Abstract
AIM
To examine the therapeutic effects of tocilizumab on experimental corneal transplantation and its effect on Treg/Th17 balance.
METHODS
Allograft corneal graft was performed between host Sprague Dawley and Wistar donor rats. The rats were randomly divided into four groups: normal, autograft, allograft, and allograft treated with tocilizumab. Kaplan-Meier was performed to draw the survival curve. The protein levels of interleukin-17A (IL-17A), vascular endothelial growth factor (VEGF), and forkhead box protein 3 (Foxp3) were measured by immunohistochemistry. The mRNA levels of IL-17A, VEGF, retinoid-related orphan receptor gammat (RORγt), interleukin-6 (IL-6) and Foxp3 were detected by reverse transcription real-time polymerase chain reaction (RT-PCR). The Treg and Th17 cells were investigated by flow cytometry.
RESULTS
The survival time of tocilizumab group was (24±1.27d) longer than that of allograft group (10±0.55d). Moreover, immunohistochemical examination revealed that IL-17A and VEGF protein levels in the allograft group were significantly higher than that of tocilizumab group (P<0.01), while Foxp3 levels in the allograft group was significantly lower than that of the tocilizumab treated group (P<0.001). Flow cytometry showed that the number of Th17 cells in allograft group was significantly higher than that in tocilizumab group (P<0.001). Meanwhile, the number of Tregs was significantly lower than in tocilizumab group (P<0.001). Simultaneously, Foxp3 mRNA expression level in corneal tissues of tocilizumab treated group was significantly higher than other groups (P<0.001).
CONCLUSION
These findings suggest that tocilizumab may promote corneal allograft survival, possibly by modulating Treg-Th17 balance.
Keywords: tocilizumab, corneal transplantation, Th17/Treg, rats
INTRODUCTION
Corneal transplantation is the most commonly used treatment for end-stage corneal diseases. Despite corneal immune privilege and anterior chamber-associated immune deviation, immune rejection is still the major limiting factor for corneal grafting. This is specifically seen in patients with high-risk corneal transplantation during corneal vascularization, repeated transplantation, alkali burn, which had almost less than 54.2% long-term survival rate[1]–[3]. Therefore, prevention and treatment of corneal graft rejection is still an ongoing and important issue in clinical research. Clinically, medicines that suppress the rejection have been applied to improve corneal transplantation success rate and long-term survival rate of the graft, but are still limited due to drug tolerance in certain patients, recurrence of rejection, high costs and also severe toxicity and side effects. Thus, there is a pressing need to screen for immunosuppressive agents with high efficiency and low toxicity. Recently, interleukin-6 (IL-6) has been considered as a key mediator in the pathogenesis of graft-versus-host diseases (GVHD)[4]–[5]. The pathway of IL-6/IL-6 receptor (IL-6/IL-6R) signaling regulates various biological process, including cell growth and differentiation, as well as immune and hematopoietic systems[6]. Tocilizumab is a recombinant monoclonal antibody specific to IL-6R, and is originally used for the treatment of rheumatoid arthritis[7]. According to previous studies, tocilizumab could reduce Th17 cell proportion, and increase Treg cell proportion, thus changing the Th17/Treg balance in patients with rheumatoid arthritis[8]–[10]. Recent reports have demonstrated that tocilizumab may reduce the severity of GVHD in steroid-refractory GVHD[11]–[12]. A previous study demonstrated that tocilizumab could prolong the survival time of islet transplantation and protect the function of islet after transplantation[13]. Some ophthalmological studies also reported that tocilizumab could reduce the formation of corneal neovascularization[14]–[15]. However, whether tocilizumab could affect the immune mediated corneal graft rejection is unknown. Hence, this study was conducted to explore this question.
MATERIALS AND METHODS
Ethical Approval
All rats were housed in a specific pathogen-free (SPF) environment. Animal experiments were approved by the Nanfang Hospital Animal Ethics Committee and adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Animals and Materials
Wistar rats served as hosts, and accepted corneal grafts from either Sprague-Dawley (SD) rats (autograft) or Wistar rats (allograft). Female rats, aged 6-8wk and weighing 180-220 g, were purchased from the Experimental Animal Centre of Southern Medical University. There are 9 Wistar rats in normal group. In each of the other 3 groups, there are 24 Wistar rats in each group respectively.
Anesthesia was carried out by injecting 3% thioethamyl (1.5 mL/kg). Tropicamide, a compound of mydriatics, eye drops and 10-0 nylon line was purchased from Ethicon (USA). Tocilizumab was purchased from Roche (Switzerland). The antibodies for immunohistochemistry were purchased from Abcam (UK) and Santa Cruz (USA). The antibodies for flow cytometry were purchased from Ebioscience (USA). RNA extraction, reverse transcription real-time polymerase chain reaction (RT-PCR) kits were purchased from Takara (Japan).
Corneal Transplantation and Post-transplant Therapies
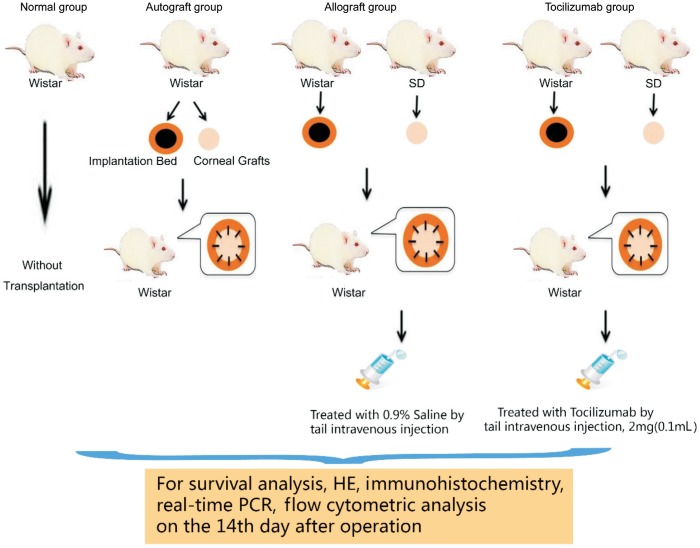
Penetrating orthotopic corneal transplantation was performed as previously described[16]. Briefly, a 3.5-mm central area of the cornea was excised from the donor and secured in the host graft bed of 3.0-mm diameter with 8-10 interrupted 10-0 nylon sutures. The occurrence of hyphema, synechia and cataract in rats during the operation were regarded as failed cases. The failed experimental animals were removed and supplemented with new ones. Four groups were included: normal, autograft, allograft, allograft treated with tocilizumab by tail vein injection intravenously, 2 mg (0.1 mL) (as group tocilizumab). In the allograft rats group, same amount of 0.9% saline was injected (as group allograft). In Wistar rats group, there was no intervention conducted, and served as normal controls. Specific grouping and schematic representation of the experiment were shown in Figure 1.
Figure 1. Schematic representation of the experiment.
Rejection Observation and Judgment Standard
After the operation, 15 grafts from each group were randomly selected for clinical evaluation of rejection. According to the scoring method of Larkin[17], the corneal rejection score was recorded. Rejection was defined by a total score of not less than 5 points or an opacity score of over 3 points. Long-term survival was defined by no sign of rejection for more than 100d.
Hematoxylin-eosin Staining and Immunohistochemistry
The eyeballs (3 rats for each group) with transplantation were taken out after the rats were sacrificed. These were then fixed with 4% polyformaldehyde solution, a gradient dehydrated with alcohol, followed by embedding in paraffin, and then were cut into 4 µm slices. Some slices were selected for staining by hematoxylin-eosin (H&E) and the corneal thickness and inflammatory cell infiltration under microscopy were observed. Some slices were used for immunohistochemistry by using rabbit anti-rat IL-17A antibody (sc7927, Santa Cruz), mouse anti-rat VEGF antibody (ab22510, abcam), mouse anti-rat Foxp3 antibody (ab22510, abcam) as primary antibodies. Goat anti-mouse antibody (ab6788, abcam), goat anti-rabbit antibody (ab97049, abcam) were used as secondary antibodies. Experiments that used phosphate buffer solution (PBS), instead of primary antibody, were set as negative controls. Using SP three step method, antigen repair by heat was performed. The specimens were observed under microscopy after BAD staining and mounting, and then photographs were taken by using the 400× field of vision (Olympus digital camera, Japan). Using Imagepro-Plus software, the cumulative integrated optical density [IOD(sum)] and corneal tissue area were calculated by the formula: IOD(mean)=IOD(sum)/area.
Quantitative Reverse Transcription Polymerase Chain Reaction
Corneal grafts (3 rats for each group) were taken out after the rats were sacrificed, then total RNA were extracted from the grafts by Trizol and RNA concentration was measure by D260/D280 value using Nanodrop. RNA were then used for cDNA synthesis using reverse transcriptase kit (Cat: RR037A, Takara). Rat GAPDH was used as endogenous control. The primer pair sequences used for the PCRs were: 5′-ACCACAGTCCATGCCATCAC-3′, and 5′-TCCACCACCCTGTTGCTGAT-3′ for rat GAPDH, 5′-TGCTGCTACTGAACCTGGAG-3′, and 5′-GCGTTTGGACACACTGAACT-3′ for rat IL-17A, 5′-GACAGGGCCCCACAGAGA-3′, 5′-TTTGTGAGGTGTGGGTCTTCTTT-3′ for rat RORγt, 5′-AGTGGCAGGGAAGGAGTGTC-3′, and 5′-TTCCAAGTCTCGTGTGAAGGC-3′ for rat Foxp3, 5′-GGCCTCTGAAACCATGAACT-3′, and 5′-TGAACTTCACCACTTGGCAT-3′ for rat VEGF, 5′-ATTCTGTCTCGAGCCCACCA-3′, and 5′-GGAAGGCAGTGGCTGTCAAC-3′ for rat IL-6. The PCR reaction solution was prepared according to the instructions of the qPCR kit (Cat: RR420A, Takara) and PCR analysis were carried out with 7500 PCR instrument (ABI, USA). Transcript quantification was performed in triplicate for each sample.
Flow Cytometric Analysis
Fourteen days after operation, 3 rats were randomly selected from each group, anaesthetized by 3% thioethamyl (1.5 mL/kg) intraperitoneally, and then 5 mL blood sample was collected from the heart by blood collection tube containing heparin. Lymphocyte was separated in the ultra clean cabinet by lymphocyte separation medium (LTS1083, TBD, China). Each lymphocyte was evenly divided into 2 samples (samples A and B). Sample A was placed in a 1640 culture medium containing 10% fetal bovine serum and 4 µL (2 µL/mL) cell stimulation cocktail (plus protein transport inhibitors; 85-00-4975-93, eBioscience, USA). The lymphocyte was cultured in cell incubation box at 37°C with 5% CO2 for 6h. Subsequently, the lymphocytes were collected by centrifugation at 2000 rpm and were divided into 3 tubes (experimental tube lymphocyte, ISO control tube lymphocyte and empty tube lymphocyte), followed by the addition of anti-rat CD4 FITC (empty tube lymphocyte were added nothing) and then placing at 4°C environment for 30min in dark. After that, Foxp3/transcription factor staining buffer (85-00-5523-00, eBioscience, USA) was added to the lymphocytes. After maintaining for 30min, the anti-mouse/rat IL-17A PE were added in the experimental tube lymphocytes, rat IgG2a K isotype control PE were added in the ISO control tube, and nothing in the empty tube lymphocytes. After 30min, the unbinding antibodies were washed by PBS. Finally, the percentage of Th17 (CD4+/IL-17A+) in CD4+ cells was tested by BD FACSCalibur (BD, USA). Sample B was also divided into 3 tubes (experimental tube lymphocyte, ISO control tube lymphocyte and empty tube lymphocyte) and no stimulation and culturing were performed. Anti-rat CD4 FITC was added into experimental tube lymphocytes and anti-rat CD4 FITC and mouse IgG1 K isotype control APC were added into anti-rat CD25 APC, ISO control tube lymphocytes, and nothing in the empty tube lymphocytes. All the tubes in sample B were maintained at 4°C for 30min in dark. Then, Foxp3/transcription factor staining buffer was added to the lymphocytes. After 30min, the anti-mouse/rat Foxp3 PE were added in the experimental tube lymphocytes, rat IgG2a K isotype control PE were added in the ISO control tube lymphocytes, and nothing in the empty tube lymphocytes. After 30min, the unbinding antibodies were washed away by PBS. Finally, the percentage of Treg (CD4+/CD25+/Foxp3+) in CD4+ cells was measured by BD FACSCalibur. All antibodies in flow cytometric analysis were purchased from eBioscience company (USA).
Statistical Analysis
The experimental data were analyzed by IBM SPSS Statistics 20 and GraphPad Prism software. One-way ANOVA was performed to test the variation of corneal neovascularization area, mRNA and protein expression levels, as well as the ratio of Th17/Treg in each group.
RESULTS
Clinical Observation of Rejection and Draw Survival Curve
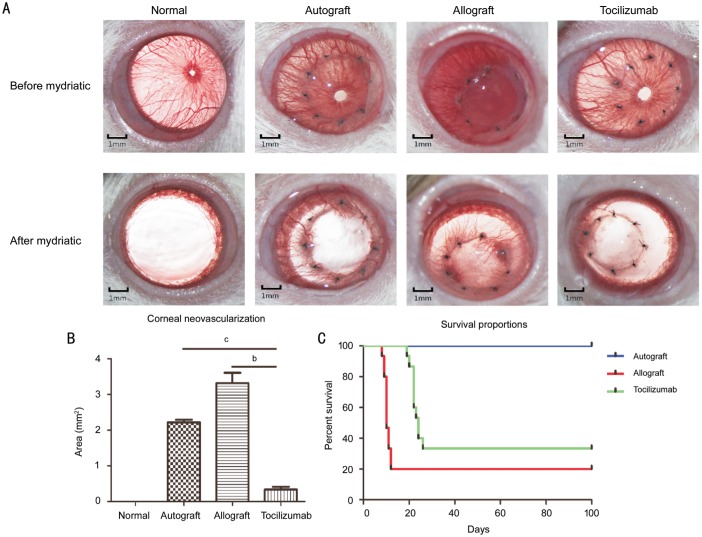
After operation, all rats were examined by slit-lamp microscopy on alternate days to calculate the rejective indices (RIs) according to opacity, edema, and neovascularization of grafts. According to the survival curve (Figure 2C), the survival time of tocilizumab group was 24±1.27d, while the survival time of allograft was 10±0.55d. After mydriasis (Figure 2A), the results showed that the neovascularization area in autograft and allograft groups were 2.21±0.19 mm2 and 3.31±0.66 mm2, respectively with Imagepro-Plus software, and were significantly higher than that of tocilizumab group (0.33±0.17 mm2, P<0.01; Figure 2B). On day 14 after operation, the intact cornea was cut from the limbus by puncturing the limbus with a bayonet, and stored at -80°C.
Figure 2. Clinical observation of corneal graft after corneal transplantation.
A: The appearance of corneal grafts after transplantation. Fourteen days after transplantation, the opacity, area of neovascularization and edema were observed under the microscopy before and after mydriasis. B: Corneal neovascularization area in each group after mydriasis (n=15). bP<0.01, cP<0.001. C: Survival percentages after surgery. On postoperative day 100, the corneal graft and the rejection score were recorded for drawing the survival curves (n=15).
Histopathological and Immunohistochemical Analysis
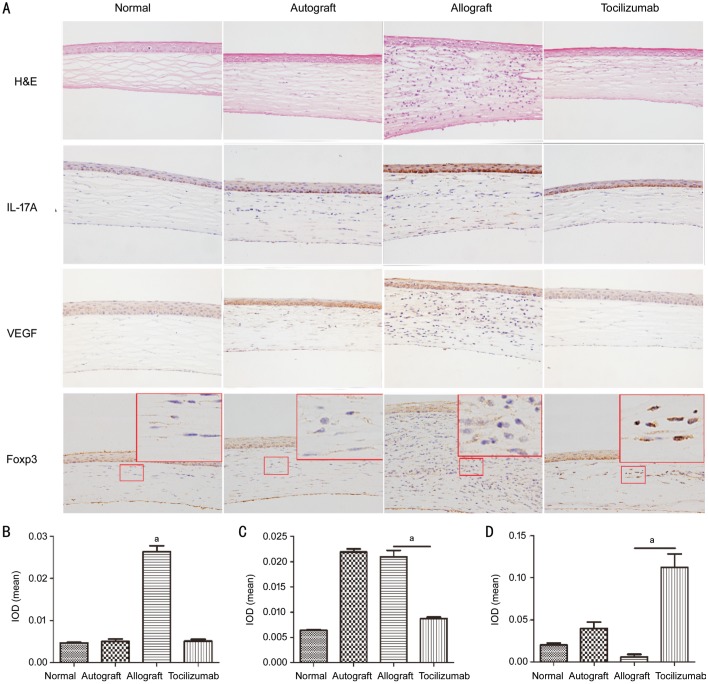
In the normal group, the structure of the cornea was clear, and there was no blood vessel and lymphocyte infiltration. As shown in Figure 3A, 14d after surgery, the corneal structures in the autograft group were clear, but showed a few new blood vessels and slight inflammatory cell infiltration in the stroma. In the allograft group, the corneal graft was thickened with a large number of lymphocytes infiltrated, and new blood vessels were observed in the stroma. More importantly, the corneal structures in the tocilizumab group were regular with individual lymphocytes, but without any obvious vascular cavity. Immunohistochemistry revealed that IL-17A and VEGF were mainly expressed in the corneal epithelial and stromal layers, and Foxp3 was predominantly expressed in the nucleus of stromal layer cells. The expressions of IL-17A and VEGF in the cornea of allograft group were significantly higher than that of the tocilizumab group, while the expression of Foxp3 was lower than that of the tocilizumab group. The corneal grafts of autograft group also exhibited high levels of VEGF expression. The results of mean IOD detected by Imagepro-Plus software were presented in Figures3B-3D, showing variations in similar expression pattern by visual observation. The mean IL-17A IOD of the allograft group and tocilizumab group were 0.026±0.002 and 0.005±0.001, respectively (P<0.05). Moreover, the mean VEGF IOD of autograft group and allograft group were 0.022±0.001 and 0.021±0.002, respectively, while that of the tocilizumab group was 0.009±0.001 (P<0.05). The mean Foxp3 IOD of allograft group was 0.006±0.006, while that of tocilizumab group was as high as 0.112±0.032 (P<0.05).
Figure 3. H&E staining and immunohistochemistry analysis of gene expression.
A: H&E staining and immunohistochemistry paraffin sections under 400× microscopy. The red box in Foxp3 immunohistochemistry means that the local areas were enlarged by ten times, showing better nuclear staining. B-D: The cartogram of mean IOD values of IL-17A, VEGF and Foxp3 expressions (n=3). aP<0.05.
The Relative mRNA Levels of IL-17A, RORγt, VEGF, IL-6 and Foxp3 in Corneal Grafts
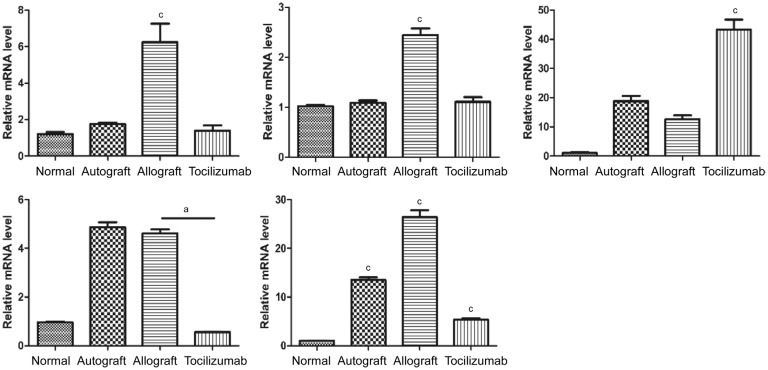
The expression levels of IL-17A, RORγt, VEGF and IL-6 in the corneal grafts of allograft group were significantly elevated when compared with those of the normal group, and autograft group also showed VEGF accumulation in the cornea. Compared to allograft group, the expressions of IL-17A, RORγt, VEGF and IL-6 in tocilizumab group were all decreased to different extents. The expression of Foxp3 gene in tocilizumab group was remarkably higher than that of the other three groups (P<0.001; Figure 4).
Figure 4. Expression levels of IL-17A, RORγt, Foxp3, VEGF and IL-6 in corneal graft.
Fourteen days after transplantation, the relative expression levels of IL-17A, RORγt, Foxp3, VEGF and IL-6 genes in each corneal graft group (n=3). aP<0.05, cP<0.001.
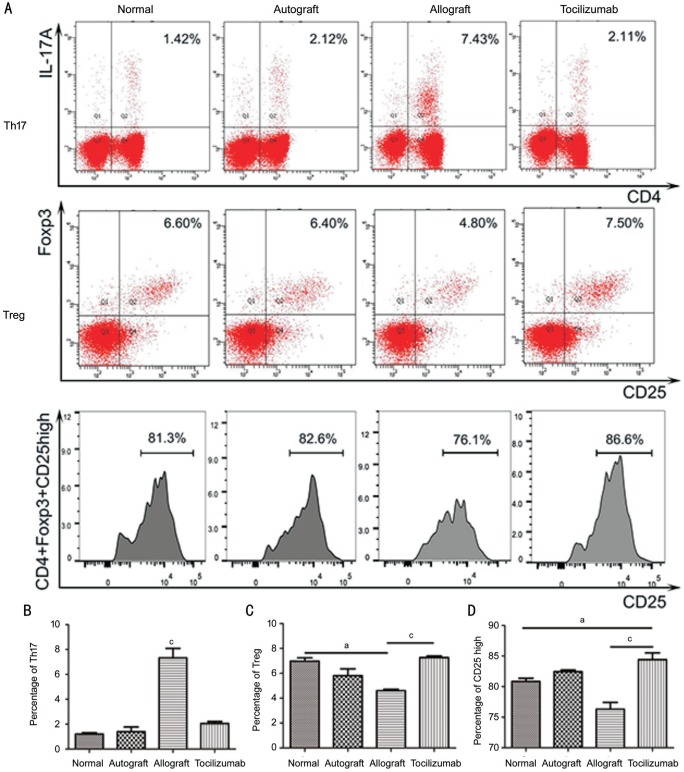
Flow Cytometry Analysis
On day 14 after transplantation, the percentages of Th17 cells in CD4+ cells of rat blood between the normal group and autograft group showed no significant difference, which were 1.20%±0.19% and 1.40%±0.66% respectively. In the allograft group, it was found to be 7.32%±1.33%, and was significantly higher than that in normal group (P<0.001), while it was only 2.05%±0.29% in tocilizumab group, showing no significant elevation (P=0.21). The tocilizumab group had the highest percentage of Treg cells in CD4+ cells of rat blood (7.27%±0.21%), followed by normal group (6.96%±0.47%) and autograft group (5.80%±0.95%). The allograft group had the lowest percentage (4.60%±0.20%), showing significant differences when compared with the tocilizumab group (P<0.001). The percentage of CD4+/CD25high/Foxp3+ cells in CD4+/CD25+/Foxp3+ cells was also different from each group with the tocilizumab group, exhibiting the highest (84.40%±1.91%), and are higher than those in the normal group (80.83%±0.89%, P<0.05) and the allograft group (76.30%±0.91%, P<0.001; Figure 5).
Figure 5. Analysis of blood lymphocytes by flow cytometry on postoperative day 14.
A: Flow type scatter plot of Th17 (CD4+/IL-17A+) and Treg (CD4+/CD25+/Foxp3+) and the cell count of CD4+/CD25high/Foxp3+ Tregs; B: Percentages of CD4+/IL-17A+ Th17 cells in CD4+ cells (n=3); C: Percentages of CD4+/CD25+/Foxp3+ Treg cells in CD4+ cells (n=3); D: Percentages of CD4+/CD25high/Foxp3+ Treg cells in CD4+/CD25+/Foxp3+ Treg cells (n=3). aP<0.05, cP<0.001.
DISCUSSION
The immune response following corneal transplantation is a complicated process, and infiltration of inflammatory cells and neovascularization at the implantation site after transplantation are major risk factors of corneal graft rejection[3]. Common immunosuppressive drugs may effectively inhibit rejection, but are associated with side effects such as renal and liver toxicities. Tocilizumab is a recombinant humanized monoclonal antibody against IL-6R that specifically binds with IL-6R and blocks signal transduction of IL-6R to signal transducers and activators of transcription 3 (STAT3). Besides, it is safe with less toxicity and side effects, no immunogenicity and no induction of immune response[7].
The present study found that tocilizumab could significantly prolong the survival time of corneal grafts, shifting the Th17/Treg balance. Th17 cells, which are a subset of CD4+ T cells with a role in autoimmunity, have been implicated as main players in the acute phase of allograft rejection[18]–[19]. Conversely, CD4+/CD25+/Foxp3+ regulatory T cells (Treg), which maintain immune homeostasis, play a vital role in protecting grafts from immune rejection[20]. Studies have shown that IL-6 and transforming growth factor β (TGF-β) regulate the differentiation of T helper cell precursors (Thp) into Th17. When IL-6 levels are low, TGF-β induces differentiation of Thp into Treg[21]. Meanwhile, due to blockage of IL-6 signaling pathway and unaffected TGF-β levels, Thp cells were inclined to differentiate into Tregs[18],[21]. Therefore, we hypothesized that tocilizumab might induce the biological activity loss of IL-6R by specifically binding with IL-6R, thus blocking the IL-6 signaling pathway. It has been observed that interruption of signalling transduction might induce downstream phosphorylation of STAT3, decrease in RORγt expression, eventually decreasing IL-17A expression and reducing Th17 cell number and activity[22]–[25]. Therefore, tocilizumab could prolong the survival time of the graft by shifting the balance of Th17/Treg cells[18].
Corneal neovascularization is an important risk factor of rejection after corneal transplantation[26]. According to a previous study, IL-17A played an important role in the formation of corneal neovascularization, as it could promote the growth of corneal neovascularization by destroying the VEGF-A/sVEGFR-1 balance in the cornea, and blocking of IL-17A could suppress corneal neovascularization and inflammatory cell infiltration as well[27]. Another study showed that tocilizumab could affect the expression of matrix metalloproteinases and basic fibroblast growth factor by reducing the phosphorylation of STAT3, and then by down-regulating the content of VEGF in the cornea, thus suppressing the formation of corneal neovascularization[14]. Therefore, the use of tocilizumab also prevents corneal graft rejection by reducing corneal neovascularization.
Interestingly, we observed that the expression of corneal IL-6 was also reduced when treated with tocilizumab to block IL-6R in this experiment. This may be due to that the Th17 cells could secrete IL-6, and so tocilizumab could reduce the secretion of IL-6 by inhibiting Th17[21]. The reduction of IL-6 in turn reduces the rejection of graft and formation of corneal neovascularization. The results showed that tocilizumab can prolong corneal allograft survival by increasing the proportion of CD4+/CD25high/Foxp3+ Treg cells.
It has been widely acknowledged that IL-17A secreted by Th17 plays a partial role in rejection of liver, kidney and other organs transplantation[28]–[31]. But in corneal transplantation, the role of IL-17A still remains controversial. Our study observed that IL-17A expression was increased in allograft group, while it decreased in tocilizumab group. Hence we supposed that IL-17A could promote corneal graft rejection. Some previous studies have claimed that IL-17A promotes transplant rejection, and anti-IL-17 therapy restricts and reverses late-term corneal allo-rejection[18],[32]–[34]. However, some studies have demonstrated that IL-17A could promote graft survival via promoting immune privilege and anterior chamber associated immune deviation[35]–[36]. Since tocilizumab can effect numerous cytokines except IL-17A in this study, further studies are required to clarify its mechanism.
In conclusion, our findings provide experimental evidences for potential clinical application of tocilizumab in corneal graft. However, the effects of tocilizumab on Th17/Treg balance in vitro were not tested, which should done in future studies.
In summary, tocilizumab may promote corneal allograft survival, possibly by modulating Treg-Th17 balance. This may be a novel approach for inhibiting transplant rejection.
Acknowledgments
Foundations: Supported by Science and Technology Planning Project of Guangdong Province (No.2017A020211005); Science and Technology Programme of Guangzhou, China 2016 (No.201607010386).
Conflicts of Interest: Wu XS, None; Lu XL, None; Wu J, None; Ma M, None; Yu J, None; Zhang ZY, None.
REFERENCES
- 1.Qazi Y, Hamrah P. Corneal allograft rejection: immunopathogenesis to therapeutics. J Clin Cell Immunol. 2013;2013(Suppl 9):006. doi: 10.4172/2155-9899.S9-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niederkorn JY. Corneal transplantation and immune privilege. Int Rev Immunol. 2013;32(1):57–67. doi: 10.3109/08830185.2012.737877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu T, Rajendran V, Griffith M, Forrester JV, Kuffová L. High-risk corneal allografts: a therapeutic challenge. World J Transplant. 2016;6(1):10–27. doi: 10.5500/wjt.v6.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, Drobyski WR. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114(4):891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tawara I, Koyama M, Liu C, Toubai T, Thomas D, Evers R, Chockley P, Nieves E, Sun YP, Lowler KP, Malter C, Nishimoto N, Hill GR, Reddy P. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011;17(1):77–88. doi: 10.1158/1078-0432.CCR-10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86(4):1243–1254. [PubMed] [Google Scholar]
- 7.Sebba A. Tocilizumab: the first interleukin-6-receptor inhibitor. Am J Health Syst Pharm. 2008;65(15):1413–1418. doi: 10.2146/ajhp070449. [DOI] [PubMed] [Google Scholar]
- 8.Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J, Ornetti P, Maillefert JF, Miossec P, Bonnotte B. Brief Report: Inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64(8):2499–2503. doi: 10.1002/art.34477. [DOI] [PubMed] [Google Scholar]
- 9.Pesce B, Soto L, Sabugo F, Wurmann P, Cuchacovich M, López MN, Sotelo PH, Molina MC, Aguillón JC, Catalán D. Effect of interleukin-6 receptor blockade on the balance between regulatory T cells and T helper type 17 cells in rheumatoid arthritis patients. Clin Exp Immunol. 2013;171(3):237–242. doi: 10.1111/cei.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi J, Hashizume M, Kaneko Y, Yoshimoto K, Nishina N, Takeuchi T. Peripheral blood CD4(+)CD25(+)CD127(low) regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: increase in regulatory T cells correlates with clinical response. Arthritis Res Ther. 2015;17:10. doi: 10.1186/s13075-015-0526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gergis U, Arnason J, Yantiss R, Shore T, Wissa U, Feldman E, Woodworth T. Effectiveness and safety of tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, in a patient with refractory GI graft-versus-host disease. J Clin Oncol. 2010;28(30):e602–e604. doi: 10.1200/JCO.2010.29.1682. [DOI] [PubMed] [Google Scholar]
- 12.Drobyski WR, Pasquini M, Kovatovic K, Palmer J, Douglas Rizzo J, Saad A, Saber W, Hari P. Tocilizumab for the treatment of steroid refractory graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17(12):1862–1868. doi: 10.1016/j.bbmt.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahraoui A, Kloster-Jensen K, Ueland T, Korsgren O, Foss A, Scholz H. Anakinra and tocilizumab enhance survival and function of human islets during culture: implications for clinical islet transplantation. Cell Transplant. 2014;23(10):1199–1211. doi: 10.3727/096368913X667529. [DOI] [PubMed] [Google Scholar]
- 14.Yoo AR, Chung SK. Effects of subconjunctival tocilizumab versus bevacizumab in treatment of corneal neovascularization in rabbits. Cornea. 2014;33(10):1088–1094. doi: 10.1097/ICO.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 15.Sari ES, Yazici A, Aksit H, Yay A, Sahin G, Yildiz O, Ermis SS, Seyrek K, Yalcin B. Inhibitory effect of sub-conjunctival tocilizumab on alkali burn induced corneal neovascularization in rats. Curr Eye Res. 2015;40(1):48–55. doi: 10.3109/02713683.2014.914541. [DOI] [PubMed] [Google Scholar]
- 16.Williams KA, Coster DJ. Penetrating corneal transplantation in the inbred rat: a new model. Invest Ophthalmol Vis Sci. 1985;26(1):23–30. [PubMed] [Google Scholar]
- 17.Larkin DF, Calder VL, Lightman SL. Identification and characterization of cells infiltrating the graft and aqueous humour in rat corneal allograft rejection. Clin Exp Immunol. 1997;107(2):381–391. doi: 10.1111/j.1365-2249.1997.279-ce1171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Wang WT, Xu JJ, Wu SQ, Le QH. All-trans retinoid acid promotes allogeneic corneal graft survival in mice by regulating Treg-Th17 balance in the presence of TGF-Β. BMC Immunol. 2015;16:17. doi: 10.1186/s12865-015-0082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan H, Li LX, Han DD, Kou JT, Li P, He Q. Increase of peripheral Th17 lymphocytes during acute cellular rejection in liver transplant recipients. HBPD INT. 2012;11(6):606–611. doi: 10.1016/s1499-3872(12)60231-8. [DOI] [PubMed] [Google Scholar]
- 20.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 21.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148(1):32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ataie-Kachoie P, Pourgholami MH, Morris DL. Inhibition of the IL-6 signaling pathway: a strategy to combat chronic inflammatory diseases and cancer. Cytokine Growth Factor Rev. 2013;24(2):163–173. doi: 10.1016/j.cytogfr.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao ZJ, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu JF, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O'Shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon JH, Sudo K, Kuroda M, Kato M, Lee IK, Han JS, Nakae S, Imamura T, Kim J, Ju JH, Kim DK, Matsuzaki K, Weinstein M, Matsumoto I, Sumida T, Mamura M. Phosphorylation status determines the opposing functions of Smad2/Smad3 as STAT3 cofactors in TH17 differentiation. Nat Commun. 2015;6:7600. doi: 10.1038/ncomms8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dohlman TH, Omoto M, Hua J, Stevenson W, Lee SM, Chauhan SK, Dana VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation. 2015;99(4):678–686. doi: 10.1097/TP.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 27.Suryawanshi A, Veiga-Parga T, Reddy PB, Rajasagi NK, Rouse BT. IL-17A differentially regulates corneal vascular endothelial growth factor (VEGF)-A and soluble VEGF receptor 1 expression and promotes corneal angiogenesis after herpes simplex virus infection. J Immunol. 2012;188(7):3434–3446. doi: 10.4049/jimmunol.1102602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung BH, Kim KW, Kim BM, Doh KC, Cho ML, Yang CW. Increase of Th17 cell phenotype in kidney transplant recipients with chronic allograft dysfunction. PLoS One. 2015;10(12):e0145258. doi: 10.1371/journal.pone.0145258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma L, Zhang HM, Hu KB, Lv G, Fu YW, Ayana DA, Zhao PW, Jiang YF. The imbalance between Tregs, Th17 cells and inflammatory cytokines among renal transplant recipients. BMC Immunol. 2015;16:56. doi: 10.1186/s12865-015-0118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serody JS, Hill GR. The IL-17 differentiation pathway and its role in transplant outcome. Biol Blood Marrow Transplant. 2012;18(1 Suppl):S56–S61. doi: 10.1016/j.bbmt.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwan T, Chadban SJ, Ma J, Bao S, Alexander SI, Wu H. IL-17 deficiency attenuates allograft injury and prolongs survival in a murine model of fully MHC-mismatched renal allograft transplantation. Am J Transplant. 2015;15(6):1555–1567. doi: 10.1111/ajt.13140. [DOI] [PubMed] [Google Scholar]
- 32.Heidt S, San D, Chadha R, Wood KJ. The impact of Th17 cells on transplant rejection and the induction of tolerance. Curr Opin Organ Transplant. 2010;15(4):456–461. doi: 10.1097/MOT.0b013e32833b9bfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen HY, Wang WL, Xie HY, Xu X, Wu J, Jiang ZJ, Zhang ML, Zhou L, Zheng SS. A pathogenic role of IL-17 at the early stage of corneal allograft rejection. Transpl Immunol. 2009;21(3):155–161. doi: 10.1016/j.trim.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Yin XT, Zobell S, Jarosz JG, Stuart PM. Anti-IL-17 therapy restricts and reverses late-term corneal allorejection. J Immunol. 2015;194(8):4029–4038. doi: 10.4049/jimmunol.1401922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunnusamy K, Chen PW, Niederkorn JY. IL-17 promotes immune privilege of corneal allografts. J Immunol. 2010;185(8):4651–4658. doi: 10.4049/jimmunol.1001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunnusamy K, Chen PW, Niederkorn JY. IL-17A-dependent CD4+CD25+ regulatory T cells promote immune privilege of corneal allografts. J Immunol. 2011;186(12):6737–6745. doi: 10.4049/jimmunol.1100101. [DOI] [PMC free article] [PubMed] [Google Scholar]