Summary
Basal forebrain (BF) is a principal source of modulation of the neocortex [1–6], and is thought to regulate cognitive-functions such as attention, motivation, and learning by broadcasting information about salience [2, 3, 5, 7–19]. However, events can be salient for multiple reasons – such as their novelty, surprise, or reward prediction-errors [20–24] – and to date, precisely which salience-related information BF broadcasts is unclear. Here, we report that primate-BF contains at least two types of neurons which often process salient-events in distinct manners: one with phasic burst responses to salient-events, and one with ramping-activity anticipating them. Bursting neurons respond to cues that convey predictions about the magnitude, probability, and timing of primary-reinforcements. They also burst to reinforcement itself, particularly when it is unexpected. However, they have little response to reinforcement-omission (the unexpected absence of an event). Thus, bursting neurons do not convey value prediction errors, but do signal surprise associated with external events. Indeed, they are not limited to processing primary-reinforcement: they discriminate fully expected novel visual objects from familiar objects, and respond to object-sequence violations. In contrast, ramping neurons predict the time of many salient, novel, and surprising events. Their ramping activity is highly sensitive to the subjects’ confidence in event timing, and on average encodes the animals’ surprise after unexpected events occur. These data suggest that primate-BF contains mechanisms to anticipate the timing of a diverse set of important external events (via ramping activity) and to rapidly deploy attentional resources when these events occur (via short latency bursting).
Blurb
Zhang et al show that cells in primate basal forebrain (BF), an area that mediates activity of the neocortex, anticipate the timing of events that capture attention, such as surprising reinforcements and novel objects. After a salient event, other BF cells’ burst-activations signal information about motivational salience, novelty, and surprise.
Results
Previous work suggests that two prominent neuronal activation patterns in the BF support its mediation of cognitive functions in response to salient events: phasic bursting that has been identified in the brains of rodents [8, 25] and tonic activations [4, 8], which in monkeys are often seen in neurons that also ramp to the time of delivery of uncertain or noxious outcomes [4]. To date, it remains unclear how these neuronal activations signal surprise and/or novelty, and how their surprise-related responses relate to errors in estimates of state values, often referred to as reward prediction errors (RPEs). Therefore, how bursting and ramping BF activations contribute to cognitive functions remains poorly understood. Here, we assessed whether phasic bursting and ramping activity occurs in distinct groups of neurons, tested whether and how BF represents prediction errors, surprise, value, novelty, and timing.
CS-related phasic and ramping activity is observed in distinct BF cell groups that differentially signal reinforcement statistics
We recorded BF neurons while 5 monkeys participated in a Pavlovian-procedure in which they experienced reward-predictions that varied in magnitude and probability [4, 26, 27]. A reward-probability block contained five conditioned stimuli (CSs) associated with five probabilistic reward-predictions (0, 25, 50, 75 and 100% of 0.25ml of juice). A reward-amount block contained five other CSs associated with certain reward-predictions of varying reward amounts (0.25, 0.1875, 0.125, 0.065 and 0ml). During neuronal recording, any neuron that displayed ramping and/or phasic burst responses in the CS-epoch of this Pavlovian-procedure was recorded (n=70; Monkey H = 15; Monkey P = 16; Monkey B = 10; Monkey R =12; Monkey Z = 17).
Example neurons are shown in Figure 1A. The first neuron (Figure 1A – top) displayed short latency bursting after the presentation of the probability and amount CSs. This phasic activation was greatest following the presentation of the CS associated with the highest EV, in either the reward probability or the reward amount block, and least following the presentation of the CSs associated with the lowest EV (no reward). In either block, the bursting activity was strongly correlated with expected value (Spearman’s rank-correlation, Probability block, ρ = 0.84, p < 0.0001, Amount block, ρ = 0.86, p < 0.0001). The second neuron (Figure 1A – bottom) had a very different response. Shortly after the CSs were presented, it displayed a consistent CS-onset related inhibition that was greatest in the low value trials and less apparent during high value trials, on average roughly scaling with EV. In the reward probability block, this initial change was followed by ramping activity to the time of the uncertain (or risky) reward delivery (following 75, 50, and 25% CSs). The neuron’s activity was significantly fit by a model of uncertainty (ρ = 0.77, p = 0.0001, measured in last 500ms) but not EV (ρ = −0.01, p = 0.94). In the reward amount block, in which all trials were certain, the neuron represented EV until the time of the reinforcement in its tonic activity (ρ = 0.70, p < 0.0001, last 500ms). These example neurons suggest that the BF may contain functionally-distinct classes of neurons: phasic bursting neurons that co-vary with the magnitude and probability of reinforcements and tonic neurons that ramp, predicting the timing of uncertain-outcomes.
Figure 1 – Two groups of BF neurons encode the magnitude and probability of reinforcement in distinct manners.
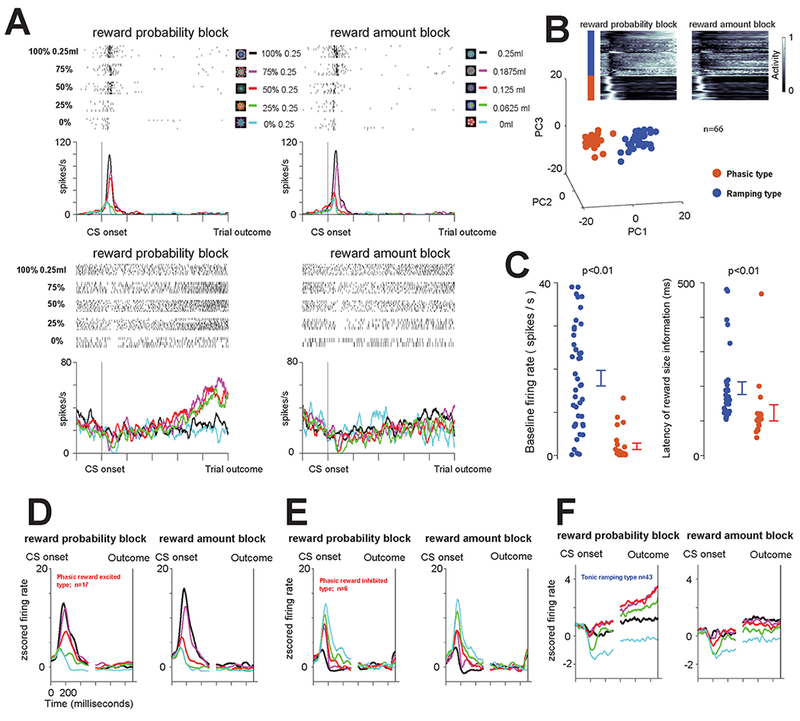
(A) Responses of two example BF neurons (top and bottom) to the presentation of 10 fractal objects associated with certain and uncertain predictions of juice reward in the reward probability block (left) and reward amount block (right). (B) Clustering of BF neurons based on average activity in the probability block. Inset heat map shows the activity of 66 BF neurons (normalized from 0 to 1 to the minimum and maximum in the reward probability block) from the time of the CS onset to the time of the trial outcome (reward or no reward) in the reward probability and reward amount blocks. Each line represents the average activity across all 5 trial types in the block for each neuron. Below are the results of PCA analyses performed on those normalized CS response functions. K-means clustering (Methods) was used to separate the neurons into two groups: red group (n=23) and blue group (n=43). The group identities of the neurons are also indicated by a color bar to the left of the heat map. (C) The two clusters of neurons (red and blue) display distinct baseline firing rates (left) and latencies of value coding in the reward amount block (right). Each dot represents data from a single neuron. Error bars around the mean show SEM. (D) Average responses of the neurons in the blue group in the reward-probability block (left) and reward amount block (right). (E-F) Average responses of the neurons in the red group in the reward-probability block (left) and reward amount block (right). E shows neurons that displayed greater activation for reward versus no reward trials, while F shows neurons that displayed greater activation for no reward trials. See also Figure S1, and Methods and their associated Figure S2 for details and anatomical locations of neuronal recordings.
To test this, we clustered BF neurons based on their average responses. Only neurons that had been recorded in every condition in both blocks were included (n=66/70). Importantly, their response-vectors were obtained by averaging the neuronal activity across all five CSs in the reward-probability block, and were subsequently normalized (Figure 1B; inset). Therefore, neuronal tuning (e.g. representation of reward probability) and baseline firing rates were not considered in the clustering analysis.
This analysis revealed two clusters (Figure 1B). The first cluster (red; n=23) showed clear bursting after the CS onset (see neurons’ response vectors in Figure 1B-inset). In contrast, the second cluster (blue; n=43) showed an initial suppression following the CS onset, and slow ramp-like increase in activity as the trial’s outcome neared.
The two clusters had different baseline firing rates (Figure 1C): one had relatively high firing rates (blue-cluster; average frequency = 18 Hz; S.D. = 12 Hz) and the other low (red-cluster; average frequency = 2.1 Hz; S.D.= 3.5 Hz). Both clusters’ initial CS-responses co-varied with the magnitude of predicted reward, initially coding EV, but the latency of this information was different among them. EV was conveyed earlier by the neurons in the phasic bursting red cluster (Figure 1C-right; ranksum test; p<0.01; blue-cluster, average = 195 ms, median = 159 ms, S.D. = 104 ms; red-cluster, average = 123 ms, median = 100 ms, S.D. = 96 ms).
These clusters differed in how they represented probability and amount of reinforcement (Figures 1D-F and S1). Phasic bursting neurons (red cluster) signaled the EV of the CSs in their bursting activations. The bursting activity was correlated with the probability in the probability block (ρ = 0.60, p<0.0001) and reward amount in the amount block (ρ = 0.71, p<0.0001). Tonic ramping neurons’ initial suppression co-varied with EV (ρ = 0.47, p < 0.0001 in probability block, p = 0.46, p <0.0001 in amount block). However, in trials in which reward was uncertain they displayed additional ramping activity to the trial outcome [4]. The activity during these 25, 50, and 75% CS trials was correlated with the probability of reinforcement delivery; Spearman’s rank correlation, ρ=0.25 p=0.0043, pre-outcome analysis window - 0.5s before outcome is delivered). And, as reported previously, on average, the pre-outcome activity in the reward probability block (across all 5 trial types) was correlated with uncertainty (ρ = 0.71, p < 0.0001; same analysis window as above).
Locations of phasic bursting and tonic ramping neurons were reconstructed using in-vivo MRI methods [28] (Figure S2). Both phasic bursting and tonic ramping neurons were found within the BF, in the diagonal band of Broca and Nucleus Basalis of Meynert [1, 4, 29].
Phasic and ramping neurons signal early-versus-late rewards under temporal uncertainty
Do ramping of BF neurons encode the estimated time of uncertain rewards? If so, then if rewards were certain but their timing was uncertain, they should display ramping activity to the time of the earliest possible reward. Second, phasic bursting neurons bursts seemed to scale with the expected values of the CSs, regardless of whether the value was manipulated by probability or amount. Might these neurons also encode the value of early versus late rewards?
To answer these questions, we designed a reward timing procedure (Figure S3). Here, five distinct visual fractal objects served as conditioned stimuli (CSs) that predicted either (1) a probabilistic delay before a reward with deterministic delivery (delays = 1.5 or 4.5 s; reward-timing-uncertain CSs); or (2) a deterministic delay before a reward with 0.5 probability of delivery (reward-probability CS). To test how phasic bursting neurons and tonic ramping neurons encode temporally-uncertain reward predictions, we first identified them using the task in Figure 1, and then recorded them in this reward timing procedure (n=52; Monkey W = 21; Monkey B = 6; Monkey R =15; Monkey Z = 10).
Tonic ramping neurons displayed ramping activity in the 0.75, 0.5, 0.25 reward-timing uncertain conditions (Figure S3). The magnitude of this activation was correlated with the probability of reinforcement delivery at 1.5 s (Spearman’s rank correlation, ρ=0.48, p<0.0001, analysis window, 1s to 1.5s). Interestingly, significant ramping was also observed to certain late reward at 4.5 s (Figure S3). Therefore, BF ramping tracks reward delivery during temporal reward uncertainty (before 1.5 s) and during relatively longer epochs in which there is temporal uncertainty due to noise in interval timing.
Phasic bursting neurons’ activity scaled with reward timing such that highest activity was evoked by CSs predicting the earliest reward (Figure S3). Their average activity was correlated with reward probability at 1.5 s (Spearman’s rank correlation, ρ=0.41, p=0.0012, analysis window, 0s to 0.5). Unlike the tonic ramping neurons, the phasic bursting neurons did not anticipate the late reward at 4.5 s (Figure S3).
BF phasic and ramping neurons signal reinforcement surprise in distinct manners
A long-standing question is whether the BF signals errors in state values, RPEs – a key signal for updating reward values and mediating economic choice [30, 31]. An alternative is that BF neurons signal a rectified or an unsigned prediction error [32, 33] rather than a value (signed) prediction error. We tested which type of prediction error is signaled by the BF by analyzing responses to reward deliveries and reward omissions after 25, 50, and 75% predictions (Figure 2).
Figure 2 -. Differential coding of surprise in ramping and bursting BF neurons.
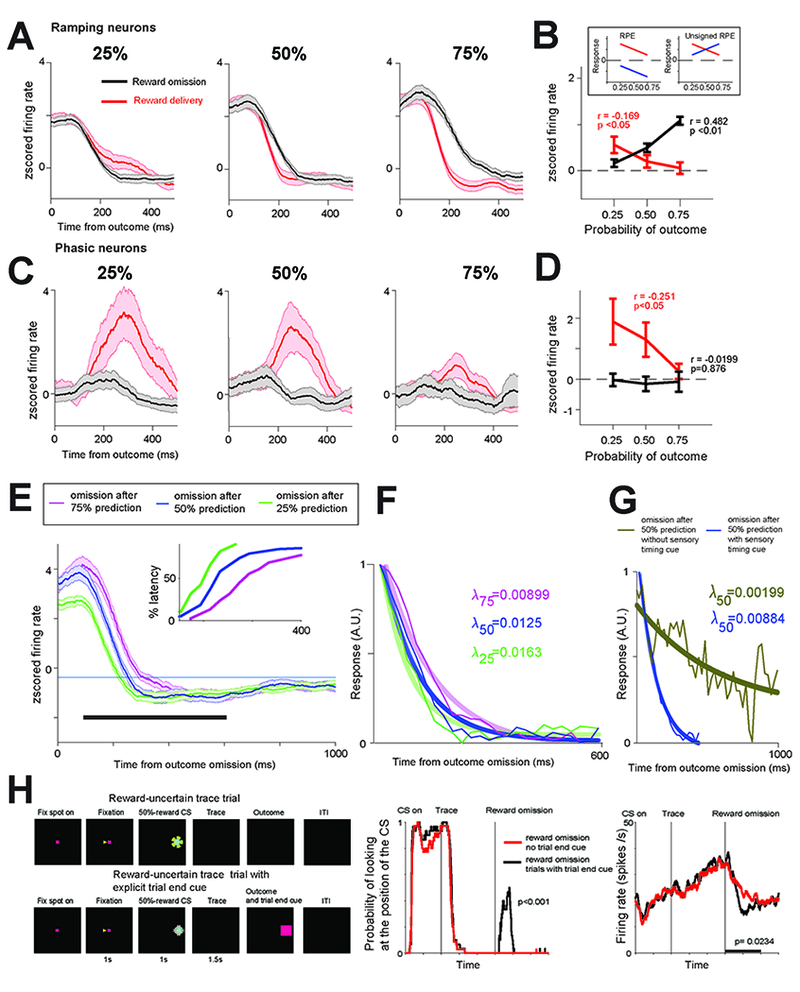
(A) Ramping neurons’ average outcome activity in 25%, 50% and 75% conditions. Red – reward delivered trials; black – no reward trials. (B) Ramping neurons’ average responses for reward delivery and no reward trials. Linear correlations of responses with reward expectancy are indicated (time window: 100ms to 400ms; p values were obtained with 10,000 permutations; Methods). The results of the correlations suggest that the activity resembles the toy model of unsigned RPE (or surprise). Inset shows cartoon models of theoretical outcome responses coding reward prediction errors (RPE; left) and unsigned RPE (right). If neurons signal unsigned reward prediction errors, then they should display greatest responses to reward deliveries following 25% reward predictions, and smallest responses following a 75% reward prediction. The same neurons should display greatest responses to reward omissions following 75% reward predictions, and smallest responses following 25% reward predictions. Alternatively, if neurons encode signed RPEs, then they will display inhibitions following omissions whose magnitude ought to be inversely related to the probability of reward. (C) Outcome activity of phasic bursting neurons. Conventions are the same as in (A). (D) Phasic bursting neurons’ responses resembled reward prediction error coding only in reward delivery trials (red; time window: 200ms to 500ms). (E) Activity of BF ramping neurons during 25, 50, and 75% reward probability trials in which the reward was omitted. The ramping activity returned to inter-trial baseline level (thin blue line) at different latencies across these three types of trials: earliest during 25% trials, and latest during 75% trials. Cumulative distributions of these latencies are shown in the inset. (F) Exponential fits (thick lines) to the population binned activity (thin lines; Methods). Fits and decay rates (right) were calculated for the population after the activity for each trial type was normalized from 0 to 1, such that for each of the tree conditions, the starting point is 1. (G) Same as F, except here we compared fit and decay rate during 50% trials in which an explicit cue indicated the end of the trial (dark blue) with fit and decay rate during 50% trials in which no explicit cue was given (and the CS remained on the screen; Methods). (H-left) Trace conditioning with and without explicit visual cues that signaled the end of the trial. (H-middle) Monkey’s gaze behavior indicated that it attended to the trial-end cue (presented at the same location as the CS; rank sum test; p<0.001). (H-right) Explicit knowledge of trial timing reduced the reward-omission related ramping activity (Monkey W; 8 neurons; p=0.0234; sign rank test). Analysis window used to study gaze behavior and neuronal activity is indicated by black bar. See also Figure S3 for activity in the temporal uncertainty procedure separately.
Ramping neurons outcome related activity on average was correlated with unsigned prediction errors (Figures 2A-B). After the trial outcome, the magnitude of their activity was greatest during reward-delivered trials following 25% reward predictions, and greatest during reward-omission trials following 75% reward predictions. Reward omission and reward delivery outcome responses were significantly correlated with expectancy (Figure 2B), albeit in opposite manners.
Phasic bursting neurons’ outcome related activity also signaled prediction errors following reward deliveries. Their delivery responses were correlated with expectancy (Figure 2D - red), displaying highest activations following reward deliveries in 25% reward trials. However, unlike the ramping neurons, these neurons did not discriminate reward omissions following different uncertain reward predictions (Figures 2C-D). To verify that the lack of relationship between reward omission related activity and reward probability was not due to firing rate normalization, we repeated the correlation analyses in Figure 2D on raw omission-related spike counts and observed the same results (p=0.89). Hence, a key feature of the value-RPE – a reward omission related suppression - was missing from phasic bursting neurons.
BF bursting can be elicited by rewarding and aversive noxious events [4, 8, 25]. Therefore, why was bursting not apparent in response to unexpected reward omissions? The most parsimonious explanation is that the lack of omission responses was due to a lack of external salient events cueing reward omissions, and due to a lack of sensitivity of phasic bursting neurons to internally generated errors in subjective-value.
Surprise has a temporal dimension, and ramping neurons clearly display ramping signals to the time of reinforcements (Figures 1–2) the magnitude of which is correlated with monkeys’ confidence in reward-delivery (Figure 1 and Figure S3; ramping responses: 0.25<0.5<0.75). Might BF ramping activity encode estimates of outcome-timing under uncertainty?
To test this, we took advantage of the fact that in our tasks, CSs co-terminated with outcomes. During omission trials, no external cues indicated that the trial has ended. If ramping reflects information about the animals’ temporal estimates, then we should see different ramping-down responses following omissions in 25, 50, and 75% trials.
BF ramping returned to baseline earliest during 25% reward trials and latest during 75% trials. Decay of the ramping also roughly scaled with reward expectation: it was greatest following omissions during 25% and least during 75% trials (Figure 2F; bootstrapping; the 95% confidence intervals of 25%, 50% and 75% decay rates exclude each other). Note that different firing rates across different trial-types could not explain these results because before obtaining the decay rates, we first normalized each trial type from 0 to 1.
Next, we studied the activity of BF ramping neurons in the reward timing procedure because it contained two distinct 50% reward predictions: one in which the CS co-terminated with the outcome and one in which the CS remained on the screen (Figure S3). In the first condition, the animals obtained a precise signal about the timing of the trial while in the other they did not. The decay rate of BF ramping neurons was again sensitive to temporal predictions: it was greater when animals did not receive an explicit temporal cue (Figure 2G; bootstrapping; the 95% confidence intervals of the decay rates exclude each other). Finally, we analyzed another task that contained two types of 50% reward CS trials with identical timing and reward statistics. The two trials differed in one way – one of them contained an external trial end cue that indicated when the trial was over. Consistent with the results of Figure 2G, when an explicit cue was given during reward omission trials the ramping down activity displayed a relatively rapid drop off (Figure 2H – right). In sum, Figures 2E-H show that BF ramping activity is strongly influenced by evidence about and confidence in the timing of reinforcements.
Object novelty and sensory surprise are signaled by the BF
The data thus far show that BF neurons are sensitive to surprise. However, surprises arise due to violations in belief states following a probabilistic prediction, when there is a deviation of the outcome from the mean of expected-outcomes [20], or as a result of novelty due to a comparison of a sensory events with representations of past experiences. To test how BF represents novelty we designed an object-sequence task in which novel objects were fully expected – they were novel but not surprising.
Monkeys experienced four sequences of object presentations (S1, S2, S3, S4). Each sequence contained 3 familiar objects and 1 novel object. The novel object was always in the second position in the sequence. If a neuron has a selective novelty response, it should respond more strongly and consistently to the novel object than to the familiar objects in the sequence. To assess if novelty-responses were dominantly due to task-relevance or reward-prediction, following S2 and S4, monkeys performed a reaction-time Delayed-Non-Matching-to-Sample task (DNMS, Figure 3A-right). During DNMS, an object that was novel during the presentation of S2 (or S4 if the DNMS trial followed S4) was presented with a novel object that has never been experienced. The trial continued until the monkeys fixated this novel object for 0.5 second to get reward (Figure 3A – right). Monkeys’ behaviors indicated that they understood the task and utilized previous experiences to increase their reward rate. Their first saccade following the presentation of the two fractals most often landed on the novel object, where gaze remained until the non-selected stimulus disappeared and reward was delivered.
Figure 3 – Object sequence task.
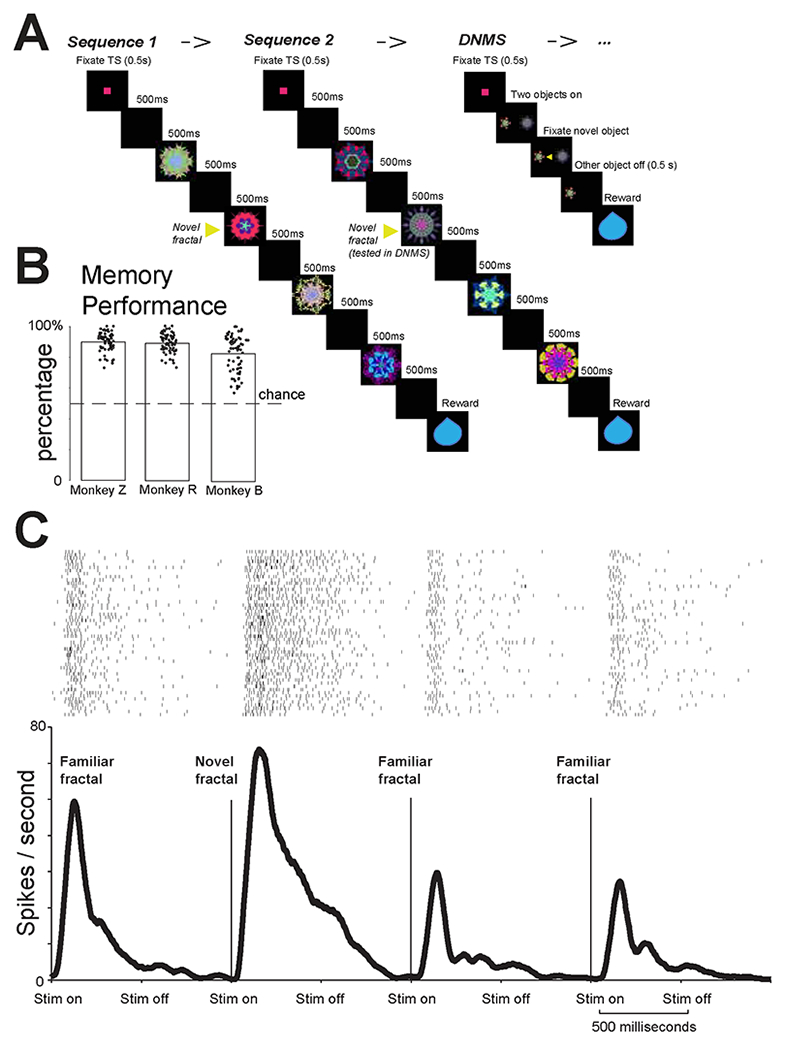
(A) The monkey was first shown two sequences of fractals. Each sequence contained 4 fractals, in which 1st, 3rd, 4th fractals were fixed familiar objects and 2nd fractal was always novel. After the two sequences, the monkeys performed a delayed non-match to sample task (DNMS) in which one object was novel and the other was the object that was previously novel in sequence 2. Monkeys fixated the novel object for reward. (B) Behavioral performance for three monkeys. Y-axis shows the percentage of first saccades to the novel object in DNMS, the percentages are significantly different from 0.5 for all three monkeys (p<0.01, sign-rank test). (C) Example BF phasic bursting neuron’s responses to the four objects in in a sequence. The response was highest for the second (novel) fractal (rank sum test; p<0.05).
We studied 39 BF neurons identified using Experiment 1 (Monkey B = 6; Monkey R =11; Monkey Z = 22). Phasic bursting neurons robustly discriminated the novel object from the familiar objects. An example phasic bursting neuron is shown in Figure 3C. This neuron responded selectively to the novel object (p<0.01; ranksum test). This selective response could not be explained by priming or reward-proximity because the novel objects always appeared in the second position in the sequence (Figure 3A) rather than the first or the last. Like the example neuron, the population of phasic bursting neurons (Figure 4A) and the single neurons (Figure 4B) selectively discriminated the novel object versus familiar objects.
Figure 4 -. Phasic bursting neurons signal novelty and surprise not directly related to reward.
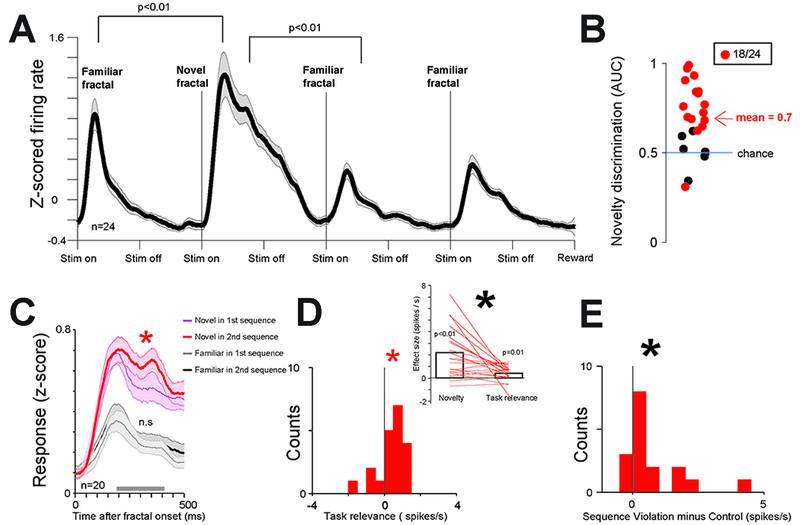
(A) Average activity of phasic bursting neurons in the object sequence task. (B) Area under the ROC curve (AUC) for each phasic neuron that assessed the ability of the neuron to discriminate novel versus familiar objects. Red dots are neurons that can significantly discriminate novel versus familiar objects (time window: 200 ms to 400 ms). (C) Phasic neuron’s group average responses to novel fractals in Sequence 1 (thin blue line), Sequence 2 (thick red line), and the to the last 2 familiar fractals in Sequence 1 (thin gray line) and Sequence 2 (thick black line). Shaded region represent SEM. Asterisk indicates significant difference (p<0.05) between novel fractal responses in Sequence 1 and 2. The results indicate phasic bursting neurons’ activity is under gain modulation by task relevance. But, this could not be explained by a general arousal increase in S2 and S4 relative to S1 and S3 because the activity for the third and fourth familiar fractals in the sequences was not different across S2 and S4 versus S1 and S3. (D) Lower left: Histogram of single neurons’ response differences for novel fractal in Sequence 2 and Sequence 1. Red asterisk indicates significant difference from 0 (p<0.05), Black asterisk indicates significant difference from 0 (p<0.01). Upper right: for each neuron, the data from the histogram (on the right) compared with the strength of novelty discrimination (left; defined as the difference between neuronal responses for X and Y). Both novelty discrimination and task-relevance effects are significant, but the novelty effect is stronger (p<0.05). (E) At low probability (11 %) one of the familiar fractal in sequence 2 (or 4) was substituted with another familiar fractal from sequence 1 (or 3) (Methods). Phasic neurons’ responses were enhanced (p<0.01) by this object sequence violation. See also Figure S4 for the activity of tonic ramping neurons.
Though all neurons in Figure 4 were identified using the task in Figure 1 and were preferentially excited by 100% reward CS, their strong and selective novelty responses in the object sequence task were present when the novel object was relevant or irrelevant for subsequent memory behaviors (Figure 4C). That is, during both S1 and S3, BF phasic neurons displayed stronger responses to novel objects than to familiar objects (signrank tests, p<0.01). Their novelty responses were also enhanced by task relevance (Figure 4C-D).
An important consideration for the interpretation of novelty responses is that novelty, in primates, is thought to exert strong influence on behavior [20, 34–36], especially on gaze behavior. However, what type of influence (attentional or motivational, or both) has been unclear. We designed a novel behavioral procedure that revealed that object novelty has a motivational value (Figure S4). This finding necessitates that future studies discriminate the role of BF bursting in attentional and motivational effects of object novelty on behavior.
In contrast to the phasic neurons, the ramping neurons had a weaker novelty-selective response (ranksum test comparing single neurons’ AUC values; p= 0.035). They displayed ramping that anticipated two critical events in the object sequence task: novel objects and rewards (occurring after a long time interval; Figure S4).
We recently showed that predictions of uncertain-rewards attract overt attention more than certain-rewards [37]. So, in sum, BF ramping may anticipate events that preferentially capture attention: novelty (Figure S4), uncertain rewards (Figure 1), and noxious stimuli (Figure S4 and [4]), and prepare the brain to receive them.
Previous work showed that temporal-cortex, a major target of BF projections [1], is sensitive to sequence-violations [38]. To test, if BF is sensitive to unexpected violations in object the sequences, we replaced an object in the S2 with an object from S1, or an object from S4 with an object from S3 on ~11 % of trials. These replacements avoided RPEs because, the proximity to reward was not changed. Sequence violations produced small but significant increases in responses of phasic and tonic neurons (Figures 4 and S4). So, the BF can broadcast information about novel and surprising sensory events that are not directly associated with primary reinforcements.
Concluding remarks
We report that primate-BF contains at least two types of neurons which often process many salient events that capture attention in distinct manners: one with phasic burst responses, and one with ramping-activity in anticipation of their occurrence.
Ramping neurons signal internal variables closely tied to confidence in the timing of surprises and novelty. Their activity may represent (or provide a readout of [39]) an internal-clock that is well-suited to guide anticipatory temporal-attention, particularly in uncertain or novel contexts. Phasic bursting neurons rapidly and precisely conveyed statistical information about the time, magnitude, and probability of reinforcement predictions, and about the surprise of reinforcement deliveries. They were highly sensitive to sensory-novelty and to errors in subjects’ beliefs about the sequences of sensory-events. Their bursting could coordinate many regions of the neocortex that receive BF projections to mediate the processing of a wide-range of external salient events, and orchestrate appropriate responses to them [8, 12, 29, 40, 41].
Phasically bursting neurons did not discriminate among expected and unexpected reinforcement omissions that monkeys had to detect internally (e.g. omissions were not cued). Thus, in contrast to many dopamine neurons, they did not convey phasic RPEs [30, 42, 43]. Notably, a set of recent studies showed that not all dopamine phasic responses signal RPEs wholly or purely. Instead, some dopamine neurons convey an alerting signal complementary to BF bursting [44–48]). Future studies must assess how the BF phasic bursting and dopamine neurons work together to mediate behavior. One possibility is that BF phasic bursting (conveyed to the neocortex in response to a salient event) is followed by the release of dopamine in the basal ganglia. This dopaminergic release would then support striatal value (or motivational-salience) assignments to events being processed by the cortex (under the mediation of the BF). How dopamine would do so, may ultimately depend on when and where it is released [44–48].
BF contains prominent groups of cholinergic, GABAergic, and glutamatergic projection neurons. Previous work in rodents has identified putative-GABAergic CS-related phasic bursting neurons, reinforcement salience related bursting cholinergic neurons, and other tonic active neurons in the BF [8, 11, 25]. It will be important to identify which neurotransmitters are released (or co-released) by phasic bursting and ramping neurons in rodents and primates.
STAR Methods
CONTACT FOR REAGENT OR RESOURCE SHARING
Further information and requests for resources, data and code should be directed to and will be fulfilled by the Lead Contact, Dr. Ilya E. Monosov (ilya.monosov@gmail.com).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Six adult sexually mature male rhesus monkeys (Monkeys B, R, Z, W, H, and P; ages: 7-10 years old) were used for recording experiments. All procedures conform to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Washington University (Monkeys B, R, W, and Z) and National Eye Institute (Monkeys P and H).
All monkeys underwent surgery under general anesthesia. For each monkey, a plastic head holder and recording chamber were fixed to the skull under general anesthesia and sterile conditions. Chambers were tilted laterally from midline by 35 degrees and aimed at the basal forebrain and anterior portion of striatum. After the monkeys recovered from surgery, they participated in behavioral and neurophysiological experiments.
METHOD DETAILS
While the monkeys participated in the behavioral procedure we recorded single neurons in the basal forebrain. The recording sites were determined with 1 mm-spacing grid system and with the aid of MR images (3T) obtained along the direction of the recording chamber. This MRI-based estimation of neuron recording locations was aided by custom-built software. Single-unit recording was performed using glass-coated electrodes (Alpha Omega). During each recording session, an electrode was inserted into the brain through a stainless-steel guide tube and advanced by an oil-driven micromanipulator (MO-97A, Narishige). Signal acquisition (including amplify cation and filtering) was performed using Alpha Omega 44 kHz SNR system. Action potential waveforms were identified online by multiple time-amplitude windows with an additional template matching algorithm (Alpha-Omega).
Neuronal recordings were restricted to single well-isolated neurons in the basal forebrain that displayed task related ramping or phasic-bursting activity following the presentation of the task conditioned stimuli in the Probability Amount procedure (described below). Basal forebrain was verified as the neuronal tissue within 2 mm relative to the bottom of the brain. Furthermore, the ventral pallidum (defined using anatomical criteria, such as the first neuronal tissue encountered following traversing the anterior commissure, and previous electrophysiological criteria, such as high and irregular firing rate) was not part of this study because its functions and anatomical projections are distinct from the medial and ventral lateral basal forebrain and because it mostly does not contain the tonic regular firing ramping neurons and phasic bursting low firing neurons. The locations of the BF recordings are detailed in Figure S2. Reconstruction procedures were detailed previously [28].
Eye position was obtained with an infrared video camera (Eyelink, SR Research). Behavioral events and visual stimuli were controlled by Matlab (Mathworks, Natick, MA) with Psychophysics Toolbox extensions. Juice, used as reward, was delivered with a solenoid delivery reward system (CRIST Instruments). Juice-related licking was measured and quantified using previously described methods. Airpuffs were delivered through a narrow tube placed ~6–8cm from the monkey’s face.
Probability Amount procedure.
To study neuronal representations of reward probability and amount, and delivery related responses following probabilistic reward deliveries, we trained monkeys on a Pavlovian conditioning procedure. Pavlovian conditioning was used to avoid fluctuations in reward rate across trials or fluctuations in outcome timing within single trials (related to action performance) which may dampen outcome prediction error signals [49].
The Pavlovian conditioning procedure contained two blocks of trials: a reward-probability block and a reward-amount block. Each trial started with the presentation of a green trial-start cue at the center. The monkeys had to maintain fixation on this trial-start cue for 1 s; then the trial start cue disappeared and one of the CSs was presented pseudo randomly. After 2.5 s (for monkeys B, Z, and R) or 1.5 s (monkeys H and P), the CS disappeared, and juice (if scheduled for that trial) was delivered. The longer duration was introduced for monkey B, Z, and R to verify that the ramping activity in the BF reaches maximum at the time of the outcome across different CS durations. The reward-probability block contained five visual fractal object CSs associated with five probabilistic reward predictions (0, 25, 50, 75 and 100% of 0.25 ml of juice). The reward-amount block contained five objects associated with certain reward predictions of varying reward amounts (0.25, 0.1875, 0.125, 0.065 and 0ml). Each block consisted of 20 trials (monkeys B, Z, and R) and 40 trials (monkeys P and H) with fixed proportions of trial types (each of the five CSs appears four times in each block). The expected values (EVs) of the five CSs in the probability block matched the expected values of the five CSs in the amount block. This two-block design removed confounds introduced by risk seeking-related changes in subjective values of the CSs [26, 27].
Before neuronal recordings began, the monkeys’ knowledge of the CSs was confirmed by a choice procedure that was detailed previously [4, 26]. Briefly, in separate experimental sessions, the monkeys’ choice preference was tested for the CSs. Each trial started with the presentation of the trial-start cue at the center, and the monkeys had to fixate it. Then two CSs appeared 10 degrees to the left and right. The monkeys had to make a saccade to one of the two CSs within 5 s and fixate it for at least 750 ms. Then the unchosen CS disappeared, and after a brief delay the outcome (associated with the chosen CS) was delivered, and the chosen CS disappeared. If the monkey failed to fixate one of the CSs, the trial was aborted and all stimuli disappeared. The trials were presented pseudo randomly, so that a block of 180 trials contained all possible combinations of the 10 CSs four times. To verify that the monkeys’ knowledge is stable during recording, we also monitor licking behavior and confirm that it, like the choices, scales with the expected values of the probability CSs and amount CSs (two separate Spearman’s correlations, threshold: p<0.05). The CS epoch responses of the 31 neurons recorded in Monkeys H and P were previously analyzed in Monosov et al., 2015.
Temporal uncertainty procedure.
To assess how monkeys’ BF neurons encoded uncertain predictions about reward timing, monkeys B, R, Z were trained on an additional Pavlovian procedure (Supplemental Figure 4). Following a trial start cue fixation period (same as above), one of five CSs were presented. These CSs predicted either (1) a probabilistic delay before a reward with deterministic delivery (reward-timing-uncertain CSs); or (2) a deterministic delay before a reward with some probability of delivery (reward-probability CS). In trials with one of the four reward-timing-uncertain CSs, reward was always delivered either 1.5 s after CS onset or 4.5 s after CS onset. Depending on the reward-timing uncertain CS, the probability that a reward was delivered at 1.5 s with 0.25, 0.50, 0.75, or 1 probability. In trials with the reward-probability CS, reward was delivered with a delay of 1.5 s after CS onset with 0.50 probability. During, the 0.25, 0.50, and 0.75 CS trials, when reward was not delivered at 1.5 s, the CS remained on the screen until reward was delivered at 4.5 s. During the 0.50 reward probability CS, the CS turned off at the time of the outcome (when reward was either delivered or omitted).
Training was verified by monkeys’ reward anticipatory licking behavior. The data suggested that they understood the meanings of the CSs and were highly sensitive to the timing and probability of reward (Figure S3B). First, during the four reward-timing-uncertain CSs, monkeys displayed increased licking behavior before 1.5 s, then a decrease in licking behavior after 1.5 s if reward was not delivered, then finally an increase in licking behavior to the time of reward at 4.5 s. During reward omissions, in 75% reward trials licking behavior remained higher than 25% and 50% trials, even 0.5 s after the reward was omitted at 1.5 s (p <0.01, rank-sum test, time window 2s to 2.5s after the onset of fractal). Also, the mean magnitude of anticipatory licking behavior before possible reward delivery at 1.5 s across all trials increased with the probability of reward delivery at 1.5 s (Spearman’s rank correlation, ρ=0.38, p=<0.0001; Figure S3). These behavioral results indicate that the magnitude and persistence of the monkeys’ anticipatory behavior were strongly influenced by reward timing conveyed by the CSs.
Object sequence procedure.
An object sequence task was used to study how BF neurons encode sensory predictions and object novelty. Monkeys B, R, and Z experienced four distinct sequences of object presentations (S1, S2, S3, S4). The object sequences began following a 0.5 s period of fixation on the trial start cue that appeared in the center of the screen. Each sequence contained 3 familiar objects and 1 novel object. These objects were presented in the center of the screen and occupied a ~3 degree visual angle. The novel object was always presented in second position in the sequence. Therefore, the novel object was surprising because it was never experienced by the monkeys, but its presentation did not deviate from the animals’ expectations. Monkeys performed more than 10,000 trials before recordings began. Following sequences S2 and S4, the monkeys performed a reaction-time Delayed Non-matching-to-Sample task (DNMS). During DNMS, an object that was novel during the presentation of S2 (or S4 if the DNMS trial followed S4) was presented with a novel object that has never been experienced. The objects were presented 10 degrees from the center, to the left and the right of the fixation point. The trial continued until the monkeys fixated the novel object for 0.5 milliseconds to get a reward. The monkeys were never penalized for looking at the previously experienced object. Therefore, the novel objects in S2 or S4 did not have an explicit reward association, but aided the monkey in subsequent DNMS trials. On ~11% of S2 or S4 presentations, the first or the third fractal was replaced by a corresponding fractal from sequences S1 and S3 (in S2 from S1; and in S4 from S3). For example, if the first fractal in S2 was replaced, the first fractal from S1 was always displayed. In this way, sequence violations did not alter the relationship of the individual fractals to the timing of reward delivery.
Reward and novelty motivated gaze task.
To test if monkeys are motivated by novelty we trained Monkeys R and Z on a saccadic task that measured their eagerness to observe a novel visual object. First, a fixation dot appeared in the center of the screen. 0.5 s after the onset of the fixation dot, a visual object fractal appeared 10 degrees to the right or the left of the fixation dot. The monkey was required to continue fixating the dot in the center. After 0.35 s the fixation spot disappeared and the monkey was free to make saccades. Reward was always delivered 3 seconds after the fractal onset. Therefore, the monkeys’ saccadic behavior after the fixation spot disappeared did not affect reward delivery. In this task, the monkeys experienced four different trial types. The first two types of trials contained a novel (type 1) or 1 of 2 familiar (type 2) visual fractal objects. Two additional trial types (3-4) tested whether the monkeys were motivated by the possibility of viewing a novel fractal. In trial type 3, 1 of 2 familiar objects appeared. After fixation spot disappeared, if the monkey fixated the familiar object, it was immediately replaced by a novel object. In trial type 4, 1 of 2 familiar objects appeared. If the monkey fixated this object, it was replaced by 1 of 2 familiar objects. If novelty is salient, we ought to observe faster target acquisition times (duration between the time when the stimulus was presented and when the monkey saccades to its location) in trial type 1 than 2. Also, if novelty exerts motivational effects on saccadic behavior, then we ought to see faster target acquisition times in trial type 3 than 4.
QUANTIFICATION AND STATISTICAL ANALYSIS
To generate spike density functions, spike times were convolved with a Gaussian kernel (σ=100 ms) across all trials. Statistical tests were two-tailed. All permutation tests used 10000 shuffles. For all analyses and figures that included deliveries and omissions of rewards, unless explicitly stated in the text, a neuron was included if it had at least 2 trials for reward delivery and omission.
To cluster the single neurons’ average responses in the probability block (Figure 1), first we performed principal component analysis (PCA). We then applied Silhouette and Calinski-Harabasz tests to estimate the optimal number of clusters (n=2). K-means clustering was used to cluster the data based on PCs into 2 clusters (for this, using the first 3 PCs and up to 10 PCs resulted in very similar group membership).
To calculate the latency of reward size coding information (Figure 1C) we performed a correlation of firing rate and value in time (in 100ms bins moving 1 ms steps) for each neuron. For each time bin we calculated the p value of the Spearman’s rank correlation of neuron’s activity with reward amount in the reward amount block. Reward size coding latency was defined as the first time p was lower than 0.01 (but similar results were obtained at p < 0.05). Importantly, such statistical latency analyses do not determine the actual latency of information coding per se because they utilize an arbitrary threshold. Instead, they are useful for demonstrating relative latencies across two groups of neurons or signals.
To calculate the baseline rate that was used to derive the latency with which ramping neurons returned to baseline (Figure 2E), we picked the time window from 1000ms to 500ms before trial start cue appeared and used the average firing rate in this time window as the baseline.
To fit the outcome related activity with exponential functions (Figure 2), we first derived spike density functions using overlapping bins of 100ms (in 50ms steps). Then we used least squares method to fit the data by the function: A*e−λt+C, in which λ is the decay rate, representing how fast the firing rate decreases. λ restrained by the interval (0,0.06). To determine if the decay rates were significantly different across the different reward-omission conditions we used bootstrapping to calculate the confidence interval of the difference between two decay rates and tested if the 95% confidence interval excluded a difference of zero. Bootstrapping was done by randomly resampling the neurons with replacement (500 times). Each time resampling was done, we obtained a set of decay rates by fitting the neurons’ average activity to the function shown above. For, Figures 2A-D, data from probability-amount and reward timing procedures were pooled (see outcome responses separately in Supplemental Figures 1 and 5).
In the DNMS object sequence task, reward was delivered as long as the monkey fixated on the novel object for 0.5 s, regardless if he had looked at the other object. To evaluate the monkey’s performance, we focused on the primary choice the monkey made, i.e., the first object he fixated for 0.5 s. To calculate performance, we obtained the percentage of trials in which the monkeys’ primary choices were the novel objects.
For single neuron analyses (Figure 4B-E) of novelty, task-relevance, and sequence-violations in the object sequence task, we subtracted the activity 100 ms before the object presentation from the activity measured after the object was presented (time window: 200ms to 400ms). In this way, changes in firing rate that were unrelated to the objects were not considered in the analyses.
Neuronal discrimination of object novelty was assessed by calculating area under the receiver operating characteristic (ROC) curve. ROC areas of 0 and 1 are equivalent statistically; both indicate that two distributions are completely separated. The analysis was structured so that ROC area values greater than 0.5 indicate that the activity during novel object presentation was greater than familiar.
Supplementary Material
Highlights.
-
-
Basal forebrain (BF) signals internal- and external- states that capture attention
-
-
Some BF cells ramp to estimated times of novel-objects and surprising-reinforcements
-
-
These ramps are sensitive to internal estimates of, and confidence in, event timing
-
-
As external events occur, other BF cells convey information about their statistics
Acknowledgements
This work was supported by the National Institute of Mental Health under Award Number R01MH110594 and the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) ElectRx program through the CMO Grant/Contract No.HR0011-16-2-0022, Edward Mallinckrodt, JR Foundation, and the McDonnell Center for Systems Neuroscience. We are grateful to Ms. Julia Pai, Dr. Noah Ledbetter and Mr. J. Kael White for assisting in data acquisition, to Ms. Kim Kocher for fantastic animal care and training, and to Dr. Okihide Hikosaka for supporting the recording experiments in Monkeys H and P. We thank Ms. Julia Pai and Drs. Ethan Bromberg-Martin and Timothy Holy for helpful discussions, and Dr. Hiroyuki Nakahara and Ms. Jamie Moffa for reading earlier versions of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The authors declare no competing financial interests.
References
- 1.Mesulam MM, Mufson EJ, Levey AI, and Wainer BH (1983). Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. The Journal of comparative neurology 214, 170–197. [DOI] [PubMed] [Google Scholar]
- 2.Everitt BJ, and Robbins TW (1997). Central cholinergic systems and cognition. Annu Rev Psychol 48, 649–684. [DOI] [PubMed] [Google Scholar]
- 3.Baxter MG, and Chiba AA (1999). Cognitive functions of the basal forebrain. Current opinion in neurobiology 9, 178–183. [DOI] [PubMed] [Google Scholar]
- 4.Monosov IE, Leopold DA, and Hikosaka O (2015). Neurons in the Primate Medial Basal Forebrain Signal Combined Information about Reward Uncertainty, Value, and Punishment Anticipation. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 7443–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, and Nadasdy Z (2013). Neurons in the Basal Forebrain Project to the Cortex in a Complex Topographic Organization that Reflects Corticocortical Connectivity Patterns: An Experimental Study Based on Retrograde Tracing and 3D Reconstruction. Cerebral cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turchi J, Saunders RC, and Mishkin M (2005). Effects of cholinergic deafferentation of the rhinal cortex on visual recognition memory in monkeys. Proceedings of the National Academy of Sciences of the United States of America 102, 2158–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin SC, Brown RE, Hussain Shuler MG, Petersen CC, and Kepecs A (2015). Optogenetic Dissection of the Basal Forebrain Neuromodulatory Control of Cortical Activation, Plasticity, and Cognition. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 13896–13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hangya B, Ranade SP, Lorenc M, and Kepecs A (2015). Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback. Cell 162, 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voytko ML (1996). Cognitive functions of the basal forebrain cholinergic system in monkeys: memory or attention? Behavioural brain research 75, 13–25. [DOI] [PubMed] [Google Scholar]
- 10.Chudasama Y, Dalley JW, Nathwani F, Bouger P, and Robbins TW (2004). Cholinergic modulation of visual attention and working memory: dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learning & memory 11, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avila I, and Lin SC (2014). Distinct neuronal populations in the basal forebrain encode motivational salience and movement. Frontiers in behavioral neuroscience 8, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raver SM, and Lin S-C (2015). Basal forebrain motivational salience signal enhances cortical processing and decision speed. Frontiers in behavioral neuroscience 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, and Dan Y (2013). Fast modulation of visual perception by basal forebrain cholinergic neurons. Nature neuroscience 16, 1857–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peck CJ, and Salzman CD (2014). The amygdala and basal forebrain as a pathway for motivationally guided attention. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 13757–13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson RT, and DeLong MR (1990). Context-dependent responses of primate nucleus basalis neurons in a go/no-go task. The Journal of neuroscience : the official journal of the Society for Neuroscience 10, 2528–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson FA, and Rolls ET (1990). Neuronal responses related to reinforcement in the primate basal forebrain. Brain research 509, 213–231. [DOI] [PubMed] [Google Scholar]
- 17.Wilson FAW, and Ma Y-Y (2004). Reinforcement-related neurons in the primate basal forebrain respond to the learned significance of task events rather than to the hedonic attributes of reward. Cognitive Brain Research 19, 74–81. [DOI] [PubMed] [Google Scholar]
- 18.Masuda R, Fukuda M, Ono T, and Endo S (1997). Neuronal Responses at the Sight of Objects in Monkey Basal Forebrain Subregions during Operant Visual Tasks. Neurobiology of Learning and Memory 67, 181–196. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda M, Masuda R, Ono T, and Tabuch E (1993). Responses of monkey basal forebrain neurons during visual discrimination task. In Progress in brain research, Volume 95 (Elsevier), pp. 359–369. [DOI] [PubMed] [Google Scholar]
- 20.Barto A, Mirolli M, and Baldassarre G (2013). Novelty or Surprise? Frontiers in psychology 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallis JD, and Rich EL (2011). Challenges of Interpreting Frontal Neurons during Value-Based Decision-Making. Frontiers in neuroscience 5, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, and Mitchell CJ (2011). Attention and relative novelty in human perceptual learning. Journal of experimental psychology. Animal behavior processes 37, 436–445. [DOI] [PubMed] [Google Scholar]
- 23.Hayden BY, Heilbronner SR, Pearson JM, and Platt ML (2011). Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 4178–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preuschoff K, t Hart BM, and Einhauser W (2011). Pupil Dilation Signals Surprise: Evidence for Noradrenaline’s Role in Decision Making. Frontiers in neuroscience 5, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SC, and Nicolelis MA (2008). Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron 59, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monosov IE, and Hikosaka O (2013). Selective and graded coding of reward uncertainty by neurons in the primate anterodorsal septal region. Nature neuroscience 16, 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White JK, and Monosov IE (2016). Neurons in the primate dorsal striatum signal the uncertainty of object–reward associations. Nature communications 7, 12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daye PM, Monosov IE, Hikosaka O, Leopold DA, and Optican LM (2013). pyElectrode: an open-source tool using structural MRI for electrode positioning and neuron mapping. Journal of neuroscience methods 213, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turchi J, Chang C, Frank QY, Russ BE, David KY, Cortes CR, Monosov IE, Duyn JH, and Leopold DA (2018). The basal forebrain regulates global resting-state fMRI fluctuations. Neuron 97, 940–952. e944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lak A, Stauffer WR, and Schultz W (2014). Dopamine prediction error responses integrate subjective value from different reward dimensions. Proceedings of the National Academy of Sciences of the United States of America 111, 2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz W (2002). Getting formal with dopamine and reward. Neuron 36, 241–263. [DOI] [PubMed] [Google Scholar]
- 32.Roesch MR, Calu DJ, Esber GR, and Schoenbaum G (2010). All that glitters… dissociating attention and outcome expectancy from prediction errors signals. Journal of neurophysiology 104, 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearce JM, and Hall G (1980). A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological review 87, 532–552. [PubMed] [Google Scholar]
- 34.Berlyne DE (1970). Novelty, complexity, and hedonic value. Perception & Psychophysics 8, 279–286. [Google Scholar]
- 35.Foley NC, Jangraw DC, Peck C, and Gottlieb J (2014). Novelty Enhances Visual Salience Independently of Reward in the Parietal Lobe. The Journal of Neuroscience 34, 7947–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiitinen H, May P, Reinikainen K, and Näätänen R (1994). Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature 372, 90. [DOI] [PubMed] [Google Scholar]
- 37.Monosov IE (2017). Anterior cingulate is a source of valence-specific information about value and uncertainty. Nature communications 8, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer T, and Olson CR (2011). Statistical learning of visual transitions in monkey inferotemporal cortex. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paton JJ, and Buonomano DV (2018). The Neural Basis of Timing: Distributed Mechanisms for Diverse Functions. Neuron 98, 687–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu R, Crawford J, Callahan PM, Terry AV Jr, Constantinidis C, and Blake DT (2017). Intermittent stimulation of the nucleus basalis of meynert improves working memory in adult monkeys. Current Biology 27, 2640–2646. e2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shuler MG, and Bear MF (2006). Reward timing in the primary visual cortex. Science 311, 1606–1609. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto M, and Hikosaka O (2009). Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris G, Arkadir D, Nevet A, Vaadia E, and Bergman H (2004). Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 43, 133–143. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi YK, Langdon AJ, Niv Y, and Schoenbaum G (2016). Temporal Specificity of Reward Prediction Errors Signaled by Putative Dopamine Neurons in Rat VTA Depends on Ventral Striatum. Neuron 91, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bromberg-Martin ES, Matsumoto M, and Hikosaka O (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto M, and Takada M (2013). Distinct Representations of Cognitive and Motivational Signals in Midbrain Dopamine Neurons. Neuron 79, 1011–1024. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi YK, Batchelor HM, Liu B, Khanna A, Morales M, and Schoenbaum G (2017). Dopamine Neurons Respond to Errors in the Prediction of Sensory Features of Expected Rewards. Neuron 95, 1395–1405. e1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babayan BM, Uchida N, and Gershman SJ (2018). Belief state representation in the dopamine system. Nature communications 9, 1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apicella P, Ravel S, Deffains M, and Legallet E (2011). The Role of Striatal Tonically Active Neurons in Reward Prediction Error Signaling during Instrumental Task Performance. The Journal of Neuroscience 31, 1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


