Abstract
BACKGROUND:
Surgical site infection is one of the major health-care-associated problems causing substantial morbidity and mortality and constituting a financial burden on hospitals as well. The wound management is one of the crucial evidence-based strategies in the reduction of surgical site infection rates
AIM:
To study the impact of standardisation of transparent semipermeable dressing procedure on the rate of surgical site infection in comparison with conventional dressing in clean and clean-contaminated surgeries.
METHODS:
The study included 100 patients who were admitted to surgical wards in Cairo university hospitals, for clean and clean-contaminated operations, in the period from February 2017 to August 2017. Immunocompromised and uncontrolled diabetic patients were excluded. Patients were randomly allocated into two groups; in the first group, patients wounds were covered using transparent semipermeable dressing, while the second group patients’ wounds were covered using conventional occlusive gauze dressing. Patients were followed up for criteria of infection every other day during the first week then at two weeks, three weeks and four weeks.
RESULTS:
In clean and clean-contaminated operations, the transparent dressing group showed a significantly lesser rate of surgical site infection at (2%), compared with the conventional occlusive gauze dressing group with a surgical site infection rate of (14%) (p-value of 0.02).
CONCLUSION:
The transparent semipermeable dressing is effective in reducing surgical site infection rate in clean and clean-contaminated operations.
Keywords: Surgical site infection, Dressings, Incision, Transparent, Occlusive
Introduction
Surgical site infections (SSIs) are those occurring in a surgically created wound within 30 days. SSIs are the commonest hospital-acquired infections. They represent a significant burden on the health care system worldwide with significant patient comorbidity and mortality. The human and financial costs of treating surgical site infections (SSIs) are rising. It is estimated that approximately half of SSIs are deemed preventable using evidence-based strategies [1], [2].
The Centers for Disease Control and Prevention (CDC) classified SSI to superficial; within the skin and subcutaneous fat, deep; musculo-facial layers or organ space; in an organ or cavity, if breached during surgery [3].
Different classifications of risk factors have been proposed to be associated with SSIs. They can be classified to Preoperative, intraoperative and postoperative risk factors. SSI risk factors can also be divided into modifiable, e.g. cigarette smoking and non-modifiable as extreme of age and severity of illness [4].
In developing countries, the distribution of Hospital-acquired infections is different from more developed, with fewer bloodstream infections since fewer devices are used and a higher proportion of SSIs; which can be redeemed preventable through evidence-based measures [5].
SSI (defined using Centers for Disease Control and Prevention’s National Healthcare Safety Network criteria) is defined as infection that occurs within 30 days after any operative procedure (where day 1 = the procedure date) and patient has at least one of the following: purulent drainage, organisms identified from an aseptically-obtained specimen from the incision by a culture or non-culture based microbiologic testing method [1]. The incision that is deliberately opened by a surgeon, and the patient has at least one of the following signs or symptoms: pain or tenderness; localised swelling; erythema; or heat and diagnosis of incisional SSI by the surgeon or attending physician [1].
In 11 Egyptian hospitals, 510 SSIs were identified following 4,246 surgeries with overall SSI rate of 12% [6]. The incidence of SSI at Cairo university hospitals was 9.2%. A significant increase was associated with a prolonged preoperative hospital stay, prolonged surgery, contaminated wounds and presence of the drain. The most common organism was Staphylococcus aureus (24.3%) then Klebsiella pneumonia (18.5%) [7].
Good wound care will minimise the inflammatory response, speed healing and minimize scarring. A dressing is a sterile pad or compresses applied to a wound to promote healing and/or prevent further harm. Most of the procedures result in wounds in which the edges are brought together to heal using stitches, staples, clips or glue to allow healing by primary intention. Afterwards, wounds are often covered with a dressing that acts as a barrier between them and the outside environment. One advantage of this may be to protect the wound from micro-organisms, and thus infection. Many different dressing types are available for use on surgical wounds [8].
The clean wound is an uninfected operative wound in which no inflammation is encountered, and the respiratory, alimentary, genital, or uninfected urinary tracts are not entered, and clean wounds are primarily closed. While Clean-Contaminated wounds are operative wounds in which the respiratory, alimentary, genital, or urinary tracts are entered under controlled conditions and without unusual contamination [1].
Low adherence dressings and wound contact materials are usually cotton pads that are placed directly in contact with the wound. They are either non-medicated (e.g. paraffin gauze dressing), or medicated (e.g. containing povidone-iodine or chlorhexidine) [9].
Transparent film dressings are semi-permeable membrane dressings; that is waterproof yet permeable to oxygen and water vapour which help in preventing bacterial contamination. They may be used as a primary or secondary dressing. They also maintain a humid wound environment, facilitate cell migration and encourage necrotic tissue autolysis by trapping moisture on the surface of the wound [10], [11].
The main aim is to study the impact of standardisation of transparent semipermeable dressing procedure on the rate of surgical site infection in comparison with a conventional occlusive gauze dressing.
Methodology
The study included 100 patients who were admitted to surgical wards in Cairo university hospitals, during the period between February 2017 to August 2017.
This study was revised and approved by the research ethics committee, Faculty of Medicine, Cairo University. The study was designed as a randomised controlled trial in which patients were allocated to two different groups, according to the chronological order of their presentation, to A and B; group A patients received the transparent dressings for their surgical wounds and group B patients had the conventional occlusive gauze dressings.
Patients were then assessed for eligibility according to the inclusion and exclusion criteria listed below. Patients were informed of the nature of the study, consented to participate.
Inclusion criteria
- Patients who presented to the plastic and general surgery department in the study period.
- Patients with clean surgeries.
- Patients with clean-contaminated surgeries.
Exclusion criteria
- Extremes of age; children under the age of 10 and adults beyond the age of 60.
- Patients with uncontrolled diabetes.
- Immuno-compromised patients.
- Any patient with a history of impaired healing.
- Patients on medication that may impede wound healing or render them susceptible to infection (eg. Steroids)
- Patient with contaminated or infected surgery.
- Patients presenting in the trauma department.
- Drop-outs from follow up.
Group A: Included fifty patients’ undergone different clean and clean-contaminated surgeries, and received postoperative semipermeable transparent wound dressing since day one and throughout their postoperative course.
Figure 1.

Gauze dressing covering abdominal incision postoperatively
Group B: Included fifty patients’ undergone different clean and clean-contaminated surgeries, and received conventional occlusive gauze dressings since day one and throughout their postoperative course.
Figure 2.

Transparent dressing covering incision postoperatively
The following data were collected from patients upon enrollment in the study: - Full medical history analysis including age, sex, cigarette smoking and medical comorbidities, regular medications that may impede healing and drug allergies; - Operative details including the use of any foreign material and previous surgical history; - Full general examination including body weight, vital signs, and skin conditions preceding the surgical operation; - Preoperative investigations including complete blood picture, fasting blood glucose and HbA1c; and - Preoperative prophylaxis was done according to hospital policy; ampicillin-sulbactam was given within minutes to one hour before incision. One dose was sufficient, yet additional doses were given for operating procedures longer than three hours.
Follow up and criteria of infection
Wounds were evaluated postoperatively every other day in the first week then weekly till the end of the month.
Group A: transparent wound dressings were applied intraoperatively. Afterwards, they were evaluated for adherence, underlying exudate, and transparency and stigmata of infection, and were only changed when a leak was detected or lost adherence. Under aseptic conditions, films were removed, and the wounds were cleaned with normal saline, and povidone-iodine was used as a disinfectant and allowed to dry, and a new film was reapplied.
Group B: basic gauze dressing was used intraoperatively, with every other day, dressing changes starting from day 2 postoperatively till the third week. The dressings were removed, the wounds were inspected and cleansed with saline, povidone-iodine was applied, and the wound was covered with gauze followed by adhesive plaster.
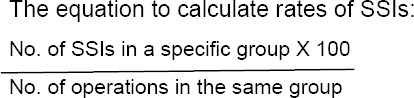
Microbiological analysis for patients with suspected wound infection: Using a sterile technique, a sterile cotton-wool swab was used to collect a sample from the infected site.
Sample processing: All samples were cultured on blood MacConkey agar incubated aerobically at 37°C for 24-48 hrs. Direct Gram-stained films were prepared from each wound swab and examined microscopically. Identification of isolated microorganisms according to standards using: Gram stain, colony morphology, Biochemical reactions for gram-positive isolates (catalase, coagulase, mannitol, DNase). Also, novobiocin disc was used for further identification of Staphylococci. Biochemical reactions for gram-negative isolates (TSI, LIA, MIO, citrate, urease, and oxidase). Antimicrobial susceptibility was done by disk diffusion method.
Statistical Method
Data were analysed using SPSS win statistical package version 20 (SPSS Inc., Chicago, IL). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test (Fisher’s exact test) was used to examine the relation between qualitative variables. For quantitative data, a comparison between the two groups was made using either student t-test or Mann-Whitney test (non-parametric t-test) as appropriate. A p-value < 0.05 was considered significant.
Results
The age of the patients included in group A ranged from 15 – 55 years, with a mean of 34 ± 10.02. The age of the patients included in group B ranged from 18 – 57 years with a mean of 34.46 ± 9.157. Group A included 40 females (80%) and 10 males (20%), while group B included 41 females (82%) and 9 males (18%) as shown in Table 1.
Table 1.
Patients’ baseline demographics and wound characteristics
| Group A Transparent dressing | Group B Conventional dressing | |
|---|---|---|
| Age: Y Mean (SD*) | 34 (10.022) | 34.46 (9.157) |
| Sex in (percent %): | ||
| Male | 20% | 18% |
| Female | 80% | 82% |
| Risk factors (per cent %): | ||
| Obesity | 42% | 34% |
| Smokers | 4% | 6% |
| Type of wounds: | ||
| Clean | 44 | 44 |
| Clean-contaminated | 6 | 6 |
Denotes standard deviation.
Our findings confirm that the rate of SSI is lower when transparent dressings were used on surgical incisions when compared to the conventional occlusive gauze dressing, as shown in Table 2 and 3, (p-value 0.02).
Table 2.
Comparison between Transparent dressing and Conventional dressing
| Dressing type | ||||||
|---|---|---|---|---|---|---|
| Transparent dressing | Conventional dressing | P value | ||||
| Number | % | Number | % | |||
| Age (Mean ± SD*) | 34 ± 10 | 34.5 ± 9.2 | 0.81 | |||
| Sex | ||||||
| Male | 10 | 52.6% | 9 | 47.4% | 0.79 | |
| Total | 50 | 50.0% | 50 | 50.0% | ||
| Obesity | ||||||
| Yes | 21 | 55.2% | 17 | 44.7 % | 0.5 | |
| Total | 50 | 50.0% | 50 | 50.0% | ||
| Smoking | ||||||
| Yes | 2 | 40.0% | 3 | 60.0% | 0.65 | |
| Total | 50 | 50.0% | 50 | 50.0% | ||
| Type of wound | Clean | 44 | 50.0% | 44 | 50.0% | |
| clean contaminated | 6 | 50.0% | 6 | 50.0% | 1 | |
| Total | 50 | 50.0% | 50 | 50.0% | ||
| Infection group | ||||||
| Infected | 1 | 12.5% | 7 | 87.5% | 0.02 | |
| Total | 50 | 50.0% | 50 | 50.0% | ||
Denotes standard deviation.
Table 3.
Comparison between infected and non-infected groups
| Non-Infected | Infected | P-value | ||||
|---|---|---|---|---|---|---|
| Number | % | number | % | |||
| Age (Mean ± SD*) | 33.9 ± 9.5 | 37.2 ± 9.5 | 0.33 | |||
| Sex | Male | 19 | 20.6% | 0 | 0.0% | 0.15 |
| Total | 92 | 92.0% | 8 | 8.0% | ||
| Obesity | Yes | 32 | 34.7% | 6 | 15.7% | 0.02 |
| Total | 92 | 92% | 8 | 8.0% | ||
| Smoking | Yes | 5 | 100.0% | 0 | 0.0% | 0.49 |
| Total | 92 | 92.0% | 8 | 8.0% | ||
| Total leucocytic count | High | 7 | 87.5% | 1 | 12.5% | 0.62 |
| Total | 92 | 92.0% | 8 | 8.0% | ||
| Type of wound | Clean | 80 | 90.9% | 8 | 9.0% | |
| clean contaminated | 12 | 100.0% | 0 | 0.0% | ||
| Total | 92 | 92.0% | 8 | 8.0% | 0.27 | |
| Dressing type | Transparent dressing | 49 | 98.0% | 1 | 2.0% | |
| Conventional dressing | 43 | 86.0% | 7 | 14.0% | 0.02 | |
| Total | 92 | 92.0% | 8 | 8.0% | ||
Denotes standard deviation.
Figure 3.
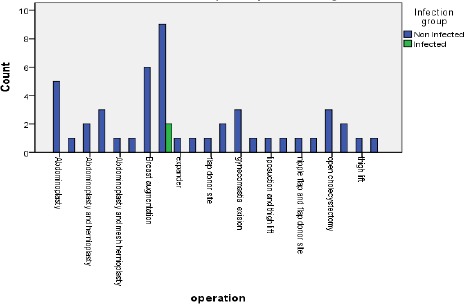
Types of operations in a transparent dressing group
To study obesity as a risk factor of SSI, we found that of the 100 patients, 38 % were obese, and 62% were non-obese. Of the 38 patients suffering from Obesity, 6 had SSI which is 15.7%, while those are not suffering from Obesity only 2 out of 62 patients suffered from SSI with 3.2% only. (p-value < 0.02) which is statistically significant.
Figure 4.

Types of operations in a conventional dressing group
Age of the patients included in our study ranged from 15 – 57 years, with a mean of 34 ± 9.579. Mean age in the infected group was 37.2 ± 9.7 in comparison to 33.9 ± 9.5 in the non-infected group.
Figure 5.
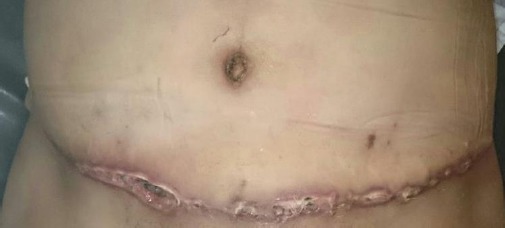
showing infected wound postoperatively after abdominoplasty operation
The incidence of smoking as a risk factor among both groups was 5%. Such an incidence was not high enough to reliably assess the impact of smoking as a risk factor of SSI.
Follow up
The transparent dressing stayed in place for a mean of 6 days (range 5 – 7), and the gauze dressing was removed after a mean of 1.5 days (range 1 – 3). The transparent dressing, therefore, stayed in place, a mean of four and a half days longer than the gauze dressing.
We gained the impression that the patient’s comfort and well-being were better in the transparent group as it made bathing possible and allowed earlier postoperative mobilisation.
The patients with transparent dressing felt more satisfied as the dressing was conformable, and the frequency of dressing change was less than the gauze dressing.
Inspection of the wound through the transparent dressing was transparent in 24 patients (48%), slightly opaque in 11 patients (22%) but the sutures could be seen, and 15 (30%) were opaque that the sutures couldn’t be seen and the dressing was replaced to visualise the sutures.
Figure 6.
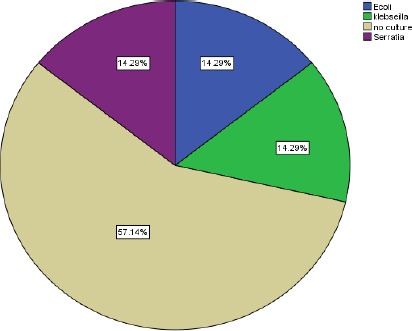
Frequency of various pathogens causing surgical site infections in the conventional group
The TLC was elevated among 7 patients 14% in the conventional dressing group and 1 patient 2% in the transparent dressing group (p-value < 0.03)
Table 4.
Frequency of pathogens causing surgical site infections in patients with occlusive transparent dressing group
| Frequency | Per cent | Per cent | |
|---|---|---|---|
| negative culture | 1 | 2.0 | 100.0 |
| Total | 1 | 2.0 | 100.0 |
Discussion
In this study, our goal was to evaluate the impact of a transparent semipermeable film dressing versus the conventional occlusive gauze dressing on rates of SSIs in the period of February 2017 to August 2017.
The age of the patients included in the study ranged from 15 – 57 years, with a mean of 34 ± 9.579. The mean age in the infected group was 37.2 ± 9.7 compared to non-infected group 33.9 ± 9.5 in the non-infected group (p-value 0.33). However, the mean age was higher in the study conducted by C. Holm et al., 1998 which was 61.3, (range 25 – 90) years, and also in the study conducted by Shinohara et al., 2008 the mean age was 63.5 years (range 31 – 91 years) [12], [13]. This may be due to the conduction of our study in a developing country where the mean age of the general population is lower than that where C. Holm and Shinohara conducted their study. Also, this could be attributed to the fact that we excluded patients above the age of 60 years old.
Our study included 81 females and 19 males, which differs from the studies conducted by C. Holm et al., 1998 and by Shinohara et al., 2008 which included equal numbers of males and females. While the study conducted by Ubbink et al., 2008 included 92 males and 50 females in the transparent dressing group and 93 males and 50 females in the conventional dressing group [12], [13], [14]. That difference, in our opinion, had no effect on the results of our study. This larger number of female patients involved in this study might be since females are more prone to go for plastic procedures. Also, on a national level, the female’s super number the males which make our finding sensible.
In our study, the type of wound dressing used had an influence on the rate of SSI; this influence was found to be statistically significant. One patient (2%) of a total of 50 patients who had used transparent semipermeable wound dressing suffered from SSI, compared to seven patients (14%) of a total of 50 patients suffered from SSI in the other group that used conventional occlusive gauze dressing. This shows the superiority of transparent semipermeable wound dressing usage following elective clean and clean-contaminated surgical procedures in reducing the rate of SSI (p-value 0.02).
Collated data from 50 controlled trials on a variety of wounds yielded infection rates of 5.37% and 3.25% rates (p < 0.001) between conventional gauze and transparent dressings, respectively [15].
In a study conducted by Maki and Ringer 1987, Cutaneous colonisation using transparent dressing was lower in level and comparable with a gauze dressing and other dressings (range, 100.58 to 100.70 colony-forming units) [16].
There is also evidence that moist wound healing results in better cosmesis, decreased pain, and improvement in the granulation tissue of the wound bed [17].
This differs from a study conducted by Sastry et al., 2015. They implemented the use of sterile gauze or a transparent semipermeable dressing to cover the wound of cardiac implantable electronic devices, with no inclination to the use of any of the fore-mentioned types [18].
This agrees with another study conducted by Cosker 2005 showing that there was no statistically significant difference in the number of SSIs in the basic wound contact-dressed group (5 / 100; 5%), compared with the transparent film-dressing group (9 / 200; 5%) [19].
The overall SSI incidence rate in the current study was 8 %. Nearly similar findings were concluded from a study conducted at Tanta University Hospital in Egypt by Afifi and Baghagho, 2010 who detected an overall SSI incidence rate of 8.3% [20]. Meanwhile lower SSI rates could be detected in France, Italy and Germany, SSI rates of 3.3%, 3.3% and 1.2% were detected in three studies conducted by Rioux et al., 2006, Moro et al., 2005 and Hirschmann et al., 2005 [21], [22], [23]. This could be attributed to the conduction of our study in a developing country which usually shows higher rates of SSIs than more developed countries.
In the current study, obesity was statistically significantly associated with an increased risk of SSI (P-value = 0.02). This was like a study of Egyptian orthopaedic patients by Abdel-Halim et al., 2010 who reported in their study that obesity was a significant risk factor for SSI (P < 0.001) [24]. To study obesity as a risk factor of SSI, we found that of the 100 patients, 38% were obese, and 62% were non-obese. Incidence of infection in the obese group was 15.7% and in the non-obese group was 3.2% (p-value < 0.02) which is statistically significant. Our study detected that the incidence of obesity among the transparent dressing group included 21 patients, 42%, while among the conventional dressing group included 17 patients, 34%.
Our study as well as Shinohara et al., 2008 gained the expression that Patients seemed more comfortable with the transparent dressings, which allowed them to move about freely and to take a shower when necessary and early postoperative mobilization was also facilitated, and studies suggested that film dressings might be less painful for patients than basic wound contact dressings [13].
AS for the SSI microbiology in our study, the organisms isolated from the infected wounds from the transparent dressing group: negative culture 2%. Organisms isolated from the infected wounds gauze dressing group: negative culture 8%, E-coli 2%, Klebsiella 2%, Serratia 2%. This differs from results of a study conducted in Japan by Shinohara published in 2008 showing the isolation of Bacteroides fragilis (3/63) 4.8% in transparent dressing group and isolation of Bacteroides fragilis and Enterococcus faecalis (4/71) 5.6% in gauze dressing group (p = 0.567) [13]. Staph aureus was the main causative organism of SSI (44.4%), all S. aureus isolates were MRSA, followed by Klebsiella pneumoniae 22/90 (24.44%) and Acinetobacter 15/90 (16.67%). The implant was highly associated with SSI cases 80 / 90 (89%) according to Helal et al., 2015 [25].
One of the limitations encountered during our study was negative cultures despite the presence of SSI that was diagnosed by the surgeons and according to the CDC criteria for SSI. The appearance of postoperative SSI in the absence of culturable bacterial pathogens is a common dilemma for surgeons. The potential causes of culture-negative SSI include prior antimicrobial therapy, the presence of fastidious or slow-growing microorganisms or infection caused by ordinary bacteria that may be dismissed as “contaminants” and performing aerobic cultures only [26]. Other limitations in our study included a lower number of clean-contaminated surgeries in our study in comparison to clean surgeries.
In conclusion, our study showed that the transparent semipermeable dressing is effective in reducing surgical site infection rate in clean and clean-contaminated operations as well as, reducing its burdens as additional hospital stay and additional costs associated with the occurrence of infection.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA surgery. 2017;152(8):784–91. doi: 10.1001/jamasurg.2017.0904. https://doi.org/10.1001/jamasurg.2017.0904 PMid:28467526. [DOI] [PubMed] [Google Scholar]
- 2.Khadilkar R, Khsirsagar V, Khadilkar S, Bendre M, Chavan S. A Comprehensive Study of 100 Patients of SSI (Surgical Site Infections) in Patients Undergoing Abdominal Surgery, Elective/Emergency, in Our Hospital. JMSCR. 2017:5. https://doi.org/10.18535/jmscr/v5i4.192. [Google Scholar]
- 3.Kiernan M. Reducing the risk of surgical site infection. Nursing times. 2012;108(27):12–4. [PubMed] [Google Scholar]
- 4.Johnson R, Jameson SS, Sanders RD, Sargant NJ, Muller SD, Meek RM, Reed MR. Reducing surgical site infection in arthroplasty of the lower limb: A multi-disciplinary approach. Bone & joint research. 2013;2(3):58–65. doi: 10.1302/2046-3758.23.2000146. https://doi.org/10.1302/2046-3758.23.2000146 PMid:23610703 PMCid:PMC3626200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhangu A, Ademuyiwa AO, Aguilera ML, Alexander P, Al-Saqqa SW, Borda-Luque G, Costas-Chavarri A, Drake TM, Ntirenganya F, Fitzgerald JE, Fergusson SJ. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. The Lancet Infectious Diseases. 2018;18(5):516–25. doi: 10.1016/S1473-3099(18)30101-4. https://doi.org/10.1016/S1473-3099(18)30101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abduo EM, El-Kholy J, Abdou S, Hafez S, Omar N, Talaat M. Incidence and Microbial Etiology of Surgical Site Infections at Select Hospitals in Egypt. American Journal of Infection Control. 2016;44(6):S52–3. https://doi.org/10.1016/j.ajic.2016.04.049. [Google Scholar]
- 7.Wassef MA, Hussein A, El-Sherif RH. A prospective surveillance of surgical site infections: Study for efficacy of preoperative antibiotic prophylaxis. African journal of microbiology research. 2012;6(12):3072–8. https://doi.org/10.5897/AJMR12.377. [Google Scholar]
- 8.Dumville JC, Keogh SJ, Liu Z, Stubbs N, Walker RM, Fortnam M. Alginate dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews. 2015:5. doi: 10.1002/14651858.CD011277.pub2. https://doi.org/10.1002/14651858.CD011277.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford C, Hamerslagh BJ. inventors; Advanced Medical Solutions Ltd, assignee. Wound dressing. United States patent application US 15/672,571. 2018 [Google Scholar]
- 10.Moshakis V, Fordyce MJ, Griffiths JD, McKinna JA. Tegadern versus gauze dressing in breast surgery. The British journal of clinical practice. 1984;38(4):149. [PubMed] [Google Scholar]
- 11.Thomas S, Loveless P, Hay NP. Comparative review of the properties of six semipermeable film dressings. Pharm J. 1988;240:785–7. [Google Scholar]
- 12.Holm C, Petersen JS, Grønbk F, Gottrup F. Effects of occlusive and conventional gauze dressings on incisional healing after abdominal operations. The European journal of surgery. 1998;164(3):179–83. doi: 10.1080/110241598750004616. https://doi.org/10.1080/110241598750004616 PMid:9562277. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara T, Yamashita Y, Satoh K, Mikami K, Yamauchi Y, Hoshino S, Noritomi A, Maekawa T. Prospective evaluation of occlusive hydrocolloid dressing versus conventional gauze dressing regarding the healing effect after abdominal operations: randomized controlled trial. Asian journal of surgery. 2008;31(1):1–5. doi: 10.1016/S1015-9584(08)60046-9. https://doi.org/10.1016/S1015-9584(08)60046-9. [DOI] [PubMed] [Google Scholar]
- 14.Ubbink DT, Vermeulen H, Goossens A, Kelner RB, Schreuder SM, Lubbers MJ. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Archives of Surgery. 2008;143(10):950–5. doi: 10.1001/archsurg.143.10.950. https://doi.org/10.1001/archsurg.143.10.950 PMid:18936373. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson JJ, Lawrence JC. Wound infection under occlusive dressings. Journal of Hospital Infection. 1991;17(2):83–94. doi: 10.1016/0195-6701(91)90172-5. https://doi.org/10.1016/0195-6701(91)90172-5. [DOI] [PubMed] [Google Scholar]
- 16.Maki DG, Ringer M. Evaluation of dressing regimens for prevention of infection with peripheral intravenous catheters: Gauze, a transparent polyurethane dressing, and an lodophor-transparent dressing. Jama. 1987;258(17):2396–403. https://doi.org/10.1001/jama.1987.03400170082027. [PubMed] [Google Scholar]
- 17.Helfman T, Ovington L, Falanga V. Occlusive dressings and wound healing. Clinics in dermatology. 1994;12(1):121–7. doi: 10.1016/0738-081x(94)90262-3. https://doi.org/10.1016/0738-081X(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 18.Sastry S, Rahman R, Yassin MH. Cardiac implantable electronic device infection: from an infection prevention perspective. Advances in preventive medicine 2015. 2015 doi: 10.1155/2015/357087. https://doi.org/10.1155/2015/357087 PMid:26550494 PMCid:PMC4621323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cosker T, Elsayed S, Gupta S, Mendonca AD, Tayton KJ. Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. Journal of wound care. 2005;14(1):27–9. doi: 10.12968/jowc.2005.14.1.26722. https://doi.org/10.12968/jowc.2005.14.1.26722 PMid:15656462. [DOI] [PubMed] [Google Scholar]
- 20.Afifi IK, Baghagho EA. Three months study of orthopaedic surgical site infections in an Egyptian University hospital. Int J Infect Control. 2010;6(1):1–6. https://doi.org/10.3396/ijic.v6i1.002.10. [Google Scholar]
- 21.Moro ML, Morsillo F, Tangenti M, Mongardi M, Pirazzini MC, Ragni P. Rates of surgical-site infection: an international comparison. Infection Control & Hospital Epidemiology. 2005;26(5):442–8. doi: 10.1086/502565. https://doi.org/10.1086/502565 PMid:15954481. [DOI] [PubMed] [Google Scholar]
- 22.Hirsemann S, Sohr D, Gastmeier K, Gastmeier P. Risk factors for surgical site infections in a free-standing outpatient setting. American journal of infection control. 2005;33(1):6–10. doi: 10.1016/j.ajic.2004.09.006. https://doi.org/10.1016/j.ajic.2004.09.006 PMid:15685128. [DOI] [PubMed] [Google Scholar]
- 23.Rioux C, Grandbastien B, Astagneau P. The standardized incidence ratio as a reliable tool for surgical site infection surveillance. Infection Control & Hospital Epidemiology. 2006;8:817–24. doi: 10.1086/506420. https://doi.org/10.1086/506420 PMid:16874641. [DOI] [PubMed] [Google Scholar]
- 24.Khaleid M, Haleim A, Zein K. ET: surgical site infections and associated risk factors in Egyptian orthopedic patients. J Am Sci. 2010;6(7):272–80. [Google Scholar]
- 25.Helal S, El Anany M, Ghaith D, Rabeea S. The Role of MDR-Acinetobacter baumannii in orthopedic surgical site infections. Surgical infections. 2015;16(5):518–22. doi: 10.1089/sur.2014.187. https://doi.org/10.1089/sur.2014.187 PMid:26114551. [DOI] [PubMed] [Google Scholar]
- 26.Rasnake MS, Dooley DP. Culture-negative surgical site infections. Surgical infections. 2006;7(6):555–65. doi: 10.1089/sur.2006.7.555. https://doi.org/10.1089/sur.2006.7.555 PMid:17233574. [DOI] [PubMed] [Google Scholar]


