This meta-analysis assesses whether intravitreal anti–vascular endothelial growth factor therapy and which variables might be associated with increased risk of mortality among patients with retinal disease.
Key Points
Question
Does intravitreal anti–vascular endothelial growth factor therapy increase the risk of mortality among patients with retinal disease?
Findings
A frequentist meta-analysis on all-cause mortality that included 8887 unique patients in 34 unique studies provided an increased odds of 1.34 between anti–vascular endothelial growth factor therapy and control groups. No definitive linear increase in risk for 1 injection more or higher mortality in studies with longer follow-up were shown by meta-regression analyses.
Meaning
This study revealed little evidence of increased mortality and cannot determine whether the increased death risk suggested from studies in which monthly injections were delivered for as long as 2 years was associated with the larger number of injections or with longer follow-up.
Abstract
Importance
Although intravitreal anti–vascular endothelial growth factor (VEGF) treatment represents the first-line therapy for many retinal diseases, the issue of their systemic safety is debatable.
Objectives
To assess whether intravitreal anti-VEGF therapy might be associated with increased risk of mortality and which variables are associated with the increase.
Data Sources
PubMed, MEDLINE, and Embase databases, the Cochrane Library, and ClinicalTrials.gov were systematically searched from inception to May 6, 2019.
Study Selection
Randomized clinical trials comparing intravitreal anti-VEGF treatment with control groups and with follow-up of at least 6 months were selected.
Data Extraction and Synthesis
Data were independently collected by 2 investigators. Meta-analyses were conducted using the frequentist and Bayesian methods. For the frequentist approach, random- and fixed-effects models were used, with random-effects models considered the primary technique. Odds ratios (ORs) with 95% CIs were computed. For the bayesian approach, uninformative and informative priors were used. Odds ratios with 95% credible intervals (CrIs) were computed. Meta-regression analyses were based on random-effects models.
Main Outcomes and Measures
The primary outcome measure was the all-cause death rate. Secondary outcomes included meta-regression analyses on the following variables: type of drug, number of injections, follow-up time, diagnosis, and cardiovascular risk.
Results
Of 2336 studies identified, 34 unique studies with 8887 unique participants were included in the present meta-analysis. For the frequentist analysis, fixed- and random-effects models yielded similar estimates (ORs, 1.34 [95% CI, 0.95-2.07; P = .09] and 1.34 [95% CI, 0.89-2.01; P = .17], respectively). For the Bayesian approach, noninformative and informative priors yielded similar results (ORs, 1.34 [95% CrI, 0.79-2.34; 0.13 probability of OR≤1.00] and 1.40 [95% CrI, 0.82-2.32; 0.11 probability of OR≤1.00], respectively). Meta-regression analyses showed the following risk for 1 injection more: frequentist OR of 1.12 (95% CI, 1.04-1.22; P = .005) and Bayesian OR of 1.06 (95% CrI, 0.98-1.15; 0.06 probability of OR≤1.00).
Conclusions and Relevance
In this study, no difference was found in the mortality rate between intravitreal anti-VEGF treatment and control groups. Additional data seem warranted to determine whether the mortality rate is increased in patients receiving a greater number of injections.
Introduction
Intravitreal therapy with anti–vascular endothelial growth factor (VEGF) agents represents the most remarkable breakthrough in treatment of many retinal diseases during the last 25 years, with 7 million estimated intravitreal injections in the United States and more than 20 million throughout the world in 2016.1 Despite outstanding clinical efficacy, some concerns have been raised about the safety profile of these agents.2,3 No risk of systemic adverse events, including mortality rate, has been found in randomized clinical trials (RCTs) that evaluated the safety profile of anti-VEGF drugs.4,5,6,7,8,9 However, assessing the risk of mortality associated with intravitreal anti-VEGF therapy is challenging, because no registered trial, to our knowledge, has been sufficiently powered to detect a significant difference between anti-VEGF treatment and control groups owing to the low incidence of the event.10 Furthermore, an increased mortality rate recently has been reported after intravitreal anti-VEGF treatment in the general population11 and patients presenting additional risk factors.12,13 Systematic reviews and meta-analyses published on this issue reported controversial outcomes.2,3,14
To date, no study, to our knowledge, has pooled data from RCTs on age-related macular degeneration (AMD), retinal vein occlusion (RVO), and diabetic macular edema (DME), including all anti-VEGF agents used to treat these diseases, with the purpose of evaluating the mortality rate. Moreover, future RCTs of anti-VEGF drugs are bound to compare a new drug with approved drugs and no longer with controls. Thus, evidence of mortality associated with intravitreal anti-VEGF treatment needs to be drawn from RCTs that have been conducted previously because of the need for a control group. Therefore, we conducted a systematic review and meta-analysis of RCTs on all anti-VEGF agents used for treating AMD, RVO, and DME with the aim of assessing whether intravitreal therapy with anti-VEGF agents is associated with an increased risk of mortality compared with controls and which clinical variables may be correlated with this event.
Methods
Sources and Search Methods
A systematic electronic search of PubMed, MEDLINE, and Embase databases, the Cochrane Library, and ClinicalTrials.gov was conducted from their inception through May 6, 2019, for RCTs of intravitreal anti-VEGF drugs in AMD, DME, and RVO. The search method was based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)15 and on the statements reported in the Cochrane Handbook.16 The PRISMA checklist and the search strategy are available in eTables 1 and 2, respectively, in the Supplement. In addition, abstracts from ophthalmology congresses such as the European Society of Retina Specialists, the American Academy of Ophthalmology, and the Association for Research in Vision and Ophthalmology were reviewed, and reference lists of all included articles were searched. Only studies published in peer-reviewed journals and in English were considered, irrespective of main outcomes, date, or publication status. Institutional review board approval and informed consent were not required for this systematic review of RCTs.
To be included in the present meta-analysis, RCTs had to meet the eligibility criteria of comparing an intravitreal anti-VEGF treatment group with a control group that did not receive any intravitreal or systemic anti-VEGF drugs. If control patients were offered to cross over to intravitreal anti-VEGF treatment at a time point in the trial, the study was considered eligible to that point. A follow-up shorter than 6 months and study design different from an RCT were considered as exclusion criteria.
Data Collection and Risk for Bias Assessment
All-cause mortality rates in the population treated with anti-VEGF and in the control population were extracted from each study by 2 independent investigators (M.F. and A.L.) as the primary outcome of interest. For each trial, data on study quality, dosing and type of drug used, number of injections, injection protocol, follow-up time, diagnosis, and demographic characteristics of groups, including sex, age, and cardiovascular risk (history of stroke or myocardial infarction), were collected independently by the 2 investigators (M.F. and A.L.). Discrepancies were addressed by a third reviewer (T.A.). When clarifications or further information were required, the authors were contacted. When more than 1 study had been published using data from the same population, we included either the most recent one or the one with the best quality in this analysis. Risk of bias was evaluated according to the recommendations of the Cochrane collaboration.17
Data Synthesis and Analysis
The clinical and methodological features of the included studies were assessed based on the guidelines of the Cochrane collaboration.16 First, we conducted a frequentist meta-analysis in which studies with no events were excluded. In this analysis, we compared the consistency of fixed and random effects in meta-analysis to explore the effects of large studies and then considered random effects as the primary technique. We did not consider the Peto odds ratio (OR) because that method is not reliable when study arms have a very different size.
Along with conventional computation of random-effects ORs with 95% CIs, we used Bayesian meta-analysis methods to account for sparse data with no events in some studies.18 We used WinBUGS, version 1.4.3,19 to conduct Bayesian meta-analysis, and the robustness of the results was tested by using 2 types of prior distributions on between-study variance of the logarithm OR: an uninformative prior distribution (flat uniform distribution between 0 and 1), and an informative prior distribution with a half-normal positive distribution with median variance of 0.8, meaning that the OR between studies could a priori vary by 5 times in 95% of the studies. The range of plausible ORs computed using the Bayesian approach was expressed as the 95% credible interval (CrI). We fitted random-effects meta-regression models that included the following variables: type of drug, number of injections, follow-up time, diagnosis, and cardiovascular risk. Because ranibizumab and bevacizumab trials could have used 2 different doses (0.30 and 0.50 mg for ranibizumab and 1.25 and 2.50 mg for bevacizumab), the analysis combined both doses for each drug in a single group, as previously reported. Heterogeneity was investigated by calculating the χ2 test and I2 statistics for frequentist meta-analyses and examining the τ2 statistic, the between-study variance, for bayesian meta-analyses. Two-sided P < .05 was considered significant.
Results
Study Selection
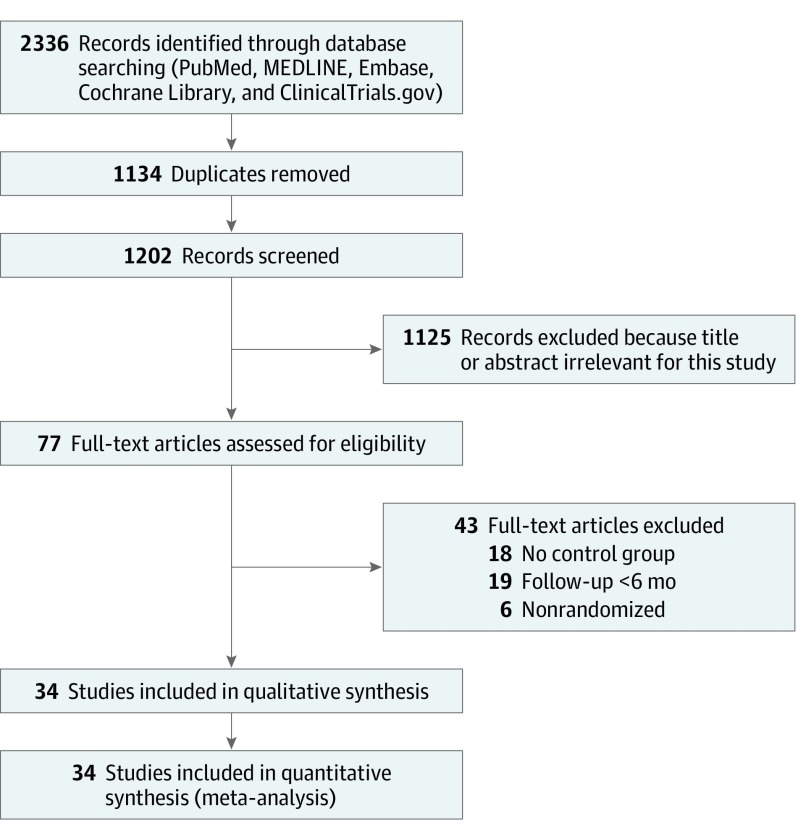
Figure 1 shows the study selection process. A total of 2336 studies were identified from the electronic database search, of which 1134 were duplicates. The remaining 1202 articles were screened by applying inclusion and exclusion criteria, and 77 potentially relevant studies were identified. Forty-three trials were ruled out after full-text assessment, of which 18 did not include a control group, 19 had less than a 6-month follow-up, and 6 were not randomized. A total of 34 unique studies with 8887 unique patients were selected and included in our analysis.5,8,9,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50
Figure 1. Study Selection Flowchart.
Of the 34 included studies, 15 were on DME,20,21,22,23,24,25,26,27,34,35,40,41,47,49,50 13 were on RVO,8,9,31,32,33,37,38,39,42,43,44,45,48 and 6 were on AMD.5,28,29,30,36,46 For the anti-VEGF agent, 18 of the included trials reported on intravitreal ranibizumab.5,8,9,20,21,22,23,24,25,26,27,28,29,30,31,32,33,44 Bevacizumab was used in 6 studies,34,35,36,37,38,39 aflibercept in 7 studies,40,41,42,43,45,49,50 and pegaptanib sodium in 3 studies.46,47,48
Among the included studies, 14 presented a follow-up ranging from 6 to 8 months and were classified as 6-month follow-up studies8,9,20,30,31,32,33,34,38,39,42,44,47,48; 15 studies featured a follow-up ranging from 11 to 12 months and were classified as 12-month follow-up studies5,21,23,24,25,26,27,28,35,36,37,43,45,46,49; and 5 trials presented a follow-up ranging from 23 to 24 months and were classified as 24-month follow-up studies.22,29,40,41,50
Quality Assessment and Risk of Bias
Risk of bias assessment is shown in eFigures 1 and 2 in the Supplement. Thirty trials5,8,9,21,22,23,24,25,26,27,28,29,30,32,33,34,35,36,37,38,40,41,42,44,45,46,47,48,49,50 featured a low risk with respect to sequence generation, whereas the risk was unclear in 4 studies.20,31,39,43 Two of these studies20,43 presented an unclear risk with respect to allocation concealment, as did 7 other trials.8,9,26,27,28,30,42 Because death was an unexpected adverse event in these studies, we suggest that there is no or little potential for bias regarding these domains in our review.
Masking of participant assessment was low risk in 23 trials,5,8,9,21,22,23,25,28,30,31,33,34,38,39,40,41,42,43,45,46,47,48,49 unclear in 6 trials,29,32,35,37,44,50 and high risk in 5 trials.20,24,26,27,36 Masking of outcome assessment was at low risk in 22 trials,22,23,25,27,28,29,31,32,38,39,40,41,42,43,44,46,47,48,50 unclear in 9 trials,21,24,30,33,34,35,37,45,49 and at high risk in 3 trials.20,26,36 We considered that masking was not an issue for an objective and well-defined outcome such as all-cause mortality.
Attrition bias was judged as low risk in 14 studies,5,21,23,24,28,32,34,36,38,39,46,47,49,50 unclear in 14 studies,8,9,20,22,27,29,31,40,41,42,43,44,45,48 and high risk in 6 studies.25,26,30,33,35,37 Even small amounts of missing data could cause important bias when estimating rare adverse events, such as death. Nonetheless, we believe that large, multicenter RCTs, such as those included in our review, actively record adverse events, and death is unlikely to be missed. Thus, we considered that there was potential for bias due to attrition in our meta-analyses, but the risk was not serious.
Reporting bias was assessed as low risk in 25 trials,5,8,9,21,22,23,26,28,29,31,32,36,38,39,40,41,42,43,44,45,46,47,48,49,50 unclear in 8 trials,24,25,27,30,33,34,35,37 and high risk in 1 trial.20 We found that reporting causes little risk of bias due to poor reporting of death, which is a serious adverse event and must be reported regardless of its inclusion as an outcome in a trial protocol.
Risk of other bias was low in 19 trials9,20,21,22,23,24,25,26,32,36,38,39,40,41,42,47,48,49,50 and unclear in 14 trials5,27,28,29,30,31,33,34,35,37,43,44,45,46; the study of Campochiaro et al8 was judged to be at high risk for other biases, mainly because the differences reported between anti-VEGF and sham groups could have been influenced by the fact that all patients could receive laser treatment.
All-Cause Mortality
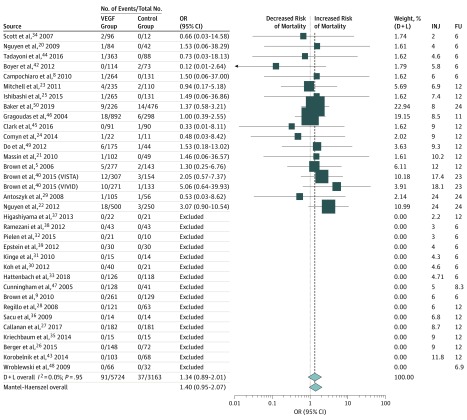
Figure 2 shows numerical results for all studies. In this forest plot, studies providing data to the analysis are sorted by increasing follow-up time and number of injections.
Figure 2. Forest Plot of All-Cause Mortality in Anti–Vascular Endothelial Growth Factor (VEGF) and Control Cohorts.
Studies with no deaths in the VEGF and control groups were excluded from the frequentist meta-analysis. OR indicates odds ratio. D+L indicates DerSimonian and Laird random-effects meta-analysis methods; FU, follow-up; and INJ, number of injections. Weights are calculated from random-effects analysis.
In the 34 included studies, all-cause death was recorded in 91 (1.6%) of 5724 patients receiving anti-VEGF treatment and in 37 (1.2%) of 3163 controls. These percentages increased to 91 (2.1%) of 4389 anti-VEGF–treated patients and 37 (1.6%) of 2291 controls in 18 studies with at least 1 death recorded in both study arms,5,8,20,21,22,23,24,25,29,34,40,41,42,44,45,46,49,50 excluding 16 studies with no deaths.9,26,27,28,30,31,32,33,35,36,37,38,39,43,47,48
The frequentist pooled estimate yielded similar fixed- and random-effects ORs (1.34 [95% CI, 0.95-2.07; P = .09] and 1.34 [95% CI, 0.89-2.01; P = .17], respectively) with no heterogeneity (I2 = 0%; P = .95). No evidence of publication bias was found using the Peters test (P = .44).
The primary bayesian meta-analysis using noninformative priors yielded an OR of 1.34 (95% CrI, 0.79-2.34) and a probability of 0.13 that the OR was 1.00 or less. The sensitivity Bayesian meta-analysis using an informative prior yielded similar results, with an OR of 1.40 (95% CrI, 0.82-2.32) and a probability of 0.11 that the OR was 1.00 or less, suggesting the robustness of the estimates.
Meta-regressions
The Table presents results of meta-regression for frequentist and bayesian meta-analyses. In the frequentist framework, a linear increase in risk was found for 1 injection more (OR, 1.12; 95% CI, 1.04-1.22; P = .005). The meta-analysis of 5 studies with follow-up longer than 12 months22,29,40,41,50 (23 or 24 months) showed a higher mortality in the anti-VEGF subgroup (OR, 1.89; 95% CI, 1.06-3.36), but not higher than that in 14 studies with follow-up of about 6 months8,9,20,30,31,32,33,34,38,39,42,44,47,48 (OR, 0.65; 95% CI, 0.16-2.66; P = .17 for the ratio of ORs). Using follow-up time as a linear covariate, odds of mortality per month increased by 9% (OR, 1.09; 95% CI, 1.00-1.18). Only the number of injections (OR, 1.10; 95% CI, 1.00-1.20; P = .04), but not follow-up time (OR, 1.03; 95% CI, 0.95-1.12; P = .44), maintained a statistically significant effect in a multivariate meta-regression. No differences were detected among drugs; however, data on bevacizumab were sparse and mostly based on small studies with no deaths. Frequentist meta-analysis in the DME subgroup showed higher mortality in the anti-VEGF group (OR, 1.68; 95% CI, 1.01-2.77; P = .04). However, it was not different compared with the AMD subgroup (OR, 2.52; 95% CI, 0.63-10.0; P = .17).
Table. Meta-regression From Frequentist and Bayesian Models.
| No. of Studies in Bayesian/Frequentist Analyses (n = 34/n = 18) | Covariate | Frequentist Model | Bayesian Model | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Valuea | OR (95% CrI) | Probability Relative OR ≤ 1.00b | ||
| Diagnosis | |||||
| 6/3 | AMD | 1.01 (0.46-2.20) | 1 [Reference] | 0.98 (0.33-3.41) | 1 [Reference] |
| 15/11 | DME | 1.68 (1.01-2.77) | .17 | 1.75 (0.94-3.85) | 0.15 |
| 13/4 | RVO | 0.45 (0.09-2.17) | .72 | 0.30 (0.03-2.25) | 0.82 |
| No. of injections | |||||
| 33/17 | Per 1 more | 1.12 (1.04-1.22) | .005 | 1.06 (0.98-1.15) | 0.06 |
| Drug | |||||
| 18/6 | Ranibizumab | 1.44 (0.72-2.87) | 1 [Reference] | 1.75 (0.74-4.82) | 1 [Reference] |
| 6/1 | Bevacizumab | 0.66 (0.03-14.6) | .65 | 24.4 (0.10-∞) | 0.24 |
| 3/1 | Pegaptanib sodium | 1.00 (0.39-2.21) | .64 | 1.03 (0.21-4.97) | 0.74 |
| 6/10 | Aflibercept | 1.47 (0.79-2.72) | .56 | 1.46 (0.61-3.23) | 0.62 |
| Follow-up, mo | |||||
| 14/5 | 6 (6-8) | 0.65 (0.16-2.66) | 1 [Reference] | 0.82 (0.15-6.53) | 1 [Reference] |
| 15/8 | 12 (11-12) | 1.02 (0.54-1.92) | .83 | 1.07 (0.51-2.46) | 0.41 |
| 5/5 | 24 (23-24) | 1.89 (1.06-3.36) | .17 | 2.07 (1.06-4.49) | 0.21 |
| Previous cardiovascular disease | |||||
| 15/7 | No | 1.12 (0.70-2.06) | 1 [Reference] | 1.23 (0.52-3.22) | 1 [Reference] |
| 18/11 | Yes | 2.12 (0.56-8.08) | .14 | 1.50 (0.41-4.72) | 0.24 |
Abbreviation: OR, odds ratio.
Calculated for the comparison of the ORs of death in the subgroup compared with that in the reference category.
Indicates probability that the relative OR for the category is more than 1.00 compared with the reference category.
The result of bayesian meta-analysis showed more extreme but, as expected, less precise estimates, with 95% CrI of meta-regressions always including equivalence and suggestive of a potential effect only for the number of injections. Although a 2.5-fold increase in mortality was seen with follow-up longer than 24 months compared with 6 months (OR, 2.07; 95% CI, 1.06-4.49), this estimate was imprecise and included no difference or a decrease (probability of a relative OR of 1.00 or less, 0.21).
Discussion
The present meta-analysis was designed to assess the risk of mortality related to intravitreal anti-VEGF treatment. Overall, frequentist and bayesian meta-analyses revealed no difference in the mortality rate between intravitreal anti-VEGF treatment and control groups. A linear increase in the risk of death for 1 injection more and higher mortality in studies with longer follow-up were shown by meta-regression analyses, but definitive evidence has not been consistently shown across 95% CIs. The findings also suggest that further studies likely are warranted to determine whether risk of death is increased when monthly injections are delivered as long as 24 months and, if so, whether this effect is associated with the larger number of injections, the longer follow-up, or both.
An association between intravitreal anti-VEGF treatment and cardiovascular accidents, including vascular death and all-cause death, has been debated since the introduction of these drugs for the treatment of many retinal diseases and remains a controversial issue. No significant association between intravitreal anti-VEGFs and arterial cardiovascular events has been revealed in RCTs, but these trials are supposed to be not powered enough to show a significantly increased risk because of the uncommon incidence of these events.10 However, systematic reviews with meta-analysis of published RCTs showed controversial results on this issue.2,3,4 Schmucker et al51 did not report an increased risk of stroke, myocardial infarction, or mortality after intravitreal anti-VEGF therapy. Similarly, Cheng et al10 did not demonstrate evidence of increased risk of arterial thromboembolic accidents associated with intravitreal anti-VEGF use. Thulliez et al14 later investigated cardiovascular accidents and bleeding risk associated with intravitreal anti-VEGF therapy, also considering mortality. The findings of their meta-analysis showed no significant increase of overall mortality, cardiovascular mortality, stroke, or myocardial infarction in patients receiving intravitreal anti-VEGF treatment compared with control participants. Conversely, Ueta et al3 found a positive association between intravitreal ranibizumab and increased risk of cerebrovascular events in patients with AMD in their meta-analysis.
In the present meta-analysis, the primary outcome was all-cause mortality in patients receiving intravitreal anti-VEGF treatment vs controls, without considering other outcomes, such as vascular death rate or arterial thromboembolic events, including cardiac and cerebral ischemia. All-cause mortality is an objective event that does not require any further assessment to be defined, whereas confounding factors may have an influence on the diagnosis of vascular death, whose definition according to the Antiplatelet Trialists’ Collaboration study also includes deaths of unknown cause.52
In our study, frequentist and bayesian meta-analyses were conducted. Both methods showed no difference in mortality rate between the anti-VEGF therapy and control groups. Even with a difference in mortality rates, this analysis suggests that the differences likely would be very small.
Differences between our bayesian and frequentist meta-analyses may be owing to the inclusion of 16 additional studies with no deaths in both treatment arms in bayesian models.9,26,27,28,30,31,32,33,35,36,37,38,39,43,47,48 In a simulation, Cheng et al53 found the inclusion of no-event studies should be considered when beneficial effects are unlikely to present, but they also suggested that such studies should be excluded when evaluating harmful events such as mortality. Thus, we think that the good concordance of the 2 types of meta-analysis we used is reassuring about the robustness of our results, specifically regarding overall mortality.
The precision of our estimates should be interpreted along with absolute mortality in the control arm, which was 1.2% with the inclusion of studies with no deaths in both arms and 2.1% with the exclusion of these studies. Considering our upper 95% CI limit, that is, an OR of 2.00, we cannot rule out harmful effects that increase mortality, doubling the baseline risk to 2.4% including studies with no deaths and 4.0% excluding them. Such absolute risk differences are compatible with no important increase in overall mortality. Moreover, patients at higher risk of death within 1 or 2 years are typically excluded from RCTs on anti-VEGF therapy for retinal diseases, whereas even a small relative increase in the risk of death could be important in patients with poor health who are sometimes treated in clinical practice. Risk estimates were very imprecise regarding bevacizumab because 5 of 6 studies using this drug were very small and recorded no deaths. Although a 2.5-fold increase in mortality was found in studies with a 24-month follow-up compared with those with a 6-month follow-up, this effect seemed to be owing to a few large studies22,29,40,41,50 characterized by intensive intravitreal therapy on a regular monthly basis for 2 years that accounted for almost 50% of the weight in the meta-analysis.
Diabetes represents a well-known risk factor for cardiovascular diseases, with the risk of myocardial infarction in patients with diabetes as high as in those patients with a previous myocardial infarction.54 Avery et al2 showed a significant higher mortality rate in patients receiving intensive intravitreal anti-VEGF treatment for DME. In particular, the authors included in their meta-analysis only RCTs reporting a 2-year outcome of monthly intravitreal anti-VEGF therapy for DME. Their findings corroborate those of the present meta-analysis, raising concerns about prolonged intravitreal anti-VEGF treatment in this category of patient. On the contrary, Cochrane meta-analysis studies on DME did not report any significant difference regarding overall mortality and arterial thromboembolic events between anti-VEGF agents and control groups (sham or laser treatment) and among the 3 anti-VEGF drugs as well.55,56 However, their estimates were imprecise regarding all-cause death, providing very low certainty evidence.55
Limitations
This study has some limitations. First, only 5 trials among the 34 included studies had a follow-up longer than 12 months. Considering that the subgroup analysis revealed a significant difference in mortality when pooling data from these 5 studies, this finding must be interpreted cautiously. Second, most included trials were sponsored by pharmaceutical companies, which may represent a risk of bias.57 However, we suggest that this is unlikely to affect all-cause mortality. Third, this meta-analysis was conducted on study-level data because individual patient data were not available. Consequently, it was not possible to investigate individual baseline characteristics that can influence mortality, particularly cardiovascular risk. However, this meta-analysis features more accurate 95% CIs and higher power compared with an individual trial.58 In addition, most of the included trials were characterized by a low risk of bias. No evidence of publication bias was found in the main frequentist meta-analysis according to the Peters test, and heterogeneity tests showed consistent outcomes across all included trials. Finally, our risk estimates may not be generalizable to patients at high cardiovascular risk because of recent arterial thromboembolic events, which were typically excluded from the RCTs used in our study.
Conclusions
Overall, we found no difference in mortality rate between intravitreal anti-VEGF treatment and the control group. This analysis cannot determine whether the increased death risk suggested from studies in which monthly injections were delivered for as long as 2 years was associated with the larger number of injections or longer follow-up. Nonrandomized comparative studies using existing electronic (real-world) data and adjusting for confounders might be conducted and might further clarify this issue.
eTable 1. PRISMA Checklist
eTable 2. Search Strategy
eFigure 1. Risk of Bias Graph
eFigure 2. Risk of Bias Summary
References
- 1.Martin DF. Evolution of intravitreal therapy for retinal diseases–from CMV to CNV: the LXXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2018;191:xli-xlviii. doi: 10.1016/j.ajo.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avery RL, Gordon GM. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol. 2016;134(1):21-29. doi: 10.1001/jamaophthalmol.2015.4070 [DOI] [PubMed] [Google Scholar]
- 3.Ueta T, Noda Y, Toyama T, Yamaguchi T, Amano S. Systemic vascular safety of ranibizumab for age-related macular degeneration: systematic review and meta-analysis of randomized trials. Ophthalmology. 2014;121(11):2193-2203.e7. doi: 10.1016/j.ophtha.2014.05.022 [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Kaiser PK, Michels M, et al. ; ANCHOR Study Group . Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444. doi: 10.1056/NEJMoa062655 [DOI] [PubMed] [Google Scholar]
- 6.Elman MJ, Aiello LP, Beck RW, et al. ; Diabetic Retinopathy Clinical Research Network . Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.e35. doi: 10.1016/j.ophtha.2010.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf S, Balciuniene VJ, Laganovska G, et al. ; RADIANCE Study Group . RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121(3):682-692.e2. doi: 10.1016/j.ophtha.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 8.Campochiaro PA, Heier JS, Feiner L, et al. ; BRAVO Investigators . Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102-1112.e1. doi: 10.1016/j.ophtha.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 9.Brown DM, Campochiaro PA, Singh RP, et al. ; CRUISE Investigators . Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124-1133.e1. doi: 10.1016/j.ophtha.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 10.Cheng JW, Cheng SW, Lu GC, Wei RL. Effect of intravitreal anti-vascular endothelial growth factor therapy on the risk of arterial thromboembolic events: a meta-analysis. PLoS One. 2012;7(7):e41325. doi: 10.1371/journal.pone.0041325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanhart J, Comaneshter DS, Freier Dror Y, Vinker S. Mortality in patients treated with intravitreal bevacizumab for age-related macular degeneration. BMC Ophthalmol. 2017;17(1):189. doi: 10.1186/s12886-017-0586-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanhart J, Comaneshter DS, Vinker S. Mortality after a cerebrovascular event in age-related macular degeneration patients treated with bevacizumab ocular injections. Acta Ophthalmol. 2018;96(6):e732-e739. doi: 10.1111/aos.13731 [DOI] [PubMed] [Google Scholar]
- 13.Hanhart J, Comaneshter DS, Freier-Dror Y, Vinker S. Mortality associated with bevacizumab intravitreal injections in age-related macular degeneration patients after acute myocardial infarct: a retrospective population-based survival analysis. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):651-663. doi: 10.1007/s00417-018-3917-9 [DOI] [PubMed] [Google Scholar]
- 14.Thulliez M, Angoulvant D, Le Lez ML, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta-analysis. JAMA Ophthalmol. 2014;132(11):1317-1326. doi: 10.1001/jamaophthalmol.2014.2333 [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Deeks JJ, Altman DG, eds. Special topics in statistics. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. https://handbook-5-1.cochrane.org/index.htm#front_page.htm. Updated March 2011. Accessed April 29, 2019.
- 17.Higgins JPT, Altman DG, Sterne JAC; Cochrane Statistical Methods Group Assessing risk of bias in included studies. In: Higgins JPT, Green S, Chandler J, et al; Cochrane Bias Methods Group, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. https://handbook-5-1.cochrane.org/index.htm#front_page.htm. Updated March 2011. Accessed April 29, 2019.
- 18.Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26(1):53-77. doi: 10.1002/sim.2528 [DOI] [PubMed] [Google Scholar]
- 19.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modeling framework: concepts, structure, and extensibility. Stat Comput. 2000;10(4):325-337. doi: 10.1023/A:1008929526011 [DOI] [Google Scholar]
- 20.Nguyen QD, Shah SM, Heier JS, et al. ; READ-2 Study Group . Primary end point (six months) results of the ranibizumab for Edema of the Macula in Diabetes (READ-2) study. Ophthalmology. 2009;116(11):2175-2181.e1. doi: 10.1016/j.ophtha.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 21.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399-2405. doi: 10.2337/dc10-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen QD, Brown DM, Marcus DM, et al. ; RISE and RIDE Research Group . Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. doi: 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 23.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. ; RESTORE study group . The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615-625. doi: 10.1016/j.ophtha.2011.01.031 [DOI] [PubMed] [Google Scholar]
- 24.Comyn O, Sivaprasad S, Peto T, et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study). Am J Ophthalmol. 2014;157(5):960-970. doi: 10.1016/j.ajo.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi T, Li X, Koh A, et al. ; REVEAL Study Group . The REVEAL Study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology. 2015;122(7):1402-1415. doi: 10.1016/j.ophtha.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 26.Berger A, Sheidow T, Cruess AF, Arbour JD, Courseau AS, de Takacsy F. Efficacy/safety of ranibizumab monotherapy or with laser versus laser monotherapy in DME. Can J Ophthalmol. 2015;50(3):209-216. doi: 10.1016/j.jcjo.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 27.Callanan DG, Loewenstein A, Patel SS, et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):463-473. doi: 10.1007/s00417-016-3472-1 [DOI] [PubMed] [Google Scholar]
- 28.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145(2):239-248. doi: 10.1016/j.ajo.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 29.Antoszyk AN, Tuomi L, Chung CY, Singh A; FOCUS Study Group . Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol. 2008;145(5):862-874. doi: 10.1016/j.ajo.2007.12.029 [DOI] [PubMed] [Google Scholar]
- 30.Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination withH ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32(8):1453-1464. doi: 10.1097/IAE.0b013e31824f91e8 [DOI] [PubMed] [Google Scholar]
- 31.Kinge B, Stordahl PB, Forsaa V, et al. Efficacy of ranibizumab in patients with macular edema secondary to central retinal vein occlusion: results from the sham-controlled ROCC study. Am J Ophthalmol. 2010;150(3):310-314. doi: 10.1016/j.ajo.2010.03.028 [DOI] [PubMed] [Google Scholar]
- 32.Pielen A, Mirshahi A, Feltgen N, et al. ; RABAMES Study Group . Ranibizumab for Branch Retinal Vein Occlusion Associated Macular Edema Study (RABAMES): six-month results of a prospective randomized clinical trial. Acta Ophthalmol. 2015;93(1):e29-e37. doi: 10.1111/aos.12488 [DOI] [PubMed] [Google Scholar]
- 33.Hattenbach LO, Feltgen N, Bertelmann T, et al. ; COMRADE-B Study Group . Head-to-head comparison of ranibizumab PRN versus single-dose dexamethasone for branch retinal vein occlusion (COMRADE-B). Acta Ophthalmol. 2018;96(1):e10-e18. doi: 10.1111/aos.13381 [DOI] [PubMed] [Google Scholar]
- 34.Scott IU, Edwards AR, Beck RW, et al. ; Diabetic Retinopathy Clinical Research Network . A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860-1867. doi: 10.1016/j.ophtha.2007.05.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriechbaum K, Prager S, Mylonas G, et al. ; Diabetic Retinopathy Research Group . Intravitreal bevacizumab (Avastin) versus triamcinolone (Volon A) for treatment of diabetic macular edema: one-year results. Eye (Lond). 2014;28(1):9-15. doi: 10.1038/eye.2013.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacu S, Michels S, Prager F, et al. Randomised clinical trial of intravitreal Avastin vs photodynamic therapy and intravitreal triamcinolone: long-term results. Eye (Lond). 2009;23(12):2223-2227. doi: 10.1038/eye.2008.423 [DOI] [PubMed] [Google Scholar]
- 37.Higashiyama T, Sawada O, Kakinoki M, Sawada T, Kawamura H, Ohji M. Prospective comparisons of intravitreal injections of triamcinolone acetonide and bevacizumab for macular oedema due to branch retinal vein occlusion. Acta Ophthalmol. 2013;91(4):318-324. doi: 10.1111/j.1755-3768.2011.02298.x [DOI] [PubMed] [Google Scholar]
- 38.Ramezani A, Esfandiari H, Entezari M, et al. Three intravitreal bevacizumab versus two intravitreal triamcinolone injections in recent-onset branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2012;250(8):1149-1160. doi: 10.1007/s00417-012-1941-8 [DOI] [PubMed] [Google Scholar]
- 39.Epstein DL, Algvere PV, von Wendt G, Seregard S, Kvanta A. Bevacizumab for macular edema in central retinal vein occlusion: a prospective, randomized, double-masked clinical study. Ophthalmology. 2012;119(6):1184-1189. doi: 10.1016/j.ophtha.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 40.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122(10):2044-2052. doi: 10.1016/j.ophtha.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 41.Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123(11):2376-2385. doi: 10.1016/j.ophtha.2016.07.032 [DOI] [PubMed] [Google Scholar]
- 42.Boyer D, Heier J, Brown DM, et al. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119(5):1024-1032. doi: 10.1016/j.ophtha.2012.01.042 [DOI] [PubMed] [Google Scholar]
- 43.Korobelnik JF, Holz FG, Roider J, et al. ; GALILEO Study Group . Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO study. Ophthalmology. 2014;121(1):202-208. doi: 10.1016/j.ophtha.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 44.Tadayoni R, Waldstein SM, Boscia F, et al. ; BRIGHTER study group . Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: six-month results of BRIGHTER. Ophthalmology. 2016;123(6):1332-1344. doi: 10.1016/j.ophtha.2016.02.030 [DOI] [PubMed] [Google Scholar]
- 45.Clark WL, Boyer DS, Heier JS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology. 2016;123(2):330-336. doi: 10.1016/j.ophtha.2015.09.035 [DOI] [PubMed] [Google Scholar]
- 46.Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR; VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group . Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805-2816. doi: 10.1056/NEJMoa042760 [DOI] [PubMed] [Google Scholar]
- 47.Cunningham ET Jr, Adamis AP, Altaweel M, et al. ; Macugen Diabetic Retinopathy Study Group . A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112(10):1747-1757. doi: 10.1016/j.ophtha.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 48.Wroblewski JJ, Wells JA III, Adamis AP, et al. ; Pegaptanib in Central Retinal Vein Occlusion Study Group . Pegaptanib sodium for macular edema secondary to central retinal vein occlusion. Arch Ophthalmol. 2009;127(4):374-380. doi: 10.1001/archophthalmol.2009.14 [DOI] [PubMed] [Google Scholar]
- 49.Do DV, Nguyen QD, Boyer D, et al. ; da Vinci Study Group . One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119(8):1658-1665. doi: 10.1016/j.ophtha.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 50.Baker CW, Glassman AR, Beaulieu WT, et al. ; DRCR Retina Network . Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880-1894. doi: 10.1001/jama.2019.5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmucker C, Loke YK, Ehlken C, et al. Intravitreal bevacizumab (Avastin) versus ranibizumab (Lucentis) for the treatment of age-related macular degeneration: a safety review. Br J Ophthalmol. 2011;95(3):308-317. doi: 10.1136/bjo.2009.178574 [DOI] [PubMed] [Google Scholar]
- 52.Antiplatelet Trialists’ Collaboration Collaborative overview of randomised trials of antiplatelet therapy, I: prevention of death, myocardial infarction, and strokes by prolonged antiplatelet therapy in various categories of patients [published correction appears in BMJ. 1994;308(6943):1540]. BMJ. 1994;308:81-106. [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng J, Pullenayegum E, Marshall JK, Iorio A, Thabane L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ Open. 2016;6(8):e010983. doi: 10.1136/bmjopen-2015-010983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229-234. doi: 10.1056/NEJM199807233390404 [DOI] [PubMed] [Google Scholar]
- 55.Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2017;6:CD007419. doi: 10.1002/14651858.CD007419.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev. 2014;(10):CD007419. doi: 10.1002/14651858.CD007419.pub4 [DOI] [PubMed] [Google Scholar]
- 57.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289(4):454-465. doi: 10.1001/jama.289.4.454 [DOI] [PubMed] [Google Scholar]
- 58.Fallico M, Russo A, Longo A, et al. Internal limiting membrane peeling versus no peeling during primary vitrectomy for rhegmatogenous retinal detachment: a systematic review and meta-analysis. PLoS One. 2018;13(7):e0201010. doi: 10.1371/journal.pone.0201010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. PRISMA Checklist
eTable 2. Search Strategy
eFigure 1. Risk of Bias Graph
eFigure 2. Risk of Bias Summary