Abstract
Background
There is increasing demand for compounds to treat antimicrobial-resistant pathogens, and essential oils have gained interest. Moreover, previous studies have demonstrated antimicrobial activity of these nonpharmaceutical products. We investigated the activity of essential oils against multiresistant bacteria and other clinical isolates to evaluate the potential of their use topically and/or internally for treatment of bacterial infections.
Methods
We studied the in vitro activity of 10 essential oils and 1 essential oil blend against clinical isolates including extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, multidrug-resistant Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus.
Results
Essential oils of oregano, thyme, cinnamon bark, and lemongrass had the largest zones of inhibition against Gram-positive organisms, whereas cinnamon bark had the largest zone of inhibition against P aeruginosa. Oregano, thyme, and cinnamon bark had the largest zones of inhibition against Enterobacteriaceae.
Conclusions
Essential oils have promising in vitro activity that warrants further study of their activity and use in the clinical setting.
Keywords: carbapenem-resistant Enterobacteriaceae, essential oils, in vitro susceptibility
Antimicrobial resistance is a major public health problem around the world. Resistance in Klebsiella pneumoniae, Escherichia coli, including carbapenem-resistant Enterobacteriaceae (CRE), and multidrug-resistant Pseudomonas aeruginosa continues to increase, and methicillin-resistant Staphylococcus aureus is widespread [1]. The WHO Global Action Plan to combat antimicrobial resistance encourages public-private partnerships for research on new antimicrobial agents (Objective 5) [2]. The National Action Plan for Combating Antibiotic-Resistant Bacteria in the United States calls for developing nontraditional therapeutics, including natural compounds such as essential oils (Goal 4.4) [3]. Previous studies have suggested the potential of essential oils for antimicrobial activity [4–7]. The global essential oils market demand has increased from 61.8 kilotons in 2014 to 226.9 kilotons in 2018 and is still increasing [8], combined with a growing inclination by consumers toward natural and holistic therapies. These factors, in addition to rising antimicrobial resistance, warrant further study of antimicrobial effects, particularly against more recent multidrug-resistant pathogens. A previous pilot study by our group showed in vitro activity of essential oils (EOs) against CRE and selected American Type Culture Collection (ATCC) strains [9] and led us to investigate activity against a larger sample of pathogens. To that end, our objective was to explore the activity of essential oils against multiresistant bacteria and other clinical isolates to evaluate the potential of their use topically and/or internally for treatment of bacterial infections.
MATERIALS AND METHODS
Essential Oils and Bacterial Isolates
Essential oils of cinnamon bark (Cinnamomun zeylanicum), clove (Szygium aromaticum), lemongrass (Cymopogon flexuosus), oregano (Origanum vulgare), rosemary (Rosmarinus officinalis), thyme (Thymus vulgaris), tea tree (Melaleuca alternifolia), manuka (Leptospermum scoparium), and the oil blend, Thieves (a proprietary blend of cinnamon, clove, lemon, eucalyptus, and rosemary), were provided by Young Living Essential Oils (Lehi, UT) who also performed gas chromatography/flame ionization detector analysis. Major constituents from the essential oils are shown in Table 1. Thirty Gram-positive, including methicillin-susceptible S aureus (MSSA) and methicillin-resistant S aureus (MRSA), and 70 Gram-negative bacterial clinical isolates, including extended-spectrum beta-lactamase and CRE strains, were tested using Clinical and Laboratory Standards Institute methods [10]. Selected ATCC quality control isolates (ATCC 29213 methicillin-susceptible S aureus, ATCC 49619 Streptococcus pneumoniae, ATCC 25922 E coli, and ATCC 27853 P aeruginosa) were also included. All isolates were maintained as frozen stocks in the Microbial Pathology Research Laboratory at UT Health San Antonio and were subcultured twice before testing.
Table 1.
Content of Each Major Constituent Was Determined From a Peak Area Relative to the Total Peak Area in Gas Chromatography/Flame Ionization Detector Analysis: The Top Three Constituents Are Listed
| Essential Oils Used and Their Major Constituents | |
| Cinnamon bark oil | trans-cinnamaldehyde 68.4%, eugenol 8.5%, cinnamyl acetate 4.1% |
| Clove oil | eugenol 78.9%, eugenyl acetate 13.0%, trans-beta-caryophyllene 6.5% |
| Lemongrass oil | geranial 39.2%, neral 32.0%, geraniol 6.9% |
| Manuka oil | leptospermone 16.4%, calamenene 12.8%, isoleptospermone 6.1% |
| Oregano oil | carvacrol methyl ether 67.4%, para-cymene 7.7%, gamma-terpinene 4.8% |
| Tea tree oil | terpinene-4-ol 37.1%, gamma-terpinene 20.8%, alpha-terpinene 11.3% |
| Thieves oil | eugenol 35.5%, limonene 16.9%, trans-cinnamaldehyde 12.7% |
| Thyme oil | thymol 43.3%, para-cymene 16.0%, gamma-terpinene 7.7% |
Susceptibility Testing
Isolates were grown overnight on tryptic soy agar, 0.5 McFarland suspensions were prepared in sterile saline, and Mueller-Hinton agar plates were inoculated by streaking for susceptibility testing using the Kirby-Bauer method. Twenty microliters of full-strength oils were pipetted onto blank paper disks in a sterile dish. Twenty microliters corresponded to approximately half of a drop of essential oil, a conservative estimate of an amount that would be feasible to apply topically when diluted in a carrier oil, and an amount that could be ingested when diluted in a carrier oil. Disks were placed aseptically onto the plates immediately after inoculating disks. In the previous pilot study, lemongrass lacked substantial Gram-negative in vitro activity, and in this study it was only tested against Gram-positive isolates. For positive control comparators and as quality controls, vancomycin was tested against Gram-positive isolates and meropenem was tested against Gram-negative isolates. Replicates were not performed. All plates were read using a benchtop desk lamp, and a ruler was used to measure zone sizes. No pop-up colonies were noted within the zone recorded.
RESULTS
Mean and median zone diameters of essential oils are shown in Tables 2–4. Representative sample disk diffusion agar plates are shown in Figure 1. Essential oils oregano, thyme, cinnamon bark, and lemongrass had the largest zones of inhibition against Gram-positive clinical isolates. Manuka and Thieves also showed significant Gram-positive activity. Manuka had greater activity against Gram positives than Gram negatives, with the exception of E coli. Oregano, thyme, and cinnamon bark had the largest zones of inhibition against Enterobacteriaceae. Cinnamon bark had the largest zone of inhibition against P aeruginosa. Zone sizes for P aeruginosa showed the most variability. This was due to 1 carbapenem-susceptible P aeruginosa isolate and 1 carbapenem-resistant P aeruginosa isolate that displayed much larger zone sizes with the essential oils than the other P aeruginosa isolates. Zone sizes for the positive control comparator vancomycin showed that the Gram-positive isolates were susceptible as expected. Zone sizes for meropenem for the Gram-negative isolates confirmed susceptibility for the non-CRE isolates and resistance for the CRE isolates, as expected. Vancomycin and meropenem zone sizes for the respective Gram-positive and Gram-negative selected ATCC strains confirmed susceptibility, as expected.
Table 2.
Mean, Standard Deviation, Median, and Range of Zone Diameters (mm) for Essential Oils Against Gram-Positive Clinical Isolates
| Species (n) | Oregano | Thyme | Cinnamon Bark | Lemongrass | Manuka | Clove | Tea Tree | Thieves | Vanco |
|---|---|---|---|---|---|---|---|---|---|
| MRSA (10) | |||||||||
| Mean (SD) | 23.6 (0.84) | 26.8 (1.69) | 29.7 (0.82) | 29.2 (2.04) | 19.7 (1.34) | 12.8 (0.63) | 9.4 (0.52) | 18.8 (1.81) | 17.8 (0.42) |
| Median, range | 23, 2 | 26, 5 | 30, 3 | 29, 5 | 20, 4 | 13, 2 | 9, 1 | 18, 1 | 18, 1 |
| MSSA (10) | |||||||||
| Mean (SD) | 26.6 (2.21) | 29.7 (1.77) | 30.2 (2.78) | 31.4 (6.40) | 18.8 (1.62) | 13.4 (1.35) | 8.9 (2.42) | 19.5 (2.72) | 18.2 (3.05) |
| Median, range | 26, 7 | 30, 6 | 29, 7 | 30, 20 | 18, 5 | 13, 5 | 8.5, 7 | 19, 8 | 19, 11 |
| GAS | |||||||||
| Mean (SD) | 18.3 (0.48) | 19.7 (1.57) | 13.1 (0.32) | 23.2 (3.33) | 14.0 (1.94) | 13.0 (0.47) | 6.9 (0.99) | 19.1 (1.60) | 21.0 (1.16) |
| Median, range | 18. 1 | 19.5, 4 | 13, 1 | 22, 11 | 13, 2 | 13, 2 | 6.5, 2 | 18.5, 5 | 20.5, 3 |
Abbreviations: GAS, group A streptococcus; Streptococcus pyogenes; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S aureus; SD, standard deviation.
Table 3.
Mean, Standard Deviation, Median, and Range of Zone Diameters (mm) for Essential Oils Against Gram-Negative, Carbapenem-Susceptible Clinical Isolates
| Species (n) | Oregano | Thyme | Manuka | Clove | Tea Tree | Thieves | Meropenem | |
|---|---|---|---|---|---|---|---|---|
| ESBL Escherichia coli (19) | Cinnamon Bark | |||||||
| Mean (SD) | 23.6 (0.84) | 26.8 (1.69) | 29.7 (0.82) | 19.7 (1.34) | 12.8 (0.63) | 9.4 (0.52) | 18.8 (1.81) | 30.0 (1.81) |
| Median, range | 23, 2 | 26, 5 | 30, 3 | 20, 4 | 13, 2 | 9, 1 | 18, 1 | 30, 7 |
| ESBL Klebsiella pneumoniae (7) | ||||||||
| Mean (SD) | 21.3 (3.50) | 15.4 (1.27) | 22.3 (1.60) | 6.0 (0) | 10.7 (1.38) | 14.4 (0.79) | 11.6 (1.41) | 30.3 (0.95) |
| Median, range | 23, 10 | 15, 19 | 22, 5 | 6, 0 | 10, 4 | 15, 2 | 12, 3 | 30, 3 |
| Enterobacter spp (10) | ||||||||
| Mean (SD) | 18.5 (5.15) | 14.8 (5.60) | 22.3 (4.08) | 6.3 (0.48) | 9.8 (2.44) | 13.6 (3.27) | 11.5 (3.10) | 26.4 (4.86) |
| Median, range | 20.5, 14 | 20.5, 14 | 21, 14 | 6, 1 | 10, 8 | 14.5, 12 | 11, 10 | 27, 17 |
| Pseudomonas aeruginosa (4) | ||||||||
| Mean (SD) | 13.0 (11.27) | 11.0 (7.81) | 28.5 (13.08) | 10.0 (6.16) | 11.0 (5.83) | 8.5 (3.0) | 13.8 (6.24) | 12.8 (2.22) |
| Median, range | 20, 7 | 7, 14 | 27.5, 27 | 7.5, 13 | 10.5, 11 | 8, 6 | 14, 13 | 13, 5 |
Abbreviations: ESBL, extended-spectrum beta-lactamase-producing; SD, standard deviation.
Table 4.
Mean, Standard Deviation, Median, and Range of Zone Diameters (mm) for Essential Oils Against Gram-Negative, Carbapenem-Resistant Clinical Isolates
| Species (n) | Oregano | Thyme | Manuka | Clove | Tea Tree | Thieves | Meropenem | |
|---|---|---|---|---|---|---|---|---|
| Klebsiella pneumoniae (13) | Cinnamon Bark | |||||||
| Mean (SD) | 19.7 (3.57) | 16.2 (3.16) | 21.2 (1.54) | 6.0 (0) | 12.8 (0.63) | 14.7 (1.93) | 12.1 (1.04) | 10.8 (2.35) |
| Median, range | 20, 12 | 15, 12 | 21, 5 | 20, 4 | 6, 0 | 15, 8 | 12, 5 | 11, 8 |
| Enterobacter spp (4) | ||||||||
| Mean (SD) | 20.0 (0) | 16.8 (2.36) | 21.3 (1.50) | 6.0 (0) | 9.0 (2.58) | 15.3 (1.26) | 10.5 (1.92) | 11.3 (2.36) |
| Median, range | 20, 0 | 16, 5 | 21, 3 | 6, 0 | 9, 6 | 15, 3 | 11, 4 | 16, 5 |
| Pseudomonas aeruginosa (11) | ||||||||
| Mean (SD) | 9.2 (10.23) | 9.2 (10.23) | 18.0 (8.12) | 6.7 (2.41) | 7.5 (4.82) | 6.7 (2.10) | 8.7 (5.06) | 6.7 (1.10) |
| Median, range | 6, 34 | 6, 34 | 16, 29 | 6, 8 | 6, 16 | 6, 7 | 6, 17 | 6, 3 |
Abbreviations: SD, standard deviation.
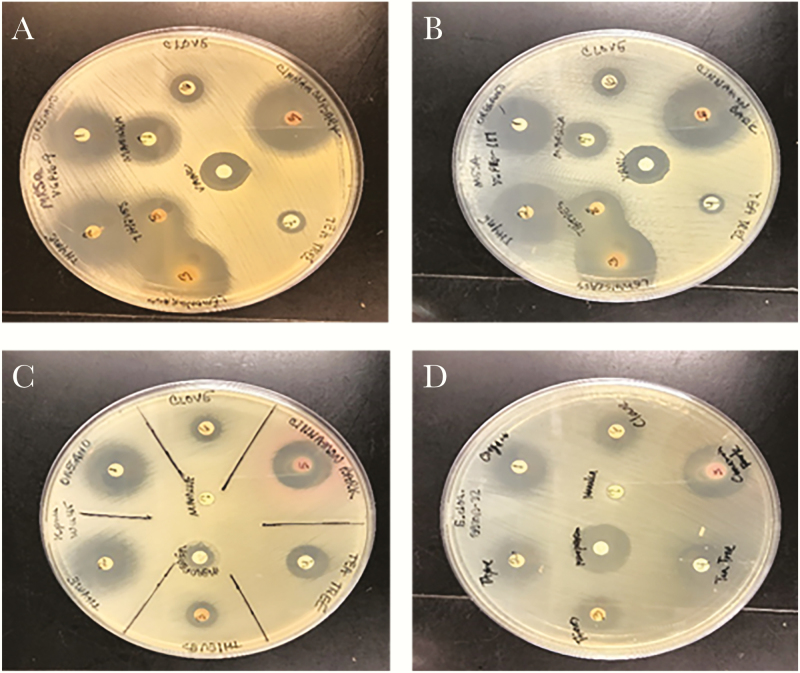
Figure 1.
Agar disk diffusion results: (A) methicillin-resistant Staphylococcus aureus; (B) methicillin-susceptible S aureus; (C) Klebsiella pneumoniae; (D) Enterobacter cloacae.
DISCUSSION
The ancient and traditional use of plants as medicine, the emergence of multidrug-resistant pathogens, and the increasing use of essential oils make the study of their antimicrobial activity timely and relevant. Essential oils have been shown to have antimicrobial activity, in particular, oregano, thyme, and tea tree oil [5–7, 11, 12]. Commonly used essential oils have not been studied against recently isolated multidrug-resistant pathogens, which prompted our study. Oregano, thyme, cinnamon bark, lemongrass, and manuka had notable activity against Gram-positive pathogens. In our pilot study, lemongrass had more Gram-positive than Gram-negative activity. In this study, manuka had more Gram-positive activity than Gram-negative activity, with the exception of E coli.
The least activity was demonstrated against P aeruginosa, but cinnamon bark oil had the largest zone of inhibition against P aeruginosa. Of note, the Thieves essential oil blend (containing clove, cinnamon, lemon, eucalyptus, and rosemary) had the next largest zone of inhibition against P aeruginosa. Oregano, thyme, and cinnamon bark had the largest zones of inhibition against Enterobacteriaceae. Cinnamon bark demonstrated the broadest spectrum of activity across Gram-positive and Gram-negative isolates, including P aeruginosa. Excluding P aeruginosa, oregano, thyme, close, tea tree oil, and Thieves had the broadest spectrum. It is of interest that 1 carbapenem-susceptible and 1 carbapenem-resistant P aeruginosa isolate showed more susceptibility to essential oils oregano, thyme, cinnamon bark, and Thieves than the other isolates. This suggests that these oils could be useful for some P aeruginosa isolates, based on susceptibility testing.
Major constituents of essential oils with antimicrobial activity include terpenoids such as the phenols thymol, carvacrol, and geraniol, phenylpropenes such as eugenol, as well as para-cymene and cinnamaldehyde [6, 7, 12]. These compounds were demonstrated as components in several of the essential oils tested. In general, essential oils are composed of 20 to 60 different compounds [7, 12]. Many of these compounds have antimicrobial activity, and the presence of the compounds together can be more powerful than the action of 1 compound alone. This combined approach is contrary to most currently used antibiotics, which are typically an expansion of 1 functional molecular structure, with activity extended by synthetic rearrangements. The presence of multiple compounds in essential oils may strengthen and prolong antimicrobial activity against microbes. Some studies have noted synergy between essential oil components and antibiotics, which could be another useful role of these agents [13].
The mechanisms of activity of essential oils against bacteria is suggested to be (1) increased cell permeability due to the hydrophobicity of the oils and (2) toxic effects on membrane structure and function [6, 7]. Similar to our study, some previous studies have observed that essential oils have more activity against Gram-positive than Gram-negative isolates [6]. This is thought to be due to the more complex, rigid outer membrane of Gram-negative bacteria with lipopolysaccharide that limits the diffusion of hydrophobic compounds. The complex outer membrane is not present in Gram-positive bacteria, and the peptidoglycan cell wall provides less resistance against the hydrophobic compounds. Some essential oils have been noted to eradicate biofilms of Pseudomonas spp and S aureus [14], which could provide additional mechanisms of antimicrobial action.
Methods used to assess antimicrobial activity of essential oils varies among publications, but agar disk diffusion is frequently used for initial studies [4] and was chosen for this study. This may be considered a limitation of the study, and it may explain some of the variability seen in results. Although broth minimum inhibitory concentration detection is more precise for testing antimicrobial effects, the hydrophobicity of essential oils necessitates the use of a solvent to allow uniform distribution for accurate broth microdilution results.
Essential oils may be able to be used topically or by ingestion for antimicrobial activity, either alone or in combination with traditional antibiotics. Although essential oil antimicrobial activity looks promising, it must be remembered that safety and toxicity studies for the ingestion of essential oils are limited. In addition, these essential oils must be significantly diluted when used topically [15]. Based on current knowledge, the safest way to use essential oils is by inhalation. Essential oil inhalation has been noted to affect the autonomic nervous system, suggesting some significant systemic absorption [16], but data on systemic levels achieved via inhalation are limited. So, although these activities are promising, clinical studies are needed to determine the practicality and applicability of use.
CONCLUSIONS
Essential oils can inhibit growth of a broad range of pathogens correlating to their presence in aromatic plants. We showed significant in vitro activity against clinical isolates, including multidrug-resistant pathogens and CRE. Further study of the clinical activity of essential oils is warranted.
Acknowledgments
We thank Dr. Richard Carlson of Young Living Essential Oils for providing the essential oils and gas chromatography/flame ionization detector analysis used in this study.
Presented in part: IDWeek 2017, October 2017, San Diego, CA.
Potential conflicts of interest. J. E. P. has received compensation from of Young Living Essential Oils as a wholesale member. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Antimicrobial resistance. Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed 2 December 2019. [Google Scholar]
- 2. World Health Organization. Global action plan on antimicrobial resistance. 2015. Available at: http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf. Accessed 2 December 2019. [DOI] [PubMed] [Google Scholar]
- 3. The White House. National Action Plan For Combating Antibiotic-Resistant Bacteria. 2015. Available at: https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed 2 December 2019. [Google Scholar]
- 4. Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 1999; 86:985–90. [DOI] [PubMed] [Google Scholar]
- 5. Teles Andrade BF, Nunes Barbosa L, da Silva Probst I, Fernandes Junior A. Antimicrobial activity of essential oils. J Essential Oil Res 2014; 26:1: 34–40. [Google Scholar]
- 6. Chouhan S, Sharma K, Guleria S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines 2017; 4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumara Swamy M, Akhtar MS, Sinniah UR. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evidence-Based Complementary and Alternative Medicine 2016; 3012462 Available at: 10.1155/2016/3012462. Accessed 2 December 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Essential Oils Market Size, Share & Trends Analysis Report By Application (Cleaning & Home, Medical, Food & Beverages, Spa & Relaxation), By Product, By Sales Channel, And Segment Forecasts, 2019–2025. Grand View Research 2019:187 https://www.grandviewresearch.com/industry-analysis/essential-oils-market. Accessed 2 December 2019.
- 9. Patterson JE, Cadena J, Traugott K, et al. In vitro susceptibility testing of essential oils against carbapenem-resistant Enterobacteriaceae and selected ATCC strains [abstract]. IDWeek (New Orleans, LA). October 2016. [Google Scholar]
- 10. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed CLSI Standard M07. Wayne, PA; Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 11. Halcón L, Milkus K. Staphylococcus aureus and wounds: a review of tea tree oil as a promising antimicrobial. Am J Infect Control 2004; 32:402–8. [DOI] [PubMed] [Google Scholar]
- 12. Nazzaro F, Fratianni F, De Martino L, et al. Effect of essential oils on pathogenic bacteria. Pharmaceuticals (Basel) 2013; 6:1451–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langeveld WT, Veldhuizen EJ, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol 2014; 40:76–94. [DOI] [PubMed] [Google Scholar]
- 14. Kavanaugh NL, Ribbeck K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl Environ Microbiol 2012; 78:4057–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tisserand R, Young R.. Essential Oil Safety. 2nd ed Edinburgh, London, New York, Oxford, Philadelphia, St Louis, Sydney, Toronto:Churchill Livingstone Elsevier; 2014. [Google Scholar]
- 16. Kim I, Kim C, Seong K, et al. Essential oil inhalation on blood pressure and salivary cortisol levels in prehypertensive and hypertensive subjects. Evidence-Based Complementary and Alternative Medicine 2012; 984203 Available at: 10.1155/2012/984203. Accessed 2 December 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]