Abstract
Archaeal sequences have been detected in human colostrum and milk, but no studies have determined whether living archaea are present in either of these fluids. Methanogenic archaea are neglected since they are not detected by usual molecular and culture methods. By using improved DNA detection protocols and microbial culture techniques associated with antioxidants previously developed in our center, we investigated the presence of methanogenic archaea using culture and specific Methanobrevibacter smithii and Methanobrevibacter oralis real-time PCR in human colostrum and milk. M. smithii was isolated from 3 colostrum and 5 milk (day 10) samples. M. oralis was isolated from 1 milk sample. For 2 strains, the genome was sequenced, and the rhizome was similar to that of strains previously isolated from the human mouth and gut. M. smithii was detected in the colostrum or milk of 5/13 (38%) and 37/127 (29%) mothers by culture and qPCR, respectively. The different distribution of maternal body mass index according to the detection of M. smithii suggested an association with maternal metabolic phenotype. M. oralis was not detected by molecular methods. Our results suggest that breastfeeding may contribute to the vertical transmission of these microorganisms and may be essential to seed the infant’s microbiota with these neglected critical commensals from the first hour of life.
Subject terms: Archaeal genomics, Archaeal physiology
Introduction
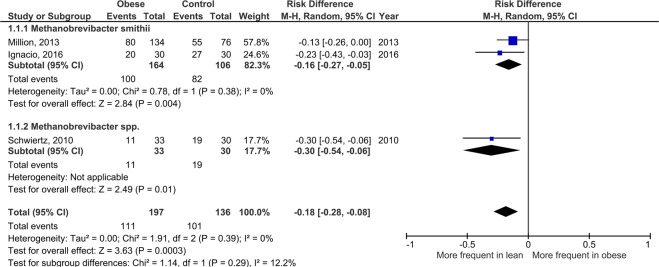
Breastfeeding is a major determinant of human health1. Breast colostrum and milk contain a very diverse bacterial microbiota that plays a key role in human health2. In this context, while archaeal sequences have been detected3,4, no studies have determined whether living archaea are present in human milk2. The human archaeome is increasingly recognized owing to dedicated molecular methods5. The genomic analysis of Methanobrevibacter smithii6, the main human methanogenic archaea, showed an evolutive adaptation to the human gut7. The emerging role of this species is notable for methanogenic archaea8, which are particularly adapted to the gut and key components of human-archaeal-bacterial mutualism through methanogenesis9. Methanogenesis improves energy harvest by consuming end products of microbial fermentation7,9. The role for human health and metabolism is supported by the fact that M. smithii was found in virtually all healthy lean human adults but was depleted in obese individuals (Fig. 1)10,11 and in those with severe acute malnutrition12.
Figure 1.
Meta-analysis comparing the frequency of Methanobrevibacter species in human feces from obese and lean individuals. The study of Million et al.52 included individuals from previous studies from the same center51,68. The study of Ignacio et al. was performed in Brazil and confirmed the decreased frequency of M. smithii in obesity61. No substantial heterogeneity was observed between the 2 studies (I2 = 0%), with a highly significant association (p = 0.004). These results were consistent with those reported by Scwhiertz et al. focusing on Methanobrevibacter species53 with very low heterogeneity (I2 = 12%) suggesting a consistent and significant (p = 0.0003) effect at the genus level. Studies with a lower taxonomical resolution did not show any consistent result. For instance, studies at the Methanobacteriales order level included M. stadtmanae15 associated with proinflammatory properties in vitro46 and in clinical studies64. These results suggest a genus-specific effect of human methanogenic Archaea on weight regulation and obesity and support that M. smithii is a neglected critical commensal for human health.
Methanogenic archaea form a co-occurrence network with other heritable Bacteria such as the family Christensenellaceae10,13, and the abundances of both are enriched in lean healthy individuals10. However, the way(s) of vertical transmission of methanogenic Archaea remain a missing piece in the puzzle of metabolic phenotype inheritance2. Indeed, while both the human genome and microbiome are inherited10, the microbiome has been found to be more informative than the human genome for predicting obesity10. In this context, we previously showed that M. smithii consistently colonizes the newborn stomach, suggesting an early gut colonization14. Accordingly, the culture of methanogenic archaea from human colostrum and milk represents an exciting challenge.
Human methanogenic archaea remain largely neglected, and their role in human health is underestimated for several reasons15. First, the culture of methanogenic archaea was, until recently, very tedious, expensive, and time-consuming. Second, metagenomics and 16S amplicon sequencing studies neglected human methanogenic Archaea due to DNA extraction bias, depth bias (majority species are more likely to be detected) and primer bias (specifically for 16S amplicon sequencing studies)5. This is supported by the fact that M. smithii is detected in 64% of human adults by 16S amplicon sequencing10 but in 96% by specific PCR6. Third, a large part of the current knowledge on the human microbiome relies on transplantation studies in germ-free mice10. Strikingly, M. smithii does not persist in mice10.
As a result, only a few studies have reported the culture of methanogenic archaea from humans (see Supplementary Tables S1 and S2)13,14,16–34. The main limitation of culture is that most human gut microbes including methanogenic archaea are extremely sensitive to oxygen and its reactive derivatives. In this context, we recently established a microbial culture technique using a patented antioxidant mixture containing high doses of ascorbic acid, glutathione35 and uric acid36–38 (see conflict of interest section). We then aerobically cultured several methanogenic archaeal strains from culture collections using a dual chamber system. All methanogenic archaea tested could be cultured29. This new antioxidant-based culture system allowed us to investigate the presence of methanogenic archaea in human colostrum and milk by culture and genome sequencing. We also used specific PCR (qPCR) as a confirmatory method and compared the characteristics of the mother and child according to the detection of M. smithii in colostrum and/or milk, identified in this study as the most prevalent methanogenic archaea in human colostrum and milk.
Results
Included mothers
A total of 128 mothers who had provided at least 1 sample (colostrum and/or milk (day 10)) were included; 138 samples including 118 colostrum and 20 milk samples were obtained and analyzed by at least one technique (culture and qPCR). The full clinical details and results are provided in Dataset 1. Among the 128 included mothers, 14 (11%) were obese before pregnancy, 33 (26%) were overweight, 74 (58%) were lean and 7 (5%) were underweight.
Culture and isolation
For the culture of methanogenic archaea, 20 samples from 13 mothers were analyzed (Table 1 and Dataset 1). The culture was positive in 9 out of 20 samples, including 3 colostrum and 6 milk samples. M. smithii was isolated from 8 samples corresponding to 5 mothers (with both colostrum and milk isolation in 3 mothers). Focusing on the 6 mothers for whom both colostrum and milk (day 10) were available, the excretion of M. smithii in colostrum was associated in all cases with excretion in milk on day 10 (3/3). In contrast, the three mothers who produced culture-negative colostrum also produced culture-negative milk. For all culture-positive samples for methanogens, colonies appeared after 9 days of incubation (Supplementary Fig. 1). M. oralis was isolated from 1 sample (milk, no colostrum available from this mother).
Table 1.
Summary of the results of the molecular and culture analyses.
| Colostrum | Milk (around day 10) | |||
|---|---|---|---|---|
| Methane production | Strain identification | Methane production | Strain identification | |
| Mother_018 | NA | NA | − | ND |
| Mother_076 | − | ND | + | M. oralis strain M2 CSUR P5920a |
| Mother_095 | + | M. smithii strain C1 CSUR P5920 | + | M. smithii strain M5 CSUR P5919 |
| Mother_096 | + | M. smithii strain C2 CSUR P5816a | + | M. smithii strain M6 CSUR P5818 |
| Mother_097 | + | M. smithii strain C3 CSUR P5922 | + | M. smithii strain M7 CSUR P5820 |
| Mother_098 | − | ND | − | NA |
| Mother_099 | − | ND | ND | ND |
| Mother_100 | − | ND | ND | ND |
| Mother_101 | − | ND | ND | ND |
| Mother_102 | − | ND | − | NA |
| Mother_103 | − | ND | − | NA |
| Mother_104 | NA | ND | + | M. smithii strain M1 CSUR P5819 |
| Mother_105 | NA | ND | + | M. smithii strain M3 CSUR P5921 |
Methane production detected by gas chromatography from a Hungate tube with SAB medium and antioxidants inoculated with maternal colostrum or milk after transport in Ae-Ana medium supplemented with antioxidants. PCR: Polymerase chain reaction detecting all archaea performed on colonies identified on Petri dishes from methane-positive samples. Strain identification was performed by 16S rRNA gene sequencing., +=positive, −=negative, ND: not done, NA: sample not available. aStrains sequenced for genome analysis.
For all 9 strains (8 M. smithii and 1 M. oralis), we determined the 16S rRNA gene sequence. For 2 strains, namely, M. smithii strain C2 CSUR P5816 (isolated from the colostrum of Mother_096) and M. oralis strain M2 CSUR P5920 (isolated from the milk of Mother_076 on day 10), the 16S rRNA gene sequence was extracted from the genome sequence (16S and genome sequences deposited in a public repository, see Data availability). For the 7 other M. smithii strains, a partial 16S rRNA gene sequence was obtained (see methods) and deposited in a public repository (see Data availability). All 9 sequences were aligned and compared with the reference sequence of the type strain for each species.
According to the list of prokaryotic names with standing in nomenclature (www.bacterio.net), the reference sequence U55233 of the type strain of M. smithii (PS = ATCC 35061 = DSM 861 = OCM 144) was used to align and compare the sequences of our 8 M. smithii strains (Table 2). The sequences of 2 strains (M2 and M6) were identical (100% identity), 3 strains (C1, C3, M3) showed 1 mismatch (99.83%) and 3 strains (M1, M5, M7) showed 2 mismatches (99.61 to 99.66%). The sequence HE654003.1 of the M. oralis type strain (DSM 7256) was used to align and compare the sequence of our M. oralis strain. A 99.39% similarity was found with 9 mismatches (1462/1471) and 3 gaps (3/1471 (0.20%)).
Table 2.
Alignment of the 16S rRNA gene sequences of 8 M. smithii strains isolated from human colostrum and milk with the reference sequence of the type strain M. smithii PS (=ATCC 35061 = DSM 861 = OCM 144) - Sequence Accession No. U55233.
| Strain | Origin | Sequence number | Length | Identities | Gaps |
|---|---|---|---|---|---|
| C2 CSUR P5816 | Colostrum | LR590664 | 1472 bp | 1343/1343 (100%) | 0/1343 (0%) |
| M6 CSUR P5818 | Milk (day 10) | LR584037 | 592 bp | 592/592 (100%) | 0/592 (0%) |
| M3 CSUR P5921 | Milk (day 10) | LR584041 | 608 bp | 604/605 (99.83%) | 1/605 (0.16%) |
| C1 CSUR P5920 | Colostrum | LR584035 | 604 bp | 603/604 (99.83%) | 0/604 (0%) |
| C3 CSUR P5922 | Colostrum | LR584038 | 594 bp | 593/594 (99.83%) | 1/594 (0.17%) |
| M5 CSUR P5919 | Milk (day 10) | LR584040 | 607 bp | 603/605 (99.66%) | 2/605 (0.33%) |
| M7 CSUR P5820 | Milk (day 10) | LR584039 | 597 bp | 595/597 (99.66%) | 2/597 (0.33%) |
| M1 CSUR P5819 | Milk (day 10) | LR584036 | 517 bp | 516/518 (99.61%) | 2/518 (0.38%) |
Bp: base pairs.
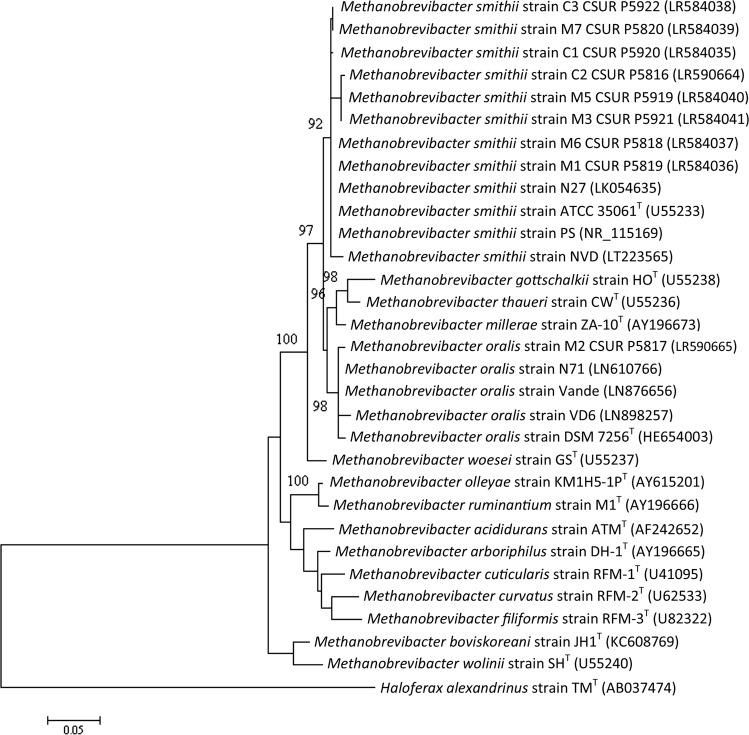
Phylogenetic analysis of the 16S rRNA gene sequence confirmed that all isolated M. smithii strains grouped together with the type strain and strains previously described and did not form a distinct cluster (Fig. 2). This result suggests that the methanogenic archaeal strains of human colostrum and milk are not different from strains previously isolated, notably from the human gut.
Figure 2.
Molecular phylogenetic analysis by maximum likelihood method of the new isolates and their closest species. Bootstrap values ≥90% indicated at nodes. The evolutionary history was inferred by using the maximum likelihood method based on the Kimura 2-parameter model69. The tree with the highest log likelihood (−3941.91) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. The initial tree for the heuristic search was obtained automatically by applying the maximum parsimony method. A discrete gamma distribution was used to model the evolutionary rate differences among sites (5 categories (+G, parameter = 0.3801)). The tree is drawn to scale, with branch lengths measured according to the number of substitutions per site. The analysis involved 18 nucleotide sequences. There were a total of 1490 positions in the final dataset. Evolutionary analyses were conducted in MEGA770.
Genome sequencing
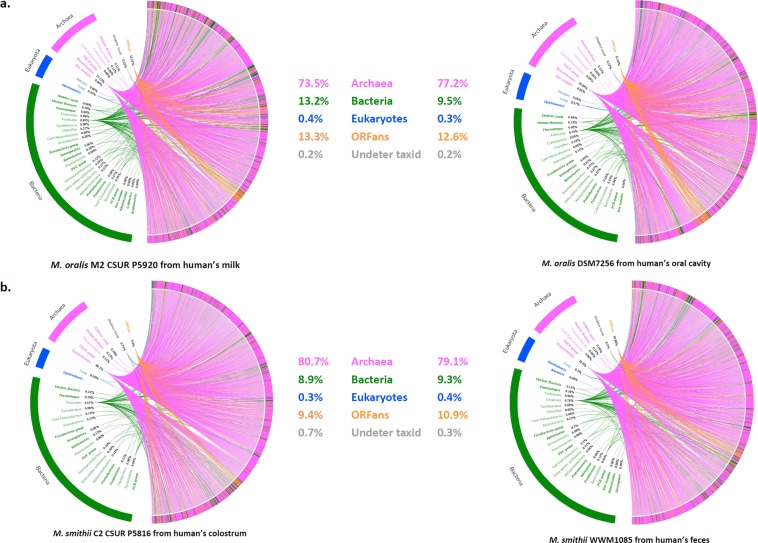
For two strains, M. smithii strain C2 (colostrum) and M. oralis strain M2 (milk, Table 1), the genome was sequenced and analyzed. To clarify whether these strains were similar to the human digestive (M. smithii) or oral (M. oralis) strains, we analyzed the rhizome as previously described39. Rhizome analysis evaluates possible sequence exchanges and their phylogenetic origin. The hypothesis is that strains living in the same microbial environment (human microbiota) share the same lateral sequence exchange profile as phylogenetic groups present in the same ecological niche. Rhizome analysis by visual examination of the global pattern of sequences shared with other prokaryotic species showed that these strains’ profiles were similar to those of M. oralis and M. smithii strains previously isolated from the human mouth and intestine (Fig. 3).
Figure 3.
Comparison of rhizomes of Methanobrevibacter smithii and Methanobrevibacter oralis isolated from human milk with archaea previously described in the digestive tract (M. smithii) and mouth (M. oralis). M. smithii strain C2 CSUR P5816 was isolated from the colostrum of mother_2 (Table 1). Its genome (GenBank number: SAMEA104570327) was compared to the genome of a strain isolated from human feces (=WWM1085, GenBank Number: NQLD000000000000). M. oralis strain M2 CSUR was isolated from the milk (day 10 after delivery) of mother_11. Its genome P5920 (GenBank Number: SAMEA10457076) was compared to the genome of the type strain of M. oralis strain ZR (Genome Number: NZ_LWMU00000000.1) isolated from the human oral cavity (=DSM7256, =JCM 30027).
Detection of M. smithii and M. oralis using real-time PCR (rt-PCR)
We performed specific M. smithii and M. oralis real-time PCR for 127 of the 128 healthy mothers included in the study. In total, 136 samples collected from these 127 mothers were analyzed by real-time PCR and included 117 colostrum and 19 milk samples. Thirty-two of 117 (27.3%) colostrum and 5/19 (26.3%) milk samples were positive for M. smithii, totaling 37 positive samples from 136 total samples (27.2%). Among the 37 positive samples, the cycle threshold was relatively high (median 38.40, interquartile range [36.75–40.00], range 31.50–40.80). Using calibration curves, we estimated the concentration and found that the abundance was very low (median, 463 copies DNA/mL for colostrum, 339 copies DNA/mL for milk at day 10, Supplementary Fig. 2). Among the 127 mothers included in this analysis, 37 were positive for M. smithii in colostrum and/or milk (29.1%). M. oralis-specific real-time PCR was negative on all 136 tested samples (127 mothers).
Comparison of clinical characteristics of mothers and newborns according to the detection of M. smithii in colostrum and/or milk
Because only two species were found by culture and M. oralis was detected solely by culture in only one milk sample, we focused on M. smithii to uncover associations between the detection of this species and clinical variables. Among the 128 mothers included in the study, 40 (31%) were positive for M. smithii by culture and/or qPCR. This result suggests that, as previously reported in the gut6,10, M. smithii is the most prevalent methanogenic archaea in human colostrum and milk.
The frequency of the detection of M. smithii by culture and/or PCR was lower in obese (14%) mothers than in overweight (45%), lean (28%) or underweight (28%) mothers, suggesting an association with maternal metabolic phenotype. The difference between obese (2/14 (14%)) and nonobese mothers (38/114 (33%)) was substantial but not significant (Odds ratio 0.33, 95% confidence interval 0.049–1.41, p = 0.08). Conversely, M. smithii was more frequent in overweight (45%) than in lean (28%) mothers (Odds ratio 2.09, 95% confidence interval 0.88–4.95, p = 0.047). No other clinical variables appeared to be associated with the detection of M. smithii in the colostrum or milk (Supplementary Table 3).
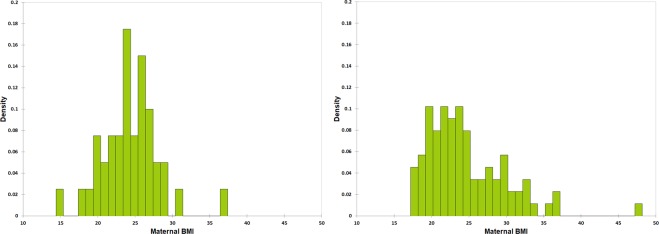
Focusing on the distribution of pre-pregnancy maternal BMI according to the detection of M. smithii in colostrum and milk (Fig. 4), we observed that the median of the two groups were similar (24.0 and 23.3 for mother with or without detection of M. smithii). However, observation of the plots and Kolmogorov-Smirnov test (KS test) suggested that the distribution of BMI values was normal in mothers with detection of M. smithii (KS test p-value > 0.10) but not in mothers without detection of M. smithii (p < 0.0001). The variances were significantly different (Levene’s test, p = 0.049). Skewness was higher in mothers without detection of M. smithii (1.38 versus 0.50 in mothers with detection of M. smithii).
Figure 4.
Distribution of maternal BMI values according to the detection of M. smithii in colostrum and/or milk. Density histograms of pre-pregnancy maternal BMI values for mothers with (left) or without (right) detection of M. smithii in colostrum and/or milk.
Discussion
Here, we have shown that methanogenic archaea are alive in colostrum and human milk. Two species have been identified, namely, M. smithii and M. oralis, and their presence was demonstrated by culture and confirmed by genome sequencing. Approximately one-third of the mothers harbored M. smithii in their colostrum and/or milk. Our results are consistent with early neonatal digestive colonization6,14,40–42 and the association between gut colonization and organic dairy consumption in children43.
The very low median abundance of M. smithii observed in this study (2 log 10 copies DNA/mL) suggests that minority microbes are important in the human microbiome, particularly in human colostrum and milk. We previously described this as the “minority paradigm44”. Moreover, primer bias is another critical limitation of the 16S amplicon sequencing in deciphering the human archaeome5,45.
Human methanogenic archaea are critical for human health. The 3 main steps of human digestion are hydrolysis, fermentation and methanogenesis. Fermentation by gut anaerobic bacteria yields short-chain fatty acids (mainly acetate, propionate and butyrate), formate, ethanol, and lactic acid but particularly dihydrogen (H2) and carbon dioxide (CO2). However, according to the Le Chatelier principle, formate and hydrogen consumption by methanogenesis is critical to accelerate the production of ATP and short-chain fatty acids, thus increasing the efficiency of the energy harvest for the host9,13. According to current scientific knowledge, no other microbes or human cells in the human gut are able to replace archaea for methanogenesis, so these symbionts can be considered critical for human health15. The very low H2-utilization threshold of M. smithii compared to that of acetogens makes it more efficient at depleting H2 from the gut environment13.
M. smithii is the dominant human gut-associated archaeon13, is almost ubiquitous in healthy adults6, is stable over time during life18, is associated with human microbiome diversity and high gene count10,11, and harbors specific features that suggest its coevolution as a commensal13, with specific interactions with host mucosal immunity46. In addition, increasing evidence has been reported for innate and adaptative immune recognition and activation by human-associated archaea, notably by archaeosomes15. Association with the absence of obesity (Fig. 1)10,11 further suggests that M. smithii is a critical commensal for human health7.
Only a few teams in the world have reported the successful culture of human archaea in the literature (Supplementary Tables S1 and S2). Working on microbiota and malnutrition12,47–49, we recently discovered the critical role of 3 major nonenzymatic human plasmatic antioxidants (ascorbate, glutathione, uric acid) in the culture of anaerobes and Archaea29,35,37. Strikingly, these 3 molecules are also the 3 major nonenzymatic antioxidants in human colostrum and milk50. This finding is not random and suggests that human methanogenic archaea are dependent on the concentration of these antioxidants in the gut. Further studies are needed to confirm this, but this discovery has been decisive in the successful culture of several archaea from the human microbiome29.
We have previously shown that M. smithii was associated with the absence of obesity51 and malnutrition12. In the literature, there has been a general confusion in the association between methanogenic archaea and obesity15. Inconclusive or contradictory results are obtained when different taxonomic levels (M. smithii51–53, Methanobacteriales54,55, Archaea56), breath methane production56–58, and overweight and obese individuals59 are evaluated together. We previously clarified this issue by a meta-analysis including only comparisons of the frequency of detection of Methanobrevibacter species between obese individuals and controls60. As a new study has been published61, we performed this meta-analysis again and confirmed the association between a decreased frequency of M. smithii and Methanobrevibacter species in obesity (Fig. 1). This consistent effect was not observed with lower taxonomic levels (Methanobacteriales, Archaea) and with breath methane production. Accordingly, a genus- and/or species-specific effect of M. smithii on weight regulation is likely, as previously reported for Lactobacillus51,52,62,63. At least two large-scale metagenomics and 16S amplicon sequencing studies corroborate the association of M. smithii with the lean phenotype10,11.
Methanosphaera stadtmanae is a representative of another human methanogenic archaeal genus and a member of the Methanobacteriales order. M. stadtmanae was associated with different roles in human health, notably with a severe proinflammatory response (TNF-α, IL-1β) not observed for M. smithii15. This species, which was less frequent than M. smithii in the human gut (approximately 30% versus >95%6), was not detected in human colostrum and milk in our study. Moreover, a 3-fold increase in the abundance of M. stadtmanae was reported in inflammatory bowel diseases (IBD) compared to that of controls, whereas M. smithii’s abundance did not differ64. This species could explain the discrepancy between studies including only Methanobrevibacter species and those including larger taxonomical groups (Methanobacteriales order, Archaea) and physiological studies analyzing breath methane. Overall, the role of M. smithii in the commensal human gut microbiota appears to be the result of evolutionary coadaptation of the human host and this archaeal species7,13,15. This is not the case for M. stadtmanae associated with in vitro proinflammatory properties46 and inflammatory bowel diseases64.
Here, we found that M. smithii was detectable in 33% of nonobese mothers. M. smithii was detected less frequently (14%) in colostrum or milk of obese mothers. The difference was not significant. However, a two-fold difference (33 versus 14%) and an Odds ratio of 0.3 should not be neglected even if the p-value is 0.0865, which is consistent with the association between M. smithii in feces and absence of obesity (Fig. 1). Strikingly, M. smithii was detected more frequently in overweight versus lean mothers. Finally, the normal distribution of maternal BMI observed only when M. smithii was detected suggested a role of M. smithii for weight regulation (Fig. 4). To clarify if detection of M. smithii in colostrum and/or milk is associated with absence of maternal obesity, we calculated that at least 88 cases and 88 controls should be included in a future study to rule out a putative association with a two-sided confidence level 95% and power of 80%.
In conclusion, we showed that human colostrum and milk contain viable M. smithii and M. oralis. This supports a key role for the early initiation of breastfeeding and sheds light on the mechanisms underlying the inheritance of methanogenic archaea, specifically M. smithii, a neglected critical commensal highly suspected to be involved in weight regulation.
Methods
Patients and samples
Healthy mothers aged over 18 years of age with a full-term pregnancy who had opted for mixed or exclusive breastfeeding were selected to participate in this study. Breast colostrum and milk samples were collected from healthy women at the second and tenth day postpartum, respectively. The sampling was performed by a pediatrician. Before sampling, the pediatrician washed his or her hands with alcoholic solution and then put gloves on before touching the sampling site. Nipples and areolas were not cleaned. The samples (250–1000 µL) were collected in sterile tubes by manual pressing at the neonatology unit of the Hôpital de la Conception and Hôpital Nord, Marseille, France. The main inclusion criteria of this study were breastfeeding and acceptance of participation in the study protocol. The main criteria for exclusion were exclusively artificial breastfeeding, refusal of participation and the presence of a disorder (mastitis or breast abscess) that may have an impact on the microbiota of the study subject. The data collected from the included mothers were recruitment center, age, weight before pregnancy, height (body mass index before pregnancy was calculated), gestity, parity, gestational diabetes, and tobacco smoking. For pregnancy, we collected gestational age, preterm delivery, delivery route (vaginal or C-section) and twin pregnancy. For newborns, we collected sex, birth weight, birth height, head circumference, exclusive or mixed breastfeeding. Body mass index, weight-for-length z-score, weight-for-age z-score, length-for-age z-score, BMI-for-age z-score, and head-circumference-for-age z-score were calculated using the software WHO Anthro (version 3.2.2, January 2011) available online (https://www.who.int/childgrowth/software/en/). All participants were informed and gave their written consent before the samples were collected. The ethics committee of the “Institut Fédératif de Pathologies Transmissibles et Pathologies Infectieuses Tropicales 48” approved the consent and study protocol under number 2016–004. The authors certified that this study was not in opposition to the Declaration of Helsinki and was in accordance with French laws (certificates available on request).
Culture and isolation
A volume of 250 µL of breast milk or colostrum sample was seeded in ambient air in a sterile Hungate tube (Dominique Dutscher, Brumath, France). The culture and isolation of methanogenic archaea were performed according to the previously published protocol29 under aerobic conditions using coculture with Bacteroides thetaiotaomicron. Each Hungate tube contained 5 mL of SAB broth supplemented with ascorbic acid (1 g/L; VWR International, Leuven, Belgium), uric acid (0.1 g/L) and glutathione (0.1 g/L; Sigma-Aldrich, Saint-Quentin Fallavier, France). The pH of the culture media was adjusted to 7.5 with KOH (10 M). Five milliliters of SAB medium and 250 µL of milk or colostrum were inoculated with B. thetaiotaomicron (105 cells/mL) for hydrogen production at 37 °C with agitation for seven days. The growth of all methanogens was inferred from the production of methane (CH4) detected by gas chromatography, as previously described66. Subcultures were seeded on a Petri dish containing SAB medium supplemented with 15 g/L agar and deposited in the upper chamber of a double chamber. A tube containing noninoculated SAB medium was used as a negative control. For solid medium culture, a noninoculated agar Petri dish was used as a negative control.
DNA extraction and 16S rRNA gene sequencing for colony identification
The identification of colonies obtained with the method described above was confirmed by the following DNA extraction and sequencing protocol. DNA was extracted using the E.Z.N.A.® Tissue DNA Kit (OMEGA Bio-tek, Norcross, GA, USA) according to the manufacturer’s instructions and the modified extraction protocol described by Dridi et al.6. PCR was performed with a PTC-200 automated thermal cycler (MJ Research, Waltham, USA) in 50 µL of PCR mixture. The archaeal 16S rRNA gene was amplified using a 40-cycle program with the archaeal primers SDArch0333aS15 (50-TCCAGGCCCTACGGG-30) and SDArch0958aA19 (5′-YCCGGCGTTGAMTCCAATT-3′) (Eurogentec, Seraing, Belgium). PCR products were purified and sequenced using a 3500xL genetic analyzer (ThermoFisher, Waltham, MA USA) and a Big-Dye Terminator, version 1.1, cycle sequencing kit DNA according to the manufacturer’s instructions (Applied Biosystems, Foster City, USA). The chromas Pro1.34 software (Technelysium Pty. Ltd) was used for sequence correction. BLASTn (nucleotide Basic Local Alignment Search Tool) searches were performed against GenBank (http://blast.ncbi.nlm.nih.gov.gate1.inist.fr/Blast.cgi) for taxonomic assignment. Two negative control samples consisting of master mix and RNase-free water were introduced for every 5 samples tested.
Genome sequencing
Genomic DNA of the isolates C2 CSUR P5816 and M2 CSUR P5920 cultured from colostrum and milk, respectively, were sequenced using MiSeq Technology (Illumina, Inc., San Diego CA 92121, USA) with a paired-end and barcode strategy with 15 other projects constructed according to the Nextera XT library kit (Illumina). The gDNA was quantified by a Qubit assay with a high-sensitivity kit (Life Technologies, Carlsbad, CA, USA) to 0.8 ng/µL and diluted to require one ng of DNA as input. The “tagmentation” step fragmented the genomic DNA. Then, PCR cycle amplification completed the tag adapters and introduced the dual-index barcodes. After purification on AMPure beads (Life Technologies), the libraries were normalized on specific beads according to the Nextera XT protocol (Illumina). Normalized libraries were pooled into a single library for sequencing via MiSeq. A pooled single-strand library was loaded onto the reagent cartridge and then onto the instrument along with the flow cell. We performed automated cluster generation and paired-end sequencing with dual-index reads in a single 39-hour run in 2 × 251-bp. We obtained total information with an 8.5 gigabase sequence with an 899 K/mm2 cluster density, with 94.9% (16,382,000 clusters) of the clusters passing quality control filters. Within this pooled run, the index representation of the isolate was determined to be 13.50%. The 2,212,330 paired-end reads were filtered according to the read qualities.
Genome assembly and construction of the rhizome
The Illumina reads obtained for both strains were assembled using SPAdes software (http://bioinf.spbau.ru/spades) helped by GapFiller v2.1.1 35 to reduce the set. Subsequently, the assembly was refined using manual finishing. BLASTp was performed on all translated coding sequences using the nr database. For each coding DNA sequence, the best BLAST hit was determined from the max bit score. Only all hits related to the Methanobrevibacter genus after data filtering were considered ORFans. The origins of all genes of the two strains C2 CSUR P5816 (M. smithii) and M2 CSUR P5920 (M. oralis) were determined according to their taxonomic affiliation. The rhizome representation was created using Circos software 36 for both strains (M. oralis M2 CSUR P5920 and M. smithii C2 CSUR P5816), as well as the M. oralis strain MBORA DSM 7256 isolated from the human mouth23 and M. smithii strain WWM1085 isolated from the human gut24.
Detection of M. smithii and M. oralis using qPCR
Following the culture and characterization of methanogenic archaeal strains obtained after direct inoculation of fresh samples from 13 mothers, we sought to estimate the proportion of archaea in the colostrum and/or milk of the whole cohort by real-time PCR. DNA was extracted using the E.Z.N.A.® Tissue DNA Kit (OMEGA Bio-tek) according to the manufacturer’s instructions and the modified extraction protocol described by Dridi et al.6. Real-time PCR assays were performed with an MX3000TM system (Stratagene, Amsterdam, The Netherlands) using the QuantiTect Probe PCR Kit (Qiagen, Courtaboeuf, France) with 5 pmol of each primer, a probe labeled with FAM, and 5 mL of DNA in a final volume of 25 mL. The PCR amplification program for M. smithii was 95 °C for 15 min, followed by 42 cycles of 95 °C for 30 s and 60 °C for 1 min, and that for M. oralis amplification was 95 °C for 15 min, followed by 42 cycles of 95 °C for 10 s, 60 °C for 45 s and 45 °C for 30 s as previously described6,67. The primers and probes used for M. oralis and M. smithii amplification were as follows: M. oralis: M-cnp602F 5′-GCTGGTGTAATCGAACCTAAACG-3′, cnp602R 5′-CACCCATACCCGGATCCATA-3′ FAM 5′-AGCAGTGCACCTGCTGATATGGAAGG-3′; M. smithii: Smit.16S-740F, 5′-CCGGGTATCTAATCCGGTTC-3′, Smit.16S-862R, 5′-CTCCCAGGGTAGAGGTGAAA-3′, Smit.16S FAM 5′-CCGTCAGAATCGTTCCAGTCAG-3′. We used calibration curves as previously described6,67. Two negative control samples consisting of master mix and RNase-free water were introduced for every 5 samples tested.
Metabolic variables and groups
As we previously associated human methanogenic archaea and specifically M. smithii with the absence of obesity (Fig. 1), we sought to test whether this association was found in the present study. A body mass index (BMI) categorical variable was defined as follows: underweight (BMI <18.5), lean (normal weight, BMI ≥18.5 and ≤25), overweight (BMI >25 and <30) and mothers who met obesity criteria (BMI ≥30).
Statistical analysis
We compared the clinical characteristics of mothers and newborns according to the detection of M. smithii in colostrum and/or milk. When comparing newborn characteristics, twins were excluded. Quantitative variables were analyzed using the unpaired t-test or Mann-Whitney test according to the distribution of the data. Qualitative variables were analyzed using the two-sided Fisher or mid-p exact test. The test used to test the previously reported association between the depletion of M. smithii and obesity (Fig. 1) was unilateral. A p-value < 0.05 was considered significant. Comparison of the distribution of maternal BMI values between mothers with or without detection of M. smithii was performed by observation of density histograms, Kolmogorov-Smirnov normality test, Levene’s test and measure of skewness. GraphPad Prism v8.1.1 (GraphPad software, San Diego, CA USA) and XLSTAT 2019.1.2 (Addinsoft, Paris, France) were used for statistical analysis.
Accession codes
The genome and 16S sequences of M. smithii strain C2 CSUR P5816 were deposited in EMBL-EBI under the accession numbers CAABOX 000000000 and LR590664, respectively. The genome and 16S sequences of M. oralis strain M2 CSUR P5920 were deposited in EMBL-EBI under the accession numbers OKQL00000000 and LR590665, respectively. The partial 16S rRNA gene sequences of the 7 other M. smithii strains C1 CSUR P5920, M1 CSUR P5819, M6 CSUR P5818, C3 CSUR P5922, M7 CSUR P5820, M5 CSUR P5919 and M3 CSUR P5921 were deposited under the Bioproject PRJEB32060 and numbered from LR584035 to LR584041.
Supplementary information
Acknowledgements
The authors thank the genomics platform for the genomic sequencing. This work has received financial support from the French Government through the Agence Nationale pour la Recherche (ANR), including the “Programme d’Investissement d’Avenir” under the reference Méditerranée Infection 10-IAHU-03.
Author contributions
A.H.T. and M.M. wrote the manuscript. A.H.T., G.G., S.K. and M.B. cultivated the archaea and carried out the PCRs. C.D.R. and V.B. recruited the mothers and collected the samples. A.C., E.B. and A.L. performed the bioinformatics analyses. M.D. and D.R. supervised the study.
Competing Interests
S.K., M.D. and D.R. are coinventors of a patent Ref. No. 1H52437 cas 32fr on the use of the three antioxidants herein reported to cultivate anaerobic bacteria and methanogenic archaea aerobically. A.H.T., M.M., G.G., M.B., C.D.R., V.B., A.C., E.B. and A.L. declare no potential conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Amadou Hamidou Togo, Ghiles Grine and Matthieu Million.
Supplementary information
is available for this paper at 10.1038/s41598-019-54759-x.
References
- 1.Victora CG, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 2.Togo A, et al. Repertoire of human breast and milk microbiota: a systematic review. Future Microbiol. 2019;14:623–641. doi: 10.2217/fmb-2018-0317. [DOI] [PubMed] [Google Scholar]
- 3.Ward TL, Hosid S, Ioshikhes I, Altosaar I. Human milk metagenome: a functional capacity analysis. BMC Microbiol. 2013;13:116. doi: 10.1186/1471-2180-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiménez, E. et al. Metagenomic analysis of milk of healthy and mastitis-suffering women. 31, 406–415 (2015). [DOI] [PubMed]
- 5.Pausan, M. R. et al. Exploring the archaeome: detection of archaeal signatures in the human body. bioRxiv 334748, 10.1101/334748 (2019). [DOI] [PMC free article] [PubMed]
- 6.Dridi B, Henry M, El Khéchine A, Raoult D, Drancourt M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS One. 2009;4:e7063. doi: 10.1371/journal.pone.0007063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuel BS, et al. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proceedings of the National Academy of Sciences. 2007;104:10643–10648. doi: 10.1073/pnas.0704189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol. 2008;6:579–591. doi: 10.1038/nrmicro1931. [DOI] [PubMed] [Google Scholar]
- 9.Samuel, B. S. & Gordon, J. I. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proceedings of the National Academy of Sciences103, 10011–10016 (2006). [DOI] [PMC free article] [PubMed]
- 10.Goodrich JK, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Chatelier E, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 12.Million M, et al. Increased gut redox and depletion of anaerobic and methanogenic prokaryotes in severe acute malnutrition. Scientific Reports. 2016;6:26051. doi: 10.1038/srep26051. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Hansen EE, et al. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl 1):4599–4606. doi: 10.1073/pnas.1000071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grine G, Boualam MA, Drancourt M. Methanobrevibacter smithii, a methanogen consistently colonising the newborn stomach. European Journal of Clinical Microbiology & Infectious Diseases. 2017;36:2449–2455. doi: 10.1007/s10096-017-3084-7. [DOI] [PubMed] [Google Scholar]
- 15.Bang C, Schmitz RA. Archaea associated with human surfaces: not to be underestimated. FEMS Microbiol. Rev. 2015;39:631–648. doi: 10.1093/femsre/fuv010. [DOI] [PubMed] [Google Scholar]
- 16.Miller TL, Weaver GA, Wolin MJ. Methanogens and anaerobes in a colon segment isolated from the normal fecal stream. Appl Environ Microbiol. 1984;48:449–450. doi: 10.1128/aem.48.2.449-450.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller TL, Wolin MJ. Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch. Microbiol. 1985;141:116–122. doi: 10.1007/BF00423270. [DOI] [PubMed] [Google Scholar]
- 18.Miller TL, Wolin MJ. Stability of Methanobrevibacter smithii populations in the microbial flora excreted from the human large bowel. Appl Environ Microbiol. 1983;45:317–318. doi: 10.1128/aem.45.1.317-318.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller TL, Wolin MJ. Enumeration of Methanobrevibacter smithii in human feces. Arch Microbiol. 1982;131:14–18. doi: 10.1007/BF00451492. [DOI] [PubMed] [Google Scholar]
- 20.Miller TL, Wolin MJ. Oxidation of hydrogen and reduction of methanol to methane is the sole energy source for a methanogen isolated from human feces. J Bacteriol. 1983;153:1051–1055. doi: 10.1128/jb.153.2.1051-1055.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller TL, Wolin MJ, Conway de Macario E, Macario AJ. Isolation of Methanobrevibacter smithii from human feces. Applied and Environmental Microbiology. 1982;43:227–232. doi: 10.1128/aem.43.1.227-232.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver GA, Krause JA, Miller TL, Wolin MJ. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986;27:698–704. doi: 10.1136/gut.27.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari A, Brusa T, Rutili A, Canzi E, Biavati B. Isolation and characterization of Methanobrevibacter oralis sp. nov. Current Microbiology. 1994;29:6. doi: 10.1007/BF01570184. [DOI] [Google Scholar]
- 24.Jennings ME, Chia N, Boardman LA, Metcalf WW. Draft genome sequence of Methanobrevibacter smithii Isolate WWM1085, obtained from a human stool sample. Genome Announc. 2017;5:e01055–17. doi: 10.1128/genomeA.01055-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dridi B, Fardeau M-L, Ollivier B, Raoult D, Drancourt M. Methanomassiliicoccus luminyensis gen. nov., sp. nov., a methanogenic archaeon isolated from human faeces. International Journal of Systematic and Evolutionary Microbiology. 2012;62:1902–1907. doi: 10.1099/ijs.0.033712-0. [DOI] [PubMed] [Google Scholar]
- 26.Huynh HT, Pignoly M, Nkamga VD, Drancourt M, Aboudharam G. The repertoire of archaea cultivated from severe periodontitis. PLoS One. 2015;10:e0121565. doi: 10.1371/journal.pone.0121565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khelaifia S, Raoult D, Drancourt M. A versatile medium for cultivating methanogenic Archaea. PLoS One. 2013;8:e61563. doi: 10.1371/journal.pone.0061563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khelaifia S, Garibal M, Robert C, Raoult D, Drancourt M. Draft genome sequence of a human-associated isolate of Methanobrevibacter arboriphilicus, the lowest-G + C-content Archaeon. Genome Announc. 2014;2:e01181–13. doi: 10.1128/genomeA.01181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khelaifia S, et al. Aerobic culture of methanogenic archaea without an external source of hydrogen. European Journal of Clinical Microbiology & Infectious Diseases. 2016;35:985–991. doi: 10.1007/s10096-016-2627-7. [DOI] [PubMed] [Google Scholar]
- 30.Nkamga VD, Lotte R, Roger PM, Drancourt M, Ruimy R. Methanobrevibacter smithii and Bacteroides thetaiotaomicron cultivated from a chronic paravertebral muscle abscess. Clinical microbiology and infection. 2016;22:1008–1009. doi: 10.1016/j.cmi.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Huynh HTT, Pignoly M, Drancourt M, Aboudharam G. A new methanogen “Methanobrevibacter massiliense” isolated in a case of severe periodontitis. BMC Res Notes. 2017;10:657. doi: 10.1186/s13104-017-2980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drancourt M, et al. Evidence of archaeal methanogens in brain abscess. Clin Infect Dis. 2017;65:1–5. doi: 10.1093/cid/cix286. [DOI] [PubMed] [Google Scholar]
- 33.Grine G, et al. Tobacco-smoking-related prevalence of methanogens in the oral fluid microbiota. Sci Rep. 2018;8:9197. doi: 10.1038/s41598-018-27372-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Seck EH, et al. Salt in stools is associated with obesity, gut halophilic microbiota and Akkermansia muciniphila depletion in humans. Int J Obes (Lond) 2019;43:862–871. doi: 10.1038/s41366-018-0201-3. [DOI] [PubMed] [Google Scholar]
- 35.La Scola B, Khelaifia S, Lagier JC, Raoult D. Aerobic culture of anaerobic bacteria using antioxidants: a preliminary report. Eur J Clin Microbiol Infect Dis. 2014;33:1781–1783. doi: 10.1007/s10096-014-2137-4. [DOI] [PubMed] [Google Scholar]
- 36.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dione N, Khelaifia S, La Scola B, Lagier JC, Raoult D. A quasi-universal medium to break the aerobic/anaerobic bacterial culture dichotomy in clinical microbiology. Clin Microbiol Infect. 2016;22:53–58. doi: 10.1016/j.cmi.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 38.Peden DB, et al. Uric acid is a major antioxidant in human nasal airway secretions. Proc Natl Acad Sci USA. 1990;87:7638–7642. doi: 10.1073/pnas.87.19.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levasseur A, et al. The rhizome of Lokiarchaeota illustrates the mosaicity of Archaeal genomes. Genome Biol Evol. 2017;9:2635–2639. doi: 10.1093/gbe/evx208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS biology. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koenig JE, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wampach L, et al. Colonization and succession within the human gut microbiome by Archaea, Bacteria, and Microeukaryotes during the First Year of Life. Front Microbiol. 2017;8:738. doi: 10.3389/fmicb.2017.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Pol JA, et al. Gut colonization by methanogenic Archaea is associated with organic dairy consumption in Children. Front Microbiol. 2017;8:355. doi: 10.3389/fmicb.2017.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagier JC, et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin. Microbiol. Infect. 2012;18:1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 45.Koskinen K, et al. First insights into the diverse human Archaeome: specific detection of Archaea in the gastrointestinal tract, lung, and nose and on skin. mBio. 2017;8:e00824–17. doi: 10.1128/mBio.00824-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bang C, Weidenbach K, Gutsmann T, Heine H, Schmitz RA. The intestinal archaea Methanosphaera stadtmanae and Methanobrevibacter smithii activate human dendritic cells. PloS one. 2014;9:e99411. doi: 10.1371/journal.pone.0099411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Million M, Diallo A, Raoult D. Gut microbiota and malnutrition. Microb. Pathog. 2017;106:127–138. doi: 10.1016/j.micpath.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Pham TP, et al. Gut microbiota alteration is characterized by a Proteobacteria and Fusobacteria bloom in kwashiorkor and a bacteroidetes paucity in marasmus. Sci Rep. 2019;9:9084. doi: 10.1038/s41598-019-45611-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Tidjani Alou M, et al. Gut bacteria missing in severe acute malnutrition, can we identify potential probiotics by culturomics? Front Microbiol. 2017;8:899. doi: 10.3389/fmicb.2017.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marinkovic V, et al. Antioxidative activity of colostrum and human milk: effects of pasteurization and storage. J Ped Gastroenterol. Nutrition. 2016;62:901–906. doi: 10.1097/MPG.0000000000001090. [DOI] [PubMed] [Google Scholar]
- 51.Million M, et al. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes (Lond) 2012;36:817–825. doi: 10.1038/ijo.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Million M, et al. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes (Lond) 2013;37:1460–1466. doi: 10.1038/ijo.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Schwiertz A, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee HS, et al. Associations among organochlorine pesticides, Methanobacteriales, and obesity in Korean women. PLoS One. 2011;6:e27773. doi: 10.1371/journal.pone.0027773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandes J, et al. Age, dietary fiber, breath methane, and fecal short chain fatty acids are interrelated in Archaea-positive humans. J Nutr. 2013;143:1269–1275. doi: 10.3945/jn.112.170894. [DOI] [PubMed] [Google Scholar]
- 57.Basseri RJ, et al. Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol Hepatol. 2012;8:22–28. [PMC free article] [PubMed] [Google Scholar]
- 58.Mathur R, et al. Methane and hydrogen positivity on breath test is associated with greater body mass index and body fat. J Clinical Endocrinol Metabol. 2013;98:E698–702. doi: 10.1210/jc.2012-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mbakwa CA, et al. Gut colonization with Methanobrevibacter smithii is associated with childhood weight development. Obesity (Silver Spring, Md) 2015;23:2508–2516. doi: 10.1002/oby.21266. [DOI] [PubMed] [Google Scholar]
- 60.Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012;7:91–109. doi: 10.2217/fmb.11.142. [DOI] [PubMed] [Google Scholar]
- 61.Ignacio A, et al. Correlation between body mass index and faecal microbiota from children. Clinical Microbiol Infect. 2016;22:258 e251–258. doi: 10.1016/j.cmi.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 62.Million M, et al. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53:100–108. doi: 10.1016/j.micpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Million M, Raoult D. Species and strain specificity of Lactobacillus probiotics effect on weight regulation. Microb Pathog. 2013;55:52–54. doi: 10.1016/j.micpath.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 64.Blais Lecours P, et al. Increased prevalence of Methanosphaera stadtmanae in inflammatory bowel diseases. PLoS One. 2014;9:e87734. doi: 10.1371/journal.pone.0087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stang A, Poole C, Kuss O. The ongoing tyranny of statistical significance testing in biomedical research. Eur J Epidemiol. 2010;25:225–230. doi: 10.1007/s10654-010-9440-x. [DOI] [PubMed] [Google Scholar]
- 66.Nkamga, V. D. & Drancourt, M. Methanomassiliicoccus in Bergey’s Manual of Systematics of Archaea and Bacteria (eds Whitman, W. B. et al.) 1–7 (John Wiley & Sons, Ltd, 2016).
- 67.Bringuier A, Khelaifia S, Richet H, Aboudharam G, Drancourt M. Real-Time PCR Quantification of Methanobrevibacter oralis in periodontitis. Journal of Clinical Microbiology. 2013;51:993–994. doi: 10.1128/JCM.02863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One. 2009;4:e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 70.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.