Abstract
Borrelia (B.) mayonii sp. nov. has recently been reported as a novel human pathogenic spirochete causing Lyme disease (LD) in North America. Previous data reveal a higher spirochaetemia in the blood compared to patients infected by LD spirochetes belonging to the B. burgdorferi sensu lato complex, suggesting that this novel genospecies must exploit strategies to overcome innate immunity, in particular complement. To elucidate the molecular mechanisms of immune evasion, we utilized various methodologies to phenotypically characterize B. mayonii and to identify determinants involved in the interaction with complement. Employing serum bactericidal assays, we demonstrated that B. mayonii resists complement-mediated killing. To further elucidate the role of the key regulators of the alternative pathway (AP), factor H (FH), and FH-like protein 1 (FHL-1) in immune evasion of B. mayonii, serum adsorption experiments were conducted. The data revealed that viable spirochetes recruit both regulators from human serum and FH retained its factor I-mediated C3b-inactivating activity when bound to the bacterial cells. In addition, two prominent FH-binding proteins of approximately 30 and 18 kDa were detected in B. mayonii strain MN14-1420. Bioinformatics identified a gene, exhibiting 60% identity at the DNA level to the cspA encoding gene of B. burgdorferi. Following PCR amplification, the gene product was produced as a His-tagged protein. The CspA-orthologous protein of B. mayonii interacted with FH and FHL-1, and both bound regulators promoted inactivation of C3b in the presence of factor I. Additionally, the CspA ortholog counteracted complement activation by inhibiting the alternative and terminal but not the classical and Lectin pathways, respectively. Increasing concentrations of CspA of B. mayonii also strongly affected C9 polymerization, terminating the formation of the membrane attack complex. To assess the role of CspA of B. mayonii in facilitating serum resistance, a gain-of-function strain was generated, harboring a shuttle vector allowing expression of the CspA encoding gene under its native promotor. Spirochetes producing the native protein on the cell surface overcame complement-mediated killing, indicating that CspA facilitates serum resistance of B. mayonii. In conclusion, here we describe the molecular mechanism utilized by B. mayonii to resists complement-mediated killing by capturing human immune regulators.
Keywords: lyme disease, spirochetes, borrelia, borrelia mayonii, innate immunity, complement, immune evasion, host cell interaction
Introduction
In 2016, Borrelia (B.) mayonii sp. nov. was identified by routine diagnostic testing of human specimens obtained from 100,595 patients in the US (1). Multi-locus sequence typing of eight housekeeping genes delineated this Borrelia species as a new member of the B. burgdorferi sensu lato (s.l.) complex (1, 2). Clinically, B. mayonii infected patients showed higher loads of spirochetes in the blood (105-106) (1) and some of them have had a focal or diffuse rash or developed neurological symptoms. So far, this species has only been identified in Ixodes (I.) scapularis ticks collected from the northeast and upper midwest of the US but not in I. ricinus from France, suggesting that B. mayonii is mainly distributed in the North America (2–4). Recently, experimental mice infection studies and field investigations revealed that B. mayonii like other Lyme disease (LD) spirochetes is maintained by transstadial transmission rather than passed by transovarial (vertical) transmission to the offspring (5). Furthermore, the probability of B. mayonii to be transmitted from infected I. scapularis ticks to the mammalian host parallels the transmission of spirochetes belonging to the B. burgdorferi s.l. complex. It has been shown that probability of host infection gradually increases over the duration of tick attachment, reaching 70% after 72 h of attachment and >90% after a complete tick blood meal (6–9). As potential reservoir hosts, white-footed mice (Peromyscus leucopus) and American red squirrel (Tamiasciurus hudsonicus) have been identified so far (10).
Complement as part of the innate immune system operates as a first line of defense against invading pathogenic microorganisms (11, 12). This system forms a cooperative network of more than 40 inactive precursors, membrane-bound, and fluid-phase regulators and inhibitors that discriminate between “self” from “non-self” and thereby plays a central role in the recognition and elimination of pathogenic intruders (13–17). Complement is activated by the classical (CP), the lectin (LP), and the alternative pathway (AP) (14, 16, 18, 19). Activation of the CP is primarily initiated through the interaction of C1q with IgM or IgG (13, 19). The LP is initiated by binding of mannose-binding lectin or mannan-binding lectin (MBL) or ficolins to carbohydrates on microbial cell surfaces and, in contrast to the CP and LP, the initialization of the AP is spontaneously triggered by charged moieties on microbial surfaces. Once activated, all three pathways act in concert to generate C3b, a highly reactive molecule that covalently binds to microbial surfaces by the formed C3 convertases C4b2a (CP and LP) or C3bBb (AP). Binding of C3b to the C3 convertases leads to the assembly of the C5 convertases of the CP and LP (C4b2a3b) or AP (C3bBb3b) that cleave C5. Further downstream activation is driven by linking C5b molecules to the microbial surface and a subsequent accumulation of C6, C7, C8, and C9 to create the bacteriolytic, pore-forming C5b-9 complex or membrane attack complex, MAC.
To protect self surfaces from complement activation and harmful attack of activated splice products, the complement cascade is controlled by fluid phase and surface-attached regulators or inhibitors, respectively. FH and FHL-1 are the main fluid phase regulators of the AP. Both regulators act as cofactors for factor I-mediated degradation of C3b and thereby support the dissociation (decay-accelerating activity) of the C3 convertase of the AP (18, 20). FH, a 150 kDa protein is structured into 20 complement control protein (CCP) domains of which CCP domains 1–4 are responsible for decay-accelerating and cofactor activities (20, 21). FHL-1, a 42 kDa glycoprotein, arises from alternative splicing of the CFH gene and is composed of the first seven CCP domains of FH (CCP 1-7) but possess four amino acids (SFTL) at the C terminus (22). Both regulators also compete with FB for binding to C3b.
Like other blood-borne pathogens, LD spirochetes have developed multiple strategies to overcome innate immunity, thereby avoiding clearance by the immune system of the respective host, e.g., by changing the surface composition or by targeting complement activation [reviewed in (23, 24)]. Two important mechanisms to combat complement activation involve the (i) recruitment of complement regulators of the AP, FH and FHL-1, to inactivate the key complement component C3b and (ii) inhibition of the assembly of the MAC by interacting with late components C7 and C9 (25–28). Concerning B. burgdorferi, the primary ligands involved in the acquisition of FH and FHL-1 are CspA (CRASP-1, BBA68) and CspZ (CRASP-2, BBH06), two outer surface molecules encoded by genes located on the linear plasmids lp54 and lp28-3, respectively (29–31). Both lipoproteins confer resistance to complement-mediated bacteriolysis, confirmed by studies with infectious ΔcspA and ΔcspZ knockout strains as well as non-pathogenic gain-of-function strains ectopically producing CspA or CspZ (29, 32–36). More recently it has been shown that CspA plays a role in B. burgdorferi survival in ticks' blood meal, resulting in tick-to-host transmission (33). This result is consistent with the fact that cspA is expressed in ticks and the biting site of skin (33, 37). In contrast to cspA, cspZ is expressed during mouse infection but the gene is downregulated in spirochetes residing in the tick midgut (33, 37). In vivo studies revealed that binding of FH to CspZ promotes hematogenous dissemination and tissue colonization of B. burgdorferi (35). Further studies also illuminated a role of CspA and CspZ in complement-driven host specificity and selective transmission which is largely based on the tailored capability of both proteins to interact with FH molecules of different animals (33, 35, 38).
Moreover, clinical examinations of positive tested specimens revealed an unusually high spirochaetemia in patients infected with B. mayonii, which implies that this particular borrelial species is able to survive in human blood by producing determinants to overcome complement-mediated killing. In the present study, we set out to elucidate the underlying principles of complement resistance of B. mayonii and to identify and functional characterize the complement interacting ligand(s).
Materials and Methods
Bacterial Strains, Biological and Geographical Origin, and Culture Conditions
Low-passage (<20) B. mayonii MN14-1420T (DSMZ No. 102811, human blood, USA, deposited to culture collection by J. Petersen, CDC, USA), B. burgdorferi LW2 (skin, Germany), B. burgdorferi B31T (ATCC No. 35210, tick, USA), B. burgdorferi Pka-1 (CSF, Germany), and B. garinii G1 (CSF, Germany) were cultured until mid-exponential phase (5 × 107 cells per ml) at 33°C in Barbour-Stoenner-Kelly (BSK-H) medium (Bio&SELL, Feucht, Germany). Transformant G1/pCspA_Bmayo was cultured in BSK-H medium supplemented with 100 μg ml−1 streptomycin. Escherichia coli strains used as a host for propagation of the vectors and for production of recombinant hexahistidine (His6)-tagged proteins were grown in yeast tryptone broth at 37°C.
Human Serum, Antibodies, and Proteins
Non-immune human serum (NHS) collected from healthy blood donors was initially tested for the presence of anti-Borrelia IgM and IgG antibodies as previously described (29). Only sera considered to be negative were combined to form a serum pool. All complement components were purchased from Complement Technology (Tyler, TX, USA). Polyclonal anti-FH and anti-C3 antisera were obtained from Merck Biosciences (Bad Soden, Germany) and the neoepitope-specific monoclonal anti-C5b-9 antibody was purchased from Quidel (San Diego, CA, USA). Purification of FHL-1, FH fragments containing different CCP domains, and the anti-CCP1-4 antiserum has been described previously (39). The monoclonal antibody L41 1C11 was used to detect the borrelial FlaB protein (40). Proteinase K and trypsin were purchased from Merck (Darmstadt, Germany). For the detection of His-tagged proteins, a mouse anti-His antiserum was used (GE Healthcare, Munich, Germany), and all horseradish peroxidase-conjugated immunoglobulins were purchased from Dako (Hamburg, Germany).
Generation of Vectors and Purification of His-Tagged Proteins
The generation of vectors producing amino-terminally hexahistidine (His6)-tagged proteins BBA69, ErpP, and CspA of B. burgdorferi LW2, and BGA66 of B. bavariensis, respectively, are described elsewhere (29, 41–43).
The CspA-like encoding gene Bmayo_04535 (44) was amplified by PCR using primers CspA_Bmayo BamHI and CspA_Bmayo SalI (Supplementary Table 1). Following PCR amplification and digestion with appropriate restriction endonucleases, the DNA fragment was cloned into the expression vector pQE-30 Xa (Qiagen, Hilden, Germany). The resulting plasmid pQE CspA_Bmayo was then used to transform E. coli JM109 cells. Plasmids were sequenced to ensure that no mutations were incorporated during PCR and the cloning procedure. The production and purification of recombinant proteins have previously been described in detail (45).
To obtain a DNA fragment encompassing the CspA-like encoding gene Bmayo_04535 and the adjacent regulatory regions, genomic DNA isolated from B. mayonii strain MN14-1420 was used as a template for PCR with primers CspA_Bmayo_2 BamHI and CspA_Bmayo SphI (Supplementary Table 1). Following amplification and digestion, the purified DNA fragment was cloned into the shuttle vector pKFSS1 (46). Plasmids were prepared from presumptive E. coli clones with the Monarch plasmid kit (New England Biolabs, Frankfurt, Germany) and DNA inserts were sequenced by a commercial provider (Eurofins Genomics, Ebersberg, Germany).
SDS-PAGE, Western Blot, and Far-Western Blot Analysis
His6-tagged proteins were purified by affinity chromatography, separated by 10% Tris/tricine SDS-PAGE, and transferred to nitrocellulose membranes as described previously (45). Briefly, the membranes were blocked with 5% non-fat dry milk in TBS containing 0.1% Tween 20 (TBS-T). After three wash steps with TBS-T, membranes were incubated with a monoclonal antibody L41 1C11 (anti-FlaB) (1:100) (40) or an anti-His antibody (1:3,000) followed by horseradish peroxidase-conjugated anti-mouse immunoglobulins (1:1,000). Protein-antigen complexes were detected by tetramethylbenzidine as substrate as described elsewhere (47). For the identification of FH and FHL-1 binding proteins in Borrelia strains and the CspA interacting domains in FH, Far-Western blotting was employed by using purified FH and FHL-1, respectively, as previously described (27). Images of the gels and nitrocellulose membranes were processed by using a GS-710 image densitometer (Bio-Rad) and the Quantity One software version 4.2.1 (Bio-Rad) (see Supplementary Figures).
Enzyme-Linked Immunosorbent Assay (ELISA)
Nunc MaxiSorp 96-well microtiter plates (Thermo Fisher Scientific) were coated with 5 μg/ml of His6-tagged proteins or BSA in PBS at 4°C as described (29). Wells were washed three times with PBS containing 0.05% (v/v) Tween 20 (PBS-T) and then blocked with Blocking Buffer III BSA (AppliChem, Darmstadt, Germany). Following three washes with PBS-T, 100 μl FH or FHL-1 (5 μg/ml) were added. Following incubation for 1 h at RT, wells were washed thoroughly with PBS-T and binding of complement regulators were then assessed by incubation of the wells with a polyclonal goat anti-FH antiserum (1:1,000). After washing, protein complexes were detected by using HRP-conjugated anti-goat immunoglobulins (1:2,000). Afterwards, o-phenylenediamine (Merck, Darmstadt, Germany) was added to the wells and the absorbance was measured at 490 nm. Additionally, CspA of B. mayonii MN14-1420 was immobilized and incubated with increasing amounts of FH to determine dose-dependency of the binding and to calculate the dissociation constant.
Binding of FH to spirochetes was also assessed by ELISA. Briefly, bacterial cells (2 × 107 cells) in 100 μl PBS were immobilized to Nunc MaxiSorp 96-well microtiter plates at 4°C overnight and binding of FH was detected as described above.
C3b Degradation Assay
Factor I-mediated C3b inactivation in the presence of spirochetes was assayed after pre-incubation of 1 × 108 borrelial cells with PBS supplemented with 1 μg/ml FH for 1 h at RT as described (27). Following incubation, cells were washed twice with PBS and resuspended in 30 μl PBS containing 10 μg/ml C3b and 20 μg/ml factor I. To analyze C3b degradation products by Western blotting, the bacterial cells were sedimented by centrifugation and supernatant were subjected to SDS-PAGE.
In addition, cofactor activity of FH bound to purified proteins was analyzed by monitoring factor I-mediated degradation of C3b using ELISA. Briefly, purified proteins (100 ng/ml) were immobilized on Nunc MaxiSorp 96-well microtiter plates at 4°C and after blocking, wells were incubated with FH (100 ng/ml) for 1 h at RT. After washing, PBS containing C3b (10 μg/ml) and factor I (20 μg/ml) was added. Following incubation, SDS-PAGE sample buffer was added. Each reaction was loaded on a 10% Tris/tricine SDS gel and C3b inactivation products were then analyzed by Western blotting using a polyclonal anti-C3 antibody.
Complement Inactivation Assays
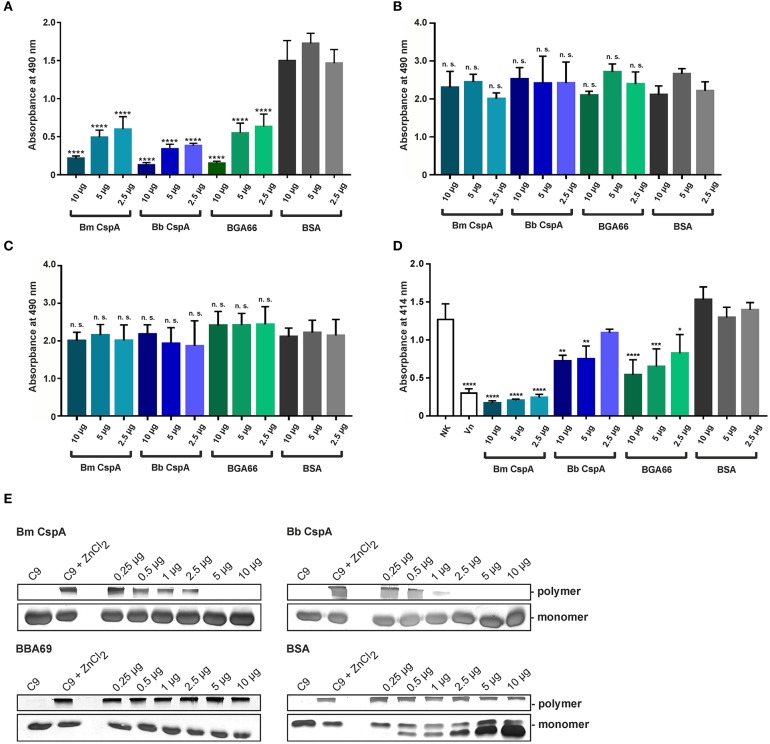
The inhibitory capacity of CspA of B. mayonii MN14-1420 on the classical (CP), Lectin (LP), and alternative pathway (AP) was assessed by ELISA. Nunc MaxiSorp 96-well microtiter plates were coated with either human IgM (30 ng/ml) (Merck, Darmstadt, Germany) for the CP, mannan (1 μg/ml) (Merck, Darmstadt, Germany) for the LP or LPS (100 ng/ml) (Hycult Biotech, Beutelsbach, Germany) for the AP at 4°C overnight. Following three wash steps with TBS containing 0.05% (v/v) Triton X-100 (TBS-T), wells were blocked with PBS-T for 2 h at RT. NHS (1% for the CP, 2% for the LP, and 15% for the AP) was then pre-incubated with increasing concentrations (2.5, 5, and 10 μg) of His6-tagged proteins for 15 min at RT before added to the wells to initiate complement activation. After washing with TBS-T, a monoclonal anti-C5b-9 antibody (1:500) (Quidel, Athens, OH; USA) was added. Following incubation for 1 h at RT, wells were washed thoroughly with TBS-T and incubated with HRP-conjugated anti-mouse immunoglobulins (1:1,000) at RT for 1 h. The reactions were developed as described above.
In order to examine the inhibitory potential of CspA of B. mayonii MN14-1420 on the terminal pathway, a hemolytic assay was performed as described previously (29). Briefly, sensitized sheep erythrocytes (1.5 × 107 cells) were pre-incubated with C5b-6 (1.5 μg/ml) for 10 min at RT. In parallel, complement C7 (2 μg/ml), C8 (0.4 μg/ml), and C9 (2 μg/ml) were pre-incubated with or without purified His6-tagged proteins (2.5, 5, or 10 μg) for 5 min at RT. The pre-incubated proteins were then added to the C5b-6 coated sheep erythrocytes. Following incubation for 30 min at 37°C, erythrocytes were sedimented by centrifugation and the supernatants were transferred to a microtiter plate. Finally, the hemolysis was determined by measuring the absorbance of the supernatant at 414 nm.
Determination of the Inhibitory Capacity of CspA of B. mayonii MN14-1420 on C9 Polymerization
To assess the inhibitory capacity of CspA of B. mayonii MN14-1420 on C9 polymerization, increasing concentrations (0.25–10 μg) of CspA of B. mayonii MN14-1420, CspA of B. burgdorferi LW2, BBA69, and BSA was incubated with C9 (3 μg) as previously described (28, 41, 48). Thereafter, auto-polymerization of C9 was induced by adding 50 μM ZnCl2 to each reaction mixture. As additional controls, purified C9 was incubated with or without ZnCl2. Reaction mixtures were then subjected to 8% Tris/Tricin-SDS gels and monomeric and polymeric C9 molecules were visualized by silver staining.
Serum Bactericidal Assay
Spirochetes grown at mid-logarithmic phase were sedimented by centrifugation and resuspended in 500 μl BSK medium. Reaction mixtures consisting of 50 μl highly viable spirochetes (1 × 107) and 50 μl of NHS were incubated at 37°C with gentle agitation. The percentage of motile and viable cells was determined by dark field microscopy after 1, 2, 4, and 6 h, respectively. Nine microscopy fields were counted for each time point per strain. Each test was performed at least three times.
Serum Adsorption Assay
Spirochetes grown at mid-logarithmic phase were harvested and resuspended in 500 μl Gelatin veronal-buffered saline (GBS, Complement Technology, Tyler, Texas) as described previously (27). Briefly, sedimented bacteria (2 × 109) were resuspended in 750 μl NHS-EDTA and incubated for 1 h at RT. After washing, the proteins bound to the bacteria surface were eluted by using 0.1 M glycine-HCl, pH 2.0. After adding 1 M Tris-HCl (pH 9.0), the cell debris were sedimented and the supernatant were analyzed by SDS-PAGE and Western blotting as previously described (27).
Pull-Down Assay for Detecting Interacting Serum Proteins
Purified His6-tagged proteins were immobilized onto magnetic beads (Dynabeads®) as described previously (49). Briefly, recombinant proteins (40 μg) bound to magnetic particles were incubated with 500 μl NHS-EDTA for 1 h on ice and after washing, bound serum proteins were eluted with 100 mM glycine-HCl (pH 2.0). The eluate and the last wash fraction were loaded onto 10% Tris/tricine gels and proteins were detected by silver staining.
Transformation and Characterization of Serum-Sensitive B. garinii Producing CspA of B. mayonii MN14-1420
A high-passage, non-infectious B. garinii strain G1 chosen as surrogate strain was grown in 100 ml BSK medium (Bio&SELL) and electrocompetent cells were prepared as described previously (30). After electroporation, spirochetes were transferred to fresh BSK medium without antibiotics (50). Following incubation for 24 h at 33°C, the cell suspension were further diluted by adding 90 ml BSK medium containing streptomycin (25 μg/ml) and 200 μl aliquots were seeded into 96-well cell culture plates. After 2–3 months, streptomycin-resistant clones could macroscopically be detected by a color change of the medium. Selected clones were then further characterized by amplifying the CspA encoding gene using primers M31 For and M31 Rev (Supplementary Table 1) as described (49).
In situ Surface Assessment of CspA of B. mayonii MN14-1420 on Spirochetes by a Protease Degradation Assay
Localization of CspA of B. mayonii MN14-1420 on the surface of viable spirochetes was assessed by an in situ protease degradation assay as previously described (30). Borrelial cells (2 × 108) were incubated with increasing concentrations of proteinase K and trypsin (6.25–50 μg/ml), respectively, for 2 h at RT. All reactions were terminated by adding 5 μl phenylmethylsulfonyl fluoride (Merck, Darmstadt, Germany) and 71 μl of cOmplete™ protease inhibitor cocktail (Merck, Darmstadt, Germany). Following sedimentation, cells were washed twice with PBS and lysed 5 times by sonication. Borrelial lysates were then subjected to 10% Tris/tricine SDS-PAGE and respective proteins were then detected by Far Western blotting (FH binding) or by Western blotting (FlaB) as described above.
Sequence Analysis
For sequence analysis, generation of phylogenetic trees and sequence alignments, we used CLC sequence Viewer 8.0 (QIAGEN Aarhus A/S, Denmark) and EMBOSS Needle (https://www.ebi.ac.uk).
Statistical Analysis
“Unless stated otherwise, data represent means from at least three independent experiments, and error bars indicate SD. One-way ANOVA analysis with Bonferroni's multiple comparison post test (95% confidence interval) was employed for statistical analysis using GraphPad Prism version 7. Results were deemed statistically significant for the following p-values: ***P < 0.001 and ****P < 0.0001” (45).
Results
B. mayonii MN14-1420 Resists Complement-Mediated Killing in Human Serum
In contrast to other Borrelia species of the B. burgdorferi s.l. complex, B. mayonii causes exceptional high spirochaetemia in human blood, suggesting that this vector-borne pathogen successfully overcomes innate immunity, in particular complement. It is well-established that spirochetes belonging to the B. burgdorferi s.l. complex differ in their serum susceptibility pattern to human serum (51–54). To gain insight into the fundamental properties of complement evasion of this pathogen, spirochetes were treated with 50% of non-immune human serum (NHS) or heat-inactivated serum (hiNHS) and survival was assessed by counting motile cells after 1, 2, 4, and 6 h of incubation at 33°C. As shown in Figure 1, the motility and viability of the cells remained unaffected over the entire incubation period when B. mayonii MN14-1420 and serum-resistant strain B. burgdorferi LW2 were employed. As expected, more than 90% of the cells of the serum-sensitive B. garinii strain G1 were immotile after 6 h. In agreement with previous findings, most of these cells showed signs of fragmentations, cell lysis, and bleb formation (53). This finding suggests that B. mayonii MN14-1420 resists complement-mediated killing.
Figure 1.
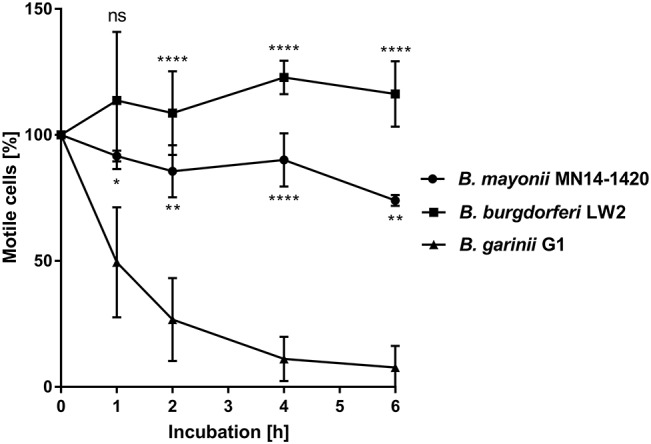
B. mayonii resists complement-mediated killing in human serum. Survival of B. mayonii MN14-1420, B. burgdorferi LW2, and B. garinii G1 in 50% NHS was monitored by dark-field microscopy. Viability and motility of borrelial cells were determined at 1, 2, 4, and 6 h. At least four independent experiments were conducted, each with very similar results. For clarity, only data from a representative experiment is shown. All experiments were performed at least four times. ****p ≤ 0.0001, **p ≤ 0.0021, *p ≤ 0.0002; one-way ANOVA with Bonferroni post test (confidence interval = 95%). ns, no statistical significance.
B. mayonii MN14-1420 Interacts With Complement Regulator FH and FHL-1
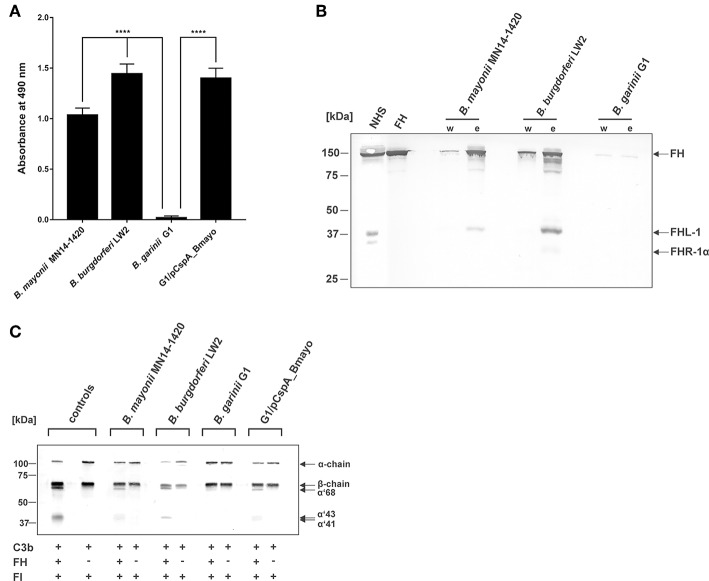
Binding of complement regulators FH and FHL-1 is a prerequisite for all serum-resistant spirochetes except B. bavariensis (41) to counteract complement and overcome bacteriolysis (25, 27, 55–57). To investigate recruitment of complement regulator FH by spirochetes, cells were immobilized onto microtiter plates and binding of purified FH was assessed by ELISA. B. mayonii MN14-1420 and B. burgdorferi LW2 significantly bound FH while B. garinii G1 did not (Figure 2A). Having demonstrated that immobilized B. mayonii interacts with FH, we next examined if viable spirochetes were able to bind native complement regulators from human serum under close-to-native conditions. Cells of B. mayonii MN14-1420, B. burgdorferi LW2, and B. garinii G1 (each 2 × 109) were incubated with 750 μl NHS-EDTA and serum proteins bound to the spirochetes were eluted by adding glycine. The reaction mixtures were then subjected to SDS-PAGE following Western blotting. As depicted in Figure 2B, B. mayonii MN14-1420 bound FH and FHL-1 as did control strain B. burgdorferi LW2. Additionally, a weak signal corresponding to FH-related protein-1α (FHR-1α) was detected in the latter borrelial strain, while B. garinii G1 did not bind any complement regulators. To further explore the functional activity of FH bound to the bacterial surface, factor I-mediated cleavage of C3b was assayed. Spirochetes immobilized onto microtiter plates were incubated with or without FH and after washing reaction mixtures consisting of purified factor I and C3b were added. After incubation, supernatants treated with sample buffer were loaded to 10% Tris/tricine SDS gels and C3b cleavage products were detected by Western blotting. Characteristic degradation products corresponding to the 68-, 43-, 41-kDa fragment of C3b (Figure 2C) were detected in B. mayonii MN14-1420 and B. burgdorferi LW2 but not in B. garinii G1, indicating that FH bound to surface of serum resistant strains retained cofactor activity. In contrast, no inactivated C3b fragments could be detected in the absence of FH.
Figure 2.
Interaction of B. mayonii with complement regulator FH. (A) Binding of purified FH to immobilized spirochetes was assessed by ELISA. Bound FH was detected using a polyclonal anti-FH antiserum (1:1,000 dilution). All experiments were performed at least four times, with each individual test carried out in triplicate. ****p ≤ 0.0001, one-way ANOVA with Bonferroni post test (confidence interval = 95%). (B) Determination of FH binding to viable spirochetes. B. mayonii MN14-1420, B. burgdorferi LW2, and B. garinii G1 were incubated in NHS-EDTA and cell-bound proteins were eluted. The last wash (w) and the eluate (e) fractions were separated and transferred to nitrocellulose. For detection of molecules of the FH protein family, a polyclonal anti-FH antibody (dilution 1:1,000) was applied. The mobilities of molecular mass standards are shown to the left of the panel. For better visualization of faint signals, the contrast has been increased as indicated in Supplementary Figure 4. (C) Determination of the regulatory activity of cell-bound FH. Factor I-mediated inactivation of C3b was assessed by the detection of C3b cleavage products. Spirochetes were incubated with purified FH and afterwards with C3b and factor I. For control purposes, all reactions were also performed in the absence of FH. The C3b fragments were visualized by Western blotting using an anti-human C3 antiserum (dilution 1:1,000). As additional controls, samples containing C3b and factor I were incubated with (+) or without (-) purified FH, respectively. The mobility of the α'- and the β-chain of C3 and the cleavage products of the α'-chain the α'68,' α'46 and α'43 fragments are indicated as well as the mobilities of molecular mass standards. A full scan of the original membrane is presented in Supplementary Figure 4.
Identification of FH-Binding Proteins in B. mayonii MN14-1420
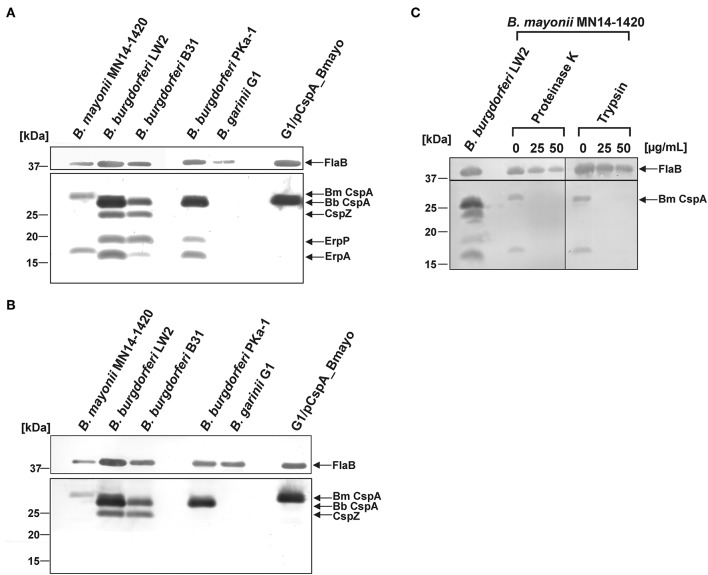
To identify potential FH-binding proteins in B. mayonii MN14-1420, Far Western blotting was employed. Two well-characterized isolates, B. burgdorferi B31 and B. burgdorferi PKa-1 (47) along with B. burgdorferi LW2 and B. garinii G1 were also included in this analysis. Cell lysates generated were separated, transferred to nitrocellulose, and incubated with NHS as a source of FH. Binding of FH was detected by a monospecific antibody. This approach identified two FH-binding proteins of approximately 30 kDa and 19 kDa produced by B. mayonii MN14-1420 (Figure 3A). In line with previous findings (47), four FH-binding proteins were detected in B. burgdorferi LW2 and B. burgdorferi B31 (CspA LW2, CspZ, ErpP, and ErpA) and three FH-binding proteins were identified in B. burgdorferi PKa-1. Of note, ErpC (18.5 kDa) is often hardly visible in B. burgdorferi LW2 and due to the similar molecular weight to ErpA (17.7 kDa) this particular CRASP protein is difficult to differentiate. To further characterize the FH-binding proteins, a Far Western blot was conducted using purified FHL-1 as a bait protein and the anti-CCP1-4 antiserum for detection. As depicted in Figure 3B, a single FHL-1 binding protein could be detected in cell lysates of B. mayonii MN14-1420 and B. burgdorferi PKa-1 while two FHL-1 binding proteins were found in the cell lysates of B. burgdorferi B31 and B. burgdorferi LW2. Owing to their capability to bind both regulators these CRASPs most likely correspond to CspA and CspZ.
Figure 3.
Identification and surface exposure of FH/FHL-1-binding proteins in B. mayonii MN14-1420. (A,B) Detection of FH/FHL-1-binding proteins by Far Western blot analysis using NHS as source of FH and purified FHL-1 (750 ng/ml). Cell lysates obtained from B. mayonii MN14-1420, B. burgdorferi LW2, B. burgdorferi B31, B. burgdorferi PKa-1, B. garinii G1, and transformant G1/pCspA_Bmayo were separated by 10% Tris/tricine-SDS-PAGE and transferred onto a nitrocellulose membrane. Flagellin (FlaB) was detected with the monoclonal antibody L41 1C11. The FH-binding proteins (A) were visualized by applying an anti-FH antiserum and FHL-1-binding proteins (B) were detected by using an anti-CCP1-4 antiserum. The corresponding to CspA protein of B. mayonii (Bm) MN14-1420, CspA of B. burgdorferi (Bb) LW2, CspZ, ErpP, and ErpA of B. burgdorferi s.s. are indicated at the right. (C) in situ protease accessibility assay. Native spirochetes were incubated with or without proteinase K or trypsin, then lysed by sonication and total proteins were separated by 10% Tris/tricine-SDS-PAGE. The band corresponding to CspA of B. mayonii is indicated on the right. The mobilities of molecular mass standards are indicated on the left. A full scan of the original membranes is presented in Supplementary Figure 5.
Additionally, an in situ protease susceptibility assay was performed to determine localization of FH-binding proteins on the outer surface of B. mayonii. Viable spirochetes were incubated with 25 and 50 μg/ml of proteinase K or trypsin following preparation of cell lysates used for detection of the 30-kDa and 18-kDa protein by Far Western blotting as described above. Both proteins were highly susceptible to any protease applied while the periplasmatic FlaB protein remains unaffected during treatment, indicating that these molecules are surface exposed (Figure 3C).
Due to the similar molecular weight of the 30-kDa protein to other CspA orthologs, a nucleotide Basic Local Alignment Search Tool (BLAST) search was conducted by using the sequence of the CspA encoding gene from B. burgdorferi B31 (Gene ID: 1194383; protein number AAC66286.1) as a reference to find potential FH-binding proteins in B. mayonii MN14-1420. Two cspA-like genes, Bmayo_04535 and Bmayo_04540 located on linear plasmid lp54 (44) encode putative outer surface proteins of 30.2 kDa and 34.8 kDa, respectively, whereby Bmayo_04535 exhibits the highest sequence identity of 61.2% and a similarity of 75.4% to the cspA gene of B. burgdorferi B31 (Supplementary Figure 1). The Bmayo_04535 encoding gene lacking the signal sequence and the lipidation motif was PCR amplified and the DNA fragment was cloned into the pQE-30 Xa expression vector to generate a molecule carrying a hexahistidine peptide at its N-terminus. His-tagged proteins including CspA of B. burgdorferi LW2 as well as BBA69, and ErpP of B. burgdorferi B31 (29, 42, 58) as well as BGA66 of B. bavariensis PBi (41) were included as controls to assess differences in the complement-binding capability of this CspA-like protein. Due to the lower molecular weight (<20 kDa) and its property to bind serum-derived FH but not FHL-1, the 18-kDa protein identified (Figure 3A) most likely belong to the OspE protein family of which two (Bmayo_05450 and Bmayo_05655) encoded on cp32-1 and cp32-3 were detected by comparative genomics (44).
CspA Orthologous Protein of B. mayonii MN14-1420 Interacts With Complement Regulator FH and FHL-1
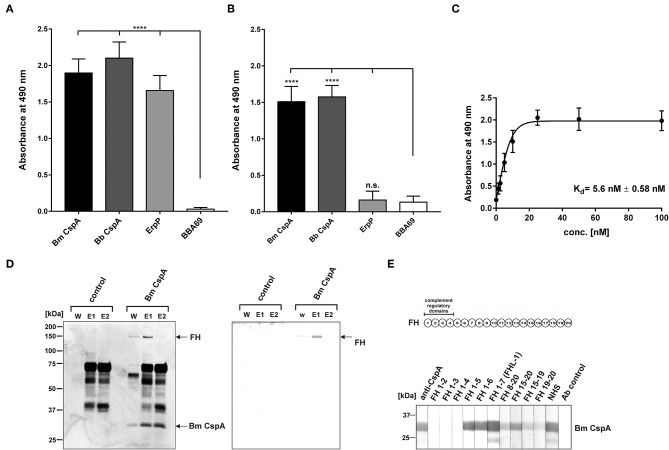
Assuming that the CspA-like protein of B. mayonii Bmayo_04535 displays binding properties similar to CspA of B. burgdorferi, we first investigated binding of FH and FHL-1 by ELISA. The CspA-like protein as well as CspA and ErpP of B. burgdorferi LW2 significantly bound FH (Figure 4A). In addition, the CspA-like protein and CspA of B. burgdorferi LW2 bound to FHL-1 while ErpP did not (Figure 4B). As expected, BBA69 neither interacted with FH nor FHL-1 (42). Further binding analysis revealed a strong binding affinity of the CspA-like protein of 5.6 nM ± 0.58 to FH (Figure 4C). Based on the identical binding properties and molecular weight to CspA of B. burgdorferi LW2, the 30 kDa protein was also termed CspA.
Figure 4.
Interaction of CspA of B. mayonii MN14-1420 with FH and FHL-1 and mapping of the FH/FHL-1 binding site. (A) Binding of recombinant borrelial proteins to FH. Microtiter plates were coated with His6-tagged proteins, incubated with purified FH, and antigen-antibody complexes were detected using an anti-FH antiserum. All experiments were performed at least three times, with each individual test carried out in triplicate. ****p ≤ 0.0001, one-way ANOVA with Bonferroni post test (confidence interval = 95%). (B) Binding of recombinant proteins to FHL-1. Binding of purified FHL-1 was assessed by ELISA as described in (A). (C) Dose-dependent binding of FH to CspA of B. mayonii MN14-1420. Recombinant CspA of B. mayonii MN14-1420 (5 μg/ml) was immobilized and incubated with increasing concentrations of FH. Binding curve and dissociation constant were approximated via non-linear regression, using a one-site, specific binding model. Data represent means and standard deviation of at least three different experiments, each conducted in triplicate. (D) FH adsorption to immobilized CspA of B. mayonii MN14-1420. Recombinant CspA of B. mayonii MN14-1420 immobilized onto magnetic particles was incubated with NHS. Empty beads were also incubated under the same conditions and used as a control to identify non-specific binding of serum proteins. After extensive washing, bound proteins were eluted with 100 mM glycine and eluate fraction e1 and e2 were separated by 10% Tris/tricine SDS-PAGE, following silver staining and Western blotting using a polyclonal anti-FH antibody. Mobilities of molecular mass standards are indicated to the left. For better visualization of faint signals, the background of the original silver stained gels was reduced as indicated in Supplementary Figure 8. (E) Mapping of the binding domain in FH. Schematic representation of the FH molecule (upper panel). The complement regulatory domains (CCP domains 1-4) are indicated. Localization of the CspA interacting domain in FH by Far Western blotting (lower panel). Purified CspA of B. mayonii (Bm) MN14-1420 was separated by 10% Tris/tricine SDS-PAGE, and transferred to nitrocellulose. The membrane strips were then incubated with constructs of FH containing different CCP domains FH1-2, FH1-3, FH1-4, FH1-5, FH1-6, FH1-7/FHL-1, FH8-20, FH15-20, FH15-19, FH19-20, NHS, and with the secondary Ab (Ab control). Bound proteins were visualized using polyclonal anti-FH antibody. A full scan of the original gel and membrane is presented in Supplementary Figure 6.
To further assess binding of serum-derived FH to the CspA protein of B. mayonii MN14-1420, a pull down assay was conducted. The purified His6-tagged CspA of B. mayonii MN14-1420 was immobilized to magnetic particles following incubation with inactivated NHS. Bound proteins eluted from the particles were separated by SDS-PAGE and detected by silver staining and Western blot analyses. As depicted in Figure 4D, immobilized CspA of B. mayonii MN14-1420 bound FH from human serum, confirming the experimental data obtained by ELISA.
Next we sought to gain further insight into the molecular interaction of the CspA protein of B. mayonii MN14-1420 with FH/FHL-1. In our previous investigation, we showed that CspA of B. burgdorferi interacts with short consensus repeats 5 through 7 of FH/FHL-1 and CCP19-20 of FH (31). In order to map the domains responsible for binding of the CspA protein of B. mayonii MN14-1420, a series of truncated FH constructs were used for Far Western blotting. The CspA-like protein bound constructs representing domains FH1-5, FH1-6, and FH1-7/FHL-1 as well as the construct comprising the C-terminal region FH15-20 (Figure 4E). Faint signals were also detected after incubation with constructs FH8-20, FH15-19, and FH 19-20. These findings suggest that CspA of B. mayonii MN14-1420 preferentially binds FH and FHL-1 by CCP5-7 and that interaction with both complement regulators did not influence their complement inhibitory activity. An additional interaction site appears to be located at the C-terminus of FH.
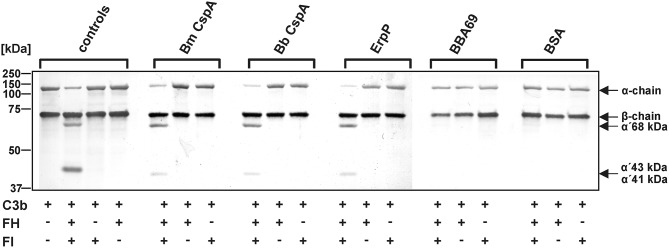
To confirm that binding of CspA of B. mayonii MN14-1420 to FH does not affect C3b inactivation mediated by factor I, an ELISA-based cofactor assay was conducted. Recombinant proteins coated onto microtiter plates were incubated with purified FH and after washing purified factor I and C3b were added. Each reaction mixture was then loaded to a 10% Tris/tricine SDS gels and C3b degradation products generated by FH cleavage were detected by a polyclonal antibody. The appearance of the 68-, 43-, 41-kDa fragments indicates that FH exerts cofactor activity when bound to CspA of B. mayonii MN14-1420, CspA of B. burgdorferi LW2, and ErpP (Figure 5). In contrast, none of the C3b cleavage products were detected when reactions with BBA69 and BSA were analyzed by Western blotting or in the absence of factor I.
Figure 5.
Determination of the C3b inactivation capacity of FH bound to CspA of B. mayonii MN14-1420. Purified proteins immobilized to microtiter plates were incubated with purified FH. After washing, C3b and factor I were added. As controls, all reactions were performed in the absence of FH or factor I (FI). All samples were subjected to Tris/tricine SDS-PAGE and transferred onto a nitrocellulose membrane. As additional controls, reaction mixtures containing different combinations of purified complement proteins were assessed. The various C3b fragments were visualized by Western blotting using an anti-human C3 antiserum (dilution 1:1,000). The mobility of the α'- and the β-chain of C3 and the cleavage products of the α'-chain the α'68,' α'46, and α'43 fragments are indicated as well as the mobilities of molecular mass standards. A full scan of the original membranes is presented in Supplementary Figure 7. For better visualization of faint signals, the contrast has been increased as indicated. Bm, B. mayonii; Bb, B. burgdorferi.
CspA Ortholog of B. mayonii MN14-1420 Inhibits Activation of the AP
To assess the inhibitory capacity of CspA of B. mayonii MN14-1420 on complement, the formation of the C5b-9 complex (MAC) following activation of the AP, CP, and LP by specific activators (LPS, IgM, and mannan) was detected by an ELISA-based assay. In this assay, generation of the C5b-9 complex after activation of the three pathways was measured by a neoepitope-specific antibody (59). Microtiter plates were coated with the specific activating substances and samples of NHS pre-incubated with increasing amounts of the respective proteins were added. As shown in Figure 6A, CspA of B. mayonii MN14-1420, CspA of B. burgdorferi LW2, BGA66 of B. bavariensis PBi inhibited the AP in a dose-dependent fashion but did not affect the CP or LP (Figures 6B,C). As expected, BSA used as control in all assays did not inhibit complement activation. These findings indicate that CspA of B. mayonii MN14-1420 specifically inhibits the AP.
Figure 6.
CspA of B. mayonii MN14-1420 terminates activation of the AP and TP and inhibits C9 polymerization. (A–C) Assessment of the inhibitory capacity of CspA of B. mayonii MN14-1420 on complement activation by an ELISA-based assay. Microtiter plates immobilized with LPS for the AP (A), IgM for the CP (B), and mannan for the LP (C) were incubated with NHS pre-incubated with increasing concentrations of recombinant proteins or BSA. After washing, formation of the MAC was detected by a monoclonal anti-C5b-9 antibody (dilution 1:500). All experiments were performed at least three times, with each individual test carried out in triplicate. ****p ≤ 0.0001, one-way ANOVA with Bonferroni post test (confidence interval = 95%). n.s., no statistical significance. (D) CspA-mediated inhibition of the TP. Sensitized sheep erythrocytes covered with the preforming C5b-6 complex were incubated with a reaction mixture containing C7, C8, and C9 that was pre-incubated with increasing concentrations of recombinant proteins or BSA. Following incubation, hemolysis of erythrocytes was detected at 414 nm. Means of three independent experiments are shown and error bars correspond to SD. Raw data were analyzed using one-way ANOVA with Bonferroni post test (confidence interval = 95%). ****p ≤ 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05. (E) CspA MN14-1420 inhibits C9 polymerization. C9 was incubated with increasing concentrations of recombinant proteins or BSA and ZnCl2 were then added to the samples to induced polymerization. C9 incubated with and without ZnCl2 was used as controls. Following incubation, reactions mixtures were separated by 7.5% SDS-PAGE and C9 monomers and high molecular weight polymers were visualized by silver staining. A full scan of the original gels is presented in Supplementary Figure 8. For better visualization of faint signals in (E), the contrast has been increased as indicated in Supplementary Figure 8. Bm, B. mayonii; Bb, B. burgdorferi.
CspA of B. mayonii MN14-1420 Terminates MAC Assembly by Inhibiting Auto-Polymerization of Complement C9
Assuming that CspA of B. mayonii MN14-1420 might also directly interfere with MAC formation, a cell-based hemolytic assay was employed. Initially, sheep erythrocytes were incubated with the pre-forming C5b-6 complex and increasing concentrations of CspA of B. mayonii MN14-1420, CspA of B. burgdorferi LW2, BGA66 of B. bavariensis PBi, and BSA, respectively, followed by being incubated with complement C7, C8, and C9. Both reaction mixtures were combined and the amount of erythrocyte lysis was measured. Among the three borrelial proteins investigated, CspA of B. mayonii MN14-1420 most efficiently inhibited the activation of the terminal pathway (TP) and protected lysis of sheep erythrocytes from complement-mediated lysis (Figure 6D). These findings suggest that CspA of B. mayonii MN14-1420 mainly affects AP- and TP-activation.
Having demonstrated that CspA of B. mayonii MN14-1420 terminates formation of the MAC, we sought to elucidate the molecular mechanism of terminal pathway inhibition by investigating interaction of CspA of B. mayonii MN14-1420 to C9. Monomeric C9 is able to auto-polymerize to SDS-resistant, high molecular weight C9 polymers in the presence of divalent Zn2+ which can be detected by SDS-PAGE (48, 60). Initially, CspA of B. mayonii MN14-1420, CspA of B. burgdorferi LW2, BGA66 of B. bavariensis PBi, and BSA were allowed to bind to C9 at increasing concentrations following initiation of C9 polymerization by adding 50 μM ZnCl2. As an additional control, purified C9 was incubated with or without Zn2+ at any reaction mixture following separation through SDS-PAGE. Monomeric and polymeric C9 were then visualized by silver staining. As shown in Figure 6E, CspA of B. mayonii MN14-1420 and CspA of B. burgdorferi LW2 inhibited C9 polymerization in a dose-dependent fashion at 5.0 and 2.5 μg, respectively. By contrast, polymerization of C9 was not affected when control proteins BBA69 and BSA were assessed under identical conditions. Consistent with previous reports, C9 polymers were readily detectable after incubation with ZnCl2 (48, 61). This finding indicates that CspA of B. mayonii MN14-1420, CspA of B. burgdorferi LW2, and BGA66 of B. bavariensis PBi interfered with C9 to terminate assembly of the MAC.
CspA of B. mayonii MN14-1420 Promotes Spirochete Binding to FH and Survival in Human Serum
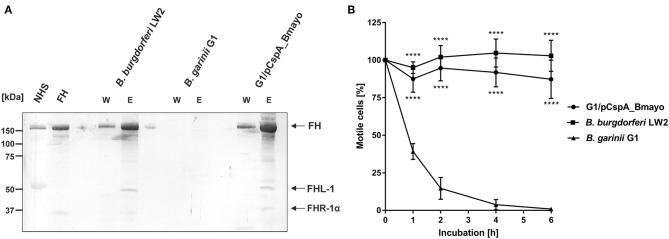
Having demonstrated that B. mayonii overcome complement-mediated bacteriolysis by interacting with complement regulator FH, we sought to generate a gain-of-function Borrelia strain to elucidate the role of CspA of B. mayonii MN14-1420 independently of the 18-kDa FH-binding protein in facilitating complement resistance. Following transformation of B. garinii G1 with a shuttle vector carrying the CspA encoding gene with the up and downstream flanking regions, the selected transformants were assessed for ectopical production of CspA. As expected, CspA of B. mayonii MN14-1420 was produced on the spirochetes' surface, supported by the proteins' protease sensitivity: CspA of B. mayonii MN14-1420 produced by B. garinii G1 was highly susceptible to proteinase K at concentrations <6.25 μg/ml and nearby completely degraded 12 μg/ml trypsin K (Supplementary Figure 2). Additionally, diverse approaches revealed that transformant G1/pCspA_Bmayo efficiently bound FH (Figures 2A, 3A, 7A) and the functional assay also revealed that FH bound to the surface of G1/pCspA_Bmayo inactivates C3b in the presence of factor I (Figure 2C).
Figure 7.
CspA of B. mayonii MN14-1420 facilitates resistance of Borrelia to complement-mediated killing. (A) Determination of FH binding to vital spirochetes. B. burgdorferi LW2, B. garinii G1, and B. garinii strain G1 producing CspA of B. mayonii MN14-1420 (G1/pCspA_Bmayo) were incubated in NHS-EDTA and cell-bound proteins were eluted using 0.1 M glycine. The last wash (w) and the eluate (e) fractions were separated by glycine-SDS-PAGE and transferred to nitrocellulose. For detection of molecules of the FH protein family, a polyclonal anti-FH antibody (dilution 1:1,000) was applied. The mobilities of molecular mass standards are shown to the left of the panel. A full scan of the original membranes is presented in Supplementary Figure 9. (B) Survival of G1/pCspA_Bmayo, B. burgdorferi LW2, and B. garinii G1 in 50% NHS was monitored by dark-field microscopy. Viability and motility of borrelial cells were determined at 1, 2, 4, and 6 h. At least four independent experiments were conducted, each with very similar results. ****p ≤ 0.0001; one-way ANOVA with Bonferroni post test (confidence interval = 95%).
Next, serum susceptibility of CspA producing B. garinii cells were assessed by challenging spirochetes with NHS. We found that the majority of cells of this spirochete strains were highly motile and viable after 6 h of human serum incubation, indicating that CspA of B. mayonii MN14-1420 promotes spirochetes to evade complement-mediated killing in human serum (Figure 7B). By contrast, cells of the wild type B. garinii G1 strain were strongly affected, showing a reduction of motile cells of more than 50% after 1 h of serum challenge and nearby all cells died after 4 h. These spirochetes showed signs of cell destruction, bleb formation, and loss of motility. When using hiNHS instead of NHS, spirochetes did not show morphological changes or impact on viability by dark-field microscopy.
Discussion
Recent studies revealed that B. mayonii achieves higher cell densities of spirochetes in the blood of infected patients, in contrast to the other LD causing genospecies such as B. burgdorferi sensu stricto (s.s.), B. afzelii, B. garinii, and B. bavariensis (1). Additionally, B. mayonii appears to exhibit a tropism for the central nervous system like B. garinii and B. bavariensis. These findings implicated that this novel human pathogenic genospecies developed means to successfully combat complement as the first line of defense of the human host and possess unique adhesins to bind to neuronal tissues. How B. mayonii resists complement-mediated bacteriolysis and what are the underlying molecular mechanisms of serum resistance are important issues that require extensive investigations. Here, we elucidated for the first time the immune evasion strategy used by B. mayonii to survive in human serum and identified the key molecule conferring serum resistance. The molecular mechanism of serum resistance involves inactivation of complement at the activation level of complement C3 by binding FH and FHL-1 in conjunction with the inhibition of MAC assembly by targeting C9 polymerization. These modes of action efficiently prevent opsonization and inhibit deposition of complement components on the cell surface of B. mayonii. Furthermore, functional analyses of a gain-of-function strain producing the key FH/FHL-1 binding protein CspA of B. mayonii MN14-1420 convincingly shown that this particular molecule rendered the surrogate B. garinii strain highly resistant to human serum.
Acquisition of fluid-phase complement regulators FH and FHL-1 are one of the prominent strategies utilized by a range of human pathogens including group A and group B streptococci as well as pneumococci (62–66), Neisseria meningitides (67), N. gonorrhoeae (68, 69), Moraxella catarrhalis (70), Staphylococcus aureus (71), Pseudomonas aeruginosa (72), and Leptospira interrogans (73) to combat the destructive effects of complement. Concerning LD spirochetes, previous investigations clearly showed that all serum-resistant Borrelia including B. burgdorferi s.s., B. afzelii, and B. spielmanii except B. bavariensis developed this immune evasion strategy for terminating complement activation at the most critical step of the cascade, thus allowing spirochetes to survive in the otherwise hostile environment of human blood (25, 27, 57). In this study, we demonstrate—to our knowledge for the first time—that B. mayonii displays a serum-resistant phenotype similar to B. burgdorferi s.s (Figure 1) and that resistance to complement strongly correlated with the acquisition of serum-derived immune regulators, FH and FHL-1 (Figures 2A,B, 7). More importantly, in the presence of factor I, FH bound to the borrelial surface clearly inactivates C3b (Figure 2C). The characteristic degradation products of inactivated C3b, α68-, α43-, and α41-kDa fragments were detectable upon incubation with FH but not in the absence of this complement regulator suggesting that B. mayonii lacks endogenous proteolytic activity to cleave C3b. This observation is in strong agreement with previous findings demonstrating that serum-resistant microorganisms degrade C3b following binding of FH and/or FHL-1 indicating that the interaction with complement regulators is of physiological relevance (25, 27, 57, 65, 71, 72).
Like other human pathogens, Borrelia developed additional means to overcome complement attack, e.g., by the interaction with (i) components of the CP namely C1r or C4b (74, 75), (ii) components of the TP (C7, C8, C9) (28, 41), and (iii) complement regulator C4Bp (76), respectively. Targeting different activation steps of the cascade might allow spirochetes to inhibit complement in the absence of, or with limited access to FH/FHL-1 as previously demonstrated for B. burgdorferi and its capacity to inhibit complement by blocking activation of the TP using FH/FHL-1 depleted human serum (28). Similar mechanisms have been described for certain parasites including Entamoeba histolytica, Schistosoma mansoni, Trichinella spiralis, and Trypanosoma cruzi, all of which are able to bind complement component of the so-called “MAC protein family” for complement inactivation (77–81). Our data strongly support the notion of a similar immune evasion strategy exploit by B. mayonii to terminate MAC assembly by counteracting C9 polymerization (Figure 6E).
A range of diverse FH-binding proteins have been identified so far from LD and relapsing fever borreliae including molecules exhibiting proclivities to interact with FH and FHL-1 (CspA LW2, CspZ, FhbA), FH and FHRs (ErpA, ErpC, ErpP, BsCRASP-3, Erp60, Erp61, Erp62, BhCRASP-1, HcpA, BpcA) or FH alone (CbiA) (30, 47, 49, 82–89). Among these Borrelia-derived proteins, CspA of B. burgdorferi s.s., B. afzelii, and B. spielmanii, CspZ of B. burgdorferi s.s., BhCRASP-1 of B. hermsii, BpcA of B. parkeri, and CbiA of B. miyamotoi facilitate resistance to complement-mediated killing as demonstrated by using gain-of-function or loss-of-function borrelial strains (30, 32, 33, 86, 88, 89). Using Far-Western blotting, we identified a FH/FHL-1-binding protein of approximately 30 kDa and a FH-binding protein of 19 kDa of which the 30 kDa molecule mostly resembled the CspA protein of B. burgdorferi and the 19 kDa protein appears to belong to the Erp protein family (Figures 3A,B). Our bioinformatic approach identified a CspA-like encoding gene located on the linear plasmid lp54 annotated as Bmayo_04535 (44) which is herein termed CspA and a gene encoding for an OspE-like protein (WP_075552645.1). The latter has a calculated molecular weight of 20.2 kDa and exhibits 77% sequence identity to the ErpA protein of B. burgdorferi B31. Recently, the FH binding site in ErpA and ErpC has been delineated by a computer-aided modeling of the ErpA/FH-complex based on the crystal structures of both proteins and CCP20 of FH (PDB ID codes: 3RJ3, 3SW0 and 2G7I) (90). Seven of nine residues involved in binding of OspE to FH (90) are also conserved in the identified OspE-like protein including positions Glu75, Asn79, Gly86, Thr90, Tyr120, and Asp126 suggesting that this particular molecule displays FH-binding properties (Supplementary Figure 3). Further studies will definitively clarify whether the OspE-like protein of B. mayonii interacts with FH and/or with other members of the FH protein family such as FHR-1, FHR-2, and FHR-5, respectively, as previously been demonstrated for ErpA, ErpC, and ErpP (49, 91).
The binding analyses showed that CspA of B. mayonii MN14-1420 displays a strong binding affinity of 5.6 nM to FH (Figure 4C) and confers serum resistance when ectopically produced in a serum-sensitive surrogate strain (Figure 7B). A similar FH-binding affinity of 28 nM have been reported for the CspA protein from B. burgdorferi s.s. (31) underscoring a preferred binding capability of these CspA orthologs to FH. Additionally, the functional characterization of the protein-protein interaction discloses the mode of action on complement. Binding of CspA of B. mayonii MN14-1420 via CCP domains 5 to 7 (Figure 4E) FH retains its cofactor activity for C3b inactivation (Figure 5) in the presence of factor I and also augmented the regulators' decay accelerating activity to rapidly eliminate the formed C3 convertase from the bacterial surface as also previously demonstrated for the CspA orthologous molecules of B. burgdorferi s.s., B. afzelii, and B. spielmanii and CspZ of B. burgdorferi s.s. (29, 30). The data collected with the CspA producing B. garinii strain confirm the findings obtained with the His-tagged protein as well as with the wild type B. mayonii strain as FH bound to the transformant displayed comparable regulatory activity (Figure 2C) and confers serum resistance (Figure 7B). Moreover, CspA of B. mayonii MN14-1420 exhibit dual inhibitory functions on complement, (i) termination of the AP by interacting with complement regulators FH/FHL-1 and (ii) blocking MAC assembly by binding to C9. As expected, CspA of B. mayonii MN14-1420 and CspA of B. burgdorferi B31 showed a similar inactivation capacity on the AP but did not target the CP and LP while CspA of B. mayonii MN14-1420 more strongly affected the TP compared to CspA B31 and BGA66 of B. bavariensis (Figure 6D). Of note, polymerization of C9 is more efficiently inhibited by CspA of B. mayonii MN14-1420 compared to CspA of B. burgdorferi LW2, thus it is tempting to speculate that CspA of B. mayonii block activation of the TP by interaction with additional terminal components or with pre-forming MAC complexes as previously elucidated for BGA66 and BGA71 of B. bavariensis PBi (41).
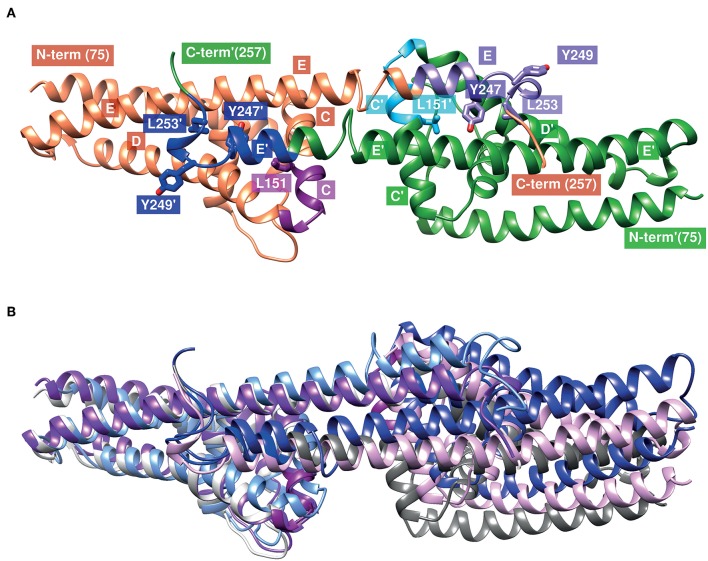
The CspA monomer consists of five α-helices (helix A to helix E) that are connected by short loop regions to forms a “lollipop”-like structure in which the C-terminal-located helix E protrudes outwards from the pyramidal core composed of helix A to D (Figure 8) (94). Two CspA monomers assembled together by the C-terminal helices by generating a large cleft at the dimer interface (Figure 8A) (94). The extensive buried surface of the interface build by helix C and the C-terminal helix E of both subunits monomers is typical for a biologically relevant dimer and for the interaction of complement regulators. It has previously been hypothesized that the interface involving amino acid residues 146–154 located at C-terminal end of helix C and amino acid residues 233–247 at the C-terminal helix E is most likely the binding site for the interaction with both complement regulators (94, 95). By modeling the structure of CspA of B. mayonii MN14-1420, the homologous regions in this CspA ortholog are represented by amino acid residues 151–159 and 240–254, respectively (Figure 8A). Using in vitro mutagenesis, we previously identified four amino acid residues, L146, Y240, D242, and L246 in CspA of B. burgdorferi (Figure 8A) which drastically influence ligand binding (96). Replacement of leucine 146 (L151 in CspA of B. mayonii MN14-1420) with a histidine and tyrosine 240 (Y247 in CspA of B. mayonii MN14-1420) with an alanine strongly affect binding of FH and FHL-1. Interestingly, the leucine residue of the first subunit (L151) and the tyrosine residue of the second subunit (Y247) point into the same hydrophobic cavity (Figure 8A), and, thus it is likely that substitutions at these sides changes the orientation of the C-terminal helix. Furthermore, the substitution L246D (L253 in CspA of B. mayonii MN14-1420) with its additional negative charge could interact with polar or hydrophobic residues, situated on the opposed helices D and E of the other subunit, thereby changing the orientation of the C-terminal helix as well. Obviously, the position of the C-terminal helix could control the orientation of the second subunit and, thus, the overall form of the cleft between the subunits and consequently the binding of FH and FHL-1 to CspA orthologs. In accordance with our conclusions, structural superimposition (92) of the known CspA structures [1w33 (94), 4bl4 (97), 5a2u (https://www.rcsb.org/structure/5a2u)] revealed different orientations of the C-terminal helix and the second subunit (Figure 8B). It has been shown that the replacement of an aspartic acid at position 242 by a tyrosine residue results in a reduced binding capability of FHL-1 to CspA of B. burgdorferi (96). The homologous residue in CspA of B. mayonii MN14-1420 is a tyrosine residue at position 249 indicating that this particular amino acid residue does not participate in the interaction with the complement regulators. Additionally, both tyrosine residues are exposed to the solvent making it rather unlikely that they are important for binding. The findings that homologous regions are conserved in CspA of B. mayonii MN14-1420 in conjunction with the binding data collected strongly suggest that the same regions are engaged in the interaction with the key complement regulators of the AP.
Figure 8.
Structural model of the CspA of B. mayonii MN14-1420 dimer and superimposed structures of CspA of B. burgdorferi. (A) The two subunits are represented as orange or green ribbons, regions involved in binding of FHL-1 or FH are colored magenta (151–159) and violet (240–246) in the orange subunit and light blue and blue in the green subunit, respectively. Helices are marked by a capital letter, mutated residues are shown in sticks, and an apostrophe denotes the second subunit. Molecular graphics images are produced using the UCSF Chimera (92). (B) Superimposed structures of the CspA homodimer of both orthologs (1w33, 4bl4, 5a2u) show the different orientations of the C-terminal helix and the second subunit. The subunits in the structures of 1w33, 4bl4, and 5a2u are colored light-and dark-gray, light- and dark-magenta and light- and dark-blue, respectively. We modeled the CspA of B. mayonii MN14-1420 structure according to the crystal structure of CspA of B. burgdorferi B31 (1w33) using the Swiss-Model automated comparative protein modeling server (93).
In conclusion, this is the first study describing an immune evasion mechanism utilized by the novel blood-borne human pathogenic LD genospecies B. mayonii. We identified the key FH/FHL-interacting ligand, CspA of B. mayonii MN14-1420 as an immune evasion molecule terminating AP and TP activation. At least two modes of action were delineated for this particular protein: (i) acquisition of FH and FHL-1 following factor I-mediated degradation of C3b and (ii) inhibition of MAC assembly by direct binding to C9. More importantly, heterologous production of CspA protects spirochetes from the deleterious effects of complement and renders serum susceptible spirochetes fully resistant. It will be interesting to determine at which time point the CspA encoding gene is expressed during the tick-to-host infection cycle and whether the expression pattern matches that of CspA of B. burgdorferi. To further explore the role of CspA within the infection process of B. mayonii, a virulent loss-of-function or a gain-of-function strain will be necessary.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics committee of the University Hospital Frankfurt (control number 160/10 and 222/14), Goethe University of Frankfurt am Main. The patients/participants provided their written informed consent to participate in this study in accordance with the Declaration of Helsinki.
Author Contributions
LW, VS, and FR: study design, experimental work, data interpretation, figure preparation, and final approval. KF-W: structure analysis, data interpretation, figure preparation, and final approval. PZ: contribution of reagents, critical reading of the manuscript, and final approval. PK: study design, data interpretation, figure and table preparation, drafting the article, wrote the manuscript, and final approval.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are greatly indebted to Michael Kirschfink and Renate Rutz (University Heidelberg, Germany) for generously providing sheep erythrocytes. We want to thank Katja Becker (Justus Liebig University Gießen, Germany) for her generous support (LOEWE Center DRUID Novel Drug Targets against Poverty-Related and Neglected Tropical Infectious Diseases, project E3) and Arno Koenigs and Yi-Pin Lin for critical review and language editing of the manuscript. Parts of the data have been presented at the Gordon Research Conference (Biology of Spirochetes, January, 21–26, 2018, Ventura, USA) and at the 13th International Symposium on Ticks and Tick-borne Diseases (March, 28–30, 2019, Weimar, Germany).
Footnotes
Funding. This work was supported by the Deutsche Forschungsgemeinschaft Funginet CRC Transregio 124 Pathogenic Fungi and their human host: Networks of Interaction, project C6 (PZ), by NIH R01AI121401 (PK), and by the LOEWE Center DRUID (Novel Drug Targets against Poverty-Related and Neglected Tropical Infectious Diseases), project C3 (PK) and E3 (KF-W).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.02722/full#supplementary-material
References
- 1.Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, Davis JP, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dise. (2016) 16:556–64. 10.1016/S1473-3099(15)00464-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pritt BS, Respicio-Kingry LB, Sloan LM, Schriefer ME, Replogle AJ, Bjork J, et al. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int J Syst Evol Microbiol. (2016) 66:4878–80. 10.1099/ijsem.0.001445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisen L, Breuner NE, Hojgaard A, Hoxmeier JC, Pilgard MA, Replogle AJ, et al. Comparison of vector efficiency of Ixodes scapularis (Acari: Ixodidae) from the northeast and upper midwest of the United States for the Lyme disease spirochete Borrelia mayonii. J Med Entomol. (2017) 54:239–42. 10.1093/jme/tjw160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer PH, De Martino SJ, Hansmann Y, Zilliox L, Boulanger N, Jaulhac B. No evidence of Borrelia mayonii in an endemic area for Lyme borreliosis in France. Parasit Vectors. (2017) 10:282. 10.1186/s13071-017-2212-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breuner NE, Hojgaard A, Eisen L. Lack of evidence for transovarial transmission of the Lyme disease spirochete Borrelia mayonii by infected female Ixodes scapularis (Acari: Ixodidae) ticks. J Med Entomol. (2018) 55:739–741. 10.1093/jme/tjx248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolan MC, Breuner NE, Hojgaard A, Boegler KA, Hoxmeier JC, Replogle AJ, et al. Transmission of the Lyme disease spirochete Borrelia mayonii in relation to duration of attachment by nymphal Ixodes scapularis (Acari: Ixodidae). J Med Entomol. (2017) 54:1360–4. 10.1093/jme/tjx089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolan MC, Breuner NE, Hojgaard A, Hoxmeier JC, Pilgard MA, Replogle AJ, et al. Duration of Borrelia mayonii infectivity in an experimental mouse model for feeding Ixodes scapularis larvae. Ticks Tick Borne Dis. (2017) 8:196–200. 10.1016/j.ttbdis.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 8.Dolan MC, Hojgaard A, Hoxmeier JC, Replogle AJ, Respicio-Kingry LB, Sexton C, et al. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick Borne Dis. (2016) 7:665–9. 10.1016/j.ttbdis.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 9.Eisen L. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis. (2018) 9:535–42. 10.1016/j.ttbdis.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson TL, Graham CB, Hojgaard A, Breuner NE, Maes SE, Boegler KA, et al. Isolation of the Lyme disease spirochete Borrelia mayonii from naturally infected rodents in Minnesota. J Med Entomol. (2017) 54:1088–92. 10.1093/jme/tjx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blom AM, Hallström T, Riesbeck K. Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol Immunol. (2009) 46:2808–17. 10.1016/j.molimm.2009.04.025 [DOI] [PubMed] [Google Scholar]
- 12.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Micro. (2008) 6:132–42. 10.1038/nrmicro1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walport MJ. Complement - second of two parts. N Engl J Med. (2001) 344:1140–4. 10.1056/NEJM200104123441506 [DOI] [PubMed] [Google Scholar]
- 14.Walport MJ. Complement - first of two parts. N Engl J Med. (2001) 344:1058–66. 10.1056/NEJM200104053441406 [DOI] [PubMed] [Google Scholar]
- 15.Zipfel PF. Complement and immune defense: from innate immunity to human diseases. Immunol Lett. (2009) 126:1–7. 10.1016/j.imlet.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 16.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. (2010) 11:785–97. 10.1038/ni.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trouw LA, Daha MR. Role of complement in innate immunity and host defense. Immunol Lett. (2011) 138:35–7. 10.1016/j.imlet.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 18.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. (2009) 9:729–40. 10.1038/nri2620 [DOI] [PubMed] [Google Scholar]
- 19.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part i - molecular mechanisms of activation and regulation. Front Immunol. (2015) 6:262. 10.3389/fimmu.2015.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zipfel PF, Skerka C, Hellwage J, Jokiranta ST, Meri S, Brade V, et al. Factor H family proteins: on complement, microbes and human diseases. Biochem. Soc. Trans. (2002) 30(Pt 6):971–8. 10.1042/bst0300971 [DOI] [PubMed] [Google Scholar]
- 21.Forneris F, Wu J, Xue X, Ricklin D, Lin Z, Sfyroera G, et al. Regulators of complement activity mediate inhibitory mechanisms through a common C3b-binding mode. EMBO J. (2016) 35:1133–49. 10.15252/embj.201593673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zipfel PF, Skerka C. FHL-1/reconectin: a human complement and immune regulator with cell-adhesive function. Immunol Today. (1999) 20:135–40. 10.1016/S0167-5699(98)01432-7 [DOI] [PubMed] [Google Scholar]
- 23.Embers ME, Ramamoorthy R, Philipp MT. Survival strategies of Borrelia burgdorferi, the etiologic agent of Lyme disease. Microbes Infect. (2004) 6:312–8. 10.1016/j.micinf.2003.11.014 [DOI] [PubMed] [Google Scholar]
- 24.Kraiczy P. Hide and seek: how Lyme disease spirochetes overcome complement attack. Front Immunol. (2016) 7:385. 10.3389/fimmu.2016.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alitalo A, Meri T, Ramo L, Jokiranta TS, Heikkila T, Seppala IJT, et al. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. (2001) 69:3685–91. 10.1128/IAI.69.6.3685-3691.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellwage J, Meri T, Heikkila T, Alitalo A, Panelius J, Lahdenne P, et al. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. (2001) 276:8427–35. 10.1074/jbc.M007994200 [DOI] [PubMed] [Google Scholar]
- 27.Kraiczy P, Skerka C, Kirschfink M, Brade V, Peter Zipfel F. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. Eur J Immunol. (2001) 31:1674–84. [DOI] [PubMed] [Google Scholar]
- 28.Hallström T, Siegel C, Morgelin M, Kraiczy P, Skerka C, Zipfel PF. CspA from Borrelia burgdorferi inhibits the terminal complement pathway. MBio. (2013) 4:e00481–13. 10.1128/mBio.00481-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammerschmidt C, Koenigs A, Siegel C, Hallstrom T, Skerka C, Wallich R, et al. Versatile roles of CspA orthologs in complement inactivation of serum-resistant Lyme disease spirochetes. Infect Immunity. (2014) 82:380–92. 10.1128/IAI.01094-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, Brade V, et al. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol. (2006) 61:1220–36. 10.1111/j.1365-2958.2006.05318.x [DOI] [PubMed] [Google Scholar]
- 31.Kraiczy P, Hellwage J, Skerka C, Becker H, Kirschfink M, Simon MM, et al. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. (2004) 279:2421–9. 10.1074/jbc.M308343200 [DOI] [PubMed] [Google Scholar]
- 32.Brooks CS, Vuppala SR, Jett AM, Alitalo A, Meri S, Akins DR. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J Immunol. (2005) 175:3299–308. 10.4049/jimmunol.175.5.3299 [DOI] [PubMed] [Google Scholar]
- 33.Hart T, Nguyen NTT, Nowak NA, Zhang F, Linhardt RJ, Diuk-Wasser M, et al. Polymorphic factor H-binding activity of CspA protects Lyme borreliae from the host complement in feeding ticks to facilitate tick-to-host transmission. PLoS Pathog. (2018) 14:e1007106. 10.1371/journal.ppat.1007106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenedy MR, Vuppala SR, Siegel C, Kraiczy P, Akins DR. CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect Immun. (2009) 77:2773–82. 10.1128/IAI.00318-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcinkiewicz AL, Dupuis AP, II, Zamba-Campero M, Nowak N, Kraiczy P, Ram S, et al. Blood treatment of Lyme borreliae demonstrates the mechanism of CspZ-mediated complement evasion to promote systemic infection in vertebrate hosts. Cell Microbiol. (2019) 21:e12998. 10.1111/cmi.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel C, Schreiber J, Haupt K, Skerka C, Brade V, Simon MM, et al. Deciphering the ligand-binding sites in the Borrelia burgdorferi complement regulator-acquiring surface protein 2 required for interactions with the human immune regulators factor H and factor H-like protein 1. J Biol Chem. (2008) 283:34855–63. 10.1074/jbc.M805844200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bykowski T, Woodman ME, Cooley AE, Brissette CA, Brade V, Wallich R, et al. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect. Immun. (2007) 75:4227–36. 10.1128/IAI.00604-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mühleip JJ, Lin YP, Kraiczy P. Further insights into the interaction of human and animal complement regulator factor H with viable Lyme disease spirochetes. Front Vet Sci. (2018) 5:346. 10.3389/fvets.2018.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kühn S, Skerka C, Zipfel PF. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H1. J Immunol. (1995) 155:5663–70. [PubMed] [Google Scholar]
- 40.Hauser U, Lehnert G, Wilske B. Validity of interpretation criteria for standardized western blots (immunoblots) for serodiagnosis of Lyme Borreliosis based on sera collected throughout Europe. J. Clin. Microbiol. (1999) 37:2241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammerschmidt C, Klevenhaus Y, Koenigs A, Hallstrom T, Fingerle V, Skerka C, et al. BGA66 and BGA71 facilitate complement resistance of Borrelia bavariensis by inhibiting assembly of the membrane attack complex. Mol Microbiol. (2016) 99:407–24. 10.1111/mmi.13239 [DOI] [PubMed] [Google Scholar]
- 42.Kraiczy P, Rossmann E, Brade V, Simon MM, Skerka C, Zipfel PF, et al. Binding of human complement regulators FHL-1 and factor H to CRASP-1 orthologs of Borrelia burgdorferi Wien Klin Wochenschr. (2006) 118:669–76. 10.1007/s00508-006-0691-1 [DOI] [PubMed] [Google Scholar]
- 43.Stevenson B, Bono JL, Schwan TG, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect. Immun. (1998) 66:2648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kingry LC, Batra D, Replogle A, Rowe LA, Pritt BS, Petersen JM. Whole genome sequence and comparative genomics of the novel Lyme borreliosis causing pathogen, Borrelia mayonii. PLoS ONE. (2016) 11:e0168994. 10.1371/journal.pone.0168994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen NTT, Rottgerding F, Devraj G, Lin YP, Koenigs A, Kraiczy P. The complement binding and inhibitory protein CbiA of Borrelia miyamotoi degrades extracellular matrix components by interacting with plasmin(ogen). Front Cell Infect Microbiol. (2018) 8:23. 10.3389/fcimb.2018.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank KL, Bundle SF, Kresge ME, Eggers CH, Samuels DS. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. (2003) 185:6723–7. 10.1128/JB.185.22.6723-6727.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kraiczy P, Skerka C, Brade V, Zipfel PF. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. (2001) 69:7800–9. 10.1128/IAI.69.12.7800-7809.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tschopp J. Circular polymerization of the membranolytic ninth component of complement. Dependence on metal ions. J Biol Chem. (1984) 259:10569–73. [PubMed] [Google Scholar]
- 49.Siegel C, Hallström T, Skerka C, Eberhardt H, Uzonyi B, Beckhaus T, et al. Complement factor H-related proteins CFHR2 and CFHR5 represent novel ligands for the infection-associated CRASP proteins of Borrelia burgdorferi. PLoS ONE. (2010) 5:e13519. 10.1371/journal.pone.0013519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samuels DS, Mach KE, Garon CF. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J Bacteriol. (1994) 176:6045–9. 10.1128/jb.176.19.6045-6049.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brade V, Kleber I, Acker G. Differences of two Borrelia burgdorferi strains in complement activation and serum resistance. Immunobiology. (1992) 185:453–65. 10.1016/S0171-2985(11)80087-2 [DOI] [PubMed] [Google Scholar]
- 52.Breitner-Ruddock S, Würzner R, Schulze J, Brade V. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med Microbiol Immunol. (1997) 185:253–60. 10.1007/s004300050038 [DOI] [PubMed] [Google Scholar]
- 53.Kraiczy P, Hunfeld KP, Breitner-Ruddock S, Wurzner R, Acker G, Brade V. Comparison of two laboratory methods for the determination of serum resistance in Borrelia burgdorferi isolates. Immunobiology. (2002) 201:406–19. 10.1016/S0171-2985(00)80094-7 [DOI] [PubMed] [Google Scholar]
- 54.van Dam AP, Oei A, Jaspars R, Fijen C, Wilske B, Spanjaard L, et al. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun. (1997) 65:1228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraiczy P, Stevenson B. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: Structure, function and regulation of gene expression. Ticks Tick Borne Dis. (2013) 4:26–34. 10.1016/j.ttbdis.2012.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDowell JV, Wolfgang J, Tran E, Metts MS, Hamilton D, Marconi RT. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect. Immun. (2003) 71:3597–602. 10.1128/IAI.71.6.3597-3602.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herzberger P, Siegel C, Skerka C, Fingerle V, Schulte-Spechtel U, van Dam A, et al. Human pathogenic Borrelia spielmanii sp. nov. resists complement-mediated killing by direct binding of immune regulators factor H and factor H-like protein 1. Infect. Immun. (2007) 75:4817–25. 10.1128/IAI.00532-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kraiczy P, Hellwage J, Skerka C, Kirschfink M, Brade V, Zipfel PF, et al. Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur J Immunol. (2003) 33:697–707. 10.1002/eji.200323571 [DOI] [PubMed] [Google Scholar]
- 59.Seelen MA, Roos A, Wieslander J, Mollnes TE, Sjoholm AG, Würzner R, et al. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods. (2005) 296:187–98. 10.1016/j.jim.2004.11.016 [DOI] [PubMed] [Google Scholar]
- 60.Tschopp J, Muller-Eberhard HJ, Podack ER. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. (1982) 298:534–8. 10.1038/298534a0 [DOI] [PubMed] [Google Scholar]
- 61.Dankert JR, Esser AF. Proteolytic modification of human complement protein C9: loss of poly(C9) and circular lesion formation without impairment of function. Proc Natl Acad Sci USA. (1985) 82:2128–32. 10.1073/pnas.82.7.2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pathak A, Bergstrand J, Sender V, Spelmink L, Aschtgen MS, Muschiol S, et al. Factor H binding proteins protect division septa on encapsulated Streptococcus pneumoniae against complement C3b deposition and amplification. Nat Commun. (2018) 9:3398. 10.1038/s41467-018-05494-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kotarsky H, Hellwage J, Johnsson E, Skerka C, Svensson HG, Lindahl G, et al. Identification of a domain in human factor H and factor H-like protein-1 required for the interaction with streptococcal M proteins. J Immunol. (1998) 160:3349–54. [PubMed] [Google Scholar]
- 64.Jarva H, Hellwage J, Jokiranta TS, Lehtinen MJ, Zipfel PF, Meri S. The group B streptococcal b and pneumococcal Hic proteins are structurally related immune evasion molecules that bind the complement inhibitor factor H in an analogous fashion. J Immunol. (2004) 172:3111–8. 10.4049/jimmunol.172.5.3111 [DOI] [PubMed] [Google Scholar]
- 65.Jarva H, Janulczyk R, Hellwage J, Zipfel PF, Bjorck L, Meri S. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8-11 of factor H. J Immunol. (2002) 168:1886–94. 10.4049/jimmunol.168.4.1886 [DOI] [PubMed] [Google Scholar]
- 66.Jarva H, Jokiranta TS, Würzner R, Meri S. Complement resistance mechanisms of streptococci. Mol Immunol. (2003) 40:95–107. 10.1016/S0161-5890(03)00108-1 [DOI] [PubMed] [Google Scholar]
- 67.Ram S, Mackinnon FG, Gulati S, McQuillen DP, Vogel U, Frosch M, et al. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol Immunol. (1999) 36:915–28. 10.1016/S0161-5890(99)00114-5 [DOI] [PubMed] [Google Scholar]
- 68.Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, Rice PA. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. (1998) 188:671–80. 10.1084/jem.188.4.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis LA, Rice PA, Ram S. Role of gonococcal neisserial surface protein A (NspA) in serum resistance and comparison of its factor H binding properties with those of its meningococcal counterpart. Infect Immun. (2019) 87:e00658–18. 10.1128/IAI.00658-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernhard S, Fleury C, Su YC, Zipfel PF, Koske I, Nordstrom T, et al. Outer membrane protein OlpA contributes to Moraxella catarrhalis serum resistance via interaction with factor H and the alternative pathway. J Infect Dis. (2014) 210:1306–10. 10.1093/infdis/jiu241 [DOI] [PubMed] [Google Scholar]
- 71.Haupt K, Reuter M, van den Elsen J, Burman J, Halbich S, Richter J, et al. The Staphylococcus aureus protein Sbi acts as a complement inhibitor and forms a tripartite complex with host complement factor H and C3b. PLoS Pathog. (2008) 4:e1000250. 10.1371/journal.ppat.1000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kunert A, Losse J, Gruszin C, Huhn M, Kaendler K, Mikkat S, et al. Immune evasion of the human pathogen Pseudomonas aeruginosa: elongation factor Tuf is a factor H and plasminogen binding protein. J Immunol. (2007) 179:2979–88. 10.4049/jimmunol.179.5.2979 [DOI] [PubMed] [Google Scholar]
- 73.Verma A, Hellwage J, Artiushin S, Zipfel PF, Kraiczy P, Timoney JF, et al. LfhA, a novel factor H-binding protein of Leptospira interrogans. Infect Immun. (2006) 74:2659–66. 10.1128/IAI.74.5.2659-2666.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caine JA, Lin YP, Kessler JR, Sato H, Leong JM, Coburn J. Borrelia burgdorferi outer surface protein C (OspC) binds complement component C4b and confers bloodstream survival. Cell Microbiol. (2017) 19:e12786. 10.1111/cmi.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia BL, Zhi H, Wager B, Hook M, Skare JT. Borrelia burgdorferi BBK32 inhibits the classical pathway by blocking activation of the C1 complement complex. PLoS Pathog. (2016) 12:e1005404. 10.1371/journal.ppat.1005404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pietikainen J, Meri T, Blom AM, Meri S. Binding of the complement inhibitor C4b-binding protein to Lyme disease Borreliae. Mol Immunol. (2010) 47:1299–305. 10.1016/j.molimm.2009.11.028 [DOI] [PubMed] [Google Scholar]
- 77.Albrecht JC, Fleckenstein B. New member of the multigene family of complement control proteins in herpesvirus saimiri. J Virol. (1992) 66:3937–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braga LL, Ninomiya H, McCoy JJ, Eacker S, Wiedmer T, Pham C, et al. Inhibition of the complement membrane attack complex by the galactose-specific adhesion of Entamoeba histolytica. J Clin Invest. (1992) 90:1131–7. 10.1172/JCI115931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng J, Gold D, LoVerde PT, Fishelson Z. Inhibition of the complement membrane attack complex by Schistosoma mansoni paramyosin. Infect Immun. (2003) 71:6402–10. 10.1128/IAI.71.11.6402-6410.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iida K, Whitlow MB, Nussenzweig V. Amastigotes of Trypanosoma cruzi escape destruction by the terminal complement components. J Exp Med. (1989) 169:881–91. 10.1084/jem.169.3.881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Z, Yang J, Wei J, Yang Y, Chen X, Zhao X, et al. Trichinella spiralis paramyosin binds to C8 and C9 and protects the tissue-dwelling nematode from being attacked by host complement. PLoS Negl Trop Dis. (2011) 5:e1225. 10.1371/journal.pntd.0001225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haupt K, Kraiczy P, Wallich R, Brade V, Skerka C, Zipfel P. Binding of human factor H-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J Infect Dis. (2007) 196:124–33. 10.1086/518509 [DOI] [PubMed] [Google Scholar]
- 83.Herzberger P, Siegel C, Skerka C, Fingerle V, Schulte-Spechtel U, Wilske B, et al. Identification and characterization of the factor H and FHL-1 binding complement regulator-acquiring surface protein 1 of the Lyme disease spirochete Borrelia spielmanii sp. nov. Int J Med Microbiol. (2009) 299:141–54. 10.1016/j.ijmm.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 84.Seling A, Siegel C, Fingerle V, Jutras BL, Brissette CA, Skerka C, et al. Functional characterization of Borrelia spielmanii outer surface proteins that interact with distinct members of the human factor H protein family and with plasminogen. Infect Immun. (2010) 78:39–48. 10.1128/IAI.00691-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grosskinsky S, Schott M, Brenner C, Cutler SJ, Kraiczy P, Zipfel PF, et al. Borrelia recurrentis employs a novel multifunctional surface protein with anti-complement, anti-opsonic and invasive potential to escape innate immunity. PLoS ONE. (2009) 4:e4858. 10.1371/journal.pone.0004858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rossmann E, Kraiczy P, Herzberger P, Skerka C, Kirschfink M, Simon MM, et al. Dual binding specificity of a Borrelia hermsii-associated complement regulator-acquiring surface protein for factor H and plasminogen discloses a putative virulence factor of relapsing fever spirochetes. J Immunol. (2007) 178:7292–301. 10.4049/jimmunol.178.11.7292 [DOI] [PubMed] [Google Scholar]
- 87.Hovis KM, Jones JP, Sadlon T, Raval G, Gordon DL, Marconi RT. Molecular analyses of the interaction of Borrelia hermsii FhbA with the complement regulatory proteins factor H and factor H-like protein 1. Infect Immun. (2006) 74:2007–14. 10.1128/IAI.74.4.2007-2014.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schott M, Grosskinsky S, Brenner C, Kraiczy P, Wallich R. Molecular characterization of the interaction of Borrelia parkeri and Borrelia turicatae with human complement regulators. Infect Immun. (2010) 78:2199–208. 10.1128/IAI.00089-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Röttgerding F, Wagemakers A, Koetsveld J, Fingerle V, Kirschfink M, Hovius JW, et al. Immune evasion of Borrelia miyamotoi: CbiA, a novel outer surface protein exhibiting complement binding and inactivating properties. Sci Rep. (2017) 7:303 10.1038/s41598-017-00412-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brangulis K, Petrovskis I, Kazaks A, Akopjana I, Tars K. Crystal structures of the Erp protein family members ErpP and ErpC from Borrelia burgdorferi reveal the reason for different affinities for complement regulator factor H. Biochim Biophys Acta. (2015) 1854:349–55. 10.1016/j.bbapap.2014.12.025 [DOI] [PubMed] [Google Scholar]
- 91.Hammerschmidt C, Hallstrom T, Skerka C, Wallich R, Stevenson B, Zipfel PF, et al. Contribution of the infection-associated complement regulator-acquiring surface protein 4 (ErpC) to complement resistance of Borrelia burgdorferi. Clin Dev Immunol. (2012) 2012:349657. 10.1155/2012/349657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. (2004) 25:1605–12. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 93.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. (2006) 22:195–201. 10.1093/bioinformatics/bti770 [DOI] [PubMed] [Google Scholar]
- 94.Cordes FS, Roversi P, Kraiczy P, Simon MM, Brade V, Jahraus O, et al. A novel fold for the factor H-binding protein BbCRASP-1 of Borrelia burgdorferi. Nat Struct Mol Biol. (2005) 12:276–7. 10.1038/nsmb902 [DOI] [PubMed] [Google Scholar]
- 95.Cordes FS, Kraiczy P, Roversi P, Simon MM, Brade V, Jahraus O, et al. Structure-function mapping of BbCRASP-1, the key complement factor H and FHL-1 binding protein of Borrelia burgdorferi. Int J Med Microbiol. (2006) 296 (Suppl. 40):177–84. 10.1016/j.ijmm.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 96.Kraiczy P, Hanssen-Hubner C, Kitiratschky V, Brenner C, Besier S, Brade V, et al. Mutational analyses of the BbCRASP-1 protein of Borrelia burgdorferi identify residues relevant for the architecture and binding of host complement regulators FHL-1 and factor H. Int J Med Microbiol. (2009) 299:255–68. 10.1016/j.ijmm.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 97.Caesar JJ, Wallich R, Kraiczy P, Zipfel PF, Lea SM. Further structural insights into the binding of complement factor H by complement regulator-acquiring surface protein 1 (CspA) of Borrelia burgdorferi. Acta Crystallogr Sect F Struct Biol Cryst Commun. (2013) 69(Pt 6):629–33. 10.1107/S1744309113012748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.