Abstract
Throughout our lives we are immersed in, and colonized by, immense and complex microbial communities. These microbiota serve as activators and early sparring partners for the progressive construction of the layers within our immune defenses and are essential to immune homeostasis. Yet, at times imbalances within the microbiota may contribute to metabolic and immune regulatory abnormalities that underlie the development of inflammatory and autoimmune diseases. Here, we review recent progress in investigations of the microbiome, with emphasis on the gut microbiota associated with systemic autoimmunity. In particular, these studies are beginning to illuminate aspects of the pathogenesis of Systemic Lupus Erythematosus, and may suggest that interconnections with specific disease-associated patterns of dysbiosis within gut communities are bidirectional and mutually reinforcing.
Introduction
Influences from our microbiome communities permeate our very essence. Integral to the birthing process itself is an intimate relationship in which maternal bacteria taxa immediately begin to colonize the newborn’s every surface, with the largest communities ultimately residing within the gut. In adults, the intestine is home to complex and dynamic communities of an estimated 100 trillion bacteria. In health, individual intestinal communities are comprised of many hundreds of phylogenetically distinct taxa. Indeed, we co-evolved with our commensal bacteria that are absolutely essential for ensuring the availability of nutrients for metabolism, including for harvesting energy sources from otherwise indigestible plant polysaccharides. These processes also facilitate the availability of a range of factors that are immune modulators. The representation of individual members of these communities may reflect continuous expansions and contractions, to adapt to dietary opportunities, stressors associated with illness, and the medications that we ingest, amongst many many potential influences. Colonization by microbiota also primes the developing innate and adaptive immune system, resulting in the calibration and immune regulation that determines triggering thresholds for subsequent immune responses. And what better agents than commensal microbes to serve as sparring partners for the developing immune system? Evidence is now mounting that the commensal populations within our internal environments, and especially the gut, can represent the greatest single class of influences for the development of autoimmune disease. In the following sections we highlight a number of key findings regarding the microbiome and both disease susceptibility and subsequent disease activity.
Observations in murine models with potential relevance for clinical disease
In mouse models, the distribution and balance of intestinal microbes have been shown to affect fundamental features of autoimmune pathogenesis, with central paradigms elucidated from studies of Candidatus Savagella, commonly known as segmented filamentous bacteria (SFB), a commensal within the gut microbiota of rodents, fish and chickens. In a predisposed murine strain, SFB has been shown to contribute to the development of inflammatory arthritis while in other mouse strains SFB expansions instead contribute to the production of anti-nuclear autoantibody (ANA) [1•]. SFB are also inducers of RORγ(+) Th17 cells that potentiate autoimmune tissue injury, whereas other taxa directly or indirectly support the development of regulatory T-cells (i.e. Tregs) that suppress inflammation. SFB has therefore become the prototype for a symbiont gut bacterial species that in some settings directly contributes to autoimmune pathogenesis, acting akin to opportunistic pathogens, hence the term pathobiont. Immune homeostasis in health therefore requires a balance between the influences of diverse commensals, or an autoimmune disease state can result.
Microbial colonization has been reported to result in the imprinting by antigens from gut commensals that is a fundamental influence required for early immune development [2••,3•]. In a recent report, bacterial orthologues of the RNA-binding protein, Ro, made by human skin, oral, and gut commensal species were shown to prime and clonally select the early human B-cell repertoire [2••]. Yet autoantibodies to the Ro antigen are also prominent in SLE and Sjogren’s syndrome patients, and sera from human anti-Ro autoantibody-positive lupus patients were shown to immunoprecipitate both commensal and human Ro ribonucleoproteins. In addition, these skin and mucosal Ro-containing bacteria were shown to activate human CD4 memory T-cell clones from lupus patients, suggesting that T-cell cross-reactivity between human and microbial Ro orthologues in lupus patients is common [2••]. These studies may rationalize the process by which microbial proteins, including primordial RNA-binding proteins, serve as xeno-antigens that commonly select for B-cell clones in the emerging B-cell repertoire, which in Lupus and Sjogren’s patients may later become recruited into pathologic autoimmune responses. Indeed there are clinical settings in which circulating IgG anti-SSA/Ro responses themselves can be pathogenic [4].
In a separate report, the obligate anaerobic gut commensal, Roseburia intestinalis, was shown to express non-orthologous mimetopes for B-cell and T-cell epitopes in β2 glycoprotein I, an autoantigen that is a target for responses in patients with anti-phospholipid syndrome [5]. If subsequently validated, these studies may outline an experimental approach by which additional commensals (and their autoantigen orthologues) may be linked to other autoimmune diseases.
Imbalances in microbiome communities (termed dysbiosis) are postulated to at times influence dysregulation of the systemic immune system. In the extreme, breaches of the intestinal barrier can result in the release of components of dead bacteria, or even translocation of live bacteria to outside of the gut. In a recent report, mice that develop a severe inflammatory Lupus-like syndrome were reported to have expansions in the small intestine of an anaerobe, Enterococcus gallinarum, detectable by direct culture of mesenteric lymph nodes, and by in situ hybridization studies of hepatic tissue [6]. This colonization was linked to triggering of the Aryl hydrocarbon receptor system, resulting in autoantibody production and a type I interferon signature, even though only low-level serum anti-E. gallinarum antibody responses were detected [6]. In a separate report colonization of gnotobiotic mice with a microbial consortium from a patient with primary biliary sclerosis resulted in Th17 expansions and hepatobiliary injury. In these colonized mice E. gallinarum, as well as Klebsiella pneumoniae and Proteus mirabilis, were recovered in cultures of mesenteric lymph nodes [7]. These latter studies therefore suggest that translocation of E. gallinarum may not have a specific association with Lupus alone, but may also occur in other inflammatory diseases that affect the liver.
Dysbiosis and impaired gut barrier function in SLE
Patients with SLE display a remarkable range of clinical manifestations, and disease activity often appears to wax and wane in the absence of obvious external precipitating influences. Recent reports have begun to consider the potential contributions of the gut microbiome [8-10]. Whereas the human gut microbiome is dominated by four bacterial phyla —Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria, in one Lupus cohort with all patients in remission, and in a second that included patients with a broader range of disease activity, overall shifts were reported with expansions of Proteobacteria (aerobes) along with reductions of Firmicutes (anaerobes) [8,9], a pattern previously seen in other inflammatory pathologic conditions. However, such patterns were not found in a third Lupus study [10]. These differences could be ascribed to different microbiomes in patients residing in different parts of the world. Or perhaps the great clinical heterogeneity within the patients in these small cohorts was contributory, as each had limited representation of clinically active patients.
To address the need for studies of larger Lupus cohorts with a broader range of disease activity, a larger cross-sectional study was recently reported with 61 female patients with comparisons to a control group of healthy female volunteers [11••]. Akin to findings associated with SFB colonization of the murine intestine [12•], Lupus fecal samples had greater than twofold overall elevations in secretory IgA than was found in healthy controls attesting to local immune activation [11••]. In addition, many Lupus patients had elevated fecal levels of calprotectin [11••], a host innate protein from neutrophils that has become an accepted biomarker for intestinal inflammation and barrier defect in patients with inflammatory bowel disease (IBD). Although serious intestinal disease is distinctly uncommon in SLE, Lupus patients also displayed significantly raised serum soluble CD14 and α1-acid glycoprotein levels, which have been attributed to gut bacterial translocation in other conditions [13,14]. Notably, the immune systems of Lupus patients are reported to commonly show evidence of chronic endotoxin exposure that has been interpreted as suggestive of leakage of the gut barrier [15]. Taken together, these findings bolster the notion that Lupus patients may at times suffer impaired gut barrier integrity, potentially enabling commensals, or their components, to escape the intestinal lumen. Yet, the actual anatomic site responsible for these postulated ‘leaks’ has not been directly studied in lupus patients, and unlike those with IBD Lupus patients do not commonly suffer from overt enteritis.
SLE patients with active disease display characteristic patterns of dysbiosis and immune responses
Studies of 16S rRNA libraries have confirmed an inverse association between Lupus disease activity and overall intestinal microbiota biodiversity [11••]. Strikingly, SLE patients with high disease activity displayed a mean fivefold overabundance of the obligate anaerobic species, Ruminococcus gnavus [11••], which has been reassigned to the Blautia genus of the Lachnospiraceae family. Furthermore, patients with high disease activity had an eightfold increase in R. gnavus abundance compared to the healthy subjects and most patients with high R. gnavus abundance had active Lupus nephritis, an often severe clinical feature of disease, at the time of biosampling [11••].
In earlier surveys, Lupus patients were also reported to have expansions of Blautia related bacteria [10]. Moreover, in two different cohorts fecal 16S rRNA surveys also detected expansions of the Veillonella species [11••, 16], which are common components of the oral microbiome. These latter findings suggest that the dysbiosis in Lupus patients can involve translocation of bacteria from the oral cavity to gut.
In a study of serum responses to oral microbiota, Bagavant et al. stratified Lupus patients based on seropositivity to the major Lupus-associated autoantigens; dsDNA, SmRNP, SSA/Ro and SSB/La. Patients with anti-dsDNA antibodies also displayed higher antibody levels to Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema denticola, and the commensal Capnocytophaga ochracea, when compared to patients lacking anti-dsDNA. Furthermore, the presence of anti-SmRNP antibodies was associated with higher anti-bacterial antibody titers against all the bacteria, while anti-SSA/Ro and anti-SSB/La failed to associate with antibodies to any of the periodontal bacteria [17•]. These studies support the notion that inflammatory responses in the gingival tissues, especially to periodontal pathogens, may contribute to Lupus disease activity.
In the above-described studies of the gut microbiome, the abundance of R. gnavus in fecal samples correlated with serum IgG anti-R. gnavus antibody levels. Anti-R. gnavus antibody levels also directly correlated with those of autoantibodies against native DNA that is a laboratory correlate of Lupus nephritis. Indeed, in three independent cohorts, the highest levels of IgG anti-R. gnavus antibodies were associated with active Lupus nephritis [11••], which suggested a link between a dysbiotic gut community dominated by high R. gnavus abundance and autoimmune pathogenesis responsible for Lupus nephritis. A cell wall lipoglycan was identified as an immunodominant antigen in a R. gnavus strain that is recognized by serum IgG responses in the Lupus patients, and purified preparations displayed TLR2-activating capacity [11••]. Cumulatively, these findings suggested that increased gut permeability in Lupus patients may result in systemic release of microbial substances, that include R. gnavus bacterial antigens and immune-activators. Furthermore, some bacterial factors can also induce type I interferon production (reviewed in Ref. [18]), which could represent a missing link in our understanding of Lupus pathogenesis. In a recent report, a secreted polysaccharide with TLR4 stimulatory capacity was isolated from a R. gnavus strain [19•], indicating this pathobiont may affect host immunity via a number of pathways
Ruminococcus gnavus outgrowths implicated in diverse pathologic conditions
In the human gut, the Lachnospiraceae family members fill a special niche and degrade complex polysaccharides including those of the intestinal mucus layer, which might contribute in some individuals to a leaky gut [20]. Yet R. gnavus strains vary greatly in their genomic composition, metabolic features and competitiveness, and some isolates produce Lantibiotic polypeptides that suppress competing species. R. gnavus strains are found generally at low levels in the intestines of an estimated 90% of adults, typically representing <0.1% of the gut microbiota. However, abundance is also greatly increased in a subset of patients with Crohn’s disease, and these RG blooms, which can represent up to 69% of communities [21•] often co-occur with disease flares. Although roles in pathogenesis are circumstantial, R. gnavus monocolonization of mice can cause expansions of IL-17 producing T-cells in the small bowel [22].
Increases in intestinal R. gnavus abundance have also been found in infants with allergic disease [23]. Patients with spondyloarthropathy with a history of IBD have also been reported to have up to threefold R. gnavus expansions that directly correlated with joint disease activity [24]. Intriguingly, patients with chronic kidney disease have been reported to have impaired intestinal barriers as well as characteristic R. gnavus expansions [25]. Based on metagenomics and culturomics studies, the R. gnavus strains in unaffected healthy individuals are reported to differ from those in IBD patients that display a unique set of genes (reviewed in Ref. [26]), including those postulated to foster adaptation to disease-associated oxidative stress in the gut [21•]. These reports highlight the diverse range of autoimmune and inflammatory diseases that have been associated with R. gnavus expansions, and rationalize the importance of investigations of how strain-associated genomic variations in candidate pathobionts may contribute to the molecular pathways responsible for pathogenesis. Most exciting, it has been reported that many strains of R. gnavus express a VH3 BCR targeted B cell superantigen [31]. This could be very important for understanding the pathogenesis of Lupus for which B cell activation is a major hallmark.
Could the influences of gut dysbiosis on Lupus immunopathogenesis be bidirectional?
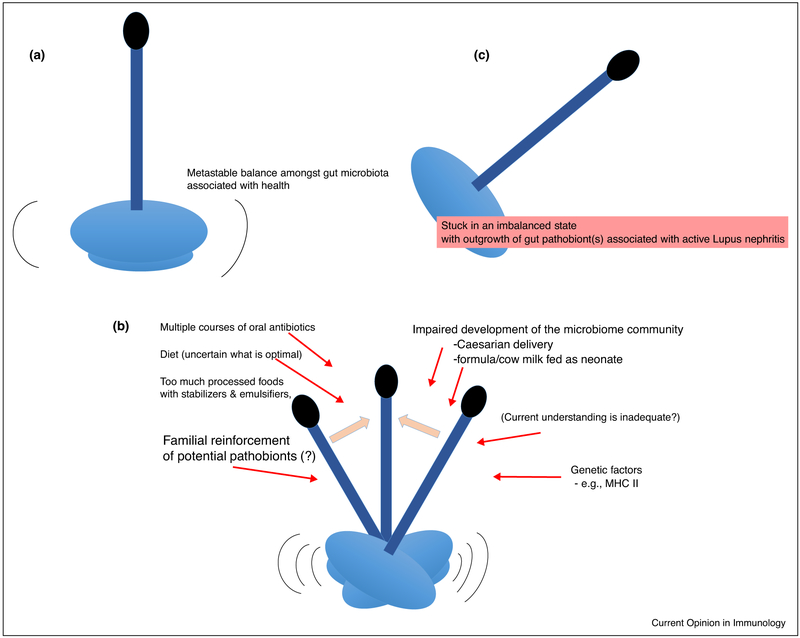
In healthy adults, the composition of the microbiome can be remarkably stable over many years, even at the level of strains [27,28]. We therefore wonder if the above-described Lupus disease-associated dysbiotic shifts are stable in an individual patient over time? The balance within gut communities is directly affected by diet and host genetics, as well as potentially by an enlarging list of medications [29]. Notably, Lupus patients often receive empiric courses of antibiotics, which potentially contribute to specific reductions and secondary expansions (or oscillations) in intestinal microbiota representation. These perturbations could result in a dysbiotic community that triggers Lupus clinical onset and/or disease flares (Figure 1). Longitudinal studies of the gut microbiome are therefore needed to understand the nature and stability of these autoimmune disease-associated microbiome shifts.
Figure 1.
Numerous perturbations in the Lupus gut microbiome may lead to dysbiosis that is supportive of autoimmune pathogenesis. In these sequential panels, we illustrate that (a) there can be a dynamic balance within the diverse gut microbiota. (b) SLE patients in particular are repeatedly subjected to stressors that may result in shifts and reductions in the diversity within the microbiome. (c) Stressors and/or adaptation to an environment resulting from the high oxidative stress of the inflammatory components of Lupus pathogenesis could result in stabilization of a community associated with R. gnavus expansions that amplify immune complex mediated pathology responsible for Lupus nephritis.
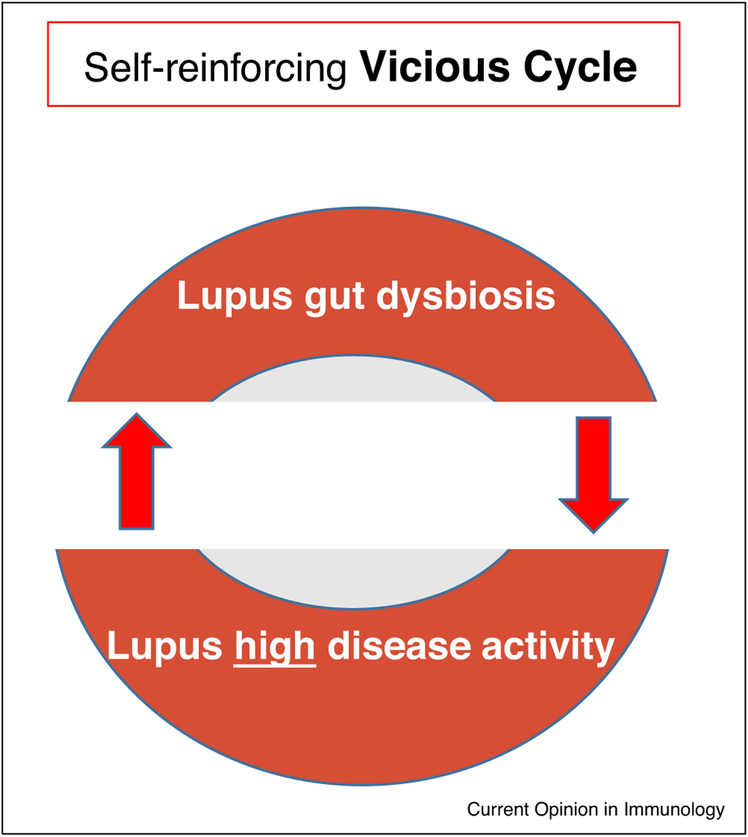
The central question is whether these shifts in the microbiome communities in Lupus are direct drivers of pathogenesis. Alternatively, these shifts may be secondary, as immune activation and systemic inflammatory milieu could alter the metabolomic environment in the gut that then drives these apparently dysbiotic shifts. However, we should also consider that these Lupus-associated shifts in the gut microbiome may reflect a bi-directional process (Figure 2). Indeed, transplantation of fecal samples from mice with a Lupus-like syndrome into germ-free C57BL/6 mice induced significant levels of anti-dsDNA antibodies and promoted an inflammatory shift in the immune systems of recipient mice [30•].
Figure 2.
The influences of Lupus disease activity and Gut dysbiosis may be bidirectional. Stabilization of a dysbiotic gut microbiome community may be an adaptation to a local gut environment arising from Lupus pathology, which had expansions and contractions of specific taxa that directly or indirectly drivers of Lupus pathogenesis.
How this process starts is unclear. In one scenario, a shift in the microbiota that follows an intercurrent infection, or drug exposure, might cause a flare of Lupus disease that may itself act to stabilize the new balance amongst the gut microbiota. Importantly, if influences from, and upon, Lupus microbiome communities are truly bidirectional, then therapeutic interventions for high-hurdle clinical responses may require an effective combinatorial approach that normalizes the immune system along with correction of the imbalances in the intestinal gut microbiome. Such a coordinated approach may attain what has so far been an elusive goal of the predictable induction of long-lasting remissions, or even a cure, for the most refractory patients.
Acknowledgements
Our work has been supported in part by National Institutes of Health Grants; 1R01AI143313-01 (GJS), P50 AR070591 (GJS), 1R01LM012517-01A1 (AVA), and the Judith and Stewart Colton Autoimmunity Center (GJS).
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.•.Van Praet JT, Donovan E, Vanassche I, Drennan MB, Windels F, Dendooven A, Allais L, Cuvelier CA, van de Loo F, Norris PS et al. : Commensal microbiota influence systemic autoimmune responses. EMBO J 2015, 34:466–474Segmented Filamentous Bacteria (SFB) pose a paradigm for understanding how a gut commensal may contribute to different autoimmune diseases in predisposed murine strains.
- 2.••.Greiling TM, Dehner C, Chen X, Hughes K, Iniguez AJ, Boccitto M, Ruiz DZ, Renfroe SC, Vieira SM, Ruff WE et al. : Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci Transl Med 2018, 10:10 10.1126/scitranslmed.aan2306 pii: eaan2306Bacterial commensal orthologues of the human RNA-binding protein, SSA/Ro, are recognized by lymphocytes in gut-associated lymphoid tissue, and may become the sources of autoimmune responses in predisposed individuals.
- 3.•.Chen Y, Chaudhary N, Yang N, Granato A, Turner JA, Howard SL, Devereaux C, Zuo T, Shrestha A, Goel RR et al. : Microbial symbionts regulate the primary Ig repertoire. J Exp Med 2018, 215:1397–1415Primary B lymphogenesis begins during early development of the murine gut in response to microbial colonization.
- 4.Cuneo BF, Buyon JP: Keeping upbeat to prevent the heartbreak of anti-Ro/SSA pregnancy. Ultrasound Obstet Gynecol 2019, 54:7–9. [DOI] [PubMed] [Google Scholar]
- 5.Ruff WE, Dehner C, Kim WJ, Pagovich O, Aguiar CL, Yu AT, Roth AS, Vieira SM, Kriegel C, Adeniyi O et al. : Pathogenic autoreactive T and B cells cross-react with mimotopes expressed by a common human gut commensal to trigger autoimmunity. Cell Host Microbe 2019, 26:100–113.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, Costa FRC, Tiniakou E, Greiling T, Ruff W et al. : Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018, 359:1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamoto N, Sasaki N, Aoki R, Miyamoto K, Suda W, Teratani T, Suzuki T, Koda Y, Chu PS, Taniki N et al. : Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol 2019, 4:492–503. [DOI] [PubMed] [Google Scholar]
- 8.Hevia A, Milani C, Lopez P, Cuervo A, Arboleya S, Duranti S, Turroni F, Gonzalez S, Suarez A, Gueimonde M et al. : Intestinal dysbiosis associated with systemic lupus erythematosus. mBio 2014, 5:e01548–01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Z, Shao T, Li H, Xie Z, Wen C: Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog 2016, 8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo XM, Edwards MR, Mu Q, Yu Y, Vieson MD, Reilly CM, Ahmed SA, Bankole AA: Gut microbiota in human systemic lupus erythematosus and a mouse model of lupus. Appl Environ Microbiol 2018, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.••.Azzouz D, Omarbekova A, Heguy A, Schwudke D, Gisch N, Rovin BH, Caricchio R, Buyon JP, Alekseyenko AV, Silverman GJ: Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann Rheum Dis 2019, 78:947–956In a large cross-sectional cohort with evidence of impaired gut barrier function, expansions of Ruminococcus gnavus, were linked to Lupus disease activity. Active Lupus nephritis correlated with systemic IgG antibody responses to a R. gnavus strain-associated cell wall lipoglycan.
- 12.•.Lecuyer E, Rakotobe S, Lengline-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ et al. : Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity 2014, 40:608–620Segmented Filamentous Bacteria (SFB) expansions induce simultaneous B-cell and T-cell responses in gut-associated lymphoid tissues.
- 13.Rainone V, Schneider L, Saulle I, Ricci C, Biasin M, Al-Daghri NM, Giani E, Zuccotti GV, Clerici M, Trabattoni D: Upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents. Int J Obes (Lond) 2016, 40:1026–1033. [DOI] [PubMed] [Google Scholar]
- 14.Fotis L, Shaikh N, Baszis KW, Samson CM, Lev-Tzion R, French AR, Tarr PI: Serologic evidence of gut-driven systemic inflammation in juvenile idiopathic arthritis. J Rheumatol 2017, 44:1624–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi L, Zhang Z, Yu AM, Wang W, Wei Z, Akhter E, Maurer K, Costa Reis P, Song L, Petri M et al. : The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS One 2014, 9:e93846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Wang H, Li X, Li H, Zhang Q, Zhou H, He Y, Li P, Fu C, Zhang X et al. : Disordered intestinal microbes are associated with the activity of Systemic Lupus Erythematosus. Clin Sci (Lond) 2019. 10.1042/CS20180841. pii: CS20180841. [DOI] [PubMed] [Google Scholar]
- 17.•.Bagavant H, Dunkleberger ML, Wolska N, Sroka M, Rasmussen A, Adrianto I, Montgomery C, Sivils K, Guthridge JM, James JA et al. : Antibodies to periodontogenic bacteria are associated with higher disease activity in lupus patients. Clin Exp Rheumatol 2019, 37:106–111Immune surveys found correlations between antibody responses to periodontal pathogens and autoimmune responses in Lupus patients.
- 18.Kovarik P, Castiglia V, Ivin M, Ebner F: Type I interferons in bacterial infections: a balancing act. Front Immunol 2016, 7:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.•.Henke MT, Kenny DJ, Cassilly CD, Vlamakis H, Xavier RJ, Clardy J: Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A 2019, 116:12672–12677A secreted polysaccharide from this candidate pathobiont is credited with TLR4 stimulatory activity.
- 20.Crost EH, Tailford LE, Monestier M, Swarbreck D, Henrissat B, Crossman LC, Juge N: The mucin-degradation strategy of Ruminococcus gnavus: the importance of intramolecular trans-sialidases. Gut Microbes 2016, 7:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.•.Hall AB, Yassour M, Sauk J, Garner A, Jiang X, Arthur T, Lagoudas GK, Vatanen T, Fornelos N, Wilson R et al. : A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med 2017, 9:103.Metagenomic analyses highlighted R. gnavus strain-associated genomic differences that may explain the molecular pathways of this candidate pathobiont.
- 22.Hoffmann TW, Pham HP, Bridonneau C, Aubry C, Lamas B, Martin-Gallausiaux C, Moroldo M, Rainteau D, Lapaque N, Six A et al. : Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. ISME J 2016,10:460–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chua HH, Chou HC, Tung YL, Chiang BL, Liao CC, Liu HH, Ni YH: Intestinal dysbiosis featuring abundance of Ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology 2018, 154:154–167. [DOI] [PubMed] [Google Scholar]
- 24.Breban M, Tap J, Leboime A, Said-Nahal R, Langella P, Chiocchia G, Furet JP, Sokol H: Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis 2017, 76:1614–1622. [DOI] [PubMed] [Google Scholar]
- 25.Lun H, Yang W, Zhao S, Jiang M, Xu M, Liu F, Wang Y: Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. Microbiologyopen 2019, 8:e00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schirmer M, Garner A, Vlamakis H, Xavier RJ: Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol 2019, 17:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL et al. : The long-term stability of the human gut microbiota. Science 2013, 341:1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie H, Guo R, Zhong H, Feng Q, Lan Z, Qin B, Ward KJ, Jackson MA, Xia Y, Chen X et al. : Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst 2016, 3:572–584.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H et al. : Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.•.Ma Y, Xu X, Li M, Cai J, Wei Q, Niu H: Gut microbiota promote the inflammatory response in the pathogenesis of systemic lupus erythematosus. Mol Med 2019, 25:35.Fecal transplants from mice with active Lupus-like syndrome induced anti-DNA antibody responses and an inflammatory internal milieu in previously germ-free C57BL/6 mice.
- 31.Bunker JJ, Drees C, Watson AR, Plunkett CH, Nagler CR, Schneewind O, Eren AM, Bendelac A: B cell superantigens in the human intestinal microbiota. Sci Transl Med 2019, 11 pii: eaau9356. doi: 10.1126eaau9356. [DOI] [PMC free article] [PubMed] [Google Scholar]