Abstract
Finding a clinically useful neuroimaging biomarker that can predict treatment response in patients with major depressive disorder (MDD) is challenging, in part because of poor reproducibility and generalizability of findings across studies. Previous work has suggested that posterior hippocampal volumes in depressed patients may be associated with antidepressant treatment outcomes. The primary purpose of this investigation was to examine further whether posterior hippocampal volumes predict remission following antidepressant treatment. Magnetic resonance imaging (MRI) scans from 196 patients with MDD and 110 healthy participants were obtained as part of the first study in the Canadian Biomarker Integration Network in Depression program (CAN-BIND 1) in which patients were treated for 16 weeks with open-label medication. Hippocampal volumes were measured using both a manual segmentation protocol and FreeSurfer 6.0. Baseline hippocampal tail (Ht) volumes were significantly smaller in patients with depression compared to healthy participants. Larger baseline Ht volumes were positively associated with remission status at weeks 8 and 16. Participants who achieved early sustained remission had significantly greater Ht volumes compared to those who did not achieve remission by week 16. Ht volume is a prognostic biomarker for antidepressant treatment outcomes in patients with MDD.
Subject terms: Predictive markers, Depression, Predictive markers, Depression, Predictive markers
Introduction
Major depressive disorder (MDD) is a leading cause of disability [1, 2]. Unfortunately, a substantial portion of patients fail to respond to first-line treatments (40–60%; [2, 3]), and only a minority achieve full remission [4]. Choosing optimal, effective treatments early in the course of illness and finding ways to predict treatment outcomes are important goals of research in MDD.
Brain measures obtained from magnetic resonance imaging (MRI) have demonstrated a potential value in differentiating healthy controls from individuals diagnosed with MDD [5, 6], and for predicting treatment responses in MDD patients [7, 8]. The anatomy and function of the hippocampus have been a particular focus of research because of work, suggesting that it is important in the pathophysiology of depression and response to treatment [9, 10]. Smaller hippocampal volumes in patients with depression compared to healthy participants are now well-documented [5, 11, 12].
While there are reports of an association between structural changes in the hippocampus and clinical variables in smaller studies [6, 9, 13], large-multi-site datasets have not had standardized clinical data to permit replication of these findings. For example, the MDD working group from The Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) study reported significant associations between MDD and alterations in subcortical gray matter volumes, including total hippocampal volumes (THV) [5]. The ENIGMA dataset includes patients who underwent a variety of treatment interventions, making it difficult to ascertain whether these changes in gray matter volumes predict treatment outcome to a specific therapeutic intervention.
More recently, several multi-site studies have applied common treatment and imaging protocols to study the relations between brain structure and function, and the outcome of specific interventions. For example, the Establishing Moderators and Biosignatures of Antidepressant Response in Clinical care (EMBARC) project examined brain changes in cortical thickness during the first week of antidepressant medication (ADM) treatment [14]. Thickening in the rostral anterior cingulate cortex (rACC) was associated with the change in symptom severity during a trial week of sertraline, but baseline THV data did not inform the ADM treatment response prediction algorithm [14]. The International Study to Predict Optimized Treatment in Depression (iSPOT) collaborative designed studies to identify pre-treatment structural MRI measures that could predict acute treatment outcomes [15, 16]. In one of these investigations, Maller et al. [16] found that larger hippocampal tail (Ht) volume predicted clinical remission, independent of total brain volume (TBV), age or THV [16].
Although there are divergent findings about whether THV predicts treatment outcomes [17–19], converging lines of evidence now suggest that subfield-specific changes in hippocampal structure may be a reliable biomarker for treatment remission [8, 16]. Several studies using manual segmentation of hippocampal subregions reported that localized hippocampal volumes might be associated with the rate, extent, and maintenance of clinical response to ADM [7, 20–22]. Notably, there are even earlier reports showing that hippocampal volumes predict treatment outcomes even on the longer term [12, 23]. However, these studies were not looking at hippocampal longitudinal changes of subfield-specific hippocampal volumes.
More recent studies employed an automated segmentation software FreeSurfer 6.0 with integrated hippocampus subfield-specific atlases (FS6.0-sf) [24]. Maller et al. [16] detected Ht volume group differences between remitters and non-remitters to ADM with the help of an atlas building algorithm that can measure 12 hippocampal subfields using T1-weighted MRI images as input [24, 25]. Cao et al. [8] employed the same segmentation pipeline to identify patients likely to achieve remission following electroconvulsive therapy and to examine hippocampal subfield volumes in patients with bipolar disorder and first episode psychosis [26–28]. Using a more advanced approach, combining T1 and T2* images, Roddy et al. [6] have also shown selective subfield-specific differences between MDD and healthy control participants. The updated FS6.0-sf segmentation pipeline (cross-sectional [25]) has outperformed previous segmentation strategies on reliability parameters [29]. The FS6.0-sf workflow can be conducted using default settings (for instructions see ref. [25]) that reduce the likelihood of methodological divergence between study sites, and consequently improve the likelihood of replication. This is important because the lack of reproducibility in neuroimaging research is a growing concern [30], and may be partially explained by methodological inconsistencies.
Given the established literature describing the connection between the hippocampal pathology and MDD, it is surprising that only a few studies have aimed to reproduce findings on hippocampal subfield-specific volumes as a predictor of ADM response/remission. In this report, we therefore aimed to replicate and extend the finding [7, 16] that Ht volumes predict remission with antidepressant treatment, in a large and strictly independent sample of MDD patients undergoing a uniform ADM treatment protocol. Therefore, we hypothesized that Ht volumes will be smaller in patients with MDD compared to controls. Furthermore, based on previous work [7, 16], we expected that larger baseline Ht volumes would be associated with an increased probability of response/remission following ADM treatment.
We incorporated outputs from both automated (FS6.0-sf) and manual segmentation protocols, analyzing data gathered from The Canadian Biomarker Integration Network in Depression (CAN-BIND), the details of which are available elsewhere [31]. We analyzed baseline neuroimaging and clinical data and assessed for response and remission status at weeks 8 and 16. As a secondary goal, we investigated whether hippocampal volumetry informs not only the degree of improvement, but the rate at which improvement occurs in patients treated with ADM.
Methods and materials
Study participants
Study participants were recruited from six academic health centers across Canada. The initial sample included 196 participants with MDD and 110 healthy comparison (HC) participants that met CANBIND-1 inclusion and exclusion criteria (see ref. [31] for details). Briefly, the Mini-International Neuropsychiatric Interview was used to confirm group assignment [32]. Patients were included if they scored ≥24 on the Montgomery-Åsberg Depression Rating Scale (MADRS) [33] at their baseline visit. Patients were either ADM naive or had a wash-out period of at least five half-lives for psychotropic medications before receiving 10–20 mg/day escitalopram. Patients whose symptoms did not improve with the initial treatment after 8 weeks of monotherapy received adjunctive aripiprazole 2–10 mg/day for an additional 8 weeks.
Treatment response was defined as a reduction in MADRS score of at least 50% from their baseline score. Remission was defined as MADRS score of ≤10. Clinical assessments were conducted by practicing psychiatrists at each study site; treatment outcomes were assessed at multiple time points, including week 8 and 16 (for the procedural details see ref. [31]). We analyzed both response and remission status at 8 and 16 weeks. For clarity, the response and remission statuses were analyzed separately. Participants who successfully responded/remitted at both 8 and 16 weeks were considered as early responders/remitters. Participants who responded/remitted only at the end of the study were considered late remitters/responders.
The CAN-BIND sample excluded participants with an Axis I diagnosis other than MDD as a primary diagnosis; significant Axis II diagnosis; substance abuse within the past 6 months; history with adverse reactions to escitalopram; history of neurologic diseases, head trauma. The healthy comparison group included participants 18–60 years of age with no history of Axis I or Axis II disorders as determined by the MINI [32]. All eligible study participants provided written informed consent for all procedures after a complete description of the study.
MRI data acquisition and processing
The CAN-BIND neuroimaging acquisition protocols have been published [34]. Briefly, all sites followed similar MRI acquisition protocols performed on 3T MR scanners. A whole-brain T1-weighted turbo gradient echo sequence was acquired at 1 mm3 resolution. The pulse sequence parameters were: repetition time (TR) = 6.4–1900 ms; echo time (TE) = 2.2–3.4 ms; flip angle = 8–15°; inversion time (TI) = 450–950 ms; field of view (FOV) = 256 mm; matrix dimensions 220 × 220 and 256 × 256; 155–192 contiguous slices at 1 mm thickness. A vitamin E pill was used as a stereotactic marker that was placed at the right side of the participant’s head. Data quality control (QC) and data quality assurance (QA) procedures for the CAN-BIND MRI protocols have been described [34].
Automated hippocampal subfield segmentations were performed using FreeSurfer version 6.0 (http://surfer.nmr.mgh.harvard.edu/). A comprehensive description of this pipeline is provided by Iglesias and colleagues [25]. The pipeline generated THV as well as 12 additional segmentations for hippocampal subregions: hippocampal tail (Ht), subiculum, fissure, presubiculum, parasubiculum, molecular layer (ML), granule cell layer and molecular layer of the dentate gyrus (GC-ML-DG), fimbria, the cornu ammonis (CA) area subdivided into CA1, CA2/3, CA4, and hippocampal amygdala transition area (HATA). Additional brain volumes were gathered via the asegstats2table FreeSurfer 6.0 command, which gathers statistics on the whole-brain segmentation routine [35]. These measurements include: an estimated total intracranial volume (eTIV), total gray matter volume, total white matter volume. Total brain volumes (TBV) were calculated from FS6.0 output (total gray matter plus total white matter volume).
Manual segmentations of THV were also obtained using the European Alzheimer’s Disease Consortium (EADC)—Alzheimer’s Disease Neuroimaging Initiative (ADNI) Harmonized Protocol (HarP) [36]. Two trained tracers (NN, MM) followed the EADC-ADNI HarP manual [37], and when necessary referred to an atlas of the human brain [38] to ascertain the correct anatomical identification. All baseline scans were manually traced by both tracers blinded to group allocation.
A three-step quality control procedure for hippocampal segmentation is described in Supplementary Material (see 1.1.).
Statistical analyses
All data were analyzed using SPSS version 25.0 (IBM SPSS Statistics for iMac, Armonk, NY, USA). Pearson correlations (r) and intraclass correlation coefficients (ICC) were used to compare automated and manually segmented hippocampal volumetry outputs. To compare hippocampal volumes between groups, simple t-tests and analysis of variance were used. A general linear model (GLM) was used for both regression and univariate analysis of covariance (ANCOVA) to account for covariate terms. Covariate terms were predetermined using a backwards multiple linear regression model. We used a receiver operating characteristic (ROC) analysis to evaluate predictive properties of variables of interest. Statistical analyses were two-tailed with significance set at the 0.05 alpha levels. The Bonferroni correction was used for multiple comparisons only.
Results
Sample characteristics
Table 1 summarizes the demographic and clinical information of the study sample across all time points. At baseline, there were no significant differences in mean ages or other demographic variables between MDD and HC participants, although healthy men were younger (mean age = 32.4) than men with MDD (mean age = 38.7). There were no significant age differences between remitters/responders and non-remitters/non-responders at week-8 nor at week 16. For completeness, a schematic flow chart illustrates the schedule of interventions and assessments for the treatment arms in the present study (see Supplementary Fig. 1).
Table 1.
Demographic and clinical characteristics of study participants across several time points
| Study site-specific sample characteristics at baseline | |||||||
|---|---|---|---|---|---|---|---|
| Study site and scanner model | Sex | Control | MDD | ||||
| N | Mean age | SD | N | Mean age | SD | ||
| Center for Addiction and Mental Health | Females | 2 | 28.0 | 7.1 | 8 | 30.4 | 12.1 |
| GE 3.0 T Discovery MR750 | Males | 5 | 32.2 | 12.9 | 1 | 33.0 | – |
| McMaster University | Females | 12 | 32.8 | 11.2 | 22 | 33.9 | 12.5 |
| GE 3.0 T Discovery MR750 | Males | 7 | 39.4 | 15.5 | 9 | 33.0 | 15.1 |
| Queen’s University | Females | 11 | 29.6 | 8.9 | 8 | 41.0 | 17.4 |
| Siemens 3.0 T TrioTim | Males | 3 | 25.0 | 4.0 | 10 | 43.1 | 13.2 |
| Toronto General Hospital | Females | 16 | 34.9 | 12.6 | 31 | 32.8 | 12.1 |
| GE 3.0 T Signa HDxt | Males | 7 | 31.4 | 6.3 | 19 | 40.9 | 11.9 |
| University of Calgary | Females | 21 | 33.3 | 11.1 | 18 | 30.3 | 11.7 |
| GE 3.0 T Discovery MR750 | Males | 14 | 30.1 | 7.0 | 13 | 35.2 | 11.6 |
| University of British Columbia | Females | 8 | 36.1 | 12.7 | 39 | 32.9 | 11.8 |
| Phillips 3.0 T Intera | Males | 4 | 35.8 | 9.2 | 18 | 39.1 | 10.8 |
| Total sample characteristics | |||||||
| Baseline | Sex | Control | MDD | ||||
| N | Mean age | SD | N | Mean age | SD | ||
| Females | 70 | 33.2 | 11.1 | 126 | 33.0 | 12.4 | |
| Males | 40 | 32.4 | 10.0 | 70 | 38.6 | 12.2 | |
| Total | 110 | 32.9 | 10.7 | 196 | 35.0 | 12.6 | |
| Week 8 | Remitters | Non-remitters | |||||
| Females | 35 | 32.1 | 11.4 | 74 | 33.8 | 12.9 | |
| Males | 18 | 36.9 | 12 | 45 | 39.3 | 12.3 | |
| Total | 53 | 33.7 | 11.7 | 119 | 35.8 | 12.9 | |
| Responders | Non-responders | ||||||
| Females | 54 | 33 | 12 | 55 | 33 | 12 | |
| Males | 27 | 36.7 | 11 | 36 | 39.6 | 13 | |
| Total | 81 | 34.2 | 23 | 91 | 36 | 13 | |
| Week 16 | Remitters | Non-remitters | |||||
| Females | 63 | 33.6 | 12 | 36 | 35.1 | 13 | |
| Males | 33 | 37.9 | 11 | 26 | 38.5 | 12 | |
| Total | 96 | 35.1 | 12 | 62 | 36.5 | 13 | |
| Responders | Non-responders | ||||||
| Females | 76 | 33.8 | 12 | 23 | 35.8 | 14 | |
| Males | 42 | 36.7 | 11 | 17 | 40 | 12 | |
| Total | 118 | 34.8 | 12 | 40 | 37.9 | 13 | |
SD standard deviation, HC healthy comparison participants, MDD participants with major depressive disorder, NS non-significant
General neuroimaging characteristics
THV measurements from FS6.0-sf and manual segmentations were correlated (Cronbach’s Alpha = 0.809, Pearson r = 0.67, ICC = 0.507, p = 0.001), as previously reported in a large longitudinal methodological study [39].
There was a significant difference between left and right THVs according to both segmentation methods (FS6.0-sf: t = −6.13, df = 298, p < 0.05; manual: t = −6.9, df = 305, p < 0.05), so further analyses were conducted separately for left (-lh) and right (-rh) hemispheres. Hemispheric asymmetry analysis was conducted as previously described [39]; the asymmetry index was used to determine the directionality of the lateral asymmetry and absolute values (ABS) were used to examine the magnitude of the asymmetry regardless of the directionality (ABS|Right-Left|). The asymmetry Index identified THV rightward asymmetry in both manual (HC: + 0.18, MDD: + 0.22) and FS6.0-sf segmentation methods (HC: + 0.14, MDD: + 0.19).
Determinants of hippocampal volumes
The automated segmentation workflow FS6.0-sf uses a probabilistic atlas to estimate hippocampal subfields with a Bayesian Inference algorithm that enables to adapt to the particular MRI image intensity characteristics of each scanner [25]. Nevertheless, differences in neuromorphometric data collected across different sites and MR hardware may still be present.
To control for possible between-site variance in hippocampal volumes we used a backward multiple regression model. We regressed THVs as dependent variable and study site, age, sex, handedness, and TBV were included as independent variables. This model retained only TBV as a covariate term for left (R2 = 0.57, F = 396.6, df = 1298, p = 0.000) and right THV R2 = 0.54, F = 356.4, df = 1298, p = 0.000). Study site differences did not significantly contribute to explaining hippocampal volume variability (lh: p = 0.963; rh: p = 0.942). A similar procedure was conducted for Ht as a variable of interest where both TBV and sex were meaningful terms for left (R2 = 0.24, F = 29.2, df = 2298, p = 0.000) and right (R2 = 0.28, F = 60.6, df = 1298, p = 0.000) Ht volumes. Therefore, further analyses were conducted using TBV (for total volumes) or TBV and sex as covariate terms for Ht volumes.
When controlled for age and sex, participants with MDD had significantly smaller TBVs as compared to HCs (F = 4.8, df = 301, p = 0.028), however, there were no significant group differences in eTIV (F = 2.2, df = 301, p = 0.13). As TBV did not include volumes of lateral and third ventricles, that are within anatomical proximity to the hippocampus, we also compared ventricular volumes between study participants. Although MDD participants had observably greater ventricle volumes as compared to HC, these differences were not statistically significant (see Supplementary Table 1).
Hippocampal volumes and clinical features of depression
Baseline
THVs did not differ between groups. Ht volumes, however, were significantly smaller bilaterally in MDD group as compared to HC (lh: F = 5.5, df = 1296, p = 0.019 η2 = 0.19, power = 0.65; rh: F = 5.3, df = 1296, p = 0.021, η2 = 0.18, power = 0.65). Interestingly, manual segmentation detected a significant difference in THV absolute asymmetry between MDD participants and HCs (F = 5.8, df = 1301, p = 0.016, η2 = 0.19, power = 0.71). Detailed information and group statistics for other hippocampal subfields are provided in Supplementary Table 1.
Treatment outcomes at week 8
Patients who achieved remission by week 8 had larger left Ht volumes at baseline (lh: mean = 566.2 mm3, SEM = 9.1 mm3) compared to patients who did not achieve remission (lh: mean = 540 mm3, SEM = 5.9 mm3) (F = 5.5, df = 1167, p = 0.020, η2 = 0.033, power = 0.64). In addition, patients who achieved remission by week 8 demonstrated significantly lower baseline Ht absolute asymmetry volumes (mean = 27 mm3, SEM = 4.7 mm3) compared to non-remitters (mean = 45.9 mm3, SEM = 3 mm3) (F = 11, df = 1167, p = 0.001, η2 = 0.063, power = 0.91).
Left Ht volumes also approached statistical significance levels for associations with the treatment response status at week 8 (F = 3.3, df = 1164, p = 0.07, η2 = 0.02, power = 0.44). Notably, non-responders were more likely to have greater absolute Ht asymmetry volumes at baseline compared to those who responded at week 8 (F = 4.8, df = 1164, p = 0.03, η2 = 0.028, power = 0.58). For other subfields, group comparisons are presented in Supplementary Table 2.
Treatment outcomes at week 16
Consistent with findings at week 8, MDD participants who achieved remission by week 16 were more likely to have larger left and right Ht volumes at baseline than patients who did not achieve remission (lh: F = 7.5, df = 1150, p = 0.007, η2 = 0.048, power = 0.77, rh: F = 6, df = 1150, p = 0.01, η2 = 0.039, power = 0.68). Of those 96 participants who achieved remission at week 16, only 42 remitted by week 8 and maintained remission until week 16 (see Supplementary Fig. 2). This cohort had significantly lower Ht absolute asymmetry (mean = 25.2 mm3, SEM = 5.3 mm3) compared to patients who only achieved remission by week 16 (mean = 47.5 mm3, SEM = 4.7 mm3, p = 0.01) or those who did not remit at all (mean = 45.4 mm3, SEM = 4.5 mm3, p = 0.018) (F = 5.2, df = 1143, p = 0.006, η2 = 0.069, power = 0.82, Bonferroni corrected; see Fig. 1).
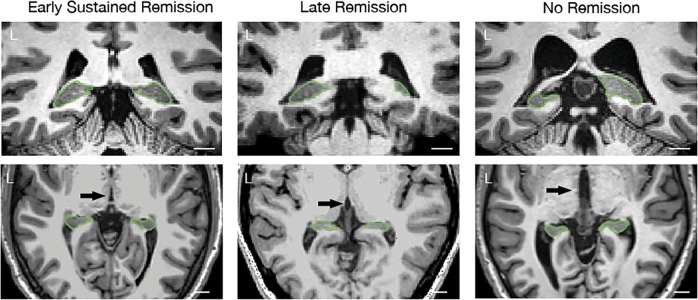
Fig. 1.
Different patterns of hippocampal tail volume disproportions in patients with MDD. Green marker shows distal zone of the hippocampal tail (coronal section). L = left side; white bar indicates 1 cm measurement. Early sustained remission = MDD participants that achieved remission at week 8 and maintained the status until week 16. Late remission = MDD participants that remitted only by the end of the study, at week 16; no remission = MDD participants who did not achieve remission of their symptoms at any point of time during the study
Responders at week 16 were more likely to exhibit larger Ht volumes at baseline as compared to participants who failed to respond (F = 11.7, df = 1150, η2 = 0.072, power = 0.92, p = 0.001) (See Fig. 2). Notably, 3rd ventricle volumes were significantly greater in both non-responders and non-remitters as compared to responders (F = 4.2, df = 1151, η2 = 0.028, power = 0.53, p = 0.04) and remitters (F = 3.9, df = 1151, η2 = 0.026, power = 0.5, p = 0.048) at week 16. (For details see Supplementary Table 3).
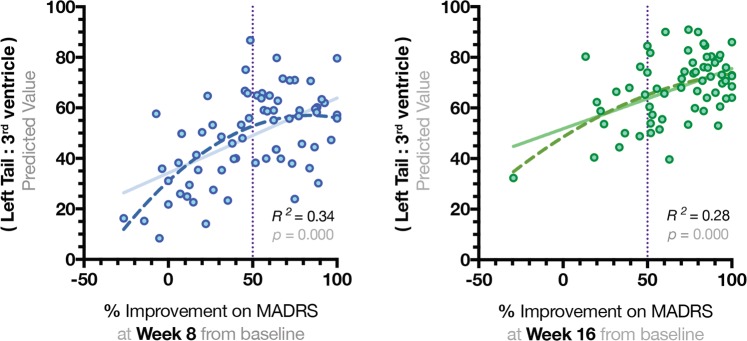
Fig. 2.
Quadratic regression model to represent significant associations between the results of total Montgomery–Åsberg Depression Rating Scale and proportional values of the left hippocampal tail. Hippocampal tail (Ht) volumes were divided by volumes of the 3rd ventricle and regressed with total MADRS scores. Quadratic non-linear function appeared to be the best fit to explain the variance in MADRS scores at weeks 8 and 16
Predictive ability of hippocampal tail volume as a proportional variable
As results from the present statistical analyses demonstrated consistently higher F-values for the left Ht volumes particularly, we specifically concentrated on predictive analyses for the left hemisphere (see Supplementary Tables 1–3). Detailed results of the ROC analyses are reported in Tables 2 and 3. The left Ht volumes had the area under the curve (AUC) of 0.56 in predicting remission status at week 8 and AUC = 0.58 in predicting remission at week 16. TBV as a separate variable was not a significant predictor for remission statuses at week 8 (p = 0.6) or week 16 (p = 0.9). However, when left Ht volumes were considered as a proportional value of the TBV (lh Ht:TBV), we observed stronger predictive characteristics for both week 8 (AUC = 0.59) and week 16 remission statuses (AUC = 0.61). Since TBV did not include the volume of the ventricles, we tested the predictive properties of Ht as a proportional value relative to the anatomically adjacent 3rd ventricle (lh Ht:3rd ventricle). The predictive ability of these proportional variables (lh Ht:3rd ventricle) were observably higher AUC = 0.64 for remission statuses at both time points; and AUC = 0.67 as a predictor of the response status at week 16. When Left Ht was divided by the absolute asymmetry, the differentiation between early and late remitters was at the level of AUC = 0.70 (CI [0.59,0.81], p = 0.001).
Table 2.
Characterizing predictive ability of hippocampal tail volume as a proportional value
| Pearson correlation | Age | MADRS | ||
|---|---|---|---|---|
| MDD | HC | Week 8 | Week 16 | |
| Left tail | −0.166* | −0.256** | −0.153* | −0.242** |
| Tail asymmetry | 0.035 | 0.108 | 0.226** | 0.14 |
| TBV | −0.183* | −0.142 | 0.034 | −0.073 |
| 3rd ventricle | 0.401** | 0.091 | 0.233** | 0.258** |
| Left lateral ventricle | 0.442** | 0.258** | 0.015 | 0.1 |
| Right lateral ventricle | 0.428** | 0.177 | 0.033 | 0.119 |
| (Left tail/TBV) | −0.026 | −0.184 | −0.198** | −0.211** |
| (Left tail/3rd ventricle) | −0.423** | −0.184 | −0.306** | −0.310** |
| (Left tail/tail asymmetry) | −0.037 | 0.193* | −0.154* | −0.137 |
| General linear model | ||||
| (Left tail/3rd ventricle) | R2 | F | h2 (partial eta) | p |
| Week 8 response | 0.37 | 10.9 | 0.063 | 0.001 |
| Week 8 remission | 0.37 | 11.1 | 0.064 | 0.001 |
| Week 16 response | 0.39 | 13.0 | 0.082 | 0.000 |
| Week 16 remission | 0.37 | 6.5 | 0.042 | 0.012 |
Upper panel reflects results obtained with Pearson Correlation analyses. Asterisks indicate that the r-value is significant at the level of 0.001 (**) or 0.05 (*). Lower panel reflects the results of generalized linear model that included (left tail /3rd ventricle) as a dependent variable controlled for age, sex, and lateral ventricles, and variable of interest as an independent term (response/remission). R2 = adjusted; h2 = partial eta as a measure of the effect size. p-values were provided for the dependent variable (left tail/ 3rd ventricle). All GLM models were statistically significant at the level <0.05.
HC healthy comparison participants, MDD participants with major depressive disorder, MADRS The Montgomery–Åsberg Depression Rating Scale
Table 3.
The results of receiver operating characteristic analyses
| Week 8 | Week 16 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | AUC | SEM | p | CI | AUC | SEM | p | CI | ||
| Remission | ||||||||||
| Left tail | 0.57 | 0.05 | 0.176 | 0.47 | 0.67 | 0.54 | 0.05 | 0.397 | 0.45 | 0.63 |
| Tail asymmetry | 0.33 | 0.04 | 0.001 | 0.25 | 0.42 | 0.39 | 0.04 | 0.016 | 0.31 | 0.48 |
| TBV | 0.48 | 0.05 | 0.64 | 0.38 | 0.57 | 0.47 | 0.05 | 0.568 | 0.39 | 0.56 |
| 3rd ventricle | 0.41 | 0.05 | 0.062 | 0.31 | 0.51 | 0.4 | 0.04 | 0.027 | 0.31 | 0.49 |
| (Left tail/ TBV) | 0.59 | 0.05 | 0.068 | 0.5 | 0.68 | 0.58 | 0.04 | 0.088 | 0.49 | 0.66 |
| (Left tail/ 3rd ventricle) | 0.64 | 0.05 | 0.005 | 0.54 | 0.73 | 0.63 | 0.04 | 0.003 | 0.55 | 0.72 |
| (Left tail/ tail asymmetry) | 0.69 | 0.04 | 0 | 0.59 | 0.76 | 0.59 | 0.04 | 0.06 | 0.49 | 0.68 |
| Response | ||||||||||
| Left tail | 0.54 | 0.05 | 0.397 | 0.45 | 0.63 | 0.65 | 0.05 | 0.006 | 0.54 | 0.75 |
| Tail asymmetry | 0.39 | 0.04 | 0.016 | 0.31 | 0.48 | 0.41 | 0.05 | 0.093 | 0.31 | 0.51 |
| TBV | 0.47 | 0.05 | 0.568 | 0.39 | 0.56 | 0.56 | 0.05 | 0.282 | 0.45 | 0.66 |
| 3rd ventricle | 0.4 | 0.04 | 0.027 | 0.31 | 0.49 | 0.39 | 0.06 | 0.034 | 0.28 | 0.49 |
| (Left tail/ TBV) | 0.58 | 0.04 | 0.088 | 0.49 | 0.66 | 0.63 | 0.05 | 0.017 | 0.53 | 0.73 |
| (Left tail/ 3rd ventricle) | 0.63 | 0.04 | 0.003 | 0.55 | 0.72 | 0.68 | 0.05 | 0.001 | 0.58 | 0.77 |
| (Left tail/ tail asymmetry) | 0.62 | 0.04 | 0.008 | 0.53 | 0.7 | 0.61 | 0.05 | 0.034 | 0.51 | 0.71 |
AUC area under the curve, SEM standard error mean, p p-value, CI confidence intervals, TBV total brain volume, Lat ventricle lateral ventricle
The ratio of lh Ht:3rd ventricle was also correlated with total MADRS scores from week 8 (Pearson r = −0.30, p = 0.001) and week 16 (Pearson r = −0.31, p = 0.001) (see Table 2, upper panel). A quadratic regression model with lh Ht:3rd ventricle as a dependent variable explained 34% variability for percent improvement in MADRS at week 8 (R2 = 0.34, F = 16.9, df = 2,68, p = 0.000) and 28% for week 16 (R2 = 0.28, F = 15.8, df = 2,61, p = 0.000) (See Fig. 2). When Lh Ht:3rd ventricle was correlated with age separately for MDD and HC groups, significant negative correlations were only within the MDD group (r = .−423, p = 0.001). Finally, a general linear model with the dependent variable of interest (lh Ht:3rd ventricle) and covariate terms that included sex, age, and lateral ventricles, explained ~40% of variance for both remission and response rates at weeks 8 and 16 (see Table 2, lower panel).
Discussion
Consistent with previous reports, left Ht volumes were reduced at baseline in depressed participants compared to healthy participants. Our primary interest, however, was in determining whether Ht volumes at baseline had the capacity to predict outcome in MDD participants receiving ADM [7, 16]. Regarding outcomes in this sample, approximately one-third of patients achieved remission following 8 weeks of escitalopram and were maintained on escitalopram. Participants who had not responded by 8 weeks had another eight weeks of escitalopram along with adjunctive aripiprazole, increasing the cumulative response and remission rates to 74.7% and 60.8%, respectively. MDD participants with larger left Ht volumes at baseline were, in fact, more likely to achieve remission at week 8 and week 16. This association was specific to Ht volumes, as we did not find a significant association between the outcome of treatment and THV measurements, using either manual or automated segmentation methods.
Consistent with Maller et al. [16], we report that TBV, but not eTIV, measures are significantly smaller in MDD participants compared to HC [16]. Indeed, TBV measurements appear more relevant and may be further evaluated as a neuroimaging indicator of structural deficits in MDD participants. In our sample, we observed another well-documented finding that cerebral ventricles of depressed patients were greater as compared to healthy participants (for example ref. [40]). As expected, aberrant patterns of ventricular enlargement were correlated primarily with age of the depressed participant. Importantly, in the present analysis this curvilinear pattern of ventricular enlargement independently coincided with a significant decrease in Ht volume in depressed participants.
We therefore examined whether the predictive power of the Ht volumes may be enhanced if Ht volume was considered as a proportion relative to the 3rd cerebral ventricular size. This proportional variable significantly correlated with MADRS total scores. When adjusted for age, sex, and lateral ventricles, the left Ht:3rd ventricle proportion explained ~40% of variance in response and remission statuses at both week 8 or 16.
Generally, late remitters were characterized by leftward Ht asymmetry and disproportionally small (58%) left Ht volume relative to 3rd ventricle volume. Depressed participants who did not achieve remission even by week 16 were characterized by rightward Ht asymmetry and pronounced disproportions in left Ht (55%). Early sustained remitters exhibited greater left Ht volumes (65%) in relation to the 3rd ventricle volumes without detectable deviations in Ht asymmetry (see Fig. 1).
Therefore, MDD participants with exaggerated patterns of Ht volume disproportion and asymmetry achieved remission later following first initiation of ADM. Disproportionally small Ht may be predictive of the rate of improvement to remission, as well as the overall likelihood of remission. Although Ht may help predict who will remit, Ht asymmetry may differentiate early and late remitters or those who are likely to benefit from atypical adjunctive antipsychotic medication.
While the role of the hippocampus and stress in the pathophysiology has been established [9], it remains unclear why the Ht in particular is the most vulnerable to MDD. Anatomically, the initial segment of the tail resembles the body of the hippocampus [41]. The posterior region of the hippocampus is vascularized by a separate group of hippocampal arteries that are prominently anastomosed [42], suggesting its distinct physiological importance. However, in the tail, the CA1 region is heavily folded where terminal segments of the arterioles are particularly vulnerable to anoxia due to stress-induced vasoconstriction [43]. As common ADM pharmacological agents may only partially ameliorate these illness-associated hippocampal deficits [44, 45], there exists the possibility that Ht volume decline may relate to a cumulative imprint of previously long-lasting untreated depressive episodes. Small Ht volumes found in non-remitting patients may be a surrogate marker of previously unmanaged depressive episodes that in turn—reduce the likelihood of treatment response to current ADM [9]. We could note, however, that MacQueen et al. [7] found smaller Ht volumes in patients presenting with a first treated episode of illness hence—it is less likely that past illness episodes are the sole explanation of the finding. It is also unclear why hippocampal and ventricular asymmetry may have pathophysiological significance for clinical outcomes in patients MDD. It is possible that the phenomenon of occipital bending (Yakovlevian torque [46]) may be relevant to the observed phenomenon that connects hippocampal tail asymmetry and remission status [47].
Many neuroimaging studies have documented global alterations in hippocampal volumes not only in depression but in psychotic disorders [48] and other stress-sensitive illness such as post-traumatic disorder (both subfield-specific [49] and global changes [50]). This may reflect a general vulnerability to stress-related changes independent of the discrete nature of the illness. Interestingly, bipolar disorder is one illness where small volumes [51] have been less reliably reported, raising questions about the neuroprotective effects of common treatments for bipolar disorder, such as lithium [52, 53].
There are some limitations to the present work. Although the analyses of covariance included sex and age, sex-specific differences in the hippocampal structure were not explored in relation to ADM outcomes, as more female participants both entered and remained in the study. The main limitation of FS6.0.-sf segmentation workflow using 1 mm T1-image as input is that the position of the internal boundaries between the hippocampal subregions heavily relies on the probabilistic estimation, thus the volumes of internal subfields of the hippocampus should be interpreted with caution. However, volume estimations of the hippocampal tail and fimbria do not suffer from this technical limitation. While the strength of the study was the consistent algorithmic approach to treatment, this feature also limits our capacity to make inferences regarding other classes of antidepressants or other adjunctive strategies. We examined response status in participants at week 8 and week 16. In general, the pattern of results was not as stable when using the response as the outcome measure, and this appears consistent with reports in the literature that favor remission as the outcome measure more reliably predicted by volumetric analyses [7, 8, 16].
To the best of our knowledge, there have been no previous attempts to replicate Ht volume as a marker of treatment outcome in a large independent sample employing a uniform, standardized image processing workflow, and a uniform intervention protocol. This study adds to an emerging body of clinical literature that consistently reports that Ht volumes predict the outcome to ADM in participants with MDD. We also show that the degree of disproportion of Ht may be more informative biomarker as compared to Ht volume on its own and may potentially surpass group-level association to predict treatment outcome. Future studies should confirm whether this prognostic feature of Ht volumes extends to other treatment modalities.
Supplementary information
Funding and disclosure
CAN-BIND is an Integrated Discovery Program carried out in partnership with, and financial support from, the Ontario Brain Institute (OBI), an independent non-profit corporation, funded partially by the Ontario government. The opinions, results, and conclusions are those of the authors and no endorsement by the OBI is intended or should be inferred. Additional funding is provided by the Canadian Institutes of Health Research (CIHR), Lundbeck, Bristol-Myers Squibb, Pfizer, and Servier. Funding and/or in kind support is also provided by the investigators’ universities and academic institutions. N Nogovitsyn, M Muller, R Souza, S Hassel, S Arnott, A Davis, G Hall, J Harris, J Addington, K Harkness, S Rotzinger, and N Zamyadi have no relevant or material financial disclosures or any other potential conflicts of interest. Dr. Metzak has received a Research Fellowship grant support from the CIHR. Dr. Ismail has received grants from CIHR, Brain Canada, Consortium on Neurodegeneration in Aging, Jansen; consulting honoraria from Jansen, Lundbeck, Otsuka, and Sunovion. Dr. Downar reports research grants from CIHR, the National Institute of Mental Health, Brain Canada, the Canadian Biomarker Integration Network in Depression, the OBI, the Weston Foundation, the Klarman Family Foundation, the Arrell Family Foundation, and the Buchan Family Foundation, travel stipends from Lundbeck and ANT Neuro, in-kind equipment support for investigator-initiated trials from MagVenture, and is an advisor for BrainCheck, TMS Neuro Solutions, and Restorative Brain Clinics. Dr. Parikh reports, in the last 36 months, consulting honoraria from Takeda, Sunovion, Assurex, and Mensante, research grants from Takeda, Assurex, OBI, Canadian Institutes of Health Research, the Ethel and James Flinn Foundation, and shares in Mensante. Dr. Soares received honoraria for ad hoc speaking or advising/consulting, or received research funds, from: Lundbeck, Merck, OBI, Ontario Research Funds—Research Excellence, Otsuka, Pfizer, Sunovion. Dr. Milev has received research funds and honoraria for speaking/consulting from: Allergan, Canadian Institutes of Health Research, Canadian Biomarker Integrated Network in Depression, Canadian Network for Mood and Anxiety Treatments, Janssen, Lundbeck, Otsuka, Pfizer, Shire, Sunovion. Dr. Frey has received research funding from Pfizer and consulting fees from Otsuka. Dr. Lam has received honoraria for ad hoc speaking or advising/consulting, or received research funds, from: Akili, Allergan, Asia-Pacific Economic Cooperation, BC Leading Edge Foundation, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Association, CME Institute, Hansoh, Healthy Minds Canada, Janssen, Lundbeck, Lundbeck Institute, Medscape, Mind.Me, MITACS, OBI, Otsuka, Pfizer, St. Jude Medical, University Health Network Foundation, and VGH-UBCH Foundation. Dr. Strother receives funding from the OBI and CIHR (MOP137097) for neuroimaging analysis in CAN-BIND and he is the Chief Scientific Officer of ADMdx, Inc., a neuroimaging consulting company. Dr. Kennedy is/or has been an advisor/consultant for: Abbott, Alkermes, Allergan, BMS, Janssen, Lundbeck, Lundbeck Institute, Otsuka, Pfizer, Servier, Sunovion; and participated in clinical trials/studies for Abbott, BMS, Janssen, Pfizer, Servier; Speaking engagements with the following organizations: BMS, Lundbeck, Pfizer, Servier, Xian-Janssen; and has received research support from: OBI, CIHR, BMS, Brain Canada, Janssen, Lundbeck, ORF, Pfizer, Servier. Dr MacQueen has received honoraria from Lundbeck, Janssen, and AllerGen in the past 36 months.
Data availability
Data for the present analyses were accessed via exclusive permission of the CAN-BIND consortium (for data inquiries: www.canbind.ca).
Code availability
Codes used for the present analyses are available from the corresponding author, NN, upon request.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0542-1).
References
- 1.Baingana F, al’Absi M, Becker AE, Pringle B. Global research challenges and opportunities for mental health and substance-use disorders. Nature. 2015;527:S172–7. doi: 10.1038/nature16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder. Can J Psychiatry. 2016;61:540–60. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord. 2009;117:Suppl:S26–43. doi: 10.1016/j.jad.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of Outcomes With citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roddy DW, Farrell C, Doolin K, Roman E, Tozzi L, Frodl T, et al. The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol Psychiatry. 2018;85:487–97. doi: 10.1016/j.biopsych.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 7.MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry. 2008;64:880–3. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Cao B, Luo Q, Fu Y, Du L, Qiu T, Yang X, et al. Predicting individual responses to the electroconvulsive therapy with hippocampal subfield volumes in major depression disorder. Sci Rep. 2018;8:5434. doi: 10.1038/s41598-018-23685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–64. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 10.Campbell S, MacQueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–26. [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 12.Frodl T, Meisenzahl EM, Zetzsche T, Höhne T, Banac S, Schorr C, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry. 2004;65:492–9. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- 13.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci. 2003;100:1387–92. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartlett EA, DeLorenzo C, Sharma P, Yang J, Zhang M, Petkova E, et al. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacology. 2018;43:2221–30. doi: 10.1038/s41386-018-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korgaonkar MS, Rekshan W, Gordon E, Rush AJ, Williams LM, Blasey C, et al. Magnetic resonance imaging measures of brain structure to predict antidepressant treatment outcome in major depressive disorder. EBioMedicine. 2015;2:37–45. doi: 10.1016/j.ebiom.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maller JJ, Broadhouse K, Rush AJ, Gordon E, Koslow S, Grieve SM. Increased hippocampal tail volume predicts depression status and remission to anti-depressant medications in major depression. Mol Psychiatry. 2018;23:1737–44. doi: 10.1038/mp.2017.224. [DOI] [PubMed] [Google Scholar]
- 17.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biol Psychiatry. 2004;56:101–12. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 19.MacQueen GM. Magnetic resonance imaging and prediction of outcome in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:343–9. [PMC free article] [PubMed] [Google Scholar]
- 20.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh M-H, McQuoid DR, Levy RM, Payne ME, MacFall JR, Steffens DC. Hippocampal volume and antidepressant response in geriatric depression. Int J Geriatr Psychiatry. 2002;17:519–25. doi: 10.1002/gps.611. [DOI] [PubMed] [Google Scholar]
- 22.Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–90. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- 23.Frodl T, Zill P, Baghai T, Schüle C, Rupprecht R, Zetzsche T, et al. Reduced hippocampal volumes associated with the long variant of the tri- and diallelic serotonin transporter polymorphism in major depression. Am J Med Genet Part B Neuropsychiatr Genet. 2008;147B:1003–7. doi: 10.1002/ajmg.b.30680. [DOI] [PubMed] [Google Scholar]
- 24.Iglesias JE, Van Leemput K, Augustinack J, Insausti R, Fischl B, Reuter M. Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject-specific atlases. Neuroimage. 2016;141:542–55. doi: 10.1016/j.neuroimage.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–37. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao B, Passos IC, Mwangi B, Amaral-Silva H, Tannous J, Wu M-JJ, et al. Hippocampal subfield volumes in mood disorders. Mol Psychiatry. 2017;22:1352–8. doi: 10.1038/mp.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baglivo V, Cao B, Mwangi B, Bellani M, Perlini C, Lasalvia A, et al. Hippocampal subfield volumes in patients with first-episode psychosis. Schizophr Bull. 2018;44:552–9. doi: 10.1093/schbul/sbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao B, Bauer IE, Sharma AN, Mwangi B, Frazier T, Lavagnino L, et al. Reduced hippocampus volume and memory performance in bipolar disorder patients carrying the BDNF val66met met allele. J Affect Disord. 2016;198:198–205. doi: 10.1016/j.jad.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worker A, Dima D, Combes A, Crum WR, Streffer J, Einstein S, et al. Test–retest reliability and longitudinal analysis of automated hippocampal subregion volumes in healthy ageing and A lzheimer’s disease populations. Hum Brain Mapp. 2018;39:1743–54. doi: 10.1002/hbm.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18:115–26. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam RW, Milev R, Rotzinger S, Andreazza AC, Blier P, Brenner C, et al. Discovering biomarkers for antidepressant response: protocol from the Canadian biomarker integration network in depression (CAN-BIND) and clinical characteristics of the first patient cohort. BMC Psychiatry. 2016;16:105. doi: 10.1186/s12888-016-0785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 33.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 34.Macqueen GM, Hassel S, Arnott SR, Addington J, Bowie CR, Bray SL, et al. The Canadian Biomarker Integration Network in Depression (CAN-BIND): magnetic resonance imaging protocols. J Psychiatry Neurosci. 2019;2019:223–36. doi: 10.1503/jpn.180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 36.Frisoni GB, Jack CR. HarP: The EADC-ADNI harmonized protocol for manual hippocampal segmentation. A standard of reference from a global working group. Alzheimer’s Dement. 2015;11:107–10. doi: 10.1016/j.jalz.2014.05.1761. [DOI] [PubMed] [Google Scholar]
- 37.Boccardi M. Eadc-adni harmonized protocol for manual 1. Introduction: hippocampal segmentation: user manual. Eadc-Adni Harmon Protoc Man Hippocampal Segmentation. 2014:1–35.
- 38.Mai JK, Paxinos G, Assheuer JK. Atlas of the Human Brain. San Diego, CA: Acad Press; 2004. [Google Scholar]
- 39.Schmidt MF, Storrs JM, Freeman KB, Jack CR, Turner ST, Griswold ME, et al. A comparison of manual tracing and FreeSurfer for estimating hippocampal volume over the adult lifespan. Hum Brain Mapp. 2018;39:2500–13. doi: 10.1002/hbm.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beats B, Levy R, Förstl H. Ventricular enlargement and caudate hyperdensity in elderly depressives. Biol Psychiatry. 1991;30:452–8. doi: 10.1016/0006-3223(91)90306-7. [DOI] [PubMed] [Google Scholar]
- 41.Duvernoy H, Cattin F, Risold P-Y. Structure, functions, and connections. In: The human hippocampus functional anatomy, vascularization and serial sections with MRI. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. pp. 5–38. [Google Scholar]
- 42.Duvernoy H, Cattin F, Risold P-Y. Vascularization. In: The human hippocampus functional anatomy, vascularization and serial sections with MRI. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. pp. 69–105. [Google Scholar]
- 43.Klosovskii B. Fundamental principles of the development, structure and function of the vaso-capillary network of the brain. n: Klosovs. Oxford: Pergamon; 1963. [Google Scholar]
- 44.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colla M, Kronenberg G, Deuschle M, Meichel K, Hagen T, Bohrer M, et al. Hippocampal volume reduction and HPA-system activity in major depression. J Psychiatr Res. 2007;41:553–60. doi: 10.1016/j.jpsychires.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 46.PI Yakovlev PR. Patterns of decussation of bulbar pyramids and distribution of pyramidal tracts on two sides of the spinal cord. Trans Am Neurol Assoc. 1966;91:366–7. [Google Scholar]
- 47.Maller JJ, Anderson R, Thomson RH, Rosenfeld JV, Daskalakis ZJ, Fitzgerald PB. Occipital bending (Yakovlevian torque) in bipolar depression. Psychiatry Res Neuroimaging. 2015;231:8–14. doi: 10.1016/j.pscychresns.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23:1764–72. doi: 10.1038/mp.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: A meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 51.Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry. 2017;22:1455–63. doi: 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yucel K, Taylor VH, McKinnon MC, MacDonald K, Alda M, Young LT, et al. Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacology. 2008;33:361–7. doi: 10.1038/sj.npp.1301405. [DOI] [PubMed] [Google Scholar]
- 53.Hajek T, Bauer M, Simhandl C, Rybakowski J, O’Donovan C, Pfennig A, et al. Neuroprotective effect of lithium on hippocampal volumes in bipolar disorder independent of long-term treatment response. Psychol Med. 2014;44:507–17. doi: 10.1017/S0033291713001165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for the present analyses were accessed via exclusive permission of the CAN-BIND consortium (for data inquiries: www.canbind.ca).
Codes used for the present analyses are available from the corresponding author, NN, upon request.