Abstract
Background: Findings by epidemiologic studies on menopausal hormone replacement therapy (HRT) and the risk of ovarian cancer are inconsistent. This study aimed to assess the association of menopausal HRT with the risk of ovarian cancer by histological subtype.
Methods: A literature search was performed in PubMed, Web of Science, and EmBase for relevant articles published from inception to August 2018. Pooled relative risk ratios (RRs) with 95% confidence intervals (CIs) were determined with a random-effects model.
Results: Thirty-six studies involving 4, 229, 061 participants were included in this meta-analysis. The pooled RR of ovarian cancer was 1.29 (95%CI 1.19–1.40, I2 = 57.4%) for menopausal HRT. In subgroup analysis by study design, pooled RRs of ovarian cancer in cohort and case-control studies were 1.35 (95%CI 1.19–1.53) and 1.24 (95%CI 1.11–1.38), respectively. In subgroup analysis by continent, association of menopausal HRT with ovarian cancer was significant for North America (1.41 [1.23–1.61]), Europe (1.22 [1.12–1.34]), and Asia (1.76 [1.09–2.85]), but not Australia (0.96 [0.57–1.61]). Association differed across histological subtypes. Increased risk was only found for two common types, including serous (1.50 [1.35–1.68]) and endometrioid (1.48 [1.13–1.94]) tumors.
Conclusion: This meta-analysis suggests that menopausal HRT may increase the risk of ovarian cancer, especially for serous and endometrioid tumors.
Keywords: ovarian cancer, menopause, hormone replacement therapy, meta-analysis, association
Introduction
Ovarian cancer is known as the most lethal genital system malignancy (1). It is also the fifth leading cause of cancer-related deaths in American women (1). In 2018, the estimated new ovarian cancer cases and deaths will be 22,240 and 14,070 in the US, respectively (1). In 2018, the age standardized incidence rate of ovarian cancer is 6.6 per 100,000 in world1. Ovarian cancer can be divided into five histologic subtypes: serous tumor, mucinous tumor, endometrioid tumor, clear cell tumor, and other type of ovarian cancer. And the different histologic types of ovarian cancer may has different protective factors or pathogenic factors. Breastfeeding (2) and oral contraceptives (3) have been confirmed as protective factors in ovarian cancer. However, other exposures such as obesity (4, 5), diabetes (6), miscarriage (7) and a family history of breast/ovarian cancer (8) are demonstrated risk factors for ovarian cancer.
Menopausal hormone replacement therapy (HRT) is widely used to improve postmenopausal symptoms and ward off bone loss. However, in the past few years, many epidemiological studies have revealed that HRT is associated with an increased risk of breast cancer (9, 10). Data regarding HRT and the risk of ovarian cancer are contradictory. According to several studies, HRT is associated with an increased risk of ovarian cancer (11–20). However, several studies found no relationship between them (21–37) and others found the positive association in individual histological subtype (38–46). Although the increased risk of ovarian cancer associated with menopausal HRT has been described previously in several meta-analyses, the histological subtype of ovarian cancer was not taken into account (47, 48). Until now, whether the effect of HRT on the risk of ovarian cancer differs by histological subtype is not completely known. Therefore, we performed the current meta-analysis to evaluate the effect of menopausal HRT on ovarian cancer risk by histological subtype.
Materials and Methods
Literature Search Strategy
We performed a literature search to identify relevant available articles from PubMed, Web of Science and EmBase from inception to August 2018 with no restrictions. Search terms included “hormone replacement therapy” (or “HRT”) and “ovarian cancer” (or “ovarian neoplasms” or “ovarian carcinoma” or “ovary cancer”). The reference lists of the included studies were also reviewed for potential relevant studies.
Inclusion Criteria
Inclusion criteria were: (1) original report from observational studies; (2) menopausal HRT as the exposure of interest; (3) ovarian cancer as the outcome of interest; (4) relative risk ratio (RR) with 95% confidence interval (CI) provided. The most recent and complete study was selected if studies from the same population were repeated.
Two investigators searched and reviewed all relevant studies independently. Any disagreement was resolved by consensus with the involvement of a third reviewer.
Data Extraction
The following information were extracted from each study by two investigators independently: first author's name, published year, country, study design, follow-up duration, age range or mean age at baseline, sample size and number of cases, histological subtype of ovarian cancer, the types of hormones used in the study population, RR (we presented all results as RR for simplicity) with 95%CI and adjustment for potential confounders. We extracted RRs adjusted for the most confounding factors in the original studies. We prioritized the RRs for highest vs. lowest duration category of HRT use. If the study did not provide RRs for highest vs. lowest duration category of HRT use, we extracted the RRs for “use vs. non-use.”
Statistical Analysis
The Newcastle–Ottawa Scale was used to assess the quality of studies included in this meta-analysis. Pooled data were obtained as the inverse variance-weighted means of the logarithm of RRs with 95%CI to assess the associations of menopausal HRT and the risk of different histological subtypes of ovarian cancer, respectively. The DerSimonian and Laird random effects model (REM) was used to combine study-specific RRs (95%CIs). The I2 statistic was adopted to assess heterogeneity among studies (I2-values of 0, 25, 50, and 75% represented no, low, moderate and high heterogeneity, respectively). Meta-regression with restricted maximum likelihood estimation was performed to explore the important covariates that might have significant impact on between-study heterogeneity. Subgroup analyses were stratified on study design, geographic location and the types of hormones used in the study population. Sensitivity analysis was performed with one study removed at a time to assess whether the results could have been affected markedly by a single study. The funnel plot and Egger's test were performed to explore the small-study effect.
All statistical analyses were performed with STATA version 14.0 (Stata Corporation, College Station, TX, United States). All reported probabilities (P-values) were two-sided, with a statistical significance level of 0.05.
Results
Literature Search Results
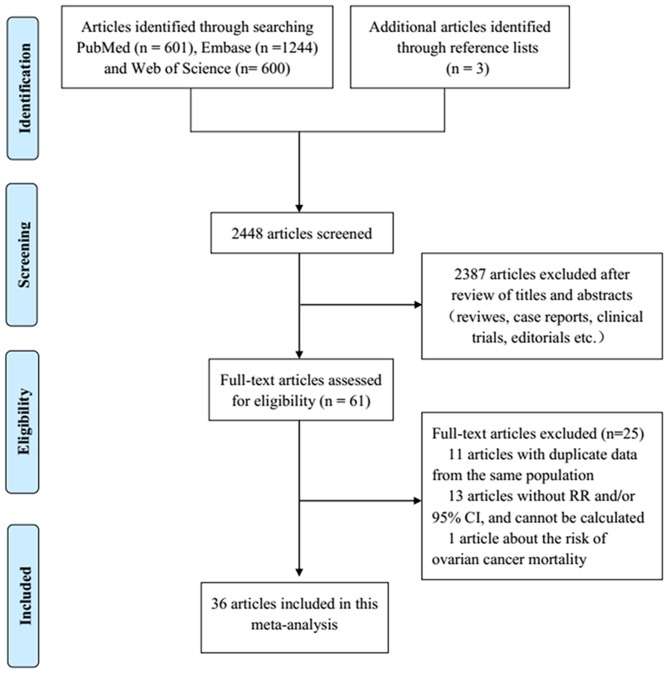
We identified 2,445 articles by literature search, of which 2,387 were excluded after title and abstract review (Figure 1). Three additional articles were found by searching the reference lists of included articles. Eleven articles with duplicate data from the same population, 13 reports without RR and/or 95%CI and one article assessing the risk of ovarian cancer mortality were excluded. Finally, 36 published articles were eligible for this meta-analysis.
Figure 1.
Flowchart of the study selection process based on PRISMA in this meta-analysis.
Characteristics of Studies
For the association of menopausal HRT with the risk of ovarian cancer, 34 articles (11–28, 30–42, 44–46) (15 cohort and 19 case-control studies) were included, involving 3,305,108 participants. The Newcastle-Ottawa Scale indicated that most of the studies included in this meta-analysis were of high quality (thirty of them scored more than seven). Among these studies, 15 were performed in Europe, 15 in North America, 2 in Asia and 2 in Australia. For the association of menopausal HRT and the risk of ovarian cancer by histological subtype, 12 studies (21, 29, 35, 38–46) assessing 1,193,201 participants were included for serous tumors, 10 reports (21, 38–46) evaluating 1,173,009 participants were included for endometrioid tumors, 9 studies (35, 38–45) assessing 1,089,421 participants were included for mucinous tumors, 5 reports (21, 39, 41, 43, 45) with 1,081,067 participants were included for clear cell tumors and 5 studies (38, 39, 41, 44, 45) evaluating 175,429 participants were included for other types of ovarian cancer. The detailed characteristics of the included studies are shown in Table 1.
Table 1.
The detailed characteristics of the included studies.
| References | Country (year) | Age | Study design | Years of follow-up | Participants (cases) | Cancer type | Hormone type | RR (95% CI) | Adjustment for covariant |
|---|---|---|---|---|---|---|---|---|---|
| Danforth et al. (46) | America (2007) | 61.2 (mean) | Cohort | 26 years | 82,950 (389) | Ovarian cancer | HRT | 1.41 (1.07, 1.86) | Age, parity, duration of oral contraceptive use, tubal ligation, age at natural menopause, age at menarche |
| Danforth et al. (46) | America (2007) | 61.2 (mean) | Cohort | 26 years | 82,950 (233) | Serous tumors | HRT | 1.66 (1.17, 2.36) | Age, parity, duration of oral contraceptive use, tubal ligation, age at natural menopause, age at menarche |
| Danforth et al. (46) | America (2007) | 61.2 (mean) | Cohort | 26 years | 82,950 (60) | Endometrioid tumors | HRT | 1.86 (0.89, 3.91) | Age, parity, duration of oral contraceptive use, tubal ligation, age at natural menopause, age at menarche |
| Bethea et al. (30) | America (2017) | 37.8 ± 10.3 | Cohort | 18 years | 59,000 (115) | Ovarian cancer | HRT | 1.42 (0.75, 2.70) | Age, questionnaire cycle, parity, lactation, age at first birth, age at last birth, hysterectomy, tubal ligation, oral contraceptive use, educational HRT attainment, and BMI |
| Li et al. (25) | 10 European countries (2015) | 52.4 (median) | Cohort | 11.7 years | 367,903 (791) | Ovarian cancer | HRT | 1.09 (0.92, 1.30) | Menopausal status, age at menopause, age at menarche, number of full-term pregnancies (FTPs), age at first FTP, duration of breast-feeding, number of miscarriages, unilateral ovariectomy, hysterectomy, HRT, OC use, IUD use, BMI, smoking status, alcohol consumption, and pre-existing diabetes |
| Soegaard et al. (38) | Denmark (2007) | 35-79 | Case control | NA | 1,614 (50) | Mucinous tumors | HRT | 0.71 (0.37, 1.36) | Age, pregnancy, additional pregnancies and duration of oral contraceptive use |
| Soegaard et al. (38) | Denmark (2007) | 35-79 | Case control | NA | 1,907 (343) | Serous tumors | HRT | 1.30 (1.00, 1.68) | Age, pregnancy, additional pregnancies and duration of oral contraceptive use |
| Soegaard et al. (38) | Denmark (2007) | 35-79 | Case control | NA | 1,639 (75) | Endometrioid tumors | HRT | 1.75 (1.07, 2.84) | Age, pregnancy, additional pregnancies and duration of oral contraceptive use |
| Soegaard et al. (38) | Denmark (2007) | 35-79 | Case control | NA | 1,650 (86) | Other types of ovarian cancer | HRT | 1.43 (0.90, 2.28) | Age, pregnancy, additional pregnancies and duration of oral contraceptive use |
| Soegaard et al. (38) | Denmark (2007) | 35-79 | Case control | NA | 2,118 (554) | Ovarian cancer | HRT | 1.30 (1.05, 1.61) | Age, pregnancy, additional pregnancies and duration of oral contraceptive use |
| Koskela-Niska et al. (39) | Finland (2013) | > 50 | Case control | NA | 15,283 (3,958) | Ovarian cancer | HRT | 1.15 (0.99, 1.32) | Age and place of residence |
| Koskela-Niska et al. (39) | Finland (2013) | > 50 | Case control | NA | 7,333 (1,903) | Serous tumors | HRT | 1.45 (1.20, 1.75) | Age and place of residence |
| Koskela-Niska et al. (39) | Finland (2013) | > 50 | Case control | NA | 2,901 (748) | Endometrioid tumors | HRT | 1.25 (0.88, 1.76) | Age and place of residence |
| Koskela-Niska et al. (39) | Finland (2013) | > 50 | Case control | NA | 1,611 (417) | Mucinous tumors | HRT | 0.35 (0.19, 0.67) | Age and place of residence |
| Koskela-Niska et al. (39) | Finland (2013) | > 50 | Case control | NA | 596 (155) | Clear cell tumors | HRT | 0.72 (0.23, 2.29) | Age and place of residence |
| Koskela-Niska et al. (39) | Finland (2013) | > 50 | Case control | NA | 2,842 (735) | Other types of ovarian cancer | HRT | 0.82 (0.56, 1.20) | Age and place of residence |
| Folsom et al. (11) | America (2004) | 55-69 | Cohort | 15 years | 31,381 (174) | Ovarian cancer | ERT | 2.53 (1.44, 4.45) | Age, family history of ovarian cancer in a first- or second-degree relative, hysterectomy, unilateral oophorectomy, number of live births, physical activity index, pack-years of smoking, waist/hip ratio, and BMI |
| Risch (40) | Canada (1996) | Case: 59.5, control: 57.5 (mean) | Case control | NA | 776 (212) | Serous tumors | ERT | 2.03 (1.04, 3.97) | Age, number of full-term pregnancies, total years of oral-contraceptive use, and average lactation/pregnancy as continuous terms, and history of tubal ligation, hysterectomy, and mother/sister with breast cancer as dichotomous terms |
| Risch (40) | Canada (1996) | Case: 59.5, control: 57.5 (mean) | Case control | NA | 637 (73) | Endometrioid tumors | ERT | 2.81 (1.15, 6.89) | Age, number of full-term pregnancies, total years of oral-contraceptive use, and average lactation/pregnancy as continuous terms, and history of tubal ligation, hysterectomy, and mother/sister with breast cancer as dichotomous terms |
| Risch (40) | Canada (1996) | Case: 59.5, control: 57.5 (mean) | Case control | NA | 604 (40) | Mucinous tumors | ERT | 0.58 (0.08, 4.21) | Age, number of full-term pregnancies, total years of oral-contraceptive use, and average lactation/pregnancy as continuous terms, and history of tubal ligation, hysterectomy, and mother/sister with breast cancer as dichotomous terms |
| Risch (40) | Canada (1996) | Case: 59.5, control: 57.5 (mean) | Case control | NA | 891 (327) | Ovarian cancer | ERT | 1.77 (0.98, 3.20) | Age, number of full-term pregnancies, total years of oral-contraceptive use, and average lactation/pregnancy as continuous terms, and history of tubal ligation, hysterectomy, and mother/sister with breast cancer as dichotomous terms |
| Perri et al. (12) | Israeli (2015) | Case: 53.6 ± 10.3, control: 49.1 ± 13.4 | Cohort | 18 years | 1,073 (175) | Ovarian cancer | HRT | 1.98 (1.21, 3.25) | Mutation type, age at menarche, oral contraceptive use, parity, age at first pregnancy |
| Bakken et al. (26) | Norway (2004) | 53.0 (mean) | Cohort | 7 years | 30,115 (74) | Ovarian cancer | HRT | 1.30 (0.80, 2.00) | Age, BMI, smoking, ever use of OCs, time since menopause, parity and age at last birth |
| Mills et al. (41) | America (2005) | NA | Case control | NA | 1,378 (256) | Ovarian cancer | HRT | 1.39 (1.01, 1.93) | Age, race/ethnicity, duration of oral contraceptive use and breastfeeding |
| Mills et al. (41) | America (2005) | NA | Case control | NA | 1,214 (92) | Serous tumors | HRT | 1.61 (0.99, 2.60) | Age, race/ethnicity, duration of oral contraceptive use and breastfeeding |
| Mills et al. (41) | America (2005) | NA | Case control | NA | 1,157 (35) | Endometrioid tumors | HRT | 0.96 (0.44, 2.10) | Age, race/ethnicity, duration of oral contraceptive use and breastfeeding |
| Mills et al. (41) | America (2005) | NA | Case control | NA | 1,138 (16) | Mucinous tumors | HRT | 1.32 (0.40, 4.40) | Age, race/ethnicity, duration of oral contraceptive use and breastfeeding |
| Mills et al. (41) | America (2005) | NA | Case control | NA | 1,134 (12) | Clear cell tumors | HRT | 1.14 (0.27, 4.84) | Age, race/ethnicity, duration of oral contraceptive use and breastfeeding |
| Mills et al. (41) | America (2005) | NA | Case control | NA | 1,149 (27) | Other types of ovarian cancer | HRT | 1.30 (0.57, 2.97) | Age, race/ethnicity, duration of oral contraceptive use and breastfeeding |
| Purdie et al. (23) | Australia (1999) | 18-79 | Case control | NA | 1,648 (793) | Ovarian cancer | HRT | 1.20 (0.90, 1.60) | Age, education, area of residence, BMI, hysterectomy, tubal sterilization, talc use in perineal region, smoking status, duration of OCP use, parity and a family history of breast or ovarian cancer |
| Riman et al. (42) | Sweden (2002) | Case: 62.4 ± 7.4, control: 63.4 ± 7.1 | Case control | NA | 4,432 (642) | Ovarian cancer | ERT | 2.10 (0.99, 4.48) | Age, parity,BMI (kg/m2), age at menopause, hysterectomy,duration of oral contraceptive use, and ever use of estrogen only (estrogen replacement therapy [ERT]) and continuous estrogen–progestin combinations (HRTcp) as categorized variables |
| Riman et al. (42) | Sweden (2002) | Case: 62.6 ± 7.3, control: 63.4 ± 7.1 | Case control | NA | 4,123 (333) | Serous tumors | ERT | 2.51 (1.00, 6.34) | Age, parity,BMI (kg/m2), age at menopause, hysterectomy,duration of oral contraceptive use, and ever use of estrogen only (estrogen replacement therapy [ERT]) and continuous estrogen–progestin combinations (HRTcp) as categorized variables |
| Riman et al. (42) | Sweden (2002) | Case: 61.6 ± 7.6, control: 63.4 ± 7.1 | Case control | NA | 3,967 (177) | Endometrioid tumors | ERT | 2.24 (0.64, 7.89) | Age, parity,BMI (kg/m2), age at menopause, hysterectomy,duration of oral contraceptive use, and ever use of estrogen only (estrogen replacement therapy [ERT]) and continuous estrogen–progestin combinations (HRTcp) as categorized variables |
| Riman et al. (42) | Sweden (2002) | Case: 62.5 ± 7.8, control: 63.4 ± 7.1 | Case control | NA | 3,850 (60) | Mucinous tumors | ERT | 1.59 (0.19, 13.33) | Age, parity,BMI (kg/m2), age at menopause, hysterectomy,duration of oral contraceptive use, and ever use of estrogen only (estrogen replacement therapy [ERT]) and continuous estrogen–progestin combinations (HRTcp) as categorized variables |
| Kotsopoulos et al. (22) | America (2006) | Case: 62.7, control: 61.2 (mean) | Case control | NA | 537 (162) | Ovarian cancer | HRT | 0.93 (0.56, 1.56) | Parity, OC use and country of residence |
| Hempling et al. (21) | America (1997) | Case: 54.9, control: 54.9 (mean) | Case control | NA | 1,255 (499) | Ovarian cancer | HRT | 0.60 (0.30, 1.40) | Age at diagnosis, parity, oral contraceptive use, smoking history, family history of epithelial ovarian cancer, age at menarche, menopausal status, income, location, and education |
| Hempling et al. (21) | America (1997) | Case: 54.9, control: 54.9 (mean) | Case control | NA | NA | Serous tumors | HRT | 1.20 (0.80, 1.70) | NA |
| Hempling et al. (21) | America (1997) | Case: 54.9, control: 54.9 (mean) | Case control | NA | NA | Clear cell tumors | HRT | 1.10 (0.40, 3.40) | NA |
| Hempling et al. (21) | America (1997) | Case: 54.9, control: 54.9 (mean) | Case control | NA | NA | Endometrioid tumors | HRT | 0.40 (0.20, 1.20) | NA |
| Sit et al. (24) | America (2002) | Case: 56.6, control: 55.7 (mean) | Case control | NA | 1,410 (848) | Ovarian cancer | HRT | 1.03 (0.69, 1.53) | Numbers of live births, family history of ovarian carcinoma,OC use, history of tubal ligation, and age at diagnosis |
| Mørch et al. (43) | Denmark (2012) | ≥50 | Cohort | 8 years | 909,946 (1,336) | Serous tumors | HRT | 1.64 (1.41, 1.89) | Age, time period, number of births, educational level, and history of hysterectomy, sterilization, unilateral oophorectomy or salpingo-oophorectomy, endometriosis, and infertility |
| Mørch et al. (43) | Denmark (2012) | ≥50 | Cohort | 8 years | 909,946 (377) | Endometrioid tumors | HRT | 1.81 (1.39, 2.36) | Age, time period, number of births, educational level, and history of hysterectomy, sterilization, unilateral oophorectomy or salpingo-oophorectomy, endometriosis, and infertility |
| Mørch et al. (43) | Denmark (2012) | ≥50 | Cohort | 8 years | 909,946 (293) | Mucinous tumors | HRT | 0.74 (0.51, 1.08) | Age, time period, number of births, educational level, and history of hysterectomy, sterilization, unilateral oophorectomy or salpingo-oophorectomy, endometriosis, and infertility |
| Mørch et al. (43) | Denmark (2012) | ≥50 | Cohort | 8 years | 909,946 (159) | Clear cell tumors | HRT | 0.81 (0.50, 1.32) | Age, time period, number of births, educational level, and history of hysterectomy, sterilization, unilateral oophorectomy or salpingo-oophorectomy, endometriosis, and infertility |
| Morch et al. (13) | Denmark (2009) | ≥50 | Cohort | 8 years | 909,946 (2,297) | Ovarian cancer | HRT | 1.57 (1.26, 1.95) | Age, period of use, number of births, hysterectomy, sterilization, unilateral oophorectomy or salpingo-oophorectomy, endometriosis, infertility, and educational status |
| Wernli et al. (27) | America (2008) | 40-79 | Case control | NA | 6,559 (751) | Ovarian cancer | HRT | 1.24 (0.97, 1.60) | BMI, oral contraceptive use, tubal ligation, parity, family history of ovarian cancer, hysterectomy, and menopausal status |
| Urban et al. (14) | America (2015) | 50-79 | Cohort | 12.3 years | 74,786 (461) | Ovarian cancer | HRT | 1.50 (1.23, 1.83) | Age and race |
| Tavani et al. (15) | Italy (2000) | Case: 54.0, control: 52.0 (mean) | Case control | NA | 232 (93) | Ovarian cancer | HRT | 1.80 (1.30, 2.60) | Age and area of residence |
| Lacey et al. (16) | America (2002) | 56.6 (mean) | Cohort | 13.4 years | 44,241 (275) | Ovarian cancer | ERT | 3.20 (1.70, 5.70) | Age, menopause type, and duration of oral contraceptive use |
| Simin et al. (17) | Sweden (2017) | ≥40 | Cohort | 7 years | 290,186 (573) | Ovarian cancer | HRT | 1.09 (1.00, 1.19) | NA |
| Yang et al. (45) | America (2012) | Case: 62.8 ± 5.3, control: 61.8 ± 5.4 | Cohort | Case: 5.1 years; control: 9.8 years | 168,323 (849) | Ovarian cancer | HRT | 1.57 (1.31, 1.89) | Age, oral contraceptive use, parity, menopausal hormone therapy |
| Yang et al. (45) | America (2012) | Case: 62.6 ± 5.4, control: 61.8 ± 5.4 | Cohort | Case: 5.1 years; control: 9.8 years | 168,323 (449) | Serous tumors | HRT | 1.64 (1.27, 2.13) | Age, oral contraceptive use, parity, menopausal hormone therapy |
| Yang et al. (45) | America (2012) | Case: 61.0 ± 6.2, control: 61.8 ± 5.4 | Cohort | Case: 5.1 years; control: 9.8 years | 168,323 (78) | Endometrioid tumors | HRT | 2.27 (1.26, 4.09) | Age, oral contraceptive use, parity, menopausal hormone therapy |
| Yang et al. (45) | America (2012) | Case: 63.5 ± 5.5, control: 61.8 ± 5.4 | Cohort | Case: 5.1 years; control: 9.8 years | 169,391 (37) | Mucinous tumors | HRT | 0.50 (0.17, 1.42) | Age, oral contraceptive use, parity, menopausal hormone therapy |
| Yang et al. (45) | America (2012) | Case: 59.7 ± 6.2, control: 61.8 ± 5.4 | Cohort | Case: 5.1 years; control: 9.8 years | 168,323 (26) | Clear cell tumors | HRT | 1.82 (0.64, 5.17) | Age, oral contraceptive use, parity, menopausal hormone therapy |
| Yang et al. (45) | America (2012) | Case: 63.9 ± 4.8, control: 61.8 ± 5.4 | Cohort | Case: 5.1 years; control: 9.8 years | 168,323 (255) | Other types of ovarian cancer | HRT | 1.53 (1.11, 2.13) | Age, oral contraceptive use, parity, menopausal hormone therapy |
| Rossing et al. (18) | America (2007) | Case: 47.0, control: 48.0 (median) | Case control | NA | 1,818 (715) | Ovarian cancer | ERT | 1.60 (1.10, 2.50) | Age, county of residence, year of diagnosis/reference date, number of full-term pregnancies, and duration of hormonal contraception |
| Moorman et al. (44) | America (2005) | 20-74 | Case control | NA | 734 (364) | Ovarian cancer | HRT | 1.20 (0.80, 1.60) | Age, race, parity, tubal ligation, hysterectomy, BMI 1 year before interview, 1st degree family history of breast or ovarian cancer, breastfeeding, oral contraceptive use, and educational level |
| Moorman et al. (44) | America (2005) | 20-74 | Case control | NA | 572 (216) | Serous tumors | HRT | 2.00 (1.30, 3.10) | Age and race |
| Moorman et al. (44) | America (2005) | 20-74 | Case control | NA | 421 (65) | Endometrioid tumors | HRT | 1.00 (0.50, 2.00) | Age and race |
| Moorman et al. (44) | America (2005) | 20-74 | Case control | NA | 382 (25) | Mucinous tumors | HRT | 0.90 (0.30, 2.50) | Age and race |
| Moorman et al. (44) | America (2005) | 20-74 | Case control | NA | 397 (40) | Other types of ovarian cancer | HRT | 1.10 (0.50, 2.70) | Age and race |
| Beral et al. (19) | United Kingdom (2007) | 57.2 ± 4.6 | Cohort | 5.3 years | 948,576 (2,273) | Ovarian cancer | HRT | 1.31 (1.12, 1.53) | Region of residence, socioeconomic group,time since menopause, parity, BMI, alcohol consumption, and use of oral contraceptives |
| Koskela-Niska et al. (39) | Finland (2013) | ≥50 | Cohort | 12 years | 224,015 (602) | Ovarian cancer | HRT | 1.13 (0.74, 1.64) | Age |
| Rasmussen et al. (29) | Denmark (2017) | NA | Case control | NA | 14,007 (885) | Ovarian cancer | HRT | 1.34 (0.86, 2.09) | Age, tubal ligation, salpingectomy, hysterectomy, endometriosis, pelvic inflammatory disease, infertility, parity, and hormone replacement therapy |
| Chiaffarino et al. (31) | Italy (2001) | Case: 56.0, control: 57.0 (median) | Case control | NA | 3,442 (1031) | Ovarian cancer | HRT | 1.40 (0.80, 2.50) | Age, center education, parity, OC use. and family history of ovarian and breast cancer in first degree relative |
| Braem et al. (32) | Netherlands (2010) | Case: 62.0, control: 61.5 (mean) | cohort | 16 years | 2,706 (375) | Ovarian cancer | HRT | 0.97 (0.69, 1.37) | Age, parity, duration of OC and HRT use |
| Pasalich et al. (33) | China (2013) | Case: 59.0 ± 5.6, control: 59.7 ± 6.4 | Case control | NA | 1,000 (500) | Ovarian cancer | HRT | 1.05 (0.35, 3.21) | Age, smoking status, alcohol drinking, education, BMI,mutually adjusted for parity, oral contraceptive use, hormone replacement therapy, menopausal status, hysterectomy and family history of ovarian and/or breast cancer |
| Salazar-Martinez et al. (34) | Mexico (1999) | Case: 52.8, control: 54.6 (mean) | Case control | NA | 752 (84) | Ovarian cancer | HRT | 1.00 (0.36, 2.70) | Age, anovulatory index, smoking, diabetes mellitus, hypertension, physical activity, menopausal status, and body build index |
| Jordan et al. (35) | Australia (2007) | 18-79 | Case control | NA | 885 (133) | Mucinous tumors | HRT | 0.67 (0.28, 1.64) | Age, state of residence, education, parity, hysterectomy, smoking status |
| Jordan et al. (35) | Australia (2007) | 18-79 | Case control | NA | 982 (230) | Serous tumors | HRT | 0.71 (0.40, 1.27) | Age, state of residence, education, parity, hysterectomy, smoking status |
| Jordan et al. (35) | Australia (2007) | 18-79 | Case control | NA | 1,115 (363) | Ovarian cancer | HRT | 0.70 (0.42, 1.17) | Age, state of residence, education, parity, hysterectomy, smoking status |
| Polychronopoulou et al. (20) | Greece (1993) | <75 | Case control | NA | 389 (189) | Ovarian cancer | HRT | 5.73 (1.07, 30.80) | Age, years of schooling, weight before the disease, age at menarche, parity and age at first birth |
| Adami et al. (36) | Sweden (1989) | 54.5 (mean) | cohort | 6.7 years | 23,244 (64) | Ovarian cancer | HRT | 0.96 (0.74, 1.23) | NA |
| Schneider et al. (37) | United Kingdom (2009) | 51.3 ± 6.1 | Case control | NA | 602 (86) | Ovarian cancer | HRT | 0.97 (0.61, 1.54) | Smoking status, BMI, use of oral contraceptives, progesterone preparations and vaginal estrogens |
RR, relative risk; CI, confidence interval; BMI, body mass index; NA, not available; HRT, hormone replacement therapy; ERT, estrogen replacement therapy.
Quantitative Synthesis
The association of menopausal HRT with the risk of ovarian cancer is summarized in Table 2.
Table 2.
Summary risk estimates of the association between hormone replacement therapy and ovarian cancer.
| Subgroup | No. of studies | Pooled RR (95% CI) | I2 (%) | Pheterogeneity |
|---|---|---|---|---|
| All studies | 34 | 1.29 (1.19–1.40) | 57.4 | <0.001 |
| After excluding two studies (RR > 3.0) | 32 | 1.27 (1.17–1.37) | 52.1 | <0.001 |
| Study design | ||||
| Cohort studies | 15 | 1.35 (1.19–1.53) | 72.9 | <0.001 |
| Case control studies | 19 | 1.24 (1.11–1.38) | 30.4 | 0.103 |
| Hormones types | ||||
| HRT | 28 | 1.24 (1.15–1.35) | 51.6 | 0.001 |
| ERT | 6 | 1.85 (1.28–2.66) | 75.1 | 0.001 |
| Geographic location | ||||
| North America | 15 | 1.41 (1.23–1.61) | 45.5 | 0.028 |
| Europe | 15 | 1.22 (1.12–1.34) | 52.6 | 0.009 |
| Asia | 2 | 1.76 (1.09–2.85) | 4.8 | 0.305 |
| Australia | 2 | 0.96 (0.57–1.61) | 69.1 | 0.072 |
RR, relative risk; CI, confidence interval; HRT, hormone replacement therapy; ERT, estrogen replacement therapy.
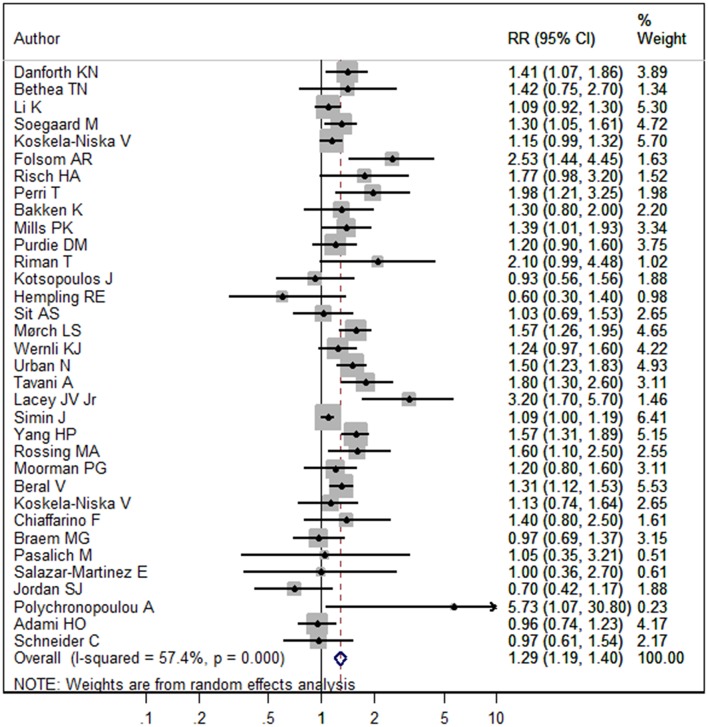
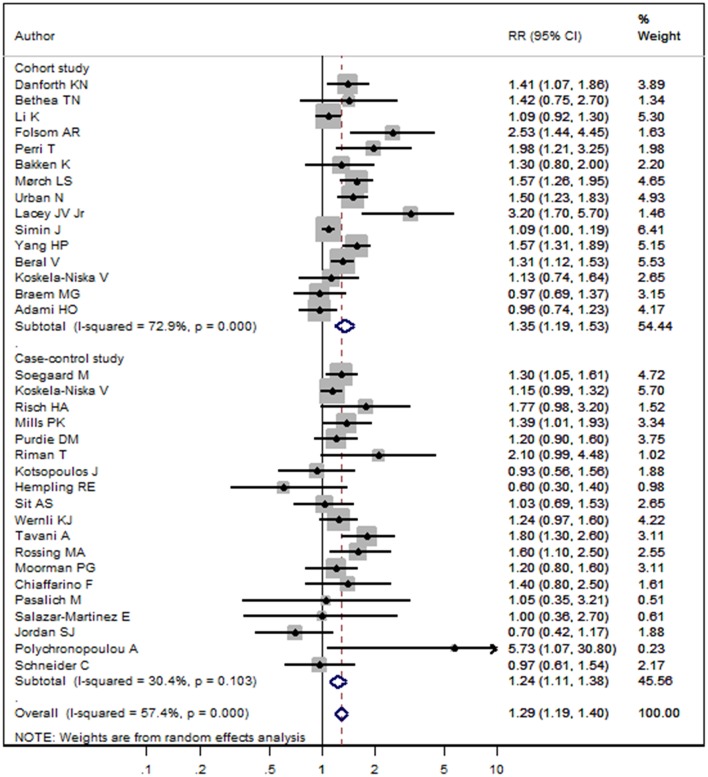
The pooled RR of menopausal HRT and the risk of ovarian cancer was 1.29 (95%CI 1.19–1.40, I2 = 57.4%, Pheterogeneity < 0.001, Figure 2). In subgroup analysis stratified by study design, pooled RRs in cohort and case-control studies were 1.35 (95%CI 1.19–1.53, I2 = 72.9%, Pheterogeneity < 0.001) and 1.24 (95%CI 1.11–1.38, I2 = 30.4%, Pheterogeneity = 0.103), respectively (Figure 3). In subgroup analysis stratified by geographic location, significant positive associations were found for North America (RR = 1.41, 95%CI 1.23–1.61, I2 = 45.5%, Pheterogeneity = 0.028), Europe (RR = 1.22, 95%CI 1.12–1.34, I2 = 52.6%, Pheterogeneity = 0.009), and Asia (RR = 1.76, 95%CI 1.09–2.85, I2 = 4.8%, Pheterogeneity = 0.305), but not Australia (RR = 0.96, 95%CI 0.57–1.61, I2 = 69.1%, Pheterogeneity = 0.072) (Figure S1). In subgroup analysis stratified by the hormones types, pooled RRs for HRT and ERT (estrogen replacement therapy) were 1.24 (95%CI 1.15–1.35, I2 = 51.6%, Pheterogeneity = 0.001) and 1.85 (95%CI 1.28–2.66, I2 = 75.1%, Pheterogeneity = 0.001), respectively (Figure S2).
Figure 2.
Forest plot of menopausal HRT and the risk of ovarian cancer. The size of a gray box is proportional to the weight assigned to the respective study, and horizontal lines represent 95% confidence intervals (CIs).
Figure 3.
Forest plot of menopausal HRT and the risk of ovarian cancer in subgroup analysis stratified by study design. The size of a gray box is proportional to the weight assigned to the respective study, and horizontal lines represent 95% confidence intervals (CIs).
The associations of menopausal HRT with the risk of ovarian cancer in various histological subtypes are summarized in Table 3.
Table 3.
Summary risk estimates of the association between hormone replacement therapy and ovarian cancer by histologic subtype.
| Histologic subtype of ovarian cancer | Subgroup | No. of studies | Pooled RR(95% CI) | I2 (%) | Pheterogeneity |
|---|---|---|---|---|---|
| Serous tumor | All studies | 12 | 1.50 (1.35–1.68) | 27.5 | 0.175 |
| Study design | |||||
| Cohort studies | 3 | 1.64 (1.46–1.85) | 0.0 | 0.998 | |
| Case control studies | 9 | 1.42 (1.20–1.67) | 33.6 | 0.149 | |
| Continent | |||||
| North America | 6 | 1.61 (1.38–1.88) | 0.0 | 0.578 | |
| Europe | 5 | 1.51 (1.36–1.68) | 2.7 | 0.391 | |
| Australia | 1 | 0.71 (0.40–1.27) | NA | NA | |
| Hormones types | |||||
| HRT | 9 | 1.48 (1.29–1.70) | 38.8 | 0.109 | |
| ERT | 3 | 1.54 (1.25–1.89) | 4.3 | 0.352 | |
| Mucinous | All studies | 9 | 0.66 (0.52–0.85) | 0.0 | 0.553 |
| tumor | Study design | ||||
| Cohort studies | 2 | 0.71 (0.50–1.01) | 0.0 | 0.495 | |
| Case control studies | 7 | 0.62 (0.44–0.89) | 1.9 | 0.410 | |
| Continent | |||||
| North America | 4 | 0.79 (0.43–1.44) | 0.0 | 0.666 | |
| Europe | 4 | 0.62 (0.40–0.94) | 38.8 | 0.179 | |
| Australia | 1 | 0.67 (0.28–1.62) | NA | NA | |
| Hormones types | |||||
| HRT | 6 | 0.74 (0.58–0.97) | 0.0 | 0.900 | |
| ERT | 3 | 0.41 (0.23–0.73) | 0.0 | 0.383 | |
| Endometrioid | All studies | 10 | 1.48 (1.13–1.94) | 51.8 | 0.028 |
| tumor | Study design | ||||
| Cohort studies | 3 | 1.88 (1.49–2.36) | 0.0 | 0.789 | |
| Case control studies | 7 | 1.25 (0.86–1.82) | 53.1 | 0.046 | |
| Continent | |||||
| North America | 6 | 1.32 (0.78–2.23) | 66.2 | 0.011 | |
| Europe | 4 | 1.61 (1.32–1.97) | 5.5 | 0.365 | |
| Hormones types | |||||
| HRT | 7 | 1.40 (0.99–1.98) | 60.2 | 0.020 | |
| ERT | 3 | 1.68 (0.97–2.91) | 38.6 | 0.196 | |
| Clear cell | All studies | 5 | 0.94 (0.65–1.36) | 0.0 | 0.689 |
| tumor | Study design | ||||
| Cohort studies | 2 | 1.06 (0.50–2.23) | 47.3 | 0.168 | |
| Case control studies | 3 | 0.95 (0.48–1.90) | 0.0 | 0.837 | |
| Continent | |||||
| North America | 3 | 1.36 (0.70–2.64) | 0.0 | 0.776 | |
| Europe | 2 | 0.80 (0.51–1.24) | 0.0 | 0.853 | |
| Hormones types | |||||
| HRT | 4 | 0.97 (0.66–1.44) | 0.0 | 0.567 | |
| ERT | 1 | 0.72 (0.23–2.27) | NA | NA | |
| Other type of | All studies | 5 | 1.21 (0.91–1.61) | 39.0 | 0.161 |
| ovarian cancer | Study design | ||||
| Cohort studies | 1 | 1.53 (1.10–2.12) | NA | NA | |
| Case control studies | 4 | 1.08 (0.80–1.45) | 16.2 | 0.311 | |
| Continent | |||||
| North America | 3 | 1.44 (1.09–1.92) | 0.0 | 0.747 | |
| Europe | 2 | 1.07 (0.62–1.84) | 69.6 | 0.070 | |
| Hormones types | |||||
| HRT | 4 | 1.44 (1.13–1.84) | 0.0 | 0.900 | |
| ERT | 1 | 0.82 (0.56–1.20) | NA | NA |
RR, relative risk; CI, confidence interval.
In the five major histologic subtypes, significant positive associations were observed in serous (RR = 1.50, 95%CI 1.35–1.68, I2 = 27.5%, Pheterogeneity = 0.175) and endometrioid (RR = 1.48, 95%CI 1.13–1.94, I2 = 51.8%, Pheterogeneity = 0.028) tumors. In subgroup analysis stratified by study design, pooled RRs for serous tumors in cohort and case-control studies were 1.64 (95%CI 1.46–1.85, I2 = 0.0%, Pheterogeneity = 0.998) and 1.42 (95%CI 1.20–1.67, I2 = 33.6%, Pheterogeneity = 0.149), respectively. In subgroup analysis stratified by continent, significant positive associations were found for North America (RR = 1.61, 95%CI 1.38–1.88, I2 = 0.0%, Pheterogeneity = 0.578) and Europe (RR = 1.51, 95%CI 1.36–1.68, I2 = 2.7%, Pheterogeneity = 0.391), respectively. In subgroup analysis stratified by the hormones types, pooled RRs for HRT and ERT were 1.48 (95%CI 1.29–1.70, I2 = 38.8%, Pheterogeneity = 0.109) and 1.54 (95%CI 1.25–1.89, I2 = 4.3%, Pheterogeneity = 0.352), respectively. In subgroup analysis for endometrioid tumors, significant positive associations were obtained in cohort studies (RR = 1.88, 95%CI 1.49–2.36, I2 = 0.0%, Pheterogeneity = 0.789) and Europe (RR = 1.61, 95%CI 1.32–1.97, I2 = 5.5%, Pheterogeneity = 0.365), respectively.
Meta-Regression and Sensitivity Analysis
To assess between-study heterogeneity, we performed univariate meta-regression with the covariates of study design, publication year and continent. However, none of these covariates was found to have a significant impact on between-study heterogeneity. After excluding two study (16, 20) (RR > 3.0) in the ovarian cancer assessment, the heterogeneity remained at a moderate level (I2 = 52.1%, Pheterogeneity < 0.001), and the pooled RR was 1.27 (95%CI 1.17–1.37).
In sensitivity analysis excluding one study at a time, pooled RRs (95%CIs) of the association of menopausal HRT with the risk of ovarian cancer ranged from 1.27 (95%CI 1.18–1.38) to 1.31 (95%CI 1.20–1.42). No individual study had excessive effect on the pooled RR.
Publication Bias
Visual inspection of the funnel plot (Figure S3) and Egger's test (Povarian cancer = 0.083) showed no evidence of significant small-study effect for the association of menopausal HRT with the risk of ovarian cancer. Egger's test also provided no evidence of significant small-study effect for the association of menopausal HRT with the risk of ovarian cancer by histologic subtype (Pserous tumor = 0.762, Pendometrioid tumor = 0.550, Pmucinous tumor = 0.655, Pclear call tumor = 0.349, Pother types of ovarian cancer = 0.892).
Discussion
The current meta-analysis assessed associations of menopausal HRT with the risk of ovarian cancer in various histologic subtypes. Findings of this meta-analysis indicated a positive association of menopausal HRT with the risk of ovarian cancer. In subgroup analysis by study design, significant positive associations were observed in both cohort and case control studies. In subgroup analysis by histologic subtypes, we found that menopausal HRT may increase the risk of serous and endometrioid tumors. In subgroup analysis by the hormones types, significant positive associations were observed for both HRT and ERT. The pooled RR indicated that there might be a stronger association in ERT users, but the result might be not true enough with the insufficient studies about ERT.
The mechanism underlying the association of menopausal HRT with ovarian cancer is not well-understood. A theory suggests that high levels of gonadotropins during menopause act as a promoter on the affected ovarian tissue (49). These findings imply that menopausal HRT might decrease the risk of cancer by reducing the levels of gonadotropins. However, these benefits might be outweighed by estrogen-induced ovarian cell proliferation (50). Estrogen and progesterone receptors are found in normal ovarian surface and most of ovarian tumors are estrogen receptor-positive (51, 52). Estrogen could stimulate the proliferation of ovarian surface epithelial cells and progesterone could promote the apoptosis of ovarian cells. The weaker risk effect of HRT than ERT may be because progesterone counteract the proliferative effect of estrogen on ovarian cells (52–54).
Between-study heterogeneity is common in meta-analysis. It is necessary to explore the potential sources of between-study heterogeneity. In this meta-analysis, a moderate between-study heterogeneity was found. However, meta-regression analysis with the covariates of study design, published year and continent revealed no source of between-study heterogeneity. After excluding two study (20) (RR > 3.0) in the analysis of menopausal HRT and ovarian cancer, between-study heterogeneity was slightly reduced, but results did not change substantially. This indicated that the results were stable and credible.
This meta-analysis had some advantages. The first is the sufficient sample size that made the study had high statistical power to detect even small associations. Secondly, we extracted RRs reflecting the highest degree of control for potential confounders in the original studies. This will help us to get a real connection between the factors and the disease. Thirdly, sensitivity analysis showed that no individual study had excessive effects on pooled data for menopausal HRT and the risk of ovarian cancer by histologic subtypes. Fourthly, after excluding two study (RR > 3.0) in ovarian cancer analysis, between-study heterogeneity was slightly reduced, and the results did not change substantially, suggesting that they were stable. Fifthly, in subgroup analysis stratified by the hormones types, we found both HRT and ERT could increase the risk of ovarian cancer.
However, there were still some deficiencies in this meta-analysis. First, the authors adjusted for confounders such as age, parity, duration of oral contraceptive use, tubal ligation, age at natural menopause and age at menarche etc. in original studies, but we dare not deny whether some unknown confounders might lead to exaggerating or underestimating the association. In addition, confounders adjusted for in various studies were different, which might affect the observed association. Some common biases such as selection bias, recall bias and lost to follow-up etc. in observational studies might also affect the authenticity of the results. Secondly, follow-up durations in various cohort studies differed. Some potential cases might not be observed due to limited follow-up in certain studies. Thirdly, menopausal HRT might be slightly different in each of the papers analyzed. In some papers, the HRT referred to estorgens + progestins, but in others, the HRT referred to only estorgens or estorgens + progestins. This might affect the observed association. Fourthly, the limited amount of studies assessing histologic subtypes made it difficult to confirm the relationship in terms of the kind of therapy and its association with the histological subtypes of ovarian cancer. Fifthly, although the age of subjects was over 50 years old in most included studies, a few studies covered the data from women from pre-menopausal age. This might bias the results of the meta-analysis. Sixthly, the insufficient available data prevented us from conducting a dose-response relationship to explore the association between length of HRT use and the risk of ovarian cancer.
In conclusion, this meta-analysis suggests that menopausal HRT may increase the risk of ovarian cancer, especially for serous and endometrioid tumors. This finding requires confirmation by further studies of associations of menopausal HRT with the risk of ovarian cancer in various histological subtypes.
Data Availability Statement
All datasets for this study are included in the article/supplementary material.
Author Contributions
YL and LM conceived and coordinated the study, designed, performed and analyzed the experiments, and wrote the paper. XY, JB, DL, CS, and JZ carried out the data collection, data analysis, and revised the paper. YM and JL designed the study, carried out the data analysis, and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This study was supported by National Natural Science Foundation (No. 81860515), Health Science and Technology Plan Project of Yunnan (Nos. 2014NS092, 2016NS286, 2016NS287, and 2017NS277), Yunnan Health Training Project of High Level Talents (No. H-201629), and Applied Basic Research Joint Special Fund Project of 2018 Yunnan Provincial Science and Technology Department-Kunming Medical University [No. 2018FE001(-055)].
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00801/full#supplementary-material
Forest plot of menopausal HRT and the risk of ovarian cancer in subgroup analysis stratified by geographic location. The size of a gray box is proportional to the weight assigned to the respective study, and horizontal lines represent 95% confidence intervals (CIs).
Forest plot of menopausal HRT and the risk of ovarian cancer in subgroup analysis stratified by the hormones types. The size of a gray box is proportional to the weight assigned to the respective study, and horizontal lines represent 95% confidence intervals (CIs).
The funnel plot of menopausal HRT and the risk of ovarian cancer. Each dot represents a distinct study.
References
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Li DP, Du C, Zhang ZM, Li GX, Yu ZF, Wang X, et al. Breastfeeding and ovarian cancer risk: a systematic review and meta-analysis of 40 epidemiological studies. Asian Pac J Cancer Prev. (2014) 15:4829–37. 10.7314/APJCP.2014.15.12.4829 [DOI] [PubMed] [Google Scholar]
- 3.Havrilesky LJ, Moorman PG, Lowery WJ, Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol. (2013) 122:139–47. 10.1097/AOG.0b013e318291c235 [DOI] [PubMed] [Google Scholar]
- 4.Bae HS, Kim HJ, Hong JH, Lee JK, Lee NW, Song JY. Obesity and epithelial ovarian cancer survival: a systematic review and meta-analysis. J Ovarian Res. (2014) 7:41. 10.1186/1757-2215-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Zhang TT, Zhao JJ, Qi SF, Du P, Liu DW, et al. The association between overweight, obesity and ovarian cancer: a meta-analysis. Jpn J Clin Oncol. (2015) 45:1107–15. 10.1093/jjco/hyv150 [DOI] [PubMed] [Google Scholar]
- 6.Lee JY, Jeon I, Kim JW, Song YS, Yoon JM, Park SM. Diabetes mellitus and ovarian cancer risk: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. (2013) 23:402–12. 10.1097/IGC.0b013e31828189b2 [DOI] [PubMed] [Google Scholar]
- 7.Braem MG, Onland-Moret NC, Schouten LJ, Kruitwagen RF, Lukanova A, Allen NE, et al. Multiple miscarriages are associated with the risk of ovarian cancer: results from the European Prospective Investigation into Cancer and Nutrition. PLoS ONE. (2012) 7:e37141. 10.1371/journal.pone.0037141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazerouni N, Greene MH, Lacey JV, Jr, Mink PJ, Schairer C. Family history of breast cancer as a risk factor for ovarian cancer in a prospective study. Cancer. (2006) 107:1075–83. 10.1002/cncr.22082 [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Li F, Chen L, Lai YM, Zhang X, Li HY. Change in risk of breast cancer after receiving hormone replacement therapy by considering effect-modifiers: a systematic review and dose-response meta-analysis of prospective studies. Oncotarget. (2017) 8:81109–24. 10.18632/oncotarget.20154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sillero-Arenas M, Delgado-Rodriguez M, Rodigues-Canteras R, Bueno-Cavanillas A, Galvez-Vargas R. Menopausal hormone replacement therapy and breast cancer: a meta-analysis. Obstet Gynecol. (1992) 79:286–94. [PubMed] [Google Scholar]
- 11.Folsom A, Anderson J, Ross J. Estrogen replacement therapy and ovarian cancer. Epidemiology. (2004) 15:100–4. 10.1097/01.ede.0000091606.31903.8e [DOI] [PubMed] [Google Scholar]
- 12.Perri T, Lifshitz D, Sadetzki S, Oberman B, Meirow D, Ben-Baruch G, et al. Fertility treatments and invasive epithelial ovarian cancer risk in Jewish Israeli BRCA1 or BRCA2 mutation carriers. Fertil Steril. (2015) 103:1305–12. 10.1016/j.fertnstert.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 13.Morch L, Lokkegaard E, Andreasen A, Kruger-Kjaer S, Lidegaard O. Hormone therapy and ovarian cancer. JAMA. (2009) 302:298–305. 10.1001/jama.2009.1052 [DOI] [PubMed] [Google Scholar]
- 14.Urban N, Hawley S, Janes H, Karlan BY, Berg CD, Drescher CW, et al. Identifying post-menopausal women at elevated risk for epithelial ovarian cancer. Gynecol Oncol. (2015) 139:253–60. 10.1016/j.ygyno.2015.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tavani A, Ricci E, La Vecchia C, Surace M, Benzi G, Parazzini F, et al. Influence of menstrual and reproductive factors on ovarian cancer risk in women with and without family history of breast or ovarian cancer. Int J Epidemiol. (2000) 29:799–802. 10.1093/ije/29.5.799 [DOI] [PubMed] [Google Scholar]
- 16.Lacey JV, Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. (2002) 288:334–41. 10.1001/jama.288.3.334 [DOI] [PubMed] [Google Scholar]
- 17.Simin J, Tamimi R, Lagergren J, Adami HO, Brusselaers N. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. (2017) 84:60–8. 10.1016/j.ejca.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 18.Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS. Menopausal hormone therapy and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. (2007) 16:2548–56. 10.1158/1055-9965.EPI-07-0550 [DOI] [PubMed] [Google Scholar]
- 19.Beral V, Million Women Study C, Bull D, Green J, Reeves G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. (2007) 369:1703–10. 10.1016/S0140-6736(07)60534-0 [DOI] [PubMed] [Google Scholar]
- 20.Polychronopoulou A, Tzonou A, Hsieh CC, Kaprinis G, Rebelakos A, Toupadaki N, et al. Reproductive variables, tobacco, ethanol, coffee and somatometry as risk factors for ovarian cancer. Int J Cancer. (1993) 55:402–7. 10.1002/ijc.2910550312 [DOI] [PubMed] [Google Scholar]
- 21.Hempling RE, Wong C, Piver MS, Natarajan N, Mettlin CJ. Hormone replacement therapy as a risk factor for epithelial ovarian cancer: results of a case-control study. Obstet Gynecol. (1997) 89:1012–6. 10.1016/S0029-7844(97)00118-X [DOI] [PubMed] [Google Scholar]
- 22.Kotsopoulos J, Lubinski J, Neuhausen SL, Lynch HT, Rosen B, Ainsworth P, et al. Hormone replacement therapy and the risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers. Gynecol Oncol. (2006) 100:83–8. 10.1016/j.ygyno.2005.07.110 [DOI] [PubMed] [Google Scholar]
- 23.Purdie DM, Bain CJ, Siskind V, Russell P, Hacker NF, Ward BG, et al. Hormone replacement therapy and risk of epithelial ovarian cancer. Br J Cancer. (1999) 81:559–63. 10.1038/sj.bjc.6690731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sit AS, Modugno F, Weissfeld JL, Berga SL, Ness RB. Hormone replacement therapy formulations and risk of epithelial ovarian carcinoma. Gynecol Oncol. (2002) 86:118–23. 10.1006/gyno.2002.6746 [DOI] [PubMed] [Google Scholar]
- 25.Li K, Husing A, Fortner RT, Tjonneland A, Hansen L, Dossus L, et al. An epidemiologic risk prediction model for ovarian cancer in Europe: the EPIC study. Br J Cancer. (2015) 112:1257–65. 10.1038/bjc.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakken K, Alsaker E, Eggen AE, Lund E. Hormone replacement therapy and incidence of hormone-dependent cancers in the Norwegian Women and Cancer study. Int J Cancer. (2004) 112:130–34. 10.1002/ijc.20389 [DOI] [PubMed] [Google Scholar]
- 27.Wernli KJ, Newcomb PA, Hampton JM, Trentham-Dietz A, Egan KM. Hormone therapy and ovarian cancer: incidence and survival. Cancer Causes Control. (2008) 19:605–13. 10.1007/s10552-008-9125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koskela-Niska V, Lyytinen H, Riska A, Pukkala E, Ylikorkala O. Ovarian cancer risk in postmenopausal women using estradiol-progestin therapy - a nationwide study. Climacteric. (2013) 16:48–53. 10.3109/13697137.2012.663818 [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen ELK, Hannibal CG, Dehlendorff C, Baandrup L, Junge J, Vang R, et al. Parity, infertility, oral contraceptives, and hormone replacement therapy and the risk of ovarian serous borderline tumors: a nationwide case-control study. Gynecol Oncol. (2017) 144:571–6. 10.1016/j.ygyno.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 30.Bethea TN, Palmer JR, Adams-Campbell LL, Rosenberg L. A prospective study of reproductive factors and exogenous hormone use in relation to ovarian cancer risk among Black women. Cancer Causes Control. (2017) 28:385–91. 10.1007/s10552-016-0840-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiaffarino F, Pelucchi C, Parazzini F, Negri E, Franceschi S. Reproductive and hormonal factors and ovarian cancer. Ann Oncol. (2001) 12:337–41. 10.1023/A:1011128408146 [DOI] [PubMed] [Google Scholar]
- 32.Braem MG, Onland-Moret NC, van den Brandt PA, Goldbohm RA, Peeters PH, Kruitwagen RF, et al. Reproductive and hormonal factors in association with ovarian cancer in the Netherlands cohort study. Am J Epidemiol. (2010) 172:1181–9. 10.1093/aje/kwq264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasalich M, Su D, Binns CW, Lee AH. Reproductive factors for ovarian cancer in southern Chinese women. J Gynecol Oncol. (2013) 24:135–40. 10.3802/jgo.2013.24.2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salazar-Martinez E, Lazcano-Ponce EC, Gonzalez Lira-Lira G, Escudero-De los Rios P, Salmeron-Castro J, Hernandez-Avila M. Reproductive factors of ovarian and endometrial cancer risk in a high fertility population in Mexico. Cancer Res. (1999) 59:3658–62. [PubMed] [Google Scholar]
- 35.Jordan SJ, Green AC, Whiteman DC, Webb PM. Risk factors for benign serous and mucinous epithelial ovarian tumors. Obstet Gynecol. (2007) 109:647–54. 10.1097/01.AOG.0000254159.75977.fa [DOI] [PubMed] [Google Scholar]
- 36.Adami HO, Persson I, Hoover R, Schairer C, Bergkvist L. Risk of cancer in women receiving hormone replacement therapy. Int J Cancer. (1989) 44:833–9. 10.1002/ijc.2910440515 [DOI] [PubMed] [Google Scholar]
- 37.Schneider C, Jick SS, Meier CR. Risk of gynecological cancers in users of estradiol/dydrogesterone or other HRT preparations. Climacteric. (2009) 12:514–24. 10.3109/13697130903075352 [DOI] [PubMed] [Google Scholar]
- 38.Soegaard M, Jensen A, Hogdall E, Christensen L, Hogdall C, Blaakaer J, et al. Different risk factor profiles for mucinous and nonmucinous ovarian cancer: results from the Danish MALOVA study. Cancer Epidemiol Biomarkers Prev. (2007) 16:1160–6. 10.1158/1055-9965.EPI-07-0089 [DOI] [PubMed] [Google Scholar]
- 39.Koskela-Niska V, Pukkala E, Lyytinen H, Ylikorkala O, Dyba T. Effect of various forms of postmenopausal hormone therapy on the risk of ovarian cancer–a population-based case control study from Finland. Int J Cancer. (2013) 133:1680–8. 10.1002/ijc.28167 [DOI] [PubMed] [Google Scholar]
- 40.Risch HA. Estrogen replacement therapy and risk of epithelial ovarian cancer. Gynecol Oncol. (1996) 63:254–7. 10.1006/gyno.1996.0315 [DOI] [PubMed] [Google Scholar]
- 41.Mills PK, Riordan DG, Cress RD, Goldsmith DF. Hormone replacement therapy and invasive and borderline epithelial ovarian cancer risk. Cancer Detect Prev. (2005) 29:124–32. 10.1016/j.cdp.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 42.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Hormone replacement therapy and the risk of invasive epithelial ovarian cancer in Swedish women. J Natl Cancer Inst. (2002) 94:497–504. 10.1093/jnci/94.7.497 [DOI] [PubMed] [Google Scholar]
- 43.Morch LS, Lokkegaard E, Andreasen AH, Kjaer SK, Lidegaard O. Hormone therapy and different ovarian cancers: a national cohort study. Am J Epidemiol. (2012) 175:1234–42. 10.1093/aje/kwr446 [DOI] [PubMed] [Google Scholar]
- 44.Moorman PG, Schildkraut JM, Calingaert B, Halabi S, Berchuck A. Menopausal hormones and risk of ovarian cancer. Am J Obstet Gynecol. (2005) 193:76–82. 10.1016/j.ajog.2004.11.013 [DOI] [PubMed] [Google Scholar]
- 45.Yang HP, Trabert B, Murphy MA, Sherman ME, Sampson JN, Brinton LA, et al. Ovarian cancer risk factors by histologic subtypes in the NIH-AARP Diet and Health Study. Int J Cancer. (2012) 131:938–48. 10.1002/ijc.26469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. A prospective study of postmenopausal hormone use and ovarian cancer risk. Br J Cancer. (2007) 96:151–6. 10.1038/sj.bjc.6603527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greiser CM, Greiser EM, Doren M. Menopausal hormone therapy and risk of ovarian cancer: systematic review and meta-analysis. Hum Reprod Update. (2007) 13:453–63. 10.1093/humupd/dmm012 [DOI] [PubMed] [Google Scholar]
- 48.Pearce CL, Chung K, Pike MC, Wu AH. Increased ovarian cancer risk associated with menopausal estrogen therapy is reduced by adding a progestin. Cancer. (2009) 115:531–9. 10.1002/cncr.23956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stadel BV. Letter: The etiology and prevention of ovarian cancer. Am J Obstet Gynecol. (1975) 123:772–4. 10.1016/0002-9378(75)90509-8 [DOI] [PubMed] [Google Scholar]
- 50.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. (1983) 71:717–21. [PubMed] [Google Scholar]
- 51.Cunat S, Hoffmann P, Pujol P. Estrogens and epithelial ovarian cancer. Gynecol Oncol. (2004) 94:25–32. 10.1016/j.ygyno.2004.03.026 [DOI] [PubMed] [Google Scholar]
- 52.Syed V, Ulinski G, Mok SC, Yiu GK, Ho SM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. (2001) 61:6768–76. [PubMed] [Google Scholar]
- 53.Lau KM, Mok SC, Ho SM. Expression of human estrogen receptor-alpha and -beta, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells. Proc Natl Acad Sci USA. (1999) 96:5722–7. 10.1073/pnas.96.10.5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukherjee K, Syed V, Ho SM. Estrogen-induced loss of progesterone receptor expression in normal and malignant ovarian surface epithelial cells. Oncogene. (2005) 24:4388–400. 10.1038/sj.onc.1208623 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot of menopausal HRT and the risk of ovarian cancer in subgroup analysis stratified by geographic location. The size of a gray box is proportional to the weight assigned to the respective study, and horizontal lines represent 95% confidence intervals (CIs).
Forest plot of menopausal HRT and the risk of ovarian cancer in subgroup analysis stratified by the hormones types. The size of a gray box is proportional to the weight assigned to the respective study, and horizontal lines represent 95% confidence intervals (CIs).
The funnel plot of menopausal HRT and the risk of ovarian cancer. Each dot represents a distinct study.
Data Availability Statement
All datasets for this study are included in the article/supplementary material.