Abstract
Background:
Recently, there is mounting evidence suggesting the efficacy of steroid eluting stents (SES) for management of chronic rhinosinusitis following endoscopic sinus surgery (ESS). This meta-analysis serves to evaluate the efficacy of SES in improving post-operative outcomes following ESS.
Methods:
A systematic literature search was performed in PubMed for articles published between 1985 and 2018. The outcome variables were reported on-average 30 days post-intervention.
Results:
Seven out of the 76 published studies, all of which were industry-sponsored, were included for a collective cohort of 444 SES and 444 control sinuses. In patients who received SES compared to controls, collective Odds Ratio (OR) for post-operative need for intervention, surgery, and oral steroid were 0.45 (95% CI, 0.33–0.62; p < 0.001), 0.30 (95% CI, 0.18–0.52; p < 0.001), and 0.58 (95% CI, 0.40–0.84; p = 0.004), respectively. Additionally, collective OR for frontal sinus ostia (FSO) patency, moderate-severe adhesion/scarring, and increase in polyp score were 2.53 (95% CI, 1.61–3.97; p < 0.001), 0.28 (95% CI, 0.13–0.59; p < 0.001), and 0.42 (95% CI, 0.25–0.74; p = 0.002), respectively. Collective mean difference for FSO/ethmoid inflammation and FSO diameter were −10.86 mm (p < 0.001) and +1.34 mm (p < 0.001), respectively.
Conclusion:
Aggregate evidence suggests that SES can improve ESS outcomes by reducing rates of post-operative intervention and recurrent polyposis and inflammation, while promoting FSO patency. All included and analyzed studies were industry-sponsored and ruling out publication bias was not possible. Future independent and non-sponsored studies to further evaluate SES’s long-term efficacy are warranted.
Keywords: chronic rhinosinusitis, steroid eluting stent, endoscopic sinus surgery, meta-analysis
Introduction
Due to its widespread prevalence, there is a great demand for proper management of chronic rhinosinusitis (CRS) which can affect up to 14–16% of the U.S. population.1,2 CRS is characterized by prolonged inflammation of sinonasal mucosa which leads to direct symptoms like nasal congestion, post-nasal drip, and facial pressure, and further leads to quality-of-life-impeding consequences like bodily pain, dysgeusia/dysosmia, and social dysfunction.3–5 Surgical treatment of CRS cases refractory to medical therapy is on the rise, with the endoscopic sinus surgery (ESS) approach markedly increasing in utilization.6 Despite surgery, local sinonasal inflammation may continue and risk scarring of the sinus ostia. This can potentially lead to recurrence in sinus stenosis, adhesion, and eventual need for further post-operative intervention.7–10 This may be because ESS does not address the underlying pathophysiology of CRS; however, it can create an effective neosinus delivery system for topical corticosteroid to address the inflammatory field.8,9 This localized and persistent steroid delivery method may offer effective penetration, minimal systemic adverse effects, and likely long-term cost effectiveness compared to alternative options of nasal saline and topical or oral steroids.7,9,11,12
Today, U.S. Food and Drug Administration (FDA) approved steroid eluting stents (SES) have become available to offer sustained and localized steroid delivery to sinus tissue. These devices can be left in either the ethmoid sinus cavity or frontal sinus ostium following ESS, where they will continuously release corticosteroid over a period of time. Once the steroid has been administered, it is either removed or bioabsorbed. Han et al. reported the first and only meta-analysis to pool results of two randomized controlled trials (RCT) of 143 patients to demonstrate the safety and efficacy of SES in ESS.13 Since this publication in 2012, several similar RCTs have further studied SES outcomes assessing more outcome variables and longer follow-up durations. This meta-analysis of all the published articles to-date aims to serve as a comprehensive updated evaluation of SES efficacy in improving ESS post-operative outcomes.
Materials and methods
We performed a thorough literature search of the published articles in PubMed, Ovid MEDLINE, and Cochrane databases using “steroid”, “sinus”, and “stent” or “implant” keywords. Each article’s abstract was independently evaluated by two authors (K.G. and M.A.) to consider for data inclusion. The methodology of this meta-analysis was compliant with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.14 Institutional Review Board consideration was not applicable as this study does not use patient information beyond those already published in the literature.
Our search criteria resulted in 78 studies published from January 1985 to December 2018. Inclusion criteria mandated that the authors report both SES and control (interpatient or intrapatient) outcomes following ESS. Extracted data included patient demographics, post-operative interventions, frontal sinus ostia (FSO) occlusion/restenosis, FSO diameter, polyp score change, middle turbinate lateralization, FSO/ethmoid inflammation, and adhesion/scarring. Other data reported by studies in scarcity or in different formatting, and thus not feasible for quantitative analysis, were Sino-Nasal Outcome Test-22, visual analog scale, and Lund-Mackay score. Post-operative intervention consisted of post-operative oral steroid or surgical intervention. FSO occlusion or restenosis ratio was inversed to calculate FSO patency ratio. Polyposis formation was in many studies defined as increase in polyp score to at least a grade 1 in a 0–3 grading system with grade 0 = none and grade 3 = extensive polyps with obstruction.15 Adhesion or scarring ratio consisted of patients whose post-operative adhesion/scarring was described as either moderate, severe, or dense.
We designated indication for post-operative intervention, FSO patency, and recurrent polyposis as primary outcome variables, whereas FSO/ethmoid inflammation (on a 100-mm visual analog scale) and adhesion/scarring were regarded as secondary outcome variables. These outcome variables, reported on average 30 days post-implementation, were compared using Review Manager v5.3 (Nordic Cochrane Centre, The Cochrane Collaboration, 2014) via binary and continuous random-effects models for Odds Ratio (OR) and Mean Difference (MD), respectively. A p value < 0.05 was considered significant and 95% confidence interval (CI) and Forest plots were obtained for outcome variables.
Results
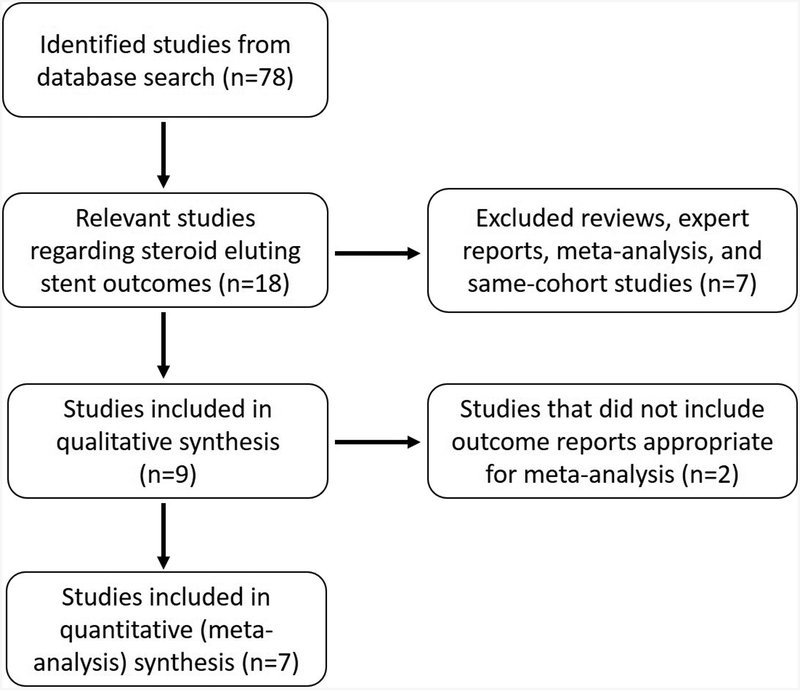
Nine out of the 78 published studies were included for qualitative analysis. Quantifiable data from seven of these studies for a total of 444 SES and 444 controlled frontal or ethmoid sinuses was utilized for meta-analysis (Figure 1). This is because Businco et al.16 (35 SES cases) and Taulu et al.17 (28 SES cases) did not provide compatible data for our meta-analysis purposes, but they were instead utilized for descriptive reports in the discussion. Patient inclusion criteria were adequately similar across the studies, including adults of age 18–65 years diagnosed with CRS via societal and previously published guidelines or computed tomographic Lund-Mackay scores (≥1 on each side), and indicated for ESS. Overall, mean age ranged between 42.0–51.0 years and females comprised 34%−68% of these cohorts. Patients’ demographics and relevant symptoms on presentation are described in Table 1.
FIGURE 1.
Flowchart of study inclusion.
Table 1.
Summary of patient demographics on presentations (all values represent percentages except for L-M Score).
| Citations | Nasal O/C | Facial P/P | Hyposmia/Anosmia | Nasal drainage | Aspirin A/I | Asthma | Smoker | HA | Prior FESS | L-M | Polyp P/E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Murr 201125 | 84% | 42% | 33% | - | - | - | - | 33% | 37% | 13.4 | 72% |
| Marple 201226 | 91% | 61% | 51% | 54% | 3% | 28% | 17% | 65% | 30% | 12.8 | 59% |
| Forwith 201618 | - | - | - | - | - | - | - | - | - | - | - |
| Smith 201627 | - | - | - | - | 8% | 38% | - | - | 51% | 15.8 | 76% |
| Businco 201616 | - | - | - | - | - | - | - | - | - | - | - |
| Taulu 201717 | 97% | 81% | 51% | 86% | - | 16% | 33% | - | - | 10.9 | 16% |
| Adriaensen 201715 | 97% | 64% | 100% | - | 22% | 67% | - | - | 92% | 19 | 19% |
| Luong 201728 | - | - | - | - | 9% | 45% | 4% | - | 51% | 14.8 | 55% |
| Singh 201829 | - | - | - | - | 8% | 41% | 33% | - | 51% | 15.3 | 63% |
FESS: functional endoscopic sinus surgery; Nasal O/C: nasal obstruction/congestion; Facial P/P: facial pain/pressure; Aspirin A/I: aspirin allergy/intolerance; HA: headache; L-M: CT Stage Lund-Mackay total; Polyp P/E: polyp present/edema.
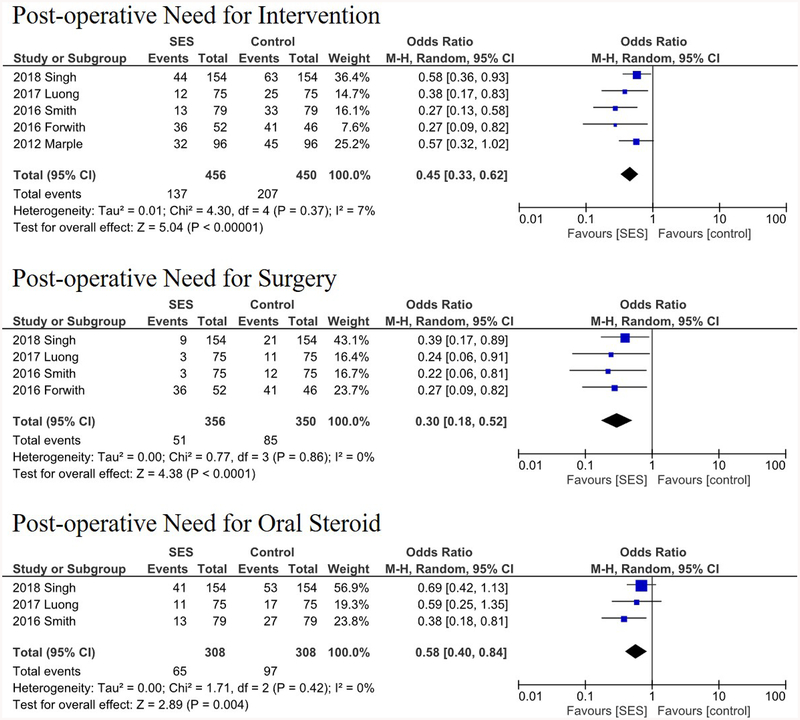
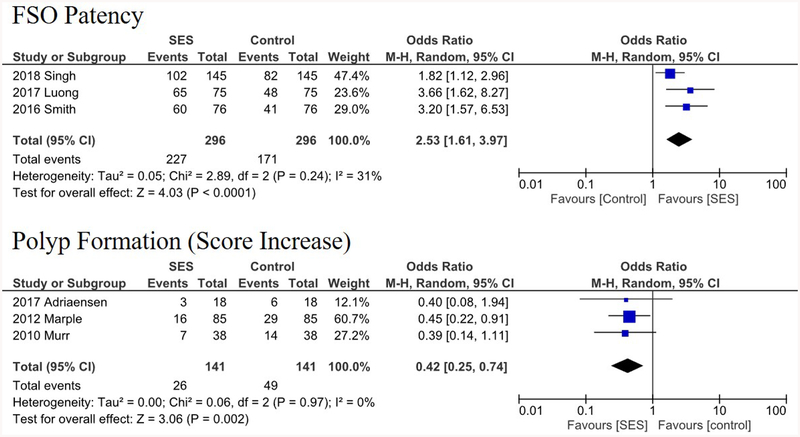
The types of implanted SESs were as follows: Intersect ENT Propel Mini or Contour,26,27,28,29 unnamed Intersect ENT-provided steroid eluting stent,18,25 SinuBand Fluticasone Propionate,25 and Relieva Stratus MicroFlow Spacer.16,17 The sites of local implantation and delivery were ethmoid sinus cavity15,16,17,18,25,26 and frontal sinus ostia.27,28,29 A summary of the analyzed studies with their respective primary and secondary outcome variables are demonstrated in Table 2. All included studies in the quantitative analyses were industry-sponsored RCTs. All seven studies utilized intrapatient controls (using different sinuses in the same patient) except Forwith et al.18 (sham surgeries). These seven studies prescribed a 10- or 14-day course of antibiotics post-operatively for all subjects, with the exception of Forwith et al. that prescribed daily doses of steroid nasal spray. Some studies permitted additional post-operative treatment, such as corticosteroids for asthma control.18 A comparison of key study characteristics is provided in Table 3. In patients who received SES compared to controls, collective OR for post-operative need for intervention, surgery, and oral steroid were 0.45 (95% CI, 0.33–0.62; p < 0.001), 0.30 (95% CI, 0.18–0.52; p < 0.001), and 0.58 (95% CI, 0.40–0.84; p = 0.004), respectively (Figure 2). Collective OR for FSO patency and polyposis formation were 2.53 (95% CI, 1.61–3.97; p < 0.001), and 0.42 (95% CI, 0.25–0.74; p = 0.002), respectively (Figure 3). Additionally, collective OR for moderate-severe adhesion/scarring was 0.28 (95% CI, 0.13–0.59; p < 0.001) and collective MD for FSO/ethmoid inflammation and FSO diameter were −10.86 mm (p < 0.001) and +1.34 mm (p < 0.001), respectively.
Table 2.
Summary of outcome variables reported by the studies utilized for meta-analysis.
| Citations | Cohorts | Need for P-op Interv. | Need for P-op Surgery | Need for P-op Oral Steroid | Patent FSO | Polyp Formation | FSO mean diameter mm (SD) | FSO/Ethmoid Inflammation** (SD) | Adhesion/Scarring |
|---|---|---|---|---|---|---|---|---|---|
| Murr 201125γ | SES=38 | - | - | - | - | 7/38 (18%) | - | 20.2 (18.5) | 2/38 (5%) |
| Control=38 | 14/38 (37%) | 30.1 (22.4) | 8/38 (21%) | ||||||
| Marple 201226γ | SES=105 | 32/96 (33%) | - | - | - | 16/85 (19%) | - | - | 5/105 (5%) |
| Control=105 | 45/96 (47%) | 29/85 (34%) | 13/105 (12%) | ||||||
| Forwith 201618χ | SES=53 | 36/52 (69%) | 36/52 (69%) | - | - | - | - | - | - |
| Control=47 | 41/46 (89%) | 41/46 (89%) | |||||||
| Smith 201627γ | SES=79 | 13/79 (16%) | 3/75 (4%) | 12/79 (15%) | 60/76 (79%) | - | 5.9 (2.8) | 24.7 (27.0) | - |
| Control=79 | 33/79 (42%) | 12/75 (16%) | 27/79 (34%) | 41/76 (54%) | 4.4 (2.4) | 41.3 (29.3) | |||
| Adriaensen 201715γ | SES=18 | - | - | - | - | 3/18 (17%) | - | - | - |
| Control=18 | 6/18 (33%) | ||||||||
| Luong 201728γ | SES=80 | 12/75 (16%) | 3/75 (4%) | 11/75 (15%) | 65/75 (87%) | - | 6.3 (2.7) | 23.1 (24.2) | 3/75 (4%) |
| Control=80 | 25/75 (33%) | 11/75 (15%) | 17/75 (23%) | 48/75 (64%) | 4.5 (3.2) | 35.6 (31.1) | 11/75 (15%) | ||
| Singh 201829* | SES=160 | 44/154 γ | 9/154 (6%) | 41/154 (27%) | 102/145 (70%) | - | 5.2 (3.3) | 29.2 (32.3) | - |
| Control=160 | 63/154 γ | 21/154 (14%) | 53/154 (34%) | 82/145 (57%) | 4.3 (3.15) | 35.5 (33.0) |
P-op: Post-operative; Interv.: Intervention; Follow-up times: γ = 1 month, χ = 6 months;
in Singh 2018, the three first columns are 1 month follow up, and the latter three columns are 3 months follow up.
Inflammation as observed endoscopically, indicated on a 100-mm visual analog scale (0=no inflammation; 100=severe inflammation).
Table 3.
Comparison of key study characteristics.
| Citations | Sponsor | Stent device (manufacturer) | Stent location | Stent duration* | Steroid** | Post-operative treatment*** | Study Groups | Maximum Follow-up |
|---|---|---|---|---|---|---|---|---|
| Murr 201125 | Intersect ENT | Unspecified (Intersect ENT) | ethmoid sinus cavity | 30 days (bioabsorbed) | 370μg mometasone furoate | 14-day ABX | SES, control (intrapatient) | 2 Months |
| Marple 201226 | Intersect ENT | Propel (Intersect ENT) | ethmoid sinus cavity | 30 days (bioabsorbed) | 370μg mometasone furoate | 14-day ABX | SES, control (intrapatient) | 3 Months |
| Forwith 201618 | Intersect ENT | Unspecified (Intersect ENT) | ethmoid sinus cavity | 60 days (removed) | 370μg mometasone furoate | Daily steroid nasal spray | SES, control | 3 Months |
| Smith 201627 | Intersect ENT | Propel Mini (Intersect ENT) | frontal sinus ostia | 21 days (removed) | 370μg mometasone furoate | 10-day ABX | SES, control (intrapatient) | 3 Months |
| Businco 201616 | - | Relieva Stratus MicroFlow Spacer (Acclarent) | ethmoid sinus cavity | 28 days (removed) | 12mg triamcinolone acetonide | - | SES, ethmoidectomy | 12 Months |
| Taulu 201717 | - | Relieva Stratus MicroFlow Spacer (Acclarent) | ethmoid sinus cavity | 28 days (removed) | 12mg triamcinolone acetonide | - | SES, nasal spray | 6 Months |
| Adriaensen 201715 | BioInspire Technologies, Inc. | SinuBand FP (BioInspire Technologies, Inc.) | ethmoid sinus cavity | Unspecified | 320μg fluticasone propionate | 14-day ABX | SES, nasal pack, control (intrapatient) | 2 Months |
| Luong 201728 | Intersect ENT | Propel Contour (Intersect ENT) | frontal sinus ostia | 21 days (removed) | 370μg mometasone furoate | 10-day ABX | SES, control (intrapatient) | 3 Months |
| Singh 201829 | Intersect ENT | Propel Mini or Contour (Intersect ENT) | frontal sinus ostia | 21 days (removed) | 370μg mometasone furoate | 10-day ABX | SES, control (intrapatient) | 3 Months |
Approximate length of time steroid was administered before stent was either removed or bioabsorbed.
Quantity is given per side.
Treatment mandated for all subjects in study. Other post-operative treatment may or may not have been permitted; see individual studies for details.
FIGURE 2.
Forest plots demonstrating an overall 0.45, 0.30, and 0.58 odds ratios of need for post-operative intervention, surgery, and oral steroids, respectively. Lines represent the 95% confidence interval and boxes represent the post-operative intervention rate with each box’s size correlating to the respective study’s effect size.
FIGURE 3.
Forest plots demonstrating an overall 2.53 and 0.42 odds ratios of FSO patency and polyposis formation, respectively. Lines represent the 95% confidence interval and boxes represent the post-operative intervention rate with each box’s size correlating to the respective study’s effect size.
Discussion
This meta-analysis demonstrates the potential efficacy of SES following ESS for CRS, leading to fewer post-operative interventions, lower rates of scarring and recurrent polyp formation, and higher rates of sinus patency. We evaluated the quantitative SES outcomes reported by 7 RCTs consisting of 444 subjects. Our analyses demonstrated that SES implants resulted in improved outcomes compared to controls. This was true in every analyzed outcome variable including need for postoperative medical or surgical interventions, adhesions/scarring, polyposis formation, sinus inflammation, and FSO diameter and patency. Similarly, the previous meta-analysis by Han et al. of two RCTs demonstrated a significantly reduced post-operative intervention by 35%, adhesion lysis by 51%, and frank polyposis reduction of 46%.13 With the utilization of 5 additional RCTs for quantitative analyses and another 2 for qualitative discussions, this manuscript presents updated evidence suggesting that SES is efficacious and appears to be associated with improved clinical outcomes.
Recently, emerging evidence has suggested the benefits of SES in ESS for CRS patients. SES’s potential efficacy can be due to its addressing of sinus inflammation and agitation caused by ESS, which can further cause polyposis, adhesion, and restenosis of the frontal recess otherwise.7–10,12,19 As a result, SES’s localized and targeted corticosteroid administration can render it safer and potentially more effective than the currently used topical and oral steroid treatments. This is because the latter two have been associated with under-penetration or detrimental short- and long-term systemic side effects, respectively.20–23 Thus, by delivering a stable and localized drug dose to the inflamed mucosa, SES implants have the potential to promote sinus patency and decrease post-ESS need-for-intervention while also limiting unnecessary high dosages or adverse effects associated with oral systemic steroid treatment. Through our quantitative meta-analysis demonstrating improved outcomes compared to controls, we also demonstrated that the outcomes reported by the utilized RCTs followed the individual study outcomes closely. This can be due to the similar study designs and patient populations utilized, as all the RCTs utilized for quantitative analysis were sponsored by two industry companies.
The comparison of pre- and post-operative SNOT-22 scores is one of the most common methods of evaluating patient-reported outcomes following CRS treatment.24 However, only three of the nine RCTs evaluated this specific outcome, and each in different formats. Adriaensen et al. reported SES patients’ overall significant improvement in SNOT-22 scores on 1-month and 3-month follow-ups (from baseline 52 to 19); though there was no control-SES comparison as the study utilized contralateral nares as controls.15 Businco et al. evaluated the five most important patient-defined SNOT-22 questions and reported a significantly increased 12-month improvement in SES compared to controls (2.7 vs. 3.9 modified SNOT).16 However, Taulu et al. reported SNOT-22 improvements in both SES and controls without anysignificant differences in 3- or 6-month follow-ups.17 Thus, it is not clear whether SES implantation leads to a significant improvement in SNOT scores, or whether some reported improvements translate to a minimal clinically important difference. Future comprehensive and standardized SNOT-22 evaluations and full reporting is warranted. Additionally, 30-day middle turbinate lateralization was reported significantly differently in two studies: 2/38 in SES patients vs. 6/38 in controls in Murr et al.,25 and 2/105 in SES patients compared to 7/105 controls in Marple et al.26 A meta-analysis of these values was not performed due to a low number of available patients. Regardless, we can speculate that these preliminary results are suggestive that decreasing the rates of middle turbinate lateralization is another potential advantage of SES utilization.
Taulu et al.17 and Businco et al.’s16 quantitative data could not be combined within the current meta-analysis due to incompatibility. Moreover, these studies differed in other significant ways from the other studies that were analyzed. First, both studies had different control populations. Specifically, control patients received ethmoidectomy and corticosteroid nasal sprays in Businco et al.16 and Taulu et al.17, respectively, distinguishing them from the rest of the studies which utilized a sham procedure or intra-patient controls. Further, these studies were the only studies in our analysis to use the Relieva Stratus Microflow spacer. This device is notably the only SES in our analysis not reported to be bioabsorbable. Nevertheless, these studies provided valuable insights regarding SES outcomes. Businco et al. demonstrated that control patients experienced significantly greater nasal secretions, synechiae, and crusting.16 Also, even though they reported no difference in polyp recurrence or Lund-Mackay (LM) score, there was improved post-operative rhinomanometry scores in SES patients.16 Contrary to the theme of most studies, however, Taulu et al. reported no significant difference in changes of SNOT-22, visual analog scale, LM scores, rhinomanometry scores, or endoscopic scores when comparing SES with control patients.17.
While we took great care in collecting correct data and performing appropriate analyses, this study is not without its limitations. The difference in studies’ patient baselines, follow-up timelines, and heterogeneity in measuring and reporting outcomes can lead to various confounding factors beyond the scope of this meta-analysis. However, we hope that the existing internal validity and inclusion of RCT level of evidence may control for confounders. If an outcome variable was not reported similarly between studies, for example in the case of SNOT or LM scores, we refrained from performing meta-analysis and only utilized them for qualitative discussions. Though the longest follow-up of these studies was 6 months, most analyses are comprised of 30-day follow-up reports and thus future studies can investigate whether SES efficacy remains superior in longer follow-up measurements. There is also potential for study and reporting bias, as it may be more likely for positive outcomes to achieve publication. However, the low number of studies available per analysis of each outcome variable precluded us from performing appropriate statistical evaluations (such as funnel plots) to assess systematic or publication bias. Further investigation of SES will lead to a higher number of published results that can appropriately be assessed for reporting bias.
Additionally, the follow-up durations were 1 to 6 months, thus making the consensus on longer term SES effects still unclear. Though most studies evaluated patients for 2–3 months or longer (Table 3), many of the reported primary outcomes were based on 1-month data. Thus, it should be highlighted that the analyzed results are based on relatively short-term outcomes and future long-term reporting can better evaluate the overall efficacy. Another notable limitation was the limited number of studies utilized per each outcome variable. This provides an opportunity for future studies to investigate SES efficacy and safety as well as its long-term effects and potential QOL improvements. Further investigation into different patient groups, namely CRS phenotype and endotype, is also warranted to better understand which patient groups respond best to SES supplementation.
The final limitation to the current understanding of SES efficacy and safety is the nature of the performed RCTs. As mentioned, all 7 studies whose data were feasible for combination and collective quantitative meta-analysis were industry-sponsored. This may raise potential issues, as desired optimal results for FDA consideration may lead to study design or patient selection criteria that may deviate from real world scenarios and patient populations. It has been previously discussed that industry sponsored clinical trials may use suboptimal controls or inappropriately generalize conclusions drawn from narrow patient populations.30 Notably, conflict of interest is likely to occur in the exploration and parameters regarding a medication or device’s safety and efficacy, which can bring the manufacturer and researcher in direct conflict.30 As such, a recent Cochrane review demonstrated that manufacturing company sponsored studies demonstrated superior efficacies compared to other trials.31 Potential bias from sponsored studies may be due to deviation from the uncertainty principle, and such studies are thus encouraged to choose appropriate controls and report all findings.32 Overall, though these limitations preclude us from making definitive generalized conclusions, the current data suggests potential benefits of SES in post-ESS outcomes for CRS.
Conclusion
Available evidence suggests steroid eluting implants may have a beneficial effect on multiple CRS outcome measures. This meta-analysis and review of current literature evaluates the overall efficacy of SES in ESS treatment of CRS and provides updated evidence for this claim. We report improvement in ESS outcomes, namely reduced rates of post-operative intervention, inflammation, and polyposis, while promoting FSO patency by maintaining FSO diameter and avoiding restenosis. All included and analyzed studies were industry-sponsored and ruling out publication bias was not possible. Future independent and non-sponsored RCTs are warranted to further evaluate the safety and efficacy of SES.
Acknowledgements
This work was supported by the National Institutes of Health under award numbers T32GM008620 and TL1TR001415–04.
Footnotes
Conflict of Interest: Edward C. Kuan is a consultant for Intersect ENT, Menlo Park, CA.
Portions of this work has been presented at the 2019 Rhinoworld Scientific Meeting, Chicago, Illinois.
References
- 1.Pleis JR, Lethbridge-Cejku M. Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Vital Health Stat 10. 2007; 235: 1–153. [PubMed] [Google Scholar]
- 2.Halawi AM, Smith SS, Chandra RK. Chronic rhinosinusitis: epidemiology and cost. Allergy Asthma Proc. 2013; 34(4): 328–334. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007; 137(3 Suppl): S1–31. [DOI] [PubMed] [Google Scholar]
- 4.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995; 113(1):104–109. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MP, Fishman P, Short SO, Sullivan SD, Yueh B, Weymuller EA. Health care utilization and cost among adults with chronic rhinosinusitis enrolled in a health maintenance organization. Otolaryngol Head Neck Surg. 2002; 127(5): 367–376. [DOI] [PubMed] [Google Scholar]
- 6.Svider PF, Sekhsaria V, Cohen DS, Eloy JA, Setzen M, Folbe AJ. Geographic and temporal trends in frontal sinus surgery. Int Forum Allergy Rhinol. 2015; 5(1): 46–54. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy DW. The PROPEL™ steroid-releasing bioabsorbable implant to improve outcomes of sinus surgery. Expert Rev Respir Med. 2012; 6(5): 493–498. [DOI] [PubMed] [Google Scholar]
- 8.Wei CC, Kennedy DW. Mometasone implant for chronic rhinosinusitis. Med Devices (Auckl). 2012; 5: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell RG, Kennedy DW. What is new and promising with drug-eluting stents in sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2014; 22(1): 2–7. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z, Hwang P, Sun Y, Zhou B. Steroid-eluting sinus stents for improving symptoms in chronic rhinosinusitis patients undergoing functional endoscopic sinus surgery. Cochrane Database Syst Rev. 2015; 6: CD010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudmik L, Soler ZM, Orlandi RR, et al. Early postoperative care following endoscopic sinus surgery: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2011; 1(6): 417–430. [DOI] [PubMed] [Google Scholar]
- 12.Bury S, Singh A. Evaluation of a steroid releasing sinus implant for the treatment of patients undergoing frontal sinus surgery for chronic rhinosinusitis. Expert Rev Med Devices. 2017; 14(2): 93–101. [DOI] [PubMed] [Google Scholar]
- 13.Han JK, Marple BF, Smith TL, et al. Effect of steroid-releasing sinus implants on postoperative medical and surgical interventions: An efficacy meta-analysis. Int Forum Allergy Rhinol. 2012; 2(4):271–279. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151(4): 264–269. [DOI] [PubMed] [Google Scholar]
- 15.Adriaensen GFJPM Lim KH, Fokkens WJ. Safety and efficacy of a bioabsorbable fluticasone propionate–eluting sinus dressing in postoperative management of endoscopic sinus surgery: a randomized clinical trial. Int Forum Allergy Rhinol. 2017; 7(8): 813–820. [DOI] [PubMed] [Google Scholar]
- 16.Businco LD, Mattei A, Laurino S, et al. Steroid-Eluting Ethmoidal Stent Versus Antero-Posterior Ethmoidectomy: Comparison Of Efficacy And Safety In Allergic Patients. Otolaryngol Pol. 2016; 70(2): 6–12. [DOI] [PubMed] [Google Scholar]
- 17.Taulu R, Bizaki AJ, Numminen J, Rautiainen M. A prospective, randomized clinical study comparing drug eluting stent therapy and intranasal corticoid steroid therapy in the treatment of patients with chronic rhinosinusitis. Rhinology. 2017; 55(3): 218–226. [DOI] [PubMed] [Google Scholar]
- 18.Forwith KD, Han JK, Stolovitzky JP, et al. RESOLVE: bioabsorbable steroid-eluting sinus implants for in-office treatment of recurrent sinonasal polyposis after sinus surgery: 6-month outcomes from a randomized, controlled, blinded study. Int Forum Allergy Rhinol. 2016; 6(6): 573–581. [DOI] [PubMed] [Google Scholar]
- 19.Naidoo Y, Wen D, Bassiouni A, Keen M, Wormald PJ. Long-term results after primary frontal sinus surgery. Int Forum Allergy Rhinol. 2012; 2(3): 185–190. [DOI] [PubMed] [Google Scholar]
- 20.Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013; 9(1): 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snidvongs K, Chaowanapanja P, Aeumjaturapat S, Chusakul S, Praweswararat P. Does nasal irrigation enter paranasal sinuses in chronic rhinosinusitis? Am J Rhinol. 2008; 22(5): 483–486. [DOI] [PubMed] [Google Scholar]
- 22.Pundir V, Pundir J, Lancaster GA, et al. Role of corticosteroids in functional endoscopic sinus surgery-a systematic review and meta-analysis. Rhinology. 2016; 54(1): 3–19. [DOI] [PubMed] [Google Scholar]
- 23.Hansen FS, Djupesland PG, Fokkens WJ. Preliminary efficacy of fluticasone delivered by a novel device in recalcitrant chronic rhinosinusitis. Rhinology. 2010; 48(3): 292–299. [DOI] [PubMed] [Google Scholar]
- 24.Le PT, Soler ZM, Jones R, Mattos JL, Nguyen SA, Schlosser RJ. Systematic Review and Meta-analysis of SNOT-22 Outcomes after Surgery for Chronic Rhinosinusitis with Nasal Polyposis. Otolaryngol Head Neck Surg. 2018; 159(3): 414–423. [DOI] [PubMed] [Google Scholar]
- 25.Murr AH, Smith TL, Hwang PH, et al. Safety and efficacy of a novel bioabsorbable, steroid-eluting sinus stent. Int Forum Allergy Rhinol. 2011; 1(1): 23–32. [DOI] [PubMed] [Google Scholar]
- 26.Marple BF, Smith TL, Han JK, et al. Advance II: A Prospective, randomized study assessing safety and efficacy of bioabsorbable steroid-releasing sinus implants. Otolaryngol Head Neck Surg. 2012; 146(6): 1004–1011. [DOI] [PubMed] [Google Scholar]
- 27.Smith TL, Singh A, Luong A, et al. Randomized controlled trial of a bioabsorbable steroid-releasing implant in the frontal sinus opening. Laryngoscope. 2016; 126(12): 2659–2664. [DOI] [PubMed] [Google Scholar]
- 28.Luong A, Ow RA, Singh A, et al. Safety and effectiveness of a bioabsorbable steroid-releasing implant for the paranasal sinus ostia: A randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2017; doi: 10.1001/jamaoto.2017.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh A, Luong AU, Fong KJ, et al. Bioabsorbable steroid-releasing implants in the frontal sinus ostia: a pooled analysis. Int Forum Allergy Rhinol. 2019; 9(2): 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montaner JS, O’Shaughnessy MV, Schechter MT. Industry-sponsored clinical research: a double-edged sword. Lancet. 2001; 358(9296): 1893–5. [DOI] [PubMed] [Google Scholar]
- 31.Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017, issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Djulbegovic B, Lacevic M, Cantor A, Fields KK, Bennett CL, Adams JR, Kuderer NM, Lyman GH. The uncertainty principle and industry-sponsored research. Lancet. 2000; 356(9230): 635–8. [DOI] [PubMed] [Google Scholar]