Abstract
BACKGROUND:
Soybean oil contains vitamin E and acts as a natural sunscreen which can absorb Ultra Violet (UV) B light and has antioxidant properties to reduce the photooxidative damage that results from UV-induced Reactive Oxygen Species production. The UV blocking from most natural oils is insufficient to obtain a high UV protection. The strategies for preparations of sunscreen products with high SPF can be done by nanoemulsion formulation and Ultra Violet filter combinations of Soybean Oil, Avobenzone and Octyl methoxycinnamate.
AIM:
The purpose of this study was to prepare and in vitro efficacy evaluation of sunscreen nanoemulsion containing Soybean oil, Avobenzone and Octyl methoxycinnamate.
METHODS:
The sunscreen nanoemulsions were prepared by the high energy emulsification method. The formulation uses a combination of 3% Avobenzone, 7.5% Octyl methoxycinnamate, with different ratio of Soybean oil and Liquid Paraffin. The nanoemulsion was evaluated for droplet sizes by using particle size analyzer, physical stability in room temperature (25 ± 2°C during experiment for 12 weeks of storage, physical stability (cycling test), phase separation by centrifugation at 3750 rpm for 5 hours, pH, viscosity, and Sun Protection Factor (SPF) value by UV spectrophotometric. The SPF value of sunscreen nanoemulsion was compared to sunscreen nanoemulsion without Soybean Oil and sunscreen emulsion. Particle morphology observation of nanoemulsion by using Transmission Electron Microscope.
RESULTS:
The sunscreen nanoemulsion formulation containing a combination of 3% Avobenzone, 7.5% Octyl methoxycinnamate with a ratio of 2.73% Soybean Oil and 0.27% Paraffin Oil resulted in the smallest average droplet size of 68.47 nm. The sunscreen nanoemulsion without Soybean Oil had an average droplet size of 384.07 nm. The globules size was increased during the experiment for 12 weeks of storage at room temperature, but there was no phase separation after centrifugation. The formulation of sunscreen emulsion, phase separation was formed after centrifugation. The nanoemulsion had a pH value of 7.23 ± 0.06 and a viscosity value of 133.33 ± 7.22 cP. The sunscreen nanoemulsion containing a combination of 3% Avobenzone, 7.5% Octyl methoxycinnamate 2.73%, Soybean Oil, 2.73% and 0.27% Liquid Paraffin had SPF value (21.57 ± 1.21) higher than sunscreen nanoemulsion without Soybean Oil (16.52 ± 0.98) and sunscreen emulsion (15.10 ± 0.22). The TEM analysis of globules morphology showed that the sunscreen nanoemulsion formed a spherical globule.
CONCLUSION:
The sunscreen nanoemulsion containing a combination of 3% Avobenzone, 7.5% Octyl Methoxycinnamate, 2.73% Soybean Oil and 0.27% Liquid Paraffin showed synergistic sunscreen efficacy on SPF. This sunscreen nanoemulsion is more stable than sunscreen emulsion formulation during the experiment for 12 weeks at room temperature.
Keywords: Soybean oil, Avobenzone, Octyl methoxycinnamate, Nanoemulsion, Sunscreen
Introduction
The excessive exposure of human skin to Ultra Violet Radiation (UVR) may cause sunburn, erythema, photoaging, and increase the risk of skin cancer. UVR causes DNA damage and genetic mutations, which subsequently lead to skin cancer. The regular use of sunscreens protects the skin from the harmful effects of UV radiation, particularly the UVB (290-320 nm) and UVA (320-400 nm). UVC (200-290 nm) radiation is filtered by the atmosphere before it reaches the earth. UVB induces photoaging and mutagenic damage to nucleic acids. UVA promotes ROS (Reactive Oxygen Species) accumulation. ROS also induce direct cell damage, carcinogenesis and contribute to photoaging [1].
UV filters (“sunscreens”) are designed to protect the skin from the harmful effects of solar radiation, particularly the UVB (290-320 nm) and UVA (320-400 nm). UVB induces photoaging and mutagenic damage to nucleic acids. UVA promotes ROS (Reactive Oxygen Species) accumulation. ROS also induce direct cell damage, carcinogenesis and contribute to photoaging [2].
Photoprotection involves both primary protective factors (sunscreens) and secondary factors (e.f., Antioxidants, osmolytes, and DNA repair enzymes) that can disrupt the photochemical cascade triggered by UV-penetration, thereby limiting skin damage. The sunscreens should provide broad-spectrum UV protection for the presence of active ingredients, which attenuate the transmission of UV radiation onto the skin by absorbing, reflecting or scattering the incident radiation [3], [4].
Nowadays, there is an increasing interest in reducing the use of synthetic UV-filters by incorporating in natural sunscreen compounds that exhibit a similar filtering activity and possess radical scavenger properties, providing broad-spectrum sunscreen product with antioxidant properties [5], [6].
The efficacy of sunscreen products usually measured in the form of sun protection factor (SPF), which can be evaluated by in vitro or in vivo techniques. UVB protection is measured by a product’s SPF, which theoretically indicates that products with high SPFs provide more protection against the hazardous effects of sunlight than those with low SPFs.
The UV blocking from most natural oils is insufficient to obtain significant UV protection [7]. The strategies for preparations of sunscreen products with high SPF are nanotechnology formulations [8] and UV filter combinations [9], [10]. In this study, soybean oil was used as a natural UV filter, avobenzone and OMC as synthetic UV filters.
Soybean oil is a vegetable oil extracted from the seeds of the soybean. It is a natural sunscreens oil with UV B filter effect by absorbing them and antioxidant effect to reduce the photooxidative damage that results from UV-induced ROS production. Soybean oil contains (71.3 ± 6.4) mg/kg alpha-tocopherol and (273.3 ± 11.1 mg/kg gamma-tocopherol [11]. The results of the analysis of Indonesian Oil Palm Research Institute showed that soybean oil used in this study contains polyunsaturated fatty acids (46.4% linoleic acid) and 554 ppm Vitamine E. Vitamin E absorbs strongly in the UV-B region (280-320 NM) [12].
Experiments in vivo showed that soybean-germ oil (SGO) possesses a remarkable protective activity against UVB-induced skin inflammation, probably due to its radical-scavenging components, mainly tocopherols and polyunsaturated fatty acids [13].
Avobenzone is among the most common UV filters present on the market, due to the broad absorption spectrum in the UVA region. It is insoluble in water but freely soluble in organic solvent and oil. However, it suffers photo-degradation, giving rise to new compounds responsible for photoallergic and phototoxic reactions. This UVA filter is commonly used in concentrations between 3.0 to 10.0%. Therefore, the concentration of Avobenzone used in this study was 3%, and paraffin liquid was used for ensuring the Photostability of Avobenzone [14], [15]. Octyl methoxycinnamate (OMC) is one of the most commonly used UVB filters in sunscreen products, due to its high absorption capacity in the short wavelength region (290–320 NM). The approved concentration of OMC is 7.5-8.5% The concentration of OMC used in this study was 7.5% [16].
Nanoemulsion is very attractive to be applied in cosmetics (sunscreen products) because nanoemulsion has droplet size (20-500 NM) smaller than conventional emulsion (0.1-100 µm), so it is more stable, can prevent creaming, sedimentation or coalescence, besides also increase the solubility of an insoluble active ingredient in water [17]. Nanoemulsion has low viscosity, and transparent visual aspect, and a high surface area allows effective delivery of the active ingredient for the skin, thereby increasing the efficacy (SPF value) of the sunscreen product [18], [19].
Thus, the aims of this study were to investigate effects of nanoemulsion formulations on SPF values of sunscreen nanoemulsions containing the blends of herbal oil (soybean oil) and organic UV filters (Avobenzone and OMC) by in vitro (spectrophotometric) method and to verify the synergistic efficacy by a combination of the UV filters.
Material and Methods
The sunscreen nanoemulsion and emulsion was formulated using Soybean Oil (CV. Surya Agung, Jakarta), Avobenzone, Octyl methoxycinnamate (India), Tween 80, Ethanol and water demineralised were purchased from PT. Bratachem, Butylated hydroxytoluene, Liquid paraffin, Methylparaben, Propylparaben, Propylene glycol, Sodium CMC, Span 80 and Glycerin were purchased from CV. Rudang Jaya Medan Indonesia.
Nanoemulsion was prepared by using variations of Soybean oil and paraffin Liquid. Tween 80 as a surfactant and ethanol as a co-surfactant were used in the preparation of nanoemulsion. Oil phase consists of Avobenzone, Soybean oil, Paraffin Liquid, Butylated hydroxytoluene, Octyl methoxycinnamate (OMC) and Propylparaben, while the water phase was prepared by dissolving Methylparaben in hot water demineralised. This solution was then cooled down and added with Tween 80. This water phase then stirred with a magnetic stirrer for 30 minutes. Nanoemulsion was obtained by adding oil phase into the water phase, then homogenised with magnetic stirrer HI 190 M (Hanna Instruments) at 3500 rpm for 6 hours and sonicated using sonicator (Branson) for 1 hour to obtain a transparent, yellowish colour nanoemulsion.
Emulsion system consists of an oil phase and water phase. The mixture of Avobenzone, OMC, Soybean oil, Liquid paraffin, Propylparaben, Butylated hydroxytoluene and Span 80 were heated in a water bath at 70°C (Oil phase). The mixture of Methylparaben, Tween 80, propylene glycol and Glycerin were heated in the water bath at 70°C (water phase). The water phase was then added to the sodium Carboxy Methylcellulose (CMC) and was stirred quickly to avoid the formation of air bubble. The oil phase was then added to the mixture, then stirred until an emulsion was produced.
The nanoemulsion and emulsion were stored in a room temperature (25 ± 2°C) for 12 weeks and evaluated the physical stability, including consistency, odour, colour and phase separation.
Nanoemulsions globule size was determined by using particle size analyser (Analysette 22 Nanotec Fritsch) at room temperature for 0, 6, 12 weeks. Observation of phase separation of nanoemulsions and the emulsion was done by using a centrifuge (Hitachi CF 16 R X II) at 3750 rpm for 5 hours.
The pH of the nanoemulsions was determined by using a pH meter (Hanna) and viscosity by using viscometer Brookfield DV-E with specific spindle (spindle 62) after the nanoemulsions were storage for 0, 4, 8, and 12 weeks at room temperature.
The Evaluation of nanoemulsions stability at low and high temperatures (cycling test) was done by storing it’s at low temperature (4 ± 2°C) in refrigerator for 24 hours, then directly stored in high temperature (40 ± 2°C) in climatic chamber for another 24 hours (1 cycle). This test was done with 6 cycle repetition.
Evaluation of sunscreen activity was performed using one gram of sunscreen nanoemulsion or emulsion diluted in ethanol 96% at a final concentration of 200 ppm analysed by UV Spectrophotometry (Shimadzu) from 290-320 nm with the interval of 5 nm and 10 nm with the interval from 320-400 nm. Calculate the average of three determinations and calculate SPF by Mansur equation [20].
The SPF determination which is the correlation between the erythemogenic effect (EE) and the radiation intensity at each wavelength (I) and is adjusted according to Eq:
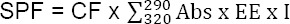
Where: Correction factor (CF) is 10, EE is the erythemogenic effect of radiation on wavelength, I is the intensity of solar light at each wavelength, and Abs is an absorption value from the sample [21].
Results
The nanoemulsions were prepared in 6 formulas, as shown in Table 1. All the nanoemulsions were yellowish, clear and transparent (Figure 1).
Table 1.
Composition of sunscreen nanoemulsion containing Soybean Oil, Avobenzone and OMC
| Material | Quantity of 100 mL (%) | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |
| Soybean oil | 0 | 0.5 | 1.5 | 2.5 | 2.73 | 3 |
| Liquid paraffin | 3 | 2.5 | 1.5 | 0.5 | 0.27 | 0 |
| Avobenzone | 3 | 3 | 3 | 3 | 3 | 3 |
| OMC | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Ethanol 96% | 26 | 26 | 26 | 26 | 26 | 26 |
| Tween 80 | 34 | 34 | 34 | 34 | 34 | 34 |
| Propylene glycol | 5 | 5 | 5 | 5 | 5 | 5 |
| Butylated hydroxytoluene | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Methylparaben | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Prophylparaben | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Water demineralized | Up to 100 mL | Up to 100 mL | Up to 100 mL | Up to 100 mL | Up to 100 mL | Up to 100 mL |
Figure 1.

Appearance of the prepared sunscreen nanoemulsions F1 (without Soybean Oil) and F5 containing Soybean Oil, Avobenzone and OMC; A) Before storage; B) After storage for 4 weeks; C) After storage for 8 weeks; D) After storage for 12 weeks at room temperature; E) After cycling test
The formula for emulsion preparation was shown in Table 2. The emulsion formed was milky-white in colour and was not transparent.
Table 2.
Formula of sunscreen emulsion containing Soybean oil, Avobenzone and OMC
| Material | Quantity of 100 mL (%) |
|---|---|
| Soybean oil | 2.73 |
| Liquid paraffin | 0.27 |
| Avobenzone | 3 |
| OMC | 7.5 |
| Tween 80 | 3.6 |
| Span 80 | 1.4 |
| Glycerin | 13 |
| Propylene glycol | 10 |
| Butylated hydroxytoluene | 0.1 |
| Methylparaben | 0.1 |
| Prophylparaben | 0.02 |
| Sodium CMC | 2 |
| Water demineralised | Up to 100 mL |
The results of physical stability evaluation of the nanoemulsions were shown in Figure 1. Nanoemulsions were stored at room temperature (25 ± 2°C) for 12 weeks.
The cycling test was done by storing it’s at low temperature (4 ± 2°C) in the refrigerator for 24 hours, then at high temperature (40 ± 2°C) in the climatic chamber for another 24 hours (1 cycle). This test was done with 6 cycle repetition. The result of physical stability evaluation of emulsion was shown in Figure 2.
Figure 2.
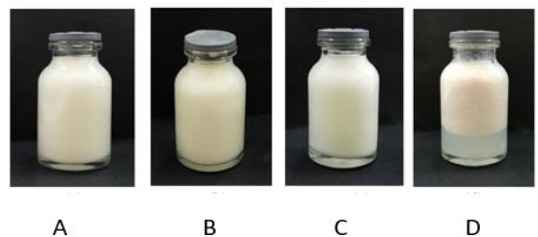
Appearance of the prepared sunscreen emulsion containing Soybean oil, Avobenzone and OMC; A) Before storage; B) After storage for 4 weeks; C) After storage for 8 weeks; E) After storage for 12 weeks) at room temperature
The results of centrifugation test showed that all the nanoemulsion were stable, there is no discolouration and phase separation or creaming after centrifugation, but the emulsion was not stable, colour changes occur and the formation of phase separation (Figure 3).
Figure 3.

Appearance of the prepared sunscreen nanoemulsion without Soybean oil (F1) and containing Soybean oil (F5); and emulsion containing Soybean oil; A) Before; B) After centrifugation
Nanoemulsion (F5) has the smallest average globule size (Table 3).
Table 3.
Average globule size of sunscreen nanoemulsions and emulsion
| Formula | The ratio of Soybean Oil and Liquid Paraffin | Average Globule Size (nm) | |
|---|---|---|---|
| Soybean Oil | Liquid Paraffin | ||
| F1 | 0 | 3 | 384.07 |
| F2 | 0.5 | 2.5 | 17100 |
| F3 | 1.5 | 1.5 | 14310 |
| F4 | 2.5 | 0.5 | 538.90 |
| F5 | 2.73 | 0.37 | 68.47 |
| F6 | 3 | 0 | 560.18 |
Globule size of nanoemulsion and emulsion were increased after storage at room temperature (Table 4).
Table 4.
Average globule size of sunscreen nanoemulsion and emulsion containing Soybean oil (F5) during storage for 12 weeks at room temperature
| Formula | Time (week) | Average Globule Size (nm) |
|---|---|---|
| Nanoemulsion (F5) | 0 | 68.47 |
| 4 | 404.09 | |
| 8 | 619.82 | |
| 12 | 863.36 | |
| Emulsion | 0 | 1294.96 |
| 4 | 2869.46 | |
| 8 | 4727.27 | |
| 12 | 7417.51 |
There was a decrease in pH and increase in viscosity from sunscreen nanoemulsion formulation after storage at room temperature for 12 weeks, but viscosity was decreased after storage at high temperature for 12 weeks (Table 5).
Table 5.
pH of sunscreen nanoemulsion containing Soybean oil (F5) during storage for 12 weeks at room and high temperature
| Formula | Time (week) | pH ± SD | Viscosity ± SD | ||
|---|---|---|---|---|---|
| 25 ± 2°C | 40 ± 2°C | 25 ± 2°C | 40 ± 2°C | ||
| Nanoemulsion (F5) | 0 | 7.23 ± 0.06 | 7.23 ± 0.06 | 133.33 ± 7.22 | 133.33 ± 7.22 |
| 4 | 6.90 ± 0.20 | 6.63 ± 0.06 | 162.50 ± 12.50 | 120.83 ± 7.22 | |
| 8 | 6.63 ± 0.06 | 6.30 ± 0.00 | 245.83 ± 7.22 | 104.17 ± 7.22 | |
| 12 | 6.20 ± 0.00 | 5.93 ± 0.06 | 383.33 ± 14.43 | 75.00 ± 0.00 | |
N = 3.
The pH and viscosity of sunscreen emulsion formulation were decreased during storage for 12 weeks at room temperature (Table 6).
Table 6.
pH and viscosity of sunscreen emulsion containing Soybean oil during storage for 12 weeks at room temperature
| Formula | Time (week) | pH ± SD | Viscosity± SD |
|---|---|---|---|
| Sunscreen Emulsion | 0 | 6.63 ± 0.06 | 8100.00 ± 0.00 |
| 4 | 6.43 ± 0.10 | 6933.33 ± 28.87 | |
| 8 | 6.30 ± 0.00 | 5800.00 ± 0.00 | |
| 12 | 5.93 ± 0.06 | 4400.00 ± 0.00 |
N = 3.
Shape and size of nanoemulsion were investigated using TEM (JEOL JEM 1400). This evaluation was performed on nanoemulsion (F5) with the smallest particle size among all nanoemulsion formulations. Figure 4 shows that sunscreen nanoemulsion containing Soybean oil, Avobenzone and OMC has a spherical morphology.
Figure 4.
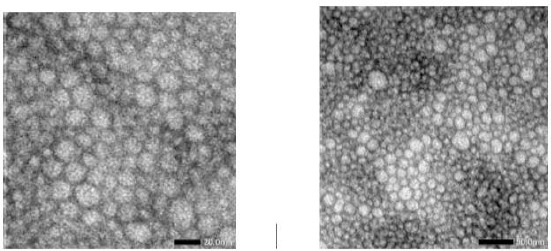
TEM Images of sunscreen nanoemulsion containing Soybean oil, Avobenzone and Octyl methoxycinnamate
The results of determination of the SPF value are shown in Table 7. The results showed that the SPF value of sunscreen nanoemulsion containing Soybean oil, Avobenzone and OMC is higher than the emulsion.
Table 7.
SPF value of sunscreen nanoemulsions (F5) and emulsion
| Formula | Sun Protection Factor (SPF) Value | Average SPF Value | |||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | ||
| Nanoemulsion F5 | 21.15 | 20.56 | 20.64 | 23.82 | 21.98 | 21.32 | 21.57 ± 1.21 |
| Emulsion | 14.81 | 14.96 | 14.98 | 15.33 | 15.15 | 15.37 | 15.10 ± 0.22 |
N = 6.
Discussion
The sunscreen nanoemulsion formulation (F5) containing a combination of 3% Avobenzone, 7.5% Octyl Methoxycinnamate with a ratio of 2.73% Soybean Oil and 0.27% Liquid Paraffin showed the smallest average droplet size of 68.47 nm. This formulation was selected for stability and sunscreen activity evaluation. The sunscreen nanoemulsion without Soybean Oil had an average droplet size of 384.07 nm. This is because avobenzone is more soluble in soybean oil. However, the optimum formulation is obtained from a combination of 2.73% soybean oil and liquid paraffin 0.275 as the oil phase compared to formulations using only soybean oil or liquid paraffin. Selection of an appropriate oil phase is very important, mainly in case of O / W nanoemulsions. Usually, the oil which has the maximum solubilising potential for active substance is selected as an oily phase for the formulation of nanoemulsions. This helps to achieve maximum active substance loading in the nanoemulsions [22]. The droplet size of nanoemulsions and emulsion were increased during 12 weeks of storage at room temperature, but the nanoemulsion formulation (F5) still in the nano-size range.
The sunscreen emulsion containing a combination of 3% Avobenzone, 7.5% Octyl Methoxycinnamate had an average droplet size of 1294.96 nm greater than sunscreen nanoemulsion formulation. The formulation of nanoemulsion was prepared based on high energy emulsification method, in which mechanical energy input is applied by High-Shear Stirring (magnetic stirrer 3500 rpm) and sonication. Thus, droplet sizes of the internal phase can be significantly decreased [23].
The sunscreen nanoemulsion was stable during the experiment for 12 weeks of storage at room temperature (25 ± 2°C), and high temperature (40 ± 2°C). There was no discolouration, changes in consistency, odour and phase separation during the experiment for 12 weeks storage at a variation temperature in the nanoemulsion, but the emulsion showed discolouration and phase separation (not stable) for 12 weeks storage at room temperature.
Centrifugation test was performed to determine the stability of nanoemulsion. The centrifugation test describes the stability of one year of storage. All of the nanoemulsions were stable with no phase separation or creaming after centrifugation at 3750 rpm for 5 hours. However, the emulsion was not stable with the formation of phase separation.
The results of pH evaluation all nanoemulsions show that there were decreases in pH value and the pH value of formula F5 is close to the neutral pH of human skin normally ranges from 4.5 to 6.0 [24]. The viscosity of all nanoemulsions was increased during storage for 12 weeks at room temperature, but the decrease in high temperature. The viscosity of emulsion was decreased during storage for 12 weeks at room temperature; this is caused by the occurrence of phase separation caused by damage to the interface layer.
The SPF value of sunscreen nanoemulsion formulation (F5) containing a combination of 3% Avobenzone, 7.5% Octyl Methoxycinnamate, 2.73% Soybean Oil and 0.27% Liquid Paraffin was higher than sunscreen nanoemulsion formulation without Soybean Oil (F1) and emulsion preparation. This is because Soybean Oil has properties that can absorb UVB rays. Nanoemulsion technologies, which is being applied to enhance the solubility of lipophilic substance (Avobenzone) and had a smaller globule size and also is more stable throughout the stability experiment. So, they absorb more ultraviolet light which results in higher SPF values.
The sunscreen nanoemulsion containing a combination of 3% Avobenzone, 7.5% Octyl Methoxycinnamate, 2.73% Soybean Oil and 0.27% Liquid Paraffin showed synergistic efficacy sunscreen on SPF and more stable compare with sunscreen emulsion formulation.
The in vitro photoprotective efficacy assessment demonstrated that there was a synergism between the formulation with the combination of 2,73% Soybean Oil, 3% Avobenzone, and 7.5% Octyl methoxycinnamate. The sunscreen nanoemulsion more stable than sunscreen emulsion during the experiment for 12 weeks at room temperature. The SPF value of this nanoemulsion more higher than nanoemulsion without Soybean oil and emulsion preparation.
Footnotes
Funding: This work was financially supported by the University of Sumatera Utara, Indonesia through the TALENTA Research, scheme of Penelitian Guru Besar 2018
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Sambandan DR, Ratner D. Journal of the American Academy of Dermatology [Internet] 4. Vol. 64. Elsevier BV; 2011. Sunscreens: An overview and update; pp. 748–58. https://doi.org/10.1016/j.jaad.2010.01.005 PMid:21292345. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez S. The Open Dermatology Journal [Internet] 1. Vol. 5. Bentham Science Publishers Ltd; 2011. Current Trends in Photoprotection - A New Generation of Oral Photoprotectors; pp. 6–14. https://doi.org/10.2174/1874372201105010006. [Google Scholar]
- 3.Rai R, Shanmuga SC, Srinivas CR. Update on photoprotection. Indian journal of dermatology. 2012;57(5):335. doi: 10.4103/0019-5154.100472. https://doi.org/10.4103/0019-5154.100472 PMid:23112351 PMCid:PMC3482794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal S, Godse K, Patil S, Nadkarni N. Knowledge and attitude of general population toward effects of sun exposure and use of sunscreens. Indian Journal of Dermatology. Medknow; 2018;63(4):285. doi: 10.4103/ijd.IJD_609_17. https://doi.org/10.4103/ijd.IJD_609_17 PMid:30078870 PMCid:PMC6052747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manikrao Donglikar M, Laxman Deore S. Sunscreens: A review. Pharmacognosy Journal. 2016;8(3):171–9. https://doi.org/10.5530/pj.2016.3.1. [Google Scholar]
- 6.Wang SQ, Osterwalder U, Jung K. Ex vivo evaluation of radical sun protection factor in popular sunscreens with antioxidants. Journal of the American Academy of Dermatology. 2011;65(3):525–30. doi: 10.1016/j.jaad.2010.07.009. https://doi.org/10.1016/j.jaad.2010.07.009 PMid:21624700. [DOI] [PubMed] [Google Scholar]
- 7.Gause S, Chauhan A. UV-blocking potential of oils and juices. International journal of cosmetic science. 2016;38(4):354–63. doi: 10.1111/ics.12296. https://doi.org/10.1111/ics.12296 PMid:26610885. [DOI] [PubMed] [Google Scholar]
- 8.Xia Q, Saupe A, Müller RH, Souto EB. Nanostructured lipid carriers as novel carrier for sunscreen formulations. International journal of cosmetic science. 2007;29(6):473–82. doi: 10.1111/j.1468-2494.2007.00410.x. https://doi.org/10.1111/j.1468-2494.2007.00410.x PMid:18489386. [DOI] [PubMed] [Google Scholar]
- 9.El-Boury S, Couteau C, Boulande L, Paparis E, Coiffard LJ. Effect of the combination of organic and inorganic filters on the Sun Protection Factor (SPF) determined by in vitro method. International journal of pharmaceutics. 2007;340(1-2):1–5. doi: 10.1016/j.ijpharm.2007.05.047. https://doi.org/10.1016/j.ijpharm.2007.05.047 PMid:17606340. [DOI] [PubMed] [Google Scholar]
- 10.Couteau C, Chammas R, Alami-El Boury S, Choquenet B, Paparis E, Coiffard LJ. Combination of UVA-filters and UVB-filters or inorganic UV filters-Influence on the sun protection factor (SPF) and the PF-UVA determined by in vitro method. Journal of dermatological science. 2008;50(2):159–61. doi: 10.1016/j.jdermsci.2007.11.007. https://doi.org/10.1016/j.jdermsci.2007.11.007 PMid:18262775. [DOI] [PubMed] [Google Scholar]
- 11.Grilo EC, Costa PN, Gurgel CS, Beserra AF, Almeida FN, Dimenstein R. Alpha-tocopherol and gamma-tocopherol concentration in vegetable oils. Food Science and Technology. 2014;34(2):379–85. https://doi.org/10.1590/S0101-20612014005000031. [Google Scholar]
- 12.Goswami PK, Samant M, Srivastava R. Natural Sunscreen Agents A Review. Sch Acad J Pharm. 2013;2(6):458–463. [Google Scholar]
- 13.Bonina F, Puglia C, Avogadro M, Baranelli E, Cravotto G. The Topical Protective Effect of Soybean-Germ Oil against UVB-Induced Cutaneous Erythema: an in vivo Evaluation. Archiv der Pharmazie: An International Journal Pharmaceutical and Medicinal Chemistry. 2005;338(12):598–601. doi: 10.1002/ardp.200500159. https://doi.org/10.1002/ardp.200500159 PMid:16281310. [DOI] [PubMed] [Google Scholar]
- 14.Banker T, Kale P, PeepliwaL A. Method Development And Validation For Simultaneous Estimation Of Oxybenzone, Octinoxate And Avobenzone In Sunscreen Lotion By Reversed Phase High Performance Liquid Chromatography. International Journal of Biomedical and Advance Research. Scholar Science Journals. 2011;2:2. https://doi.org/10.7439/ijbar.v2i2.25. [Google Scholar]
- 15.Vallejo JJ, Mesa M, Gallardo C. Evaluation of the Avobenzone Photostability in Solvents Used in Cosmetic Formulations. Vitae, Revista De La Facultad De Quimica Farmaceutica. 2011;18(1):63–71. [Google Scholar]
- 16.Latha MS, Martis J, Shobha V, Shinde RS, Bangera S, Krishnankutty B, Bellary S, Varughese S, Rao P, Kumar BRN. Sunscreening Agents: A Review. J Clin Aesthet Dermatol. 2013;6(1):16–26. [PMC free article] [PubMed] [Google Scholar]
- 17.Debnath S, Satayanarayana Kumar VG. Nanoemulsion-A Method to Improve The solubility of Lipophilic Drugs. Pharmanest - An International Journal of Advances In Pharmaceutical Sciences. 2010;2(2 - 3):72–83. [Google Scholar]
- 18.Devarajan V, Ravichandran V. Nanoemulsions: as modified drug delivery tool. Int J Compr Pharm. 2011;2(4):1–6. [Google Scholar]
- 19.Koroleva MY, Yurtov EV. Russian Chemical Reviews. 1. Vol. 81. IOP Publishing; 2012. Nanoemulsions: the properties, methods of preparation and promising applications; pp. 21–43. https://doi.org/10.1070/RC2012v081n01ABEH004219. [Google Scholar]
- 20.Mansur JS, Breder MNR, Mansur MCA, Azulay RD. Determinação do fator de proteção solar por espectrofotometria. An Bras Dermatol Rio De Janeiro. 1986;61:121–24. [Google Scholar]
- 21.Dutra EA, Oliveira DAG da C, Kedor-Hackmann ERM, Santoro MIRM. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Revista Brasileira de Ciências Farmacêuticas [Internet]. FapUNIFESP (SciELO); 2004;40(3):381–5. https://doi.org/10.1590/S1516-93322004000300014. [Google Scholar]
- 22.Date AA, Nagarsenker MS. International Journal of Pharmaceutics [Internet] 1-2. Vol. 355. Elsevier BV; 2008. Parenteral microemulsions: An overview; pp. 19–30. https://doi.org/10.1016/j.ijpharm.2008.01.004 PMid:18295991. [DOI] [PubMed] [Google Scholar]
- 23.Solè I, Solans C, Maestro A, González C, Gutiérrez JM. Journal of Colloid and Interface Science. 1. Vol. 376. Elsevier BV; 2012. Study of nano-emulsion formation by dilution of microemulsions; pp. 133–9. https://doi.org/10.1016/j.jcis.2012.02.063 PMid:22480397. [DOI] [PubMed] [Google Scholar]
- 24.Chikakane K, Takahashi H. Measurement of skin pH and its significance in cutaneous diseases. Clinics in dermatology. 1995;13(4):299–306. doi: 10.1016/0738-081x(95)00076-r. https://doi.org/10.1016/0738-081X(95)00076-R. [DOI] [PubMed] [Google Scholar]


