Abstract
Background:
Multiple risk factors predict temporomandibular disorders (TMD) onset, but temporal changes in risk factors and their contribution to risk of TMD have not been evaluated. The study aims were to (1) describe changes occurring in premorbid TMD risk factors when re-measured at TMD onset and six months later; and (2) determine if measures of change improve accuracy in predicting TMD incidence compared to premorbid measures alone.
Methods:
In this observational prospective cohort study at 4 university research clinics, 3258 community-based, 18–44 year-olds without TMD were enrolled. During the 3-year median follow-up, 260 incident cases of first-onset TMD were identified, and 196 TMD-free subjects were selected as matched controls. Six-months later, 147/260 incident cases (56.6%) were re-examined revealing 72 (49%) with “persistent TMD” and 75 (51%) whose condition had resolved (“transient TMD”). Virtually all (126) of the 127 re-examined controls remained without TMD. Questionnaires and clinical measurements evaluated risk factors from clinical, health, psychological and behavioral, and neurosensory domains.
Results:
Most risk factors across all 4 domains increased with TMD onset, remained elevated in the persistent group, and declined in the transient group (i.e., significant ANOVA interactions, p<0.05). Accuracy in predicting first-onset TMD, quantified as area under the receiver operating characteristic curve was 0.71 (95%CL 0.68, 0.73) using only premorbid measures of risk factors, which increased to 0.91 (95%CL 0.89, 0.94) after addition of change measures.
Conclusions:
TMD pain onset and persistence appear to be determined by enduring characteristics of the person as well as mutually interactive with temporally evolving variables.
Keywords: Temporomandibular disorders, prediction, risk factors, multivariate, chronic pain
1. Introduction
Temporomandibular disorders (TMD), primarily characterized by pain in the jaw joints and muscles, affect ~10% of the U.S. population (Drangsholt, 1999). Individuals with both acute and chronic TMD seek consultation in primary care settings, with greater utilization by those with ill-defined conditions and greater psychological dysfunction (Von Korff, 2007). Risk factors for TMD range from upstream genetic influences to intermediate phenotypes of experimental pain sensitivity, making TMD a complex biopsychosocial disorder (Diatchenko, 2006; Maixner, 2016). Overlapping comorbidity is common and includes other primarily-painful conditions, such as headache, and other physical or psychological conditions, such as depression (Slade, 2013c; Smith, 2013). TMD exhibits many clinical characteristics similar to other musculoskeletal pain conditions (Von Korff, 1988).
When evaluating risk factors for TMD, different study designs produce seemingly inconsistent findings. For example, using a case-control study design, the OPPERA (Orofacial Pain: Prospective Evaluation and Risk Assessment) project reported that increased psychological distress and greater sensitivity to experimental pain were associated with higher odds of chronic TMD (Fillingim, 2011b). However, OPPERA’s prospective study of first-onset TMD found that experimental pain sensitivity was only a weak predictor of TMD incidence, while indicators of poor health status emerged as independent risk factors for development of TMD (Slade, 2013c).
These apparent inconsistences might be due to underlying differences between acute and chronic TMD, particularly the factors contributing to each condition, how the factors change over time, and how the emerging condition influences the factors. A longitudinal design that examines individuals before, at the time of, and after onset can address this question. The fact that only a subset of those factors associated with chronic TMD predict acute TMD should not be surprising given that both TMD and the biopsychosocial context in which it arises evolve over time (Slade, 2013c). Thus, some factors associated with chronic TMD in cross-sectional studies may reflect changes that emerge concurrent with development or persistence of TMD and thereby do not affect risk of acute TMD but could, in principle, be causal for the transition to chronic TMD. In contrast, other factors are likely consequences of TMD pain once it becomes persistent, in which case no differences would likely be detectable in such factors until chronic TMD develops.
This paper has two aims: (1) describe changes in TMD risk factors from the premorbid period, before TMD develops, to the time of TMD onset, and then six months later; and (2) determine if accuracy in predicting incidence of TMD can be improved using measures of change in risk factors from enrollment to the time of TMD onset compared to enrollment measures alone. The change measures reflect both longer-term processes, well before TMD onset, and near-term processes, likely influenced by the progressively developing condition. This approach allowed us to test two hypotheses: (1) While many measures represent premorbid risk factors for TMD onset, some measures will show maladaptive changes coincident with TMD onset. And, (2) some measures will change differentially from enrollment to TMD onset for participants who develop persistent TMD compared to those whose first-onset TMD is transient.
2. Methods
2.1. Overview
The data presented in this paper derive from a nested case-control study of first-onset TMD in the OPPERA project. Methods and findings from other studies that are part of the OPPERA project have been previously described (Bair, 2013a; Fillingim, 2011b; Slade, 2011; Slade, 2013c). Institutional Review Boards at each study site approved the OPPERA project’s methods, and all study participants provided signed, informed consent to participate.
2.2. Participants
The OPPERA prospective cohort study enrolled 3,258 adults who had no significant history of TMD at four U.S. study sites: Baltimore, MD; Buffalo, NY; Chapel Hill, NC; and Gainesville, FL. A negative significant history of TMD meant that no TMD diagnosis had ever been provided, that any prior facial pain had never exceeded 4 days in a month, and that the month prior to enrollment was pain-free. Details of the study settings and study participants are reported elsewhere (Bair, 2013a). Eligibility criteria included: aged 18–44 years; English language fluency; fewer than five headaches/month in the three months before enrollment; no history of significant TMD symptoms; no prior diagnosis or treatment for TMD; and absence of 13 specific health conditions: 1) traumatic facial injury or surgery on the face or jaw within the 6 months preceding enrollment, 2) currently receiving orthodontic treatments, 3) pregnant or nursing, 4) kidney failure or renal dialysis, 5) heart disease or heart failure, 6) chronic respiratory disease that is not controlled with medication, 7) hypertension that is not controlled with medication, 8) epilepsy or medication to control grand mal seizures, 9) hyperthyroidism, 10) diabetes that is not controlled with medication or diet, 11) drug or alcohol abuse, 12) psychiatric disorders or conditions that have required hospitalization, or (13) chemotherapy or radiation therapy.
2.3. Study design
At enrollment (Visit1), health and psychological questionnaires were completed, a standardized clinical examination was conducted, and quantitative sensory testing (QST) was performed. For up to five years after enrollment, participants completed quarterly health questionnaires that screened for emergence of TMD symptoms. Subjects reporting symptoms were invited to return to research clinics for a follow-up assessment (Visit2), which included the same clinical examination protocol to determine presence or absence of TMD. For each confirmed incident case, one control participant matched for time since enrollment, gender, and study site completed an in-clinic visit where examiners verified absence of clinical TMD. The median period between Visit1 and Visit2 examinations was 17 months (interquartile range=10–26 months). Approximately six months after the Visit2 examination, participants were asked to return for a third examination (Visit3) to determine the persistence of TMD symptoms among incident cases. The median period between Visit2 and Visit3 examinations was 8 months (interquartile range (IQR)=6–15 months).
A flowchart of data collection is provided in Figure S1. Visit2 confirmed 260 incident cases of first-onset TMD and 196 TMD-free controls. Of the 260 incident cases examined at Visit2, 147 (56.6%) were re-examined at Visit3: 72 (49%) had examiner-verified TMD and were labeled “persistent TMD” cases and the remaining 75 (51%) who no longer had examiner-verified TMD at Visit3 were labeled “transient TMD” cases. Virtually all (126/127) of the controls re-examined at Visit 3 remained free of clinical TMD.
2.4. Measures
Risk factors measured at enrollment were repeated at Visits 2, 3 or both according to the schedule shown in Table 1. These measures are described briefly below and in greater detail in previous publications (Fillingim, 2013; Greenspan, 2013; Ohrbach, 2013; Sanders, 2013; Slade, 2013b).
Table 1.
Domains and measures administered premorbid, concurrent, and following TMD onset. The selected variables were identified based on prior univariate analyses [5; 16; 19; 32; 38]. Grayed box indicates administration of measure, and empty box indicates variable was not measured. Visit 1 refers to enrollment, Visit 2 refers to the clinic visit associated with TMD pain onset (cases) or by invitation (matched controls), and Visit 3 refers to the approximate 6-month follow-up.
| Domain, sub-domain, instrument, and measure | Visit 1 | Visit 2 | Visit 3 |
|---|---|---|---|
| Clinical Measures | |||
|
Examination Findings Research Diagnostic Criteria Exam: Pain-free jaw opening (mm), Maximum unassisted jaw opening (mm) [15] Clinical Exam: Painful body (non-jaw) sites from palpation (#) |
|||
|
Pain & Physical Symptoms Comprehensive Pain & Symptom Questionnaire: Non-specific Orofacial Symptoms (#), Headache Types (#), Comorbid Conditions (#) [32; 33] |
|||
|
Function and Parafunction Jaw Functional Limitation Scale (JFLS): Scores from JFLS-Chew, JFLS-Open, JFLS-Verbal and Emotional Expression subscales [34] Oral Behaviors Checklist (OBC): Total Score for oral parafunctional behavior [28; 31] |
|||
| Health Status Measures | |||
|
Pittsburgh Sleep Quality Index: Global Score [7] Medical Outcomes Study Short Form 12 Item Version 2 (SF-12v2): Physical Composite Score, Mental Composite Score [51] |
|||
| Psychological Measures | |||
|
Stress and Affect
Perceived Stress Scale: Total Score [10] Life Experiences Survey: Negative Impact Score [16; 17; 39] Lifetime Stressor List: PTSD Checklist-Civilian: Total Symptom Score [52] State Anxiety Inventory: Total Score [46] Profile of Mood States-Bipolar: Positive Affect, Negative Affect [25] |
|||
|
Somatic Symptoms and Other Psychological Characteristics Pennebaker Inventory of Limbic Languidness (PILL): Total Score [35] Kohn Reactivity Scale: Total Score [23] Symptom Checklist-90 Revised: Somatization subscale score, Depression subscale score [12] Trait Anxiety Inventory: Total Score [46] Pain Catastrophizing Scale: Total Score [47] |
|||
| Quantitative Sensory Testing Measures | |||
|
Pressure Pain Thresholds: Temporalis, Masseter, TMJ, Trapezius, Epicondyle [20] |
|||
|
Heat Pain: Pain Tolerance (°C), Peak Pain Rating and Pain After- sensation (15s) to temporal summation (48°C) [19] |
|||
|
Mechanical Cutaneous Pain: Pain Threshold (mN), Pain Rating and Pain After-sensation (15s) to temporal summation @512 mN [19] |
|||
2.4.1. Clinical Examination
The clinical examination protocol was adapted from the Research Diagnostic Criteria (RDC) for TMD (Dworkin, 1992). TMD classification required both criteria: 1) pain in the orofacial region for ≥5 days in the prior 30 days; and 2) evoked pain in ≥3 muscle locations (myalgia) or in ≥1 temporomandibular joints (arthralgia). For the second criterion, study participants performed standardized jaw movements, and examiners bilaterally manually palpated ten masticatory locations. Examiners were trained in the examination procedures prior to study initiation and maintained excellent inter-examiner reliability, assessed annually at Kappa=0.8–1.0 (Slade, 2011).
2.4.2. Questionnaire Administration
Questionnaires were administered to assess multiple domains of physical and psychological functioning, as detailed in our previous publications (Fillingim, 2011a; Ohrbach, 2011). Briefly, these questionnaires included assessment of the following constructs: non-specific non-pain orofacial symptoms, number of headache types, number of comorbid conditions, functional limitation of the jaw, oral parafunctional behaviors (that is, over-use behaviors), sleep, health-related quality of life, perceived stress, life experiences, life stressors, state and trait anxiety, mood states, depression, non-specific physical symptoms, physical reactivity, and pain catastrophizing. These constructs were selected for this study due to prior evidence for their value, using univariate models, to have either predictive power for TMD onset or to respond to that onset (Fillingim, 2013; Ohrbach, 2013; Sanders, 2013). Table 1 summarizes the questionnaires used to measure each construct.
2.4.3. Quantitative Sensory Testing
The QST battery included assessments for three types of experimental pain sensitivity: pressure pain (temporalis, masseter, TMJ, trapezius, and epicondyle), heat pain in the forms of pain tolerance, peak pain rating, and pain after-sensations at 15sec to temporal summation at 48°C (to the forearm), and cutaneous mechanical pain in the forms of pain threshold (mN), and pain rating and pain after-sensation (15s) to temporal summation @512 mN (assessed at the dorsum of fingers). This selected set of QST measures was shown in a prior publication to predict or be associated with TMD onset (Greenspan, 2013). While the full QST protocol was performed only at Visit1 and Visit2, pressure pain threshold assessment was conducted as part of the clinical examination procedures and therefore obtained at all three visits. QST measures are summarized in Table 1 and are detailed in our previous publication (Greenspan, 2011).
2.4.4. Control Variables
Demographic characteristics (gender, age, race/ethnicity) were self-reported in screening interviews at enrollment. Three racial/ethnic groups were formed: non-Hispanic White, African-American, and other or unstated racial-ethnic groups. Study site was a categorical variable (i.e., FL, MD, NC, or NY).
2.5. Potential bidirectional influence of the change score
In order to address potential influence on the change score (from baseline to TMD onset) by circumstances specific to the time of onset, variables closely matching our predictor variables measured at the two clinic visits were identified from the Quarterly Health Update (QHU) questionnaires. The QHU were administered every three months after enrollment and thereby provide longitudinal data for those selected variables. Separate analyses for each of those identified variables could therefore assess the timing of change with respect to TMD onset within the nested case-control study. QHU variables included mood, stress, jaw pain intensity, number of body pain sites, number of comorbid conditions, and number of non-painful jaw symptoms; collectively, this set of proxy variables is similar to the change variables found to be significant predictors. Data reduction, following a method previously published (Slade, 2015), used the last QHU, the penultimate QHU (i.e., the 3-month period prior to the final 3-month period in which potential cases typically first reported jaw pain meeting threshold for becoming a case), the first QHU, and the mean of the QHUs intervening between first and penultimate, thereby providing an ordinal time-series that was consistent for all participants despite unequal periods of time under observation. First-onset TMD was compared with the non-TMD controls using mixed-model analyses of each predictor variable in turn, adjusted for study site, age, gender, and race.
2.6. Data analysis
The measured risk factors are of two types: those measured at enrollment, and those derived as change from enrollment to TMD-onset. Collectively, both types are referred to as predictors in the statistical models, but only one type – measures collected at enrollment – represents an unambiguous predictor variable.
2.6.1. Univariate mixed models
For the first aim (describe changes in TMD risk factors from the premorbid period, before TMD develops, to the time of TMD onset, and then six months later), separate mixed models for repeated measures were created, each using a continuous measurement of one risk factor as the dependent variable. Predictor variables included study group (non-cases, transient TMD, and persistent TMD); time (enrollment, onset, and 6-month follow-up); and a study group-time interaction term. Adjustment variables included age, gender, race, and study site. Graphical displays used the adjusted means and standard error.
2.6.2. Multivariate LASSO models
For the second aim (determine if accuracy in predicting incidence of TMD can be improved using measures of change in risk factors from enrollment to the time of TMD onset compared to enrollment measures alone), multivariable models determined whether change scores from Visit1 to Visit2 predicted odds of first-onset TMD (i.e., TMD vs. control) after accounting for baseline values of each variable. Multivariable modeling was performed using LASSO regression(Tibshirani, 1996), a penalized regression method that provided estimate of direction as well as estimate of magnitude for area under the curve (AUC) computation, part of our model-building approach. LASSO also performs variable selection and is consequently less prone to over-fitting than more conventional stepwise regression procedures (Babyak, 2004; McNeish, 2015). Adjustment variables included age, gender, race, and study site. The tuning parameter, which determined the number of predictors identified by the model, was set based on the lowest cross-validation error rate.
Two pairs of LASSO models were calculated; variables entered into each pair of models are listed in Table S1. Model_1A used all study variables collected at Visit1. Variables that are part of the case definition for TMD were not included in any models. Model_1B used all Visit1 variables and the Visit2-Visit1 difference. The final pair of models used the same structure, but they excluded all variables from Model_1 that were closely related to symptoms of TMD. The rationale for calculating the more restricted models was to avoid a potentially tautological model that showed that participants with symptoms more closely tied to the masticatory system at Visit2 are more likely to have TMD at Visit2. All models included age as a potential predictor as well as dummy variables for gender, race and OPPERA study site.
Prior to fitting the LASSO models, all variables (with the exception of the dummy variables for gender, race and study site) were normalized to have mean=0 and standard deviation=1. For each of the models, variables with nonzero LASSO coefficients are reported as well as the area under the receiver operating characteristic AUC for the model estimated using cross-validation. Cross-validation consisted of dividing the sample into 10 partitions, estimating the model using data from 90% of the sample, and testing the model in the remaining 10%; this was repeated 10 times.
3. Results
3.1. Descriptive characteristics
Table S2 lists the descriptive characteristics for all three groups at enrollment. Age and gender are similar across groups. Non-Hispanic Whites are more likely to have persistent TMD while African Americans have double the proportion of transient TMD and the “Other” racial/ethnic group have half the proportion of both transient TMD and persistent TMD. Table 2 lists the clinical characteristics of transient and persistent TMD cases at the time of TMD onset as well as 8 months (IQR=6–15 months) later. At time of onset, transient and persistent cases were similar on most TMD-relevant clinical measures; cases destined for persistence had slightly higher characteristic pain intensity and pain-interference, a substantially greater number of muscles that were painful on palpation or jaw movement, and more frequent and longer episode duration.
Table 2.
Descriptive characteristics of pertinent findings associated with individuals with new-onset TMD.
| Characteristic | Visit 2 (onset) |
Visit 3 (6-month follow-up) |
||
|---|---|---|---|---|
| Transient | Persistent | Transient | Persistent | |
| n | 74 | 72 | 74 | 72 |
| Characteristic pain intensity [mean, (SD)] | 30.9 (26.5) | 38.5 (22.1) | 15.6 (23.3) | 30.5 (23.7) |
| Pain-interference score [mean, (SD)] | 13.7 (22.8) | 16.9 (20.4) | 5.8 (16.4) | 11.9 (19.3) |
| Number days efficiency<50% [mean, (SD)] | 10.5 (24.4) | 15.1 (32.4) | 3.7 (07.3) | 5.2 (12.2) |
| Number masticatory muscles or TMJs with pain from palpation [mean, (SD)] | 16.4 (12.1) | 26.0 (13.8) | 7.1 (09.0) | 20.6 (14.7) |
| Diagnostic distribution (%)* | ||||
| ○ myalgia only | 23 (32.9) | 14 (19.4) | 0 (0.0) | 18 (25.0) |
| ○ arthralgia only | 3 (04.3) | 1 (01.4) | 0 (0.0) | 7 (09.7) |
| ○ mixed | 44 (62.9) | 57 (79.2) | 0 (0.0) | 47 (65.3) |
| Frequency (%) | ||||
| ● None | 28 (42.4) | 12 (17.4) | 46 (70.8) | 22 (32.8) |
| ● Rarely | 8 (12.1) | 6 (08.7) | 9 (13.9) | 6 (09.0) |
| ● Less than half the days | 15 (22.7) | 22 (31.9) | 8 (12.3) | 20 (29.9) |
| ● Half or more than half | 13 (19.7) | 19 (27.5) | 2 (03.1) | 15 (22.4) |
| ● Daily | 2 (03.0) | 10 (14.5) | 0 (00.0) | 4 (06.0) |
| Episode duration (%) | ||||
| ● None | 28 (42.4) | 12 (17.4) | 46 (69.7) | 22 (32.8) |
| ● Less than 1 minutes | 5 (07.6) | 2 (02.9) | 2 (03.0) | 4 (06.0) |
| ● 1min to 1day | 16 (24.2) | 26 (37.7) | 13 (19.7) | 24 (35.8) |
| ● >1day to 1 week | 9 (13.6) | 18 (26.1) | 2 (03.0) | 13 (19.4) |
| ● >1week but not constant | 6 (09.1) | 7 (10.1) | 2 (03.0) | 4 (06.0) |
| ● Constant | 2 (03.0) | 4 (05.8) | 1 (01.5) | 0 (00.0) |
At visit 2, sub-type diagnostic classification of n=4 within the transient group is missing; final classification as a case was made by expert review of the available raw data.
3.2. Time course of data collection and potential bidirectionality
The time between pain onset and case ascertainment varied considerably, ranging 0–85 days (median=14 days) for cases and 0–85 days for non-cases (median=23 days) (Bair, 2013a). The set of proxy variables for the assessment of confounding exhibited the following significant patterns across time for cases, as compared to controls (Figure S2). Mood, stress, jaw pain intensity, number of body pain sites, number of comorbid conditions, and number of non-pain jaw symptoms were consistently worse for cases at all 4 time points. Stress and jaw pain intensity worsened for both cases and controls at each time point. Number of body pain sites and number of comorbid conditions further worsened for cases during the final time point. Number of non-pain jaw symptoms further worsened in cases during the penultimate time point.
3.3. Univariate analysis of risk factors: Changes across time
To describe changes in TMD risk factors from the premorbid period, Figures 1 and 2 show patterns across time for the three study groups for several illustrative variables. Tables S3–S6 provide the full analyses for each variable, organized by 4 domains: clinical, general health, psychological, and QST.
Figure 1. Change across time by TMD-case group for selected variables measured by self-report.
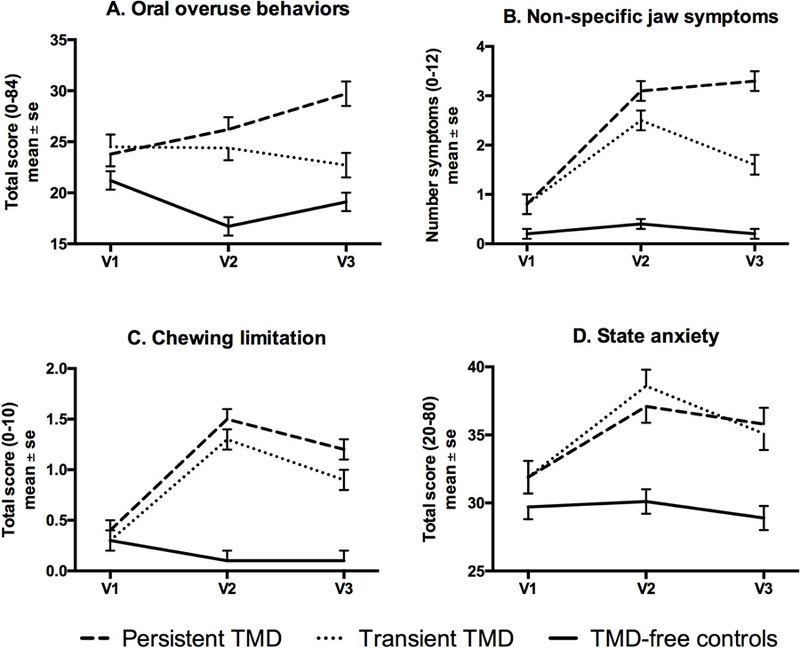
The line-plot for each Figure displays mean and standard error at enrollment (v1), onset visit for cases or clinic visit for matched controls (v2), and 6-month recall (v3), for each of the three identified groups: persistent TMD, transient TMD that remits after onset, and TMD-free controls. For all 4 plots, the interaction effect was significant, p<0.001, and the group effect was significant, p<0.0001. For plot A, the visit effect exhibited P=0.09 while for plots B-D, the visit effect was significant, P<0.0001.
Figure 2. Change across time by TMD-case group for selected variables measured clinically.
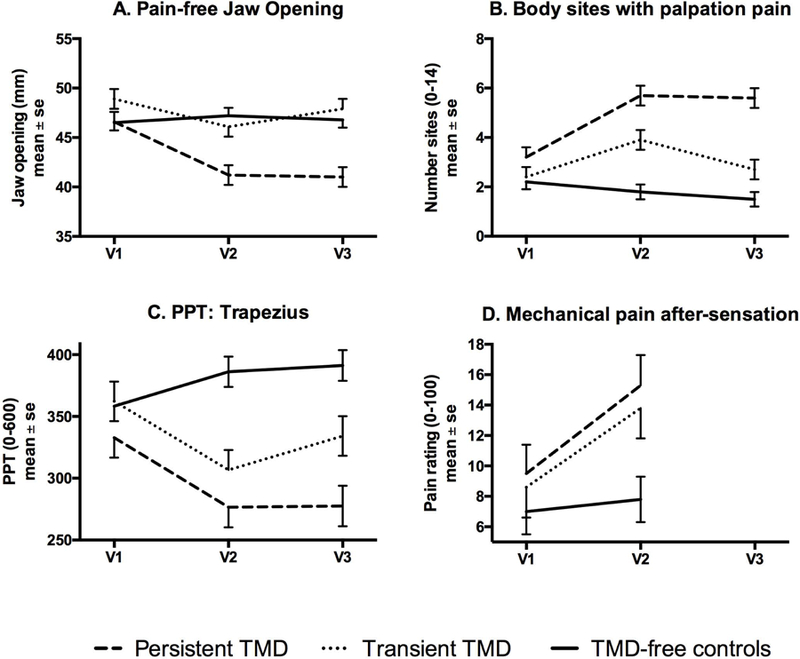
The line-plot for each Figure displays mean and standard error at enrollment (v1), onset visit for cases or clinic visit for matched controls (v2), and 6-month recall (v3), for each of the three identified groups: persistent TMD, transient TMD that remits after onset, and TMD-free controls. For plots A-C, the interaction effect was significant, p<0.0001, the group effect was significant, p<0.001, and the visit effect was significant, P<0.004. For plot D, the interaction effect exhibited P=0.075, while the group effect was significant, p=0.034 and the visit effect was significant, P=0.0003.
For the clinical domain, nearly all variables exhibited significant interaction terms, indicating differential change across time by study group. Only number of headache types failed to reach significance for the interaction term, although the pattern of change in mean values was consistent with most of the other clinical variables, namely: 1) elevations at onset (Visit2) for both TMD case groups, 2) continued elevation in the persistent cases and return toward pre-onset levels in the transient cases at 8 months (IQR=6–15 months) follow-up, and 3) no meaningful change for the TMD-free controls across time (Table S3).
General health measures showed less change over time for persistent versus transient TMD. While overall sleep quality showed a significant interaction, both persistent and transient cases showed worsening of sleep quality at 8-month follow up, whereas controls showed continued good sleep quality. Short Form-12v2 physical health and mental health composite scores showed poorer scores for both persistent and transient cases and worsened slightly across time for all groups (Table S4).
Among psychological variables, those assessing state negative affect showed increases from enrollment to onset in both persistent and transient cases, with persistent cases showing continued elevation in symptoms at 6-month follow-up, while transient cases returned toward enrollment levels. Somatic symptoms showed slightly different patterns depending on the measure. For Pennebaker Inventory of Limbic Languidness (PILL) scores, a measure of physical symptom reporting, persistent cases showed increases at 6-month follow-up, whereas controls and transient cases remained stable. In contrast, for the SCL-90R somatization scale, both persistent and transient cases showed increases from enrollment to 6-month follow-up, whereas controls remained consistently low over time. Stress measures and depression did not exhibit differential effects across time by group; however, each of these measures did differ across groups: lower in controls, and approximately equal for transient and persistent groups (Table S5).
Among QST variables, only PPT from all four regions changed differentially across time by group: while TMD-free controls and both case groups were similar at enrollment, both case groups exhibited a decrease in PPT at the time of TMD onset and the persistent cases exhibited greater pressure pain sensitivity compared to the transient cases. Heat pain tolerance and peak heat pain ratings did not distinguish groups but decreased (minimally) at Visit2 for cases. Heat pain after-sensations distinguished groups, with persistent TMD at enrollment exhibiting the highest pain rating, and values remained relatively stable at Visit2. For mechanical cutaneous pain, only the after-sensations following repetitive stimulation distinguished groups, with both persistent and transient cases showing higher values and substantial increases at onset, whereas controls remained stable at Visit2 (Table S6).
For all plots in Figures 1–2, both case groups exhibited worsening at the time of onset, and the plots generally demonstrated that individuals with persistent TMD continued to have worse levels of the variable at follow-up while the individuals with transient TMD generally started to recover by 8 months (IQR=6–15 months) post-onset.
3.4. Multivariate analysis of risk factors: Changes across time
Aim 2 attempted to improve accuracy in predicting incidence of TMD. Table 3 shows the prediction model for first-onset TMD that utilized only values at enrollment (Model_1A). It contains seven explanatory variables: stress, trait anxiety, sleep quality, depression, physical symptoms, and a small (non-zero) effect of after-sensation from repetitive heat stimuli. However, when adding change scores from Visit1 to Visit2 to the model, nine additional variables contributed to TMD onset (Model_1B). Two variables (number of non-specific jaw symptoms, number body sites painful to palpation) contributed from both baseline and change to TMD onset. The AUC increased from 0.71 for Model_1A to 0.91 for Model_1B, showing significantly increased predictive ability when adding change scores.
Table 3.
Parameter estimates from multivariable LASSO model predicting first-onset TMD. Model_1 is based on all variables listed in Table 1; see supplemental materials for detailed results of the more restrictive models. In Model_1A, enrollment (v1) status of each variable was entered into the LASSO regression, whereas in Model_1B, both enrollment status (v1) and the respective change score from enrollment to TMD onset visit or corresponding clinic visit for matched controls (v2-v1) were entered into the LASSO regression. Results represent ln(SOR); each predictor variable was standardized to z-scores. Results are adjusted for race (African-American, Asian) and for Study site (Maryland). Shaded cells refer to variables that were not measured at v2, and therefore no change score was available for entry into the LASSO regression. Blank cells indicate non-contributory variable to the respective LASSO model.
| Predictor | Model_1A | Model_1B | |
|---|---|---|---|
| Enrollment | Enrollment | Change | |
| Non-specific face and jaw symptoms (#) | 0.144 | 0.488 | 1.003 |
| Stress and coping (PSS total score) | 0.044 | 0.066 | |
| Trait anxiety (STAI) | 0.078 | 0.051 | |
| Sleep quality (PSQI) | 0.070 | 0.032 | |
| Depression (SCL90R) | 0.128 | 0.099 | |
| Physical symptoms (SCL90R) | 0.279 | 0.228 | |
| Heat pain after-sensation @15s (48°C) | 0.001 | 0.017 | |
| Positive affect score (POMS) | −0.003 | ||
| Maximum unassisted jaw opening (mm) | 0.109 | ||
| Body sites painful to palpation (#) | 0.231 | 0.284 | |
| Different headache types (#), past year | 0.099 | ||
| Oral parafunction (OBC total score) | 0.157 | ||
| Chewing limitation (JFLS subscale score) | 0.319 | ||
| Jaw opening limitation (JFLS subscale score) | 0.019 | ||
| Pain-free opening (mm) | −0.071 | ||
| State anxiety (STAI) | 0.096 | ||
| PPT: Masseter | −0.186 | ||
| PPT: Trapezius | −0.217 | ||
| Mechanical pain after-sensation @15s (512 nM) | 0.087 | ||
| Area under the curve (95%CL) | 0.71 (0.68, 0.73) |
0.91 (0.89, 0.94) |
|
Table 4 shows the results from the second pair of LASSO models, which removed all variables with any possible linkage to the case definition. Only physical symptoms (measured with SCL90R) from enrollment predicted TMD onset, whereas when change from Visit1 to Visit2 is added to the model, another 4 variables that change across time (representing a subset of the variables in Table 3: number of body sites painful to palpation, state anxiety, and PPTs from trapezius and lateral epicondyle) also contributed to enrollment prediction. In addition, another 5 variables measured at enrollment enter the model to predict TMD onset. The respective AUCs for these models are 0.67 and 0.80, again significantly different from each other.
Table 4.
Multivariable results for Model 2.
Model 2 is based on excluding the following variables as listed in Table 1 and Appendix Table 1: pain-free jaw opening, maximum unassisted jaw opening, non-specific orofacial symptoms, chewing and opening limitation, oral parafunction, number of types of headaches, and PPT measured at temporalis, masseter, and TMJ. In Model 2a, enrollment (v1) status of each variable was entered into the LASSO regression, whereas in Model 2b, both enrollment status (v1) and the respective change score from enrollment to TMD onset visit or corresponding clinic visit for matched controls (v2-v1) were entered into the LASSO regression. Results represent ln(SOR); each predictor variable was standardized to z-scores. Results are adjusted for race and for Study site. Shaded cells refer to variables that were not measured at v2, and therefore no change score was available for entry into the LASSO regression.
| Predictor (Instrument; units) | Model 2a | Model 2b | |
|---|---|---|---|
| Enrollment | Enrollment | Change | |
| Physical symptoms (SCL90R; score) | 0.058 | 0.264 | |
| Stress and coping (PSS; score) | 0.086 | ||
| Body sites painful to palpation (#) | 0.100 | 0.366 | |
| Trait anxiety (STAI; score) | 0.045 | ||
| Sleep quality (PSQI; score) | 0.046 | ||
| Depression (SCL90R; score) | 0.130 | ||
| State anxiety (STAI; score) | 0.193 | ||
| PPT: Trapezius (kPa) | −0.219 | ||
| PPT: Lateral epicondyle (kPa) | −0.008 | ||
| Mechanical pain after-sensation @15s, 512 nM | 0.001 | ||
| Area under the curve (95%CL) | 0.67 (0.64, 0.70) |
0.80 (0.78, 0.83) |
|
Figure 3 displays estimates and 95%CL for AUC for each of the two pairs of models, illustrating that when only enrollment values are used for prediction, removal of the variables with possible links to the case definition minimally affects the prediction, whereas removal of variables from the second model significantly reduces the prediction. However, equally notable is that prediction based on both enrollment status and change status remains significantly better than only considering status at enrollment.
Figure 3. Area under the curve associated with prediction of TMD onset from multivariate cross-domain models.
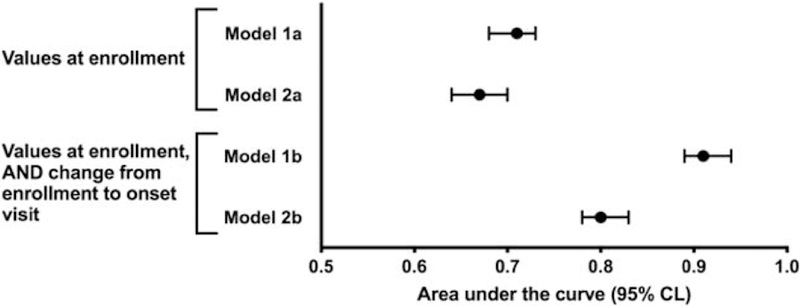
Model 1 included all variables as listed in Table 1 and also listed in Table S1. Model A refers to enrollment values only, while Model B refers to enrollment values and change values from v1 to v2. Model 2 was restricted by removing variables closely related to the masticatory system: pain-free jaw opening, maximum unassisted jaw opening, non-specific orofacial symptoms, chewing and opening limitation variables as consequences of having a TMD, oral parafunctional behaviors, number of types of headaches, and PPT measured at temporalis, masseter, and TMJ. In all models, the inclusion of change from v1 to v2, in addition to the variables measured at enrollment, improved the model’s predictive value, as measured by area under the curve (AUC). The additional restrictions in Model 2 have no impact on AUC of the enrollment variables but they do affect the AUC associated with the change scores.
4. Discussion
The findings support both hypotheses and both aims. We hypothesized that some of the measures that represent premorbid risk factors for TMD onset will also show maladaptive changes across time leading to TMD onset, and that some of the measures will exhibit differential maladaptive change across time for those who eventually develop persistent TMD vs transient TMD. These hypotheses were addressed through two aims: (1) describe changes in TMD risk factors across time from pre-morbid to post-onset, and (2) determine if predictive accuracy regarding TMD incidence can be improved using measures of change in risk factors from enrollment to the time of TMD onset compared to enrollment measures alone.
Regarding changes in individual TMD risk factors over time (aim 1), multiple variables worsened in transient and persistent cases from the time of enrollment to TMD onset; these variables then diminished at six-month follow-up in transient cases while remaining elevated among persistent cases. The changes from enrollment to TMD onset in these variables were, collectively, stronger predictors of TMD onset than the variables when measured at enrollment (aim 2), yet bidirectional change between the variable and TMD onset as it emerges over some time period is also potentially reflected in the change score. It is noteworthy that in the controls only a few variables (specifically, SF12v2 physical health and mental health composite scores, and trait anxiety) worsened parallel with cases, whereas most variables remained the same or showed tendency towards improving. The findings highlight the potential value of re-evaluating mutable risk factors when trying to predict who will develop chronic pain. In addition, as we have demonstrated for TMD onset (Slade, 2013c), it is unlikely that a single cause would be discovered as responsible for a complex disease; consequently, component causes – where a given putative cause acts as one component among many – is more appropriate and plausible (Rothman, 2005). The component causes model implies that some combination of risk factors, likely idiosyncratic to the individual, among a group of identified risk factors that, at the population level, are capable of contributing to a disease state, reduces resilience, and increases central dysregulation in pain processing systems.
Whether the changes observed in the selected temporally varying predictors from enrollment to TMD onset represent risk factors for TMD versus consequences of TMD at onset is critical to the validity of our approach. Using QHUs to examine self-reported symptoms between enrollment and onset, we found a generally consistent pattern of gradual change across time that was largely consistent for each variable. Notably, the change across time in the cases was, with one exception, not limited to only the final 3-month period during which TMD-related pain was reported. As observed in our controls, we previously reported that a majority of persons report across time intermittent fluctuations of jaw pain that is mild and does not lead to TMD onset (Slade, 2013a).
At first appearance, it is not surprising that accuracy of risk prediction can be improved by measuring change in risk factors. However, inspection of the largely non-overlapping variable sets that comprise those predicting from enrollment vs those predicting from change reveals largely separate domains. Significant premorbid predictors reflect distress, sleep, functional symptoms, and other pain disorders. Temporally varying predictors reflect behavior, function, state anxiety, and experimental pain sensitivity. The temporal pattern of phenotypic characteristics suggests that certain variables, even when their premorbid values confer increased TMD risk, change over time in parallel with developing TMD symptomatology. Specifically, increases in non-specific face and jaw symptoms (e.g., stiffness, cramping, fatigue), chewing limitations, palpation sensitivity, pressure pain sensitivity, and oral parafunction (e.g., clenching teeth while awake, bracing the jaw, touching teeth together, as assessed via the Oral Behaviors Checklist) were particularly strong temporally varying predictors of incident TMD. Factors that were not premorbid predictors became contributors to incident TMD when their change over time was considered, representing complex and dynamic contributions to TMD onset.
We interpret problems in stress and coping, general body tenderness, anxiety, sleep, and depression (enrollment variables from Model 2b, and all present in Models 1a and 1b) to be indicators of disrupted self-regulation (Chapman, 2008; Zeidan, 2011). For example, feed-forward mechanisms as part of a cascading vicious cycle related to dysregulated and maladaptive coping responses have been recently proposed as central for migraine pathogenesis (Borsook, 2012). Notably, change scores that were significant temporally varying predictors of TMD onset included not only measures of TMD-related function and facial symptoms but also more general risk factors not specific to orofacial pain (e.g. trapezius PPT, bodily palpation sensitivity, state anxiety). Consequently, we regard the observed findings as representing pathophysiological mechanisms that are potentially relevant to multiple musculoskeletal pain conditions (Apkarian, 2009; Wessely, 1999b), not just TMD.
Two variables predictive from both enrollment and change reflect experimental pain sensitivity and body symptoms. The role of experimental pain sensitivity has been well-studied (Clark, 1974; Maixner, 1995; Slade, 2007; Woolf, 2011) and its role here is consistent with our earlier heuristic (Diatchenko, 2006). Body symptoms, extremely common in the general population (Hiller, 2006), have strong associations with chronic pain (Aaron, 2001; Macfarlane, 2005; Rief, 2007; Wessely, 1999a); indeed, we have reported that the number of body symptoms exhibits extremely strong associations with chronic TMD (Ohrbach, 2011) and is among the strongest predictors of new TMD onset (Bair, 2013b; Sanders, 2013). Although central pain sensitivity, the process inferred as response to experimental pain sensitivity procedures and measures, and body symptoms represent statistically and conceptually separable constructs, it seems plausible that their parallel contributions to TMD onset could be mediated by overlapping biopsychosocial mechanisms since both are influenced by cognitive-affective processes, including pain catastrophizing and negative affect (Campbell, 2009).
Notably, these psychological processes have a neurobiological footprint that could confer both enhanced central pain sensitivity and increased body symptoms. For example, pain catastrophizing can promote systemic inflammatory responses, may interfere with endogenous pain inhibitory systems, and can amplify pain-related responses in brain regions that subserve emotional aspects of pain (Campbell, 2009), all of which have also been linked with increased somatic symptoms (Perez, 2015). And, reorganization of brain networks involved in emotional responses (i.e., the cortico-limbic system) may contribute to development and persistence of pain (Vachon-Presseau, 2016). Similarly, the experiences of body symptoms and emotional states are likely mediated through interoceptive brain networks (e.g. anterior insular, anterior cingulate cortices), and the link between body symptoms, emotions and pain could be mediated through these same mechanisms (Craig, 2003; Garfinkel, 2013). Despite our speculation regarding these mechanisms, increased understanding of the neurobiological processes underlying both central pain sensitivity and body symptoms may inform insights into comorbidity as a whole (Mayer, 2009). Referring to Figure 4, the pattern emerging from the variables identified by our LASSO model points to pain as an emergent process from dysregulation of multiple systems including central pain sensitivity and body symptoms as part of their shared pathways.
Figure 4. Heuristic biopsychosocial model for development and persistence of musculoskeletal pain disorder.
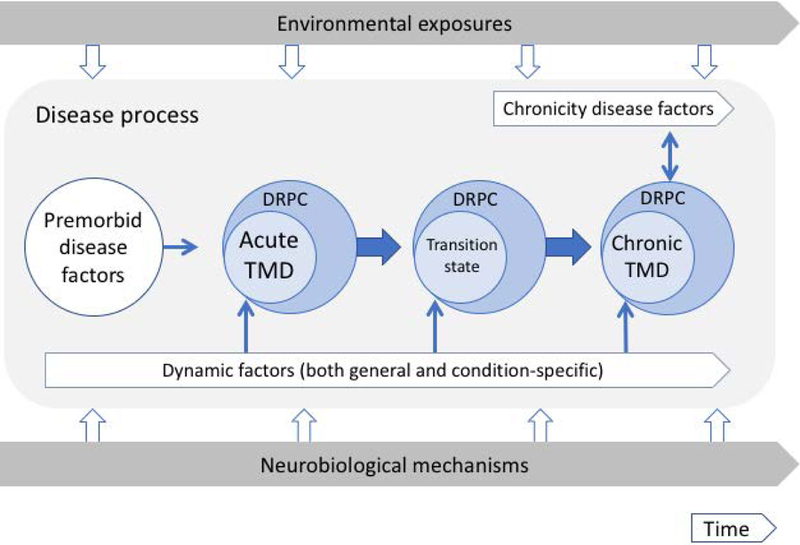
The model depicts premorbid disease factors and dynamic factors converging into both TMD symptom development and changes in the person occurring within the ongoing influence of bottom-up neurobiological mechanisms and top-down environmental exposures. The model is comprised of three major elements: environmental exposures and neurobiological mechanisms which impact on a disease process. The environmental exposures are ongoing across time and the downward open arrows highlight intermittent challenges from the external environment to the collective risk factors that comprise the disease process; the other main part of the frame is the ongoing neurobiological mechanisms of the individual, and the upward open arrows point to intermittent challenges from the internal environment into those collective risk factors. The disease process, in turn, is comprised of a number of elements that unfold across time: these elements include (1) premorbid disease factors that represent pre-existing trait-like characteristics; (2) dynamic factors and chronicity disease factors, both of which are active across time; (3) thin solid arrows that indicate contributions from the stated factors into potential disease onset, transition, or chronicity; (4) fat solid arrows that indicate the state shift from pre-morbid to acute, to transition state, and to chronic state; and (5) the specific identification of disease-related person characteristics (DRPC) – particular aspects of the “person” attached to each phase of the pain disorder, in order to emphasize that the disorder exists not alone but in the context of a person-state related to that disorder at that phase. This model builds on previously published work; see (Fillingim, 2011b; Slade, 2013c; Slade, 2016) for summaries and results related to identified nodes in the Figure. In addition, the model extends into the post-onset period, thereby suggesting hypothesized patterns of interaction across time with moderating and mediating variables. Premorbid factors are comprised of: person-level variables of problems with stress and coping, psychological distress, general body tenderness, anxiety, impaired sleep, functional symptoms, other pain disorders, and pain sensitivity. Dynamic factors are comprised of: generic person-level variables of body symptoms, state anxiety, and pain sensitivity; and condition-specific variables of increases in non-specific face and jaw symptoms, chewing limitations, local palpation sensitivity, and oral parafunction. Chronicity factors are comprised of variables from multiple domains: sociodemographic, clinical, psychological, pain sensitivity, autonomic, and genetic. Environmental exposures include physical and psychological stressors and local injury. Neurobiological mechanisms include factors such as genetic predisposition, epigenetic changes, and up-regulation and down-regulation of CNS ion channels. The thin blue lines with arrowheads depict component cause influences on the targets of acute TMD, transition state, and chronic TMD, and on the person more broadly as the effect of person-level influences. For example, the depression within the person affects the person; this is not circular, but rather reflects different levels of organization and how we understand that. The double-headed solid thin arrow depicts reciprocal influences between a chronic condition and the associated factors, and the intention, though not shown within this limited illustration, is that each continues to influence the other further across time.
The identified predictor variables are consistent with our other published research (Slade, 2013c), but the present findings are novel with respect to multivariable modeling and the demonstration that modeling the temporal course of risk factors at enrollment provides valuable new information concerning onset and persistence of TMD. Indeed, in contrast to many other major diseases in which common immutable factors (gender, age, race) have substantial contributions, we note that for acute TMD the major predictor variables from the multivariable model are not immutable but rather are alterable (Lakhan, 2013). This suggests that interventions revolving around these factors for pain disorders should address those potential changes and, moreover, capitalize on the contribution that changes in a given factor can, through additive and feed-forward processes (Borsook, 2012), have on the individual.
The findings from both models highlight the importance of the change process beyond state characteristics of the individual. Musculoskeletal disorders are the most common cause of pain impacting daily living (Woolf, 2003). In particular, investigating risk factors for development and persistence of TMD may yield findings that inform understanding of chronic musculoskeletal pain etiology more broadly. Figure 4 illustrates a heuristic model depicting the present findings with respect to change in dynamic factors across time in parallel with emergence of acute TMD; both vectors are hypothesized to converge into the transition to chronic pain, augmented by yet other factors (for example, gender, race, and PPTs at cranial sites), we have shown (Slade, 2016) to contribute to chronic TMD but not acute TMD. Clinicians should inquire into such change from one consultation to the next, but such inquiry is expensive in terms of time and can require considerable clinical skill; in contrast, predictive research models for the most part, and our medical care reimbursement systems, gravitate towards simpler assumptions about disease causation. The reality, as indicated by Models 1 and 2, is that the process of change represents critically important characteristics of the person, and the pattern of that change across time determines whether TMD (and other pain disorders not adequately measured here) emerges. More granular data are needed to assess this possibility and its implications for treatment.
Our findings should be interpreted in light of several study limitations. A major limitation is that while the transient and persistent groups were sufficiently large for the univariate analyses, the sample size of each group was too small for meaningful entry into the multivariable model. A second limitation is non-measurement of some variables at the onset or follow-up visits. A third limitation is the longer time gap for some participants between pain onset and case ascertainment, raising a question regarding the boundary (i.e., number of months) between acute and chronic pain and how such participants fit within the acute framework. A final limitation is the 43% loss to follow-up after TMD onset which has been examined in a variety of ways in prior OPPERA publications and deemed relatively non-consequential (Bair, 2013a). Extensive analyses indicate that loss-to-followup was only predicted by being a white male, who returned at a lower frequency compared to other demographic groups and perhaps explained by factors known at enrollment – that of being a college student (Meloto, 2019). Nevertheless, the concern remains regarding the unexamined ways in which those who remained in the study may differ, warranting caution towards full generalizability.
In summary, TMD pain onset is determined by both enduring characteristics of the person as well as changes in biopsychosocial functioning across time. Clinical implications of these findings suggest that assessment of patients include attention to these multiple risk determinants and recognize that both condition-specific as well as more person-centered interventions are needed. For care settings, both condition-specific and more general biopsychosocial variables are important, as is reassessment of these factors over routine intervals of follow-up. Future research is needed to replicate these findings and to extend them to other musculoskeletal conditions.
Supplementary Material
Significance.
TMD is known to be a complex disorder, in which onset and persistence are associated with disease-related variables in multiple domains, including environmental exposure, clinical, psychological, health status, and pain processing variables. Using a more dynamic approach in order to capture change across time, many aspects of those domains were found to worsen prior to the reporting of pain, with bidirectional influences between domains and pain emergence likely. TMD onset appears to represent the cumulative effect of multiple system dysregulation.
Acknowledgments
The OPPERA program also acknowledges resources specifically provided for this project by participating institutions: Battelle Memorial Institute, University at Buffalo, University of Florida, University of Maryland, and University of North Carolina at Chapel Hill.
Funding sources
This research was supported by National Institutes of Health grant U01DE017018.
Footnotes
Conflicts of interest
William Maixner and Luda Diatchenko are equity shareholders and consultants to Proove Biosciences, INC, a company that is involved in developing methods for improving the diagnosis and treatment of chronic pain conditions. William Maixner is also on the Board of Directors and a consultant to Orthogen GmB, which markets a biologic for the treatment of musculoskeletal disorders. The remaining authors declare no potential conflicts of interest.
References
- Aaron LA, & Buchwald D (2001). A review of the evidence for overlap among unexplained clinical conditions. Annals of Internal Medicine, 134(9 Pt 2), 868–881. doi: 10.7326/0003-4819-134-9_Part_2-200105011-00011 [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, & Geha PY (2009). Towards a theory of chronic pain. Progress in Neurobiology, 87(2), 81–97. doi: 10.1016/j.pneurobio.2008.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babyak MA (2004). What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosomatic Medicine, 66(3), 411–421. [DOI] [PubMed] [Google Scholar]
- Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, … Slade GD (2013a). Study protocol, sample characteristics and loss-to-follow-up: the OPPERA prospective cohort study. Journal of Pain, 14(12, supplement 2), T2–19. doi: 10.1016/j.jpain.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair E, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Diatchenko L, … Slade GD (2013b). Multivariable modeling of phenotypic risk factors for first-onset TMD: the OPPERA prospective cohort study. Journal of Pain, 14(12, supplement 2), T102–115. doi: 10.1016/j.jpain.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Maleki N, Becerra L, & McEwen B (2012). Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron, 73(2), 219–234. doi: 10.1016/j.neuron.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Campbell CM, & Edwards RR (2009). Mind–body interactions in pain: the neurophysiology of anxious and catastrophic pain-related thoughts. Translational Research, 153(3), 97–101. doi: 10.1016/j.trsl.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CR, Tuckett RP, & Song CW (2008). Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. Journal of Pain, 9(2), 122–145. doi: 10.1016/j.jpain.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WC (1974). Pain sensitivity and the report of pain: an introduction to sensory decision theory. Anesthesiology, 40, 272–287. [DOI] [PubMed] [Google Scholar]
- Craig AD (2003). A new view of pain as a homeostatic emotion. Trends in Neurosciences, 26(6), 303–307. doi: 10.1016/S0166-2236(03)00123-1 [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Nackley AG, Slade GD, Fillingim RB, & Maixner W (2006). Idiopathic pain disorders - pathways of vulnerability. Pain, 123(3), 226–230. doi: 10.1016/j.pain.2006.04.015 [DOI] [PubMed] [Google Scholar]
- Drangsholt M, & LeResche L (1999). Epidemiology of temporomandibular disorders. In Crombie IK, Croft PR, Linton SJ, LeResche L, & Von Korff M (Eds.), Epidemiology of Pain (pp. 203–233). Seattle: IASP Press; (Reprinted from: In File). [Google Scholar]
- Dworkin SF, & LeResche L (1992). Research Diagnostic Criteria for Temporomandibular Disorders: Review, Criteria, Examinations and Specifications, Critique. Journal of Craniomandibular Disorders, Facial and Oral Pain, 6(4), 301–355. [PubMed] [Google Scholar]
- Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, … Maixner W (2013). Psychosocial factors associated with development of TMD: the OPPERA prospective cohort study. Journal of Pain, 14(12, supplement 2), T75–90. doi: 10.1016/j.jpain.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, … Maixner W (2011a). Potential psychosocial risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. Journal of Pain, 12(11, supplement 3), T46–T60. doi: 10.1016/j.jpain.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Slade GD, Diatchenko L, Dubner R, Greenspan JD, Knott C, … Maixner W (2011b). Summary of Findings from the OPPERA Baseline Case-Control Study: Implications and Future Directions. Journal of Pain, 12(11, supplement 3), T102–T107. doi: 10.1016/j.jpain.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, & Critchley HD (2013). Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary on: “Anterior insular cortex mediates bodily sensibility and social anxiety” by Terasawa et al.(2012). Social cognitive and affective neuroscience, 8(3), 231–234. doi: 10.1093/scan/nss140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, … Maixner W (2013). Pain sensitivity and autonomic factors associated wtih development of TMD: the OPPERA prospective cohort study. Journal of Pain, 14(12, supplement 2), T63–74. doi: 10.1016/j.jpain.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, … Maixner W (2011). Pain sensitivity risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. Journal of Pain, 12(11, supplement 3), T61–T74. doi: 10.1016/j.jpain.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller W, Rief W, & Brähler E (2006). Somatization in the population: from mild bodily misperceptions to disabling symptoms. Social Psychiatry & Psychiatric Epidemiology, 41(9), 704–712. doi: 10.1007/s00127-006-0082-y [DOI] [PubMed] [Google Scholar]
- Lakhan SE, & Schofield KL (2013). Mindfulness-Based Therapies in the Treatment of Somatization Disorders: A Systematic Review and Meta-Analysis. PLoS ONE, 8(8), e71834. doi: 10.1371/journal.pone.0071834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane GJ (2005). Looking back: developments in our understanding of the occurrence, aetiology and prognosis of chronic pain 1954–2004. Rheumatology, 44 Suppl 4, iv23–iv26. doi: 10.1093/rheumatology/kei057 [DOI] [PubMed] [Google Scholar]
- Maixner W, Fillingim R, Booker D, & Sigurdsson A (1995). Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain, 63, 341–351. doi: 10.1016/0304-3959(95)00068-2 [DOI] [PubMed] [Google Scholar]
- Maixner W, Fillingim RB, Williams DA, Smith SB, & Slade GD (2016). Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. The Journal of Pain, 17(9), T93–T107. doi: 10.1016/j.jpain.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, & Bushnell MC (2009). Functional pain disorders: time for a paradigm shift. In Mayer EA & Bushnell MC (Eds.), Functional Pain Syndromes: Presentation and Pathophysiology (pp. 531–565). Seattle: IASP Press; (Reprinted from: In File; ). [Google Scholar]
- McNeish DM (2015). Using lasso for predictor selection and to assuage overfitting: A method long overlooked in behavioral sciences. Multivariate Behavioral Research, 50(5), 471–484. doi: 10.1080/00273171.2015.1036965 [DOI] [PubMed] [Google Scholar]
- Meloto CB, Lichtenwalter RN, Bair E, Rathnayaka N, Diatchenko L, Greenspan JD, … Ohrbach R (2019). Clinical Predictors of Persistent TMD in People With First-onset TMD: a Prospective Case-Control Study. JADA, 150(7), 572–581. doi: 10.1016/j.adaj.2019.03.023 [DOI] [PubMed] [Google Scholar]
- Ohrbach R, Bair E, Fillingim RB, Gonzalez Y, Gordon SM, Lim PF, … Slade GD (2013). Clinical orofacial characteristics associated with risk of first-onset TMD: the OPPERA prospective cohort study. Journal of Pain, 14(12, supplement 2), T33–T50. doi: 10.1016/j.jpain.2013.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, … Slade GD (2011). Clinical findings and pain symptoms as potential risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. Journal of Pain, 12(11, Supplement 3), T27–T45. doi: 10.1016/j.jpain.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, Barsky AJ, Vago DR, Baslet G, & Silbersweig DA (2015). A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci, 27(1), e40–50. doi: 10.1176/appi.neuropsych.13070170 [DOI] [PubMed] [Google Scholar]
- Rief W, & Broadbent E (2007). Explaining medically unexplained symptoms-models and mechanisms. Clinical Psychology Review, 27(7), 821–841. doi: 10.1016/j.cpr.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Rothman KJ, & Greenland S (2005). Causation and causal inference in epidemiology. American Journal of Public Health, 95(S1), S144–S150. doi: 10.2105/AJPH.2004.059204 [DOI] [PubMed] [Google Scholar]
- Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, … Ohrbach R (2013). General health status and incidence of first-onset temporomandibular disorder: the OPPERA prospective cohort study. Journal of Pain, 14(12, supplement 2), T51–62. doi: 10.1016/j.jpain.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade G, Sanders A, Bair E, Brownstein NC, Dampier D, Knott C, … Ohrbach R (2013a). Preclinical episodes of orofacial pain symptoms and their association with healthcare behaviors in the OPPERA prospective cohort study. Pain, 154(3), 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade G, Sanders A, Ohrbach R, Bair E, Maixner W, Greenspan J, … Diatchenko L (2015). COMT diplotype amplifies effect of stress on risk of temporomandibular pain. Journal of Dental Research, 94(9), 1187–1195. doi: 10.1177/0022034515595043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Bair E, By K, Mulkey F, Baraian C, Gonzalez Y, … Ohrbach R (2011). Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. Journal of Pain, 12(11, supplement 3), T12–T26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, … Ohrbach R (2013b). Signs and symptoms of first-onset TMD and socio-demographic predictors of its development: the OPPERA prospective cohort study. Journal of Pain, 14(12, supplement 2), T20–32. doi: 10.1016/j.jpain.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Diatchenko L, Bhalang K, Sigurdsson A, Fillingim RB, Belfer I, … Maixner W (2007). Influence of Psychological Factors on Risk of Temporomandibular Disorders. Journal of Dental Research, 86(11), 1120–1125. doi: 10.1177/154405910708601119 [DOI] [PubMed] [Google Scholar]
- Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, … Maixner W (2013c). Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibualr disorder: implications and future directions. Journal of Pain, 14(12, supplement 2), T116–T124. doi: 10.1016/j.jpain.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, … Maixner W (2016). Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. Journal of Dental Research, 95(10), 1084–1092. doi: 10.1177/0022034516653743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Mir E, Bair E, Slade GD, Dubner R, Fillingim RB, … Diatchenko L (2013). Genetic variants associated with development of TMD and its intermediate phenotypes: the genetic architecture of TMD in the OPPERA prospective cohort study. Journal of Pain, 14(12, supplement 2), T91–T101. doi: 10.1016/j.jpain.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R (1996). Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society. Series B (Methodological), 267–288. doi:www.jstor.org/stable/2346178
- Vachon-Presseau E, Tétreault P, Petre B, Huang L, Berger SE, Torbey S, … Griffith JW (2016). Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain, 139(Pt 7), 1958–1970. doi: 10.1093/brain/aww100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Dworkin SF, LeResche L, & Kruger A (1988). An epidemiologic comparison of pain complaints. Pain, 32(2), 173–183. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Lin EH, Fenton JJ, & Saunders K (2007). Frequency and priority of pain patients’ health care use. Clinical Journal of Pain, 23(5), 400–408. [DOI] [PubMed] [Google Scholar]
- Wessely S, & Hotopf M (1999a). Is fibromyalgia a distinct clinical entity? Historical and epidemiological evidence. Best Practice & Research Clinical Rheumatology, 13(3), 427–436. [DOI] [PubMed] [Google Scholar]
- Wessely S, Nimnuan C, & Sharpe M (1999b). Functional somatic syndromes: one or many? The Lancet, 354(9182), 936–939. doi: 10.1016/S0140-6736(98)08320-2 [DOI] [PubMed] [Google Scholar]
- Woolf AD, & Pfleger B (2003). Burden of major musculoskeletal conditions. Bulletin of the World Health Organization, 81(9), 646–656. [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain, 152(3 Suppl), S2–S15. doi: 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, & Coghill RC (2011). Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience, 31(14), 5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


