Abstract
The medial habenula (MHb) is considered a brain center regulating aversive states. The mu opioid receptor (MOR) has been traditionally studied at the level of nociceptive and mesolimbic circuits, for key roles in pain relief and reward processing. MOR is also densely expressed in MHb, however, MOR function at this brain site is virtually unknown. Here we tested the hypothesis that MOR in the MHb (MHb-MOR) also regulates aversion processing. We used chnrb4-Cre driver mice to delete the Oprm1 gene in chnrb4-neurons, predominantly expressed in the MHb. Conditional mutant (B4MOR) mice showed habenula-specific reduction of MOR expression, restricted to chnrb4-neurons (50% MHb-MORs). We tested B4MOR mice in behavioral assays to evaluate effects of MOR activation by morphine, and MOR blockade by naloxone. Locomotor, analgesic, rewarding, and motivational effects of morphine were preserved in conditional mutants. In contrast, conditioned place aversion (CPA) elicited by naloxone was reduced in both naïve (high dose) and morphine-dependent (low dose) B4MOR mice. Further, physical signs of withdrawal precipitated by either MOR (naloxone) or nicotinic receptor (mecamylamine) blockade were attenuated. These data suggest that MORs expressed in MHb B4-neurons contribute to aversive effects of naloxone, including negative effect and aversive effects of opioid withdrawal. MORs are inhibitory receptors, therefore we propose that endogenous MOR signaling normally inhibits chnrb4-neurons of the MHb and moderates their known aversive activity, which is unmasked upon receptor blockade. Thus, in addition to facilitating reward at several brain sites, tonic MOR activity may also limit aversion within the MHb circuitry.
Subject terms: Neuroscience, Molecular biology
Introduction
The habenula (Hb) is a small epithalamic structure connecting forebrain to midbrain regions [1, 2]. It is composed of two sub-structures, the lateral Hb and the medial habenula (MHb) [3]. The MHb receives inputs mainly from the septum through the stria medularis and projects massively to the interpeduncular nucleus (IPN) through the fasciculus retroflexus [1].
Recent preclinical data describe the MHb as a brain center for aversion [4] and evidence suggests that the MHb encodes negative affective states and aversive memories [5, 6]. In addiction research, most studies have focused on nicotine [7] with emphasis on the B4 nicotinic receptor subunit (hereafter called B4) that shows an expression pattern mostly restricted to the MHb [8]. Among key findings, the overexpression of B4 single nucleotide polymorphisms associated to nicotine dependence in the mouse MHb altered nicotine consumption [9], and subunit rescue in the IPN of B4 knockout mice restored intravenous nicotine self-administration [10, 11]. B4 knockouts also showed milder physical withdrawal symptoms precipitated upon nicotinic receptor blockade (mecamylamine), after chronic nicotine [12, 13], whereas transgenic mice with targeted overexpression of B4 displayed strong aversion to nicotine [14, 15]. All these data thus converge towards a role for MHb B4-expressing neurons in addiction-related behaviors, at least with regards to nicotine [16–18].
The MHb also expresses the highest density of mu opioid receptors (MORs) [2, 19] in the brain. MORs mediate both strong analgesic and addictive properties of opiates [20] and also contribute to the rewarding effects of other drugs of abuse [21] and natural rewards [22–24]. Brain sites for MOR-mediated reward have been extensively investigated both genetically [15, 21] and pharmacologically [25], and the implication of MORs in dopaminergic mesolimbic circuitry is largely demonstrated [26]. Overall, MORs are typically associated with reward processing and positive hedonic states, and much less is known about the contribution of MOR signaling in regulating negative affect. Preclinical studies have shown that pharmacological blockade of MORs produces aversive states, either emotional in naïve animals [27, 28] or both emotional and somatic in opioid-dependent animals [20, 29–31], and a study also suggested a role for MORs in emotional deficits that develop upon protracted abstinence to opiates [32]. In the latter model, the dorsal raphe nucleus was shown to be one brain site responsible for some aspects of MOR-mediated mood impairment, notably social withdrawal [33]. MORs, however, are present in several aversion brain centers, and circuit mechanisms underlying the potential influence of MOR activity on negative affect remain poorly investigated.
Here we hypothesized that MORs expressed in MHb neurons, particularly in B4-expressing neurons, may contribute to the regulation of a number of aversive responses. To test this, we genetically inactivated MORs specifically in MHb B4-expressing neurons (B4MOR mice) and characterized the contribution of MORs expressed in MHb B4-positive neurons in best-known morphine effects and food reward, as well as aversive responses to naloxone. We demonstrate that the targeted manipulation reduces conditioned place aversion elicited by MOR blockade (naloxone) in both naïve and morphine-dependent mice, whereas reward-related behaviors are unchanged. We therefore demonstrate that this small MOR population is strategically located to mediate aversive effects of naloxone, suggesting that tonic MOR signaling in the MHb normally alleviates aversive processes.
Materials and methods
Animals
For MOR/B4 colocalization, we crossed B4-Cre mice (RRID:MMRRC_036203-UCD) with MORmCherry mice [34] and the resulting MORmcherryKI/KIB4-Cre+ mice were used for the tracing experiments. For all other experiments, we generated conditional B4MOR−/− knockout mice and their B4MOR+/+ littermate controls by crossing B4-Cre mice with MORfl/fl mice [35]. Crossing was performed on the hybrid 50% 129SvPas-50% C57Bl/6J background, and male littermates (3–5 months) were used for behavioral testing. Further details are in Supplemental information. All animal procedures were conducted in accordance with the guidelines set forth by the Canadian Council of Animal Care and by the Animal Care Committees of McGill University/Douglas Mental Health University Institute (Animal protocol 7466).
See Supplemental information for drugs, molecular and histochemical methods, and behavioral testing.
Statistical analysis
All data are presented as mean ± SEM. Data were analyzed with (GraphPad Prism) unpaired t-test or two-way ANOVA with or without repeated measures (RM-ANOVA). Significant main effects and interactions of the ANOVAs were further investigated with the Bonferroni post-hoc test or method of contrast analysis. Statistical significance was set at p < 0.05. Detailed statistics information related to all figures are shown in Supplementary Table S1.
Results
B4MOR−/− mice lack MORs specifically in B4 neurons of the MHb
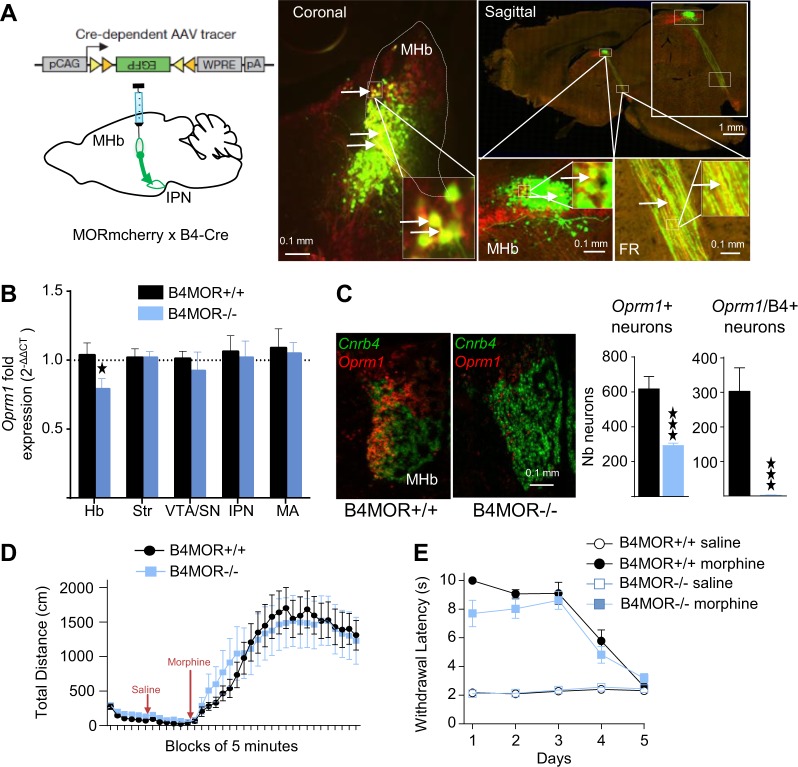
We first identified a Cre driver transgenic line that would allow specific Oprm1 gene deletion in the MHb. The Chnrb4-Cre line was a good candidate, as B4 is expressed predominantly at this brain site [8, 36]. Neuronal tracing using Chnrb4-Cre mice crossed with knock-in MORmCherry mice that express a detectable fluorescently-tagged MOR protein [34], combined with a Cre-dependent anterograde eGFP viral tracer (Fig. 1a left panel, AAV2-FlexGFP), revealed substantial overlap of MORmCherry (receptor) and eGFP (B4MOR fluorescent signals in the MHb; Fig. 1a, right image, coronal view), and along tracks forming the IPN (Fig. 1a, right image, sagittal view). We quantified coexpression of MOR and B4 at cellular level, using in situ hybridization (ISH). The Oprm1 transcript was distributed throughout the brain [25] with prominent expression in the MHb detected in both apical and lateral parts, concordant with the reported MOR protein distribution [19]. The B4 transcript was restricted to the MHb as expected (GENSAT RRID:MMRRC_036203-UCD), mainly localized in ventromedial and ventrolateral areas (Fig. 1c, left image), and was found in 48.85 ± 11.1% MHb Oprm1-positive neurons (Fig. 1c, Right panels), suggesting that the B4-Cre driver mouse line should produce a significant decrease of MORs in the MHb.
Fig. 1.
Deletion of Mu Opioid Receptors (MORs) in B4 neurons of the medial habenula (MHb). a MOR is expressed in B4-positive neurons (or B4 neurons). Left. Strategy. The AAV2-FlexGFP was injected in the MHb of MORmcherryKI/KIB4-Cre+ mice in order to visualize both the MOR protein and B4 neurons. Right. MOR and eGFP signals partially overlap. Representative images show coronal and sagittal sections with B4 neurons labeled in green and MOR labeled in red, at low and high magnification (see scales bars). FR: fasciculus retroflexus. b Anatomical specificity of the MOR deletion in B4MOR−/− mice. mRNA levels were quantified using RT-qPCR in microdissected habenula (Hb), striatrum (Str), ventral tegmental area/substantia nigra (VTA/SN), interpeduncular nucleus (IPN), and medulla (MA) samples from B4MOR−/− mutant mice (blue) and B4MOR+/+ controls (black). Data are expressed as mean of FOLD ± SEM (n = 10, in triplicates, t-test, *p < 0.05). Analysis of the Oprm1 mRNA shows significant reduction of MOR expression in the Hb sample only. c Cellular characterization of the conditional Oprm1 gene deletion using in situ hybridization (ISH, ACDbio®). Left. Representative images show both Oprm1 (red) and Cnrb4 (green) transcripts in the MHb of mutant and control mice. The Oprm1 signal is strongly reduced in the MHb of B4MOR−/− mice, mainly in the ventromedial part. Right. Quantification of single Oprm1-positive and double Oprm1/B4-positive cells in the MHb of mutant (blue) and control (black) mice. VS_DESKTOP measurement tools were applied to RNAscope results. Data are expressed as mean ± SEM number of neurons per area (manually defined in VS-DESKTOP) and show a reduction (49.85 ± 11.1%) and almost complete ablation (99.39 ± 0,21%) of single and double Oprm1-positive in mutant mice, respectively (n = 3 per genotype with 5 slides per animal and 5 slices per slide, ***p < 0.001). d Locomotion in the open field. Data are expressed as mean ± SEM total distance in cm, and show that basal locomotor activity (first 30 min), or locomotor activity after saline (next 30 min), and morphine (40 mg/kg) injection (last 120 min) do not differ between the two genotypes (n = 14–17, two-way RM-ANOVA, ns). e Tail immersion to assess morphine analgesia. Pain sensitivity was measured at 52 °C after morphine (10 mg/kg) injection in mutant and control mice, and the experiment was repeated for 5 days. The effect of saline injection on the tail immersion was also measured. Data are expressed as mean ± SEM withdrawal latency in seconds. Statistical analysis showed no difference between the two genotypes (n = 9; three-way RM-ANOVA, ns)
Next, we inactivated the Oprm1 gene in the MHb by crossing MOR floxed [35] and B4-Cre mice. To assess the extent of Oprm1 deletion in the MHb, we first quantified Oprm1 mRNA levels by RT-qPCR in microdissected brain samples from B4MOR conditional KO mice (B4MOR−/−) and their control littermates (B4MOR+/+). As shown in Fig. 1b, Oprm1 expression was significantly decreased in the Hb of B4MOR−/− mice compared to B4MOR+/+ controls, consistent with the observed Oprm1-B4 transcript overlap and also the high Cre expression predominantly in this sample (Supplementary Fig. S1). Oprm1 expression was otherwise intact in the striatum, the ventral tegmental area/substantia nigra, the IPN, and the medulla that all express high Oprm1 levels, and possibly also Chnrb4 during development [37], providing evidence that the Oprm1 deletion occurred mainly in the habenula. We further examined spatial distribution of Oprm1 deletion in the MHb using ISH analysis (Fig. 1c), and observed substantial decrease of Oprm1 mRNA mainly in the MHb lateral part of mutant brains. Semiquantification of ISH signals (Fig. 1c) revealed an overall 47.3 ± 2.2% reduction of Oprm1-positive neurons (t(4) = 4.59, p = 0.01), and an almost complete disappearance (99.4 ± 0.31%) of double positive Oprm1-B4 neurons (t(4) = 4.38, p = 0.01) in the MHb of B4MOR−/− mice, indicating selective Oprm1 mRNA loss in B4-positive neurons. Further analysis of Oprm1 transcript distribution in the two main MHb projection neuron populations (Supplementary Fig. S2), i.e., Substance P (SP) and cholinergic (Ach) neurons, showed an almost equal distribution of Oprm1 mRNAs in the two neuronal types in control mice, consistent with the known MOR protein distribution in these cell types [19]. This analysis also showed that the Oprm1 mRNA was deleted in both SP (44.3 ± 4.8%) and Ach (68.7 ± 1.8%) neurons of B4MOR−/− mice, suggesting the MOR deletion may affect activity of both neuronal types.
Finally, we tested well-described behavioral morphine effects. Morphine-induced locomotor activation in an open field was intact in mutant mice (Fig. 1d). Acute morphine analgesia is significantly reduced on day 1 only (Supplementary Fig. S3). Two-way ANOVA showed significant effect of genotype × treatment interaction (F(1, 32) = 5.489, p = 0.025). Bonferroni post-hoc test indicates a significant reduction of morphine analgesic effects on mutant mice compared to controls on day 1. On subsequent testing days, morphine analgesia was comparable and analgesic tolerance developed similarly in mutant and control mice (Fig. 1e), suggesting that MORs in B4 neurons subtly contribute to morphine analgesia but do not influence adaptations to repeated morphine exposure, at least in pathways relevant to the tail withdrawal responses (for statistical analysis see Supplementary Table S1).
B4MOR−/− mice show intact morphine and palatable food reward
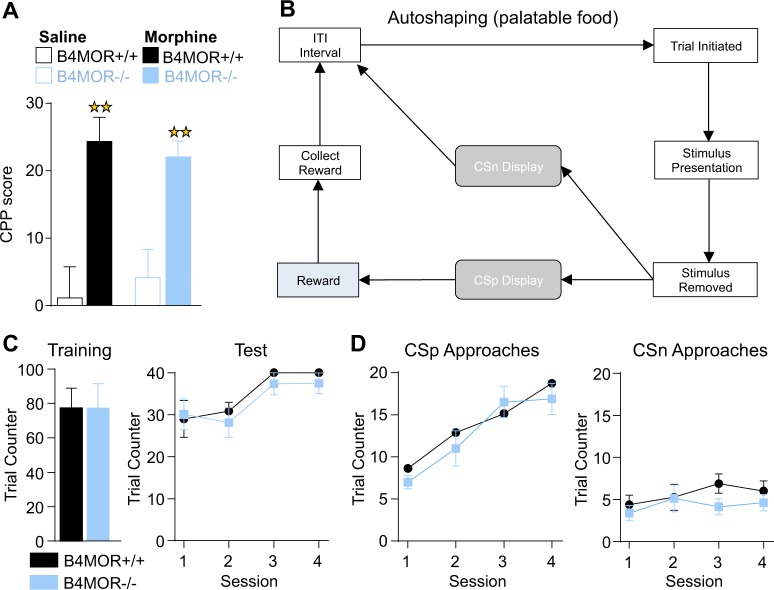
MORs are most widely associated with reward-related processes [24], which classically involve the dopaminergic mesolimbic circuit but may also involve the Hb [2]. Thus, we next investigated whether MHb-MORs expressed in B4 neurons contribute to reward processing, and tested reward-dependent pavlovian learning with both morphine and highly palatable food. Both rewarding effects of morphine in a conditioned place preference task (Fig. 2a), and stimulus-reward association in a touchscreen-based autoshaping task involving palatable food (Fig. 2b–d), were preserved (for statistical analysis see Supplementary Table S1). We also tested mutant mice in a 5-CSRT task. Results confirmed the preserved stimulus-reward association learning observed in the autoshaping task, and further revealed intact attention and inhibitory controls (see Supplementary Figs. S4 and S5). Note that in similar tasks, total MOR knockout mice showed reduced motivation, and delayed learning to gain the appetitive stimulus [38]. These data together suggest that MORs expressed in MHb-B4 neurons do not contribute to motivational and/or hedonic aspects of reward processing.
Fig. 2.
Preserved reward-related responses in B4MOR−/− mice. a Mice were tested in a morphine-conditioned place preference (CPP) paradigm. Data are expressed as mean ± SEM % CPP score defined as % of time spent in drug-paired compartment during post-conditioning − % of time spent in drug-paired compartment during preconditioning. Morphine-conditioned B4MOR−/− and B4MOR+/+ mice showed significant morphine CPP, which did not differ across genotypes. Saline-saline groups from both genotypes showed no place conditioning (n = 10–11; performed on compartment per treatment (morphine or saline) per genotype, **p < 0.01). b Mice were tested in an autoshaping test in the Touchscreen system, and the protocol is shown. Trial is initiated when the mouse inserts its head in the food tray. A stimulus is presented on one of the two sides of the screen. One side is always followed by reward presentation (positive Conditioned Stimulus or CSp) whereas the other is not (negative Conditioned Stimulus or CSn). If CSp is displayed and the mouse collects the reward, an inter-trial interval (ITI) starts, at the end of which another trial starts, until mice reach the criterion (40 trials per 1 h session). c, Left panel. Number of trials for the training phase (mice learn the association between the food tray and the reward apparition) was similar for the two genotypes (n = 8/group, t-test, nonsignificant—ns). c, Right panel. Number of trials for the testing phase (mice associate the apparition of a CSp with the apparition of a reward) was also similar for the two genotypes (n = 8/group, t-test, ns). d Number of CSp and CSn approaches during the testing phase. CSp, but not CSn, approaches increased across sessions for both genotypes, with no significant difference across genotypes. Data are expressed as mean ± SEM number of trials per session (n = 8, two-way RM-ANOVA performed on genotype per session, ns). All data are expressed as mean ± SEM
B4MOR−/− mice show reduced naloxone aversion
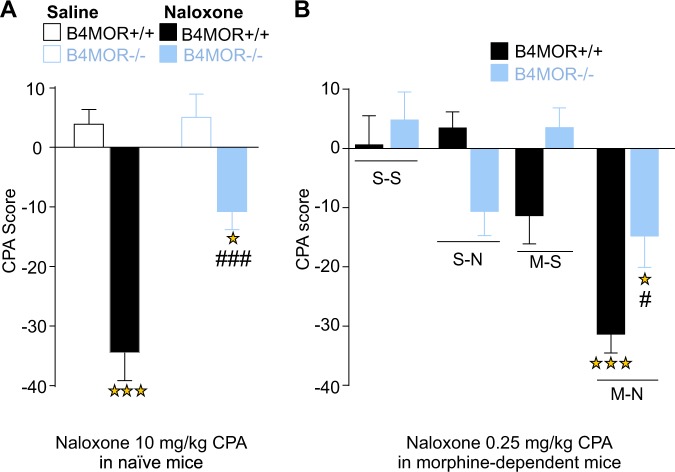
Prior evidence indicates that MOR blockade is aversive in mice, as demonstrated by conditioned place aversion (CPA) experiments using naloxone [27]. We thus tested whether MOR express in B4 neurons could contribute to this effect. B4MOR−/− and B4MOR+/+ mice were conditioned to associate a context with either naloxone (10 mg/kg; s.c.) or saline administration [39] (and see Supplementary Fig. S6), and results are shown in Fig. 3a. Two-way ANOVA revealed significant genotype (F(1, 49) = 11.39, p = 0.001), treatment (F(1, 49) = 54.3, p < 0.001) and genotype x treatment interaction effect (F(1, 49) = 9.37, p = 0.003). Subsequent post-hoc analysis showed significant aversion to the naloxone-paired compartment in B4MOR+/+mice, compared to their saline–saline controls (p < 0.001), confirming the previously reported existence of a positive hedonic tone, mediated by MORs and revealed by MORs blockade. Naloxone-induced place aversion was also observed in B4MOR−/− mice (p < 0.05). However, the magnitude of CPA was lower compared to B4MOR+/+mice (p < 0.001), demonstrating that MHb-MORs expressed in B4 neurons contribute to this activity, and mediate the aversive effect of naloxone.
Fig. 3.
Reduced aversion to naloxone in naïve and morphine-dependent B4MOR−/− mice. Mice were tested in a naloxone-conditioned place aversion paradigm. a Naïve mice. Avoidance is expressed as a CPA score defined as % of time spent in drug-paired compartment during post conditioning − % of time spent in drug-paired compartment during preconditioning. Both B4MOR+/+ controls and B4MOR−/− mutant mice showed naloxone (10 mg/kg) CPA (n = 12–14, two-way ANOVA performed on genotype per treatment (saline or naloxone), however naloxone CPA was lower in mutant mice as observed by lower significance level and a strong genotype effect. Stars, naloxone effect: ***p < 0.001 B4MOR+/+ saline versus B4MOR+/+ Naloxone; *p < 0.05 B4MOR−/− saline versus B4MOR−/− Naloxone. Squares, genotype effect, ###p < 0.001 B4MOR+/+ Naloxone versus B4MOR−/− Naloxone). b Morphine-dependent mice. Again, both B4MOR+/+ controls and B4MOR−/− mutant mice showed naloxone (0.25 mg/kg) CPA. However, as for naïve mice, the CPA score of morphine-dependent B4MOR−/− mice conditioned with naloxone (M-N) was significantly lower compared to B4MOR+/+ mice, and there was a significant genotype effect. Morphine Saline (M-S) groups did not develop any place preference or avoidance compared to Saline–Saline (S–S) group (n = 11, 12, three-way ANOVA performed on genotype per treatment (saline or naloxone) per drug (saline or morphine)). All data are expressed as mean ± SEM. Stars, naloxone effect: ***p < 0.001 B4MOR+/+ M-N versus B4MOR+/+ S–S, *p < 0.05 B4MOR−/− M-N versus B4MOR−/− S–S; Squares, genotype effect, #p < 0.05 B4MOR+/+ M-N versus B4MOR−/− M-N
We further tested B4MOR−/− mice and their controls in conditioned taste aversion. Taste aversion was similar for the two genotypes (Supplementary Fig. S7), suggesting that the naloxone CPA phenotype in B4MOR−/− mice does not generalize to other aversive responses.
Morphine-dependent B4MOR−/− mice show reduced affective signs of opioid withdrawal
Next, we examined aversive aspects of chronic opioid exposure. Opioid withdrawal is recognized as a highly aversive state, which includes both affective and somatic components [40]. We first tested the negative emotional experience of morphine withdrawal, using the CPA paradigm as before. In this case, we used a low naloxone dose (0.25 mg/kg; s.c.) that elicits place aversion, yet fails to produce physical withdrawal signs in morphine-dependent mice [41].
We adapted the procedure described in [42] (and see Supplementary Fig. S6). B4MOR−/− and B4MOR+/+ mice were injected with escalating doses of morphine (20, 40, 60, and 80 mg/kg) or saline, twice daily for 4 days and received a single morphine injection (100 mg/kg) on day 5. Preconditioning was performed 5 h after the morning injection on day 3, where mice were given free access to the two compartments. Place conditioning was performed on days 4 (saline) and 5 (naloxone 0.25 mg/kg s.c.), 5 h after the morning injection. Place conditioning was tested on day 6 and data are shown in Fig. 3b. Three-way ANOVA conducted on genotype per drug (morphine or saline) per treatment (naloxone or saline) revealed significant drug (F(1, 85) = 18.83, p < 0.001), treatment (F(1, 85) = 17.69, p < 0.001), as well as significant drug × genotype interaction (F(1, 85) = 11.81, p < 0.001) and drug × treatment interaction effect (F(1, 85) = 4.54, p = 0.04) but no significant difference for genotype (p = 0.07), genotype × treatment interaction effect (p = 0.18) or genotype × acute × chronic treatment interaction (p = 0.09). Subsequent analysis using the method of contrasts revealed that for both genotypes, low dose naloxone induced significant place aversion in morphine-treated groups compared to all the control groups (S–S, M–S; p < 0.05). However, this aversion was significantly lower in B4MOR−/− morphine-dependent mice compared to B4MOR+/+ controls (p < 0.05). Morphine-saline groups were not statistically different from saline-saline groups and did not develop any place preference or avoidance.
This result demonstrates that, as for naïve mice, MHb-MORs expressed in B4 neurons are necessary for naloxone aversion, and in this case, are involved in the aversive emotional state of withdrawal.
Morphine-dependent B4MOR−/− mice show reduced somatic signs of opioid withdrawal
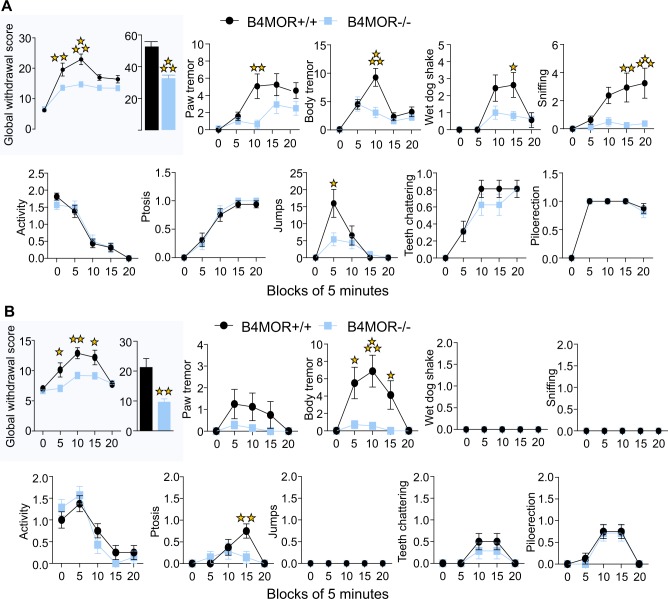
To examine whether the role of MORs in MHb-B4 neurons would apply to somatic opioid withdrawal, we further precipitated physical withdrawal in morphine-dependent mice.
We first assessed somatic withdrawal precipitated by direct MOR blockade. B4MOR−/− mice and their controls were subjected to a chronic morphine regimen using ascending morphine doses (20, 40, 60, 80, 100 mg/kg) twice daily for 5 days and received a single morphine injection (100 mg/kg) on day 6. Withdrawal was precipitated by naloxone (1 mg/kg, s.c.) [20]. Withdrawal signs were scored 5 min before, then every 5 min immediately after naloxone injection for 20 min, and a global withdrawal score was calculated (as in [43]). As shown in Fig. 4a, upper left panel, we observed a significant difference in the evolution of the global score over time between the two genotypes. Two-way RM ANOVA showed significant effect of genotype (F(1, 30) = 27.5, p < 0.001), block (F(4, 120) = 38.25, p < 0.001) and genotype x block interaction (F(4, 120) = 4.42, p < 0.001). Subsequent Bonferroni post-hoc analysis showed a significant reduction of global score in mutants compared to controls at block 5 (p < 0.01) and 10 (p < 0.001). Overall, the global score was significantly decreased in B4MOR−/− mice compared to control littermates (Fig. 4b, inset; t(30) = 4.45, p < 0.001). Specific withdrawal signs responsible for the decreased global withdrawal score are also shown in Fig. 4a (all other panels). Notable are paw and body tremors, wet dog shakes sniffing and jumps, which were significantly decreased in B4MOR−/− mice (detailed statistical analysis is in Supplementary Table S1). These results demonstrate that MOR blockade in the MHb is involved in physical withdrawal from morphine.
Fig. 4.
Reduced somatic withdrawal from chronic morphine in B4MOR−/− mice. a Naloxone-precipitated withdrawal. Withdrawal was precipitated in morphine-dependent mice by 1 mg/kg of Naloxone. Upper left inset. The global withdrawal score was significantly lower in B4MOR−/− compared to B4MOR+/+ control mice across blocks of 5 min and over the entire session (n = 16). Other panels: Scores for each withdrawal symptom monitored during the 25 min session. First row. Paw tremor, body tremor, wet dog shakes, and sniffing were significantly reduced in mutant mice compared to controls. Second row. Activity, ptosis, jumps, teeth chattering, and piloerection did not differ across genotypes. b Mecamylamine-precipitated withdrawal. Upper left inset. The global withdrawal score was significantly lower in B4MOR−/− compared to B4MOR+/+ control mice across blocks of 5 min and over the entire session (n = 7, 8). Other panels. Body tremor and ptosis were significantly reduced in mutant mice compared to controls. Paw tremor, wet dog shakes, sniffing, activity, jumps, teeth chattering, and piloerection did not differ across genotypes. All data are expressed as mean ± SEM; a, b Upper right inset. t-test, **p < 0.01 ***p < 0.001. a, b Upper left inset and Other panels. Two-way RM-ANOVA performed on genotype per block of 5 min, ***p < 0.001 and *p < 0.05; **p < 0.01; ***p < 0.001
We next measured somatic withdrawal induced by nicotinic receptor blockade. B4MOR−/− mice and their controls were subjected to the same morphine regimen, and withdrawal was precipitated by blockade of nicotinic receptors with mecamylamine. As expected [44], withdrawal signs were generally lower, compared to naloxone-precipitated withdrawal, and respective importance of the different withdrawal signs differed (Fig. 4a, b). Further, as for the naloxone experiment, there was a significant difference in the evolution of the global score over time between the two genotypes (Fig. 4b; upper left panel). Two-way ANOVA per 5-min blocks showed a significant effect of genotype (F(1, 13) = 10.28, p = 0.007), block (F(4, 52) = 18.45, p < 0.001) and genotype × block interaction (F(4, 52) = 4.33, p = 0.004). Subsequent Bonferroni post-hoc analysis showed a significant reduction of global score in B4MOR−/− mice compared to controls at block 5, 10, and 15 (p < 0.05). Overall, the global score (Fig. 4b, inset) was significantly decreased in conditional knockouts compared to control mice (t(13) = 3.48, p = 0.004). Scoring of all the signs (Fig. 4b, all other panels) shows that body tremors and ptosis were strong in controls, but absent in mutant mice, and represent the main factor explaining the genotype difference (for statistical analysis see Supplementary Table S1). Control analyses showed that in saline injected groups (B4MOR−/− and B4MOR+/+), injection of naloxone or mecamylamine did not trigger any sign of withdrawal (Supplementary Fig. S8).
This result definitely implicates MHb-MORs expressed in B4 neurons in somatic withdrawal to chronic opioids, and further reveals an opioid-nicotine cross-talk in these neurons.
Discussion
MOR function has been extensively associated to reward processes, but whether MORs also modulate aversive states is essentially unknown. In sum, our data show reduced morphine analgesia upon a first administration, consistent with our previous study showing that RSK2 signaling in the MHb partially contributes to morphine analgesia [45], but further morphine injections led to normal analgesic efficacy and tolerance. Further, most other well-described effects of morphine, including the development of analgesic tolerance, morphine locomotor activation, and conditioned place preference were intact upon Oprm1 gene deletion in MHb-B4 neurons. Also, palatable food reward-related processes, including context-dependent reward learning and stimulus-reward association, were unchanged. In contrast, the conditional mutation strongly reduces place avoidance behavior elicited by naloxone in naïve mice. In morphine-dependent mice, the conditional mutation also reduces place avoidance to naloxone and further, diminishes several signs of naloxone-precipitated somatic withdrawal. This is the first report demonstrating that MORs mediate several aversive effects of naloxone specifically at the level of MHb-B4 neurons.
Targeting MOR in the MHb
B4 nicotinic receptor subunit shows expression enriched in the MHb [8, 36], and we therefore used the Chnrb4 promoter to produce an MHb-specific Oprm1 gene knockout. We obtained an almost 50% Oprm1 gene deletion in B4 neurons of the MHb, with intact expression in other main MOR-enriched regions of the brain. Because the chnrb4 transcript is also expressed during development [37], we cannot exclude that some Oprm1 deletion has also occurred in brain regions that were not tested here, or are too small to be detected within the tested brain samples.
B4 neurons of the MHb include both SP and Ach neurons. Whether SP and Ach neuron subpopulations play distinguishable roles in the observed phenotypes is an open question. Gene targeting using Cre driver lines for SP and cholinergic neurons may complete this analysis, however we did not use these approaches, as MOR colocalization with these neurotransmitters at other brain sites would drastically complicate data interpretation. In the future, it may be interesting to know whether the remaining 50% of MORs expressed in non-B4 neurons of the MHb are also involved in aversive effects of naloxone, or play other roles.
MOR in MHb-B4 neurons limits aversive states induced by naloxone
A novel and most striking finding of this study is the observation that, in contrast to control mice, B4MOR mutant mice showed lower avoidance for the naloxone-paired context in the CPA procedure. This result demonstrates that MHb-MORs expressed in B4 contribute to naloxone aversion. As the MOR is an inhibitory G protein coupled receptor, we propose that this receptor normally inhibits MHb function by reducing the activity of B4 neurons, and thereby tempers activity of this aversive pathway. In control mice, this aversion-reducing MOR activity is revealed by naloxone blockade, and this effect is reduced in mutant mice that lack this particular receptor population. Thus, in addition to a well-known role in promoting reward and approach behavior across several brain circuits [25], MOR signaling would also limit aversive states and avoidance behavior at the level of the MHb circuitry. That MOR signaling indeed reduces the activity of B4-MHb neurons remains to be demonstrated by electrophysiological measures. Also, future experiments will determine the exact nature of this endogenous MOR activity in the MHb, which may involve either endogenous opioid peptides whose identity and origin remains to be determined, or constitutive MOR activity as was previously suggested [27, 28, 46].
Our study confirms that naloxone triggers CPA in control animals under both naïve [47] and morphine-dependent states, as previously shown [27, 29, 40]. Interestingly, naloxone CPA was strongly reduced in mutant mice, and this was observed for the two experimental settings. This result further supports our hypothesis that MHb-MORs tonically inhibit MHb-B4 neurons, and aversive consequences of their activity, whether or not animals are morphine-dependent. This observation suggests that no tolerance develops to the aversion-limiting function of MOR in MHb-B4 neurons, and that these receptors, therefore, remain efficacious even upon chronic stimulation. Notably also, some naloxone CPA remained detectable in both situations (naïve and morphine-dependent). The latter observation is in accordance with the notion that other brain sites may be engaged in this response, as proposed for basolateral amygdala [48, 49] or lateral Hb [50].
MOR in MHb-B4 neurons contributes to somatic withdrawal
A second main finding of the study is the observation that morphine-dependent B4MOR mutant mice showed a lower physical withdrawal syndrome upon naloxone challenge. This result demonstrates that MHb-MORs in B4 neurons is key to both emotional and somatic naloxone effects. Thus, beyond a role in alleviating emotional aversive states, the proposed tonic MHb-MOR activity in B4 neurons may also contribute to modulating physical signs of withdrawal. Mechanisms are unknown, but prior evidence has highlighted the importance of cholinergic transmission in the MHb-IPN pathway as chronic morphine reduces acetylcholinesterase activity in the MHb [51]. Important to note, shakes and sniffing but not jumps were abolished in mutant mice, indicating that MHb-MORs in B4 neurons do not modulate the full spectrum of somatic withdrawal signs. Other brain sites possibly contributing to this effect is the locus coeruleus, which has long been proposed as a main center for somatic drug withdrawal [52–54].
Our observation that mecamylamine-induced withdrawal was also reduced in morphine-dependent mutant mice supports the notion of a nicotinic/opioid neurotransmission cross-talk in the MHb. Growing literature shows a role for the B4 nicotinic receptor subunit in somatic aversive processes associated with nicotine withdrawal syndrome [9, 12, 13], and together, current data position both B4 and MORs in the MHb as critical actors in physical withdrawal syndromes. Both preclinical and clinical studies have already explored the existence of an opioid-nicotinic cross-induction of withdrawal [55] with emphasis on α3B4 nicotinic receptors [56]. Specifically, knockdown of B4 in the MHb attenuates cfos activation during morphine withdrawal in mice [51] indicating a role for the B4 subunit in morphine withdrawal. In humans, a recent resting state fMRI study also revealed increased habenular connectivity in opiate users, and further demonstrates that this change in connectivity is associated with subunit nicotinic gene variants [57]. At clinical level also, the MHb is not only considered a center for aversion [58] but was also associated to the high comorbidity of nicotine and opiate use disorders [59, 60]. Because MORs and B4 are the direct targets for morphine and nicotine, and MOR deletion from MHb-B4 neurons in our study reduced naloxone-precipitated somatic withdrawal, we suggest that activity of the two receptors concurrently modulate this neuronal population presumably in the same direction, providing a cross-talk mechanism at cellular level that deserves further investigation.
MOR in MHb-B4 neurons does not seem to regulate reward processing
The last important aspect of our study is the apparent lack of reward-related phenotype in mutant mice, suggesting that reward processing is preserved. MORs largely contribute to drug reward as classically measured by CPP and self-administration [24, 61], as well as to more complex reward-related behavior such as discrete stimulus-reward learning [62] and impulsivity [63]. Given the high density of habenular MORs and the proposed role of the Hb in reward mechanisms [64, 65] we explored the contribution of MHb-B4 MORs in reward processes. The absence of genotype difference throughout all reward-related testing led us to conclude that MHb-MORs expressed in B4 neurons are not involved in traditionally measured reward-related behaviors. This is consistent with the fact that receptor expression is preserved in B4MOR mutant mice at the level of the ventral tegmental area and striatum, forming the dopamine mesolimbic circuitry and considered a main center for reward processing and motivation [24, 66]. We cannot exclude that further self-administration studies may reveal a potential contribution of habenular MORs in reward processing, and/or reveal an indirect impact of MHb-MOR activity on dopamine transmission, however our current rather study favors the notion of a main role for MHb-MORs on aversive processing.
Conclusion
A fundamental task of the brain is to assign affective valence to environmental stimuli by determining whether these are rewarding and should be approached, or aversive and should be avoided [67]. Together with previous studies, our data suggest that, in addition to facilitating reward at the level of mesocorticolimbic networks, MORs limit aversion within the MHb-IPN circuitry, and the two mechanisms together contribute to increase approach and decrease avoidance, respectively. Interestingly and similarly, the conditional deletion of CB1 receptors in MHb neurons also abolished conditioned aversion without affecting appetitive memories and associations, a contribution that depends on cholinergic transmission into the IPN [5]. Together therefore, current knowledge now implicates opioid, cannabinoid and cholinergic transmission in the MHb to regulate the essential balance of reward/aversive behaviors.
Funding and disclosure
This work was supported by National Institute of Health (NIDA Grant no. 05010 to BLK and National Institute on Alcohol Abuse and Alcoholism, Grant no. 16658 to BLK), the Canada Fund for Innovation and the Canada Research Chairs to BLK, by Frame program Investissements d’Avenir ANR-10-IDEX-0002-02 ANR-10-LABX-0030-INRT (BLK, CG-R) and by the Ministère de l’Education Nationale, de la Recherche et de la Technologie (LJB).
Supplementary information
Acknowledgements
We thank the staff at the animal facility of the Neurophenotyping Center Douglas Research Center (Montréal, Canada), as well as Aude Villemain, Eujin Kim, Annie Salesse, Karine Lachapelle, Aimee Lee Luco, and DaWoon Park for animal care and genotyping. We thank the National Institute of Drug Abuse (NIDA) Drug Supply Program for providing us Morphine and the Molecular and Cellular Microscopy Platform (MCMP) of the Douglas Research center.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0395-7).
References
- 1.Aizawa H, Amo R, Okamoto H. Phylogeny and ontogeny of the habenular structure. Front Neurosci. 2011;5:138. doi: 10.3389/fnins.2011.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulos LJ, Darcq E, Kieffer BL. Translating the habenula-from rodents to humans. Biol Psychiatry. 2017;81:296–305. doi: 10.1016/j.biopsych.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beretta CA, Dross N, Guiterrez-Triana JA, Ryu S, Carl M. Habenula circuit development: past, present, and future. Front Neurosci. 2012;6:51. doi: 10.3389/fnins.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin I, Dani JA, De Biasi M. The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation. J Neurochem. 2017;142(Suppl 2):130–43. doi: 10.1111/jnc.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soria-Gomez E, Busquets-Garcia A, Hu F, Mehidi A, Cannich A, Roux L, et al. Habenular CB1 receptors control the expression of aversive memories. Neuron. 2015;88:306–13. doi: 10.1016/j.neuron.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Zhao-Shea R, DeGroot SR, Liu L, Vallaster M, Pang X, Su Q, et al. Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nat Commun. 2015;6:6770. doi: 10.1038/ncomms7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler CD, Kenny PJ. Nicotine aversion: neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology. 2014;76(Pt B):533–44. doi: 10.1016/j.neuropharm.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shih PY, Engle SE, Oh G, Deshpande P, Puskar NL, Lester HA, et al. Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. J Neurosci. 2014;34:9789–802. doi: 10.1523/JNEUROSCI.0476-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slimak MA, Ables JL, Frahm S, Antolin-Fontes B, Santos-Torres J, Moretti M, et al. Habenular expression of rare missense variants of the beta4 nicotinic receptor subunit alters nicotine consumption. Front Hum Neurosci. 2014;8:12. doi: 10.3389/fnhum.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler CD, Kenny PJ. Habenular signaling in nicotine reinforcement. Neuropsychopharmacology. 2012;37:306–7. doi: 10.1038/npp.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington L, Vinals X, Herrera-Solis A, Flores A, Morel C, Tolu S, et al. Role of beta4* nicotinic acetylcholine receptors in the habenulo-interpeduncular pathway in nicotine reinforcement in mice. Neuropsychopharmacology. 2016;41:1790–802. doi: 10.1038/npp.2015.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–9. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–8. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, et al. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–35. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Antolin-Fontes B, Ables JL, Gorlich A, Ibanez-Tallon I. The habenulo-interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology. 2015;96:213–22. doi: 10.1016/j.neuropharm.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldwin PR, Alanis R, Salas R. The role of the habenula in nicotine addiction. J Addict Res Ther. 2011;S1. https://www.frontiersin.org/articles/10.3389/fnhum.2014.00174/full. [DOI] [PMC free article] [PubMed]
- 17.Leslie FM, Mojica CY, Reynaga DD. Nicotinic receptors in addiction pathways. Mol Pharmacol. 2013;83:753–8. doi: 10.1124/mol.112.083659. [DOI] [PubMed] [Google Scholar]
- 18.Velasquez KM, Molfese DL, Salas R. The role of the habenula in drug addiction. Am J Addict. 2014;8:174. doi: 10.3389/fnhum.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardon O, Faget L, Chu Sin Chung P, Matifas A, Massotte D, Kieffer BL. Expression of mu opioid receptor in dorsal diencephalic conduction system: new insights for the medial habenula. Neuroscience. 2014;277:595–609. doi: 10.1016/j.neuroscience.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–23. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 21.Ben Hamida S, Boulos LJ, McNicholas M, Charbogne P, Kieffer BL. Mu opioid receptors in GABAergic neurons of the forebrain promote alcohol reward and drinking. Addict Biol. 2017;24:28–39. doi: 10.1111/adb.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–6. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 23.Rabiner EA, Beaver J, Makwana A, Searle G, Long C, Nathan PJ, et al. Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans. Mol Psychiatry. 2011;16:826–35, 785. doi: 10.1038/mp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci. 2015;38:217–25. doi: 10.1016/j.tins.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darcq E, Kieffer BL. Opioid receptors: drivers to addiction? Nat Rev Neurosci. 2018;19:499–514. doi: 10.1038/s41583-018-0028-x. [DOI] [PubMed] [Google Scholar]
- 27.Shoblock JR, Maidment NT. Constitutively active micro opioid receptors mediate the enhanced conditioned aversive effect of naloxone in morphine-dependent mice. Neuropsychopharmacology. 2006;31:171–7. doi: 10.1038/sj.npp.1300782. [DOI] [PubMed] [Google Scholar]
- 28.Shoblock JR, Maidment NT. Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience. 2007;149:642–9. doi: 10.1016/j.neuroscience.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Frenois F, Le Moine C, Cador M. The motivational component of withdrawal in opiate addiction: role of associative learning and aversive memory in opiate addiction from a behavioral, anatomical and functional perspective. Rev Neurosci. 2005;16:255–76. doi: 10.1515/revneuro.2005.16.3.255. [DOI] [PubMed] [Google Scholar]
- 30.Lucas M, Frenois F, Cador M, Le Moine C. Remodeling of the neuronal circuits underlying opiate-withdrawal memories following remote retrieval. Neurobiol Learn Mem. 2012;97:47–53. doi: 10.1016/j.nlm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT. Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron. 2014;82:1346–56. doi: 10.1016/j.neuron.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, et al. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–44. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutz PE, Ayranci G, Chu-Sin-Chung P, Matifas A, Koebel P, Filliol D, et al. Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive-like behaviors during heroin abstinence. Neuropsychopharmacology. 2014;39:2694–705. doi: 10.1038/npp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. 2015;220:677–702. doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weibel R, Reiss D, Karchewski L, Gardon O, Matifas A, Filliol D, et al. Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: insight from conditional knockout mice. PLoS One. 2013;8:e74706. doi: 10.1371/journal.pone.0074706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci. 2014;17:1146–52. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visel A, Carson J, Oldekamp J, Warnecke M, Jakubcakova V, Zhou X, et al. Regulatory pathway analysis by high-throughput in situ hybridization. PLoS Genet. 2007;3:1867–83. doi: 10.1371/journal.pgen.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boulos LJ, Nasseef MT, McNicholas M, Mechling A, Harsan LA, Darcq E, et al. Touchscreen-based phenotyping: altered stimulus/reward association and lower perseveration to gain a reward in mu opioid receptor knockout mice. Sci Rep. 2019;9:4044. doi: 10.1038/s41598-019-40622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakoori K, Murphy NP. Maintenance of conditioned place preferences and aversion in C57BL6 mice: effects of repeated and drug state testing. Behav Brain Res. 2005;160:34–43. doi: 10.1016/j.bbr.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Frenois F, Cador M, Caille S, Stinus L, Le Moine C. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur J Neurosci. 2002;16:1377–89. doi: 10.1046/j.1460-9568.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- 41.Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology. 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Milanes I, Almela P, Garcia-Carmona JA, Garcia-Gutierrez MS, Aracil-Fernandez A, Manzanares J, et al. Accumbal dopamine, noradrenaline and serotonin activity after naloxone-conditioned place aversion in morphine-dependent mice. Neurochem Int. 2012;61:433–40. doi: 10.1016/j.neuint.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D, et al. Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol. 2012;17:1–12. doi: 10.1111/j.1369-1600.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 44.Panchal V, Taraschenko OD, Maisonneuve IM, Glick SD. Attenuation of morphine withdrawal signs by intracerebral administration of 18-methoxycoronaridine. Eur J Pharmacol. 2005;525:98–104. doi: 10.1016/j.ejphar.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 45.Darcq E, Befort K, Koebel P, Pannetier S, Mahoney MK, Gaveriaux-Ruff C, et al. RSK2 signaling in medial habenula contributes to acute morphine analgesia. Neuropsychopharmacology. 2012;37:1288–96. doi: 10.1038/npp.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skoubis PD, Matthes HW, Walwyn WM, Kieffer BL, Maidment NT. Naloxone fails to produce conditioned place aversion in mu-opioid receptor knock-out mice. Neuroscience. 2001;106:757–63. doi: 10.1016/s0306-4522(01)00333-5. [DOI] [PubMed] [Google Scholar]
- 47.Kirkpatrick SL, Bryant CD. Behavioral architecture of opioid reward and aversion in C57BL/6 substrains. Front Behav Neurosci. 2014;8:450. doi: 10.3389/fnbeh.2014.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valero E, Gomez-Milanes I, Almela P, Ribeiro Do Couto B, Laorden ML, Milanes MV, et al. The involvement of CRF1 receptor within the basolateral amygdala and dentate gyrus in the naloxone-induced conditioned place aversion in morphine-dependent mice. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:102–14. doi: 10.1016/j.pnpbp.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 49.Ju YY, Long JD, Liu Y, Liu JG. Formation of aversive memories associated with conditioned drug withdrawal requires BDNF expression in the amygdala in acute morphine-dependent rats. Acta Pharmacol Sin. 2015;36:1437–43. doi: 10.1038/aps.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Li M, Wang P, Zha Y, He Z, Li Z. Inhibition of the lateral habenular CaMK abolishes naloxone-precipitated conditioned place aversion in morphine-dependent mice. Neurosci Lett. 2017;653:64–70. doi: 10.1016/j.neulet.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 51.Neugebauer NM, Einstein EB, Lopez MB, McClure-Begley TD, Mineur YS, Picciotto MR. Morphine dependence and withdrawal induced changes in cholinergic signaling. Pharmacol Biochem Behav. 2013;109:77–83. doi: 10.1016/j.pbb.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasmussen K. The role of the locus coeruleus and N-methyl-D-aspartic acid (NMDA) and AMPA receptors in opiate withdrawal. Neuropsychopharmacology. 1995;13:295–300. doi: 10.1016/0893-133X(95)00082-O. [DOI] [PubMed] [Google Scholar]
- 53.Van Bockstaele EJ, Menko AS, Drolet G. Neuroadaptive responses in brainstem noradrenergic nuclei following chronic morphine exposure. Mol Neurobiol. 2001;23:155–71. doi: 10.1385/mn:23:2-3:155. [DOI] [PubMed] [Google Scholar]
- 54.Fakhari M, Azizi H, Semnanian S. Central antagonism of orexin type-1 receptors attenuates the development of morphine dependence in rat locus coeruleus neurons. Neuroscience. 2017;363:1–10. doi: 10.1016/j.neuroscience.2017.08.054. [DOI] [PubMed] [Google Scholar]
- 55.Haghparast A, Khani A, Naderi N, Alizadeh AM, Motamedi F. Repeated administration of nicotine attenuates the development of morphine tolerance and dependence in mice. Pharmacol Biochem Behav. 2008;88:385–92. doi: 10.1016/j.pbb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Jackson KJ, Muldoon PP, De Biasi M, Damaj MI. New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology. 2015;96:223–34. doi: 10.1016/j.neuropharm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curtis K, Viswanath H, Velasquez KM, Molfese DL, Harding MJ, Aramayo E, et al. Increased habenular connectivity in opioid users is associated with an alpha5 subunit nicotinic receptor genetic variant. Am J Addict. 2017;26:751–9. doi: 10.1111/ajad.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hennigan K, D’Ardenne K, McClure SM. Distinct midbrain and habenula pathways are involved in processing aversive events in humans. J Neurosci. 2015;35:198–208. doi: 10.1523/JNEUROSCI.0927-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Souza MS. Neuroscience of nicotine for addiction medicine: novel targets for smoking cessation medications. Prog Brain Res. 2016;223:191–214. doi: 10.1016/bs.pbr.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Erlich PM, Hoffman SN, Rukstalis M, Han JJ, Chu X, Linda Kao WH, et al. Nicotinic acetylcholine receptor genes on chromosome 15q25.1 are associated with nicotine and opioid dependence severity. Hum Genet. 2010;128:491–9. doi: 10.1007/s00439-010-0876-6. [DOI] [PubMed] [Google Scholar]
- 61.Charbogne P, Gardon O, Martin-Garcia E, Keyworth HL, Matsui A, Mechling AE, et al. Mu opioid receptors in gamma-aminobutyric acidergic forebrain neurons moderate motivation for heroin and palatable food. Biol Psychiatry. 2017;81:778–88. doi: 10.1016/j.biopsych.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laurent V, Leung B, Maidment N, Balleine BW. Mu- and delta-opioid-related processes in the accumbens core and shell differentially mediate the influence of reward-guided and stimulus-guided decisions on choice. J Neurosci. 2012;32:1875–83. doi: 10.1523/JNEUROSCI.4688-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olmstead MC, Ouagazzal AM, Kieffer BL. Mu and delta opioid receptors oppositely regulate motor impulsivity in the signaled nose poke task. PLoS One. 2009;4:e4410. doi: 10.1371/journal.pone.0004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi Y, Sano Y, Vannoni E, Goto H, Suzuki H, Oba A, et al. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Front Behav Neurosci. 2013;7:17. doi: 10.3389/fnbeh.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker PM, Jhou T, Li B, Matsumoto M, Mizumori SJ, Stephenson-Jones M, et al. The lateral habenula circuitry: reward processing and cognitive control. J Neurosci. 2016;36:11482–8. doi: 10.1523/JNEUROSCI.2350-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology. 2014;76(Pt B):204–17. doi: 10.1016/j.neuropharm.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, et al. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33:17569–76. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.