Abstract
AIM
To evaluate the relative efficacy and safety of besifloxacin for treatment of acute bacterial conjunctivitis.
METHODS
A comprehensive search in PubMed, EMBASE Web of Science, Cochrane Central Database and CNKI was undertaken for randomized controlled trials (RCTs) comparing besifloxacin with other treatments or placebo. The primary outcome measures were clinical resolution, rates of bacterial eradication, individual clinical outcomes, cure rates, and bacterial eradication rates of different kinds of pathogens. Safety outcomes were the number of adverse effects (AEs). The final search was performed on August 2018.
RESULTS
Six RCTs were included. Four studies compared the efficacy and safety of besifloxacin with placebo, 1 study compared besifloxacin with moxifloxacin, and 1 study compared besifloxacin with gatifloxacin. A total of 2780 patients met the inclusion criteria. Besifloxacin presented higher efficacy and safety than did placebo in clinical resolution, rates of bacterial eradication, individual clinical outcomes, cure rates, bacterial eradication rates of different kinds of pathogens and the number of AEs. There was no significant difference between besifloxacin and moxifloxacin or gatifloxacin in the comparison items mentioned above.
CONCLUSION
Besifloxacin is highly effective and safe for treatment of acute bacterial conjunctivitis. Further comparative trials regarding the effect of besifloxacin for treatment of acute bacterial conjunctivitis will aid in treatment decisions.
Keywords: besifloxacin, acute bacterial conjunctivitis, Meta-analysis, randomized controlled trials
INTRODUCTION
Acute conjunctivitis, which is characterized by a self-limited course of inflammation of the conjunctiva with persistent mucopurulent discharge, erythema and discomfort, is a contagious infection of the ocular surface that affects individuals ranging from neonates to the elderly. As one of the most common eye disorders, acute conjunctivitis can easily spread from one person to another, especially in situations in which individuals are in close personal contact, such as schools, daycare centers, and chronic health care facilities[1]–[2]. The pathogens of acute conjunctivitis can be viral or fungal in nature; however, approximately 78% of cases in children and half of cases in adults are caused by bacteria. The most common causative bacterial species are Haemophilus influenza (H. influenza), Streptococcus pneumoniae (S. pneumoniae), Staphylococcus aureus (S. aureus), and Staphylococcus epidermidis (S. epidermidis)[3]–[4]. In fact, most acute conjunctivitis cases are caused by several bacterial species simultaneously, therefore, treatment often relies on clinical experience and is usually initiated with a broad-spectrum ophthalmic antibacterial treatment. Although acute bacterial conjunctivitis is a self-limited disease and can resolve spontaneously due to the host's immune factors in 1-2wk[5], topical ophthalmic antibiotics are warranted as they hasten clinical resolution and microbiological remission, decreasing the risk of relapse and the development of complications such as keratitis, orbital cellulitis, and panophthalmitis[1],[6]. Classical antibacterials options include tobramycin, trimethoprim, ciprofloxacin, gatifloxacin and moxifloxacin[7]. However, the widespread use of broad-spectrum antibiotics has resulted in the emergence of resistance to those typical antibiotics[8]–[9]. Therefore, developing new antibiotics with high efficacy and safety against some resistant bacteria is necessary.
Besifloxacin is an advanced-generation fluoroquinolone and represents the first chlorofluoroquinolone developed specifically for ophthalmic use. Unlike older fluoroquinolones that selectively target either DNA gyrase or topoisomerase IV, besifloxacin has balanced activity against both of those enzymes[10]–[12]. In vitro studies have demonstrated that its antibacterial capacity exceeds that of most other fluoroquinolones and nonfluoroquinolones, especially against multidrug resistant Staphylococci[13]–[14]. Several in vivo studies have also drawn optimistic conclusions regarding the antibacterial potency of besifloxacin[15]–[16]. At present, besifloxacin ophthalmic suspension 0.6%, a long-acting topical formulation using DuraSite technology (InSite Vision, Alameda, California) that helps retain therapeutic doses of a drug on the surface of the eye, has been approved in the United States, Canada, and various countries in Latin America, Europe, and Asia for the treatment of acute bacterial conjunctivitis[17]. However, some data among in vivo studies are contradictory, for example, Karpecki et al[15] found that besifloxacin can eradicate S. pneumoniae more efficiently than placebo, while Silverstein et al[18] argued that the eradication rate of S. pneumoniae is not better than vehicle. Therefore, summarizing the data of published studies and drawing a general conclusion to guide the clinical application of besifloxacin are necessary. This review demonstrates the efficacy and safety of besifloxacin for treatment of acute bacterial conjunctivitis via Meta-analysis of randomized placebo-controlled trials. We also compare the effect of besifloxacin with other antibiotics if necessary.
MATERIALS AND METHODS
Search Strategy
Two trained investigators performed an electronic literature search of major online databases, including PubMed, Embase, Web of Science, Cochrane Central Database and CNKI (all relevant studies were published in English or Chinese with the date range from inception to August 31, 2018). Key terms for searching the title and abstract included “besifloxacin”, “synaphymenitis”, “epipephysitis” and “conjunctivitis”.
Eligibility Criteria
Articles were included if they met the following criteria: 1) target population: individuals with acute bacterial conjunctivitis; 2) intervention: besifloxacin and placebo or other antibiotics as controls; 3) outcome: evaluated the clinical resolution, rates of bacterial eradication and adverse effects (AEs); 4) type of studies: prospective, randomized controlled trials (RCTs); and 5) full text published in English or Chinese.
Study Identification
Two investigators independently identified articles using the eligibility criteria listed above. After reading the title and the abstract, if the investigators considered the articles potentially eligible, they would subsequently read the full text. If there was any disagreement between the investigators, they discussed the issue with a third investigator until they reached an agreement.
Risk of Bias and Assessment of Study Quality
The methodological quality of each eligible study was independently determined by two investigators by using the Cochrane Risk of Bias tool, provided in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.3.0). The Cochrane risk of bias assessment tool includes the following items: sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias. The authors' judgment is categorized as “low risk”, “high risk”, or “unclear risk” of bias.
Data Extraction
The two investigators analyzed the full text of all eligible articles and then extracted the following information: study characteristics, publication years, number of participants allocated to each group, the mean age of each group, number of males and females in each group, the method of intervention and the assessment time. If the two investigators disagreed with each other, they would ask for an opinion from a third investigator until they finally came to a consensus.
Statistical Analysis
The two investigators found and recorded parameters for following outcomes: clinical resolution, rates of bacterial eradication, individual clinical outcomes, cure rates, bacterial eradication rates of different kinds of pathogens, and the number of AEs.
Statistical analyses were carried out using RevMan 5.3 software. For all comparisons, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated as summary statistics for dichotomous variables. The mean difference (MD) and 95%CI were calculated as summary statistics for continuous variables. P<0.05 was regarded as statistically significant. Statistical heterogeneity was quantified with the use of Chi-square (χ2) and I2 tests. Pooled summary statistics were calculated using a fixed-effect model if significant heterogeneity was not detected. If heterogeneity existed after determining by a statistically significant P<0.05 and I2>50%, a random effect model was applied to unsolved heterogeneity. Otherwise, a fixed effect model was used. We also performed subgroup analysis and sensitivity analysis to identify the source of heterogeneity.
RESULTS
Literature Search
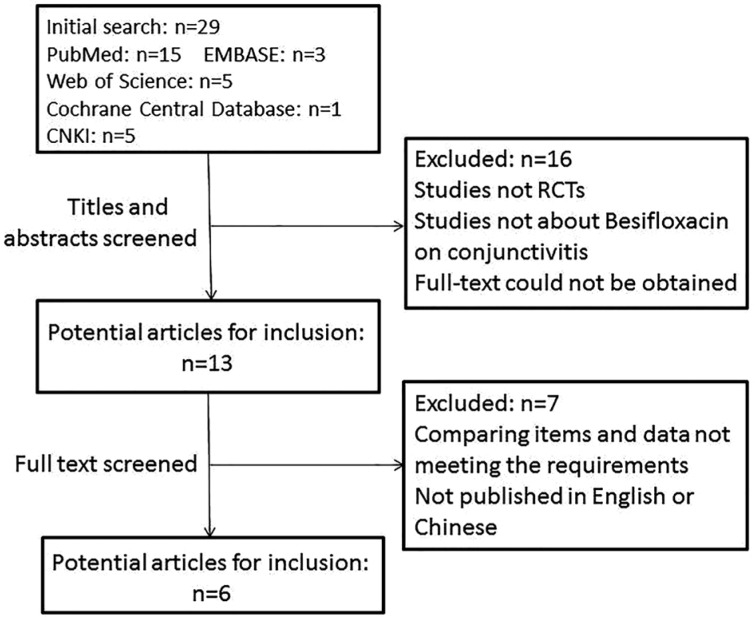
By using our search strategy, we identified 29 citations from online databases, the same articles had been taken away. Of those 29 studies, 15 articles were from PubMed, 3 from EMBASE, 5 from Web of Science, 1 from the Cochrane Central Database and 5 from CNKI. Figure 1 describes the flow of candidate and eligible articles. After reading the titles and abstracts of the 29 articles, we found that 12 studies were not randomized controlled trials, the major focus of 3 articles was the effect of Besifloxacin on conjunctivitis, and the full text of 1 article could not be acquired. After reading the full text of the remaining 13 articles, the compared items of 5 articles did not meet our requirements, and the data of 2 studies were not presented in the form of mean values and standard deviations. Ultimately, 6 articles were considered eligible for our study.
Figure 1. Flow diagram of studies included in this Meta-analysis.
Study Characteristics
The main characteristics of the 6 eligible studies are summarized in Table 1. Four studies compared the efficacy and safety of Besifloxacin with placebo, 1 studies compared Besifloxacin with Moxifloxacin, and 1 study compared Besifloxacin with Gatifloxacin. The earliest study was published in 2009, while the latest study was accepted in 2017. The number of participants varied from 16 to 482. A total of 2780 patients met the inclusion criteria. The mean age of each group ranged from 15.2 days to 38.3 years old (the subjects of one study were neonates). The assessment time can be regarded as identical, the first visit day was the 4th day after intervention, while the second visit time was the 8th day after treatment. The patterns of intervention varied slightly among those studies.
Table 1. Characteristics of randomized controlled trials included in this Meta-analysis.
| Author(s), year | Groups | No. of patients | Mean age, y | Male/female | Intervention methods |
| Karpecki et al[15], 2009 | Besifloxacin | 137 | 33.3 | 51/86 | Besifloxacin (0.6%) or vehicle 3 times daily for 5d |
| Contrast | 132 | 35.1 | 56/76 | ||
| Tepedino et al[19], 2009 | Besifloxacin | 475 | 27.3 | 173/302 | Besifloxacin (0.6%) or vehicle 3 times daily for 5d |
| Contrast | 482 | 27.3 | 182/300 | ||
| DeLeon et al[20], 2012 | Besifloxacin | 231 | 29.4 | 89/142 | Besifloxacin (0.6%) or vehicle twice daily for 3d |
| Contrast | 243 | 26.4 | 110/133 | ||
| Malhotra et al[21], 2013 | Besifloxacin | 344 | 29.6 | 140/204 | Besifloxacin (0.6%) or vehicle 3 times daily for 7d |
| Contrast | 170 | 30.5 | 75/95 | ||
| McDonald et al[22], 2009 | Besifloxacin | 252 | 31.6 | 109/143 | Besifloxacin (0.6%) or moxifloxacin (0.5%) 3 times daily for 5d |
| Contrast | 281 | 38.3 | 139/142 | ||
| Sanfilippo et al[16], 2017 | Besifloxacin | 16 | 15.8d | 4/12 | Besifloxacin (0.6%) or gatifloxacin (0.3%) 3 times daily for 7d |
| Contrast | 17 | 15.2d | 10/7 |
Study Quality
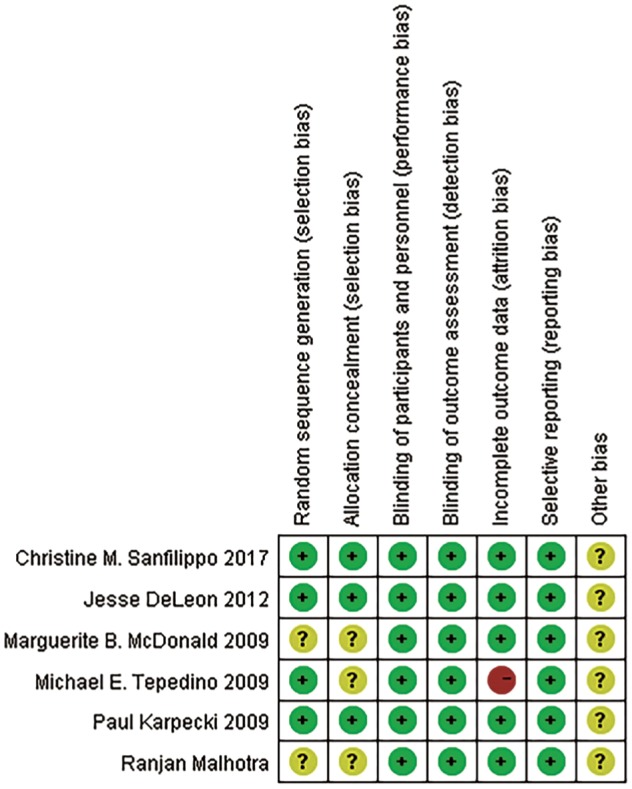
Figure 2 shows the study quality assessment of the included studies. Two studies did not provide detailed information about randomization. Three studies did not mention allocation concealment. All included studies had a low risk of bias in terms of selective reporting and blinding method. One studies had a high risk of attrition bias.
Figure 2. Risk of bias of included studies.
According to a guideline of the Cochrane library[23], assessment for publication bias is not reliable for fewer than 10 pooled studies. Therefore, we did not evaluate the existence of publication bias by the Egger's test for funnel plot asymmetry.
Results of Forest Plots
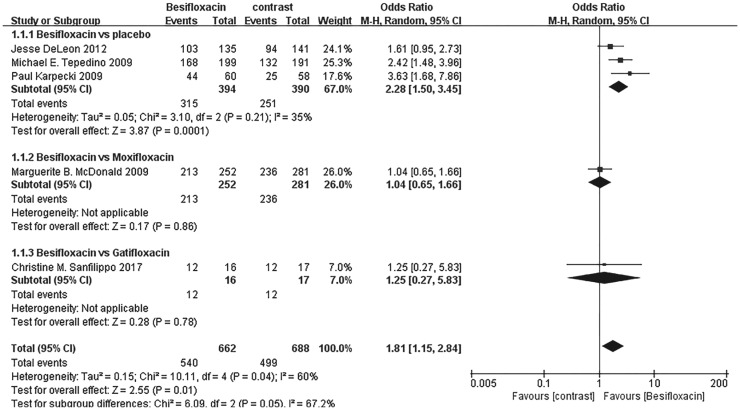
Clinical resolution
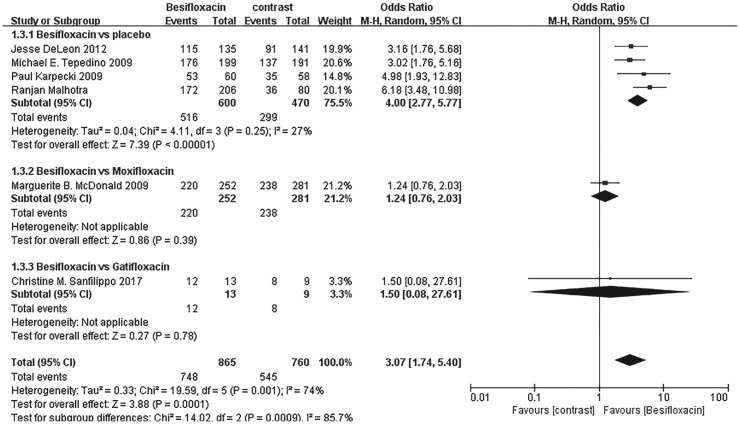
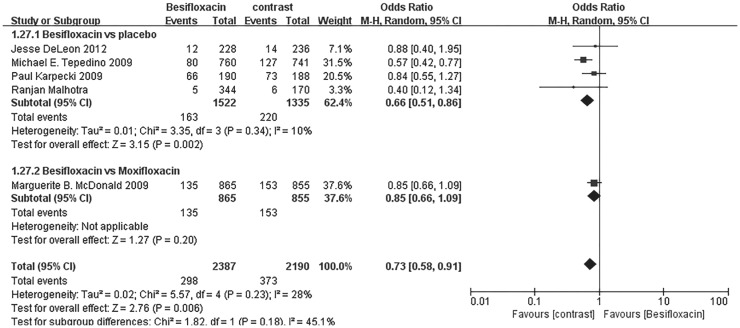
Significantly more patients receiving besifloxacin than placebo had clinical resolution of the baseline infection at days 4 and 8 after intervention, and there was no significant difference between besifloxacin and moxifloxacin or gatifloxacin (Figures 3 and 4).
Figure 3. Estimated odds ratio for changes in clinical resolution at day 4.
Figure 4. Estimated odds ratio for changes in clinical resolution at day 8.
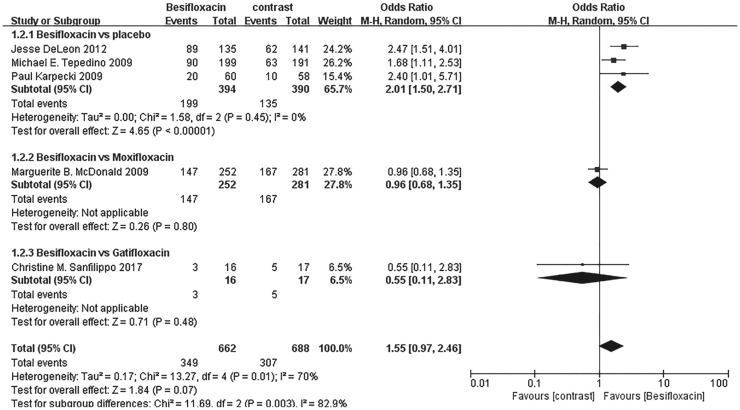
Rates of bacterial eradication
The rates of bacterial eradication were also significantly greater in those using besifloxacin than using vehicle on days 4 and 8, and no significant difference was observed between besifloxacin and moxifloxacin or gatifloxacin (Figures 5 and 6).
Figure 5. Estimated odds ratio for changes in bacterial eradication rates at day 4.
Figure 6. Estimated odds ratio for changes in bacterial eradication rates at day 8.
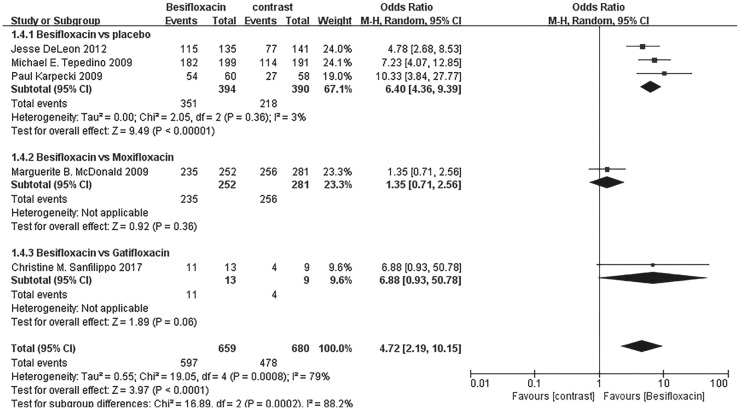
Individual clinical outcomes
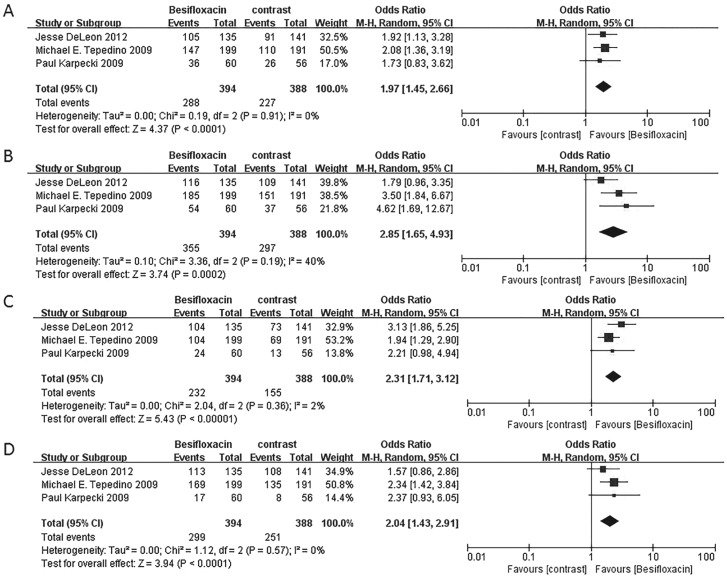
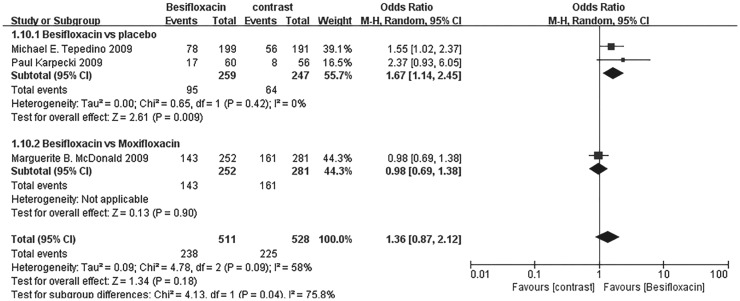
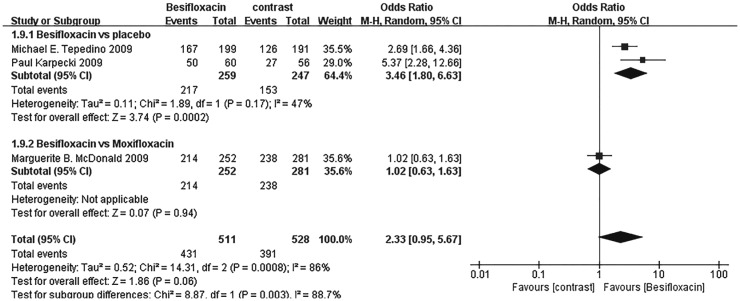
The percentage of patients treated with Besifloxacin who had resolution of ocular discharge was significantly greater at days 4 and 8 compared with that of treated with placebo, and significantly greater percentages of patients treated with Besifloxacin had normal bulbar conjunctival injection than did those treated with vehicle at days 4 and 8 (Figure 7). In addition, Besifloxacin cured more patients at day 4 and 8 than did placebo (Figures 8 and 9). No significant difference was observed in cure rates between Besifloxacin and Moxifloxacin (Figures 8 and 9).
Figure 7. Estimated odds ratio for changes in ocular discharge and bulbar conjunctival injection.
A: Resolution of ocular discharge at day 4; B: Resolution of ocular discharge at day 8; C: Normal bulbar conjunctival injection at day 4; D: Normal bulbar conjunctival injection at day 8.
Figure 8. Estimated odds ratio for changes in cure rates at day 4.
Figure 9. Estimated odds ratio for changes in cure rates at day 8.
Pathogens
Besifloxacin-treated subjects had a higher rate of bacterial eradication and clinical resolution in Gram-positive bacteria at days 4 and 8, and Gram-negative bacteria at day 8 than did placebo-treated subjects. Bacterial eradication rates were significantly better in Besifloxacin-treated eyes than in placebo-treated eyes for infections caused by H. influenza, S. pneumoniae, S. aureus and S. epidermidis at days 4 and 8. There was no overall significant difference in those comparison items between Besifloxacin and Moxifloxacin or Gatifloxacin (Table 2).
Table 2. Clinical resolution and bacterial eradication rates of different species.
| Parameters | Treatments | No. of patients | OR | 95%CI | P | χ2 | I2 | Effect model | |
| Rates of bacterial eradication | |||||||||
| 4d | |||||||||
| Gram-positive | Besifloxacin vs placebo | 755 | 7.33 | 4.18-12.85 | <0.00001 | 0.11 | 50% | Random effect | |
| Besifloxacin vs moxifloxacin | 368 | 1.26 | 0.63-2.55 | 0.51 | - | - | Random effect | ||
| Besifloxacin vs gatifloxacin | 31 | 9.33 | 1.50-58.20 | 0.02 | - | - | Random effect | ||
| Gram-negative | Besifloxacin vs placebo | 438 | 4.29 | 2.52-7.28 | <0.00001 | 0.92 | 0 | Random effect | |
| Besifloxacin vs moxifloxacin | 214 | 1.26 | 0.34-4.59 | 0.73 | - | - | Random effect | ||
| Besifloxacin vs gatifloxacin | 10 | 5.57 | 0.18-176.26 | 0.33 | - | - | Random effect | ||
| H. influenzae | Besifloxacin vs placebo | 281 | 4.01 | 2.19-7.32 | <0.00001 | 0.90 | 0 | Random effect | |
| Besifloxacin vs moxifloxacin | 169 | 1.16 | 0.30-4.48 | 0.83 | - | - | Random effect | ||
| S. pneumoniae | Besifloxacin vs placebo | 215 | 2.21 | 1.14-4.29 | 0.02 | 0.60 | 0 | Random effect | |
| Besifloxacin vs moxifloxacin | 122 | 1.02 | 0.37-2.85 | 0.97 | - | - | Random effect | ||
| S. aureus | Besifloxacin vs placebo | 141 | 9.11 | 3.50-23.72 | <0.00001 | 0.39 | 0 | Random effect | |
| Besifloxacin vs moxifloxacin | 113 | 1.55 | 0.55-4.40 | 0.41 | - | - | Random effect | ||
| Besifloxacin vs gatifloxacin | 5 | 7.00 | 0.17-291.34 | 0.31 | - | - | Random effect | ||
| S. epidermidis | Besifloxacin vs placebo | 109 | 4.58 | 1.74-12.10 | 0.002 | 0.36 | 3% | Random effect | |
| Besifloxacin vs moxifloxacin | 63 | 4.8 | 0.55-42.23 | 0.16 | - | - | Random effect | ||
| Besifloxacin vs gatifloxacin | 7 | 7.00 | 0.22-218.95 | 0.27 | - | - | Random effect | ||
| 8d | |||||||||
| Gram-positive | Besifloxacin vs placebo | 753 | 4.23 | 2.90-6.17 | <0.00001 | 0.75 | 0 | Random effect | |
| Besifloxacin vs moxifloxacin | 368 | 1.04 | 0.58-1.87 | 0.88 | - | - | Random effect | ||
| Besifloxacin vs gatifloxacin | 31 | 1.42 | 0.08-24.95 | 0.81 | - | - | Random effect | ||
| Gram-negative | Besifloxacin vs placebo | 437 | 2.14 | 1.29-3.57 | 0.003 | 0.61 | 0 | Random effect | |
| Besifloxacin vs moxifloxacin | 214 | 1.9 | 0.82-4.41 | 0.14 | - | - | Random effect | ||
| Besifloxacin vs gatifloxacin | 10 | 2.13 | 1.41-3.21 | - | - | - | Random effect | ||
| H. influenzae | Besifloxacin vs placebo | 369 | 2.36 | 1.31-4.23 | 0.004 | 0.34 | 11% | Random effect | |
| Besifloxacin vs moxifloxacin | 169 | 1.17 | 0.44-3.13 | 0.75 | - | - | Random effect | ||
| S. pneumoniae | Besifloxacin vs placebo | 215 | 2.21 | 1.14,4.29 | 0.02 | 0.6 | 0 | Random effect | |
| Besifloxacin vs moxifloxacin | 122 | 1.02 | 0.37-2.85 | 0.97 | - | - | Random effect | ||
| S. aureus | Besifloxacin vs placebo | 210 | 9.53 | 4.47-20.32 | <0.00001 | 0.74 | 0 | Random effect | |
| Besifloxacin vs moxifloxacin | 115 | 1.15 | 0.45-2.96 | 0.78 | - | - | Random effect | ||
| S. epidermidis | Besifloxacin vs placebo | 225 | 9.86 | 3.80-25.59 | <0.0001 | 0.25 | 27% | Random effect | |
| Besifloxacin vs moxifloxacin | 70 | 2.02 | 0.56-7.21 | 0.28 | - | - | Random effect | ||
| Besifloxacin vs gatifloxacin | 7 | 1.67 | 0.05-58.28 | 0.78 | - | - | Random effect | ||
| Clinical resolution | |||||||||
| 4d | |||||||||
| Gram-positive | Besifloxacin vs placebo | 532 | 2.12 | 1.47-3.06 | <0.0001 | 0.82 | 0 | Random effect | |
| Besifloxacin vs moxifloxacin | 368 | 0.91 | 0.60-1.37 | 0.65 | - | - | Random effect | ||
| Gram-negative | Besifloxacin vs placebo | 328 | 1.63 | 0.98-2.69 | 0.06 | 0.31 | 15% | Random effect | |
| Besifloxacin vs moxifloxacin | 214 | 1.2 | 0.68-2.14 | 0.52 | - | - | Random effect | ||
| 8d | |||||||||
| Gram-positive | Besifloxacin vs placebo | 532 | 1.97 | 1.34-2.90 | 0.0005 | 0.4 | 0 | Random effect | |
| Besifloxacin vs moxifloxacin | 368 | 1.14 | 0.67-1.94 | 0.64 | - | - | Random effect | ||
| Gram-negative | Besifloxacin vs placebo | 331 | 2.47 | 1.19-5.13 | 0.02 | 0.18 | 41% | Random effect | |
| Besifloxacin vs moxifloxacin | 214 | 0.81 | 0.35-1.89 | 0.63 | - | - | Random effect | ||
Safety
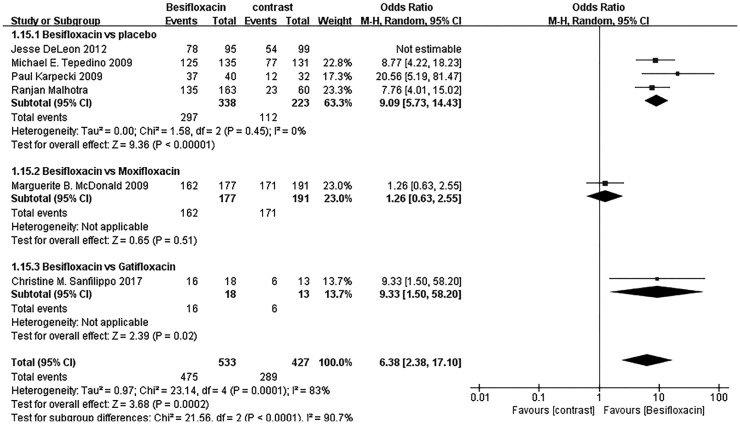
Figure 10 demonstrates there were more people in the placebo group suffering from AEs than those of Besifloxacin group, and no significant difference in AEs risk between Besifloxacin and Moxifloxacin was found.
Figure 10. Estimated odds ratio for the risk of AEs.
Sensitivity Analysis
To find the source of heterogeneity, we performed sensitivity analysis. Studies by Jesse DeLeon et al. was omitted to achieve lower heterogeneity in the comparison of the bacterial eradication rates of Gram-positive bacteria at day 4 (Figure 11).
Figure 11. Sensitivity analysis for the bacterial eradication rates of Gram-positive bacteria at day 4.
DISCUSSION
To our knowledge, this Meta-analysis is the first to summarize the efficacy and safety of besifloxacin for treatment of acute bacterial conjunctivitis. We have extensively searched electronic databases, including PubMed, EMBASE, Web of Science, Cochrane Central Database and CNKI, and 6 RCTs were ultimately included in our Meta-analysis. The forest plot results suggest that compared with placebo, besifloxacin can significantly promote clinical resolution, eradication of bacteria, improved clinical signs, symptoms and cure rates. Moreover, there is no significant difference in the occurrence of AEs. Compared with moxifloxacin and gatifloxacin, besifloxacin showed no significant difference in efficacy and safety.
Clinical resolution is defined as the absence of conjunctival discharge and bulbar conjunctival injection on the assessment days, and the rates of bacterial eradication indicate the absence of all bacterial species on assessment days that were present at or above the threshold before intervention. These two parameters are the most important clinical outcomes used to evaluate the efficacy of treatments for conjunctivitis. We recorded the number of participants who achieved clinical resolution and bacterial eradication. Pooled analyses indicate that besifloxacin is highly effective in enhancing clinical resolution and rates of bacterial eradication, and its efficacy is as high as that of moxifloxacin and gatifloxacin. Nonetheless, we noticed a declining trend in bacterial eradication rates in 3 studies from day 4 to day 8[15],[19],[22], which suggests bacterial resistance to besifloxacin. Since all 3 studies were conducted in 2009, we inferred that the reason may lie in the development of pharmaceutical and study design. Bacterial resistance is of great importance for antibiotics, and its results can change with time. Recent data should be updated to assess the effect of besifloxacin on bacterial eradication rates.
Individual clinical outcomes include ocular conjunctival discharge grading (0=absent, 1=mild, 2=moderate, and 3=severe), bulbar conjunctival injection grading (0=normal, 1=mild, 2=moderate, 3=severe) and cure rates on assessment days. We compared the number of patients who were cured and graded 0. The results of forest plots suggest that besifloxacin can significantly improve individual signs and symptoms and cure acute bacterial conjunctivitis. The cure rates of besifloxacin can be considered the same as those of moxifloxacin. There was relatively high heterogeneity in the comparison of cure rates between besifloxacin versus placebo, and we hypothesized that two reasons may account for this heterogeneity. The sample size and percentage of male participants were obviously different between the two studies. Tepedino et al[19] used data from a modified intent-to-treat population instead of all patients or culture-confirmed patients who completed the study. These two reasons may have influenced our Meta-analysis. However, more studies are necessary to verify our hypothesis. Considering that there were only two studies in this comparison, further high quality studies are needed to lower the heterogeneity and confirm our results.
Our Meta-analysis demonstrates that the clinical resolution and bacterial eradication rates were significantly higher on days 4 and 8 for Gram-positive and Gram-negative species in Besifloxacin-treated patients than in placebo-treated patients, with the exception of Gram-negative species at day 4. Further studies are needed to confirm this phenomenon. One study also presented the results on day 11, which were mostly consistent with those on days 4 and 8[21]. Compared with Moxifloxacin and Gatifloxacin, Besifloxacin presents no overall significant difference in these comparison items, which suggests that the efficacy of Besifloxacin is similar to that of Moxifloxacin and Gatifloxacin. S. epidermidis, H. influenzae, S. aureus, S. treptococcus mitis and S. pneumoniae were the most common species isolated from eyes in eligible studies. We analyzed the eradication rates of four species, and pooled analyses showed that treatment with Besifloxacin is associated with high rates of bacterial eradication of each of these species, the rates is approximate to that of moxifloxacin and gatifloxacin. Even though the clinical resolution and bacterial eradication rates of Besifloxacin were simiar to those of moxifloxacin and gatifloxacin, several included studies determined that 90% of the minimal inhibitory concentration (MIC90) values for Besifloxacin against these clinical species were lower (0.06-0.5 µg/mL) than those for moxifloxacin and gatifloxacin[15],[18], which may reflect besifloxacin's potency against isolates that were resistant to other kinds of antibiotics. Reports have already suggested that there is emerging resistance to the fourth-generation fluoroquinolones Moxifloxacin and Gatifloxacin among ocular pathogens, therefore, it is necessary to develop new antibiotics with improved activity against resistant strains[24]–[25]. Unlike other fluoroquinolones, besifloxacin is being developed exclusively for ophthalmic use. Hence, selective pressure for resistance stemming from systemic use of besifloxacin is not expected to be a factor. This factor, along with its activity against drug-resistant strains and balanced activity against topoisomerase IV and DNA gyrase, may be an important property of besifloxacin in the fight against emerging antibacterial resistance. Moreover, one study suggested that besifloxacin can eradicate bacteria more rapidly than gatifloxacin[14]. However, the sample size in current trials remains somewhat small, further studies are required to verify these properties of besifloxacin. Compared with moxifloxacin and gatifloxacin, besifloxacin has an advantage for the treatment of acute bacterial conjunctivitis in the long interval between its application to eyes. This characteristic makes besifloxacin more convenient for patients. Current studies lack direct comparisons between besifloxacin and other drugs for the efficacy of acute bacterial conjunctivitis, thus, future studies are needed to explore this subject, which is important for clinical decision making.
Common AEs caused by besifloxacin include conjunctivitis, eye pain, blurred vision, and eyelid erythema, however, their occurrence rates were relatively low (all were lower than 5%, and most were lower than 1%). A pooled analysis of safety data from three included clinical studies reported that blurred vision, eye irritation, and conjunctivitis were significantly less frequent in patients treated with besifloxacin than in patients treated with placebo or moxifloxacin[26]. Our Meta-analysis further demonstrates that AE frequency was significantly less in patients treated with Besifloxacin than in patients treated with placebo and there was no significant difference between the Besifloxacin and Moxifloxacin groups in the AE frequency. Further studies are needed to verify this hypothesis.
Even though the methodological quality of nearly all included studies is relatively high, this Meta-analysis has some limitations. First, the intervention methods in the included studies varied, which may influence the results of forest plots. Second, the number of included studies was somewhat small, and there were only 6 included articles. One study compared besifloxacin with moxifloxacin, and 1 study compared besifloxacin with gatifloxacin. The small sample size of the included studies can influence the results of our analysis. In addition, we cannot test the publication bias via funnel plots. Third, there was no recent research that compares besifloxacin and placebo, which may interfere with the results of our Meta-analysis. Fourth, because of our limitations, we could screen only English and Chinese articles.
This Meta-analysis demonstrates the high efficacy and safety of besifloxacin for treatment of acute bacterial conjunctivitis. Besifloxacin can promote the recovery of acute bacterial conjunctivitis and the eradication of bacteria with few AEs and high convenience. However, compared with moxifloxacin and gatifloxacin, current studies do not provide enough evidence for the efficacy of besifloxacin in managing antibiotic-resistant species. Moreover, the small sample size may influence the results of our analysis, and further comparative trials on the efficacy and safety of besifloxacin compared with placebo and other drugs for treatment of acute bacterial conjunctivitis are needed.
Acknowledgments
Conflicts of Interest: Wang JJ, None; Gao XY, None; Li HZ, None; Du SS, None.
REFERENCES
- 1.Sheikh A, Hurwitz B, van Schayck CP, McLean S, Nurmatov U. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2012(9):CD001211. doi: 10.1002/14651858.CD001211.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Alfonso SA, Fawley JD, Alexa Lu X. Conjunctivitis. Prim Care. 2015;42(3):325–345. doi: 10.1016/j.pop.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Haas W, Gearinger LS, Usner DW, Decory HH, Morris TW. Integrated analysis of three bacterial conjunctivitis trials of besifloxacin ophthalmic suspension, 0.6%: etiology of bacterial conjunctivitis and antibacterial susceptibility profile. Clin Ophthalmol. 2011;5:1369–1379. doi: 10.2147/OPTH.S23519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chikviladze D, Nikuradze N, Gachechiladze Kh, Miqeladze M, Metreveli D. Microbial structure of acute bacterial conjunctivitis. Georgian Med News. 2013(216):12–15. [PubMed] [Google Scholar]
- 5.Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86(1):5–17. doi: 10.1111/j.1600-0420.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 6.Andersson J, Hofsli M, Gade UL, Heegaard S, Pottegård A. Use of topical ocular antibiotics in young children: a Scandinavian drug utilization study. Acta Ophthalmol. 2018;96(8):789–794. doi: 10.1111/aos.13813. [DOI] [PubMed] [Google Scholar]
- 7.Ryder EC, Benson S. Conjunctivitis. StatPearls. Treasure Island (FL): StatPearls Publishing; 2019. [Google Scholar]
- 8.Haas W, Pillar CM, Torres M, Morris TW, Sahm DF. Monitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol. 2011;152(4):567–574.e3. doi: 10.1016/j.ajo.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. Antibiotic resistance among ocular pathogens in the United States: five-year results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. JAMA Ophthalmol. 2015;133(12):1445–1454. doi: 10.1001/jamaophthalmol.2015.3888. [DOI] [PubMed] [Google Scholar]
- 10.Bertino JS, Zhang JZ. Besifloxacin, a new ophthalmic fluoroquinolone for the treatment of bacterial conjunctivitis. Expert Opin Pharmacother. 2009;10(15):2545–2554. doi: 10.1517/14656560903213413. [DOI] [PubMed] [Google Scholar]
- 11.Cambau E, Matrat S, Pan XS, Roth Dit Bettoni R, Corbel C, Aubry A, Lascols C, Driot JY, Fisher LM. Target specificity of the new fluoroquinolone besifloxacin in Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli. J Antimicrob Chemother. 2009;63(3):443–450. doi: 10.1093/jac/dkn528. [DOI] [PubMed] [Google Scholar]
- 12.Mah FS, Sanfilippo CM. Besifloxacin: efficacy and safety in treatment and prevention of ocular bacterial infections. Ophthalmol Ther. 2016;5(1):1–20. doi: 10.1007/s40123-016-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller D, Chang JS, Flynn HW, Alfonso EC. Comparative in vitro susceptibility of besifloxacin and seven comparators against ciprofloxacin- and methicillin-susceptible/nonsusceptible staphylococci. J Ocul Pharmacol Ther. 2013;29(3):339–344. doi: 10.1089/jop.2012.0081. [DOI] [PubMed] [Google Scholar]
- 14.Haas W, Gearinger LS, Hesje CK, Sanfilippo CM, Morris TW. Microbiological etiology and susceptibility of bacterial conjunctivitis isolates from clinical trials with ophthalmic, twice-daily besifloxacin. Adv Ther. 2012;29(5):442–455. doi: 10.1007/s12325-012-0023-y. [DOI] [PubMed] [Google Scholar]
- 15.Karpecki P, Depaolis M, Hunter JA, White EM, Rigel L, Brunner LS, Usner DW, Paterno MR, Comstock TL. Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: A multicenter, prospective, randomized, double-masked, vehicle-controlled, 5-day efficacy and safety study. Clin Ther. 2009;31(3):514–526. doi: 10.1016/j.clinthera.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Sanfilippo CM, Allaire CM, DeCory HH. Besifloxacin ophthalmic suspension 0.6% compared with gatifloxacin ophthalmic solution 0.3% for the treatment of bacterial conjunctivitis in neonates. Drugs R D. 2017;17(1):167–175. doi: 10.1007/s40268-016-0164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter NJ, Scott LJ. Besifloxacin ophthalmic suspension 0.6% Drugs. 2010;70(1):83–97. doi: 10.2165/11203820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Silverstein BE, Allaire C, Bateman KM, Gearinger LS, Morris TW, Comstock TL. Efficacy and tolerability of besifloxacin ophthalmic suspension 0.6% administered twice daily for 3 days in the treatment of bacterial conjunctivitis: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study in adults and children. Clin Ther. 2011;33(1):13–26. doi: 10.1016/j.clinthera.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Tepedino ME, Heller WH, Usner DW, Brunner LS, Morris TW, Haas W, Paterno MR, Comstock TL. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr Med Res Opin. 2009;25(5):1159–1169. doi: 10.1185/03007990902837919. [DOI] [PubMed] [Google Scholar]
- 20.DeLeon J, Silverstein BE, Allaire C, Gearinger LS, Bateman KM, Morris TW, Comstock TL. Besifloxacin ophthalmic suspension 0.6% administered twice daily for 3 days in the treatment of bacterial conjunctivitis in adults and children. Clin Drug Investig. 2012;32(5):303–317. doi: 10.2165/11632470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra R, Ackerman S, Gearinger LS, Morris TW, Allaire C. The safety of besifloxacin ophthalmic suspension 0.6 % used three times daily for 7 days in the treatment of bacterial conjunctivitis. Drugs R D. 2013;13(4):243–252. doi: 10.1007/s40268-013-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald MB, Protzko EE, Brunner LS, Morris TW, Haas W, Paterno MR, Comstock TL, Usner DW. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. Ophthalmology. 2009;116(9):1615–1623.e1. doi: 10.1016/j.ophtha.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvester A, Neal T, Czanner G, Briggs M, Harding S, Kaye S. Adult bacterial conjunctivitis: resistance patterns over 12 years in patients attending a large primary eye care centre in the UK. BMJ Open Ophthalmol. 2017;1(1):e000006. doi: 10.1136/bmjophth-2016-000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrestha SP, Khadka J, Pokhrel AK, Sathian B. Acute bacterial conjunctivitis - antibiotic susceptibility and resistance to commercially available topical antibiotics in Nepal. Nepal J Ophthalmol. 2016;8(15):23–35. doi: 10.3126/nepjoph.v8i1.16153. [DOI] [PubMed] [Google Scholar]
- 26.Comstock TL, Paterno MR, Bateman KM, Decory HH, Gearinger M. Safety and tolerability of loteprednol etabonate 0.5% and tobramycin 0.3% ophthalmic suspension in pediatric subjects. Paediatr Drugs. 2012;14(2):119–130. doi: 10.2165/11596320-000000000-00000. [DOI] [PubMed] [Google Scholar]