This cross-sectional study of 9- and 10-year-old children examines whether body mass index is associated with cortical thickness and whether this association interacts with executive functioning.
Key Points
Questions
Is body mass index associated with cortical thickness in 9- and 10-year-old children, and does this association interact with executive functioning?
Findings
In this cross-sectional study, higher body mass index was associated with thinner cortex, especially in the prefrontal cortex. The association between body mass index and working memory was partially mediated by prefrontal cortex thickness.
Meaning
These findings suggest that body mass index is associated with cortical development and diminished executive functions, such as working memory.
Abstract
Importance
A total of 25.7 million children in the United States are classified as overweight or obese. Obesity is associated with deficits in executive function, which may contribute to poor dietary decision-making. Less is known about the associations between being overweight or obese and brain development.
Objective
To examine whether body mass index (BMI) is associated with thickness of the cerebral cortex and whether cortical thickness mediates the association between BMI and executive function in children.
Design, Setting, and Participants
In this cross-sectional study, cortical thickness maps were derived from T1-weighted structural magnetic resonance images of a large, diverse sample of 9 and 10-year-old children from 21 US sites. List sorting, flanker, matrix reasoning, and Wisconsin card sorting tasks were used to assess executive function.
Main Outcomes and Measures
A 10-fold nested cross-validation general linear model was used to assess mean cortical thickness from BMI across cortical brain regions. Associations between BMI and executive function were explored with Pearson partial correlations. Mediation analysis examined whether mean prefrontal cortex thickness mediated the association between BMI and executive function.
Results
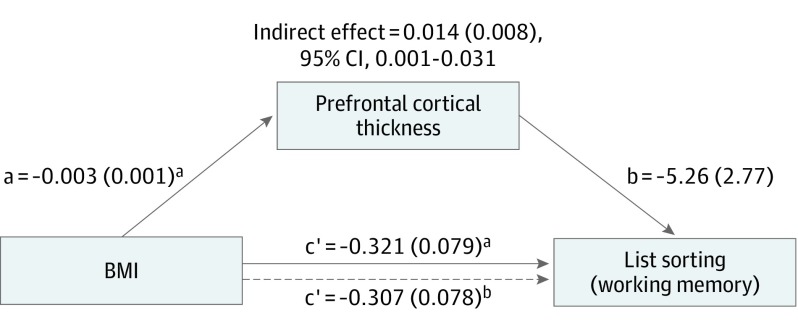
Among 3190 individuals (mean [SD] age, 10.0 [0.61] years; 1627 [51.0%] male), those with higher BMI exhibited lower cortical thickness. Eighteen cortical regions were significantly inversely associated with BMI. The greatest correlations were observed in the prefrontal cortex. The BMI was inversely correlated with dimensional card sorting (r = −0.088, P < .001), list sorting (r = −0.061, P < .003), and matrix reasoning (r = −0.095, P < .001) but not the flanker task. Mean prefrontal cortex thickness mediated the association between BMI and list sorting (mean [SE] indirect effect, 0.014 [0.008]; 95% CI, 0.001-0.031) but not the matrix reasoning or card sorting task.
Conclusions and Relevance
These results suggest that BMI is associated with prefrontal cortex development and diminished executive functions, such as working memory.
Introduction
A total of 13.7 million children in the United States younger than 18 years are obese, and an additional 12 million children are classified as overweight.1 The percentage of extreme obesity among children has continued to increase in the past decade and is now estimated to be approximately 750 000, or 6%, of children who meet the diagnostic threshold for obesity.2 The negative metabolic and cardiovascular sequelae of increasing adiposity, obesity, and chronic obesity in children is well established. Children with obesity are more likely to develop early-onset type 2 diabetes and heart disease,3,4,5 tend to have more severe risk factors and disease burden,6 and are at greater risk for premature mortality than their healthy weight peers.7,8
Higher body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared) has also been associated with poorer cognitive performance across the lifespan, particularly in the domain of higher executive functions.9,10,11,12 Less is known about the effects of obesity and being overweight on brain development and how this might interact with cognitive ability. Many neuroimaging studies13,14,15,16 have found structural alterations in cortical regions involved in executive control in obese children compared with lean children. However, limited sample sizes, insufficient statistical power, differences in sample populations, and differing magnetic resonance imaging (MRI) modalities have yielded mixed results. Maayan et al13 reported that obese children performed worse on working memory tasks compared with healthy control individuals and had less orbitofrontal cortical volume, a brain region associated with appetite control. By contrast, Saute et al14 found an association between increased visceral adiposity, but not BMI, and cortical thickness in a range of cortical regions outside the orbitofrontal cortex, and Sharkey et al15 found no statistically significant association between BMI and cortical thickness in healthy children 12 to 18 years of age.
Strong evidence supports metabolic dysregulation and dysfunction associated with escalating levels of adiposity. Chronic cellular stress and excessive inflammatory mediators in concert with extracellular adipocyte remodeling17 related to excessive adiposity are early pathophysiologic changes that lead to insulin resistance and thus cerebral and cardiovascular structural alterations.18 Neuroimaging studies have observed lower gray matter volume in association with increasing levels of adiposity in otherwise healthy individuals19 during the transition from childhood to early adolescence, a critical developmental period for brain development.20 Metabolic aberrations early in life may hamper optimal brain development and maturation, which, in turn, may affect key areas of cognition early in life. Thus, our working hypothesis was that children with increasing levels of adiposity (ie, BMI) have a thinner cortex than do children who are leaner and that this alteration mediates executive functioning.
To clarify the association among cortical thickness, cognition, and adiposity in children, we examined a large sample of 9- and 10-year-old children from the Adolescent Brain Cognitive Development (ABCD). The aims of the study were to (1) evaluate regional associations between BMI and cortical thickness, (2) assess whether higher BMI was associated with lower executive functioning, and (3) examine whether cortical thickness in brain regions associated with BMI mediated the association between BMI and executive functioning.
Methods
Data Source
This cross-sectional study used data from the ABCD study, which was designed to examine the association of brain development with childhood experiences and examine how these experiences are associated with social, emotional, and physical health; the development of risky behaviors; and substance use prospectively. A large cohort of 9- and 10-year-old children were recruited at 21 US sites in 2017.21 Children were extensively assessed with regard to mental health, cognitive function, and social, cultural, and physical environments. The assessments included structural and functional MRI using a standardized multisite protocol. Analyses were conducted on data from the ABCD study’s first (1.0) curated release, which included deidentified data from 4524 children aged 9 to 10 years. The local institutional review boards at each consortium site were responsible for ensuring protection of human subjects in accordance with the Declaration of Helsinki.22 Parents provided written informed consent, and children provided verbal assent.
Design and Sample
The study design, sample stratification, recruitment, and data collection procedures are detailed elsewhere.23 Exclusion criteria for the ABCD study included moderate to severe intellectual disability; current substance use disorder; noncorrectable vision, hearing, or sensorimotor impairments; major neurologic disorders; gestational age younger than 28 weeks; birth weight less than 1.2 kg; birth complications requiring more than 1 month of hospitalization; history of traumatic brain injury; and standard MRI contraindications (eg, implanted metals, claustrophobia, and orthodonture).23 In addition, we excluded children with a current or past diagnosis of attention-deficit/hyperactivity disorder, any neurologic condition (eg, seizures, head injury, and/or cerebral palsy), diabetes type 1 or type 2, lead exposure, muscular dystrophy, schizophrenia, autism spectrum disorder, and a BMI less than 10. Only individuals with complete data on relevant variables and assessments were included in the analysis.
Nonimaging Measures
Body Mass Index
Heights and weights were objectively measured with individuals in light clothing. The BMI percentiles for age and sex were used to classify individuals as underweight (ie, <5%), within acceptable limits (ie, 5%-85%), overweight (ie, 85%-95%), and obese (ie, ≥95%).24
Pubertal Status
Child pubertal status was assessed subjectively by parent report. Parents’ rating of child physical development yielded a categorical maturation score similar to that of Tanner staging. Scores ranged from prepubertal (score of 1) to postpubertal (score of 5).25
Cognitive Battery
The National Institutes of Health Toolbox Cognition Battery26 evaluates cognitive domains of memory, language, and other higher-order executive processes. This study focused on measures of higher executive functions dependent on the prefrontal cortex. The flanker, dimensional Wisconsin card sorting, and list sorting working memory tasks assessed cognitive control and working memory. The matrix reasoning task, a subtest of the Weschler Intelligence Test for Children-V,27 assessed fluid intelligence. Raw scores were corrected for age to yield a final age-corrected score. All instruments were administered and completed by individuals in 1 visit. A comprehensive overview of the ABCD cognitive battery can be found in the article by Luciana et al.28
Structural Neuroimaging
Imaging Protocol
Structural MRI was performed at 21 sites in the United States using a standardized protocol29 for imaging acquisition, processing, reconstruction, and quality control. All structural MRI findings were screened for incidental findings by a neuroradiologist.
Acquisition Parameters
Whole brain coverage was achieved using isotropic voxel resolution of 1 × 1 × 1 × mm, 256 × 256 matrix, flip angle of 8°, inversion delay of 1060 milliseconds, 176 to 225 sections, field of view of 256 × 240 to 256, field of view phase of 93.75% to 100%, repetition time of 2400 to 2500 milliseconds, echo time of 2 to 2.9 milliseconds, and parallel imaging of 1.5 × 2.2. The total acquisition time was 5 minutes 38 seconds to 7 minutes 12 seconds.
Image Reconstruction
Structural MRIs were generated from T1-weighted and T2-weighted images that were processed and corrected for gradient nonlinearity distortions to ensure reliability across multiple imaging sites.30 T2-weighted images were volume registered to T1-weighted images by adjusting and maximizing the relative position and orientation of mutual information among images.31 Intensity nonuniformity correction was based on tissue segmentation and sparse spatial smoothing. Images were resampled with 1-mm isotropic voxels into rigid alignment within the brain atlas. Cortical reconstruction and volumetric segmentation were performed using FreeSurfer software, version 5.3.0 (Harvard University). Images were stripped of skull and nonbrain material32 followed by white matter segmentation and initial mesh creation.33 Correction of topologic defects followed procedures described by Fischl et al34 and Ségonne et al.35 Images underwent surface optimization36,37,38 and nonlinear registration to a spherical surface-based atlas.39 Cortical regions were parcellated and labeled with a surface-based atlas classification that provides brain region of interest–level results that are easily replicable and freely available within the data release.40
Quality Control
Protocol adherence was performed among imaging sites to ensure integrity and completeness.41 Images were manually reviewed for data quality. Images with the most severe artifact, irregularities, and/or poor image quality were rejected and excluded from processing and analysis. Cortical surface reconstruction images were reviewed for motion, intensity inhomogeneity, white matter underestimation, pial overestimation, magnetic susceptibility artifact, and susceptibility artifact.
Statistical Analysis
The analytic approach tested the model that higher levels of BMI (ie, adiposity) is associated with alteration of the integrity of the cortex and that these changes within the cortex are associated with impairments within the domains of executive functioning.
Regional Associations Between BMI and Cortical Thickness
A general linear model (GLM) was used to examine associations between BMI and mean cortical thickness in each of the 66 cortical brain regions parcellated according to the Desikan-Killiany atlas.40 The BMI was used as a proxy for adiposity for all analytic procedures to better quantify associations between cortical thickness brain regions and more severe levels of obesity that would be obscured with the use of BMI z scores or BMI percentiles for age and sex.42 Cortical thickness was the primary response variable, and BMI was the primary explanatory variable of interest. Additional covariates included in the GLM were intracranial volume (ICV), age, sex, handedness, MRI scanner serial number, puberty, and race. The 2-tailed α was set at .05. The Bonferroni method was used to adjust for multiple comparisons.
Associations Between BMI and Whole Cortex
To complement our analysis that explored associations between the thickness of individual cortical regions and BMI, models to predict the converse association (ie, to predict BMI based on the thickness of all cortical regions with and without demographic measures) were created using elastic net regularized regression.43 The initial variable set included 66 cortical measures and 7 additional covariates (ie, age, ICV, puberty, handedness, race, age, MRI scanner, and sex). This allowed us to explore how much variance in BMI was associated with the potential contribution of all cortical regions. Elastic net regularization provides a robust, parsimonious model to explain associations between variables of interest that are highly collinear by using both L1 and L2 penalties to combine feature selection with inclusion of correlated features.44 To prevent bias and overfitting associated with the large number of variables in our prediction model approach, we used 10-fold nested cross-validation (giving a total of 100 model fits) to enable tuning of the 2 regularization parameters (ie, L1 and L2).45 In any single fold, the training set was composed of 90% of the individuals with testing performed on the remaining 10% of individuals. In addition, the entire fitting was repeated 5 times to estimate uncertainty in the resulting scores and further improve the models’ predictive power. The elastic net fitting and nested cross-validation were implemented using Scikit-Learn, version 0.19 in Python software, version 3.7 (Python Software Foundation).46 The quality of each model fit was reported as the Pearson correlation between the fit estimates (ie, testing model) and the true BMI (ie, training model) and as the percentage of variance in BMI explained by the model. The performance of the model was evaluated by how well it performed on aspects of the data not included in the initial model construction, thereby quantifying the generalizability and reproducibility of the results. The 2-tailed α was set at .05.
Association Between BMI and Executive Function
Pearson partial correlations were computed to investigate any significant associations between BMI and the 4 cognitive measures of interest while controlling for age, sex, race, ICV, handedness, and puberty. Cognitive measures that were significantly associated with BMI were used for mediation analysis to further explain the association among BMI, executive function, and cortex. Data were analyzed using SPSS software, version 2347 with a 2-tailed α = .05.
Prefrontal Cortex, BMI, and Executive Function Mediation Analysis
Because the results of the initial GLM revealed that the strongest association between BMI and cortical thickness was in the prefrontal cortex, a mediation analysis was performed to assess whether mean prefrontal cortical thickness mediated the association between BMI and executive function. The mean thickness of all cortical regions located within the prefrontal cortex was calculated. Only individuals who had structural imaging and complete cognitive data were included. Mediation models were tested using PROCESS, a macro developed for SPSS software, version 23 (SPSS Inc)47 by Andrew Hayes (http://processmacro.org/). PROCESS uses observed variable ordinary least squares path analysis to estimate direct and indirect effects.48,49 Significance of indirect effects of the mean prefrontal cortical thickness was assessed by 1000 bootstrap 95% CIs. Simulation research indicates that the bootstrap method is more robust to nonnormality and has better type I error control than the causal steps method and the Sobel test.49 Covariates used in the models included age, sex, ICV, race, puberty, handedness, and MRI scanner. All paths are reported as unstandardized ordinary least squares regression coefficients. Concretely, the analyses were based on model 4 in the macro. The BMI was modeled as the associated variable, mean prefrontal cortical thickness was included as the mediating variable, and each of the cognitive measures were assessed separately as the outcome variable. That is, 4 models were tested corresponding to each of the 4 cognitive measures. Each cognitive measure was tested because multiple factors may contribute to cortical thickness, some in opposing directions. Unknown or unaccounted factors that have not been expressly included in the mediation model could have obscured the apparent association between BMI and cognitive function.
Results
Of the 4524 individuals in the ABCD study curated release 1.0, 4329 had complete imaging data. Imaging data from 767 individuals were excluded because of excessive head motion and/or failure to pass acceptable study quality control measures. Therefore, 3190 individuals (mean [SD] age, 10.0 [0.61] years; 1627 [51.0%] male) had complete demographic data and were included in the analysis. The demographics of the study participants are presented in Table 1.
Table 1. Sample Characteristicsa.
| Characteristic | Aggregate (n = 3190) | Subsample (n = 2418) |
|---|---|---|
| Age, mean (SD), mo | 120.2 (7.3) | 120.1 (7.2) |
| Race | ||
| White | 2663 (84) | 2026 (83.8) |
| Black | 435 (13.6) | 321 (13.3) |
| Asian | 179 (5.6) | 127 (5.3) |
| Female | 1563 (49.0) | 1166 (48.2) |
| BMIb | ||
| Mean (SD) | 18.64 (3.9) | 18.65 (3.9) |
| 85% to <95% | 429 (13.4) | 322 (13.2) |
| ≥95% | 491 (15.4) | 357 (14.8) |
| Pubertal stage by sex | ||
| Before | ||
| Female | 511 (16.0) | 388 (16.0) |
| Male | 1166 (36.6) | 907 (37.5) |
| Early | ||
| Female | 373 (11.7) | 297 (12.3) |
| Male | 375 (11.8) | 284 (11.7) |
| Mid | ||
| Female | 604 (18.9) | 427 (17.7) |
| Male | 62 (1.9) | 44 (1.8) |
| Late | ||
| Female | 37 (1.2) | 28 (1.2) |
| Male | 6 (0.2) | 4 (0.2) |
| After | ||
| Female | 0 | 0 |
| Male | 0 | 0 |
| Right-handed | 2532 (79.4) | 1926 (79.7) |
| Total parent income, $ | ||
| ≤25 000 | 508 (15.9) | 356 (14.7) |
| >25 000 to <50 000 | 714 (22.4) | 556 (23.0) |
| >50 000 to <75 000 | 517 (16.2) | 400 (16.6) |
| >75 000 to <100 000 | 1046 (32.8) | 799 (33.0) |
| ≥200 000 | 405 (12.7) | 307 (12.7) |
| Highest parental education | ||
| Less than high school | 95 (3.0) | 65 (2.7) |
| High school | 282 (8.9) | 293 (8.0) |
| Some college | 502 (15.7) | 387 (16.0) |
| Associate’s degree | 380 (11.9) | 301 (12.4) |
| Bachelor’s degree | 1011 (31.7) | 764 (31.6) |
| Master’s degree | 708 (22.2) | 539 (22.3) |
| Doctoral level | 212 (6.6) | 169 (7.0) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters).
Data are presented as number (percentage) of patients unless otherwise indicated.
BMI according to Centers for Disease Control and Prevention criteria: 85th to 95th percentile for age and sex is considered overweight; 95th percentile and greater is considered obese.
Regional Associations Between BMI and Cortical Thickness
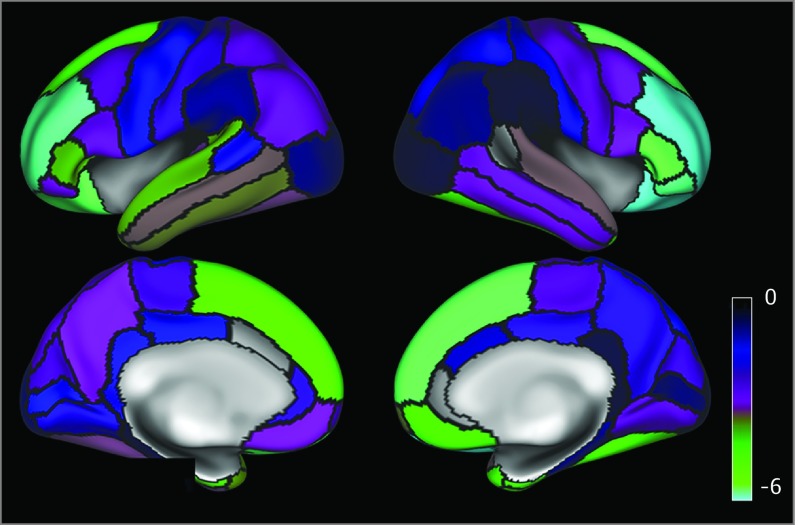
Overall, higher BMI was associated with lower cortical thickness (eTable 1 in the Supplement). Figure 1 provides a t statistic brain map that shows the overall change in cortical thickness associated with BMI without statistical thresholding. eTable 2 in the Supplement gives the mean cortical thickness for each cortex region. Eighteen cortical regions were significantly and inversely associated with BMI after adjustment for multiple comparisons (Table 2). The strongest associations were observed in the prefrontal cortex.
Figure 1. t Statistic Map Demonstrating the Associations Between Regional Parcellations of Cortical Thickness and Body Mass Index (BMI).
Brain map parcellation according to the Desikan-Killiany atlas. Regions are outlined in black. Cyan brain regions (t statistic, −6) demonstrate the strongest association between higher BMI and thinner brain cortical region. Indigo regions demonstrate weaker associations (t = −3.5) between BMI and brain cortical region.
Table 2. Cortical Brain Regions Demonstrating an Association With Body Mass Index.
| Cortical Region | t Statistica |
|---|---|
| Left cortical hemisphere | |
| Rostral middle frontal gyrus | −5.77 |
| Lateral orbitofrontal | −5.58 |
| Superior frontal gyrus | −4.86 |
| Entorhinal | −4.19 |
| Pars triangularis | −3.96 |
| Superior temporal lobe | −3.87 |
| Temporal pole | −3.74 |
| Inferior temporal lobe | −3.58 |
| Right cortical hemisphere | |
| Rostral middle frontal gyrus | −7.55 |
| Lateral orbitofrontal | −5.95 |
| Pars orbitalis | −5.75 |
| Superior frontal gyrus | −5.58 |
| Pars triangularis | −5.55 |
| Temporal pole | −4.61 |
| Fusiform gyrus | −4.53 |
| Entorhinal | −4.49 |
| Medial orbitofrontal | −4.45 |
| Frontal pole | −3.6 |
Significant with a Bonferroni threshold of P < .001 for all.
Associations Between BMI and Whole Brain Cortex
The 10-fold nested cross-validation model using only demographic data (ie, no cortex) indicated a mean r of 0.359 between the fit estimates (ie, testing model) and the BMI (ie, training model) and explained a mean (SD) of 12.9% (0.1%) of the variance between BMI and demographics (eg, sex, race, and age). The model allowing demographic data and all cortical regions indicated a mean r of 0.388, with a mean (SD) of 14.94% (0.1%) of the variance among BMI, demographics, and cortical thickness.
BMI and Neurocognitive Partial Correlates
Of the 3190 individuals, 772 lacked complete data for the full cognitive task battery, further restricting the sample size to 2418 (75.8%). The BMI was significantly and inversely correlated with dimensional card sorting (r = −0.088, P < .001), list sorting (r = −0.061, P < .003), and matrix reasoning (r = −0.095, P < .001) but not the flanker task. eTable 3 in the Supplement details the descriptive statistics of neurocognitive scores for each task.
Mediation Analysis of Prefrontal Cortex, BMI, and Executive Functioning
Mediation analysis was used to test the hypothesis that BMI is associated with alterations in prefrontal cortical thickness and diminished cognitive function. The reported coefficients are unstandardized. After partialing out the influence of the covariates, BMI was significantly associated with list sorting alone and when mean prefrontal cortical thickness was included as a mediator (Figure 2). In the PROCESS analysis toolbox, a significant indirect effect is indicated when the bootstrap CI does not include zero. There was a significant positive indirect effect of BMI associated with list sorting through prefrontal cortical thickness (mean [SE] indirect effect, 0.014 [0.008]; 95% CI, 0.001-0.031). No significant indirect effects of prefrontal cortical thickness were found for the matrix reasoning (mean [SE] indirect effect, 0.001 [0.002]; 95% CI, −0.002 to 0.004), dimensional card sorting (mean [SE] indirect effect, 0.008 [0.081]; 95% CI, −0.008 to 0.025), or the flanker task (mean [SE] indirect effect, 0.002 [0.008]; 95% CI, −0.012 to 0.018) (eFigure in the Supplement).
Figure 2. Mediation Model Demonstrating Associations Among Body Mass Index (BMI), Prefrontal Cortex, and Working Memory.
Dotted line indicates the association of BMI with working memory when the mediating variable (prefrontal cortex) is included in the model. All paths are reported as unstandardized ordinary least squares regression coefficients.
aP < .05.
bP < .001.
Discussion
Higher BMI was associated with thinner cortex in widespread parts of the brain in a large sample of 3190 children aged 9 and 10 years. These associations were significant in 18 of the 66 cortical regions examined individually. In all but 3 of the remaining 45 regions, the thinner cortical thickness was associated with higher BMI (eTable 1 in the Supplement), although these associations did not individually pass the threshold of significance for an exploratory analysis.
The strongest of the BMI associations were observed in the prefrontal cortex (Figure 1), which represents mental processes critical to decision-making and the planning of complex behavior.9,10 Greater BMI was significantly associated with poorer performance on several executive functions, including list sorting,50 card sorting,51,52 and matrix reasoning,51 which are known to depend on the integrity of the prefrontal cortex. Further analysis indicated that the association between BMI and list sorting, an index of working memory, was partially mediated by mean prefrontal cortex thickness. This finding is consistent with the hypothesis that BMI affects cortical development in a way that is detrimental to cognitive function.
Several other studies12,50 have observed a similar association between BMI and executive function, generating speculation that dysregulation of these cognitive functions could exacerbate poor decision-making with regard to diet and thus contribute to negative health outcomes, including excessive weight gain. Goldschmidt et al,53 using a similar cognitive battery, reported that children who were overweight with and without loss of control eating had deficits in working memory compared with lean children. Riggs et al54 found that alterations in working memory appeared to be antecedent to weight gain, with such deficits being associated with increased risk of becoming overweight among children.55 The present study advances our working hypothesis by identifying a plausible brain mechanism underlying this association. Additional prospective studies will be required to determine how lower cognitive functioning in these domains might contribute to higher BMI, either as a direct influence on dietary choices or perhaps an indirect influence related to higher general stress resulting from diminished ability to succeed at age-specific challenges relative to peers.
The current findings are based on a larger sample than any previous study, to our knowledge. Several of these studies15,56,57 did not find associations between otherwise healthy children with obesity and cortical abnormalities. However, brain alterations have been found in obese youth with metabolic syndrome, insulin resistance, and/or type 2 diabetes.58 Lower gray matter volume predominantly in the orbitofrontal cortex and anterior cingulate as well as lower hippocampal volumes have been reported in obese adolescents with metabolic syndrome. Obese children with early-onset type 2 diabetes were reported to have lower white matter tract integrity59 and prefrontal volume and global cerebral atrophy.60 In the present study, the right and left medial and orbitofrontal cortex areas were among the cortical areas most strongly associated with BMI (t statistic, ≥3.54 to −5.92) (Figure 1). The orbitofrontal cortex has been associated with salience attribution, hedonic valuation, and food choice.61,62,63 Thus, maladaptive valuation processes may also contribute to poor dietary decision-making. In addition, the explanatory contribution of cortical thickness to BMI is not large. In the current analyses, when demographic factors and all brain regions were included in a single model predicting BMI, only an additional 2% of the variance was explained beyond a simple model that included demographic factors alone.
A medical history of metabolic syndrome, insulin resistance, and/or metabolic markers (ie, insulin levels, C-reactive protein, and lipid analysis) was not obtained in this sample. As a consequence, it is not possible to determine the extent to which lower cortical thickness and poorer working memory in this sample are attributable to the unknown presence of metabolic syndrome and/or insulin resistance.12,64 Many pathophysiologic manifestations of obesity could produce brain abnormalities during development. Escalating levels and persistence of adiposity are associated with subclinical oxidative stress, inflammation, metabolic dysfunction, and vascular reactivity that may disrupt cellular development, vessel integrity, and neuronal architecture within the brain early in childhood. Excessive circulating inflammatory biomarkers, such as interleukin 1β, interleukin 6, and C-reactive protein, have been associated with increasing white adipose tissue and BMI. Intimal thickening, vascular stiffness, and fatty streaking have been reported in otherwise healthy children who were obese, both of which may be associated with altered cerebral blood flow and changes in neuronal activity.65,66 Microstructural changes in dendritic spine density, synaptic proteins, and microglial alterations in the prefrontal cortex have been found in rodent models after diet-induced obesity (ie, weight gain of 25% of body weight) in as early as 8 weeks, suggesting that the transition from lean to obesity may cause cellular changes within the brain.67 The prefrontal cortex may be more vulnerable to the negative effects of obesity because of its later maturity compared with other brain regions during adolescence, accounting for the particularly robust association of BMI with the prefrontal cortex in the present study.68,69
Limitations
Several factors limit the interpretation of the current findings. Metabolic information was not collected for participants. Body mass index is an indirect measure of adiposity, and as such, the use of BMI as a marker for metabolic derangement and the lack of metabolic information limits inferences and requires further investigation. However, BMI is strongly associated with total body adiposity in otherwise healthy pediatric populations.70,71 An alternate association for the present findings cannot be ruled out. It is possible that thinner cortex interferes with working memory in a way that is associated with higher BMI. The cross-sectional nature of the first ABCD study data release does not permit inferences about whether cortical thickness decreased as a result of higher BMI or whether lower cortical thickness facilitated higher BMI.54 In addition, the automated estimation of cortical thickness may conflate absolute thickness with changes in the distinctness of the boundary between the cortex and the underlying white matter during development. Mean prefrontal cortical thickness might not be sensitive enough to capture neurocognitive changes that were mediated by specific brain areas within the whole frontal lobe. The exploratory nature of the current study will refine this question for future time points in the ABCD study data set. In addition, cortical parcellation of the insula was not available and thus not included in the present analysis. Consequently, the current study does not provide negative evidence about the interaction of BMI with gray matter volume in the insula.
Conclusions
In this study, greater BMI was associated with lower cortical thickness in children. This association was strongest in the prefrontal cortex. Furthermore, prefrontal cortex thickness appeared to mediate the association between BMI and working memory. Although it is not possible to determine from a cross-sectional sample the causal relationship among BMI, cortical thickness, and cognitive ability, these findings suggest that BMI is associated with alterations in prefrontal cortex development and diminished executive function, such as working memory. Deficits in working memory may in turn contribute to poor dietary decision-making. Once established, these associations may become mutually reinforcing and contribute to ongoing health issues that persist into adulthood. Autoregressive modeling of future data releases from the longitudinal ABCD study will clarify the potential causal interactions among BMI, brain structure, and executive function over time.
eTable 1. Associations Between BMI and Cortical Regions According to t Statistic (n = 3190).
eTable 2. Mean Cortical Thickness for Brain Regions
eTable 3. Neurocognition Task Battery Scores of Executive Functioning (n = 2418)
eFigure. Mediation Analysis Model
References
- 1.Centers for Disease Control and Prevention. Childhood obesity facts: overweight & obesity. https://www.cdc.gov/obesity/data/childhood.html. Published January 31, 2019. Accessed June 18, 2019.
- 2.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292-2299. doi: 10.1001/jama.2016.6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101(3, pt 2):518-525. doi: 10.1542/peds.101.3.S1.518 [DOI] [PubMed] [Google Scholar]
- 4.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485-493. doi: 10.1056/NEJMoa0904130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents: a follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327(19):1350-1355. doi: 10.1056/NEJM199211053271904 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Causes and consequences of childhood obesity. https://www.cdc.gov/obesity/childhood/causes.html. Published December 15, 2016. Accessed April 3, 2018.
- 7.Grover SA, Kaouache M, Rempel P, et al. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3(2):114-122. doi: 10.1016/S2213-8587(14)70229-3 [DOI] [PubMed] [Google Scholar]
- 8.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011;35(7):891-898. doi: 10.1038/ijo.2010.222 [DOI] [PubMed] [Google Scholar]
- 9.Zelazo PD. Executive function: reflection, iterative reprocessing, complexity, and the developing brain. Dev Rev. 2015;38:55-68. doi: 10.1016/j.dr.2015.07.001 [DOI] [Google Scholar]
- 10.Crone EA, Steinbeis N. Neural perspectives on cognitive control development during childhood and adolescence. Trends Cogn Sci. 2017;21(3):205-215. doi: 10.1016/j.tics.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 11.Cornier MA, McFadden KL, Thomas EA, et al. Differences in the neuronal response to food in obesity-resistant as compared to obesity-prone individuals. Physiol Behav. 2013;110-111:122-128. doi: 10.1016/j.physbeh.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12(9):740-755. doi: 10.1111/j.1467-789X.2011.00920.x [DOI] [PubMed] [Google Scholar]
- 13.Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring). 2011;19(7):1382-1387. doi: 10.1038/oby.2011.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saute RL, Soder RB, Alves Filho JO, Baldisserotto M, Franco AR. Increased brain cortical thickness associated with visceral fat in adolescents. Pediatr Obes. 2018;13(1):74-77. doi: 10.1111/ijpo.12190 [DOI] [PubMed] [Google Scholar]
- 15.Sharkey RJ, Karama S, Dagher A. Overweight is not associated with cortical thickness alterations in children. Front Neurosci. 2015;9:24. doi: 10.3389/fnins.2015.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veit R, Kullmann S, Heni M, et al. Reduced cortical thickness associated with visceral fat and BMI. Neuroimage Clin. 2014;6:307-311. doi: 10.1016/j.nicl.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M-J, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13(4):371-376. doi: 10.1097/MCO.0b013e32833aabef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48(9):e12997. doi: 10.1111/eci.12997 [DOI] [PubMed] [Google Scholar]
- 19.García-García I, Michaud A, Dadar M, et al. Neuroanatomical differences in obesity: meta-analytic findings and their validation in an independent dataset. Int J Obes (Lond). 2019;43(5):943-951. doi: 10.1038/s41366-018-0164-4 [DOI] [PubMed] [Google Scholar]
- 20.Spear L. The Behavioral Neuroscience of Adolescence. New York, NY: WW Norton; 2010. [Google Scholar]
- 21.Jernigan TL, Brown SA; ABCD Consortium Coordinators . Introduction. Dev Cogn Neurosci. 2018;32:1-3. doi: 10.1016/j.dcn.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark DB, Fisher CB, Bookheimer S, et al. Biomedical ethics and clinical oversight in multisite observational neuroimaging studies with children and adolescents: the ABCD experience. Dev Cogn Neurosci. 2018;32:143-154. doi: 10.1016/j.dcn.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garavan H, Bartsch H, Conway K, et al. Recruiting the ABCD sample: design considerations and procedures. Dev Cogn Neurosci. 2018;32:16-22. doi: 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention Overweight and obesity: data, trends and maps. 2017. https://www.cdc.gov/obesity/data/databases.html. Accessed March 14, 2018.
- 25.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14(3):190-195. doi: 10.1016/1054-139X(93)90004-9 [DOI] [PubMed] [Google Scholar]
- 26.ABCD Human Subjects Study: Release Notes NDA 2017: Neurocognition. Bethesda, MD: National Institute of Mental Health; February 2018.
- 27.Wechsler D. Wechsler Intelligence Scale for Children. San Antonio, TX: Psychological Corporation; 1949. [Google Scholar]
- 28.Luciana M, Bjork JM, Nagel BJ, et al. Adolescent neurocognitive development and impacts of substance use: overview of the Adolescent Brain Cognitive Development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 2018;32:67-79. doi: 10.1016/j.dcn.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casey BJ, Cannonier T, Conley MI, et al. ; ABCD Imaging Acquisition Workgroup . The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43-54. doi: 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30(2):436-443. doi: 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- 31.Wells WM III, Viola P, Atsumi H, Nakajima S, Kikinis R. Multi-modal volume registration by maximization of mutual information. Med Image Anal. 1996;1(1):35-51. doi: 10.1016/S1361-8415(01)80004-9 [DOI] [PubMed] [Google Scholar]
- 32.Ségonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060-1075. doi: 10.1016/j.neuroimage.2004.03.032 [DOI] [PubMed] [Google Scholar]
- 33.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage. 1999;9(2):179-194. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 34.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70-80. doi: 10.1109/42.906426 [DOI] [PubMed] [Google Scholar]
- 35.Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518-529. doi: 10.1109/TMI.2006.887364 [DOI] [PubMed] [Google Scholar]
- 36.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis, II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195-207. doi: 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- 37.Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5(2):162-176. doi: 10.1162/jocn.1993.5.2.162 [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050-11055. doi: 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272-284. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 41.ABCD Human Subjects Study. Release Notes NDA 2017: MRI quality control (QC). Bethesda, MD: National Institute of Mental Health; February 2018.
- 42.Freedman DS, Butte NF, Taveras EM, et al. BMI z-scores are a poor indicator of adiposity among 2- to 19-year-olds with very high BMIs, NHANES 1999-2000 to 2013-2014. Obesity (Silver Spring). 2017;25(4):739-746. doi: 10.1002/oby.21782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385-395. doi: [DOI] [PubMed] [Google Scholar]
- 44.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol. 2017;67(2):301-320. doi: 10.1111/j.1467-9868.2005.00503.x [DOI] [Google Scholar]
- 45.Varoquaux G, Raamana PR, Engemann DA, Hoyos-Idrobo A, Schwartz Y, Thirion B. Assessing and tuning brain decoders: cross-validation, caveats, and guidelines. Neuroimage. 2017;145(pt B):166-179. doi: 10.1016/j.neuroimage.2016.10.038 [DOI] [PubMed] [Google Scholar]
- 46.Abraham A, Pedregosa F, Eickenberg M, et al. Machine learning for neuroimaging with Scikit-Learn. Front Neuroinform. 2014;8:14. doi: 10.3389/fninf.2014.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Statistics SPSS. Computer Software. Armonk, NY: IBM Corp; 2015. [Google Scholar]
- 48.Hayes A. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Vol 51 New York, NY: The Guilford Press; 2013. [Google Scholar]
- 49.Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408-420. doi: 10.1080/03637750903310360 [DOI] [Google Scholar]
- 50.Cournot M, Marquié JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67(7):1208-1214. doi: 10.1212/01.wnl.0000238082.13860.50 [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Shields GS, Guo C, Liu Y. Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neurosci Biobehav Rev. 2018;84:225-244. doi: 10.1016/j.neubiorev.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 52.Perpiñá C, Segura M, Sánchez-Reales S. Cognitive flexibility and decision-making in eating disorders and obesity. Eat Weight Disord. 2017;22(3):435-444. doi: 10.1007/s40519-016-0331-3 [DOI] [PubMed] [Google Scholar]
- 53.Goldschmidt AB, O’Brien S, Lavender JM, Pearson CM, Le Grange D, Hunter SJ. Executive functioning in a racially diverse sample of children who are overweight and at risk for eating disorders. Appetite. 2018;124:43-49. doi: 10.1016/j.appet.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riggs N, Chou C-P, Spruijt-Metz D, Pentz MA. Executive cognitive function as a correlate and predictor of child food intake and physical activity. Child Neuropsychol. 2010;16(3):279-292. doi: 10.1080/09297041003601488 [DOI] [PubMed] [Google Scholar]
- 55.Groppe K, Elsner B. Executive function and weight status in children: a one-year longitudinal perspective. Child Neuropsychol. 2017;23(2):129-147. doi: 10.1080/09297049.2015.1089981 [DOI] [PubMed] [Google Scholar]
- 56.de Groot CJ, van den Akker ELT, Rings EHHM, Delemarre-van de Waal HA, van der Grond J. Brain structure, executive function and appetitive traits in adolescent obesity. Pediatr Obes. 2017;12(4):e33-e36. doi: 10.1111/ijpo.12149 [DOI] [PubMed] [Google Scholar]
- 57.Moreno-López L, Soriano-Mas C, Delgado-Rico E, Rio-Valle JS, Verdejo-García A. Brain structural correlates of reward sensitivity and impulsivity in adolescents with normal and excess weight. PLoS One. 2012;7(11):e49185. doi: 10.1371/journal.pone.0049185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130(4):e856-e864. doi: 10.1542/peds.2012-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yau PL, Javier DC, Ryan CM, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53(11):2298-2306. doi: 10.1007/s00125-010-1857-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruehl H, Sweat V, Tirsi A, Shah B, Convit A. Obese adolescents with type 2 diabetes mellitus have hippocampal and frontal lobe volume reductions. Neurosci Med. 2011;2(1):34-42. doi: 10.4236/nm.2011.21005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318-325. doi: 10.1093/cercor/10.3.318 [DOI] [PubMed] [Google Scholar]
- 62.Volkow ND, Wang G-J, Tomasi D, Baler RD. The addictive dimensionality of obesity. Biol Psychiatry. 2013;73(9):811-818. doi: 10.1016/j.biopsych.2012.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6(9):691-702. doi: 10.1038/nrn1747 [DOI] [PubMed] [Google Scholar]
- 64.Gonzales MM, Tarumi T, Miles SC, Tanaka H, Shah F, Haley AP. Insulin sensitivity as a mediator of the relationship between BMI and working memory-related brain activation. Obesity (Silver Spring). 2010;18(11):2131-2137. doi: 10.1038/oby.2010.183 [DOI] [PubMed] [Google Scholar]
- 65.Yau PL, Kang EH, Javier DC, Convit A. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring). 2014;22(8):1865-1871. doi: 10.1002/oby.20801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farello G, Antenucci A, Stagi S, Mazzocchetti C, Ciocca F, Verrotti A. Metabolically healthy and metabolically unhealthy obese children both have increased carotid intima-media thickness: a case control study. BMC Cardiovasc Disord. 2018;18(1):140. doi: 10.1186/s12872-018-0874-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bocarsly ME, Fasolino M, Kane GA, et al. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc Natl Acad Sci U S A. 2015;112(51):15731-15736. doi: 10.1073/pnas.1511593112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718-729. doi: 10.1016/j.neubiorev.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 69.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223-8231. doi: 10.1523/JNEUROSCI.1798-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boeke CE, Oken E, Kleinman KP, Rifas-Shiman SL, Taveras EM, Gillman MW. Correlations among adiposity measures in school-aged children. BMC Pediatr. 2013;13:99. doi: 10.1186/1471-2431-13-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr. 1998;132(2):204-210. doi: 10.1016/S0022-3476(98)70433-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Associations Between BMI and Cortical Regions According to t Statistic (n = 3190).
eTable 2. Mean Cortical Thickness for Brain Regions
eTable 3. Neurocognition Task Battery Scores of Executive Functioning (n = 2418)
eFigure. Mediation Analysis Model