Abstract
Objective:
Despite increased focus on opioid prescribing, little is known about the influence of prescription opioid medication information given to patients in the emergency department (ED). The study objective was to evaluate the effect of an Electronic Medication Complete Communication (EMC2) Opioid Strategy on patients’ safe use of opioids and knowledge about opioids.
Methods:
This was a three-arm prospective, randomized controlled pragmatic trial with randomization occurring at the physician level. Consecutive discharged patients at an urban academic ED (>88,000 visits) with new hydrocodone-acetaminophen prescriptions received one of three care pathways: (1) usual care, (2) EMC2 intervention, or (3) EMC2 + SMS text messaging. The ED EMC2 intervention triggered two patient-facing educational tools (MedSheet, literacy-appropriate prescription wording [Take-Wait-Stop]) and three provider-facing reminders to counsel (directed to: ED physician, dispensing pharmacist, follow-up physician). Patients in the EMC2+SMS arm additionally received one text message/day for 1-week. Follow-up at 1–2 weeks assessed “demonstrated safe use” (primary outcome). Secondary outcomes including patient knowledge and actual safe use (via medication diaries) were assessed 2–4 days and 1-month following enrollment.
Results:
Among the 652 enrolled, 343 completed follow-up (57% women; mean age 42 years). Demonstrated safe opioid use occurred more often in the EMC2 group (adjusted odds ratio (aOR), 2.46; 95% CI, 1.19, 5.06), but not the EMC2 + SMS group (aOR 1.87; 95% CI 0.90, 3.90) compared with usual care. Neither intervention arm improved medication safe use as measured by medication diary data. Medication knowledge, measured by a 10-point composite knowledge score, was greater in the EMC2 + SMS group (Beta 0.57; 95% CI, 0.09, 1.06) than usual care.
Conclusion:
The study found that the EMC2 tools improved demonstrated safe dosing, but these benefits did not translate into actual use based on medication dairies. The text-messaging intervention did result in improved patient knowledge.
Clinical Trial registration:
Keywords: opioid analgesics, health literacy, text messaging, emergency
INTRODUCTION
The United States continues to face an epidemic of opioid addiction and overdose, the scale of which is unprecedented and has prompted the declaration of a public health emergency.1 Accordingly, efforts are being made in all parts of the opioid use continuum to decrease morbidity and mortality (e.g., decreasing initial prescribing, promoting alternatives to opioid therapy, identifying and treating opioid use disorder). Many interventions and guidelines appropriately target decreasing the number of opioid prescriptions and the number of pills per prescription.2–5 However, in settings such as the emergency department (ED), where acute or chronic pain account for almost two-thirds of ED visits,6 use of opioids may be appropriate at times, if prescribed safely.2
In those moments of appropriate prescribing, patient education and the physician patient interaction at the time of prescribing must be optimized to ensure patients are aware both of the risks of opioids and how to take them safely. The ED is widely recognized as a difficult environment for ensuring adequate communication due to factors including time constraints, unpredicted interruptions, shift changes, overcrowding, and the lack of a preexisting relationship with patients.7–10 In this setting, existing ED discharge processes may more often than not be inadequate for confirming patient understanding; increasing the risk that self-care instructions were not appropriately conveyed or followed.11 This lack of knowledge for patients newly prescribed opioids is potentially dangerous, as preliminary research indicates patients rarely recall counseling about opioid pain relievers.12,13 When counseling does occur it is highly variable and the potential for addiction is very rarely addressed.13–16
The Agency of Healthcare Quality and Research (AHRQ) has made recommendations for topics to be covered in conversations with patients about any new prescriptions (not limited to opioids) to ensure safe and appropriate use.17,18 Responsible ED prescribing of opioid analgesics should include both spoken counseling and written information for patients. These instructions should not only fulfill new medication counseling recommendations, but also directly address the potential for opioid abuse and misuse so patients can be informed consumers. With other classes of medications, one-on-one educational counseling efforts have been demonstrated to improve patient adherence to physician recommendations about medications and to improve medication related outcomes.19
Our team developed an Electronic Medication Complete Communication (EMC2) Opioid Strategy with a goal of supporting and improving one-on-one communication to ED patients about newly prescribed opioid pain relievers.20 This intervention is an EHR-integrated strategy that activates a series of two patient-facing educational tools (a hard copy one-page MedSheet about opioids, patient-centered wording on prescriptions [Take-Wait-Stop]) and three automated provider-facing EHR-based reminders to counsel patients (directed to ED physician, pharmacist, and follow-up physician) when a new prescription for an opioid is written. The strategy was designed to not only meet AHRQ recommendations for counseling, but also to support patients in all phases of the National Academy of Medicine’s Medication Use Process (prescribing, dispensing, self-administration, and monitoring).21 Herein we describe our intervention and its ability to improve safe use and patient knowledge of newly prescribed opioids.
METHODS
We conducted a three-arm, physician randomized pragmatic trial of the ED EMC2 Opioid Strategy to improve safe use and patient knowledge of newly prescribed opioids. A description of the study protocol and trial is described in greater detail in a previous publication.20 In brief, the primary goal of the study was to determine if the EMC2 intervention could improve demonstrated safe use of newly prescribed opioids. As a ‘point of care’ intervention, the need for ‘post visit’ reinforcement of the safe use educational messages was also considered. Thus, our approach was tested with and without a short message service (SMS) text messaging promotional component, understanding the need for a scalable follow-up component within a clinical context that does not routinely track patients beyond acute visits. Secondarily, we sought to determine if the EMC2 intervention could increase patient knowledge about opioid pain relievers. We hypothesized that compared to patients in the usual care arm, patients receiving EMC2 interventions would demonstrate higher rates of safe use of their prescribed opioid as measured by a demonstrated dosing task and secondarily demonstrate higher rates of opioid-related medication knowledge. The institutional review board at Northwestern University approved all study procedures, a data safety and monitoring board was established and met annually, and the trial was registered at clinicaltrials.gov [].
Randomized controlled trial study design
The trial employed a cluster-randomized design wherein the prescribing provider was the unit of randomization. This choice was made because of the automated nature of the EHR-based interventions. To increase the chances of having similar patient populations in each intervention arm, providers were placed into strata based on their roles (attending physician, resident physician and Advanced Practice Providers (APPs)), and historical volume of opioid prescriptions dispensed (high, medium, low) over the preceding two years (physician, APP) or by post-graduate year (residents). Providers from each strata were then randomized to one of three study arms; provider identities were revealed to study personnel after randomization was complete. Patients participating in the study received one of three care pathways based on the randomization allocation of the treating provider was who was ordering their discharge prescription within the EHR.
Participants
All attending and resident physicians and APPs based in the ED were approached for study inclusion. Those who agreed to be in the study were randomized as described. After consent, physician participants were informed of the changes that would be made to their EHR, but were not otherwise given targeted education and were not aware of study outcome measures.
Study participants were patients being discharged from an urban academic ED (annual volume >88,000) with a new prescription for hydrocodone-acetaminophen between July 2015 and August 2017. Patients were eligible for enrollment if they met the following five conditions: 1) 18 years of age or older, 2) English speaking, 3) prescribed a tablet form of hydrocodone-acetaminophen (non-liquid formulation), 4) responsible for self-administering their own medication, and 5) were discharged by a provider who consented to the study. Patients were excluded if they were clinically unstable, psychologically impaired or intoxicated as judged by the research staff or ED provider, chronically taking opioids (as defined by self-report of “daily or near daily” use of opioids for the past 90 days), being admitted to the hospital, unable to complete study follow-up, or pregnant. Hydrocodone-acetaminophen was selected for study because, at the time of study initiation, it was the most frequently prescribed medication at the both the study site and nationally.22 Additionally, it is commonly abused,23–25 frequently results in ED visits for overdose,26 and is among the top prescription opioids related to overdose death.27
Study Arms
The trial had three arms: Usual Care and two intervention arms named EMC2 and EMC2+ SMS Text Messaging. In the usual care (control) arm, providers had no modifications to their EHR interface, and their patients received discharge instructions, prescription instructions, and counseling about safe use per that provider’s customary practice. Of note, usual practice of the trial ED did not automatically include medication information sheets with discharge paperwork, as is done in some EDs; however, medication information documents could be manually added to the discharge documents at providers’ discretion. For providers assigned to the EMC2 arm, the three provider facing functions were ‘turned on’ and their patients were eligible to receive two of the educational materials from the ED EMC2 Opioid Strategy. Patients of providers in the EMC2 + SMS arm also received daily text messages to prompt safe use for one week following their ED visit.
Intervention
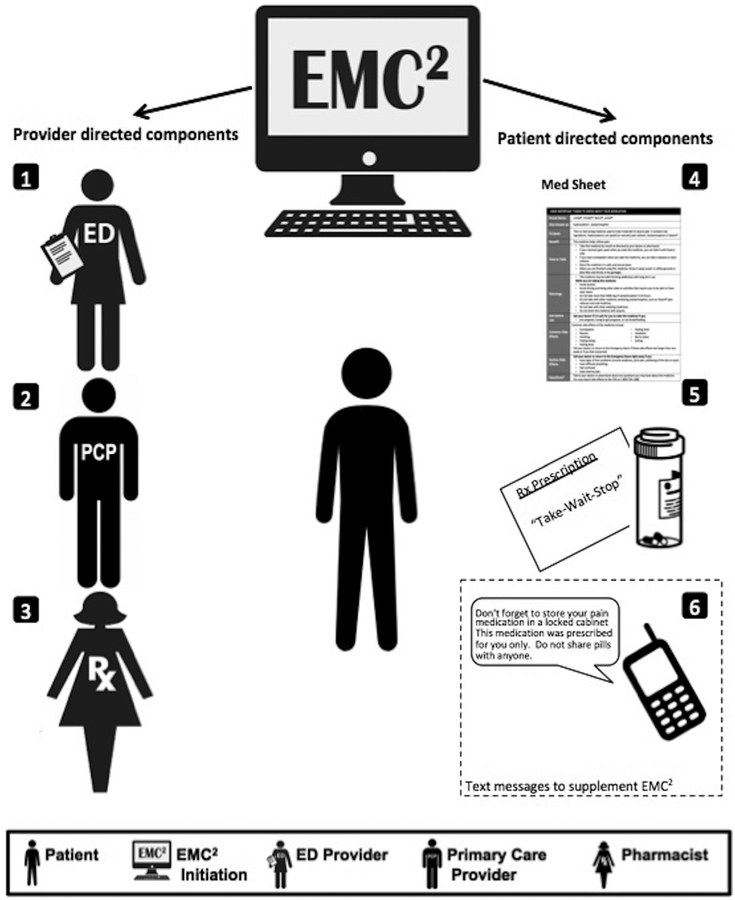
The ED EMC2 Opioid Strategy comprised five changes to the EHR that automatically triggered when any prescription for hydrocodone-acetaminophen was signed electronically.(Figure 1) The first three components targeted providers, whereas the latter two components targeted the patient directly. The three prescriber facing components included: 1) a provider medication alert reminding the prescribing ED physician to counsel the patient about safe use of opioids; 2) an inbox message delivered to the primary care outpatient provider informing them of the new prescription, pill quantity, and requesting that they follow-up with the patient to provide additional counseling about safe use; and 3) a request to the dispensing pharmacist to counsel the patient about safe use (printed automatically on the paper prescription requisition). In the event that there was no primary care provider, the inbox message could not be sent; however, the other two provider-facing components applied to all patients. The last two components of the ED EMC2 Opioid Strategy, both patient facing, were plain language MedSheets about hydrocodone-acetaminophen and “Take-Wait-Stop” patient-centered medication labeling changes made to the print prescription requisition.
Fig1.
ED EMC Opioid Strategy.
The MedSheets were previously developed by our team to provide the patient with understandable, actionable information written at an 8th grade reading level or below and formatted to result in higher recall of drug information compared to current FDA standard Medication Guides.28,29 Preliminary data demonstrated that inclusion of an opioid MedSheet in ED discharge instructions increased some aspects of patient knowledge.30
The Take-Wait-Stop prescription wording translates medications prescribed “as needed” into plain language with three deconstructed, actionable steps.31,32(Figure 2) The Take-Wait-Stop label was first developed by members of our team as an extension of the Universal Medication Schedule,31,32 and based on tenets of patient-centered prescription label design.33–37 The prescription wording places emphasis on action terms (“Take-Wait-Stop”) and deconstructs the components of PRN instructions to support understanding and recall. Anticipating the wording would be unfamiliar to community pharmacists, we additionally worked with a national community pharmacy chain manager to ensure the wording fit on a standard label. Further, the default Sig (i.e., signetur which is Latin for “let it be labeled”) on the print requisitions was changed to “Special Sig” and each prescription contained a “Note to Pharmacist: Please print the ‘Take-Wait-Stop’ instructions on the medication label” to draw attention to the wording change. This study was a pragmatic trial, so we did not ensure that each pharmacy could print the prescriptions, but had a planned analysis of prescription bottle implementation.38
Fig2.
Take-Wait-Stop prescription wording.
The sixth intervention component, only available to patients in the EMC2+SMS study arm, was SMS text messaging. Patients in this arm followed instructions on a pre-printed card and self-enrolled into an automated texting program that sent one text message per day for the week following enrollment. These educational messages focused on safe use, side effects, and safe behaviors related to prescription opioids. (Appendix 1)
The intervention was designed with the dual goal of maximizing communication whilst providing a scalable intervention with minimal workflow interruption. Further detail related to the pilot work supporting the design of this intervention and detail of each intervention component can be found in previous publications.20,30,31
Outcomes and Measures
Patients completed a baseline assessment and follow-up assessments at 2–4 days, 1–2 weeks and 1 month after enrollment and completed a paper medication diary for 10 days following discharge (returned via prepaid envelope). Baseline questions assessed sociodemographic characteristics, including health literacy (measured by the Newest Vital Sign (NVS)39) and self-report of prior use of hydrocodone. Visit characteristics (e.g. diagnosis, medications received in ED, pain scores) were obtained from the EHR. The 2–4 day and 1 month assessments were via telephone. The 1–2 week assessment was initially conducted in-person. However, due to low retention the assessment was switched to telephone.
The primary outcome was the patients’ ability to safely dose their opioid medication in a “demonstrated” dosing task. As part of this task, patients were asked to tell the RA how they would take their medication if they were in pain (“starting at 8am”). Participants were continually prompted as to when and how they would take their next dose if they were still in pain with prompting continuing until either 24 hours was reached or the patient reported that they would not take any additional pills. The prompt was left intentionally vague as to the degree of hypothetical pain to allow more freedom of response, better simulate the medication taking experience at home, and avoid patients feeling as if they had to “take” the medication because of “severe” pain. Three error types were assessed in a binary fashion: proper number of pills per dose, correct spacing of doses (recommended minimum number of hours between doses), and total pills per day (not exceeding the recommended/safe number of pills per day). As the prescription details varied patient-to-patient, each individual’s performance on the dosing task was assessed according to the wording of their prescription at discharge. For patients who did not have a “do not exceed’ statement on their prescription, the manufacturer’s recommendation was used (8 tabs daily for hydrocodone/acetaminophen 5/325 mg strength and 6 tabs daily for the 10/325 mg strength). Each error type was reported separately and in aggregate, with the primary outcome being the aggregate assessment.
The outcome measure of patient’s demonstrating dosing and frequency was informed by nearly decade of research by Michael Wolf and colleagues.35,40–42 Tasks of patient demonstration of medications dosing have been applied in studies examining inhaler technique in COPD,43 immunosupressants post-transplant,44,45 pediatric liquid dosing,46 non-prescription acetaminophen use,47 and among patients with complex regimens.48 These studies have consistently found that regimen dosing is associated with lower health literacy, cognitive function and visual acuity and, in some contexts, predictive of healthcare utilization.44
Secondary outcomes assessed included medication knowledge and actual safe hydrocodone-acetaminophen use. Medication knowledge was assessed using open-ended questions (e.g. “Do you know what ingredients are in this medicine?,” “Do you think the type of pain medicine that you were prescribed can be addictive?”). Responses were scored by trained RAs and reviewed by the study PI. A composite knowledge score (scale 0–10) was created comprised of ten items [(1) medication brand name, (2) acetaminophen as ingredient (3) hydrocodone as ingredient, (4) classification as opioid (or narcotic or controlled substance), (5) safe amount of alcohol to drink, (6) need to avoid sedating medicines, (7) potential for addiction, (8) need to avoid acetaminophen, (9) at least one GI side effect (e.g., nausea, constipation), (10) at least one sedating side effect (e.g., sleepy, dizzy, tired)]. Baseline knowledge was not assessed to avoid priming patients to knowledge items (e.g., the assessment would have been an intervention in and of itself); instead a randomized controlled study design was chosen. The EMC2 intervention was expected to optimize knowledge on all of the above topics; messages related to # 2, 5, 6, 8, and 9 were additionally targeted specifically by text messages.
Safe use in actual practice was assessed from medication diary data. The outcome was assessed in two parts: 1) an aggregate of the same three error types for the demonstrated use assessment described above (“actual safe dosing”); 2) “actual safe dosing + no sedating medications” comprised of the 3 error types above and a binary assessment of concomitant use with sedating medications. For the assessment of concomitant use of hydrocodone-acetaminophen with sedating medications, a list of drug categories was determined a priori by an experienced ED Clinical Pharmacist and Doctor of Pharmacy (AEL) based on a review of risk level and interaction classification within Micromedex, Lexi-Drugs, Clinical Pharmacology and primary literature. This list was reviewed and modified by a board certified toxicologist and addiction medicine specialist (PML). (Appendix 2) Patients were considered to have concomitant use with a sedating medication if they took a medication on the list within the same day as taking their hydrocodone-acetaminophen as it was beyond the scope of this study to adjust for other factors that would influence the likelihood of an adverse event from concomitant use (e.g., duration of medication action, chronicity of use, half-life of individual drugs).
The study was conducted as a pragmatic trial to assess how the intervention would operate in the real world if it were “turned on” and left to run without interference or repeated instruction. We measured the rate of successful printing of the MedSheet, the prescription requisition printing with the Take-Wait-Stop instructions, commercial pharmacies filling the pill bottles per the Take-Wait-Stop directions, and successful enrollment in the texting platform.
Analysis
Descriptive statistics were calculated for all socio-demographic, ED visit, and opioid prescription characteristics. Chi-square, one-way Analysis of Variance (ANOVA), and Wilcoxon rank-sum tests were used, as appropriate, to test for balanced randomization. An intercluster correlation assessment was conducted to determine the degree of independence among individuals in the same cluster. We used generalized linear mixed models on all outcomes adjusting for physician clustering. Models were run for dichotomous dosing outcomes (adjusted odds rations [aORs] reported) and for continuous composite knowledge score (adjusted mean differences reported). Examination for possible associations between patient characteristics and outcome variables were conducted and all models controlled for covariates related to that specific outcome. To isolate the additive benefit of the text messaging, the two intervention arms were compared to each other on the primary and secondary outcomes as well as the individual knowledge questions that comprised the knowledge score. Significance for all analyses was set at p < 0.025 to adjust for multiple testing.
All analyses were first conducted using an intent-to-treat approach. Subsequently, because some patients did not receive one or more pieces of the intervention, the primary outcome was analyzed per-protocol, where the same methodology described previously was applied only to patients who successfully received all patient-facing pieces of the intervention. Patients in the EMC2 arm were included in the per-protocol analysis if they received the MedSheet and the patient-centered TWS prescription wording on their bottle. Those in the EMC2 +SMS arm were included if they received the MedSheet, TWS prescription wording on bottle, successfully enrolled in SMS texting, and received all seven text messages.
The planned enrollment target was for 816 patients to complete the 1–2 week follow-up interval, which would have provided 80% power to detect a difference of 11.8% (from pilot data)31 between each of the intervention arms and the usual care arm for the primary outcome of any error on the demonstrated dosing task. All analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
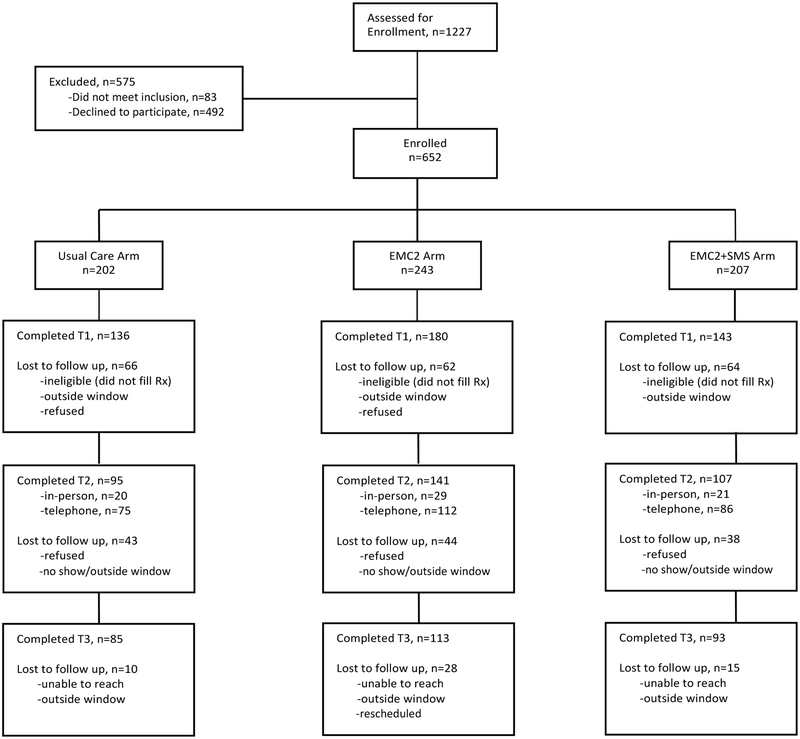
Participant Flow
Among 126 providers at the study site, 116 (92%) consented and were eligible for their patients to be enrolled. A total of 652 patients were enrolled, for an overall cooperation rate of 57% of those approached (n=1144). At the first follow up call (2–4 days) retention rates were 67.3%, 74.1% and 69.1% in the usual care, EMC2, and EMC2 +SMS groups, respectively. The primary outcome was assessed at the second time point (1–2 weeks post enrollment); at that time the retention rates were 47.0%, 58.0%, and 51.7%, respectively. By the third follow-up call (1 month post enrollment), retention rates were 42.1%, 46.5%, and 44.9%. A total of 260 (39.8%) medication diaries were returned, including 223 diaries from patients who also completed the primary outcome assessment. Attrition rates for follow-up calls and medication diary return did not differ by arm; however, they did differ by other characteristics with participants completing the study being older (mean age 45 years versus 40 years among those who dropped out) and higher literacy (48% adequate literacy and 37.9% limited or marginal literacy completed). For medication diaries, those returning diaries were older, with higher educational attainment, literacy and household earnings and were less likely to be uninsured or from a racial/ethnic minority (data not shown).
Baseline Data
Participant characteristics, overall and by arm, are summarized in Table 1. At baseline, participants had a mean age of 42 years and slightly more than half were female. Overall, the sample had a high degree of educational attainment and a similarly high rate of adequate literacy (66.4%). The majority of patients had not been previously prescribed hydrocodone, but did receive opioids in the course of their ED visit. The most common diagnosis was back pain, and the prescriptions provided were of small pill quantity (mean 15 tabs).
Table 1:
Participant Demographic, ED Visit, and Prescription Characteristics Overall and by Study Arm
| Characteristic | Total | Usual Care Arm | EMC2 Intervention Arm | EMC2 +SMS Intervention Arm |
|---|---|---|---|---|
| N=652 | n=202 | n=243 | n=207 | |
| Demographic Characteristics | ||||
| Age, mean years (SD) | 42.2 (14.0) | 43.3 (14.2) | 42.3 (14.4) | 41.3 (13.3) |
| Female gender, % | 57.1 | 55.9 | 55.6 | 59.9 |
| Race, % | ||||
| White | 46.9 | 47.3 | 45.5 | 48.3 |
| African American | 30.8 | 32.8 | 29.3 | 30.4 |
| Other | 22.3 | 19.9 | 25.2 | 21.3 |
| Education, % | ||||
| High school grad or less | 18.0 | 17.3 | 18.1 | 18.4 |
| Some college | 31.8 | 34.7 | 31.3 | 29.6 |
| College graduate | 31.3 | 26.2 | 33.7 | 33.5 |
| Graduate degree | 18.9 | 21.8 | 16.9 | 18.4 |
| Income Level, % | ||||
| <=$40,000 | 30.6 | 32.6 | 30.9 | 28.0 |
| >$40,000-$100,000 | 34.7 | 37.0 | 35.0 | 32.0 |
| >$100,000 | 34.7 | 30.4 | 34.1 | 40.0 |
| Health Literacy, % | ||||
| Low+Marginal | 33.6 | 35.1 | 32.9 | 32.9 |
| Adequate | 66.4 | 64.9 | 67.1 | 67.1 |
| Primary Insurance, % | ||||
| Medicaid | 18.0 | 17.9 | 19.2 | 16.7 |
| Medicare | 7.6 | 10.9 | 6.3 | 5.9 |
| Private/Managed Care | 63.5 | 58.7 | 65.7 | 65.5 |
| Self or no insurance | 6.4 | 6.5 | 5.9 | 6.9 |
| Other | 4.5 | 6.0 | 2.9 | 4.9 |
| Self-Reported Health Status, % | ||||
| Excellent | 14.9 | 14.9 | 16.1 | 13.6 |
| Very Good | 35.9 | 31.8 | 38.0 | 37.4 |
| Good | 31.7 | 33.3 | 29.8 | 32.5 |
| Fair | 15.3 | 18.9 | 12.8 | 14.6 |
| Poor | 2.2 | 1.0 | 3.3 | 1.9 |
| Previously Prescribed Hydrocodone, % | 38.9 | 36.9 | 39.1 | 40.6 |
| ED Visit characteristics | ||||
| Triage Acuity, % | ||||
| 1 & 2 | 8.3 | 7.5 | 7.9 | 10.0 |
| 3 | 54.7 | 53.7 | 55.6 | 56.5 |
| 4 & 5 | 35.9 | 38.8 | 36.4 | 33.5 |
| Triage pain score, mean (sd) | 7.7 (2.3) | 7.6 (2.2) | 7.7 (2.4) | 7.7 (2.3) |
| Total Length of Stay (hours), median (IQR) | 3.9 (2.9–5.2) | 3.6 (2.6–5) | 3.9 (2.8–5) | 4.1 (3.1–5.8) |
| Exposure to Opioids in the ED, % | 86.4 | 86.6 | 84.4 | 88.2 |
| Diagnosis category | ||||
| Back Pain | 18.9 | 21.8 | 16.5 | 18.8 |
| Fractures/Dislocations | 15.5 | 14.4 | 17.7 | 14.0 |
| Extremity injuries (non-fracture) | 16.9 | 14.4 | 20.6 | 15.0 |
| Kidney Stone | 14.7 | 14.9 | 16.1 | 13.0 |
| Other | 34.1 | 34.7 | 29.2 | 39.1 |
| Opioid Prescription characteristics | ||||
| Daily MME prescribed, mean (sd) | 30.8 (13.4) | 29.4 (12.3) | 30.2 (12.2) | 32.7 (15.3) |
| Tabs of opioid prescribed, mean (sd) | 15 (6.9) | 14.7 (6.4) | 16 (7.8) | 14 (5.9) |
Outcomes
Overall 76.4% of patients demonstrated safe use of their newly prescribed opioid with the highest rate of safe use in the EMC2 arm (82.0%). Demonstrated safe use occurred more often in the EMC2 group (adjusted odds ratio (aOR), 2.46; 95% CI, 1.19, 5.06), but not the EMC2 + SMS group (aOR 1.87; 95% CI 0.90, 3.90) compared with usual care.
Less than half (39.8%, n=260) of patients returned medication diaries. There were no differences between arms in either of the actual use aggregate assessments as measured by medication diary data (Table 2). Notably, among participants who both returned the medication dairy and completed the demonstrated dosing task (n=223), the overall error rate on the demonstrated dosing task was 19.7% (23.4% control, 16.5% EMC2, 20.3% EMC2+SMS, p=0.57). In contrast, those who did not return medication dairies (n=74) had higher demonstrated dosing error rates across all arms (35.1%), but particularly the control arm (66.7% control, 21.6% EMC2, 36.4% EMC2+SMS, p=0.01).
Table 2:
Outcome Measures by study arm
| EMC2 vs Usual Care | EMC2 +SMS vs Usual Care | |||||
|---|---|---|---|---|---|---|
| Usual Care Arm | EMC2 Arm | EMC2+SMS Arm | Adjusted Model Outcome | Adjusted Model Outcome | ||
| % | % | % | aOR (95% CI) | aOR (95% CI) | ||
| PRIMARY SAFE USE OUTCOME | ||||||
| Outcome of Demonstrated Dosing Activity | ||||||
| Exceeded maximum daily dose | 1.2 | 6.5 | 3.1 | -- | -- | |
| More than prescribed pills per dose | 3.6 | 4.0 | 2.0 | -- | -- | |
| Shorter interval than prescribed | 26.6 | 15.6 | 22.9 | -- | -- | |
| Demonstrated Safe Use (no errors)a | 68.4 | 82.0 | 76.0 | 2.46 (1.19, 5.06)* | 1.87 (0.90, 3.90) | |
| SECONDARY SAFE USE OUTCOMES | ||||||
| Actual Use based on Medication Diary (N=260) | ||||||
| Exceeded maximum daily dose | 1.3 | 2.0 | 0.0 | -- | -- | |
| More than prescribed pills per dose | 10.7 | 8.0 | 7.4 | -- | -- | |
| Shorter interval than prescribed | 9.3 | 10.0 | 5.9 | -- | -- | |
| Actual Safe Dosing (no errors)b | 84.0 | 85.0 | 89.4 | 1.04 (0.44, 2.44) | 1.59 (0.61, 4.15) | |
| Use with sedating medication | 37.3 | 24.0 | 28.2 | -- | -- | |
| Actual Safe Dosing + No Sedating Medications (no errors)b | 52.0 | 67.0 | 65.9 | 1.82 (0.95, 3.48) | 1.75 (0.89, 3.44) | |
| KNOWLEDGE | Beta (95% CI) | Beta (95% CI) | ||||
| Knowledge Composite Score, Mean (SD)c | 5.6 (1.5) | 5.6 (1.8) | 6.2 (1.7) | −0.02 (−0.47, 0.42) | 0.57 (0.09, 1.06)* | |
aOR: adjusted Odds Ratio
adjusted for health literacy and clustering
adjusted for clustering
adjusted for race, income, health literacy, and clustering
P value <0.025 compared to Usual Care Arm
Participants in the usual care arm had the highest rates of concomitant use of sedating medications (30.7%) compared to the EMC2 and EMC2 +SMS (21.0% and 25.9%, respectively) yet again this difference was not statistically significant. (Table 2) The most frequently used class of sedating medication was benzodiazepines (13.1%). (additional results of co-ingested sedating medications available Appendix 3)
Patients in the EMC2 + SMS arm had higher composite knowledge scores [mean (sd), 6.2 (1.7); beta (95% CI) 0.57 (0.09, 1.06)] than usual care [mean (sd) 5.6 (1.5)] or EMC2 participants [5.6 (1.8)]. Specifically evaluating the strength of the deconstructed components of the intervention (EMC2 versus EMC2+SMS) revealed that the text messages were significantly linked to three knowledge items (able to name acetaminophen as ingredient, aware of need to avoid acetaminophen, aware of need to avoid sedating medications) (Table 3).
Table 3:
Outcome Measures – By Intervention Component
| Texting vs. EMC2 | ||
|---|---|---|
| aOR (95% CI) | P-value | |
| PRIMARY SAFE USE OUTCOME | ||
| Demonstrated Safe Usea | 0.81 (0.36, 1.82) | 0.60 |
| SECONDARY SAFE USE OUTCOME | ||
| Actual Safe Dosing (no errors)+b | 0.96 (0.50, 1.85) | 0.90 |
| Actual Safe Dosing +No Sedating Medications+b | 1.53 (0.63, 3.72) | 0.35 |
| KNOWLEDGEc | ||
| Knowledge of Medication Details (N=459) | ||
| Able to identify by brand name | 1.70 (0.82, 3.49) | 0.15 |
| Able to name Acetaminophen as an active ingredient+ | 2.46 (1.16, 5.22) | 0.02 |
| Able to name Hydrocodone as an active ingredient | 1.26 (0.66, 2.39) | 0.49 |
| Aware that medication is a narcotic/controlled substance | 0.82 (0.38, 1.78) | 0.62 |
| Awareness of Precautions (N=343) | ||
| Aware of safe amount of alcohol to drink+ | 0.88 (0.38, 2.04) | 0.77 |
| Aware of need to avoid other sedating medicines+ | 2.17 (1.20, 3.91) | 0.01 |
| Aware that prescribed medication can be addictive | 0.57 (0.25, 1.29) | 0.18 |
| Aware that you should avoid Acetaminophen+ | 2.72 (1.49, 4.96) | 0.001 |
| Side Effects (N=343) | ||
| Recognized at least one GI side effect (vomiting, nausea, constipation)+ | 1.28 (0.69, 2.35) | 0.43 |
| Recognized at least one sedating side effect (sleepy, dizzy, tired) | 1.01 (0.58, 1.74) | 0.98 |
| Beta (95% CI) | ||
| Knowledge Composite Scorec | 0.61 (0.13, 1.08) | 0.014 |
aOR: adjusted Odds Ratio
adjusted for health literacy and clustering
adjusted for clustering
adjusted for race, income, health literacy, and clustering
Indicates questions that were part of the text messages
Process measures
The processes that occurred at the time of the ED visit had a high level of successful inclusion in the discharge documents, with 78% of patients in the intervention arms receiving printed MedSheets automatically (91% ultimately received the MedSheet after it was noted to be missing and the discharge documents were reprinted). The printing failure was a computer programming issue wherein the MedSheet was included if the prescription was written through the “orders” interface, but not the “discharge” interface. Sixty two percent of intervention patients responded they still had their information sheet. Within the EMC2 +SMS arm 93% of patients successfully enrolled in texting. Only 19 patients (10%) opted out of the texting intervention before all messages were delivered. Although the Take-Wait-Stop prescription requisition successfully printed in the ED for 95% of patients in the intervention arms, when those same patients were re-assessed at 1–2 week follow-up (n=211), only 38 (18.0%) had a label on their prescription bottle that corresponded with the Take-Wait-Stop (verbatim or near verbatim) instructions as written on the requisition. An additional 93 patients had wording with three action steps that was considered “adequate” implementation of the label, but not per-protocol. In contrast, 96.4% of control arm patients had labels with traditional PRN wording. A separate manuscript further evaluates the variations in the prescription filling in this sample.38
Per Protocol Analysis
The low rates of the prescription being filled per the protocol prompted a per-protocol analysis for the primary outcome. There were no significant differences by arm in baseline demographics for participants when analyzed per protocol (data not shown). Compared to participants in the usual care arm, those who completed EMC2 and EMC2+SMS “per protocol” had 1.48 (95% CI: 0.5, 4.6) and 2.48 (95% CI: 0.6, 9.6) higher odds of demonstrating proper dosing respectively when adjusting for physician clustering and health literacy level.
DISCUSSION
The ED EMC2 Opioid Strategy, designed to support patient education and counseling about safe opioid use, while minimizing burden of providers, had some significant, but overall variable influence on the outcomes studied. We evaluated outcomes in multiple domains, including demonstrated and actual medication safe use and medication knowledge. The EMC2 intervention led to higher rates of safe use when patients were asked to objectively demonstrate how they would take medication, yet our strategy did not show any improvement in actual use based on how participants recorded their medication taking behaviors via daily diaries. In light of the differences on the demonstrated dosing task between those who did/did not return the medication diaries, it is possible that the lack of difference in actual dosing is related to both low power and low rate of return among patients more prone to dosing errors rather than the strength of the intervention. While it is unclear to what extent either of these two measures validly represents a patient’s true opioid use, there is inherent value in ability of the EMC2 intervention - and specifically the Take-Wait-Stop label – to assist patients in understanding how to most appropriately dose out their medication.
Interestingly, patients were able to report more awareness of the need to avoid sedating medicines, but there were no differences in actual concomitant use between the groups. This finding supports the notion that possessing knowledge about medication risks is likely necessary but not sufficient to ensure safe use, as medication taking behaviors are often influenced by complex factors in addition to knowledge such as health literacy, self-efficacy, and attitudes.49 Although not influenced by the intervention, the data reveal the prevalence in this population of using opioids with sedating medications. Nearly one-third of the overall sample were taking their newly prescribed opioid during the same day as a sedating medication [both newly prescribed (e.g., cyclobenzaprine) and chronically used (e.g., alprazolam)]. Concomitant use of opioids with sedating medications, particularly benzodiazepines, is emerging as a significant contributor to opioid-related mortality50 and underscores the need to translate the knowledge gains we achieved with the intervention into action. The physician writing the newly prescribed opioid may be the better target for eliminating this form of “misuse.” Although the data are reported on a patient level, the root cause of the error is more likely on the prescriber side as the physician ordering the opioid should be aware of the risks of concomitant use with both chronically used and newly prescribed sedating medications and consider alternate analgesics.
Patients in the EMC2 + SMS arm had higher composite knowledge scores than both patients in the usual care and EMC2 arms supporting the use of SMS text message delivery after the visit to increase knowledge. Although the score was higher, it is unclear if this finding is clinically meaningful. The higher knowledge on the need to avoid both sedating medications and acetaminophen are arguably among the more “important” components of the score because co-ingestion of opioids with both sedating medications and acetaminophen are linked to mortality.50–52 Several other recent studies have employed a variety of interventions to achieve knowledge gain about opioids in the ED including nurse led teach-back,53 medication information sheets,30 web-based education (delivered in the ED)54 and video discharge instructions.55 However, the studies have used different knowledge metrics, making it difficult to compare the interventions’ relative strength.
The variable success of the text messages in our trial raises questions as to why some messages changed knowledge and others did not. Effectively communicating risk is challenging, particularly in the ED, and additional research is needed to determine the best way to communicate risk,56 particularly the risk of addiction—an item on which our intervention was not successful. While SMS texting has been used previously in the setting of medication adherence to encourage routine use from the ED (e.g., diabetes, hypertension, antibiotics);57–59 the goal of our text messages was not to encourage routine use, but rather to reinforce the salience of educational messages delivered at the time of the ED visit or provide a cue to action. The success of the SMS texting component is one of several recent examples of extending the reach of emergency care into the post-visit time period through technology (text messaging, mobile applications, telehealth), a growing and promising avenue for behavioral interventions.59–67 We believe that technology delivered interventions such EMC2 + SMS have great potential in the context of pain management, not only because of their reach into the post-visit space wherein the patient is less distracted, but also because the interventions are scalable and can be delivered at the time of the behavior being targeted (e.g. medication taking, pill disposal).68
LIMITATIONS
Patients were recruited from a single site in an urban area with a patient population that was relatively well educated and earned a high household income, limiting generalizability. Due to the study design and intervention delivery, patients needed to be consented post randomization, introducing selection bias. Additionally, the trial met with several recruitment challenges that we have detailed in Appendix 4 along with steps taken to improve recruitment, retention and medication diary return. Although overall recruitment and retention did improve, some of the changes, including the switch from in-person to telephone interview may have influenced measurement. Despite these attempts to improve recruitment, ultimately, the biggest limitation of the study was not reaching the planned enrollment target. In addition to the recruitment and retention challenges noted above, we had a low rate of return of the medication diaries limiting generalizability from that data source.
An additional limitation is the use of dose demonstration and medical diaries. Demonstrated dosing is an abstraction of actual dosing that may overestimate errors by prompting the patient to take the medications maximally for a 24 hour “day.” Medication diaries are subject to patient recall bias and had a low rate of return, particularly in patients who performed more poorly on the demonstrated dosing task and may be at higher risk for label misunderstanding due to lower literacy.
Further, a minority of the intervention patients received the verbatim Take-Wait-Stop label on their pill bottle. The reasons for this low implementation are likely multiple, including: pharmacist unfamiliarity with the label and additional time and workflow interruption to manually type the label rather than using preprogramed “quick codes.”
CONCLUSION
We found that the intervention improved demonstrated safe dosing of opioids and increased patient knowledge. However, there was no influence of the intervention on actual safe medication use amongst the portion of the sample returning medication diaries. While not discounting the importance of bedside communication, future ED interventions may opt to focus on post-discharge communication as the greatest increases in knowledge in this sample were amongst patients receiving the text-messaging portion of the intervention.
Supplementary Material
Fig. 3.
Consort flowchart. SMS = short message service.
Acknowledgments:
We would like to acknowledge the research assistants, project coordinators, and analysts who assisted with data collection and preliminary analysis.
Funding sources: This project was supported by grant number R18HS023459 (PI: McCarthy) from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
REDCap is supported at FSM by the Northwestern University Clinical and Translational Science (NUCATS) Institute. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Michael S Wolf has served as a consultant on health literacy measurement with Merck, Sharp & Dohme Corp. Andrea M Russell has received support from a pre-doctoral risk communication fellowship from Amgen Inc. All other authors have no conflicts to disclose.
Footnotes
Presentations: oral presentation at Society for Academic Emergency Medicine 2019
REFERENCES
- 1.Kuehn B Opioid Emergency Declared. JAMA 2017;318:2418. [DOI] [PubMed] [Google Scholar]
- 2.Lowenstein M, Grande D, Delgado MK. Opioid Prescribing Limits for Acute Pain - Striking the Right Balance. N Engl J Med 2018;379:504–6. [DOI] [PubMed] [Google Scholar]
- 3.Michael SS, Babu KM, Androski C Jr., Reznek MA. Effect of a Data-driven Intervention on Opioid Prescribing Intensity Among Emergency Department Providers: A Randomized Controlled Trial. Acad Emerg Med 2018;25:482–93. [DOI] [PubMed] [Google Scholar]
- 4.Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ Jr., An Educational Intervention Decreases Opioid Prescribing After General Surgical Operations. Ann Surg 2018;267:468–72. [DOI] [PubMed] [Google Scholar]
- 5.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- 6.Rui P, Kang K, Albert M. National Hospital Ambulatory Medical Care Survey: 2013 Emergency Department Summary Tables. 2013. (Accessed June 7, 2019, at http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf)
- 7.Eisenberg EM, Murphy AG, Sutcliffe K, et al. Communication in emergency medicine: Implications for patient safety. Communication Monographs 2005;72:390–413. [Google Scholar]
- 8.Engel KG, Buckley BA, McCarthy DM, Forth VE, Adams JG. Communication Amidst Chaos: Challenges to Patient Communication in the Emergency Department. Journal of clinical outcomes management: JCOM 2010;Vol. 17. [Google Scholar]
- 9.Knopp R, Rosenzweig S, Bernstein E, Totten V. Physician-patient communication in the emergency department, part 1. Acad Emerg Med 1996;3:1065–9. [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig S “Emergency rapport”. J Emerg Med 1993;11:775–8. [DOI] [PubMed] [Google Scholar]
- 11.Engel KG, Heisler M, Smith DM, Robinson CH, Forman JH, Ubel PA. Patient comprehension of emergency department care and instructions: are patients aware of when they do not understand? Ann Emerg Med 2009;53:454–61 e15. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy DM, Cameron KA, King JP, et al. Patient recall of health care provider counseling for opioid-acetaminophen prescriptions. Pain Med 2014;15:1750–6. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy DM, Engel KG, Cameron KA. Conversations about analgesics in the emergency department: A qualitative study. Patient Educ Couns 2016;99:1130–7. [DOI] [PubMed] [Google Scholar]
- 14.Conrardy M, Lank P, Cameron KA, et al. Emergency Department Patient Perspectives on the Risk of Addiction to Prescription Opioids. Pain Med 2016;17:114–21. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy DM, Cameron KA, Courtney DM, Adams JG, Engel KG. Communication about opioid versus nonopioid analgesics in the emergency department. J Opioid Manag 2015;11:229–36. [DOI] [PubMed] [Google Scholar]
- 16.Smith RJ, Rhodes K, Paciotti B, Kelly S, Perrone J, Meisel ZF. Patient Perspectives of Acute Pain Management in the Era of the Opioid Epidemic. Ann Emerg Med 2015;66:246–52 e1. [DOI] [PubMed] [Google Scholar]
- 17.20 Tips to Help Prevent Medical Errors. Patient Fact Sheet. Agency for Healthcare Research and Quality, 2018. (Accessed June 7, 2019, at https://www.ahrq.gov/patients-consumers/care-planning/errors/20tips/index.html) [Google Scholar]
- 18.Quick Tips—When Getting a Prescription. Agency for Healthcare Research and Quality, 2002. (Accessed June 7, 2019, at https://archive.ahrq.gov/patients-consumers/diagnosis-treatment/treatments/tipprescrip/tipprescrip.html)
- 19.Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care 1998;36:1138–61. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy DM, Courtney DM, Lank PM, et al. Electronic medication complete communication strategy for opioid prescriptions in the emergency department: Rationale and design for a three-arm provider randomized trial. Contemp Clin Trials 2017;59:22–9. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine Preventing Medication Errors. Washington DC: The National Academies Press; 2007. [Google Scholar]
- 22.Bartholow M Top 200 Drugs of 2010. 2011. (Accessed May 1, 2019, at https://www.pharmacytimes.com/publications/issue/2011/may2011/top-200-drugs-of-2010)
- 23.Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J 2011;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young AM, Havens JR, Leukefeld CG. A comparison of rural and urban nonmedical prescription opioid users’ lifetime and recent drug use. Am J Drug Alcohol Abuse 2012;38:220–7. [DOI] [PubMed] [Google Scholar]
- 25.Rosenblum A, Parrino M, Schnoll SH, et al. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend 2007;90:64–71. [DOI] [PubMed] [Google Scholar]
- 26.Crane EH. The CBHSQ Report: Emergency Department Visits Involving Narcotic Pain Relievers. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2015. (Accessed June 7, 2019, at https://www.samhsa.gov/data/sites/default/files/report_2083/ShortReport-2083.html) [PubMed] [Google Scholar]
- 27.Hedegaard H, Bastian BA, Trinidad JP, Spencer M, Warner M. Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2011–2016. Natl Vital Stat Rep 2018;67:1–14. [PubMed] [Google Scholar]
- 28.Wolf MS, King J, Wilson EA, et al. Usability of FDA-approved medication guides. J Gen Intern Med 2012;27:1714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf MS, Bailey SC, Serper M, et al. Comparative effectiveness of patient-centered strategies to improve FDA medication guides. Med Care 2014;52:781–9. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy DM, Wolf MS, McConnell R, et al. Improving patient knowledge and safe use of opioids: a randomized controlled trial. Acad Emerg Med 2015;22:331–9. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy DM, Davis TC, King JP, et al. Take-Wait-Stop: a patient-centered strategy for writing PRN medication instructions. J Health Commun 2013;18 Suppl 1:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey SC, Wolf MS, Lopez A, et al. Expanding the Universal Medication Schedule: a patient-centred approach. BMJ Open 2014;4:e003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Council for Prescription Drug Programs. Universal Medication Schedule White Paper. 2013. (Accessed June 7, 2019, at https://www.ncpdp.org/members/pdf/201304.UMS.WhitePaper.pdf)
- 34.Sahm LJ, Wolf MS, Curtis LM, et al. What’s in a label? An exploratory study of patient-centered drug instructions. Eur J Clin Pharmacol 2012;68:777–82. [DOI] [PubMed] [Google Scholar]
- 35.Shrank WH, Parker R, Davis T, et al. Rationale and design of a randomized trial to evaluate an evidence-based prescription drug label on actual medication use. Contemp Clin Trials 2010;31:564–71. [DOI] [PubMed] [Google Scholar]
- 36.Wolf MS, Davis TC, Curtis LM, et al. Effect of standardized, patient-centered label instructions to improve comprehension of prescription drug use. Med Care 2011;49:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King JP, Davis TC, Bailey SC, et al. Developing consumer-centered, nonprescription drug labeling a study in acetaminophen. Am J Prev Med 2011;40:593–8. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy DM, Russell AM, Eifler MR, et al. Implementation fidelity of patient-centered prescription label to promote opioid safe use. Pharmacoepidemiol Drug Saf 2019;(in press). [DOI] [PubMed] [Google Scholar]
- 39.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005;3:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf MS, Davis TC, Curtis LM, et al. A Patient-Centered Prescription Drug Label to Promote Appropriate Medication Use and Adherence. J Gen Intern Med 2016;31:1482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf MS, Curtis LM, Waite K, et al. Helping patients simplify and safely use complex prescription regimens. Arch Intern Med 2011;171:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis TC, Federman AD, Bass PF 3rd, et al. Improving patient understanding of prescription drug label instructions. J Gen Intern Med 2009;24:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Conor R, Muellers K, Arvanitis M, et al. Effects of health literacy and cognitive abilities on COPD self-management behaviors: A prospective cohort study. Respir Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serper M, Patzer RE, Reese PP, et al. Medication misuse, nonadherence, and clinical outcomes among liver transplant recipients. Liver Transpl 2015;21:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patzer RE, Serper M, Reese PP, et al. Medication understanding, non-adherence, and clinical outcomes among adult kidney transplant recipients. Clin Transplant 2016;30:1294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin HS, Parker RM, Sanders LM, et al. Liquid Medication Errors and Dosing Tools: A Randomized Controlled Experiment. Pediatrics 2016;138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mullen RJ, Curtis LM, O’Conor R, et al. Visual acuity, literacy, and unintentional misuse of nonprescription medications. Am J Health Syst Pharm 2018;75:e213–e20. [DOI] [PubMed] [Google Scholar]
- 48.Bailey SC, Sarkar U, Chen AH, Schillinger D, Wolf MS. Evaluation of Language Concordant, Patient-Centered Drug Label Instructions. J Gen Intern Med 2012;27:1707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey SC, Oramasionwu CU, Wolf MS. Rethinking adherence: a health literacy-informed model of medication self-management. J Health Commun 2013;18 Suppl 1:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones CM, McAninch JK. Emergency Department Visits and Overdose Deaths From Combined Use of Opioids and Benzodiazepines. Am J Prev Med 2015;49:493–501. [DOI] [PubMed] [Google Scholar]
- 51.Abrahamsson T, Berge J, Ojehagen A, Hakansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend 2017;174:58–64. [DOI] [PubMed] [Google Scholar]
- 52.Serper M, Wolf MS, Parikh NA, Tillman H, Lee WM, Ganger DR. Risk Factors, Clinical Presentation, and Outcomes in Overdose With Acetaminophen Alone or With Combination Products: Results From the Acute Liver Failure Study Group. J Clin Gastroenterol 2016;50:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waszak DL, Mitchell AM, Ren D, Fennimore LA. A Quality Improvement Project to Improve Education Provided by Nurses to ED Patients Prescribed Opioid Analgesics at Discharge. J Emerg Nurs 2018;44:336–44. [DOI] [PubMed] [Google Scholar]
- 54.McCauley JL, Back SE, Brady KT. Pilot of a brief, web-based educational intervention targeting safe storage and disposal of prescription opioids. Addict Behav 2013;38:2230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakravarthy B, Somasundaram S, Mogi J, et al. Randomized pilot trial measuring knowledge acquisition of opioid education in emergency department patients using a novel media platform. Subst Abus 2018;39:27–31. [DOI] [PubMed] [Google Scholar]
- 56.Meisel ZF, Smith RJ. Engaging patients around the risks of opioid misuse in the emergency department. Pain Manag 2015;5:323–6. [DOI] [PubMed] [Google Scholar]
- 57.Buis L, Hirzel L, Dawood RM, et al. Text Messaging to Improve Hypertension Medication Adherence in African Americans From Primary Care and Emergency Department Settings: Results From Two Randomized Feasibility Studies. JMIR Mhealth Uhealth 2017;5:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arora S, Peters AL, Burner E, Lam CN, Menchine M. Trial to examine text message-based mHealth in emergency department patients with diabetes (TExT-MED): a randomized controlled trial. Ann Emerg Med 2014;63:745–54 e6. [DOI] [PubMed] [Google Scholar]
- 59.Suffoletto B, Calabria J, Ross A, Callaway C, Yealy DM. A mobile phone text message program to measure oral antibiotic use and provide feedback on adherence to patients discharged from the emergency department. Acad Emerg Med 2012;19:949–58. [DOI] [PubMed] [Google Scholar]
- 60.Ranney ML, Suffoletto B. Extending our reach: use of mHealth to support patients after emergency care. Ann Emerg Med 2014;63:755–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choo EK, Ranney ML, Aggarwal N, Boudreaux ED. A systematic review of emergency department technology-based behavioral health interventions. Acad Emerg Med 2012;19:318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ranney ML, Pittman SK, Dunsiger S, et al. Emergency department text messaging for adolescent violence and depression prevention: A pilot randomized controlled trial. Psychol Serv 2018;15:419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mello MJ, Bromberg JR, Baird J, et al. Feasibility and Acceptability of an Electronic Parenting Skills Intervention for Parents of Alcohol-Using Adolescent Trauma Patients. Telemed J E Health 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ranney ML, Freeman JR, Connell G, et al. A Depression Prevention Intervention for Adolescents in the Emergency Department. J Adolesc Health 2016;59:401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kmiec J, Suffoletto B. Implementations of a text-message intervention to increase linkage from the emergency department to outpatient treatment for substance use disorders. J Subst Abuse Treat 2019;100:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mastroleo NR, Celio MA, Barnett NP, et al. Feasibility and Acceptability of a Motivational Intervention Combined with Text Messaging for Alcohol and Sex Risk Reduction with Emergency Department Patients: A Pilot Trial. Addict Res Theory 2019;27:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suffoletto B, Kristan J, Chung T, et al. An Interactive Text Message Intervention to Reduce Binge Drinking in Young Adults: A Randomized Controlled Trial with 9-Month Outcomes. PLoS One 2015;10:e0142877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neill LA, Kim HS, Cameron KA, et al. Who Is Keeping Their Unused Opioids and Why? Pain Med 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.