Abstract
This phase 2 clinical trial in patients with high-risk sickle cell disease assesses the feasibility, safety, and 1-year event-free survival after myeloimmunoablative conditioning and familial haploidentical stem cell transplant.
Sickle cell disease (SCD) is an autosomal recessive disorder associated with cerebral vasculopathy, stroke, acute chest syndrome, pulmonary hypertension and/or frequent vaso-occlusive crises, and a high risk of early mortality.1,2 Studies have reported 90% to 100% event-free survival (EFS) following human leukocyte antigen matched sibling allogeneic stem cell transplant.3,4 Reported results have used unrelated allogeneic donor sources; however, a paucity of unrelated matched donors and a higher than expected rate of graft failure and chronic graft-vs-host disease (GVHD) were notable disadvantages.5 The CD34+ enrichment and mononuclear cell (MNC) addback (2 × 105 CD3 cells/kg of recipient body weight) have been reported following matched unrelated donor transplant that resulted in 100% engraftment and a low cumulative incidence of acute GVHD and chronic GVHD.6
Methods
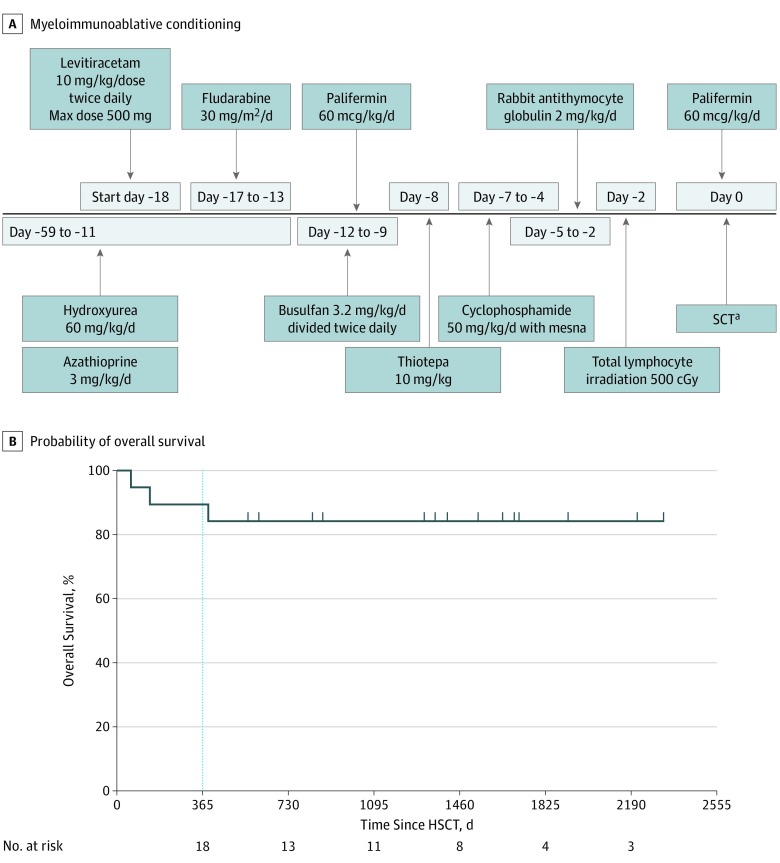
A total of 21 patients met eligibility. Two patients withdrew consent prior to the start of conditioning and the remaining 19 patients proceeded to haploidentical (HAPLO) stem cell transplant. We conducted a single-arm, open-label, prospective phase 2, multicenter trial in patients with high-risk SCD to determine the feasibility, safety, and 1-year EFS following myeloimmunoablative conditioning (Figure, A) and familial HAPLO stem cell transplant.4 Patients were enrolled from September 2012 to September 2017, and data were analyzed from December 2018 to July 2019. Patients 2 years or older and 20.99 years or younger who were homozygous for hemoglobin S were eligible and had to have one or more high-risk features5 and adequate organ function.6 The study was approved by the institutional review board at each institution (New York Medical College, University of California, Los Angeles, Medical College of Wisconsin, Washington University, Oakland Children’s Hospital/University of California, San Francisco, Tufts University, and New York Blood Center). Patients and/or their guardians signed written informed consents and/or assents. A target of 10 × 106 CD34 cells/kg of recipient body weight and MNC (2 × 105 CD3 cells/kg of recipient body weight) was cryopreserved on day −59 prior to the start of myeloimmunoablative conditioning. The CD34+ enrichment was performed using a CD34+ reagent system (CliniMACS; Miltenyi Biotec). Mononuclear cells (2 × 105 CD3 cells/kg of recipient body weight) were removed from the leukapheresis collection prior to CD34+ enrichment and were cryopreserved as a source of MNC addback (T cells). The study was designed to accrue 20 eligible patients with an estimated 1-year EFS greater than or equal to 75% by Kaplan-Meier method. Analyses were conducted using Prism, version 8.2 (GraphPad Software LLC).
Figure. Myeloimmunoablative Conditioning and Event-Free and Overall Survival.
A. Myeloimmunoablative conditioning regimen and supportive care. B, Probability of overall survival before and after health-related quality of life and processing speed. The probability of event-free survival (event defined as death or recurrent sickle cell disease symptoms) and overall survival in 19 enrolled patients following familial haploidentical stem cell transplant using donor CD34+ enrichment and mononuclear cell addback determined by the Kaplan-Meier product limit method. HSCT indicates haploidentical stem cell transplant; Max, maximum; and SCT, stem cell transplant.
aPatients who underwent stem cell transplant received hydroxyurea and azathioprine starting day –59 to day –11, fludarabine on days −17, −16, −15, −14, and −13; busulfan twice daily on days −12, −11, −10, and −9; thiotepa on day −8; cyclophosphamide on days −7, −6, −5, and −4; total lymphocyte irradiation on day −2; and rabbit antithymocyte globulin on day −5,−4,−3, and −2.
Results
The median number of days of follow-up was 1409 (range, 59-2330). The Table summarizes the major high-risk criteria for study entry and other pertinent clinical outcome information. The mean (SEM) age of donors was 41.7 (1.8) years (range, 30-55 years) with a female to male sex ratio of 15:3. The mean (SEM) CD34+ peripheral blood stem cells collected, processed, and cryopreserved was 12.9 (0.8 × 106) (range, 7.4-18.0) cells/kg of recipient body weight. The percent CD34 recovery (mean [SEM]) was 66.0% (3.4%) (range, 39.7-96) and the depletion (CD3 cells/kg of body weight) was mean (SD) 4.8 (0.1) (range, 4.0-6.0) logs. The median day post-HAPLO stem cell transplant to neutrophil engraftment was +9, and the median day post-HAPLO stem cell transplant to platelet engraftment was +19. Peripheral blood and red blood cell count (estimated by percentage donor DNA in erythroid lineage [CD71] enriched fraction) mean (SEM) donor chimerism at 1 year post-HAPLO stem cell transplant was 97.1 (1.4) and 96.4 (2.0%), respectively. The cumulative incidence of grades 2 to 4 acute GVHD and late acute GVHD was 6.2% and moderate and/or severe chronic GVHD was 6.7%. The probability of 1-year EFS or overall survival was 90% (95% CI, 64.1%-97.3%) and of 2-year EFS or overall survival was 84% (95% CI, 57.0%-94.4%), and no patient had residual SCD symptoms (Figure, B). One patient died of sinusoidal obstruction syndrome (day +59), 1 of steroid refractory grade 3 acute GVHD (day +141), and 1 of severe chronic GVHD (day +390). Pulmonary and cardiac functions were stable to improved at 2 years (mean [SD] forced vital capacity, baseline: 90 [12.5] vs at 2 years: 83.8 [16.9]; P = .50; mean [SD] fractional shortening, baseline: 33.3 [9.0] vs at 2 years: 36.1 [4.0]; P = .79). Using the Child Health Ratings Inventory (range, 0-100; higher scores indicate better functioning), significant improvements were seen at 2 years in emotional functioning (baseline, 73.7 [95% CI, 65.0-82.3] vs 2 years, 87.5 [95% CI, 74.8-100.2]; P < .03) and physical functioning (baseline, 74.2 [95% CI, 65.1-83.3] vs 2 years, 96.7 [95% CI, 88.1-105.2]; P < .01). Using the Processing Speed Index from the Wechsler Intelligence Scale for Children (range, 50-150; scores between 90-109 indicate the average range; between 80-89, the low average range; and between 70-79, the borderline range), significant improvement was seen in neurocognitive processing speed (baseline mean [SEM], 86.5 [5.1] vs 2 years mean [SEM], 93.7 [4.2]; P < .03).
Table. Clinical Characteristics and Clinical Outcome Results of 19 HAPLO Stem Cell Transplant Recipients.
| Characteristics and Outcomes | Data Results |
|---|---|
| Characteristic | |
| Age, mean (SEM) [range], y | 13.12 (1.2) [3.3-20] |
| Sex, No. | |
| Male | 12 |
| Female | 7 |
| High-risk features (primary), No. | |
| Stroke | 11 |
| Acute chest syndrome | 4 |
| Veno-occlusive crisis | 2 |
| Abnormal transcranial Doppler results | 2 |
| Outcome | |
| CD34/kg infused, mean (SEM), ×106/kg recipient | 10.9 (0.4) |
| Busulfan at steady state, mean (SEM), ng/mL | 648 (33) |
| 1-y Donor chimerism, mean (SEM), % | |
| Whole blood cell count | 97 (1.6) |
| Red blood cell count | 96.4 (1.7) |
Abbreviation: HAPLO, haploidentical.
Discussion
Our results at 2 years show full donor engraftment and low incidence of acute GVHD and chronic GVDH, stable to improved pulmonary and cardiac function, stable to improved brain magnetic resonance imaging results and significant improvement in quality of life and neurocognitive processing speed. Long-term follow-up is currently underway to assess future risks of chronic GVHD, neurocognitive function, health-related quality of life, and fertility (NCT02675959). Furthermore, alternative allogeneic donor approaches must be balanced in the future with the safety and efficacy of gene therapy in patients with high-risk SCD.4
References
- 1.Gladwin MT. Cardiovascular complications and risk of death in sickle-cell disease. Lancet. 2016;387(10037):2565-2574. doi: 10.1016/S0140-6736(16)00647-4 [DOI] [PubMed] [Google Scholar]
- 2.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639-1644. doi: 10.1056/NEJM199406093302303 [DOI] [PubMed] [Google Scholar]
- 3.Gluckman E, Cappelli B, Bernaudin F, et al. ; Eurocord, the Pediatric Working Party of the European Society for Blood and Marrow Transplantation, and the Center for International Blood and Marrow Transplant Research . Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129(11):1548-1556. doi: 10.1182/blood-2016-10-745711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talano JA, Cairo MS. Hematopoietic stem cell transplantation for sickle cell disease: state of the science. Eur J Haematol. 2015;94(5):391-399. doi: 10.1111/ejh.12447 [DOI] [PubMed] [Google Scholar]
- 5.Shenoy S, Eapen M, Panepinto JA, et al. A trial of unrelated donor marrow transplantation for children with severe sickle cell disease. Blood. 2016;128(21):2561-2567. doi: 10.1182/blood-2016-05-715870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geyer MB, Ricci AM, Jacobson JS, et al. T cell depletion utilizing CD34(+) stem cell selection and CD3(+) addback from unrelated adult donors in paediatric allogeneic stem cell transplantation recipients. Br J Haematol. 2012;157(2):205-219. doi: 10.1111/j.1365-2141.2012.09048.x [DOI] [PubMed] [Google Scholar]