebig I treated subject and aggregate data for the frequencies of the three memory subsets in 5 Tx Fiebig I-II treated subjects, (b) Representative flow plots for a Fiebig III treated subject and aggregate data for the frequencies of the three memory subsets in 5 Tx Fiebig III-V treated subjects, (c) Representative flow plots for one untreated subject and aggregate data for the frequencies of the three memory subsets in 4 UnTx subjects. The whiskers represents minimum and maximum values, P values were adjusted using Bonferroni-Dunn method.
The above transcriptional profiling of HIV-specific CD8+ T cells coupled with phenotypic TCR clonotypic analysis and functional data by flow cytometry collectively demonstrate that immune responses from early treated individuals had profiles consistent with establishing long term persistence. Therefore, we investigated if higher expression of survival genes translated into long-lived memory responses. We first longitudinally assessed responses in untreated individuals. Tetramer analysis for a representative untreated donor who maintained high plasma viremia of more than 4 log10 RNA copies/ml had two persisting responses and two responses that diminished overtime (Supplementary Fig. S3A), as has been previously observed (9, 40). Overall, most responses in untreated donors diminished over time in spite of persistently high viral loads (Supplementary Fig. S3B). Of 6 subjects evaluated, 3 had responses that disappeared, whereas 1 had newly emerging responses that never reached the magnitude of the initial responses and two had responses that diminished over time. With prompt viral load suppression most responses in early treated individuals remained low in magnitude but persisted over time, as shown by tetramer analysis in representative Fiebig stage III (Supplementary Fig. S3C) and Fiebig stage I (Supplementary Fig. S3D) treated individuals and summarized in Supplementary Fig. S3E.
Immediate cART in hyperacute infection preserves HIV-specific CD4+ T helper cells and enhances HIV-specific CD8+ T cell proliferation
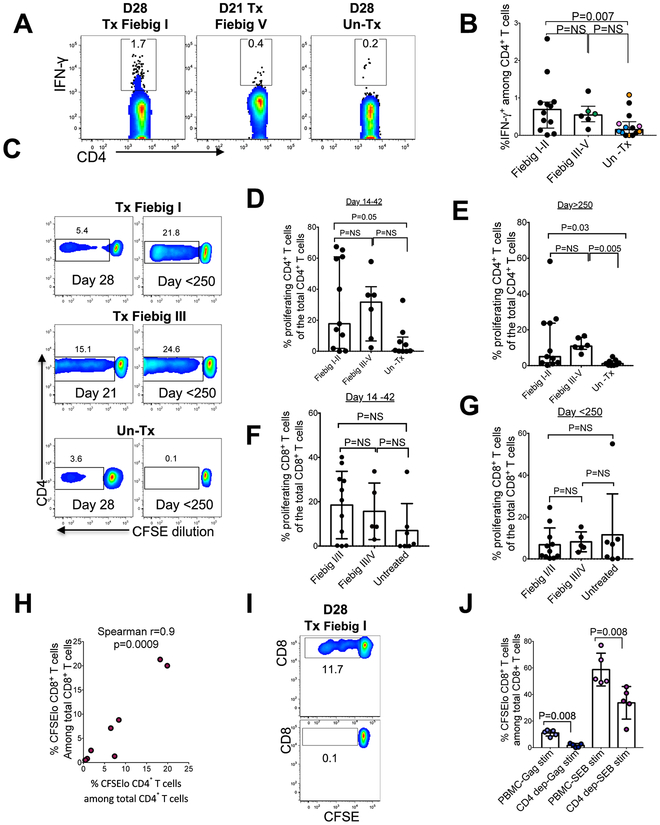
Acute HIV infection is associated with profound HIV-specific CD4+ T cell abnormalities due to selective depletion and severely impaired effector functions, whereas treatment at or following peak viremia leads to robust antigen-specific responses (41–43). Given that treatment of hyperacute infection abrogates induction of B cell responses (26, 44), we next investigated the impact of treatment prior to peak viremia on induction of HIV-specific CD4+ T cell responses. Intracellular cytokine staining (ICS) analysis between 21 and 28 days after diagnosis (Fig. 8a, 8b) shows that early treatment was associated with significantly higher frequencies of IFN-γ-secreting HIV-specific CD4+ T cells compared to untreated individuals (mixed-effects linear regression analysis: p=0.007). Immediate cART was associated with strong HIV-specific CD4+ T cell proliferative responses that were otherwise diminished in untreated infection (Fig. 8c, 8d). Moreover, early treated donors maintained robust CD4+ T cell proliferative responses for more than 250 days post diagnosis despite sustained viral suppression, whereas samples from untreated donors had no notable improvement in CD4+ T cell proliferative capacity even when durable cART mediated viral suppressed was achieved later during chronic infection (Fig 8c, 8e). There was no detectable difference in CD8+ T cell proliferation in early treated individuals at acute time points, possibly due to small sample size (Fig 8f, 8g).
Figure 8: Early treatment preserves HIV-specific CD4+ T cell responses.
HIV-specific CD4+ T cell responses were measured by IFN-γ ICS after overnight incubation in the presence of overlapping HIV-1 clade C peptide pools. (a) Representative flow plots gated on IFN-γ-secreting CD4+ T cells. (b) Aggregate data for frequencies of IFN-γ-producing CD4+ T cells in response to Gag, Nef, and Env peptide pools in 12 Fiebig I-II, 5 Fiebig II-III and 9 Un Tx are shown. Black dots denote a single measurement per donor, same coloured dots denote multiple measurements within a donor. Statistical significance was determined using multilevel mixed-effects linear regression analyses when comparing between groups to account for multiple measurements within some individuals. Horizontal lines represent median with interquartile range. (c) Flow cytometry of CFSE-labelled CD4+ T cells for 1 representative donor from each group measured at day 21 and day >250. (d) Aggregate CD4+ T cell proliferative responses measured between 14 and 42 days after diagnosis. (e) Aggregate CD4+ T cell proliferative responses measured after 120 days after diagnosis Data in panel d and e were generated 11 Fiebig I-II, 6 Fiebig III-V and 9 UnTx. (f) Aggregate CD8+ T cell proliferative responses measured between 14 and 42 days after diagnosis. (g) Aggregate CD8+ T cell proliferative responses measured 120 days after diagnosis. Data in panels f and g were generated from 11 Fiebig I-II, 5 Fiebig III-V and 7 UnTx persons. Statistical significance was determined using two tailed Mann-Whitney test. (h) Correlation between CD4+ and CD8+ T cell proliferative responses in 8 Fiebig I-II treated donors. Spearman’s rank correlation test was used. Two tailed p values are reported. (i) Representative flow plot depicting CD8+ T cell proliferation in unfractionated PBMC and in CD4-depleted PBMC. (j) Aggregate CD8+ T cell proliferative responses to HIV antigens with and without CD4+ T cells in 5 Tx Fiebig I-II donors. Statistical significance was determined using two tailed Mann-Whitney test. Horizontal lines represent median with interquartile range.
Next, we used samples from Fiebig I-II treated individuals sampled 14 to 35 days after diagnosis, which had large proliferative responses in prior experiments to investigate the effect of CD4+ T cell responses on CD8+ T cell proliferative responses. CD4+ T cell proliferation was positively correlated with CD8+ T cell proliferation following stimulation with HIV Gag antigens (Spearman r=0.9, p=0.0009) (Fig. 8h), suggesting that proliferative responses of the two cell subsets could be interdependent. In addition, in vitro depletion of CD4+ T cells prior to stimulation with HIV peptides or with SEB severely blunted CD8+ T cell proliferation (Mann Whiney test p=0.008) (Fig 8i, 8j). Together, these data indicate that proliferative CD4+ T cells sustain functional CD8+ T cell responses following immediate treatment of hyperacute infection.
Discussion
Untreated hyperacute HIV infection leads to robust induction of virus-specific CD8+ T cell responses, but these cells rapidly become poorly functional. Here we conducted a detailed analysis of the impact of treatment initiated prior to peak viremia on HIV-specific CD8+ T cell responses, which curtailed overall acute viral antigen exposure by more than two orders of magnitude in some individuals. This approach enabled us to examine the host response to HIV while limiting concurrent immune destruction, which typifies untreated acute and chronic infection. We show that immediate therapy leads to rapid suppression of plasma viremia, lowers the absolute magnitude of HIV-specific CD8+ T cell responses, promotes HIV-specific CD4+ T cell responses, and abrogates the dysfunction of CD8+ T cells that is usually seen following acute infection. These findings provide insight into features associated with natural priming of the immune system by HIV infection in the absence of CD4+ T cell depletion. These studies are relevant to vaccine design because, the several antiviral functions observed in early treated donors such as, improved CD8 effector function robust CD4 proliferative responses and maintenance of long-lived T cell memory responses are key antiviral functions that will be desirable to induce with a vaccine.
Several studies of early treatment of HIV infection have examined the effect of treatment initiation after peak viremia (17, 45, 46). Because of the design of the FRESH cohort, we were able to identify infection during Fiebig Stage I-II and initiate therapy within one to two days of detectable viremia, in some cases with peak viremia less than 1000 RNA copies/ml. Despite this limited exposure to detectable viremia, which has been shown to diminish HIV-specific B cell responses (26, 44), the majority of individuals developed detectable HIV-specific CD8+ T cell responses as measured by tetramer staining, IFN-γ ELISPOT or by proliferation in response to HIV peptides. However, the overall magnitude of responses was clearly dependent on the relative exposure to virus, as measured by viremia copy days (VCD) analogous to viremia copy years described in chronic HIV infection (47) and was shown to correlate with magnitude and breadth of HIV-specific CD8+ T cell responses. This suggests that the degree of HIV antigen burden is a key driver of acute phase CTL responses. Whether some of the early treated individuals lacked HIV-specific CD8+ T cell responses altogether (48), or whether they were not cross reactive with the reference strain of virus used, or simply below the limits of detection as shown previously in elite controllers, is not clear (49).
Recent studies have highlighted the role of metabolic state in shaping immune cell differentiation and function (50, 51). Through global RNA-seq analysis, we show that during untreated acute HIV infection, HIV-specific CD8+ T cells are metabolically hyperactive, functionally impaired and have a skewed maturation phenotype. Early cART comparatively diminished these immune dysfunctions by relative coordinated down-regulation of the stress response genes, with a concurrent up-regulation of anti-apoptotic and pro-survival genes, which are intricately involved in shaping the overall long-term survival of immune responses (35). Notably, few transcriptional differences were found in bystander and CMV-specific CD8+ T cells in both untreated and treated individuals, highlighting the direct effect of treatment on only those cells targeting HIV infected cells. Importantly, this study shows that the key benefit of initiating cART very early is the generation of transcriptionally quiescent immune responses. It is plausible that, the less stressed state of the responses in early treated people could provide better immune priming amenable to rapid boosting with therapeutic vaccines. Moreover, our data provide new insights to focus mechanistic studies into the biology of immune responses in early treated people and identify key molecular targets such as the BCL-2, AXL and SRC genes and cellular pathways that could be manipulated to enhance the generation of long-lived immune responses by future therapeutic interventions.
Furthermore, we observed divergent differentiation states of HIV-specific CD8+ T cell responses at both the peak of the response and in the chronic phase of infection, an intermediate transitional memory (Ttm) population dominated untreated responses at both the peak and chronic time points. These data suggest that the skewing of the response to this intermediate phenotype happens very early in infection and remains stable over time. In contrast, responses in Fiebig stage I-II treated individuals were heavily skewed towards the effector memory phenotype (Tem), classically superior at responding to immune stimuli (52). Interestingly, sustained viral suppression in early treated participants converted the responses to central memory (Tcm) phenotype. These data further support the notion that it will be easier to boost preexisting central memory responses with a therapeutic vaccine in early treated individuals because of the inherent ability of Tcm to rapidly expand and acquire effector functions upon restimulation (37).
Lastly, we investigated whether loss of CD4+ T cell help contributed to the observed differences in CD8+ T cell function and phenotype. CD4+ T cell responses play an important role in influencing the quality of virus-specific CD8+ T cells in chronic viral infections (53–55). Untreated HIV infection results in massive depletion of CD4+ T cells, whereas early treatment preserves this population (13, 56, 57). In this study, we observed several CD4+ T cell functional abnormalities in untreated persons that were not apparent in early treated donors. Consistent with previous reports (41), untreated HIV infection was associated with a paucity of HIV-specific CD4+ T cell responses. In addition, we observed diminished responses to HIV antigens as well as mitogens signifying HIV-induced generalized CD4+ T cell functional impairment. In contrast, robust CD4+ T cells proliferative responses were induced in early treated donors and were maintained despite prolonged cART-mediated viral suppression. Together, these results highlight the ability of CD4+ T cell helper function to enhance CD8+ T cell responses and underscore the need for more mechanistic work focused on defining the nature of CD4+ T cell help to CD8+ T cells.
Notable limitations of the study include variability in the number of days between diagnosis and sample collection, which was due to sample availability constraints. However, difference in number of days between diagnosis and sample collection did not substantially affect the immune parameters measured in the early treated groups. Secondly, tetramer analysis was not done for some of study participants due to non-availability of appropriate tetramers. Nonetheless, the wide range of tetramers used in this study covered most of the immunodominant responses in our cohort. Thirdly, in spite of differences in the phenotypic composition of memory responses between experimental groups later in infection, RNA-Seq only detected significant differences at the two-week time point, which is around the peak of activation in untreated samples. The cells at later time-points where more quiescent which makes difficult to detected transcriptional differences without prior ex-vivo stimulation. Increasing sample size, read depth, and introducing ex-vivo stimulation might overcome this limitation (58). Finally, we have not investigated the impact of viral sequence diversity, which may affect antigen exposure in an epitope-specific manner that is not captured by VCD measurements. Early treatment will prevent viral evolution, but escape mutations have been observed in conjunction with the development of CD8+ T cell responses during the first weeks of infection (59, 60). Therefore, additional analyses will be necessary to fully assess the contribution of viral sequence adaptation to differences in TCR clonotype frequencies and transcriptional profiles, particularly for untreated individuals at later time points.
There is compelling evidence that early diagnosis and treatment of HIV infection has the benefits of reducing the HIV reservoir size and decreasing transmission of the virus to sexual partners (61). However, the impact of extremely early cART on induction of adaptive cellular immune responses with the potential to suppress HIV infection was unknown. In this study, we showed that treatment initiated during hyperacute infection induces HIV-specific CD4+ and CD8+ T cell responses that differ in magnitude, phenotype, and function from responses generated in people who delay treatment. We show that preservation of CD4+ T cells by early cART initiation may have a critical role in shaping CD8+ T cell function. We also identified key molecules that could be potential targets for manipulation to restore CD8+ T cell function, such as BCL-2 and CD127. Overall, our results show that early cART leads to persistent functional T cell responses that are generated in most individuals with hyperacute infection. These data open up the possibility of harnessing immune responses generated under early treatment as a first critical step in the path towards immune-mediated HIV cure or remission strategies.
Materials and Methods
Study design
Characteristics of the study population and study samples
Study participants were recruited from the FRESH Cohort, a longitudinal study in which women aged 18–23 at enrollment are seen twice weekly for empowerment and life skills training as well as HIV prevention education. At each visit they are tested for HIV RNA by finger prick blood draw to detect acute infection, and offered immediate treatment when infection is detected (13). In this study, we included 12 HIV infected subjects identified in Fiebig I-II who delayed treatment until they met the South African guidelines at the time (CD4 counts below 350 cells/mm3), who we refer to as untreated. 26 participants were identified in Fiebig I-II and initiated on cART within 24 to 48-h of detection of plasma HIV RNA and 8 individuals were identified in Fiebig III-V who were similarly immediately initiated on cART. Sample size for each experimental group and selection of sample time points was based on the availability of PBMC samples rather than a prespecified sample effect size. Class I and II HLA typing, longitudinal CD4+ T cell counts, and viral loads were obtained, and longitudinal blood samples were collected starting prior to infection. Clinical characteristics of the study participants are shown in Supplementary Table S1. Written informed consent was obtained from each study participant in accordance with a protocol # BF298/14 approved by the Biomedical Research Ethics Committee (BREC) of the University of KwaZulu-Natal and protocol# 201P001018/PHS approved by the Institutional Review Board for Massachusetts General Hospital, Partners Human Research Committee (PHRC).
Statistical analyses
Comparisons of continuous variables between groups were done using the non-parametric Mann-Whitney test and the Dunn’s test was used to adjust for multiple comparisons were appropriate. Pearson’s chi-square test was used to analyze categorical data. Relationships between continuous variables were assessed using the Spearman’s rank-order correlation. In instances where multiple measurements were available on a single individual, resulting in correlated responses, linear mixed-effects regression models (with random intercepts at the individual level) were used to compare outcomes between groups. linear regression analyses and multilevel mixed-effects linear regression analyses were done in the treated groups (Fiebig I-II and Fiebig III-V) to assess the relationship between immune responses and the duration of treatment. Cumulative HIV exposure, here referred to as viremia copy days (VCD), was determined by calculating the area under curve of viral load over time with a baseline of 20 RNA copies/ml (limit of detection for the quantification of plasma viral load). P values <0.05 were considered significant. Analyses were done on GraphPad Prism Version 7 (GraphPad Software, Inc) and Stata version 15 (StataCorp).
RNA-Seq data were analyzed as follows: Differential expression was performed using edgeR (62) with default dispersion calculation settings. Significance for differential expression was set at a False Discovery Ratio adjusted p-value, q<0.05. Gene set analysis was performed using all differentially expressed genes within each comparison in Ingenuity Pathway Analysis (IPA, QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis). Upstream drivers and canonical pathways were extracted directly from IPA and reorganized for visual clarity.
Supplementary Material
Data file S1. Primary data
Materials and Methods
Fig. S1. Transcriptional analysis reveals predicted dysfunctional responses in CD8+ T cells from untreated individuals vs early treated individuals
Fig. S2. TCR clonotype dynamics in early treated and untreated HIV
Fig. S3. Longitudinal characterization of HIV-specific CD8+ T cell responses in treated and untreated hyperacute HIV infection.
Table S1. Baseline characteristics of study participants
Table S2. Effect of the interval between treatment initiation and sample collection on immune parameters in Fiebig I-II and Fiebig III-V
Table S3. Immunodominant responses measured by HLA-class I tetramers in individuals treated in Fiebig III-V and untreated donors
Table S8. TCR ß clonotype sequences
Table S4. Results from the RNA-seq differential expression tests in HIV-specific, CMV-specific, and total CD8+ T cells (excluding the tetramer-specific cells) between the time points assayed in treated individuals.
Table S5. Results from the RNA-seq differential expression tests in HIV-specific, CMV-specific, and total CD8+ T cells (excluding the tetramer-specific cells) between the time points assayed in untreated individuals.
Table S6. Results from the RNA-seq differential expression tests between CMV-specific vs. HIV-specific CD8+ T cells at the late and long-term time points, and the difference between those cells between the two time points.
Table S7. Results from the RNA-seq differential expression tests between HIV-specific CD8+ T cells from treated vs. untreated individuals at each time point.
Table S9: Table contains alignment statistics and metadata on the RNA-seq samples in this study.
Table 1:
Baseline parameters and sample collection timepoints for the three arms of the study
| Number in group | Group | Days from diagnosis to Txa | Days to sample collectiona | Viral load At sample collectiona | CD4 counts at sample collectiona |
|---|---|---|---|---|---|
| 26 | 1: Fiebig I-II | 1.0 (1.0–2.0) | 28.0 (20.3–30.0) | 20 (20–20) | 862.0 (669–977) |
| 8 | 2: Fiebig III-V | 1.0 (1.0–23.0) | 27.0 (21.0–28.0) | 20 (20–20) | 486 (423–599) |
| 12 | 3: Untreated | - | 28 (24.0–30.0) | 665,000 (18,750–1,357,500) | 547 (502–623) |
Values reported as median (interquartile range)
Acknowledgements:
We thank the FRESH participants, as well as the FRESH and HIV Pathogenesis Programme (HPP) laboratory staff.
Funding: This work was supported by funding from the Bill and Melinda Gates Foundation (OPP1066973 and OPP1146433); the Collaboration for AIDS Vaccine Discovery; the Howard Hughes Medical Institute (HHMI) and an HHMI International Research Scholar Award (HHMI grant # 55008743); the Witten Family Foundation; Dan and Marjorie Sullivan; the Mark and Lisa Schwartz Foundation; Ursula Brunner; Gilead Sciences (grant ID #00406); the NIAID (R01AI145305 and R37AI067073); the NIH funded Harvard University Center for AIDS Research (P30 AI060354), the International AIDS Vaccine Initiative (IAVI) (UKZNRSA1001). This work was also supported in part by the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative (grant # DEL-15-006). T.N. holds the South African Research Chair in Systems Biology of HIV/AIDS. A.K.S. was supported by the Searle Scholars Program, the Beckman Young Investigator Program, the NIH (5U24AI118672, 1U54CA217377, 1R33CA202820, 2U19AI089992, 1R01HL134539, 2RM1HG006193, 2R01HL095791, P01AI039671), and the Bill & Melinda Gates Foundation (OPP1139972). S.W.K. was supported by a National Science Foundation Graduate Student Fellowship.
Footnotes
Competing interests: None.
Data and Materials availability: All data associated with this study are present in the paper or Supplementary Materials. TCR β sequences have been deposited in GenBank, accession number MK088532-MK088568.
References and Notes
- 1.Siliciano JD, Siliciano RF. 2016. Recent developments in the effort to cure HIV infection: going beyond N = 1. J Clin Invest 126:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauci AS, Marston HD. 2015. Ending the HIV-AIDS Pandemic--Follow the Science. N Engl J Med 373:2197–2199. [DOI] [PubMed] [Google Scholar]
- 3.Mellors JW, Rinaldo CR Jr., Gupta P, White RM, Todd JA, Kingsley LA. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167–1170. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, Boswell SL, Mathews WC, Bangsberg DR, Martin J, Whalen CC, Sieg S, Yadavalli S, Deeks SG, Lederman MM. 2006. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. Jama 296:1498–1506. [DOI] [PubMed] [Google Scholar]
- 5.Lyles RH, Munoz A, Yamashita TE, Bazmi H, Detels R, Rinaldo CR, Margolick JB, Phair JP, Mellors JW. 2000. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis 181:872–880. [DOI] [PubMed] [Google Scholar]
- 6.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 68:4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freel SA, Picking RA, Ferrari G, Ding H, Ochsenbauer C, Kappes JC, Kirchherr JL, Soderberg KA, Weinhold KJ, Cunningham CK, Denny TN, Crump JA, Cohen MS, McMichael AJ, Haynes BF, Tomaras GD. 2012. Initial HIV-1 antigen-specific CD8+ T cells in acute HIV-1 infection inhibit transmitted/founder virus replication. J Virol 86:6835–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndhlovu ZM, Kamya P, Mewalal N, Kloverpris HN, Nkosi T, Pretorius K, Laher F, Ogunshola F, Chopera D, Shekhar K, Ghebremichael M, Ismail N, Moodley A, Malik A, Leslie A, Goulder PJ, Buus S, Chakraborty A, Dong K, Ndung’u T, Walker BD. 2015. Magnitude and Kinetics of CD8+ T Cell Activation during Hyperacute HIV Infection Impact Viral Set Point. Immunity 43:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radebe M, Gounder K, Mokgoro M, Ndhlovu ZM, Mncube Z, Mkhize L, van der Stok M, Jaggernath M, Walker BD, Ndung’u T. 2015. Broad and persistent Gag-specific CD8+ T-cell responses are associated with viral control but rarely drive viral escape during primary HIV-1 infection. AIDS 29:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 68:6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med 3:205–211. [DOI] [PubMed] [Google Scholar]
- 12.Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 206:1273–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong KL, Moodley A, Kwon DS, Ghebremichael MS, Dong M, Ismail N, Ndhlovu ZM, Mabuka JM, Muema DM, Pretorius K, Lin N, Walker BD, Ndung’u T. 2017. Detection and treatment of Fiebig stage I HIV-1 infection in young at-risk women in South Africa: a prospective cohort study. Lancet HIV doi: 10.1016/S2352-3018(17)30146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ndung’u T, Dong KL, Kwon DS, Walker BD. 2018. A FRESH approach: Combining basic science and social good. Sci Immunol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robb ML, Eller LA, Kibuuka H, Rono K, Maganga L, Nitayaphan S, Kroon E, Sawe FK, Sinei S, Sriplienchan S, Jagodzinski LL, Malia J, Manak M, de Souza MS, Tovanabutra S, Sanders-Buell E, Rolland M, Dorsey-Spitz J, Eller MA, Milazzo M, Li Q, Lewandowski A, Wu H, Swann E, O’Connell RJ, Peel S, Dawson P, Kim JH, Michael NL, Team RVS. 2016. Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. N Engl J Med 374:2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takata H, Buranapraditkun S, Kessing C, Fletcher JL, Muir R, Tardif V, Cartwright P, Vandergeeten C, Bakeman W, Nichols CN, Pinyakorn S, Hansasuta P, Kroon E, Chalermchai T, O’Connell R, Kim J, Phanuphak N, Robb ML, Michael NL, Chomont N, Haddad EK, Ananworanich J, Trautmann L, Rv254/Search, the RVSSG. 2017. Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, Epling L, Lee TH, Busch MP, McCune JM, Pilcher CD, Hecht FM, Deeks SG. 2013. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 208:1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C, Group AVS. 2013. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M Jr., Chun TW, Strain M, Richman D, Luzuriaga K. 2013. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 369:1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cartwright EK, Spicer L, Smith SA, Lee D, Fast R, Paganini S, Lawson BO, Nega M, Easley K, Schmitz JE, Bosinger SE, Paiardini M, Chahroudi A, Vanderford TH, Estes JD, Lifson JD, Derdeyn CA, Silvestri G. 2016. CD8(+) Lymphocytes Are Required for Maintaining Viral Suppression in SIV-Infected Macaques Treated with Short-Term Antiretroviral Therapy. Immunity 45:656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Ng’ang’a D, Nkolola JP, Brinkman AL, Peter L, Lee BC, Jimenez J, Jetton D, Mondesir J, Mojta S, Chandrashekar A, Molloy K, Alter G, Gerold JM, Hill AL, Lewis MG, Pau MG, Schuitemaker H, Hesselgesser J, Geleziunas R, Kim JH, Robb ML, Michael NL, Barouch DH. 2016. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 540:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henrich TJ, Hatano H, Bacon O, Hogan LE, Rutishauser R, Hill A, Kearney MF, Anderson EM, Buchbinder SP, Cohen SE, Abdel-Mohsen M, Pohlmeyer CW, Fromentin R, Hoh R, Liu AY, McCune JM, Spindler J, Metcalf-Pate K, Hobbs KS, Thanh C, Gibson EA, Kuritzkes DR, Siliciano RF, Price RW, Richman DD, Chomont N, Siliciano JD, Mellors JW, Yukl SA, Blankson JN, Liegler T, Deeks SG. 2017. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med 14:e1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Namazi G, Fajnzylber JM, Aga E, Bosch R, Acosta EP, Sharaf R, Hartogensis W, Jacobson JM, Connick E, Volberding P, Skiest D, Margolis D, Sneller MC, Little SJ, Gianella S, Smith D, Kuritzkes DR, Gulick RM, Mellors JW, Mehraj V, Gandhi RT, Mitsuyasu R, Schooley RT, Henry K, Tebas P, Deeks S, Chun TW, Collier AC, Routy JP, Hecht FM, Walker BD, Li JZ. 2018. The Control of HIV after Antiretroviral Medication Pause (CHAMP) study: post-treatment controllers identified from 14 clinical studies. J Infect Dis doi: 10.1093/infdis/jiy479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okoye AA, Picker LJ. 2013. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev 254:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananworanich J, Sacdalan CP, Pinyakorn S, Chomont N, de Souza M, Luekasemsuk T, Schuetz A, Krebs SJ, Dewar R, Jagodzinski L, Ubolyam S, Trichavaroj R, Tovanabutra S, Spudich S, Valcour V, Sereti I, Michael N, Robb M, Phanuphak P, Kim JH, Phanuphak N. 2016. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J Virus Erad 2:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Souza MS, Pinyakorn S, Akapirat S, Pattanachaiwit S, Fletcher JL, Chomchey N, Kroon ED, Ubolyam S, Michael NL, Robb ML, Phanuphak P, Kim JH, Phanuphak N, Ananworanich J, Group RSS. 2016. Initiation of Antiretroviral Therapy During Acute HIV-1 Infection Leads to a High Rate of Nonreactive HIV Serology. Clin Infect Dis 63:555–561. [DOI] [PubMed] [Google Scholar]
- 27.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ Jr., Saag MS. 2010. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol 171:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo J, Xiong C. 2013. Youden index and Associated Cut-points for Three Ordinal Diagnostic Groups. Commun Stat Simul Comput 42:1213–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndhlovu ZM, Piechocka-Trocha A, Vine S, McMullen A, Koofhethile KC, Goulder PJ, Ndung’u T, Barouch DH, Walker BD. 2011. Mosaic HIV-1 Gag antigens can be processed and presented to human HIV-specific CD8+ T cells. J Immunol 186:6914–6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lecuroux C, Girault I, Boutboul F, Urrutia A, Goujard C, Meyer L, Lambotte O, Chaix ML, Martinez V, Autran B, Sinet M, Venet A, Anrs Primo Cohort AHICSG, Cohort AA, Group AHS. 2009. Antiretroviral therapy initiation during primary HIV infection enhances both CD127 expression and the proliferative capacity of HIV-specific CD8+ T cells. AIDS 23:1649–1658. [DOI] [PubMed] [Google Scholar]
- 31.Wherry EJ, Day CL, Draenert R, Miller JD, Kiepiela P, Woodberry T, Brander C, Addo M, Klenerman P, Ahmed R, Walker BD. 2006. HIV-specific CD8 T cells express low levels of IL-7Ralpha: implications for HIV-specific T cell memory. Virology 353:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schluns KS, Lefrancois L. 2003. Cytokine control of memory T-cell development and survival. Nat Rev Immunol 3:269–279. [DOI] [PubMed] [Google Scholar]
- 33.Trautmann L, Mbitikon-Kobo FM, Goulet JP, Peretz Y, Shi Y, Van Grevenynghe J, Procopio FA, Boulassel MR, Routy JP, Chomont N, Haddad EK, Sekaly RP. 2012. Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood 120:3466–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seddon B, Zamoyska R. 2002. TCR signals mediated by Src family kinases are essential for the survival of naive T cells. J Immunol 169:2997–3005. [DOI] [PubMed] [Google Scholar]
- 35.Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL, Hildeman DA. 2011. Bcl-2 allows effector and memory CD8+ T cells to tolerate higher expression of Bim. J Immunol 186:5729–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Axelrod H, Pienta KJ. 2014. Axl as a mediator of cellular growth and survival. Oncotarget 5:8818–8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaech SM, Wherry EJ, Ahmed R. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol 2:251–262. [DOI] [PubMed] [Google Scholar]
- 38.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106–111. [DOI] [PubMed] [Google Scholar]
- 39.Burgers WA, Riou C, Mlotshwa M, Maenetje P, de Assis Rosa D, Brenchley J, Mlisana K, Douek DC, Koup R, Roederer M, de Bruyn G, Karim SA, Williamson C, Gray CM, Team CAIS. 2009. Association of HIV-specific and total CD8+ T memory phenotypes in subtype C HIV-1 infection with viral set point. J Immunol 182:4751–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buonaguro L, Tornesello ML, Buonaguro FM. 2007. Human immunodeficiency virus type 1 subtype distribution in the worldwide epidemic: pathogenetic and therapeutic implications. J Virol 81:10209–10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447–1450. [DOI] [PubMed] [Google Scholar]
- 42.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95–98. [DOI] [PubMed] [Google Scholar]
- 43.Iyasere C, Tilton JC, Johnson AJ, Younes S, Yassine-Diab B, Sekaly RP, Kwok WW, Migueles SA, Laborico AC, Shupert WL, Hallahan CW, Davey RT Jr., Dybul M, Vogel S, Metcalf J, Connors M. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J Virol 77:10900–10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong KL, Moodley A, Kwon DS, Ghebremichael MS, Dong M, Ismail N, Ndhlovu ZM, Mabuka JMds, Muema DM, Pretorius K, Lin N, Walker BD, Ndung’u T. 2018. Detection and treatment of Fiebig stage I HIV-1 infection in young at-risk women in South Africa: a prospective cohort study. Lancet HIV 5:e35–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, Daly OA, Nguyen J, Ignacio CC, Spina CA, Richman DD, Wong JK. 2005. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis 191:1410–1418. [DOI] [PubMed] [Google Scholar]
- 46.Sabbaj S, Heath SL, Bansal A, Vohra S, Kilby JM, Zajac AJ, Goepfert PA. 2007. Functionally competent antigen-specific CD127(hi) memory CD8+ T cells are preserved only in HIV-infected individuals receiving early treatment. J Infect Dis 195:108–117. [DOI] [PubMed] [Google Scholar]
- 47.Mugavero MJ, Napravnik S, Cole SR, Eron JJ, Lau B, Crane HM, Kitahata MM, Willig JH, Moore RD, Deeks SG, Saag MS, Centers for ARNoICSCS. 2011. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis 53:927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emu B, Sinclair E, Hatano H, Ferre A, Shacklett B, Martin JN, McCune JM, Deeks SG. 2008. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J Virol 82:5398–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ndhlovu ZM, Proudfoot J, Cesa K, Alvino DM, McMullen A, Vine S, Stampouloglou E, Piechocka-Trocha A, Walker BD, Pereyra F. 2012. Elite controllers with low to absent effector CD8+ T cell responses maintain highly functional, broadly directed central memory responses. J Virol 86:6959–6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, Walsh DA, Block KE, Fonseca R, Yan Y, Hippen KL, Blazar BR, Masopust D, Kelekar A, Vulchanova L, Hogquist KA, Jameson SC. 2018. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature 559:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pilipow K, Scamardella E, Puccio S, Gautam S, De Paoli F, Mazza EM, De Simone G, Polletti S, Buccilli M, Zanon V, Di Lucia P, Iannacone M, Gattinoni L, Lugli E. 2018. Antioxidant metabolism regulates CD8+ T memory stem cell formation and antitumor immunity. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M Jr., Lifson JD, Nelson JA, Jarvis MA, Picker LJ. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 15:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swain SL, McKinstry KK, Strutt TM. 2012. Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol 12:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, Cohen D, Robbins GK, Pae E, Alter G, Wurcel A, Stone D, Rosenberg ES, Walker BD, Altfeld M. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med 200:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, Cutler S, McAndrew E, Jessen H, Pereyra F, Rosenberg ES, Altfeld M, Walker BD, Streeck H. 2011. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol 85:733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 77:11708–11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med 8:319–323. [DOI] [PubMed] [Google Scholar]
- 58.Ching T, Huang S, Garmire LX. 2014. Power analysis and sample size estimation for RNA-Seq differential expression. RNA 20:1684–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, Keele BF, Learn GH, Turnbull EL, Salazar MG, Weinhold KJ, Moore S, B CCC, Letvin N, Haynes BF, Cohen MS, Hraber P, Bhattacharya T, Borrow P, Perelson AS, Hahn BH, Shaw GM, Korber BT, McMichael AJ. 2009. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med 206:1253–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fischer W, Ganusov VV, Giorgi EE, Hraber PT, Keele BF, Leitner T, Han CS, Gleasner CD, Green L, Lo CC, Nag A, Wallstrom TC, Wang S, McMichael AJ, Haynes BF, Hahn BH, Perelson AS, Borrow P, Shaw GM, Bhattacharya T, Korber BT. 2010. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One 5:e12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR, Team HS. 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarthy DJ, Chen Y, Smyth GK. 2012. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Research 40:4288–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data file S1. Primary data
Materials and Methods
Fig. S1. Transcriptional analysis reveals predicted dysfunctional responses in CD8+ T cells from untreated individuals vs early treated individuals
Fig. S2. TCR clonotype dynamics in early treated and untreated HIV
Fig. S3. Longitudinal characterization of HIV-specific CD8+ T cell responses in treated and untreated hyperacute HIV infection.
Table S1. Baseline characteristics of study participants
Table S2. Effect of the interval between treatment initiation and sample collection on immune parameters in Fiebig I-II and Fiebig III-V
Table S3. Immunodominant responses measured by HLA-class I tetramers in individuals treated in Fiebig III-V and untreated donors
Table S8. TCR ß clonotype sequences
Table S4. Results from the RNA-seq differential expression tests in HIV-specific, CMV-specific, and total CD8+ T cells (excluding the tetramer-specific cells) between the time points assayed in treated individuals.
Table S5. Results from the RNA-seq differential expression tests in HIV-specific, CMV-specific, and total CD8+ T cells (excluding the tetramer-specific cells) between the time points assayed in untreated individuals.
Table S6. Results from the RNA-seq differential expression tests between CMV-specific vs. HIV-specific CD8+ T cells at the late and long-term time points, and the difference between those cells between the two time points.
Table S7. Results from the RNA-seq differential expression tests between HIV-specific CD8+ T cells from treated vs. untreated individuals at each time point.
Table S9: Table contains alignment statistics and metadata on the RNA-seq samples in this study.