Abstract
Continuing investigations of corticostriatal connections in rodents emphasize an intricate architecture where striatal projections originate from different combinations of cortical layers, include an inhibitory component, and form terminal arborizations which are cell-type dependent, extensive or compact. Here, we report that in macaque monkeys, deep and superficial cortical white matter neurons (WMNs), peri-claustral WMNs, and the claustrum proper project to the putamen. WMNs retrogradely labeled by injections in the putamen (4 injections in 3 macaques) were widely distributed, up to 10mm antero-posterior from the injection site, mainly dorsal to the putamen in the external capsule, and below the premotor cortex. Striatally projecting labeled WMNs (WMNsST) were heterogeneous in size and shape, including a small GABAergic component. We compared the number of WMNsST with labeled claustral and cortical neurons and also estimated their proportion in relation to total WMNs. Since some WMNsST were located adjoining the claustrum, we wanted to compare results for density and distribution of striatally projecting claustral neurons (ClaST). ClaST neurons were morphologically heterogeneous and mainly located in the dorsal and anterior claustrum, in regions known to project to frontal, motor, and cingulate cortical areas. The ratio of ClaST to WMNsST was about 4:1 averaged across the four injections. These results provide new specifics on the connectional networks of WMNs in non-human primates, and delineate additional loops in the corticostriatal architecture, consisting of interconnections across cortex, claustralstriatal and striatally projecting WMNs.
Keywords: interstitial neurons, striatum, non-human primate, GABAergic projection neurons, RRID: AB_221544, RRID: AB_2630395, RRID: AB_477019
Graphical abstract
We report that retrograde tracer injections in the motor putamen of macaque monkeys labeled neurons in the deep and superficial cortical WM, in the peri-claustral WM, and in the claustrum (a). Striatally projecting WMNs (WMNsST) were widely distributed, up to 10 mm antero-posterior from the injection site (b: sections are spaced 1200 μm), and morphologically heterogeneous (c). Labeled neurons in the claustrum (ClaST) were mainly located in the dorsal and anterior claustrum, in regions known to project to frontal, motor, and cingulate cortical areas. These results delineate additional loops in the corticostriatal architecture, consisting of interconnections across cortex, claustralstriatal and striatally projecting WMNs.
INTRODUCTION
The general organization of corticostriatal projections is well-established for both rodents and non-human primates (NHP) (Haber, 2016; Alloway et al., 2017; Shipp, 2017). These originate from pyramidal neurons mainly in layer 5 but also in layers 3 or 6 (Reiner et al., 2010; Haber, 2016). Interconnected cortical areas form parallel circuits (Shipp, 2017). Recent studies in mice, however, have revealed several new features demonstrating a high degree of complexity of the striatal architecture. First, a direct inhibitory circuit has been identified from somatostatin-positive neurons in auditory and motor areas to spiny projection neurons (Rock et al., 2016, and see their discussion of other, parvalbumin+ and VIP+ GABAergic corticostriatal projections). Second, the degree of topographic organization of corticostriatal connectivity is shown to differ as a function of pyramidal projecting cell type, where intratelencephalic layer 5 neurons have widely divergent striatal projections, in contrast with more compact arbors of the pyramidal-tract neurons in layer 5 (Hooks et al., 2018; and Table 1 in Shipp, 2017). Third, it was demonstrated that white matter neurons (WMNs) send collaterals to the striatum (von Engelhardt et al., 2011; Frazer et al., 2017).
Especially about the third point, it is noteworthy that no studies in monkey have focused on the possible involvement of WMNs to the striatal network. To address this gap, in the present study we examined WMN putaminal projections in rhesus monkey, after injections of retrograde tracers in the dorsal (motor-related) putamen. The results showed widespread labeling of both superficial and deep WMNs, demonstrating that this circuit is phylogenetically conserved across species and extending our knowledge concerning the local and long-distance projections of these neurons. In fact, although several publications have characterized WMNs in terms of density, morphology, and neurochemical heterogeneity (rat: Clancy et al., 2001; human: Meyer et al., 1992; Garcia-Marin et al., 2010; NHP: Mortazavi et al., 2016, 2017; Swieger et al. 2018 and references therein), only scant attention has been paid to their connectivity.
Furthemore, we also examined the distribution and density of striatally projecting claustral neurons (ClaST) since 1) some of the retrogradely labeled WMNs are closely adjacent to the claustrum, and 2) the existence of claustral projections to non-cortical targets has been under discussion (in rodents: Mathur et al., 2009). Altogether, the present study demonstrates that the striatal network should be extended to the WMNs and claustrum.
METHODS
Subjects, surgical procedures, and selection of the injection sites
The experiments were carried out in 3 Macaca mulatta (Cases 61, 71, and 75), in which retrograde neural tracers were injected in the putamen (Table 1). Animal handling as well as surgical and experimental procedures complied with the European law on the humane care and use of laboratory animals (directives 86/609/EEC, 2003/65/CE, and 2010/63/EU) and Italian laws in force regarding the care and use of laboratory animals (D.L. 116/92 and 26/2014), and were periodically approved by the Veterinarian Animal Care and Use Committee of the University of Parma and authorized by the Italian Ministry of Health.
Table 1.
Animals used, location of injection sites, and type and amount of injected tracers
| Case | Species | Sex | Age (years) | Weight (kg) | Hemisph. | Location* | Tracer | Amount |
|---|---|---|---|---|---|---|---|---|
| 61 | M. mulatta | F | 6 | 4.5 | R | AC | CTBg 1% | 2 μl |
| 71 | M. mulatta | F | 6.5 | 3.3 | L | −2 mm | FB 3% | 0.3 μl |
| R | +1 mm | CTBg 1% | 1 μl | |||||
| 75 | M. mulatta | M | 6 | 3.5 | R | AC | CTBg1% | 1 μl |
All injections are in the putamen. AC: injections at the level of the crossing of the anterior commissure; mm from the AC crossing.
Before the injection of neural tracers, we obtained scans of each brain using magnetic resonance imaging (MRI; Case 71 and 75: 7T General Electric, Boston, MA; Case 61: 0.22 T Paramed Medical Systems, Genova, Italy) to calculate the stereotaxic coordinates of the putaminal target region and the best trajectory of the needle to reach it.
Under general anesthesia (in Cases 61 and 71: Zoletil®, initial dose: 20 mg/kg, i.m.; supplemental: 5–7 mg/kg/h, i.m., or Ketamine, 5 mg/kg i.m. and Medetomidine, 0.08–0.1 mg/kg i.m. In Case 75: induction with Ketamine 10 mg/kg, i.m. followed by intubation, isoflurane 1.5–2%) and aseptic conditions, each animal was placed in a stereotaxic apparatus and an incision was made in the scalp. The skull was trephined to remove the bone and the dura was opened to expose a small cortical region. After tracer injections, the dural flap was sutured, the bone was replaced, and the superficial tissues were sutured in layers. During surgery, hydration was maintained with saline, and heart rate, blood pressure, respiratory depth, and body temperature were continuously monitored. Upon recovery from anesthesia, the animals were returned to their home cages and closely observed. Dexamethasone (0.5 mg/kg, i.m.) and prophylactic broad-spectrum antibiotics (e.g., Ceftriaxone 80 mg/kg, i.m.) were administered pre- and postoperatively, as were analgesics (e.g., Ketoprofen 5 mg/kg i.m.)
Tracer injections and histological procedures
Based on stereotaxic coordinates, the neural tracers Fast Blue (FB, 3% in distilled water, Dr Illing Plastics GmbH, Breuberg, Germany) and Cholera Toxin B subunit, conjugated with Alexa 488 (CTB green, CTBg; 1% in 0.01 M phosphate-buffered saline at pH 7.4, Molecular Probes, Thermo Fisher Scientific, Waltham, MA, USA) were slowly pressure-injected through a stainless steel beveled needle (tip diameter: 100-150 μm) attached through a polyethylene tubing to a Hamilton syringe (Hamilton Company, Reno NV). In cases 71 and 75, the injection needle was lowered to the putamen within a guiding tube, to avoid tracer spill-over in the WM. Table 1 summarizes the locations of the injections, the injected tracers, and the amounts injected.
After appropriate post-injection survival periods (28 days for FB and 14 days for CTBg), each animal was deeply anesthetized with an overdose of sodium thiopental and perfused through the left cardiac ventricle consecutively with saline (about 2l in 10 min), 3.5% formaldehyde (5l in 30 min), and 5% glycerol (3l in 20 min), all prepared in 0.1 M phosphate buffer, pH 7.4. Each brain was then blocked coronally on a stereotaxic apparatus, removed from the skull, photographed, and placed in 10% buffered glycerol for 3 days and 20% buffered glycerol for 4 days. In Case 75, the right inferotemporal cortex was removed for other experimental purposes. Finally, each brain was cut frozen into coronal sections of 60-μm (Cases 61 and 75) or 50-μm (Case 71) thickness.
In all cases, sections spaced 300 μm apart –that is one section in each repeating series of 5 in Cases 61 and 75 and one in series of 6 in Case 71– were mounted, air-dried, and quickly coverslipped for fluorescence microscopy. Another series of each fifth section (sixth section in Case 71) was processed for visualizing CTBg with immunohistochemistry. Specifically, endogenous peroxidase activity was eliminated by incubation in a solution of 0.6% hydrogen peroxide and 80% methanol for 15 minutes at room temperature. The sections were then incubated for 72 h at 4°C in a primary antibody solution of rabbit anti-Alexa 488 (1:15000, Thermo Fisher Scientific, RRID:AB_221544) in 0.5% Triton, 5% normal goat serum in PBS, and for 1 h in biotinylated secondary antibody (1:200, Vector Laboratories, Burlingame, CA) in 0.3% Triton, 5% normal goat serum in PBS. Finally, CTBg labeling was visualized using the Vectastain ABC kit and 3,3’-diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO) as a chromogen and 0.01% hydrogen peroxide. The reaction was intensified with cobalt chloride and nickel ammonium sulfate. In Case 75, a subset of sections spaced 1200 μm from this series, were then incubated overnight at room temperature in a primary antibody solution of rabbit anti-NeuN (1:5000, Cell Signalling Technology, Danvers, MA, RRID:AB_2630395) in 0.3% Triton, 5% normal goat serum in PBS, and for 1 h in biotinylated secondary antibody (1:100, Vector Laboratories) in 0.3% Triton, 5% normal goat serum in PBS. Finally, NeuN positive cells were visualized using the Vectastain ABC kit and DAB as a chromogen. With this protocol, in the same tissue sections CTBg labeling was stained black and NeuN positive cells were stained brown.
In Case 75, some sections in the proximity of the injection site, from a series of each fifth section, were processed for Glutamic acid decarboxylase 65/67 (GAD65/67) immunofluorescence. Specifically, the sections were incubated overnight in a primary antibody solution of rabbit anti-GAD65/67 (1:1000, Sigma-Aldrich, RRID:AB_477019) in 0.3% Triton, 2% normal goat serum in PBS, and subsequently, for 2 h in secondary antibody conjugated with Alexa-568 (1:350, Vector Laboratories) in 0.3% Triton, 2% normal goat serum in PBS. With this protocol, CTBg and GAD65/67 positive cells were visible in the same tissue section as green and red fluorescent cells, respectively.
In all cases, one series of each fifth section (sixth section in Case 71) was stained with the Nissl method (0.1% thionin in 0.1 M acetate buffer, pH 3.7).
Antibody characterization and specificity
The antibodies used and associated details are listed in Table 2.
Table 2.
Sources and dilution of antibodies used in the current study.
| Antibody | Host | Mono/polyclonal | Immunogen | Manufacturer | Cat. n. | Dilution | RRID |
|---|---|---|---|---|---|---|---|
| Alexa-fluor 488 | rabbit | polyclonal | Alexa Fluor 488 | Thermo Fisher Scientific | A-11094 | 1:15000 | RRID:AB_221544 |
| NeuN | rabbit | monoclonal | NeuN protein | Cell Signalling Technology | 12943 | 1:5000 | RRID:AB_2630395 |
| GAD65/67 | rabbit | polyclonal | human GAD 67and 65 | Sigma-Aldrich | G5163 | 1:1000 | RRID:AB_477019 |
Data analysis
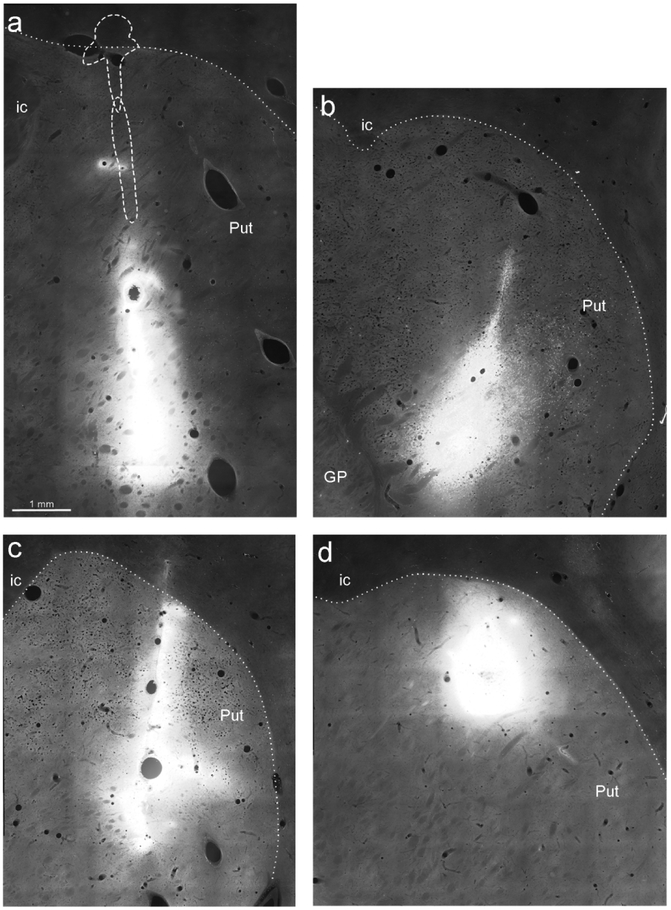
The criteria used for defining the injection site core and halo, and identifying FB and CTBg labeling have been described in earlier studies (Luppino et al., 2003; Rozzi et al., 2006). The injection sites of Cases 71 and 75 were completely restricted to the putamen, while the injection of CTBg of Case 61 had some minimal involvement of the white matter just above it (Figure 1). All injections involved a putaminal region overlying the crossing of the anterior commissure (AC) at different dorso-ventral levels (Table 1 and Figure 1). The injection sites of Cases 61r CTBg and 71l FB were more ventral with respect to the CTBg injections of Cases 75r and 71r, located in the dorsal and mid-dorsal lateral putamen, respectively.
Figure 1:
Fluorescence photomicrographs of the injection sites in the Putamen. (a) CTBg in Case 61r (section 91), the dashed line indicates the border of the injection site observed in two sections located 600 μm and 900 μm rostral, respectively. (b) FB in Case 71l (section 90, rotated for sake of comparison). (c) CTBg in Case 71r (section 77). (d) CTBg in Case 75r (section 86). Dotted lines denote the external border of the putamen. Scale bar in a applies also to b-d. GP= globus pallidus; ic= internal capsule; Put=putamen.
The distribution of retrograde labeling in the cortex, the claustrum, and the WM was analyzed in sections at an interval of every 300 μm and plotted, using a computer-based charting system, at an interval of every 1200 μm.
For identifying the border of WM with overlying layer 6 the criteria used in Mortazavi et al. (2016) were adopted; that is, the boundary of layer 6 was assessed by a fall-off in cell density and a change in soma morphology in the NeuN immunostained or in the Nissl stained sections. In fluorescent sections or in sections immunostained for tracers, the WM borders (with cortical layer 6, claustrum, and putamen) were identified by using a dark field to compare with those observed in the adjacent Nissl or NeuN section.
Quantitative analysis of labeled neurons.
In all the cases, we counted the number of retrogradely labeled neurons plotted in the hemisphere ipsilateral to the injection (cortex, claustrum, and WM) in sections at 1200 μm interval. Temporal cortex was excluded from analysis, as in Case 75 the temporal cortex was partially removed for the purpose of another study, and in the other two cases, only sparse labeling was observed in this region. Cortical afferents were expressed in terms of percentage of labeled neurons found in a given cortical region or area, with respect to the overall cortical labeling. Afferent populations to the putamen from the claustrum (ClaST) and the WM (WMNsST) were expressed in terms of ratio of labeled neurons in each structure with respect to the labeling found in the cortex (WMNsST:corticostriatal; ClaST:corticostriatal). We also calculated the ratio of labeled neurons in the WM with respect to the claustrum (WMNsST:ClaST).
In Case 75, the WMNsST were quantitatively analyzed with respect to the general distribution of WMNs (NeuN positive). Specifically, representative sections (74, 83, 90, 98) processed for NeuN were photographed with the 10× objective through a digital camera incorporated into the microscope with an automatic acquisition system (NIS-Element; Nikon Co., Tokyo, Japan), the WM was subdivided into squared (500×500 μm) or rectangular (1000×250 μm) bins (“counting box”) of 0,25 mm2 and labeled and unlabeled neurons in each bin were counted. In these sections, we noticed that brown staining (NeuN) could hide some light black staining (CTBg). Thus, for each analyzed section, an adjacent section processed only for CTBg was photographed and the two sections were digitally overlaid in order to confidently attribute retrogradely labeled neurons to a specific counting box.
A similar procedure was used for obtaining the labeled/total neuron ratio in the claustrum.
In selected sections (Case 61r CTBg section 83; Case 71r CTBg section 72; Case 75r CTBg, sections 75, 79, 91), soma area of labeled cells (63 WMNsST; 126 ClaST) was measured on digitalized images acquired with the 10× or the 20× objective using NIS-Element software.
RESULTS
Retrograde labeling in the cortex
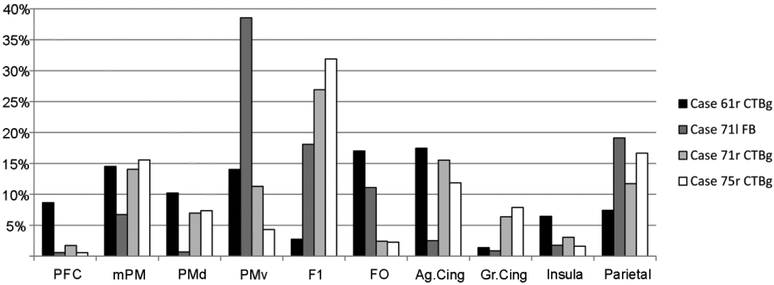
In accord with previous work, putamen injections resulted in retrogradely labeled neurons in subdivisions of the frontal, cingulate, insular, and parietal cortex (Figure 2). The primary motor, premotor, and cingulate motor cortex altogether accounted for 60-75% of the total cortical labeling, consistent with the location of the injection sites in the motor putamen. In Case 61r CTBg labeling was located more anteriorly, mainly in the premotor and cingulate motor areas, and only partially in the primary motor area. After the three other injections, a relatively high proportion of labeled cells was found in the primary motor area. In Cases 61r (CTBg) and 71l (FB) the labeled focus tended to involve more the ventral premotor and the frontal opercular cortex as compared to the dorsal and mesial premotor cortex. These results are consistent with previous reports (see, e.g., Nambu 2011; Takada et al. 2013) and serve as baseline comparison for the density of labeled neurons in the WM and in the claustrum.
Figure 2:
Percent distribution of retrograde cortical labeling. Ag.Cing: agranular cingulate cortex; FO: frontal opercular cortex; Gr.Cing= granular cingulate cortex; mPM: mesial premotor cortex; PFC: prefrontal cortex; PMd: dorsal premotor cortex; PMv: ventral premotor cortex.
Retrograde labeling in the WM
Distribution of WMNsST
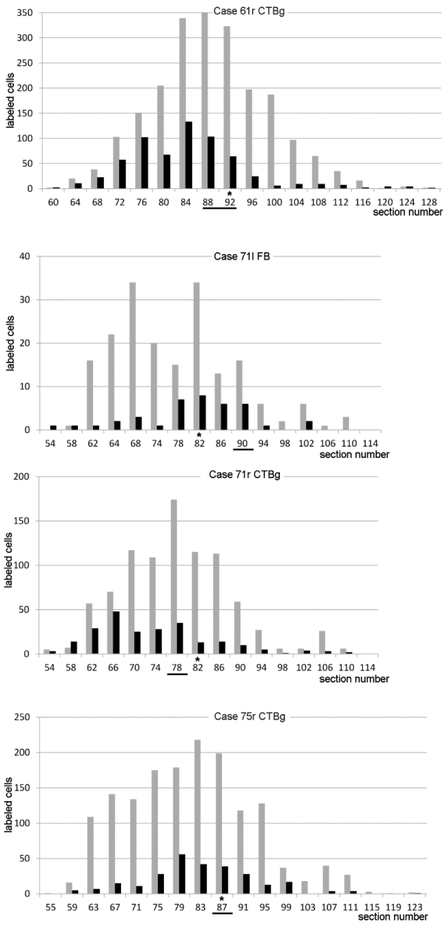
The distribution of the WMNsST after injections in the putamen is shown in Figures 3-6. In the antero-posterior (AP) plane, WMNsST were located up to 10 mm from the injection site; thus, up to 20 mm total in the AP direction (Fig. 6; Case 61r CTBg=20,4 mm; Case 71l FB=14,4 mm; Case 71r CTBg=16,8 mm; Case 75r CTBg=19,2 mm). The number of WMNsST fell off beyond 9-10 mm from the injection site.
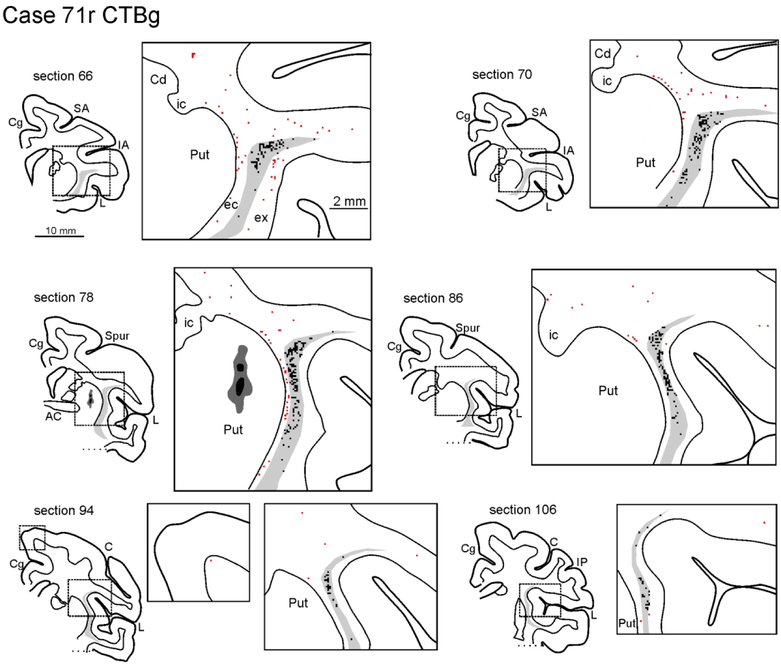
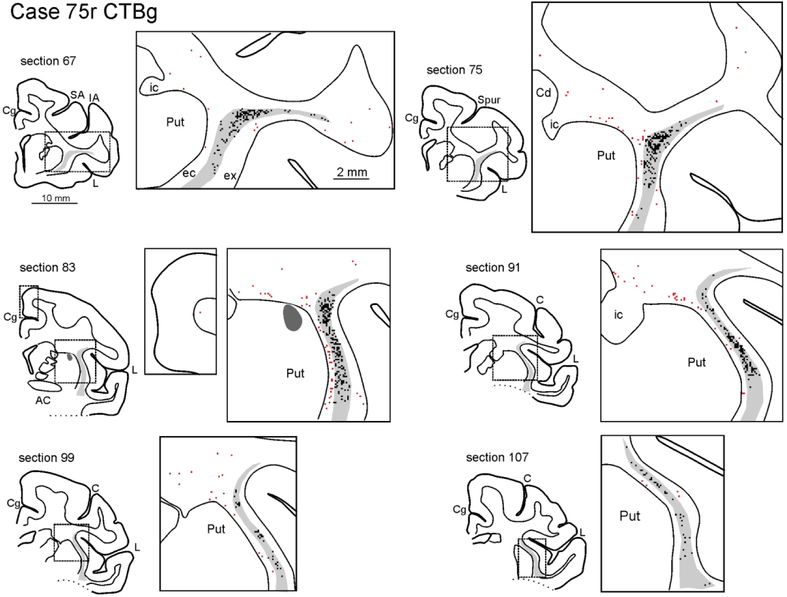
Figure 3:
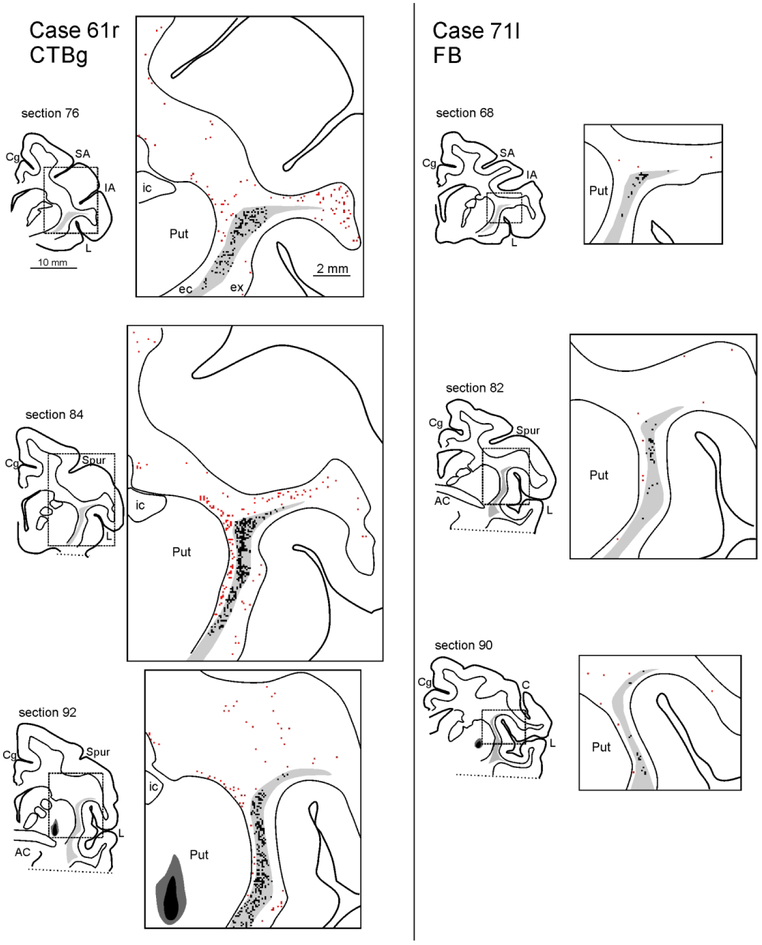
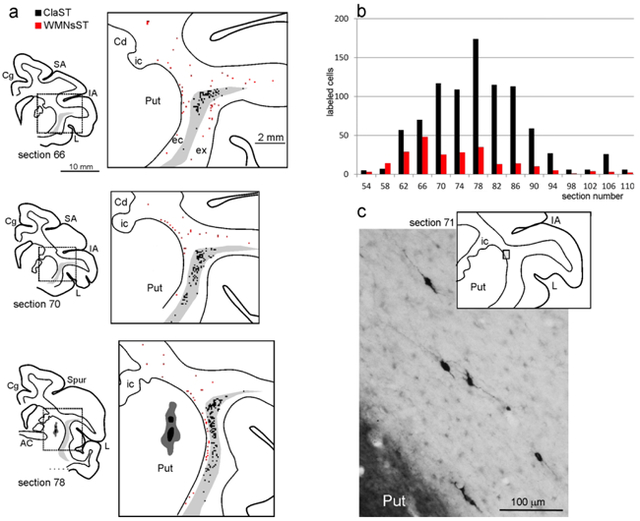
Drawings of coronal sections showing the distribution of the labeled neurons observed in the WM and the claustrum in Case 71r CTBg. The sections are shown in an anterior (section 66) to posterior (section 106) order. For each AP level, the section outline shows from where the enlargement was taken (box). In the enlargements, red dots correspond to retrogradely labeled WMNs (WMNsST) and black dots to labeled neurons within the claustrum (ClaST neurons). The claustrum is indicated with a light gray shading. In section 78 the dark grey and black regions correspond to the injection core and halo, respectively (and see Figure 1c). Because of the limited space, the location of the external (ec) and the extreme (ex) capsule are indicated only in the first section. AC: anterior commissure. C: central sulcus; Cd: caudate nucleus; Cg: cingulate sulcus; IA: inferior arcuate sulcus; IP: intraparietal sulcus; L: lateral fissure; SA: superior arcuate sulcus; Spur: spur of the arcuate sulci. Other abbreviations as in Figure 1.
Figure 6:
Antero-posterior distribution of labeled cells. A bar graph for each case shows the number of WMNsST (black) and ClaST neurons (grey) in coronal sections spaced 1.2 mm. The section/sections in which the injection site (see Figure 1) and the AC crossing were observed are highligted by a black line and an asterisk, respectively.
The focus of WMNsST was mainly dorsal to the putamen, located in the external capsule close to the claustrum, and in the WM below the ventral premotor cortex. Labeled neurons were also found between the insula and the claustrum in the extreme capsule, and below the dorsal premotor cortex.
A portion of WMNsST were identified as “superficial,” within 0.5 mm from the layer 6 border. The percentage of superficial WMNsST, with respect to the overall number of WMNsST, was high in cases 61r CTBg and 71l FB (32.7 and 30.8%, respectively) and relatively low in cases 71r CTBg and 75r CTBg (13,7% and 12,6% respectively). This difference could be related to the zonal distribution of the cortical labeling, where the percentage of superficial WMNsST correlates with more or less abundant labeling in the overlying cortex in PMv and the frontal operculum.
Topographical differences of WMNs density.
WMNs are not distributed evenly in the WM (Meyer et al., 1992; Suarez-Sola et al., 2009; Garcia-Marin et al., 2010; Mortazavi et al., 2016, 2017; Swiegers et al., 2018) and in the macaque monkey, average density varies among different lobes, being low in the cingulate and frontal regions, and (Meyer et al., 1992) higher in the motor cortical region. In our material, WMNsST, concentrated in the proximity of the injection site (10 mm anterior or posterior), were preferentially located in frontal regions. In counting boxes of 0.25 mm2, the number of WMNsST was from 1 to 6.
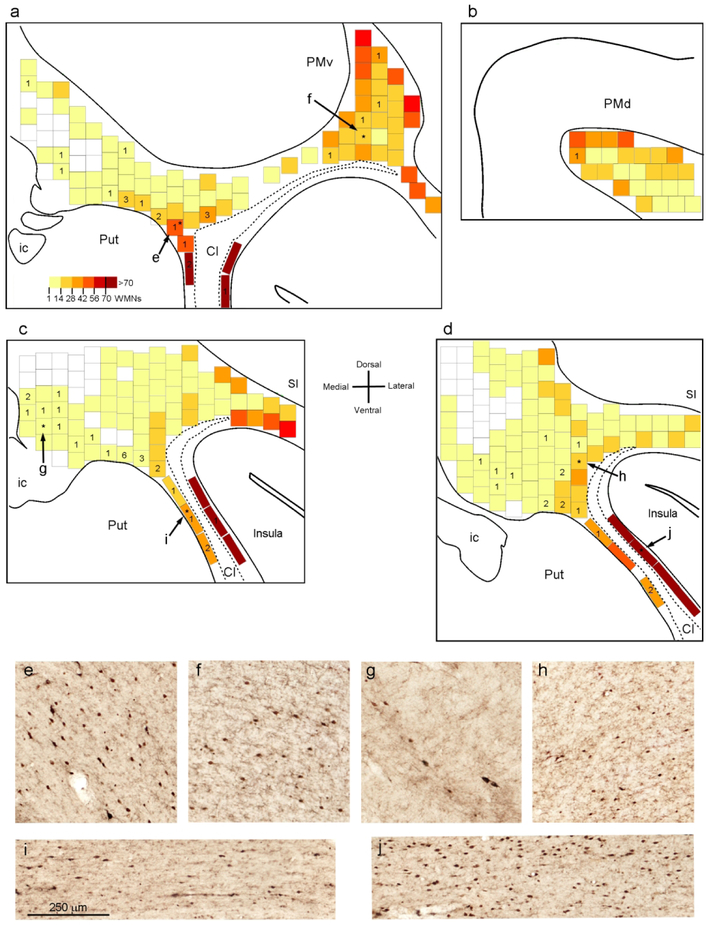
WMNsST occurred both in zones of high and low numbers of non-labeled WMNs (Fig. 7). In order to determine the percentage of WMNsST in relation to total WMNs, we first determined, from NeuN counterstained sections, the total number of labeled and unlabeled WMNs in a counting box of 0.25 mm2 in four sections (Fig. 7). This varied from zero to more than 60. In the extreme capsule, the density of these neurons could be even higher (mean=110 WMNs/0.25mm2, Fig. 7 j). Counting boxes located in the deep WM lateral to the caudate nucleus were often devoid of WMNs, those above the internal capsule showed only a few WMNs (up to 10; Fig. 7 g), and those just above the putamen and the claustrum had higher numbers of WMNs (mean=20 WMNs/0.25 mm2; Fig. 7 e and h). For the external capsule between the putamen and the claustrum there was a relatively high density of WMNs (mean=44 WMNs/0.25 mm2; Fig. 7 i). In the WM below the premotor cortex, the number of WMNs increased laterally, and progressing toward the frontal operculum (Fig. 7 a and f). A mean of 23 and 44 WMNs/0.25 mm2 were found in more deep and superficial counting boxes, respectively. Below the dorsal premotor cortex (PMd), the number of WMNs/0.25 mm2 was less, with a mean of 13 and 32 WMNs/0.25 mm2 in more deep and superficial counting boxes, respectively. Considering differences in AP and frontal location, these data are in good accord with those reported in macaque by Mortazavi et al. (2016), who found a 4:1 ratio of superficial: deep WMNs per 0.16mm2 of WM.
Figure 7:
Distribution of WMNs in three representative sections processed for NeuN (Case 75r). (a-d) Heatmaps showing the density of WMNs in 0.25 mm2 counting boxes in sections 74 (a), 82 (b), 90 (c) and 98 (d). In counting boxes near or slightly overlapping grey matter borders, only neurons that were clearly in the WM were counted. Numbers indicate WMNsST in the adjacent sections that, by overlying the two sections, could be attributed to a specific counting box. WMNsST that could not be attributed to a specific counting box are not shown. Asterisks indicate the boxes showed in e-j. (e) WM just above the putamen and (f) deep WM below the PMv (both from a); (g) WM at the dorsal entrance of the internal capsule (from c); (h) WM near the claustrum (from d); (i) external capsule (from c); (j) extreme capsule (from d). Cl: claustrum; SI: primary somatosensory cortex. Other abbreviations as in Figures 1, 2, and 3.
Proportion of WMNsST
The overall number of WMNsST varied among the different cases, depending on the amount and type of injected tracer. In comparison with corticostriatally projecting neurons, the number of WMNsST was low, not more than 0.6% and with a ratio ranging from 1:460 (Case 71l FB) to 1:165 (Case 61r CTBg). Higher proportions might result if the comparison was between WMNs and one cortical area, or between superficial WMNs and overlying cortex. The ratio of WMNsST to ClaST neurons was greater (see below and Table 3).
Table 3.
A: Number of labeled neurons projecting to the striatum (putamen) observed in the cortex (corticostriatal, CorticoSt), the WM (WMNsST), and the claustrum (ClaST). B: Ratio of striatal projecting Cla vs WMNs.
| A | B | |||
|---|---|---|---|---|
| Case | CorticoSt* | WMNsST | ClaST | ClaST:WMNsST |
| 61r CTBg | 103506 | 626 | 2136 | 3:1 |
| 71l FB | 17973 | 39 | 189 | 5:1 |
| 71r CTBg | 60757 | 234 | 897 | 4:1 |
| 75r CTBg | 59610 | 270 | 1546 | 6:1 |
all cortex except the temporal, see text for details
We next compared the numbers of labeled/unlabeled WMNs, relevant for estimating the proportion of WMNsST projections in relation to other projections of WMNs or local WM-WM connectivity. For this, we used the portion of defined counting boxes (16%) that contained WMNs and hosted at least one WMN-ST. We then calculated the percentage of WMNsST relative to the total number of WMNs in each counting box. The percentage of WMNsST was higher in the central deep white matter (mean 50%) where often 1 WMN-ST was located in counting boxes which had a total of 1-3 WMNs. In counting boxes located dorsally around the putamen, WMNsST were on average 20% (1-5 WMNsST/4-28 WMNs), while in the external capsule and in the deep white matter below PMv, WMNsST were on average 5% (1-2 WMNsST/20-40 WMNs). The lowest percentage was found in the extreme capsule, where the WMNsST were 1% of the more numerous population of total WMNs.
Heterogeneity of WMNsST.
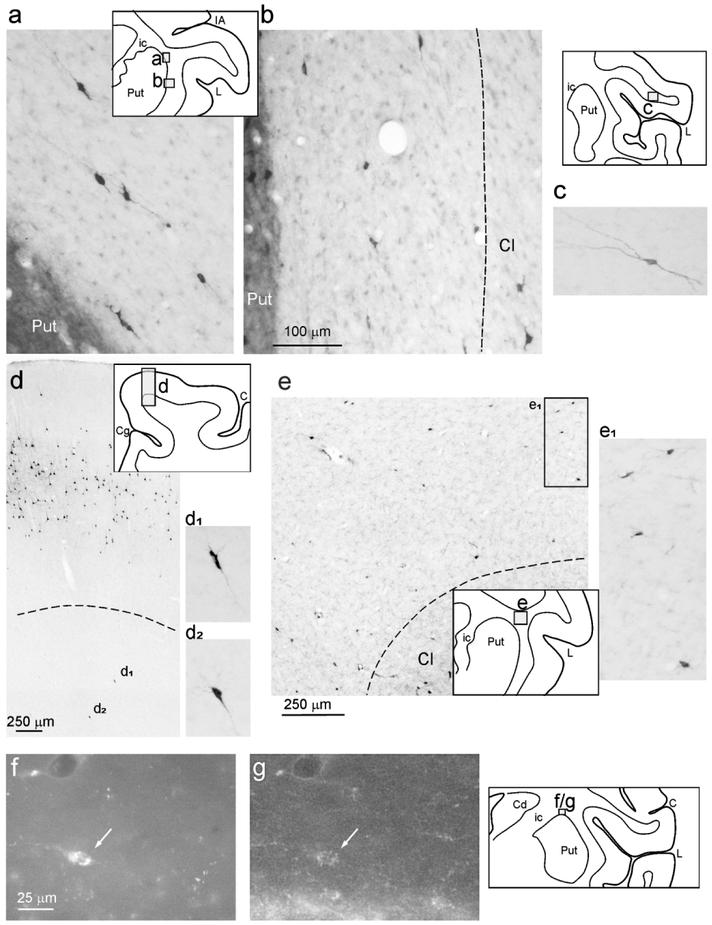
WMNsST were heterogeneous in soma size and shape, including rounded, fusiform, and multipolar neurons (Figs. 8 and 9). The range of soma size (soma area, min= 60 μm2, max= 250 μm2, mean=115 μm2) was in line with that described for WMNs in a previous report in macaque (mean=130 μm2 in Mortazavi et al., 2016).
Figure 8:
Examples of retrogradely labeled WMNs. For each photo, a drawing of the section is shown for orientation. (a-d) are taken from Case 71r CTBg (sections 71, 94, and 112), (e) is taken from Case 61r CTBg (section 83). Dashed lines indicate the border of the WM with the claustrum (b and e) and with layer 6 (d). Scale bar in (b) applies also to (a), (c), (d1), (d2), and (e1). Note that labeled cells have a different soma area and circularity. (f) and (g) show an example (arrow) from Case 75r CTBg (section 91) of a CTBg WMNsST (f) that is GAD65/67 positive (g). Abbreviations as in Figures 1 and 3.
Figure 9:
Examples of WMNsST from Case 61r CTBg (section 76). Scale bar in (b) applies also to (c-e). Conventions and abbreviations as in Figures 1, 3, and 8.
In selected sections reacted for GAD65/67 immunofluorescence it was possible to find some WMNsST that were GAD positive (Fig. 8, f and g), but the majority appeared to be GAD negative.
Retrograde labeling in the claustrum
ClaST neurons were located in almost the whole extent of the ipsilateral claustrum, except its ventralmost part. Labeling was denser in the dorsal and anterior parts, from the AP level of the injection site to more anterior levels (Figs. 3-6). Specifically, in Cases 61r CTBg and 75r CTBg, labeled foci were concentrated laterally at the most anterior claustrum but, more posteriorly, involved progressively more central and ventral sectors. The posteriormost levels (3-4 mm) had only very sparse labeling. In Cases 71l FB and 71r CTBg the labeling was weaker, less widespread and concentrated in a dorsal/central sector of the claustrum, almost avoiding the ventral half.
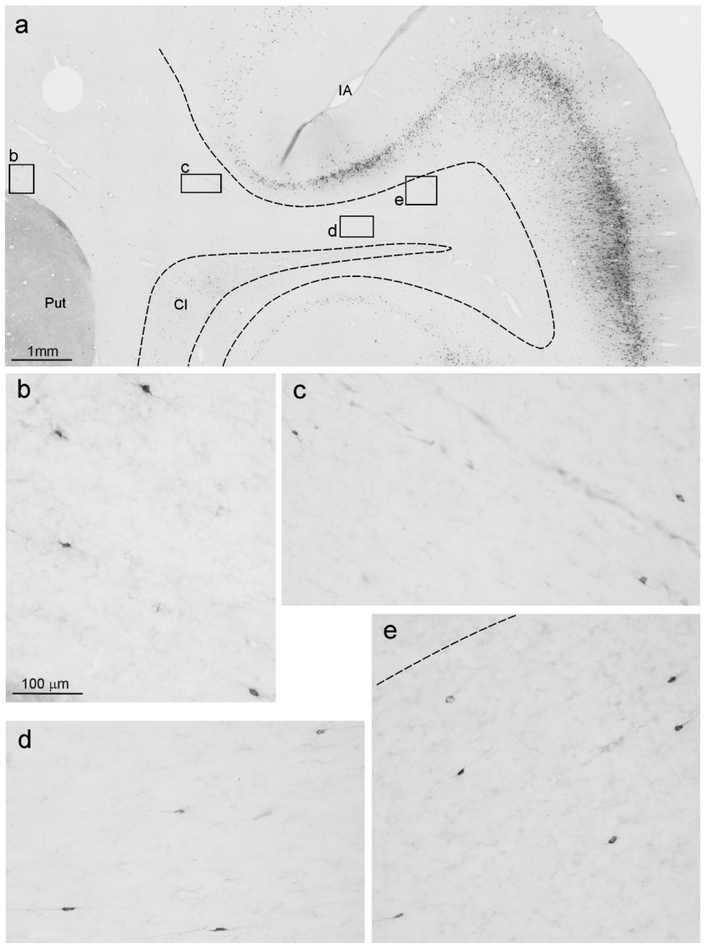
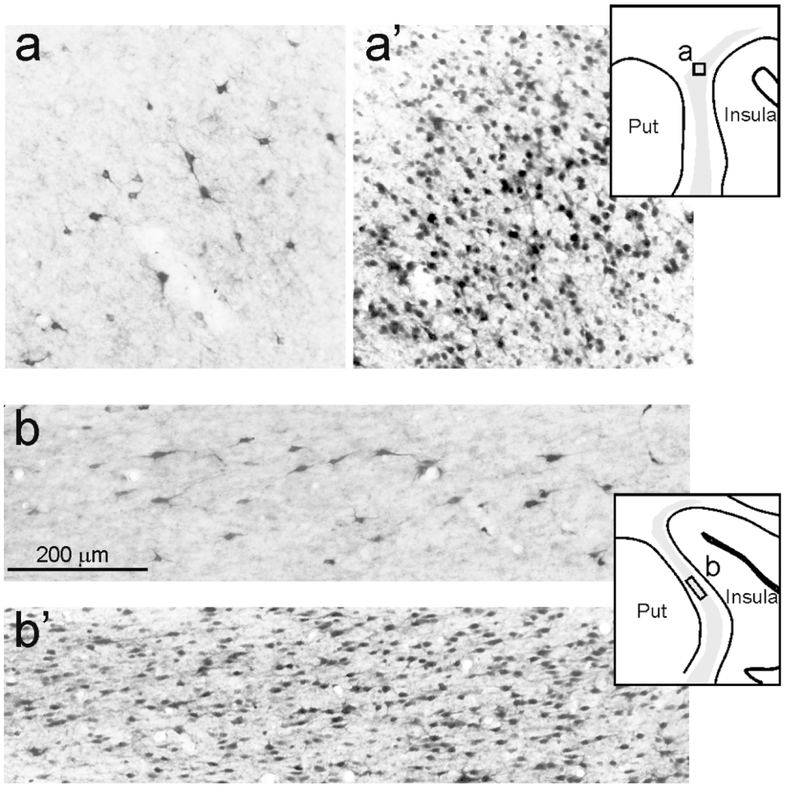
In Case 75, we determined the number of labeled ClaST neurons and total number (NeuN positive) of neurons. For this, we used a counting box of 0.25 mm2, positioned where the labeling was relatively dense (sections 75 and 79= dorsal claustrum, and 91=middle claustrum, see Figs. 4 and 10). In these boxes, the percentage of ClaST with respect to the total number of claustral neurons was from 3% to 10% (mean=6.5%). The ratio of labeled neurons found in the claustrum (ClaST) with respect to corticostriatal neurons was about 1:60, ranging from 1:95 of Case 71l FB to 1:38 of Case 75r CTBg (Table 3). The ratio of total numbers of ClaST to WMNsST was about 4:1 (Table 3).
Figure 4.
Drawings of coronal sections showing the distribution of WMNsST and ClaST neurons in Case 75r CTBg. In section 83 the dark grey region corresponds to the injection halo (see Figure 1d). Conventions and abbreviations as in Figures 1 and 3.
Figure 10:
Examples of ClaST neurons vs total neurons in counting boxes of 0.25 mm2 in the claustrum, from Case 75r. For each level, a drawing of the section is shown for orientation. (a) and (b) are from sections 79 and 91 processed for CTBg; (a’) and (b’) are from adjacent sections processed for NeuN.
Retrogradely labeled claustral neurons were heterogeneous in soma size and shape, including mainly multipolar neurons and, especially in the central part of the claustrum, fusiform neurons. The soma area varied from a minimum of 40 μm2 to a maximim of 190 μm2 (mean=100 μm2).
DISCUSSION
Here we report that, in addition to the cortical labeling expected from previous studies, retrograde tracer injections in the putamen of macaque monkeys labeled neurons in the deep and superficial cortical WM, in the peri-claustral WM, and in the claustrum. This confirms and extends, for the in vivo condition in NHP, previous in vitro experiments in mice reporting 5HTr3 WMNs having collaterals to both overlying cortex and striatum (von Engelhardt et al., 2011; Frazer et al., 2017), and provides new data about the looped architecture interconnecting the striatum, cortex, WMNs, and claustrum. WMNsST are a numerically small component, but, like other numerically small elements having widespread connections, may still have significant functional roles.
Extended network of striatal projections
Corticostriatal connections, along with thalamostriatal projections, are a major source of input to the basal ganglia; and, as is increasingly apparent, these have an intricate architecture. The projections originate from different combinations of cortical layers (layers 3 or 6, in addition to layer 5; e.g. Griggs et al., 2017; Shipp, 2017), include an inhibitory GABAergic component (in mice: Melzer et al., 2017; Rock et al., 2016), and have terminal arborizations with distinctive spatial extents (divergent from intratelencephalic vs. more compact from pyramidal-tract type neurons in layer 5, in mice: Alloway et al., 2017; Hooks et al., 2018; in macaque: Parent and Parent, 2006). The present study emphasizes the rich layering of recurrent connections across the striatum, cortical areas, WMNs, and claustrum. Each of these latter three have direct projections to the putamen, and may themselves be interconnected via collaterals. Single-axon reconstructions of motor corticostriatal axons showed 3 of 21 intratelencephalic axons with collaterals to the claustrum (Parent and Parent, 2006). Paired recordings from WMNs and cortical pyramidal neurons establish a direct synaptic connectivity (Clancy et al., 2001; Torres-Reveron and Friedlander, 2007; von Engelhardt et al., 2011; Case and Broberger, 2018). A multi-looped, recurrent architecture might mediate different aspects of motor control (as per Melzer et al., 2017). The specific significance of these results depends on further details relating to the network of WMNs, and this is briefly discussed below.
WMNs in rodents and primates
WMNs are phylogenetically conserved, although more numerous in primates than in rodents and more numerous in prenatal development than in the adult brain (Kanold and Luhmann, 2010). They are morphologically, neurochemically, and probably functionally heterogeneous (Meyer et al., 1992; Gabbott and Bacon, 1996; Suarez-Sola et al., 2009). A subpopulation positive for neuropeptide Y and nitric oxide synthase (NOS) is implicated in neurovascular regulation (NHP: Rockland and Nayyar, 2012) and a role in sleep has been proposed because WMNs, below layer 6B in rats, were strongly depolarized by neurotensin application in in vitro preparations (Case et al., 2017). WMNs in rodents are generally thought to consist of neurons in layer 6B and a second component deeper to layer 6. That WMNs have a separate identity from overlying layer 6 is supported, for mice, by the specific enrichment of the transcription factor Meis2 in a subtype of 5HTr3a-GFP interneurons largely confined to the cortical WM (Frazer et al., 2016).
The present results establish an involvement of some WMNs in striatal connectivity (and see below). Worth commenting on is the fact that, as shown here, WMNs projecting to the striatum are a mixed population of excitatory and inhibitory neurons, and that these are located in both the superficial and deeper WM. The location in the deep WM partially addresses the concern that these neurons might be corticostriatal neurons displaced from layer 6 (as does the fact that corticostriatal neurons are more typically situated in layer 5). Homology with WMNs in primates continues under discussion (Meyer et al, 1992; Wang et al., 2010; Molnar and Hoerder-Suabedissen, 2016).
General features of WMNs in NHP
Numerous quantitative studies have provided estimates for human and NHP brains of the number, distribution, and subtypes of WMNs. These are mainly excitatory (human: Meyer et al., 1992; Garcia-Marin et al., 2010; lars gibbon: Swiegers et al., 2018), with species- and area-specific distributions of GABAergic subtypes. Of these, the majority are positive for calretinin or NOS (macaque PFC: Gabbott and Bacon, 1996; human: Garcia-Marin et al., 2010; gibbon: Swiegers et al., 2018). The functional significance of this small population of WMNs is hard to evaluate directly, and will in part depend on the degree of collateralization and number and nature of synaptic connections. The currently best available data as to bouton number and arbor extent are from intracellular biocytin fills in young rats, which demonstrate an extensive axon arborization (Figure 6 in Clancy et al., 2001).
From in vitro electrophysiological experiments in rodents, WMNs are known to have neuronal firing properties; and electron microscopic studies confirm that WMNs receive excitatory and inhibitory synapses (monkeys: Kostovic and Rakic, 1990; mice: Tomioka et al. 2005; human: Garcia-Marin et al., 2010), but neither the specific presynaptic neurons nor postsynaptic targets, important for further understanding of functional circuitry, have as yet been investigated anatomically.
WMNs as projection neurons - subcortical
In the macaque, WMNs projecting to the caudate have been remarked previously, but mainly in the immediate neighborhood of the claustrum (Arikuni and Kubota, 1985; and see Figures 3, 4, and 9 in Griggs et al., 2017). Our results, from three adult monkeys, establish the differential and wide distribution of WMNs projecting to the putamen, over a 20 mm AP extent. In particular, we found an elevated density (i.e., WMNsST/WMNs in 0.25 mm2) in the WM dorsal to the putamen and in the external capsule, 9-10 mm AP from the injection site, but a relatively lower density in the extreme capsule, and in both the deep and superficial WM more distant from the injection site, below the frontal and anterior parietal regions.
We further establish that these neurons include only a small GABAergic component. Additional experiments might determine whether the GABAergic WMNs are somatostatin-positive (in accord with the phenotype of cortically projecting WMNs; Tomioka and Rockland, 2007) or calretinin-positive (reflecting the most numerous GABAergic subpopulation; Garcia-Marin et al., 2010). Inhibitory corticostriatal projections have been identified in mice as somatostatin- or parvalbumin-positive (Melzer et al., 2017; Rock et al., 2016). Another population, of NADPH-d/M2 positive WMNs, has been identified and proposed as a major relay by which cholinergic innervation from the basal forebrain could regulate the release of nitric oxide. M2 positive WMNs have been found throughout the WM, including the region around the putamen and external capsule (Smiley et al., 1998); that is, corresponding to the region where we found a high density of WMNs projecting to the putamen. Finally, some WMNs are positive for tyrosine hydroxylase (Benavides-Piccione and DeFelipe, 2003; 2007). These are abundant in the external capsule of the newborn human brain (Sousa et al., 2017), and, if found to be striatally projecting, might be an additional source of dopamine to the basal ganglia.
In macaque, there are no full reports of WMNs projecting to the thalamus, but several papers include observations or figures appearing to indicate retrogradely filled WMNs after injections in the mediodorsal thalamus (Figures 10-12 in Giguere and Goldman-Rakic, 1988; Figures1-4 in Xiao et al., 2009; Erickson and Lewis, 2004; and discussion in Meyer et al., 1992). In mice, a subset of cortical layer 6B neurons (arguably homologous to WMNs or superficial WMNs in primates) is reported to innervate higher order thalamic nuclei (Hoerder-Suabedissen et al., 2018), or to collateralize to both thalamic recipient cortical layers and the thalamus (Viswanathan et al., 2017).
WMNs as projection neurons - cortical
In the macaque brain, cortically projecting WMNs have been demonstrated by retrograde tracer injections in vivo. Those giving rise to long-distance projections to occipito-temporal areas are GABAergic, of which 90% co-localize somatostatin, nitric oxide, and M2 muscarinic receptor (Tomioka and Rockland, 2007). Cortical injections also result in retrogradely labeled neurons more anteriorly, in the extreme capsule lateral to the claustrum, in both macaque monkeys (Miyashita et al., 2005) and marmosets (Reser et al., 2017). Since these are immunonegative for GAD (Miyashita et al., 2005), it is likely that there are area-specific differences in the proportion of excitatory and inhibitory WMNs giving rise to long-distance cortical projections. GABAergic WMNs have been visualized in GAD67-GFP mice and subsequently double-labeled by retrograde tracers (Tomioka et al., 2005) or by intracellular fills in 5HTr3a-GFP mice (von Engelhardt et al., 2011; Frazer et al., 2017). Their proportion in relation to the total WMN population has not been established, and similarly unknown is whether this projection has an excitatory component in rodents.
Claustrostriatal projections
In monkeys, retrograde tracer injections in the premotor, primary motor (macaque: Tanné-Gariépy et al., 2002), and agranular cingulate motor areas (marmoset: Reser et al., 2017) show labeled cells in almost the entire AP extent of the claustrum, mainly in the dorsal and intermediate parts. Topographically placed injections in the tail or head of the caudate reveal topographically organized claustral projections, where the anterior-dorsal claustrum projects to the head of the caudate, and the posterior-ventral claustrum preferentially projects to the tail, regions respectively associated with flexible or stable value-coding (Griggs et al., 2017).
In the present study, the distribution of labeled cells in the claustrum after retrograde tracer injections in the motor putamen is similar to that described after cortical injections in frontal motor and cingulate areas. These combined results suggest a parallel, recurrent architecture of claustral connections, where direct claustrostriatal projections are in parallel with the indirect claustral-cortical-striatal connections.
Our material is relevant to distinguishing claustral borders in relation to the peri-claustral “shell,” as demarcated by Crym-ir neurons (Mathur et al., 2009). In particular, cortical injections of retrograde tracers reveal filled neurons scattered outside the apparent boundary of the claustrum, most commonly in the extreme capsule (macaque: Miyashita et al., 2005; marmoset: Reser et al., 2017). Our retrograde tracer injections in the putamen, however, produced labeled neurons mainly in the external capsule, between the claustrum and medially adjoining putamen. In the discussion as to whether these cells are part of the claustrum, insula, or both, this dissociation seems to favor the interpretation that these neurons – which extend beyond the borders of the claustrum – represent a separate population and are more appropriately considered within the broader population of WMNs. Furthermore, differently from the neurons of the claustrum proper, these cells are characterized by the absence of both VGLUT1 and Nurr 1 (Watakabe et al. 2014).
Conclusions
Telencephalic input to the striatum originates not only from cortical areas, but also from WMNs and the claustrum, which are heterogeneous and, as established for WMNs, include a GABAergic component. These results 1) inform the complex architecture of striatal connectivity, 2) contribute new data on the long-distance connectional network of WMNs in non-human primates, and 3) raise questions about the role of the claustrum in this connectional triad. In addition, from these results we can suggest that recurrent connectivity across cortex, striatum, WMNs, and possibly claustrum is conserved in both NHP and rodents. At a general level, functional consequences have been discussed as related to an active coordination across structures (as, per WMNs, Suarez-Sola et al., 2009) and/or a network reconfiguration toward a decorrelated state (neurotensin results in Case et al., 2017). WMNs in particular have been discussed in the context of neuropsychiatric disorders in humans (Connor et al., 2011), but details will require further experiments to elucidate anatomical and dynamic aspects of these and their interconnected networks.
Figure 5.
Drawings of coronal sections showing the distribution of WMNsST and ClaST neurons in Cases 61r CTBg (at left) and 71l FB (at right). For each level, a drawing of the section shows from where the enlargement was taken. In section 92 of Case 61r and section 90 of Case 71l the dark grey and black regions correspond to the injection core and halo, respectively, drawn in fluorescence (see Figure 1a and b). Conventions and abbreviations as in Figures 1 and 3.
Acknowledgments
Grant support to KSR: MH107456; local grant support from the University of Parma (fondo locale di ateneo, FIL) to GL and EB.
Footnotes
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alloway KD, Smith JB, Mowery TM, Watson GDR. 2017. Sensory Processing in the Dorsolateral Striatum: The Contribution of Thalamostriatal Pathways. Front Syst Neurosci. 11:53. doi: 10.3389/fnsys.2017.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikuni T, Kubota K. 1985. Claustral and amygdaloid afferents to the head of the caudate nucleus in macaque monkeys. Neurosci Res. 2:239–254. [DOI] [PubMed] [Google Scholar]
- Benavides-Piccione R, DeFelipe J. 2003. Different populations of tyrosine-hydroxylase-immunoreactive neurons defined by differential expression of nitric oxide synthase in the human temporal cortex. Cereb Cortex. 13:297–307. [DOI] [PubMed] [Google Scholar]
- Benavides-Piccione R, DeFelipe J. 2007. Distribution of neurons expressing tyrosine hydroxylase in the human cerebral cortex. J Anat. 211:212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case L, Broberger C. 2018. Neurotensin broadly recruits inhibition via white matter neurons in the mouse cerebral cortex: synaptic mechanisms for decorrelation. Cereb Cortex. 28:2711–2724. doi: 10.1093/cercor/bhx149. [DOI] [PubMed] [Google Scholar]
- Case L, Lyons DJ, Broberger C. 2017. Desynchronization of the rat cortical network and excitation of white matter neurons by neurotensin. Cereb Cortex. 27:2671–2685. doi: 10.1093/cercor/bhw100. [DOI] [PubMed] [Google Scholar]
- Clancy B, Silva-Filho M, and Friedlander MJ. 2001. Structure and projections of white matter neurons in the postnatal rat visual cortex. J. Comp. Neurol 434, 232–252. doi: 10.1002/cne.1174 [DOI] [PubMed] [Google Scholar]
- Connor CM, Crawford BC, Akbarian S. 2011. White matter neuron alterations in schizophrenia and related disorders. Int J Dev Neurosci. 29:325–334. doi: 10.1016/j.ijdevneu.2010.07.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. 2004. Cortical connections of the lateral mediodorsal thalamus in cynomolgus monkeys. J Comp Neurol. 473:107–27. [DOI] [PubMed] [Google Scholar]
- Frazer S, Prados J, Niquille M, Cadilhac C, Markopoulos F, Gomez L, Tomasello U, Telley L, Holtmaat A, Jabaudon D, Dayer A. 2017. Transcriptomic and anatomic parcellation of 5-HT(3A)R expressing cortical interneuron subtypes revealed by single-cell RNA sequencing. Nat Commun. 8:14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. 1996. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol. 364:609–36. [DOI] [PubMed] [Google Scholar]
- García-Marín V, Blazquez-Llorca L, Rodriguez JR, Gonzalez-Soriano J, DeFelipe J. 2010. Differential distribution of neurons in the gyral white matter of the human cerebral cortex. J Comp Neurol. 518:4740–4759. [DOI] [PubMed] [Google Scholar]
- Giguere M, Goldman-Rakic PS. 1988. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 277:195–213. [DOI] [PubMed] [Google Scholar]
- Griggs WS, Kim HF, Ghazizadeh A, Gabriela Costello M, Wall KM, Hikosaka O. 2017. Flexible and stable value coding areas in caudate head and tail receive anatomically distinct cortical and subcortical inputs. Front Neuroanat. 11:106. doi: 10.3389/fnana.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. 2016. Corticostriatal circuitry. Dialogues Clin Neurosci. 18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Hayashi S, Upton L, Nolan Z, Casas-Torremocha D, Grant E, Viswanathan S, Kanold PO, Clasca F, Kim Y, Molnár Z. 2018. Subset of cortical layer 6b neurons selectively innervates higher order thalamic nuclei in Mice. Cereb Cortex. 28:1882–1897. doi: 10.1093/cercor/bhy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Papale AE, Paletzki RF, Feroze MW, Eastwood BS, Couey JJ, Winnubst J, Chandrashekar J, Gerfen CR. 2018. Topographic precision in sensory and motor corticostriatal projections varies across cell type and cortical area. Nat Commun. 9:3549. doi: 10.1038/s41467-018-05780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. 2010. The subplate and early cortical circuits. Annu Rev Neurosci. 33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. 1990. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 297:441–470. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rozzi S, Calzavara R, Matelli M. 2003. Prefrontal and agranular cingulate projections to the dorsal premotor areas F2 and F7 in the macaque monkey. Eur J Neurosci 17:559–578 [DOI] [PubMed] [Google Scholar]
- Mathur BN, Caprioli RM, Deutch AY. 2009. Proteomic analysis illuminates a novel structura definition of the claustrum and insula. Cereb Cortex 19:2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer S, Gil M, Koser DE, Michael M, Huang KW, Monyer H. 2017. Distinct corticostriatal GABAergic neurons modulate striatal output neurons and motor activity. Cell Rep. 19:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Wahle P, Castaneyra-Perdomo A, Ferres-Torres R. 1992. Morphology of neurons in the white matter of the adult human neocortex. Exp Brain Res. 88:204–212. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Nishimura-Akiyoshi S, Itohara S, Rockland KS. 2005. Strong expression of NETRIN-G2 in the monkey claustrum. Neuroscience 136: 487–496. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Hoerder-Suabedissen A. 2016. Regional scattering of primate subplate. Proc Natl Acad Sci U S A. 113:9676–9678. doi: 10.1073/pnas.1611194113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi F, Romano SE, Rosene DL, Rockland KS. 2017. A survey of white matter neurons at the gyral crowns and sulcal depths in the rhesus monkey. Front Neuroanat. 11:69. doi: 10.3389/fnana.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi F, Wang X, Rosene DL, Rockland KS. 2016. White matter neurons in young adult and aged rhesus monkey. Front Neuroanat. 10:15. doi: 10.3389/fnana.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A 2011. Somatotopic organization of the primate basal ganglia. Front Neuroanat. 5:26. doi: 10.3389/fnana.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent M, Parent A. 2006. Single-axon tracing study of corticostriatal projections arising from primary motor cortex in primates. J Comp Neurol. 496:202–13. [DOI] [PubMed] [Google Scholar]
- Reiner A, Hart NM, Lei W, Deng Y. 2010. Corticostriatal projection neurons - dichotomous types and dichotomous functions. Front Neuroanat. 4:142. doi: 10.3389/fnana.2010.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reser DH, Majka P, Snell S, Chan JM, Watkins K, Worthy K, Quiroga MD, Rosa MG. 2017. Topography of claustrum and insula projections to medial prefrontal and anterior cingulate cortices of the common marmoset (Callithrix jacchus). J Comp Neurol. 525:1421–1441. doi: 10.1002/cne.24009. [DOI] [PubMed] [Google Scholar]
- Rock C, Zurita H, Wilson C, Apicella AJ. 2016. An inhibitory corticostriatal pathway. Elife. 5. pii: e15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS, Nayyar N. 2012. Association of type I neurons positive for NADPH-diaphorase with blood vessels in the adult monkey corpus callosum. Front Neural Circuits. 6:4. doi: 10.3389/fncir.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G. 2006. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex. 16:1389–1417 [DOI] [PubMed] [Google Scholar]
- Shipp S 2017. The functional logic of corticostriatal connections. Brain Struct Funct. 222:669–706. doi: 10.1007/s00429-016-1250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Mesulam MM. 1998. Infracortical interstitial cells concurrently expressing m2-muscarinic receptors, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate-diaphorase in the human and monkey cerebral cortex. Neuroscience. 84:755–769. Erratum in: Neuroscience 87:317. [DOI] [PubMed] [Google Scholar]
- Sousa AMM, Zhu Y, Raghanti MA, Kitchen RR, Onorati M, Tebbenkamp ATN, Stutz B, Meyer KA, Li M, Kawasawa YI, Liu F, Perez RG, Mele M, Carvalho T, Skarica M, Gulden FO, Pletikos M, Shibata A, Stephenson AR, Edler MK, Ely JJ, Elsworth JD, Horvath TL, Hof PR, Hyde TM, Kleinman JE, Weinberger DR, Reimers M, Lifton RP, Mane SM, Noonan JP, State MW, Lein ES, Knowles JA, Marques-Bonet T, Sherwood CC, Gerstein MB, Sestan N. 2017. Molecular and cellular reorganization of neural circuits in the human lineage. Science. 358:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Solá ML, González-Delgado FJ, Pueyo-Morlans M, Medina-Bolívar OC, Hernández-Acosta NC, González-Gómez M, Meyer G. 2009. Neurons in the white matter of the adult human neocortex. Front Neuroanat. 3:7. doi: 10.3389/neuro.05.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiegers J, Bhagwandin A, Sherwood CC, Bertelsen MF, Maseko BC, Hemingway J, Rockland KS, Molnár Z, Manger PR. 2018. The distribution, number, and certain neurochemical identities of infracortical white matter neurons in a lar gibbon (Hylobates lar) brain. J Comp Neurol. October 30. doi: 10.1002/cne.24545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Hoshi E, Saga Y, Inoue K, Miyachi S, Hatanaka N, Inase M, Nambu A. 2013. Organization of two corticobasal ganglia loop circuits that arise from distinct sectors of the monkey dorsal premotor cortex In: Barrios FA, Bauer C, editors. Basal ganglia—an integrative view. InTech; p. 103–116. [Google Scholar]
- Tanné-Gariépy J, Boussaoud D, Rouiller EM. 2002. Projections of the claustrum to the primary motor, premotor, and prefrontal cortices in the macaque monkey. J Comp Neurol. 454:140–157. [DOI] [PubMed] [Google Scholar]
- Tomioka R, Okamoto K, Furuta T, Fujiyama F, Iwasato T, Yanagawa Y, Obata K, Kaneko T, and Tamamaki N. 2005. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci 21:1587–1600. doi: 10.3389/fnins.2010.00202 [DOI] [PubMed] [Google Scholar]
- Tomioka R, and Rockland KS. 2007. Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. J. Comp. Neurol 505:526–538. doi: 10.1002/cne.21504 [DOI] [PubMed] [Google Scholar]
- Torres-Reveron J, Friedlander MJ. 2007. Properties of persistent postnatal cortical subplate neurons. J Neurosci. 27:9962–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S, Sheikh A, Looger LL, Kanold PO. 2017. Molecularly defined subplate neurons project both to thalamocortical recipient layers and thalamus. Cereb Cortex. 27:4759–4768. doi: 10.1093/cercor/bhw271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Khrulev S, Eliava M, Wahlster S, and Monyer H. 2011. 5-HT(3A) receptor-bearing white matter interstitial GABAergic neurons are functionally integrated into cortical and subcortical networks. J. Neurosci 31:16844–16854. doi: 10.1523/jneurosci.0310-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WZ, Hoerder-Suabedissen A, Oeschger FM, Bayatti N, Ip BK, Lindsay S, Supramaniam V, Srinivasan L, Rutherford M, Møllgård K, Clowry GJ, Molnár Z. 2010. Subplate in the developing cortex of mouse and human. J Anat. 217:368–380. doi: 10.1111/j.1469-7580.2010.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A, Ohsawa S, Ichinohe N, Rockland KS, Yamamori T. 2014. Characterization of claustral neurons by comparative gene expression profiling and dye-injection analyses. Front Syst Neurosci. 8:98. doi: 10.3389/fnsys.2014.00098. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Zikopoulos B, Barbas H. 2009. Laminar and modular organization of prefrontal projections to multiple thalamic nuclei. Neuroscience. 161:1067–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]