Key Points
Question
Are genetically predicted lipid levels associated with the extent of coronary atherosclerosis?
Findings
This study found that a genetic score for non–high-density lipoprotein cholesterol was significantly associated with the extent of coronary atherosclerosis, as estimated by coronary angiography or coronary calcium scanning in Icelandic adults. The association persists after accounting for low-density lipoprotein cholesterol.
Meaning
Elevated non–high-density lipoprotein cholesterol is associated with the development of coronary atherosclerosis and may be a better marker for atherogenic lipoproteins than low-density lipoprotein cholesterol.
This genetic study uses data from Icelandic adults who have been genotyped to estimate the contributions of genetically predicted blood lipid levels on the extent of coronary atherosclerosis.
Abstract
Importance
Genetic studies have evaluated the influence of blood lipid levels on the risk of coronary artery disease (CAD), but less is known about how they are associated with the extent of coronary atherosclerosis.
Objective
To estimate the contributions of genetically predicted blood lipid levels on the extent of coronary atherosclerosis.
Design, Setting, and Participants
This genetic study included Icelandic adults who had undergone coronary angiography or assessment of coronary artery calcium using cardiac computed tomography. The study incorporates data collected from January 1987 to December 2017 in Iceland in the Swedish Coronary Angiography and Angioplasty Registry and 2 registries of individuals who had undergone percutaneous coronary interventions and coronary artery bypass grafting. For each participant, genetic scores were calculated for levels of non–high-density lipoprotein cholesterol (non–HDL-C), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides, based on reported effect sizes of 345 independent, lipid-associated variants. The genetic scores’ predictive ability for lipid levels was assessed in more than 87 000 Icelandic adults. A mendelian randomization approach was used to estimate the contribution of each lipid trait.
Exposures
Genetic scores for levels of non–HDL-C, LDL-C, HDL-C, and triglycerides.
Main Outcomes and Measures
The extent of angiographic CAD and coronary artery calcium quantity.
Results
A total of 12 460 adults (mean [SD] age, 65.1 [10.7] years; 8383 men [67.3%]) underwent coronary angiography, and 4837 had coronary artery calcium assessed by computed tomography. A genetically predicted increase in non–HDL-C levels by 1 SD (38 mg/dL [to convert to millimoles per liter, multiply by 0.0259]) was associated with greater odds of obstructive CAD (odds ratio [OR], 1.83 [95% CI, 1.63-2.07]; P = 2.8 × 10−23). Among patients with obstructive CAD, there were significant associations with multivessel disease (OR, 1.26 [95% CI, 1.11-1.44]; P = 4.1 × 10−4) and 3-vessel disease (OR, 1.47 [95% CI, 1.26-1.72]; P = 9.2 × 10−7). There were also significant associations with the presence of coronary artery calcium (OR, 2.04 [95% CI, 1.70-2.44]; P = 5.3 × 10−15) and loge-transformed coronary artery calcium (effect, 0.70 [95% CI, 0.53-0.87]; P = 1.0 × 10−15). Genetically predicted levels of non–HDL-C remained associated with obstructive CAD and coronary artery calcium extent even after accounting for the association with LDL-C. Genetically predicted levels of HDL-C and triglycerides were associated individually with the extent of coronary atherosclerosis, but not after accounting for the association with non–HDL cholesterol.
Conclusions and Relevance
In this study, genetically predicted levels of non–HDL-C were associated with the extent of coronary atherosclerosis as estimated by 2 different methods. The association was stronger than for genetically predicted levels of LDL-C. These findings further support the notion that non–HDL-C may be a better marker of the overall burden of atherogenic lipoproteins than LDL-C.
Introduction
Low-density lipoprotein cholesterol (LDL-C) is an established risk factor for coronary artery disease (CAD).1 Traditionally, LDL-C has been regarded as the primary marker of atherogenic lipoproteins and a treatment target for lipid-lowering therapies.1 However, there is growing evidence that LDL-C may not be the best marker of the cardiovascular risk conferred by atherogenic lipoproteins. Epidemiological studies2,3,4,5,6 have shown that non–high-density lipoprotein cholesterol (non–HDL-C) and apolipoprotein B, which are highly correlated, are superior to LDL-C for cardiovascular risk prediction in healthy individuals and patients with coronary disease. Experimental evidence from clinical trials7,8,9 shows that aggressive lowering of LDL-C can slow progression and even induce regression of coronary atherosclerosis, as assessed by intravascular ultrasonography. A recent analysis of clinical trial data,10 however, showed that changes in coronary atheroma volume may be more closely associated with levels of non–HDL-C than LDL-C.
Mendelian randomization is a method in which genetic information is used to infer whether an exposure is causally associated with an outcome (eg, a disease).11 Using this method, genetic studies1 have supported a potential causal role of LDL-C in coronary disease, but few have assessed non–HDL-C directly.12 In a previous study using mendelian randomization,13 we provided evidence to support a potential causal role of non–HDL-C in coronary diseases and showed that genetically predicted non–HDL-C levels were more significantly associated than LDL-C was with risk of CAD. Although genetic scores for lipid levels have been widely studied in the context of cardiovascular risk, less is known about their association with measures of the extent of coronary atherosclerosis. To our knowledge, 2 studies14,15 have associated genetic scores for lipid levels with the extent of coronary atherosclerosis. Both studies evaluated associations with coronary artery calcium (CAC), a noninvasive marker of the overall coronary atherosclerotic burden.16 A genetic score for LDL-C was associated with higher CAC in one study14 but not the other.15 In addition, one of the studies14 evaluated genetic scores for HDL-C and triglyceride levels and did not find significant associations with CAC. In the present study, we used mendelian randomization to evaluate the contributions of individual lipid traits on the extent of coronary atherosclerosis in a data set of Icelandic adults undergoing coronary angiography and evaluation of CAC.
Methods
Study Participants
We identified Icelandic adults who had undergone coronary angiography for any indication at Landspítali–The National University Hospital in Reykjavík, the only interventional cardiology center in Iceland. The data were obtained from 3 clinical registries, as described previously.17 First was the Swedish Coronary Angiography and Angioplasty Registry (SCAAR), which holds data on all consecutive individuals undergoing coronary angiography and percutaneous coronary intervention in Iceland since January 1, 2007.18,19 From SCAAR, we obtained data collected prospectively between January 1, 2007, and December 31, 2017 (including 13 437 procedures for 9885 adults who had been genotyped). Second, we used a registry of all percutaneous coronary intervention procedures performed in Iceland between January 1, 1987, and December 31, 2006 (including 5386 procedures for 3743 patients who had been genotyped). Finally, we used a registry of coronary-artery bypass grafting procedures performed in Iceland, which holds data on patients who underwent preprocedural coronary angiography between January 1, 2001, and December 31, 2013 (1309 procedures for 1309 patients who had been genotyped).20 For the main analyses, the 3 data sources were combined into a single data set (eFigure 1 in the Supplement); for individuals with multiple procedures, we only used the earliest record (n = 12 728 unique individuals). Information on cardiovascular risk factors was obtained from these registries. In the combined data set, hypertension, diabetes, and hyperlipidemia were defined by previous diagnosis of the respective condition or medical treatment at the time of angiography (with antihypertensive, antidiabetic, or lipid-lowering medication, respectively). Individuals with missing data were removed prior to analyses (n = 268), resulting in a total sample size of 12 460.
We identified Icelandic adults who underwent cardiac computed tomography for any indication at Röntgen Domus, the largest privately operated medical imaging clinic in the country. Imaging was performed between January 4, 2009, and October 31, 2017. A CAC score (Agatston score)21 was available for 4837 individuals who had been genotyped. For each individual, we used the earliest record only. Information on cardiovascular risk factors, other than age at the time of procedure and sex, was not available. All participants donated samples for genotyping and provided informed consent as part of various genetic programs at deCODE genetics. The study was approved by the Data Protection Authority of Iceland and the National Bioethics Committee of Iceland. Personal identities of the participants were encrypted with a third-party system provided by the Data Protection Authority of Iceland.
Coronary Angiography
Coronary angiograms were evaluated by the interventional cardiologists performing the procedures. Angiographic extent of CAD was quantified as the number of major epicardial coronary arteries (the left anterior descending artery, the circumflex artery, or the right coronary artery) with at least 50% luminal diameter stenosis (significant stenosis), ranging from 0 to 3 diseased coronary arteries. Obstructive CAD was defined as having 1 to 3 coronary arteries with significant stenosis or significant stenosis in the left main coronary artery. No or nonobstructive CAD was defined as having less than 50% stenosis in all 3 major coronary arteries and the left main coronary artery. Patients with obstructive CAD and without left main disease were categorized as having 1-vessel, 2-vessel, or 3-vessel disease, based on the number of coronary arteries with significant stenosis. Those with left main disease were categorized separately. Multivessel disease was defined as having 2-vessel or 3-vessel disease or left main disease.
Quantification of Coronary Artery Calcium
Coronary artery calcium was assessed using cardiac-gated multidetector computed tomography scanners (Aquilion [Toshiba Medical Systems]) with a slice thickness of 0.5 to 3 mm. Scans were read by radiologists, and CAC was quantified using a CAC score (Agatston score).21
Genotyping and Imputation
Genotyping and imputation methods were as previously described.22,23 Briefly, DNA sequence variants identified in the genomes of 28 075 Icelandic adults whose whole genomes have been sequenced were imputed into 155 250 Icelanders who had been genotyped using various Illumina single-nucleotide polymorphism chips and their genotypes phased using long-range phasing.22,23,24
Genetic Scores
We constructed individual-level genetic scores for levels of non–HDL-C, LDL-C, HDL-C, and triglycerides based on variants identified in a recent large-scale, exome-wide association study of lipid levels.25 That study reported 444 single-nucleotide polymorphisms in 250 loci with minor allele frequency ranging from 6.7 × 10−6 to 0.49. Each variant was reported to associate independently with at least 1 lipid level (total cholesterol, LDL-C, HDL-C, or triglycerides) at P < 2.1 × 10−7, a Bonferroni correction for the testing of 242 289 variants. In our study, a total of 414 variants were observed in the population that had been genotyped (n = 155 250), of which 412 had good imputation quality (imputation information of at least 0.90); 2 variants with imputation information less than 0.90 were excluded (eTable 1 in the Supplement). For the calculation of the genetic scores, to minimize potential bias associated with including correlated variants, we used a subset of 345 variants with pairwise r2 less than 0.20 (eTable 1 in the Supplement). Based on this set, we calculated the genetic scores by summing the product of the allele count and the corresponding effect size for each variant. A flowchart summarizing the selection of variants and calculation of the genetic scores is presented in eFigure 2 in the Supplement.
For the genetic scores for LDL-C, HDL-C, and triglycerides, we used the effect sizes (with SDs) as previously reported25; these were estimated by using data from more than 300 000 Europeans, of whom less than 1% were Icelandic. Because association results for non–HDL-C were not available from this resource, we used the reported effect sizes for total cholesterol and HDL-C to derive effect sizes for non–HDL-C. To generate an equation to estimate the association with non–HDL-C, we analyzed the lipid effect sizes of 48 463 variants with minor allele frequency greater than 1.0% that were associated with each lipid trait at P values less than .05 in the Icelandic population (sample sizes: 93 556, 103 599, and 93 746 individuals for non–HDL-C, total cholesterol, and HDL-C, respectively). Ordinary least-squares regression estimated the non–HDL-C effect size as 0.979 × total cholesterol effect size – 0.354 × HDL-C effect size (with all effect sizes in SDs, estimated on inverse normal-transformed values). There was a high correlation between estimated and observed effect sizes on non–HDL-C (R2 = 0.99). This equation had high predictive accuracy (R2 = 0.93) in an external validation sample from the UK Biobank26 (eMethods in the Supplement), using a set of 50 000 randomly selected variants with minor allele frequency greater than 1.0% and effect sizes based on cholesterol measurements for more than 358 000 individuals. Thus, the effect sizes of variants on non–HDL-C can be reliably predicted from their associations with total cholesterol and HDL-C, consistent with the fact that non–HDL-C is calculated directly from these lipid measurements (total cholesterol level − HDL-C level). We used this equation to estimate effect sizes for non–HDL-C from the effect sizes for total cholesterol and HDL-C levels, as previously reported,25 and used the estimated effect sizes for calculation of the genetic score for non–HDL-C. Effect sizes used for the calculation of the genetic scores are shown in eTable 1 in the Supplement.
Each genetic score was associated with its respective lipid level in samples of more than 87 000 Icelandic adults for whom genotyping was performed (eMethods and eTable 2 in the Supplement). The genetic score for non–HDL-C explained 12.8% of the variance in non–HDL-C levels. For LDL-C, HDL-C, and triglycerides, the variance explained by the corresponding genetic score was 13.1%, 11.3%, and 8.9%, respectively (eTable 2 in the Supplement). The genetic scores showed weak to moderate pairwise correlation, with the exception of the genetic scores for non–HDL-C and LDL-C, which were highly correlated (r = 0.95; eTable 3 in the Supplement).
Statistical Analysis
Mendelian Randomization
We used a mendelian randomization approach involving genetic scores as instrumental variables to infer potential causal contributions of individual lipid traits. Because of the pleiotropy of lipid-associated variants (ie, each variant being commonly associated with more than 1 lipid level25), the association of a genetic score for a given lipid level may be confounded by its correlation with other lipid levels. To account for these pleiotropic effects, we conducted joint analyses in which the association of a given genetic score (eg, for LDL-C) was adjusted for genetic scores for other lipid levels (eg, for HDL-C and triglycerides) by including them as covariates in the model. Recently, we applied this approach in another mendelian randomization study to infer the potential causal role of lipid levels in the context of risk of coronary disease.13
Association Analyses
We used logistic regression models to test for associations with dichotomized measures of angiographic extent of CAD and CAC. Linear regression models were used to test for associations with CAC as a continuous variable as the natural logarithm of the CAC score plus 1 (loge[CAC score +1]). Association analyses involving angiographic extent of CAD were adjusted for age, age2, sex, diabetes, hypertension, and current and former smoking status. Results did not differ materially when adjusted only for age, age2, and sex (eTable 4 in the Supplement). Association analyses of CAC were adjusted for age, age2, and sex. Unless otherwise noted, effect size estimates for the genetic scores were scaled to correspond to a 1-SD increase in multivariate-adjusted residuals of the respective lipid level (for non–HDL-C, LDL-C, and HDL-C) or doubling of triglyceride levels, as estimated in more than 87 000 samples from Icelandic adults (eTable 5 in the Supplement).
For all tests, a 2-tailed P < .05 was considered statistically significant. Analyses were conducted using R version 3.3.2 (R Project for Statistical Computing).
Results
A total of 12 460 Icelandic adults who had been genotyped and had angiographic data available were identified (Table 1). The mean (SD) age was 65.1 (10.7) years; 8383 (67.3%) were men, and 8984 (72.1%) had obstructive CAD (at least 50% diameter stenosis in at least 1 coronary artery). Among patients with obstructive CAD, 5289 (58.9%) had multivessel disease (at least 2-vessel disease or left main disease).
Table 1. Characteristics of the Coronary Angiography Sample.
| Characteristic | No. (%) |
|---|---|
| Patients, No. | 12 460 |
| Age, mean (SD), y | 65.1 (10.7) |
| Male | 8383 (67.3) |
| Diabetes mellitusa | 1467 (11.8) |
| Hypertensionb | 7184 (57.7) |
| Hyperlipidemiac | 6599 (53.2) |
| Current smoking | 2693 (21.6) |
| Former smoking | 6191 (49.7) |
| Medical history | |
| Myocardial infarction | 2221 (18.2) |
| Percutaneous coronary intervention | 330 (2.7) |
| Coronary artery bypass grafting | 659 (5.3) |
| Angiographic findings | |
| No or nonobstructive coronary artery disease | 3476 (27.9) |
| Obstructive coronary artery disease | 8984 (72.1) |
| 1-Vessel diseased | 3695 (31.6) |
| 2-Vessel diseased | 2462 (21.0) |
| 3-Vessel diseased | 2072 (17.7) |
| Left main disease | 755 (6.1) |
Previous diagnosis of diabetes mellitus or the use of antidiabetic medication.
Previous diagnosis of hypertension or the use of antihypertensive medication.
Previous diagnosis of hypercholesterolemia or the use of lipid-lowering medication.
Without left main disease.
Genetic Scores for Lipid Levels and Obstructive CAD
We assessed whether the genetic scores for levels of non–HDL-C, LDL-C, HDL-C, and triglycerides were associated with the presence of obstructive CAD vs no or nonobstructive CAD (Table 2). The genetic scores for non–HDL-C, HDL-C, LDL-C, and triglycerides were all associated individually with obstructive CAD. A genetically predicted 1-SD increase in non–HDL-C levels (38 mg/dL [to convert to millimoles per liter, multiply by 0.0259]) was associated with an 83% higher risk of having obstructive CAD (OR, 1.83 [95% CI, 1.63-2.07]; P = 2.8 × 10−23; Table 2). Similarly, a genetically predicted 1-SD increase in LDL-C level (34 mg/dL [to convert to millimoles per liter, multiply by 0.0259]) was associated with a 73% higher risk of obstructive CAD (OR, 1.73 [95% CI, 1.54-1.95]; P = 6.4 × 10−20; Table 2).
Table 2. Genetic Scores for Lipid Levels and Angiographic Extent of Coronary Artery Disease.
| Covariates (Genetic Scores)a | Obstructive Coronary Artery Disease in Overall Sample (N = 12 460) | Patients With Obstructive Coronary Artery Disease (n = 8984) | ||||
|---|---|---|---|---|---|---|
| Multivessel Disease | 3-Vessel Disease | |||||
| Odds Ratio (95% CI) | P Value | Odds Ratiob (95% CI) | P Value | Odds Ratiob (95% CI) | P Value | |
| Non–HDL-C | 1.83 (1.63-2.07) | 2.8 × 10−23 | 1.26 (1.11-1.44) | 4.1 × 10−4 | 1.47 (1.26-1.72) | 9.2 × 10−7 |
| HDL-C | 1.74 (1.54-1.98) | 3.7 × 10−18 | 1.26 (1.10-1.45) | 6.5 × 10−4 | 1.46 (1.24-1.72) | 4.1 × 10−6 |
| HDL-C and triglycerides | 1.75 (1.52-2.01) | 3.2 × 10−15 | 1.31 (1.13-1.52) | 4.6 × 10−4 | 1.44 (1.21-1.73) | 5.8 × 10−5 |
| LDL-C | 2.13 (1.47-3.10) | 6.4 × 10−5 | 1.01 (0.68-1.50) | .97 | 1.49 (0.93-2.38) | .10 |
| LDL-C | 1.73 (1.54-1.95) | 6.4 × 10−20 | 1.28 (1.12-1.45) | 1.9 × 10−4 | 1.43 (1.23-1.67) | 3.8 × 10−6 |
| HDL-C and triglycerides | 1.63 (1.44-1.84) | 3.0 × 10−15 | 1.27 (1.11-1.45) | 3.5 × 10−4 | 1.39 (1.19-1.63) | 4.1 × 10−5 |
| Non–HDL-C | 0.85 (0.59-1.23) | .40 | 1.27 (0.86-1.87) | .24 | 0.99 (0.62-1.58) | .96 |
| HDL-C | 0.71 (0.62-0.80) | 3.0 × 10−8 | 0.94 (0.82-1.07) | .35 | 0.87 (0.75-1.02) | .09 |
| LDL-C and triglycerides | 0.83 (0.72-0.96) | .01 | 0.98 (0.85-1.13) | .77 | 0.99 (0.83-1.17) | .89 |
| Non–HDL-C and triglycerides | 0.83 (0.72-0.96) | .011 | 0.98 (0.85-1.13) | .78 | 0.99 (0.83-1.18) | .90 |
| Triglycerides | 1.86 (1.51-2.29) | 6.4 × 10−9 | 1.12 (0.89-1.39) | .34 | 1.45 (1.12-1.89) | .005 |
| LDL-C and HDL-C | 1.35 (1.06-1.71) | .014 | 1.01 (0.78-1.29) | .96 | 1.28 (0.95-1.73) | .10 |
| Non–HDL-C and HDL-C | 0.99 (0.76-1.29) | .94 | 0.87 (0.66-1.15) | .32 | 1.05 (0.75-1.46) | .78 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; non–HDL-C, non–high-density lipoprotein cholesterol.
SI conversion factor: To convert HDL-C, non–HDL-C, and LDL-C to mmol/L, multiply by 0.0259.
In all models, age, age2, sex, diabetes, hypertension, and current smoking and former smoking status were included as covariates in addition to the genetic scores. Sample sizes: obstructive coronary artery disease (8984 affected individuals and 3476 control individuals), multivessel disease (5289 affected individuals and 3695 control individuals), and 3-vessel disease (2072 affected individuals and 6157 control participants).
Odds ratios are scaled to correspond to a 1-SD increase in the respective cholesterol trait or doubling of triglyceride levels. For non–HDL-C, LDL-C, and HDL-C, this corresponds to 38 mg/dL (0.97 mmol/L), 34 mg/dL (0.87 mmol/L), and 15 mg/dL (0.38 mmol/L), respectively.
The association of the genetic score for non–HDL-C remained significant after accounting for the genetic scores for HDL-C and triglycerides (OR, 1.75 [95% CI, 1.52-2.01]; P = 3.2 × 10−15), as were the association of the genetic score of LDL-C after accounting for the genetic scores for HDL-C and triglycerides (OR, 1.63 [95% CI, 1.44-1.84]; P = 3.0 × 10−15). However, the genetic score for non–HDL-C conferred additional risk of obstructive CAD after accounting for the LDL-C genetic score (OR, 2.13 [95% CI, 1.47-3.10]; P = 6.4 × 10−5) while the association of the LDL-C genetic score was fully explained by the non–HDL-C genetic score (OR, 0.85 [95% CI, 0.59-1.23]; P = .40 after adjustment for the non–HDL-C genetic score; Table 2).
The genetic score for HDL-C showed a nominal association with obstructive CAD when adjusting for the genetic scores for non–HDL-C and triglycerides (OR, 0.83 [95% CI, 0.72-0.96]; P = .01; Table 2). The genetic score for triglycerides was nominally associated with obstructive CAD when adjusting for the genetic scores for LDL-C and HDL-C (OR, 1.35 [95% CI, 1.06-1.71]; P = .01) but not after adjustment for the non–HDL-C genetic score (OR, 0.99 [95% CI, 0.76-1.29]; P = .94; Table 2).
Genetic Scores for Lipid Levels and CAD Extent in Patients With Obstructive CAD
We tested associations with multivessel disease and 3-vessel disease among patients with obstructive CAD (n = 8984) (Table 2). A genetically predicted 1-SD increase in non–HDL-C was associated with a 26% higher risk of multivessel disease (OR, 1.26 [95% CI, 1.11-1.44]; P = 4.1 × 10−4) and a 47% higher risk of 3-vessel disease (OR, 1.47 [95% CI, 1.26-1.72]; P = 9.2 × 10−7). The association persisted after adjusting for the genetic scores for HDL-C and triglycerides (OR, 1.44 [95% CI, 1.21-1.73]; P = 5.8 × 10−5; Table 2). The genetic score for LDL-C showed similar associations (for multivessel disease: OR, 1.28 [95% CI, 1.12-1.45]; P = 1.9 × 10−4; after adjustment for the genetic scores for HDL-C and triglycerides: OR, 1.27 [95% CI, 1.11-1.45]; P = 3.5 × 10−4; for 3-vessel disease: OR, 1.43 [95% CI, 1.23-1.67]; P = 3.8 × 10−6; after adjustment: OR, 1.39 [95% CI, 1.19-1.63]; P = 4.1 × 10−5) (Table 2). Neither non–HDL-C nor LDL-C remained significant after adjusting for of the other. The genetic scores for HDL-C and triglycerides were not associated with multivessel disease or 3-vessel disease in adjusted models.
Genetic Scores for Lipid Levels and Coronary Artery Calcium
In addition to angiographic measures of CAD extent, we tested whether the genetic scores were associated with the presence and extent of CAC as assessed by cardiac computed tomography. A CAC score was available for 4837 individuals on whom genotype data were available. The mean (SD) age was 58.4 (9.7) years, and 2377 (49.1%) were men. The median CAC score was 3.8 (range, 0-5223; mean [SD], 134 [353]). Coronary artery calcium was present in 2598 (53.7%) (CAC score >0), indicating the presence of coronary atherosclerosis, and 1211 (25.0%) had moderate to extensive CAC, defined16 as a CAC score greater than 100.
Association results for the presence and extent of CAC were similar to those for angiographic extent of CAD (Table 3). The genetic score for non–HDL-C was associated with the presence of CAC (OR, 2.04 [95% CI, 1.70-2.44]; P = 5.3 × 10−15) and loge-transformed CAC score (0.70 [95% CI, 0.53-0.87]; P = 1.0 × 10−15). The genetic score for non–HDL-C was associated with the presence and extent of CAC after accounting for the LDL-C genetic score (OR, 2.06 [95% CI, 1.18-3.60]; P = .01; loge-transformed CAC score, 0.80 [95% CI, 0.27-1.33]; P = .003, respectively), but no association remained for the genetic score for LDL-C after adjusting for the non–HDL-C genetic score (Table 3). The genetic scores for HDL-C and triglycerides were not associated with CAC in adjusted models (Table 3).
Table 3. Genetic Scores for Lipid Levels and Coronary Artery Calcium.
| Covariates (Genetic Scores)a | Coronary Artery Calcium Score Greater Than 0 (n = 4837) | loge-Transformed Coronary Artery Calcium Scoreb (n = 4837) | ||
|---|---|---|---|---|
| Odds Ratioc (95% CI) | P Value | Effect Size (95% CI) | P Value | |
| non–HDL-C | 2.04 (1.70-2.44) | 5.3 × 10−15 | 0.70 (0.53-0.87) | 1.0 × 10−15 |
| HDL-C | 2.05 (1.70-2.48) | 4.2 × 10−14 | 0.67 (0.50-0.85) | 1.4 × 10−13 |
| HDL-C and triglycerides | 2.07 (1.69-2.55) | 3.9 × 10−12 | 0.70 (0.50-0.90) | 3.5 × 10−12 |
| LDL-C | 2.06 (1.18-3.60) | .01 | 0.80 (0.27-1.33) | .003 |
| LDL-C | 1.91 (1.60-2.27) | 1.4 × 10−13 | 0.62 (0.46-0.79) | 7.6 × 10−14 |
| HDL-C and triglycerides | 1.84 (1.54-2.19) | 9.8 × 10−12 | 0.58 (0.41-0.74) | 1.4 × 10−11 |
| Non–HDL-C | 0.99 (0.58-1.69) | .97 | –0.10 (–0.61 to 0.41) | .70 |
| HDL-C | 0.87 (0.76-0.99) | .04 | –0.22 (–0.35 to –0.09) | .001 |
| LDL-C and triglycerides | 1.01 (0.86-1.18) | .91 | –0.09 (–0.23 to 0.06) | .26 |
| Non–HDL-C and triglycerides | 1.01 (0.87-1.18) | .88 | –0.07 (–0.20 to 0.07) | .34 |
| Triglycerides | 1.43 (1.18-1.74) | 3.2 × 10−4 | 0.37 (0.18-0.56) | 1.4 × 10−4 |
| LDL-C and HDL-C | 1.26 (1.00-1.57) | .047 | 0.18 (–0.04 to 0.39) | .10 |
| Non–HDL-C and HDL-C | 0.97 (0.76-1.24) | .83 | –0.07 (–0.3 to 0.17) | .57 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
SI conversion factor: To convert HDL-C, non–HDL-C, and LDL-C to mmol/L, multiply by 0.0259.
In all models, age, age2, and sex were included as covariates, in addition to the genetic scores.
loge(CAC score + 1).
Odds ratios and linear regression coefficients are scaled to correspond to a 1-SD increase in the respective cholesterol trait or doubling of triglyceride levels. For non–HDL-C, LDL-C, and HDL-C, this corresponds to 37 mg/dL (0.97 mmol/L), 37 mg/dL (0.87 mmol/L), and 15 mg/dL (0.38 mmol/L), respectively.
Non–HDL-C Genetic Score and Risk of CAD
Previously, we demonstrated a robust association between genetically predicted levels of non–HDL-C and risk of CAD.13 We sought to validate the association of the current non–HDL-C genetic score with CAD among Icelandic adults with replication in the UK Biobank (eMethods and eTable 6 in the Supplement). The non–HDL-C genetic score was associated with increased risk of CAD in the Icelandic population (OR, 1.61 [95% CI, 1.51-1.71]; P = 1.2 × 10−49; 19 123 affected individuals and 124 461 control individuals) and the UK Biobank (OR, 1.52 [95% CI, 1.47-1.57]; P = 4.4 × 10−138; 28 110 affected individuals and 380 455 control individuals). Furthermore, the association remained significant after adjustment for the LDL-C genetic score in both samples (OR, 1.79 [95% CI, 1.47-2.18]; P = 7.8 × 10−9 in Icelandic adults and OR, 1.81 [95% CI, 1.63-2.01]; P = 5.9 × 10−28 in the UK Biobank), in line with our previous findings.13
Discussion
In this study, we used genetic scores to evaluate the associations of commonly measured lipid levels with the extent of coronary atherosclerosis, as assessed by 2 different methods. The main findings of this study were that (1) genetically predicted levels of non–HDL-C and LDL-C were consistently associated with greater extents of coronary atherosclerosis, (2) the genetic score for non–HDL-C was most significantly associated with the extent of CAD and provides additional predictive value beyond the LDL-C genetic score, and (3) genetically predicted levels of HDL-C and triglycerides were not significantly associated with the extent of coronary atherosclerosis after accounting for the contribution of non–HDL-C.
The non–HDL-C fraction represents the sum of cholesterol carried by all atherogenic, apolipoprotein B–containing lipoproteins. Most non–HDL-C is found within LDL particles (as LDL-C), while the remainder is carried by triglyceride-rich lipoproteins (intermediate-density lipoproteins, very-low-density lipoproteins, and chylomicron remnants) and, to a lesser degree, lipoprotein(a).27 Recently, we undertook a mendelian randomization analysis that supported a direct involvement of non–HDL-C, but not triglycerides or HDL-C, in the development of CAD.13 In that analysis, non–HDL-C was associated with CAD risk and provided predictive power beyond that of a genetic score for LDL-C.
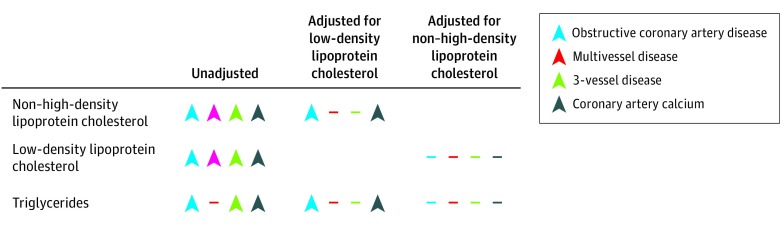
In contrast with the previous study, which compared individuals with CAD to population controls, in the present study, we studied measures of CAD extent in a population undergoing invasive or noninvasive assessment of CAD. We found that a genetic score for non–HDL-C was significantly associated with multiple measures of coronary atherosclerotic burden. In line with our previous findings, the genetic score for non–HDL-C was associated significantly with the risk of obstructive CAD and presence of coronary calcium, even after accounting for the contribution of LDL-C. This residual association may reflect the influence of the cholesterol carried within triglyceride-rich lipoproteins, also known as remnant cholesterol, which is a subfraction of non–HDL-C. Accumulating evidence suggests that triglyceride-rich lipoproteins are associated with the risk of CAD,28,29,30,31,32,33,34,35,36,37 most likely because of their content of cholesteryl esters.13,38,39 In line with this, the association of the genetic score for triglycerides with the extent of angiographic CAD is fully explained by the genetic score for non–HDL-C. These findings, summarized in the Figure, together with our previous results,13 suggest that among the commonly measured lipid fractions in clinical practice, non–HDL-C may be the best overall marker of atherogenic lipoproteins and cardiovascular risk.
Figure. Summary of Main Findings.
Color-coded arrowheads denote the direction of the association of the respective genetic score with phenotypes of coronary artery disease extent. Dashes indicate nonsignificant associations.
Mendelian randomization studies have consistently shown that HDL-C levels are not likely to contribute to the pathogenesis of CAD.13,29,40,41 We observed a nominal association between the HDL-C genetic score and obstructive CAD after accounting for the genetic scores for non–HDL-C and triglycerides. However, there was no association with other measures of angiographic extent of CAD or the extent of coronary calcium. A possible explanation for these results is residual confounding due to the pleiotropic effects of multiple HDL-C variants on other lipid fractions that may not be accounted for in the adjusted models. Taken together, these results do not support the hypothesis that HDL-C contributes to the extent of coronary atherosclerosis.
These findings have implications for predicting potential cardiovascular benefit from lipid-lowering therapies, especially with respect to non–HDL-C and triglycerides. Consistent with a potential causal role of non–HDL-C and its major component LDL-C, reduction in these lipid fractions lowers cardiovascular risk in a dose-dependent manner.42,43 On the other hand, trials of triglyceride-lowering therapies have produced variable results.44 Thus, it is unlikely that lowering triglycerides per se reduces cardiovascular risk, consistent with a likely noncausal role in atherogenesis. However, triglyceride-lowering therapies that also lower non–HDL-C and/or have nonlipid-associated vascular benefits would be expected to reduce cardiovascular risk.
The main strengths of this study include the large sample size of individuals with genotype, angiographic, and coronary calcium data available and consistent effect sizes of the genetic scores on different measures of the extent of coronary atherosclerosis. In addition, angiographic data were obtained from large nationwide angiography registries, reducing the risk of selection bias.
Limitations
This study has several limitations. We attempted to disentangle the contributions of each lipid level by evaluating the respective genetic score while adjusting for genetic scores for other lipid levels. However, unmeasured pleiotropy of the genetic scores may not be accounted for in the adjusted models. In turn, this may limit the interpretation of causality in the mendelian randomization analyses. Another limitation is the use of estimated variant effect sizes on the calculation of the non–HDL-C genetic score. However, as evident by the high agreement between calculated and observed effect sizes on non–HDL-C in Iceland and the UK Biobank, this approach provides a reliable estimate of non–HDL-C effect sizes.
Conclusions
In this study, we have demonstrated that genetically predicted levels of non–HDL-C were associated with the extent of coronary atherosclerosis and provide predictive power beyond genetically predicted LDL-C levels. These results support the notion that non–HDL-C may be a better measure of the overall burden of atherogenic lipoproteins and cardiovascular risk than LDL-C.
eMethods. Methods.
eTable 1. Reported Lipid-Associated Variants Used for Calculation of the Genetic Scores.
eTable 2. Relationship of Genetic Scores with Their Respective Lipid Traits.
eTable 3. Pairwise-Correlations for Genetic Scores (Blue) and Lipid Traits (Green).
eTable 4. Genetic Scores for Lipid Levels and Angiographic Extent of CAD.
eTable 5. Lipid levels in Genotyped Icelanders.
eTable 6. Association of the non-HDL-C Genetic Score with Coronary Artery Disease.
eFigure 1. Generation of the Combined Coronary Angiography Dataset.
eFigure 2. Selection of Lipid Variants and Calculation of Genetic Scores.
eReferences. References.
References
- 1.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease, 1: evidence from genetic, epidemiologic, and clinical studies, a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-2472. doi: 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112(22):3375-3383. doi: 10.1161/CIRCULATIONAHA.104.532499 [DOI] [PubMed] [Google Scholar]
- 3.Di Angelantonio E, Sarwar N, Perry P, et al. ; Emerging Risk Factors Collaboration . Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000. doi: 10.1001/jama.2009.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arsenault BJ, Rana JS, Stroes ESG, et al. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol. 2009;55(1):35-41. doi: 10.1016/j.jacc.2009.07.057 [DOI] [PubMed] [Google Scholar]
- 5.Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4(3):337-345. doi: 10.1161/CIRCOUTCOMES.110.959247 [DOI] [PubMed] [Google Scholar]
- 6.Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012;307(12):1302-1309. doi: 10.1001/jama.2012.366 [DOI] [PubMed] [Google Scholar]
- 7.Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297(5):499-508. doi: 10.1001/jama.297.5.499 [DOI] [PubMed] [Google Scholar]
- 8.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078-2087. doi: 10.1056/NEJMoa1110874 [DOI] [PubMed] [Google Scholar]
- 9.Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373-2384. doi: 10.1001/jama.2016.16951 [DOI] [PubMed] [Google Scholar]
- 10.Puri R, Nissen SE, Shao M, et al. Non-HDL cholesterol and triglycerides: implications for coronary atheroma progression and clinical events. Arterioscler Thromb Vasc Biol. 2016;36(11):2220-2228. doi: 10.1161/ATVBAHA.116.307601 [DOI] [PubMed] [Google Scholar]
- 11.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-R98. doi: 10.1093/hmg/ddu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stitziel NO. Human genetic insights into lipoproteins and risk of cardiometabolic disease. Curr Opin Lipidol. 2017;28(2):113-119. doi: 10.1097/MOL.0000000000000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helgadottir A, Gretarsdottir S, Thorleifsson G, et al. Variants with large effects on blood lipids and the role of cholesterol and triglycerides in coronary disease. Nat Genet. 2016;48(6):634-639. doi: 10.1038/ng.3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Setten J, Išgum I, Pechlivanis S, et al. Serum lipid levels, body mass index, and their role in coronary artery calcification: a polygenic analysis. Circ Cardiovasc Genet. 2015;8(2):327-333. doi: 10.1161/CIRCGENETICS.114.000496 [DOI] [PubMed] [Google Scholar]
- 15.Tsao CW, Preis SR, Peloso GM, et al. Relations of long-term and contemporary lipid levels and lipid genetic risk scores with coronary artery calcium in the Framingham Heart Study. J Am Coll Cardiol. 2012;60(23):2364-2371. doi: 10.1016/j.jacc.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budoff MJ, Nasir K, McClelland RL, et al. ; MESA (Multi-Ethnic Study of Atherosclerosis) . Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2009;53(4):345-352. doi: 10.1016/j.jacc.2008.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjornsson E, Gudbjartsson DF, Helgadottir A, et al. Common sequence variants associated with coronary artery disease correlate with the extent of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(6):1526-1531. doi: 10.1161/ATVBAHA.114.304985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudnason T, Gudnadottir GS, Lagerqvist B, et al. Comparison of interventional cardiology in two European countries: a nationwide Internet based registry study. Int J Cardiol. 2013;168(2):1237-1242. doi: 10.1016/j.ijcard.2012.11.054 [DOI] [PubMed] [Google Scholar]
- 19.Fröbert O, Lagerqvist B, Olivecrona GK, et al. ; TASTE Trial . Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369(17):1587-1597. doi: 10.1056/NEJMoa1308789 [DOI] [PubMed] [Google Scholar]
- 20.Johannesdottir H, Arnadottir LO, Adalsteinsson JA, et al. Favourable long-term outcome after coronary artery bypass grafting in a nationwide cohort. Scand Cardiovasc J. 2017;51(6):327-333. doi: 10.1080/14017431.2017.1364418 [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi: 10.1016/0735-1097(90)90282-T [DOI] [PubMed] [Google Scholar]
- 22.Gudbjartsson DF, Helgason H, Gudjonsson SA, et al. Large-scale whole-genome sequencing of the Icelandic population. Nat Genet. 2015;47(5):435-444. doi: 10.1038/ng.3247 [DOI] [PubMed] [Google Scholar]
- 23.Jónsson H, Sulem P, Kehr B, et al. Whole genome characterization of sequence diversity of 15,220 Icelanders. Sci Data. 2017;4:170115. doi: 10.1038/sdata.2017.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong A, Masson G, Frigge ML, et al. Detection of sharing by descent, long-range phasing and haplotype imputation. Nat Genet. 2008;40(9):1068-1075. doi: 10.1038/ng.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu DJ, Peloso GM, Yu H, et al. ; Charge Diabetes Working Group; EPIC-InterAct Consortium; EPIC-CVD Consortium; GOLD Consortium; VA Million Veteran Program . Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49(12):1758-1766. doi: 10.1038/ng.3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller M, Ginsberg HN, Schaefer EJ. Relative atherogenicity and predictive value of non-high-density lipoprotein cholesterol for coronary heart disease. Am J Cardiol. 2008;101(7):1003-1008. doi: 10.1016/j.amjcard.2007.11.046 [DOI] [PubMed] [Google Scholar]
- 28.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118(4):547-563. doi: 10.1161/CIRCRESAHA.115.306249 [DOI] [PubMed] [Google Scholar]
- 29.Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45(11):1345-1352. doi: 10.1038/ng.2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61(4):427-436. doi: 10.1016/j.jacc.2012.08.1026 [DOI] [PubMed] [Google Scholar]
- 31.Holmes MV, Asselbergs FW, Palmer TM, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36(9):539-550. doi: 10.1093/eurheartj/eht571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Do R, Stitziel NO, Won HH, et al. ; NHLBI Exome Sequencing Project . Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518(7537):102-106. doi: 10.1038/nature13917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crosby J, Peloso GM, Auer PL, et al. ; TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute . Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22-31. doi: 10.1056/NEJMoa1307095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32-41. doi: 10.1056/NEJMoa1308027 [DOI] [PubMed] [Google Scholar]
- 35.Dewey FE, Gusarova V, O’Dushlaine C, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med. 2016;374(12):1123-1133. doi: 10.1056/NEJMoa1510926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stitziel NO, Stirrups KE, Masca NG, et al. ; Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators . Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. 2016;374(12):1134-1144. doi: 10.1056/NEJMoa1507652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khera AV, Won H-H, Peloso GM, et al. ; Myocardial Infarction Genetics Consortium, DiscovEHR Study Group, CARDIoGRAM Exome Consortium, and Global Lipids Genetics Consortium . Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA. 2017;317(9):937-946. doi: 10.1001/jama.2017.0972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626-635. doi: 10.1016/S0140-6736(14)61177-6 [DOI] [PubMed] [Google Scholar]
- 39.Lawler PR, Akinkuolie AO, Chu AY, et al. Atherogenic lipoprotein determinants of cardiovascular disease and residual risk among individuals with low low-density lipoprotein cholesterol. J Am Heart Assoc. 2017;6(7):1-18. doi: 10.1161/JAHA.117.005549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572-580. doi: 10.1016/S0140-6736(12)60312-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helgadottir A, Sulem P, Thorgeirsson G, et al. Rare SCARB1 mutations associate with high-density lipoprotein cholesterol but not with coronary artery disease. Eur Heart J. 2018;39(23):2172-2178. doi: 10.1093/eurheartj/ehy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson JG, Wang S, Smith BJ, Jacobson TA. Meta-analysis of the relationship between non-high-density lipoprotein cholesterol reduction and coronary heart disease risk. J Am Coll Cardiol. 2009;53(4):316-322. doi: 10.1016/j.jacc.2008.10.024 [DOI] [PubMed] [Google Scholar]
- 43.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289-1297. doi: 10.1001/jama.2016.13985 [DOI] [PubMed] [Google Scholar]
- 44.Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. 2019;40(2):537-557. doi: 10.1210/er.2018-00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Methods.
eTable 1. Reported Lipid-Associated Variants Used for Calculation of the Genetic Scores.
eTable 2. Relationship of Genetic Scores with Their Respective Lipid Traits.
eTable 3. Pairwise-Correlations for Genetic Scores (Blue) and Lipid Traits (Green).
eTable 4. Genetic Scores for Lipid Levels and Angiographic Extent of CAD.
eTable 5. Lipid levels in Genotyped Icelanders.
eTable 6. Association of the non-HDL-C Genetic Score with Coronary Artery Disease.
eFigure 1. Generation of the Combined Coronary Angiography Dataset.
eFigure 2. Selection of Lipid Variants and Calculation of Genetic Scores.
eReferences. References.