Abstract
Background
ESKAPE bacteria are thought to be especially resistant to antibiotics, and their resistance and prevalence in bloodstream infections are rising. Large studies are needed to better characterize the clinical impact of these bacteria and to develop algorithms that alert clinicians when patients are at high risk of an ESKAPE infection.
Methods
From a US data set of >1.1 M patient encounters, we evaluated if ESKAPE pathogens produced worse outcomes than non-ESKAPE pathogens and if an ESKAPE infection could be predicted using simple word group algorithms built from decision trees.
Results
We found that ESKAPE pathogens represented 42.2% of species isolated from bloodstream infections and, compared with non-ESKAPE pathogens, were associated with a 3.3-day increase in length of stay, a $5500 increase in cost of care, and a 2.1% absolute increase in mortality (P < 1e-99). ESKAPE pathogens were not universally more resistant to antibiotics, but only to select antibiotics (P < 5e-6), particularly against common empiric therapies. In addition, simple word group algorithms predicted ESKAPE pathogens with a positive predictive value of 7.9% to 56.2%, exceeding 4.8% by random guessing (P < 1e-99).
Conclusions
Taken together, these data highlight the pathogenicity of ESKAPE bacteria, potential mechanisms of their pathogenicity, and the potential to predict ESKAPE infections upon admission. Implementing word group algorithms could enable earlier and targeted therapies against ESKAPE bacteria and thus reduce their burden on the health care system.
Keywords: antimicrobial resistance, bacteremia, ESKAPE
Despite over 100 years of innovation, bloodstream infections (BSIs) remain challenging to treat and manage effectively. Difficulties include an incomplete understanding of the invading species, limited antimicrobial options, and a dysregulated host response to infection known as sepsis [1]. Sepsis contributes to >35% of inpatient deaths and is the most expensive US hospital–treated condition, representing $23.7 billion in annual health care costs [2]. Because of the high burden of an untreated infection, clinicians administer antimicrobial drugs in patients suspected of BSI at rates of 50%–70% [3–5], far exceeding the actual BSI infection rate of 10%–15% [6–10]. A consequence of overprescribing antibiotics is the emergence and spread of antibiotic resistance. In a 2019 report to the United Nations, an international committee concluded that by 2050 antibiotic-resistant infections could cause 10 million deaths per year and an economic collapse comparable to the 2008–2009 global financial crisis [11]. Therefore, new technologies and better scientific understanding are needed to counter the growing threat of sepsis and antimicrobial resistance.
The “ESKAPE” pathogens are a group of infectious bacteria that have garnered particular attention for their ability to escape or evade common therapies through antimicrobial resistance. The ESKAPE pathogens were first defined in 2008 and consist of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. [12]. In 2009, the Infectious Diseases Society of America found a lack of new antibiotics in pharmaceutical pipelines to combat ESKAPE pathogens and made recommendations to incentivize innovation [13]. Over the past 10 years, many novel therapies to treat ESKAPE infections have been developed, including new antibiotics, bacteriophages, antimicrobial peptides, and nanoparticles [14]. In addition, a meta-analysis of 83 studies comparing antibiotic-susceptible vs -resistant bacteria highlighted that a major factor driving the economic burden of ESKAPE pathogens is antibiotic resistance [15]. But despite these advancements, ESKAPE pathogens remain a major health care burden, and recent studies suggest upward trends of ESKAPE prevalence [16], economic cost [17], and resistance [18, 19]. We hypothesize that these challenges arise in part from a paucity of large-scale studies that evaluate the true impact of ESKAPE pathogens relative to non-ESKAPE pathogens and a paucity of simple algorithms to identify patients at risk of an ESKAPE infection.
To improve our understanding and management of ESKAPE pathogens, our goal in this study was 2-fold. First, across a data set of >400K patient encounters, we evaluated if ESKAPE pathogens were associated with worse outcomes and higher costs than non-ESKAPE pathogens. Second, across a data set of >1.1M patient encounters, we assessed if simple word group algorithms based on admission code text could predict an ESKAPE BSI.
METHODS
Patient Data Set
The data source was a US database of inpatient hospital encounters comprised of 193 US hospitals, 6235171 patients, and 4.16 years from May 2014 to June 2018 (Premier, Inc., Charlotte, NC, USA). This database was previously employed in disease areas including myocardial infarction [20], pneumonia [21], gram-negative infections [22], and cancer [23]. A hospital encounter was defined as a discrete patient stay from admission to discharge. To compare the impact of ESKAPE and non-ESKAPE pathogens, we only included patients with a positive blood culture for a bacterium during the hospital encounter. To develop an algorithm predictive of ESKAPE BSI, we included all patient encounters with a blood culture result (ie, negative or positive) and at least 1 code upon admission from the International Classification of Diseases, Clinical Modification, Ninth (ICD-9-CM) or Tenth (ICD-10-CM) Revision [24]. For algorithm development, if an ESKAPE and non-ESKAPE BSI were detected within the same hospital encounter, the encounter was classified as ESKAPE.
Definition of ESKAPE and Antibiotic Resistance
We defined ESKAPE based on the original 2008 definition [12]. Resistance was defined directly from hospital reporting of antibiotic susceptibility testing. Only “resistant” or “susceptible” results were included; that is, “intermediate” and “indeterminate” results were excluded.
Algorithm Development
To predict ESKAPE infections, we parsed the ICD codes upon admission for unique words. Each word was employed as a binarized feature representing the presence or absence of the word. We developed 3 sets of word groups using different stopwords that could not be used. We defined the most predictive words of ESKAPE infection using decision trees grown with splits to minimize the Gini impurity criterion [25]. Algorithm performance was compared against random Bernoulli draws with probability of 0.5. To compare performance against a more complex algorithm, we used a dense neural network with 2 hidden layers and a cross-entropy loss function weighted 50:1 in favor of ESKAPE infections.
To evaluate model performance, we used a stratified random split to divide the data set into 3 parts: training, validation, and testing sets, representing 60%, 20%, and 20% of the data, respectively. The algorithms were fit to the training set, and the hyperparameters (eg, tree depth) were selected based on the validation set. Final model performance was evaluated with the holdout testing set not used for fitting or hyperparameter selection.
Statistical Testing and Software
All data preprocessing, analysis, statistical testing, and machine learning used the Julia programming language [26]. To compare means, medians, 2-proportions, 1-proportions, and survival curves, we used the unequal variance t test, Mann-Whitney U test, Fisher exact test, binomial test, and log-rank test, respectively.
RESULTS
Prevalence of ESKAPE Pathogens
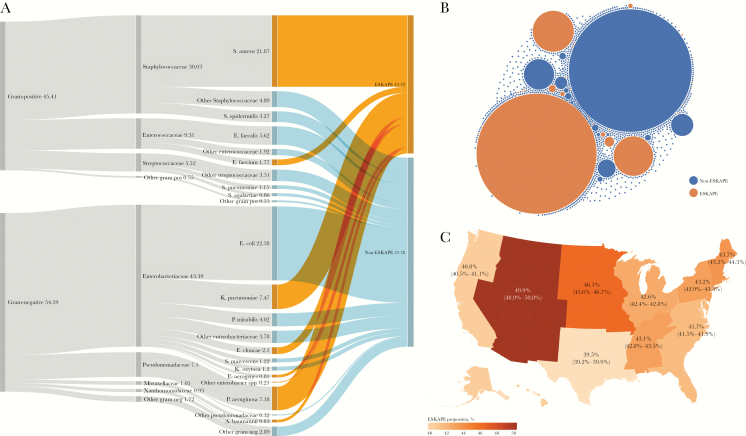
We first evaluated the prevalence of ESKAPE pathogens in positive blood cultures. In total, there were 403437 unique patients across 494817 hospital encounters, with 825529 unique isolates and 36.6M antibiotic susceptibility results. Patients were 50.9% female with a median age of 64.0 (25th–75th percentiles, 50.0–76.0) years. The most prevalent ESKAPE pathogens were S. aureus, K. pneumoniae, and P. aeruginosa, which represented 21.9%, 7.5%, and 7.2% of the total, respectively. The most prevalent non-ESKAPE pathogens were E. coli, E. faecalis, and P. mirabilis, which represented 22.6%, 5.6%, and 4.0% of the total, respectively. Overall, the proportion of isolated species classified as ESKAPE pathogens was 42.2% (Figure 1A). The isolates were comprised of 874 unique species, but the majority of the species were rare, such that the top 20 most prevalent species represented 86.4% of the total (Figure 1B). Further, the proportion of ESKAPE isolates depended on geographic region, with the lowest proportion in the West South Central region (39.5%; 95% confidence interval [CI], 39.2%–39.9%) and the highest proportion in the Mountain region (49.9%; 95% CI, 48.9%–50.8%). All regional ESKAPE proportions differed from the overall US proportion of 42.2% (P < .003) (Figure 1C).
Figure 1.
Prevalence of ESKAPE species in blood cultures in the United States. A, From left to right, relative proportions of gram staining, families, species, and ESKAPE from isolated bacteria. B, Relative prevalence of all 874 unique isolated species. The area of the circle is proportional to prevalence. C, Geographic distribution of ESKAPE pathogen prevalence in the United States. Values represent the proportion of ESKAPE isolates relative to non-ESKAPE, and range represents 95% confidence interval by the Wilson method.
Impact of ESKAPE Pathogen Infections on Patient Outcome
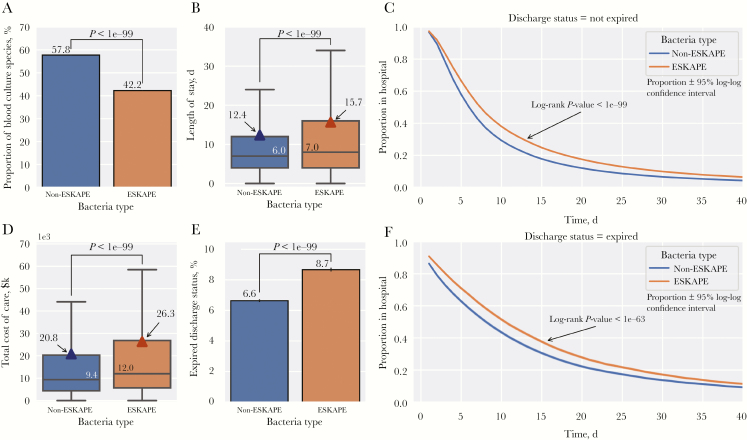
We next examined how ESKAPE pathogens impacted patient care. First, we found that the relative proportion of ESKAPE pathogens was 15.6% lower than non-ESKAPE pathogens (P < 1e-99) (Figure 2A). However, compared with patients with non-ESKAPE pathogens, patients with ESKAPE pathogens showed a longer length of stay by a median of 1.0 days (P < 1e-99) or a mean of 3.3 days (P < 1e-99) (Figure 2B). Among surviving patients at discharge, a higher proportion of patients with ESKAPE pathogens remained in the hospital for all lengths of stay (P < 1e-99) (Figure 2C). Further, the total cost of care for patients with ESKAPE pathogens was higher than for those with non-ESKAPE pathogens by a median of $2600 (P < 1e-99) or a mean of $5500 (P < 1e-99) (Figure 2D). In addition, at discharge, 6.6% of patients with non-ESKAPE pathogens expired, whereas 8.7% of patients with ESKAPE pathogens expired, an absolute increase in all-cause mortality of 2.1% (P < 1e-99) (Figure 2E). Among these expired patients, a higher proportion of patients with ESKAPE pathogens remained in the hospital for all lengths of stay (P < 1e-63) (Figure 2F).
Figure 2.
Impact of ESKAPE pathogens on patient outcomes. A, Relative proportions (±95% Wilson confidence intervals) of ESKAPE and non-ESKAPE pathogens. B, Patient length of stay by bacteria type with boxplot (median ± interquartile range [IQR]) overlaid with means (triangles). C, Proportion of patients in the hospital by length of stay and bacteria type for surviving patients. D, Total cost of care by bacteria type with boxplot (median ± IQR) overlaid with means (triangles). E, Proportion of patients expired at discharge by bacteria type (±95% confidence interval). F, Proportion of patients in hospital by length of stay by bacteria type for expired patients.
Comparison of Antibiotic Resistance
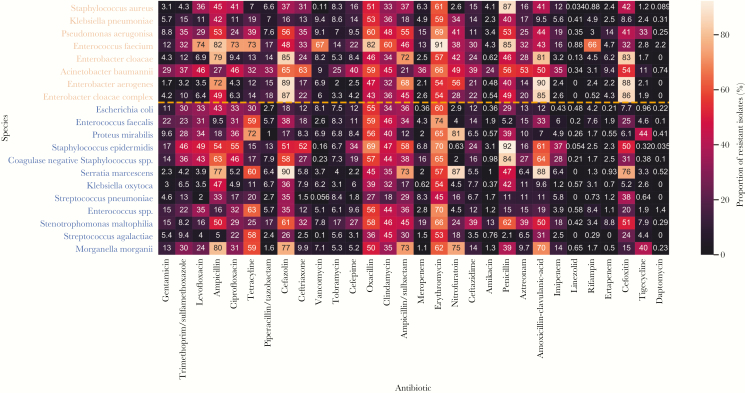
We next evaluated the proportion of isolated species with antibiotic resistance. We first considered only the top 20 species (8 ESKAPE and 12 non-ESKAPE) and top 30 tested antibiotics to provide a qualitative comparison of the most common species and antibiotics. The median number of isolates tested for each combination of species and antibiotics was 3911 (25th–75th percentiles, 1250–9290), and 99.5% (597/600) of the combinations had at least 100 tested isolates. Compared with non-ESKAPE pathogens, ESKAPE pathogens qualitatively showed similar resistance patterns as the same antibiotics (Figure 3).
Figure 3.
Proportion of isolates reported resistant for combinations of the top 20 most prevalent species and top 30 most frequently tested antibiotics. Species are ordered first by ESKAPE (orange) and non-ESKAPE (blue) and then by prevalence, and antibiotics are ordered by frequency of resistance testing (ie, gentamicin was most frequently tested).
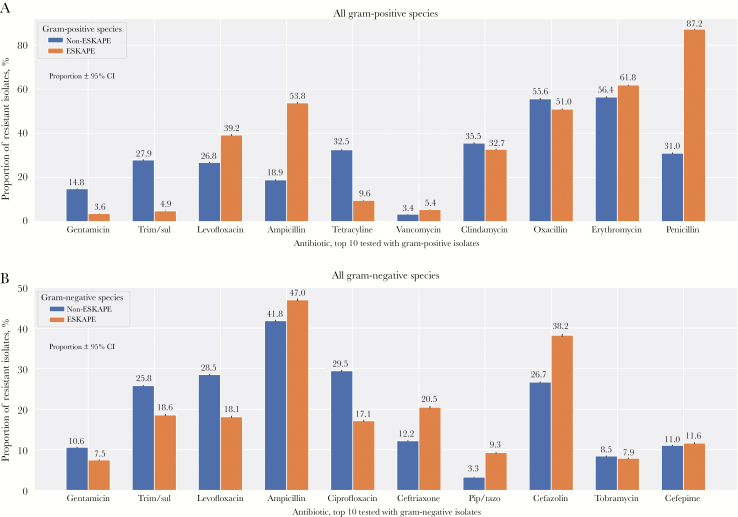
To quantitatively evaluate if ESKAPE pathogens are more resistant than non-ESKAPE pathogens, we aggregated reported resistance within all ESKAPE and non-ESKAPE species, and then stratified by gram staining to employ a common clinical grouping (Figure 4). Non-ESKAPE species showed higher resistance for gentamicin, trimethoprim/sulfamethoxazole, tetracycline, and ciprofloxacin. In contrast, ESKAPE species showed higher resistance for vancomycin, penicillin, cefazolin, ceftriaxone, and piperacillin/tazobactam. In addition, we observed relatively high resistance against ampicillin for gram-positive ESKAPE species (53.6%) and gram-negative ESKAPE species (47.0%) and relatively high resistance in gram-positive ESKAPE species against levofloxacin (39.2%) and erythromycin (61.8%). All differences in reported resistance between ESKAPE and non-ESKAPE species were significant within the gram-positive (P < 1e-45) (Figure 4A) and gram-negative (P < 5e-6) (Figure 4B) groups.
Figure 4.
Comparison of resistant isolates for all ESKAPE and non-ESKAPE species. Data are aggregated such that species with higher prevalence have higher weight and are stratified by gram-positive (A) and gram-negative (B) species. The evaluated antibiotics represent the top 10 antibiotics tested for gram-positive and gram-negative species, respectively. Bars represent proportion and 95% Wilson confidence interval. Abbreviations: pip/tazo, piperacillin/tazobactam; trim/sul, trimethoprim/sulfamethoxazole.
Predicting ESKAPE BSIs With ICD Codes Upon Admission
To predict ESKAPE BSIs, we included all patients with a blood culture result, representing 1499550 patient encounters. Of these encounters, 28.8% had a positive blood culture. To be comparable with the reported 10%–15% blood culture positivity [6–10], we randomly downsampled positive blood culture encounters, resulting in a final count of 916508 unique patients, where patients were 50.1% female, with median age of 63.0 (25th–75th percentiles, 47.0–76.0) years. These patients comprised 1185682 patient encounters, where 10.01% (118660) had a positive blood culture and 4.85% (57564) had a positive blood culture with an ESKAPE species. The total number of unique diagnosis words upon admission was 15769. We fit decision trees to these words and developed 3 word groups to predict ESKAPE infections (Table 1).
Table 1.
Selected Words to Predict ESKAPE Using ICD Code Text Upon Admission; if Any of the Words Are Present in an ICD Code Upon Admission, the Algorithm Predicts a Blood Culture Positive With an ESKAPE Species
| Word Group No. | Words in Group |
|---|---|
| 1 | staph, pseudomonas, pneumoniae |
| 2 | tract, ulcer, abscess |
| 3 | foot, leg, cutaneous, shock, pressure, anemia |
Abbreviation: ICD, International Classification of Diseases.
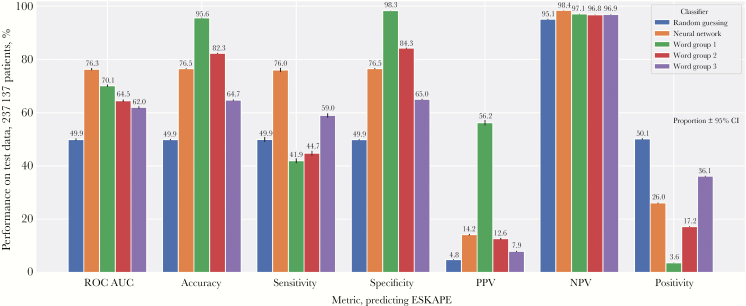
We next evaluated the performance of the word groups to predict ESKAPE BSIs with a holdout test data set of 237137 patient encounters with a blood culture result, where 11493 were positive for ESKAPE species (Figure 5). The neural network showed the best predictive performance, with an area under the receiver operating characteristics curve (ROC AUC) of 0.763 (95% CI = 0.759–0.767). Within the decision tree algorithms, all 3 word groups significantly exceeded the performance of random guessing, with absolute increases in ROC AUC of 12.1%–20.2% (P < 1e-99) and absolute increases in accuracy of 14.8%–48.4% (P < 1e-99). Compared with random guessing, all differences in ROC AUC, accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and positivity were significant for all 3 word groups (P < 5e-16). In addition, we observed a negative correlation between PPV and positivity. From word group 1 to word group 3, the PPV decreased from 56.2%, 12.6%, and 7.9%, whereas positivity increased from 3.6%, 17.2%, and 36.1%, respectively. Thus, as models became less conservative (higher positivity), the ability to correctly predict a positive ESKAPE infection decreased (lower PPV).
Figure 5. .
Performance of word group classifiers using admission International Classification of Diseases text to predict ESKAPE bloodstream infections on a holdout test set of 237137 patients. All bars represent value ±95% confidence interval using the Wilson method, except for area under the receiver operating characteristics curve, which was assessed using 1000 bootstrapped samples. Abbreviations: NPV, negative predictive value; PPV, positive predictive value; ROC AUC, area under the receiver operating characteristics curve.
Discussion
In this study, we found that ESKAPE pathogens make up nearly half the species in bloodstream infections and are associated with higher lengths of stay, cost of care, and mortality compared with non-ESKAPE pathogens. Administering effective therapy is a critical yet challenging task for patients with life-threatening bloodstream infections. For example, a meta-analysis of 70 studies found that 46.5% of patients with a bloodstream infection were given empiric therapy that was not effective, and those patients had 2-times higher odds of death [27]. In addition, in previous studies of sepsis and septic shock, for every hour delay in delivery of effective therapy, survival decreased by 7.6% [28] and odds of death increased by 4.0% [29]. Taken together, there is an urgent need to rapidly identify patients at highest risk of sepsis and triage them onto effective therapy. Our results contribute to this goal by demonstrating that patients with ESKAPE pathogens are at higher risk of poor outcomes, and implementing simple word group algorithms can identify patients at risk for an ESKAPE pathogen infection.
As this was an observational study, it was not possible to assess whether implementing a word group algorithm improves identification of patients with ESKAPE pathogens or patient outcomes relative to standard of care. To answer that question, a multicenter randomized controlled study is needed. But given that this study used >1M patient encounters, we were able to identify features of patients most associated with ESKAPE pathogens based on robust statistics. In addition, the raw data set of patients with an ICD admission code and a negative or positive blood culture produced a 28.8% blood culture positivity rate, higher than the 10%–15% positivity previously reported [6–10]. Thus, the data set may be biased relative to the native population. We attempted to account for this in 2 ways. First, in all comparisons of ESKAPE vs non-ESKAPE, we excluded patient encounters without positive blood culture results, so those comparisons were independent of positivity. Second, in the development of algorithms, we randomly downsampled the positive group to yield a 10% positivity rate and evaluated algorithms on a test data set not previously seen by the algorithms. Although a bias may still exist, we found that the algorithms generalize well to an unseen data set, with 10% blood culture positivity, and thus show promise to be implemented in practice.
Despite these limitations, our results support findings of previous studies and highlight why ESKAPE pathogens are important. The original ESKAPE papers [12, 13] called attention to the antibiotic resistance of the ESKAPE species and the need for new therapies but did not delineate differences in patient outcome between ESKAPE and non-ESKAPE species. Across >400K patient encounters, we found that the ESKAPE pathogens represented 42.2% of all detected species, with significant regional differences across the United States (Figure 1). In addition, compared with non-ESKAPE species, we found that on average ESKAPE pathogens were associated with a 3.3-day increase in length of stay, $5500 increase in cost of care, and 2.1% increase in mortality (Figure 2). Based on the reported resistance by species, it was not immediately evident why ESKAPE pathogens should result in worse outcomes, as resistance was clearly reported across the most prevalent ESKAPE and non-ESKAPE species (Figure 3). But after aggregating species by ESKAPE and non-ESKAPE, we found differences in resistance by antibiotic, where ESKAPE pathogens were significantly more resistant to vancomycin, cefazolin, ceftriaxone, and piperacillin/tazobactam (Figure 4). Given that the Surviving Sepsis guidelines recommend vancomycin, cefazolin, ceftriaxone, and piperacillin/tazobactam as empiric therapies [30], we hypothesize that the enhanced pathogenicity of ESKAPE pathogens may not be due to an overall higher resistance to antibiotics, but rather higher resistance to the most commonly used empiric therapies.
Algorithms to identify patients at highest risk of poor outcomes can augment human intuition and improve patient care. Previous studies include time-varying auto-adaptive algorithms to forecast hospital-wide incidence of ESKAPE pathogens [31], which, while accurate, were not designed to predict an ESKAPE infection within a single patient. Similarly, >200 studies have modeled antimicrobial resistance at the population level [32]. At the individual patient level, models to predict bacteremia have all utilized rapid diagnostic tests as inputs, such as procalcitonin, albumin, and bilirubin [33], procalcitonin alone [34], or blood measurements such as platelet counts and creatinine [35]. In addition, while a 2015 review found 15 published models to predict bacteremia, the authors found that none of the models were in active clinical use [36]. We postulate that one reason for a lack of implementation may be that some of the models are too time-consuming and complicated, requiring software, mathematics, and diagnostic blood tests. We therefore focused on ICD admission text as the only input, producing a simple mental model that can be easily applied by a busy clinician.
Based on this sparse history of successfully implemented bacteremia algorithms, and given that the neural network requires software to use, we focused primarily on the word groups. The selected word groups to predict ESKAPE showed tradeoffs in PPV and positivity, providing options for different levels of conservatism (Figure 5). Word group 1 was the most predictive but was also the most conservative (lowest positivity). This is perhaps not surprising given that the words include the genus and species of 3 ESKAPE pathogens (Table 1). Word group 2 is perhaps more interesting because the 3 words refer to interfaces of the body with the outside world: “tract,” “ulcer,” and “abscess.” Finally, word group 3 consisted of words associated with the lower body, blood, and skin and was the least predictive and conservative. Word group 3 used “anemia” as a predictor, which was previously associated with surgical site infection after hysterectomy [37], pneumonia after stroke [38], and general infection with aplastic anemia [39]. Similarly, the lower body words of “leg” and “foot” can refer to lower body infections associated with abscesses or ulcers, which can manifest as a comorbidity of diabetes [40]. Overall, as all 3 word groups exceeded the predictive capacity of random guessing, they could prove useful as part of workflows to triage patients with a blood culture order and 1 of these words in an admission diagnosis. Specifically, we envision algorithm usage by nurses and physicians in the emergency department or intensive care unit through word prompts, alerts configured in an electronic medical record system, and physicians and administrative staff when configuring guidelines.
In conclusion, our results from a relatively large US data set highlight the pathogenicity of ESKAPE bacteria and quantify their impact on patient length of stay, cost of care, and mortality. We also found that among all blood culture orders, employing simple word algorithms can predict ESKAPE infections better than random guessing. To our knowledge, this is the first study to compare the impact of ESKAPE vs non-ESKAPE bacteria on a nationwide data set, and the first to predict ESKAPE pathogens or bacteremia using admission diagnosis words. Given the steady rise of antibiotic resistance and the high cost of treating sepsis, rapidly identifying patients infected with ESKAPE pathogens will continue to be a major health care priority. We show here that by employing simple algorithms, major gains in our ability to predict ESKAPE pathogens can be realized, which can potentially reduce their pathogenic effects.
Acknowledgments
We thank Dr. Sandy Estrada and Jason Kniebel for discussions and critical review.
Potential conflicts of interest . All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014; 312:90–2. [DOI] [PubMed] [Google Scholar]
- 3. Suberviola B, Márquez-López A, Castellanos-Ortega A, et al. Microbiological diagnosis of sepsis: polymerase chain reaction system versus blood cultures. Am J Crit Care 2016; 25:68–75. [DOI] [PubMed] [Google Scholar]
- 4. Karlsson S, Varpula M, Ruokonen E, et al. Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive Care Med 2007; 33:435–43. [DOI] [PubMed] [Google Scholar]
- 5. Castellanos-Ortega A, Suberviola B, García-Astudillo LA, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med 2010; 38:1036–43. [DOI] [PubMed] [Google Scholar]
- 6. Tabak YP, Vankeepuram L, Ye G, Jeffers K, Gupta V, Murray PR. Blood culture turnaround time in US acute care hospitals and implications for laboratory process optimization. J Clin Microbiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mountain D, Bailey PM, O’Brien D, Jelinek GA. Blood cultures ordered in the adult emergency department are rarely useful. Eur J Emerg Med 2006; 13:76–9. [DOI] [PubMed] [Google Scholar]
- 8. Chotirmall SH, Callaly E, Lyons J, et al. Blood cultures in emergency medical admissions: a key patient cohort. Eur J Emerg Med 2016; 23:38–43. [DOI] [PubMed] [Google Scholar]
- 9. Gander RM, Byrd L, DeCrescenzo M, et al. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol 2009; 47:1021–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zwang O, Albert RK. Analysis of strategies to improve cost effectiveness of blood cultures. J Hosp Med 2006; 1:272–6. [DOI] [PubMed] [Google Scholar]
- 11. Interagency Coordination Group. No time to wait: securing the future from drug-resistant infections. Report to the Secretary-General of the United Nations.2019. https://www.who.int/antimicrobial-resistance/interagency-coordination-group/final-report/en/. Accessed April 30, 2019.
- 12. Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 2008; 197:1079–81. [DOI] [PubMed] [Google Scholar]
- 13. Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1–12. [DOI] [PubMed] [Google Scholar]
- 14. Mulani MS, Kamble EE, Kumkar SN, et al. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol 2019; 10:539–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhen X, Lundborg CS, Sun X, et al. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob Resist Infect Control 2019; 8:137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Angelis G, Fiori B, Menchinelli G, et al. Incidence and antimicrobial resistance trends in bloodstream infections caused by ESKAPE and Escherichia coli at a large teaching hospital in Rome, a 9-year analysis (2007-2015). Eur J Clin Microbiol Infect Dis 2018; 37:1627–36. [DOI] [PubMed] [Google Scholar]
- 17. Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One 2017; 12:e0189621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Socio GV, Rubbioni P, Botta D, et al. Measurement and prediction of antimicrobial resistance in bloodstream infections by ESKAPE pathogens and Escherichia coli. J Glob Antimicrob Resist 2019; 19:154–60. [DOI] [PubMed] [Google Scholar]
- 19. Ramsamy Y, Essack SY, Sartorius B, et al. Antibiotic resistance trends of ESKAPE pathogens in Kwazulu-Natal, South Africa: a five-year retrospective analysis. Afr J Lab Med 2018; 7:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pack QR, Priya A, Lagu T, et al. Association between inpatient echocardiography use and outcomes in adult patients with acute myocardial infarction. JAMA Intern Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta NM, Lindenauer PK, Yu PC, et al. Association between alcohol use disorders and outcomes of patients hospitalized with community-acquired pneumonia. JAMA Netw Open 2019; 2:e195172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonine NG, Berger A, Altincatal A, et al. Impact of delayed appropriate antibiotic therapy on patient outcomes by antibiotic resistance status from serious gram-negative bacterial infections. Am J Med Sci 2019; 357:103–10. [DOI] [PubMed] [Google Scholar]
- 23. Kalsekar I, Hsiao CW, Cheng H, et al. Economic burden of cancer among patients with surgical resections of the lung, rectum, liver and uterus: results from a US hospital database claims analysis. Health Econ Rev 2017; 7:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Centers for Disease Control and Prevention. Classification of diseases, functioning, and disability. 2019. https://www.cdc.gov/nchs/icd/index.htm. Accessed April 30, 2019. [Google Scholar]
- 25. Brieman L, Friedman JH, Olshen RA, Stone CJ.. Classification and Regression Trees. Belmont, CA: Wadsworth, Inc.; 1984. [Google Scholar]
- 26. Bezanson J, Karpinski S, Shah V, Edelman A. Julia: A Fast Dynamic Language for Technical Computing Ithaca, NY: arXiv, Cornell University, : 2012. https://ui.adsabs.harvard.edu/abs/2012arXiv1209.5145B. Accessed May 30, 2019. [Google Scholar]
- 27. Paul M, Shani V, Muchtar E, et al. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 2010; 54:4851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–96. [DOI] [PubMed] [Google Scholar]
- 29. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43:304–77. [DOI] [PubMed] [Google Scholar]
- 31. Ballarin A, Posteraro B, Demartis G, et al. Forecasting ESKAPE infections through a time-varying auto-adaptive algorithm using laboratory-based surveillance data. BMC Infect Dis 2014; 14:634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niewiadomska AM, Jayabalasingham B, Seidman JC, et al. Population-level mathematical modeling of antimicrobial resistance: a systematic review. BMC Med 2019; 17:81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ratzinger F, Haslacher H, Perkmann T, et al. Machine learning for fast identification of bacteraemia in SIRS patients treated on standard care wards: a cohort study. Sci Rep 2018; 8:12233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Müller F, Christ-Crain M, Bregenzer T, et al. ; ProHOSP Study Group Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest 2010; 138:121–9. [DOI] [PubMed] [Google Scholar]
- 35. Shapiro NI, Wolfe RE, Wright SB, et al. Who needs a blood culture? A prospectively derived and validated prediction rule. J Emerg Med 2008; 35:255–64. [DOI] [PubMed] [Google Scholar]
- 36. Eliakim-Raz N, Bates DW, Leibovici L. Predicting bacteraemia in validated models—a systematic review. Clin Microbiol Infect 2015; 21:295–301. [DOI] [PubMed] [Google Scholar]
- 37. Göksever Çelik H, Çelik E, Turan G, et al. Risk factors for surgical site infection after hysterectomy. J Infect Dev Ctries 2017; 11:355–60. [DOI] [PubMed] [Google Scholar]
- 38. Wei CC, Zhang ST, Tan G, et al. Impact of anemia on in-hospital complications after ischemic stroke. Eur J Neurol 2018; 25:768–74. [DOI] [PubMed] [Google Scholar]
- 39. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol 2009; 46:269–76. [DOI] [PubMed] [Google Scholar]
- 40. Peters EJ, Lipsky BA, Aragón-Sánchez J, et al. ; International Working Group on the Diabetic Foot Interventions in the management of infection in the foot in diabetes: a systematic review. Diabetes Metab Res Rev 2016; 32(Suppl 1):145–53. [DOI] [PubMed] [Google Scholar]