Key Points
Question
What are the characteristics of and risk factors associated with diagnosed and undiagnosed symptomatic dry eye?
Findings
In this cross-sectional study using crowdsourced data on 4454 participants, risk factors for symptomatic vs no symptomatic dry eye included younger age, female sex, pollinosis, mental illnesses, current contact lens use, extended computer and digital device screen exposure, and smoking. For individuals with undiagnosed vs diagnosed symptomatic dry eye, risk factors included younger age, male sex, absence of collagen disease, mental illnesses, ophthalmic surgery, and current and past contact lens use.
Meaning
These results suggest that detecting undiagnosed, symptomatic dry eye in at-risk populations could lead to earlier prevention or more effective interventions.
Abstract
Importance
The incidence of dry eye disease has increased; the potential for crowdsource data to help identify undiagnosed dry eye in symptomatic individuals remains unknown.
Objective
To assess the characteristics and risk factors associated with diagnosed and undiagnosed symptomatic dry eye using the smartphone app DryEyeRhythm.
Design, Setting, and Participants
A cross-sectional study using crowdsourced data was conducted including individuals in Japan who downloaded DryEyeRhythm and completed the entire questionnaire; duplicate users were excluded. DryEyeRhythm was released on November 2, 2016; the study was conducted from November 2, 2016, to January 12, 2018.
Exposures
DryEyeRhythm data were collected on demographics, medical history, lifestyle, subjective symptoms, and disease-specific symptoms, using the Ocular Surface Disease Index (100-point scale; scores 0-12 indicate normal, healthy eyes; 13-22, mild dry eye; 23-32, moderate dry eye; 33-100, severe dry eye symptoms), and the Zung Self-Rating Depression Scale (total of 20 items, total score ranging from 20-80, with ≥40 highly suggestive of depression).
Main Outcomes and Measures
Multivariate-adjusted logistic regression analysis was used to identify risk factors for symptomatic dry eye and to identify risk factors for undiagnosed symptomatic dry eye.
Results
A total of 21 394 records were identified in our database; 4454 users, included 899 participants (27.3%) with diagnosed and 2395 participants (72.7%) with undiagnosed symptomatic dry eye, completed all questionnaires and their data were analyzed. A total of 2972 participants (66.7%) were women; mean (SD) age was 27.9 (12.6) years. The identified risk factors for symptomatic vs no symptomatic dry eye included younger age (odds ratio [OR], 0.99; 95% CI, 0.987-0.999, P = .02), female sex (OR, 1.99; 95% CI, 1.61-2.46; P < .001), pollinosis (termed hay fever on the questionnaire) (OR, 1.35; 95% CI, 1.18-1.55; P < .001), depression (OR, 1.78; 95% CI, 1.18-2.69; P = .006), mental illnesses other than depression or schizophrenia (OR, 1.87; 95% CI, 1.24-2.82; P = .003), current contact lens use (OR, 1.27; 95% CI, 1.09-1.48; P = .002), extended screen exposure (OR, 1.55; 95% CI, 1.25-1.91; P < .001), and smoking (OR, 1.65; 95% CI, 1.37-1.98; P < .001). The risk factors for undiagnosed vs diagnosed symptomatic dry eye included younger age (OR, 0.96; 95% CI, 0.95-0.97; P < .001), male sex (OR, 0.55; 95% CI, 0.42-0.72; P < .001), as well as absence of collagen disease (OR, 95% CI, 0.23; 0.09-0.60; P = .003), mental illnesses other than depression or schizophrenia (OR, 0.50; 95% CI, 0.36-0.69; P < .001), ophthalmic surgery other than cataract surgery and laser-assisted in situ keratomileusis (OR, 0.41; 95% CI, 0.27-0.64; P < .001), and current (OR, 0.64; 95% CI, 0.54-0.77; P < .001) or past (OR, 0.45; 95% CI, 0.34-0.58; P < .001) contact lens use.
Conclusions and Relevance
This study’s findings suggest that crowdsourced research identified individuals with diagnosed and undiagnosed symptomatic dry eye and the associated risk factors. These findings could play a role in earlier prevention or more effective interventions for dry eye disease.
This cross-sectional study evaluates the incidence of symptomatic vs no symptomatic and diagnosed vs undiagnosed dry eye disease throughout Japan.
Introduction
Dry eye disease is the most common ocular disease worldwide1 and is becoming more prevalent with the aging society, increased digital device use, and an increasingly stressful social environment.2,3,4,5 This disease causes ocular discomfort, fatigue, and visual disturbances; interferes with the quality of life and vision; and reduces work productivity.6,7,8,9 However, dry eye disease may remain undiagnosed in many individuals who experience dry eye disease symptoms.10,11
The Internet of Medical Things amalgamation of medical devices that connect health care information technology through networks has been increasingly incorporated into clinical practice and daily life.12,13,14 The advent of big data and artificial intelligence is transforming clinical research through Internet of Medical Things functions by improving and simplifying participant recruitment, communication, real-time data acquisition, and cost-effectiveness.15 To identify dry eye disease and document the symptoms, medical history, and lifestyle habits associated with dry eye disease abnormalities, we designed the DryEyeRhythm application with Apple’s ResearchKit. The app was developed by Ohako Inc (Tokyo, Japan) under consignment contract with Juntendo University Faculty of Medicine, Department of Ophthalmology.11 Smartphone applications are expected to play a substantial role in self-help interventions for dry eye disease screening.
The aim of this study was to identify the characteristics and risk factors of symptomatic dry eye, defined as current evidence of dry eye disease based on an Ocular Surface Disease Index (OSDI), a dry eye–specific questionnaire (total score ≥13)16 among voluntary users of DryEyeRhythm. In addition, we aimed to identify the characteristics and risk factors of undiagnosed symptomatic dry eye (defined as current evidence of dry eye disease based on an OSDI total score ≥13 and no history of clinically diagnosed dry eye disease) compared with diagnosed symptomatic dry eye (defined as current evidence of dry eye disease based on an OSDI total score ≥13 and a history of clinically diagnosed dry eye disease).
Methods
Study Enrollment and Participants
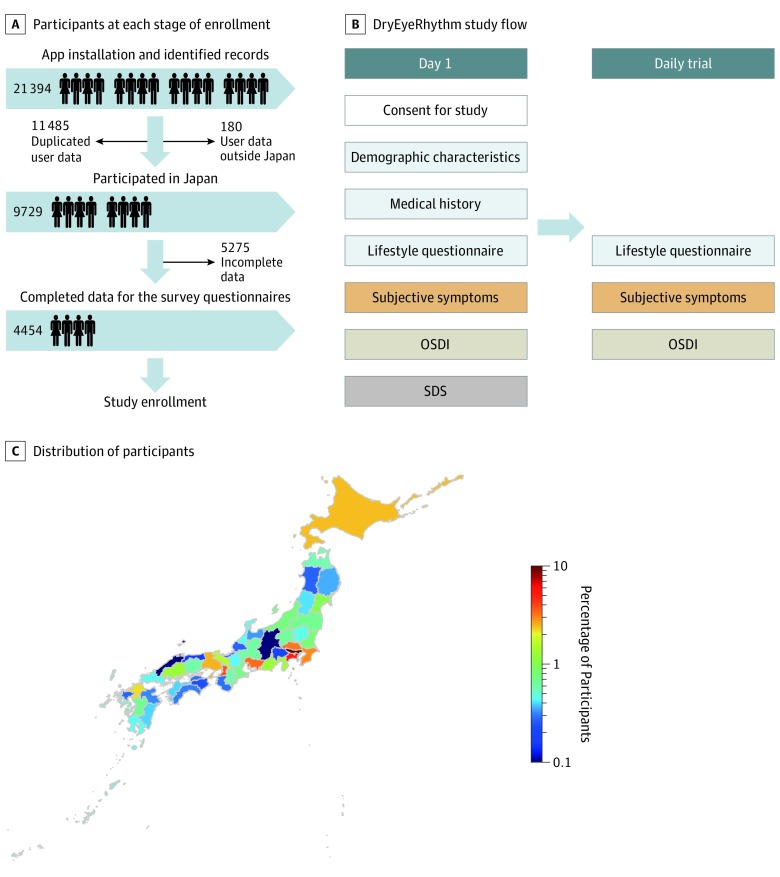
This cross-sectional crowdsourced research was conducted using DryEyeRhythm, a free smartphone application developed in Japanese and English using Apple’s ResearchKit, which was released in the Apple App Store in Japan in November 2016 and the United States in April 2018.11 Electronic informed consent for participation was obtained from all users. Figure, A shows the study cohort description. The study included participants who downloaded and used DryEyeRhythm in Japan and completed the entire questionnaire (Figure, A); the app is free and there was no financial compensation. Duplicate users were excluded. This study was conducted with approval from the independent ethics committee of Juntendo University Faculty of Medicine and adhered to the tenets of the Declaration of Helsinki.17
Figure. DryEyeRhythm Study Cohort Description.
A, Number of participants at each stage of study enrollment; B, Flowchart of the study and tasks comprising the smartphone application DryEyeRhythm; C, Geographic distribution of the participants in Japan. The percentages of the participants in this study are displayed as colors in the map. OSDI indicates Ocular Surface Disease Index (100-point scale; scores 0-12 indicate normal, healthy eyes; 13-22, mild dry eye; 23-32, moderate dry eye; 33-100, severe dry eye symptoms); SDS, Zung Self-Rating Depression Scale (total of 20 items, total score ranging from 20-80, with ≥40 highly suggestive of depression).
Data Collection
DryEyeRhythm collected a range of study components (Figure, B), including participant demographic characteristics, medical history, and lifestyle information (eTable 1 in the Supplement). Participants also reported daily subjective symptoms (eTable 2 in the Supplement) and completed disease-specific questionnaires, including the OSDI for dry eye disease and the Zung Self-Rating Depression Scale (SDS; total of 20 items, total score ranging from 20-80, with ≥40 highly suggestive of depression) for depression.18 Daily subjective symptoms, including stress level, headache, and eye itching, were collected using a 10-point visual analog scale (0, none; 10, most severe symptoms). Figure, B shows the flow of the study participants and tasks included in the DryEyeRhythm questionnaire. After electronically written informed consent was obtained, the participants completed the questionnaires in the following order: demographic characteristics, medical history, lifestyle questionnaire, daily subjective symptoms, OSDI, and SDS.
Symptomatic Dry Eye and Depressive Symptom Ascertainment
Dry eye subjective symptoms were assessed using the OSDI questionnaire, a 12-item questionnaire that evaluates the severity of the dry eye symptoms on the basis of ocular symptoms, visual functioning, and environmental triggers.19 The OSDI total score was determined based on a 100-point scale correlated with the severity of symptoms, with scores 0 to 12 representing normal, healthy eyes; 13 to 22, mild dry eye; 23 to 32, moderate dry eye; and 33 to 100, severe dry eye symptoms.16 The DryEyeRhythm-based OSDI questionnaire was validated based on the paper-based OSDI questionnaire as previously described.11
Depressive symptoms were evaluated using the SDS,18 which is an internationally used 20-item self-rating depression scale validated in Japan.20,21 Each item is rated on a 4-point Likert scale, with a total score ranging from 20 to 80. An SDS score of 40 or above is highly suggestive of depression.
First, we classified the study participants into 2 groups: no symptomatic dry eye (defined as an OSDI total score <13) and symptomatic dry eye (defined as an OSDI total score ≥13) groups. Then, the symptomatic dry eye group was further divided into those having diagnosed and undiagnosed symptomatic dry eye (eTable 3 in the Supplement). Those who had an OSDI total score of 13 or above and reported yes for a history of clinically diagnosed dry eye disease were included in the diagnosed symptomatic dry eye group. Those who had an OSDI total score of 13 or above and reported no for a history of clinically diagnosed dry eye disease were included in the undiagnosed symptomatic dry eye group.
Statistical Analysis
We compared patient characteristics between individuals with and without symptomatic dry eye and between individuals with diagnosed and undiagnosed symptomatic dry eye. Continuous variables are presented as median (interquartile range [IQR]) for factors not normally distributed, based on Shapiro-Wilk tests, and categorical variables are presented as a percentage. We conducted Mann-Whitney tests for continuous variables not normally distributed and χ2 tests for categorical variables. In addition to the unadjusted P values, we calculated the false discovery rate–adjusted P values (ie, Q values) to account for multiple comparisons.22
Next, we conducted multivariable-adjusted logistic regression analyses to identify risk factors for symptomatic vs no symptomatic dry eye, using data of all study participants, and risk factors for participants who reported undiagnosed vs diagnosed symptomatic dry eye. In the multivariable-adjusted logistic regression models, we included factors that were significantly associated with the outcome (ie, any symptomatic dry eye and undiagnosed symptomatic dry eye) in the univariable logistic regression analyses, based on a threshold 2-tailed, unpaired P value of .05.
We conducted a post hoc power calculation. It was expected that we could identify risk factors for symptomatic (n = 3294) vs no symptomatic dry eye (n = 1160) with an odds ratio (OR) of 1.31 or greater or 0.72 or less and risk for undiagnosed (n = 2395) vs diagnosed symptomatic dry eye (n = 899) with an OR of 1.36 or greater or 0.68 or less, assuming an α error of .05, power of 80%, and risk factor prevalence in the comparison group of 10%.
All data were analyzed with Stata, version 15 (StataCorp). In addition, a heatmap for the geographic distribution of the participants in Japan was constructed using the heatmap function of the matplotlib module (Python 3, version 0.9.0; Python Software Foundation). The study was conducted from November 2, 2016, to January 12, 2018.
Results
Application Downloads and Study Enrollment
Figure, A shows the study cohort description. DryEyeRhythm was downloaded 18 991 times in Japan and the United States between November 2, 2016, and January 12, 2018; 21 394 records were identified in our database and 11 485 records were excluded because of duplicate user data, 180 records of data from users outside of Japan, and 5275 incomplete data records (45.9% [4454 of 9729] users completed the survey questionnaires). A total of 4454 participants (2972 [66.7%] women; mean [SD] age, 27.9 [12.6] years) were enrolled following the completion of the entire questionnaire (Figure, B). Only 4.2% of the participants were older than 60 years. This study cohort includes data from participants across Japan (Figure, C).
Participant Characteristics
Table 1 presents the demographics, medical history, and lifestyle habits of 3294 individuals (74.0%) with and 1160 individuals (26.0%) without symptomatic dry eye. Individuals with symptomatic dry eye were younger (median [IQR], 23.0 years [18-34] vs 25.5 [18-38] years) and more likely to be women (2347 [71.3%] vs 625 [53.9%]). Height (median [IQR], 160 cm [156-167] vs 164 [158-170] cm), body weight (median [IQR], 55 [49-64] kg vs 58 [50-67] kg), and body mass index (median [IQR], 21.1 [19.2-23.4] vs 21.5 [19.5-23.9], calculated as weight in kilograms divided by height in meters squared) were lower in the symptomatic dry eye group, probably owing to the higher prevalence of women in this group. In medical history, pollinosis (termed hay fever on the questionnaire) (1726 [52.4%] vs 523 [45.1%]), depression (151 [4.6%] vs 30 [2.6%]), and mental illnesses other than depression and schizophrenia (181 [5.5%] vs 29 [2.5%]) were more common in individuals with than in those without symptomatic dry eye. In lifestyle habits, participants with symptomatic dry eye were more likely to report current contact lens use (1447 [43.9%] vs 397 (34.2%]), and extended computer or electronic device screen exposure (median [IQR], 6 [4-10] vs 6 [4-9] hours per day) and smoking (817 [24.8%] vs 241 [20.8%]). The prevalence of eyedrop use was significantly different between participants with (737 [22.4%]) and without (140 [12.1%]) symptomatic dry eye (P < .001). All measured subjective symptoms were significantly worse in participants with than in those without symptomatic dry eye, for example, stress (median [IQR], 5 [3-7] vs 4 [2-6] on a 10-point visual analog scale; P < .001).
Table 1. Characteristics of Patients With and Without Symptomatic Dry Eye.
| Characteristic | Symptomatic Dry Eye, No. (%) | P Value | Q Value | |
|---|---|---|---|---|
| Without (n = 1160) | With (n = 3294) | |||
| Age, median (IQR), y | 25.5 (18-38) | 23.0 (18-34) | <.001 | .001 |
| Age category, y | ||||
| <20 | 353 (30.4) | 1145 (34.8) | <.001 | .001 |
| 20-30 | 331 (28.5) | 1100 (33.4) | ||
| 30-40 | 208 (17.9) | 439 (13.3) | ||
| 40-50 | 155 (13.4) | 365 (11.1) | ||
| 50-60 | 85 (7.3) | 185 (5.6) | ||
| ≥60 | 28 (2.4) | 60 (1.8) | ||
| Women | 625 (53.9) | 2347 (71.3) | <.001 | <.001 |
| Height, median (IQR), cm | 164 (158-170) | 160 (156-167) | <.001 | <.001 |
| Body weight, median (IQR), kg | 58 (50-67) | 55 (49-64) | <.001 | <.001 |
| BMI, median (IQR) | 21.5 (19.5-23.9) | 21.1 (19.2-23.4) | <.001 | .001 |
| Medical survey | ||||
| Medicated hypertension | 42 (3.6) | 95 (2.9) | .55 | .27 |
| Diabetes | 21 (1.8) | 41 (1.2) | .16 | .22 |
| Systemic diseases | ||||
| Blood disease | 7 (0.6) | 25 (0.8) | .59 | .65 |
| Brain disease | 8 (0.7) | 23 (0.7) | .98 | .98 |
| Collagen disease | 6 (0.5) | 25 (0.8) | .39 | .44 |
| Heart disease | 14 (1.2) | 55 (1.7) | .27 | .33 |
| Kidney disease | 12 (1.0) | 48 (1.5) | .28 | .34 |
| Liver disease | 12 (1.0) | 33 (1.0) | .92 | .95 |
| Malignant tumor | 6 (0.5) | 21 (0.6) | .65 | .70 |
| Respiratory tract disease | 60 (5.2) | 216 (6.6) | .09 | .13 |
| Pollinosisa | 523 (45.1) | 1726 (52.4) | <.001 | <.001 |
| Mental illness | ||||
| Depression | 30 (2.6) | 151 (4.6) | .003 | .005 |
| Schizophrenia | 9 (0.8) | 27 (0.8) | .89 | .93 |
| Other | 29 (2.5) | 181 (5.5) | <.001 | <.001 |
| Ophthalmic surgery | ||||
| Cataract surgery | 9 (0.8) | 11 (0.3) | .05 | .09 |
| LASIK | 21 (1.8) | 41 (1.2) | .16 | .22 |
| Others | 26 (2.2) | 90 (2.7) | .37 | .42 |
| Lifestyle habits | ||||
| Coffee intake, median (IQR), cups/d | 0 (0-2) | 0 (0-1) | .27 | .33 |
| Contact lens use | ||||
| Negative | 633 (54.6) | 1513 (45.9) | <.001 | <.001 |
| Current use | 397 (34.2) | 1447 (43.9) | ||
| Past use | 130 (11.2) | 334 (10.1) | ||
| Eyedrop use | 140 (12.1) | 737 (22.4) | <.001 | <.001 |
| Screen exposure, median (IQR), h/d | 6 (4-9) | 6 (4-10) | <.001 | <.001 |
| Periodic exercise | 786 (67.8) | 2133 (64.8) | .06 | .10 |
| Median (IQR), h/wk | 1 (0-4) | 1 (0-3) | .07 | .11 |
| Sleep time, median (IQR), h/d | 7.0 (6.0-8.5) | 7.0 (6.0-8.5) | .16 | .21 |
| Smoking | 241 (20.8) | 817 (24.8) | .006 | .01 |
| Water intake, median (IQR), mL/d | 800 (400-1000) | 800 (400-1000) | .03 | .06 |
| Daily subjective symptoms, median (IQR)b | ||||
| Stress (0-10) | 4 (2-6) | 5 (3-7) | <.001 | <.001 |
| Headache (0-10) | 0 (0-2) | 1 (0-4) | <.001 | <.001 |
| Eye itching (0-10) | 0 (0-2) | 2 (0-5) | <.001 | <.001 |
| Asthenopia, yes | 449 (38.7) | 2144 (65.1) | <.001 | <.001 |
| Stiffness, yes | 444 (38.3) | 1796 (54.5) | <.001 | <.001 |
| Mental fatigue, yes | 190 (16.4) | 997 (30.3) | <.001 | <.001 |
| OSDI total score, median (IQR)c | 8.3 (4.2-10.4) | 29.2 (20.8-41.7) | <.001 | <.001 |
| Ocular symptoms | 10 (5-15) | 35 (25-45) | <.001 | <.001 |
| Vision-related function | 0 (0-6.3) | 16.7 (6.3-31.3) | <.001 | <.001 |
| Environmental triggers | 0 (0-16.7) | 33.3 (16.7-58.3) | <.001 | <.001 |
| OSDI total score categoryc | ||||
| 0-12 | 1160 (100) | 0 | <.001 | <.001 |
| 13-22 | 0 | 1139 (34.6) | ||
| 23-32 | 0 | 778 (23.6) | ||
| 33-100 | 0 | 1377 (41.8) | ||
| SDS score, median (IQR)d | 42 (35-49) | 48 (41-55) | <.001 | <.001 |
| SDS score >40d | 675 (58.2) | 2596 (78.8) | <.001 | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; LASIK, laser-assisted in situ keratomileusis; OSDI, Ocular Surface Disease Index; SDS, the Zung Self-Rating Depression Scale.
Termed hay fever on the questionnaire.
Measured on a visual analog scale, with 0 indicating none and 10 indicating the most severe symptoms.
Twelve-item questionnaire evaluating severity of dry eye symptoms, with the total score determined on a 100-point scale: 0 to 12, normal, healthy eyes; 13 to 22, mild dry eye disease (dry eye disease); 23 to 32, moderate dry eye disease; 33 to 100, severe dry eye disease.
Twenty-item self-rating depression scale, with each item rated on a 4-point Likert scale; total score ranges from 20 to 80. A score of 40 or above is highly suggestive of depression.
Table 2 reports the characteristics of the 899 participants (27.3%) with diagnosed and 2395 participants (72.7%) with undiagnosed symptomatic dry eye. Individuals with undiagnosed symptomatic dry eye were younger (median [IQR], 22 [18-31] vs 28 [20-42] years) and less likely to be women (1663 [69.4%] vs 684 [76.1%]) than those with diagnosed symptomatic dry eye were (Table 2; eFigure in the Supplement). Diabetes (23 [1.0%] vs 18 [2.0%]), blood disease (13 [0.5%] vs 12 [1.3%]), collagen disease (6 [0.3%] vs 19 [2.1%]), malignant tumor (8 [0.3%] vs 13 [1.4%]), depression (96 [4.0%] vs 55 [6.1%]), mental illnesses other than depression and schizophrenia (101 [4.2%] vs 80 [8.9%]), and history of laser-assisted in situ keratomileusis (22 [0.9%] vs 19 [2.1%]) and other ophthalmic surgery (43 [1.8%] vs 47 [5.2%]) were less frequently reported by individuals with undiagnosed symptomatic dry eye than in those with diagnosed symptomatic dry eye. In lifestyle habits, individuals with undiagnosed symptomatic dry eye reported less coffee intake (median [IQR], 0 cups per day [0-1] vs 0 [0-2] cups per day), more frequent periodic exercise (1586 [66.2%] vs 547 [60.8%]), and longer sleeping time (median [IQR], 7.2 [6.0-8.6] hours per day vs 7.0 [6.0-8.0] hours per day). Proportions of current (1030 [43.0%] vs 417 [46.4%]) and past (188 [7.8%] vs 146 [16.2%]) contact lens use were lower in individuals with undiagnosed symptomatic dry eye. The prevalence of eyedrop use was significantly different between individuals with diagnosed (360 [40.0%]) and undiagnosed (377 [15.7%]) symptomatic dry eye. Most measured daily subjective symptoms (except headache, eye itching, and SDS score) were worse in people with diagnosed symptomatic dry eye (eg, mental fatigue: 321 [35.7%] vs 676 [28.2%]).
Table 2. Characteristics of Patients With Diagnosed and Undiagnosed Symptomatic Dry Eye.
| Characteristic | Symptomatic Dry Eye, No. (%) | P Value | Q Value | |
|---|---|---|---|---|
| Diagnosed (n = 899 [27.3%]) | Undiagnosed (n = 2395 [72.7%]) | |||
| Age, median (IQR), y | 28 (20-42) | 22 (18-31) | <.001 | <.001 |
| Age category, y | ||||
| <20 | 198 (22.0) | 947 (39.5) | <.001 | <.001 |
| 20-30 | 294 (32.7) | 806 (33.7) | ||
| 30-40 | 157 (17.5) | 282 (11.8) | ||
| 40-50 | 145 (16.1) | 220 (9.2) | ||
| 50-60 | 78 (8.7) | 107 (4.5) | ||
| ≥60 | 27 (3.0) | 33 (1.4) | ||
| Women | 684 (76.1) | 1663 (69.4) | <.001 | <.001 |
| Height, median (IQR), cm | 160 (156-173) | 160 (156-168) | .05 | .08 |
| Weight, median (IQR), kg | 55 (49-64) | 55 (48-63) | .70 | .73 |
| BMI, median (IQR) | 21.3 (19.4-23.5) | 21.0 (19.2-23.4) | .048 | .08 |
| Medical history | ||||
| Medicated hypertension | 32 (3.6) | 63 (2.6) | .16 | .21 |
| Diabetes | 18 (2.0) | 23 (1.0) | .02 | .03 |
| Systemic diseases | ||||
| Blood disease | 12 (1.3) | 13 (0.5) | .02 | .03 |
| Brain disease | 10 (1.1) | 13 (0.5) | .08 | .12 |
| Collagen disease | 19 (2.1) | 6 (0.3) | <.001 | <.001 |
| Heart disease | 18 (2.0) | 37 (1.5) | .36 | .41 |
| Kidney disease | 13 (1.5) | 35 (1.5) | .97 | .97 |
| Liver disease | 10 (1.1) | 23 (1.0) | .70 | .71 |
| Malignant tumor | 13 (1.4) | 8 (0.3) | <.001 | <.001 |
| Respiratory tract disease | 71 (7.9) | 145 (6.1) | .06 | .09 |
| Pollinosisa | 477 (53.1) | 1249 (52.2) | .64 | .69 |
| Mental illness | ||||
| Depression | 55 (6.1) | 96 (4.0) | .01 | .02 |
| Schizophrenia | 4 (0.4) | 23 (1.0) | .14 | .20 |
| Others | 80 (8.9) | 101 (4.2) | <.001 | <.001 |
| Ophthalmic surgery | ||||
| Cataract surgery | 4 (0.4) | 7 (0.3) | .50 | .55 |
| LASIK | 19 (2.1) | 22 (0.9) | .006 | .01 |
| Other | 47 (5.2) | 43 (1.8) | <.001 | <.001 |
| Lifestyle habits | ||||
| Coffee intake, median (IQR), cups/d | 0 (0-2) | 0 (0-1) | <.001 | .002 |
| Contact lens use | ||||
| Never | 336 (37.4) | 1177 (49.1) | <.001 | <.001 |
| Current | 417 (46.4) | 1030 (43.0) | ||
| Past | 146 (16.2) | 188 (7.8) | ||
| Eyedrop use | 360 (40.0) | 377 (15.7) | <.001 | <.001 |
| Screen exposure, median (IQR), h/d | 6 (4-10) | 6 (4-10) | .19 | .23 |
| Periodic exercise | 547 (60.8) | 1586 (66.2) | .004 | .009 |
| Median (IQR), h/wk | 1 (0-3) | 1 (0-3) | <.001 | .002 |
| Sleeping time, median (IQR), h/d | 7.0 (6.0-8.0) | 7.2 (6.0-8.6) | .003 | .007 |
| Smoking | 238 (26.5) | 579 (24.2) | .17 | .23 |
| Water intake, median (IQR), mL/d | 800 (400-1000) | 800 (400-1000) | .19 | .23 |
| Daily subjective symptoms, median (IQR) | ||||
| Stress (0-10) | 5 (3-7) | 5 (3-7) | .006 | .01 |
| Headache (0-10) | 1 (0-4) | 1 (0-4) | .18 | .23 |
| Eye itching (0-10) | 2 (0-5) | 2 (0-5) | .18 | .22 |
| Asthenopia, yes | 672 (74.7) | 1472 (61.5) | <.001 | <.001 |
| Stiffness, yes | 564 (62.7) | 1232 (51.4) | <.001 | <.001 |
| Mental fatigue, yes | 321 (35.7) | 676 (28.2) | <.001 | <.001 |
| J-OSDI total score, median (IQR)b | 34.1 (25.0-47.9) | 27.3 (19.4-39.6) | <.001 | <.001 |
| Ocular symptoms | 35 (25-50) | 35 (25-45) | <.001 | <.001 |
| Vision-related function | 18.8 (6.3-37.5) | 12.5 (6.3-25.0) | <.001 | <.001 |
| Environmental triggers | 50.0 (25.0-75.0) | 33.3 (16.7-58.3) | <.001 | <.001 |
| OSDI total score categoryb | ||||
| 0-12 | NA | NA | <.001 | <.001 |
| 13-22 | 217 (24.1) | 922 (38.5) | ||
| 23-32 | 199 (22.1) | 579 (24.2) | ||
| 33-100 | 483 (53.7) | 894 (37.3) | ||
| SDS score [0-80], median (IQR)c | 33.3 (16.7-58.3) | 48.0 (41.0-55.0) | .21 | .24 |
| SDS score >40c | 692 (77.0) | 1904 (79.5) | .11 | .17 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; LASIK, laser-assisted in situ keratomileusis; OSDI, Ocular Surface Disease Index; SDS, the Zung Self-Rating Depression Scale.
Termed hay fever on the questionnaire.
Twelve-item questionnaire evaluating severity of dry eye symptoms, with the total score determined on a 100-point scale: 0 to 12, normal, healthy eyes; 13 to 22, mild dry eye disease (dry eye disease); 23 to 32, moderate dry eye disease; 33 to 100, severe dry eye disease.
Twenty-item self-rating depression scale, with each item rated on a 4-point Likert scale; total score ranges from 20 to 80. A score of 40 or above is highly suggestive of depression.
Risk Factors
Table 3 reports the results of multivariate-adjusted logistic regression analysis for symptomatic compared with no symptomatic dry eye. The multivariate-adjusted ORs of statistically significant factors for symptomatic dry eye were 0.99 (95% CI, 0.987-0.999, P = .02) for age, 1.99 (95% CI, 1.61-2.46, P < .001) for women, 1.35 (1.18-1.55, P < .001) for pollinosis, 1.78 (95% CI, 1.18-2.69, P = .006) for depression, 1.87 (95% CI, 1.24-2.82, P = .003) for mental illnesses other than depression or schizophrenia, 1.27 (95% CI, 1.09-1.48, P = .002) for current vs never use of contact lens, 1.55 (95% CI, 1.25-1.91, P < .001) for more than 8 vs less than 4 hours of screen exposure per day, and 1.65 (95% CI, 1.37-1.98, P < .001) for smoking.
Table 3. Risk Factors for Symptomatic Compared With No Symptomatic Dry Eye.
| Risk Factor | Symptomatic vs No Symptomatic Dry Eye | |||
|---|---|---|---|---|
| Univariate OR (95% CI) | P Value | Multivariate-Adjusted OR (95% CI) | P Value | |
| Demographic characteristics | ||||
| Age, every 1 y | 0.99 (0.98-0.99) | <.001 | 0.99 (0.99-0.10) | .02 |
| Sex (women vs men) | 2.12 (1.84-2.44) | <.001 | 1.99 (1.61-2.46) | <.001 |
| Height, cm | 0.97 (0.96-0.98) | <.001 | 1.00 (0.99-1.01) | .91 |
| Weight, kg | 0.98 (0.98-0.99) | <.001 | 1.00 (0.99-1.00) | .37 |
| Medical history | ||||
| Medicated hypertension (yes vs no) | 0.73 (0.47-1.12) | .16 | NA | NA |
| Diabetes (yes vs no) | 0.68 (0.40-1.16) | .16 | NA | NA |
| Systemic diseases (yes vs no) | ||||
| Blood disease | 1.26 (0.54-2.92) | .59 | NA | NA |
| Brain disease | 1.01 (0.45-2.27) | .98 | NA | NA |
| Collagen disease | 1.47 (0.60-3.59) | .40 | NA | NA |
| Heart disease | 1.39 (0.77-2.51) | .27 | NA | NA |
| Kidney disease | 1.41 (0.75-2.67) | .29 | NA | NA |
| Liver disease | 0.97 (0.50-1.88) | .92 | NA | NA |
| Malignant tumor | 1.23 (0.50-3.07) | .65 | NA | NA |
| Respiratory tract disease | 1.29 (0.96-1.73) | .09 | NA | NA |
| Pollinosis (yes vs no)a | 1.34 (1.17-1.53) | <.001 | 1.35 (1.18-1.55) | <.001 |
| Mental illness (yes vs no) | ||||
| Depression | 1.81 (1.22-2.69) | .003 | 1.78 (1.18-2.69) | .01 |
| Schizophrenia | 1.06 (0.50-2.25) | .89 | NA | NA |
| Other | 2.27 (1.52-3.38) | <.001 | 1.87 (1.24-2.82) | .003 |
| Ophthalmic surgery (yes vs no) | ||||
| Cataract surgery | 0.43 (0.18-1.04) | .06 | NA | NA |
| LASIK | 0.68 (0.40-1.16) | .16 | NA | NA |
| Others | 1.22 (0.79-1.91) | .37 | NA | NA |
| Lifestyle habits | ||||
| Coffee intake, every 1-cup/d increase | 0.99 (0.94-1.04) | .66 | NA | NA |
| Contact lens use | ||||
| Never | 1 [Reference] | NA | 1 [Reference] | NA |
| Current | 1.52 (1.32-1.76) | <.001 | 1.27 (1.09-1.48) | .002 |
| Past | 1.07 (0.86-1.34) | .53 | 1.03 (0.82-1.30) | .78 |
| Screen exposure, h | ||||
| <4 | 1 [Reference] | NA | 1 [Reference] | NA |
| 4-8 | 1.16 (0.97-1.39) | .09 | 1.15 (0.96-1.39) | .13 |
| >8 | 1.55 (1.27-1.90) | <.001 | 1.55 (1.25-1.91) | <.001 |
| Periodic exercise (yes vs no) | 0.87 (0.76-1.01) | .06 | NA | NA |
| Sleep time, every 1-h/day increase | 1.00 (0.98-1.02) | .77 | NA | NA |
| Smoking (yes vs no) | 1.26 (1.07-1.48) | .006 | 1.65 (1.37-1.98) | <.001 |
| Water intake, 100 mL/d | 0.98 (0.965-0.995) | .01 | 0.99 (0.97-1.01) | .19 |
Abbreviations: LASIK, laser in situ keratomileusis; NA, not applicable; OR, odds ratio.
Termed hay fever on the questionnaire.
Table 4 reports the results of multivariate-adjusted logistic regression analysis for undiagnosed compared with diagnosed symptomatic dry eye. The multivariate-adjusted ORs of statistically significant factors for undiagnosed symptomatic dry eye were 0.96 (95% CI, 0.95-0.97, P < .001) for age, 0.55 (95% CI, 0.42-0.72; P < .001) for women, 0.23 (95% CI, 0.09-0.60, P = .003) for collagen disease, 0.50 (95% CI, 0.36-0.69, P < .001) for mental illnesses other than depression or schizophrenia, 0.41 (95% CI, 0.27-0.64; P < .001), for ophthalmic surgery other than cataract surgery and laser-assisted in situ keratomileusis, 0.64 (95% CI, 0.54-0.77, P < .001) for current vs never contact lens use, and 0.45 (95% CI, 0.34-0.58, P < .001) for past vs never contact lens use.
Table 4. Risk Factors for Undiagnosed Compared With Diagnosed Symptomatic Dry Eye.
| Risk Factor | Undiagnosed vs Diagnosed Symptomatic Dry Eye | |||
|---|---|---|---|---|
| Univariate OR (95% CI) | P Value | Multivariate-Adjusted OR (95% CI) | P Value | |
| Demographic characteristics | ||||
| Age, every 1 y | 0.97 (0.96-0.97) | <.001 | 0.96 (0.95-0.97) | <.001 |
| Sex (women vs men) | 0.71 (0.60-0.85) | <.001 | 0.55 (0.42-0.72) | <.001 |
| Height, cm | 1.01 (1.00-1.02) | .02 | 1.00 (0.99-1.01) | >.99 |
| Weight, kg | 1.00 (0.99-1.01) | .92 | NA | NA |
| Medical history | ||||
| Medicated hypertension (yes vs no) | 0.79 (0.55-1.14) | .21 | NA | NA |
| Diabetes (yes vs no) | 0.47 (0.25-0.88) | .02 | 0.82 (0.42-1.63) | .57 |
| Systemic diseases (yes vs no) | ||||
| Blood disease | 0.40 (0.18-0.89) | .02 | 0.75 (0.31-1.82) | .52 |
| Brain disease | 0.49 (0.21-1.11) | .09 | NA | NA |
| Collagen disease | 0.12 (0.05-0.29) | <.001 | 0.23 (0.09-0.60) | .003 |
| Heart disease | 0.77 (0.43-1.36) | .36 | NA | NA |
| Kidney disease | 1.01 (0.53-1.92) | .97 | NA | NA |
| Liver disease | 0.86 (0.41-1.82) | .70 | NA | NA |
| Malignant tumor | 0.23 (0.09-0.55) | .001 | 0.46 (0.18-1.19) | .11 |
| Respiratory tract disease | 0.75 (0.56-1.01) | .06 | NA | NA |
| Pollinosis (yes vs no)a | 0.96 (0.83-1.12) | .64 | NA | NA |
| Mental illness (yes vs no) | ||||
| Depression | 0.64 (0.46-0.90) | .01 | 0.90 (0.62-1.31) | .59 |
| Schizophrenia | 2.17 (0.75-6.29) | .15 | NA | NA |
| Other | 0.45 (0.33-0.61) | <.001 | 0.50 (0.36-0.69) | <.001 |
| Ophthalmic surgery (yes vs no) | ||||
| Cataract surgery | 0.66 (0.19-2.25) | .50 | NA | NA |
| LASIK | 0.43 (0.23-0.80) | .007 | 0.84 (0.43-1.68) | .64 |
| Other | 0.33 (0.22-0.50) | <.001 | 0.41 (0.27-0.64) | <.001 |
| Lifestyle habits | ||||
| Coffee intake, every 1-cup/d increase | 0.94 (0.90-0.99) | .03 | 1.03 (0.97-1.10) | .29 |
| Contact lens use | ||||
| Never | 1 [Reference] | NA | 1 [Reference] | NA |
| Current | 0.71 (0.60-0.83) | <.001 | 0.64 (0.54-0.77) | <.001 |
| Past | 0.37 (0.29-0.47) | <.001 | 0.45 (0.34-0.58) | <.001 |
| Screen exposure, h | ||||
| <4 | 1 [Reference] | NA | NA | |
| 4-8 | 1.06 (0.85-1.31) | .61 | NA | NA |
| >8 | 1.18 (0.93-1.49) | .17 | NA | NA |
| Periodic exercise (yes vs no) | 1.26 (1.08-1.48) | .004 | 1.02 (0.87-1.21) | .78 |
| Sleep time, every 1-h/d increase | 1.02 (0.99-1.04) | .15 | NA | NA |
| Smoking (yes vs no) | 0.89 (0.74-1.06) | .17 | NA | NA |
| Water intake, mL/d | 0.99 (0.97-1.00) | .12 | NA | NA |
Abbreviations: LASIK, laser-assisted in situ keratomileusis; NA, not applicable; OR, odds ratio.
Termed hay fever on the questionnaire.
Discussion
In this study, using a large number of data collected with a novel smartphone application (DryEyeRhythm), we compared the characteristics of individuals with and without symptomatic dry eye, as well as the characteristics of individuals with diagnosed and undiagnosed dry eye. Risk factors for symptomatic vs no symptomatic dry eye included younger age, female sex, pollinosis, mental illnesses, current contact lens use, extended screen exposure, and smoking; risk factors for undiagnosed vs diagnosed symptomatic dry eye included younger age and male sex, as well as absence of collagen disease, mental illnesses other than depression and schizophrenia, schizophrenia, ophthalmic surgery other than cataract surgery and laser-assisted in situ keratomileusis, and current and past contact lens use.
Mobile health technologies could be used for the detection and management of chronic disease as well as for research to advance our understanding of dry eye disease.11,12,13,14 In this crowdsourced research, DryEyeRhythm identified many individuals with diagnosed and undiagnosed symptomatic dry eye who had dry eye symptoms. Clarifying the characteristics of symptomatic dry eye, especially undiagnosed symptomatic dry eye, using the extensive medical data collected with DryEyeRhythm may help raise awareness and prevent the exacerbation of dry eye disease.
DryEyeRhythm was downloaded by 18 991 users between November 2, 2016, and January 12, 2018. We were able to recruit a diverse cohort of participants throughout Japan and collect real-world data (Figure and Table 1). In most previous clinical studies, participants were limited to those recruited for traditional surveys, including individuals with dry eye disease symptoms and hospital follow-up and those who were physically able to participate.23 The use of DryEyeRhythm addresses these limitations by expanding the population eligible for participation to anyone with access to an iPhone. In our study, the mean participant age was 27.9 (12.6) years. Only 4.2% of the participants were older than 60 years; participation of older individuals in our study was similar to that in previous studies (0%-6.0%), as participation of older individuals in mobile health studies tends to be low.24,25,26,27,28 Therefore, the target age group and disease were considered when designing this mobile health study.
In terms of study adherence, 4454 of 9729 users (45.8%) completed the survey questionnaires (Figure, A). The adherence rate is an important factor in designing research using mobile health applications. The overall user experience affects the completion of a study by the participants; thus, developing a user-friendly interface, linking interactive voluntary posting functions to social media, offering feedback functions, and other incentives might help to improve study adherence and completion.
Although aging was identified as a risk factor for dry eye disease in previous studies,2,23,29 the present study suggested that younger age instead of older age was a risk factor for symptomatic dry eye. This finding is in accordance with those of other studies,30,31 indicating that younger individuals may be more sensitive to self-reported ocular symptoms than are older individuals. Furthermore, the increased opportunities of digital device use since childhood may contribute to dry eye symptoms in younger people. Corneal sensitivity decreases with aging, accompanied by decreased nerve density,32,33 results in perceptual stimulation to the cornea by dry eye disease symptoms that become difficult to feel with aging.
This study suggests that extended screen exposure (>8 hours per day) was positively associated with symptomatic dry eye. Currently, the increasing popularity and use of digital devices, especially among younger generations, can lead to and exacerbate dry eye disease. Therefore, the use of computers and digital devices requires careful monitoring for early dry eye disease diagnosis and prevention of severe dry eye disease. Moreover, recent studies have shown a relationship between pollinosis and dry eye disease,11,34 suggesting the need for simultaneous therapeutic intervention for both pollinosis and dry eye disease.
Few studies to date have revealed the characteristics of undiagnosed dry eye disease.35 Our crowdsourced clinical research using DryEyeRhythm identified 2395 individuals with undiagnosed symptomatic dry eye. The rate of eyedrop use was only 15.7% for individuals with undiagnosed symptomatic dry eye, in contrast to 40.0% for those with diagnosed symptomatic dry eye. Many individuals with undiagnosed symptomatic dry eye were suggested to have various symptoms without their awareness or medical treatment. Therefore, early intervention is important to prevent the development of severe dry eye disease. DryEyeRhythm may help to detect undiagnosed symptomatic dry eye in these individuals.
By comparing people with diagnosed and undiagnosed symptomatic dry eye, we believe this study identified possible risk factors for undiagnosed symptomatic dry eye. First, younger age was the common risk factor for symptomatic vs no symptomatic dry eye and undiagnosed vs diagnosed symptomatic dry eye in the present study. However, regarding sex, we found that female sex was a risk factor for symptomatic dry eye and male sex for undiagnosed symptomatic dry eye. Our interpretation of this finding is that female sex is an intrinsic risk factor for dry eye, in line with the results of previous studies,23 while sex is also associated with health-seeking behavior, ie, women may be more likely to visit clinics or hospitals for their eye symptoms. To our knowledge, this is the first study to suggest that dry eye in men might have been overlooked.
Similar to sex, we consider that collagen disease, mental illnesses, ophthalmic surgery, and contact lens use were associated with health-seeking behavior. We speculate that individuals with collagen disease, mental illnesses, ophthalmic surgery, and history of contact lens use had more opportunities to visit clinics and/or hospitals (directly or after referral) and to be diagnosed with dry eye disease. Conversely, individuals without these conditions might be less likely to be diagnosed even if they have symptomatic dry eye. From a public health perspective, identifying barriers for the identification and intervention of a disease is important. In the context of dry eye disease, the present study suggests that dry eye disease in younger men without collagen disease, mental illnesses, ophthalmic surgery, and history of contact lens use may remain undiagnosed. To reduce the burden of dry eye disease in the community, greater awareness is needed for this particular patient group with undiagnosed symptomatic dry eye.
Limitations
This crowdsourced clinical research had several limitations. First, the study is characterized by selection bias for age, socioeconomic factors, and user characteristics because the application was released only for iOS and iPhone. In addition, it is likely that volunteer bias existed. Although we believe that the internal validity of the study was good, the external validity or generalizability of the findings remains unknown, considering the difference in health-seeking behavior and socioeconomic and cultural factors in Japan. Socioeconomic, educational level, and cultural background data were not collected in this study. Further updates and android version development, as well as recruitment of individuals from the United States using the more recently released US version, will reduce this bias. Second, self-reporting bias might be present, as self-administered questionnaires were used. We speculate that the type of misclassification is more likely to be nondifferential, and therefore, the OR of each risk factor might have been underestimated. The OSDI questionnaire (ie, the most important information means used to define and classify the outcomes in the present study) was validated using the scores of the paper-based and DryEyeRhythm-based questionnaires,11 inferring that the results of the other self-administered questionnaires were also valid. Third, this mobile health application study was able to identify only symptomatic dry eye based on the OSDI questionnaire and was unable to identify no symptomatic dry eye disease based on clinical examinations, such as the Schirmer test and measurement of tear film break-up time. However, to counterbalance this limitation, this crowdsourced clinical research has overcome several common participant recruitment-related issues, resulting in successful recruitment of a diverse cohort, collection of a large data set, and overcoming geographic restrictions. We believe that it would be difficult to identify undiagnosed symptomatic dry eye without using our mobile application. In addition, although we conducted the analysis after the sample size was greatly increased since the release of this mobile application, the sample size of the present study may still be insufficient to identify risk factors weakly associated with diagnosed and undiagnosed symptomatic dry eye. With our post hoc power calculation, we found that we could identify risk factors for symptomatic vs no symptomatic dry eye with an OR of 1.31 or greater or 0.72 or less and risk for undiagnosed vs diagnosed symptomatic dry eye with an OR of 1.36 or greater or 0.68 or less, assuming an α error of .05, power of 80%, and risk factor prevalence in the comparison group of 10%.
Conclusions
This crowdsourced research using DryEyeRhythm identified individuals who reported diagnosed and undiagnosed symptomatic dry eye. We identified risk factors possibly associated with undiagnosed symptomatic dry eye, including younger age, male sex, and absence of collagen disease, mental illnesses, ophthalmic surgery, and history of contact lens use. This study may lead to further understanding of dry eye symptoms and identify at-risk individuals who should be clinically evaluated, potentially improving prevention or early treatment of dry eye disease.
eTable 1. Survey Questions
eTable 2. Daily Subjective Symptom Questions
eTable 3. Classification of Dry Eye
eFigure. Age Distribution Based on Subgroup Analysis
References
- 1.Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):163-178. doi: 10.1016/S1542-0124(12)70085-X [DOI] [PubMed] [Google Scholar]
- 2.Ding J, Sullivan DA. Aging and dry eye disease. Exp Gerontol. 2012;47(7):483-490. doi: 10.1016/j.exger.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courtin R, Pereira B, Naughton G, et al. Prevalence of dry eye disease in visual display terminal workers: a systematic review and meta-analysis. BMJ Open. 2016;6(1):e009675. doi: 10.1136/bmjopen-2015-009675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yilmaz U, Gokler ME, Unsal A. Dry eye disease and depression-anxiety-stress: a hospital-based case control study in Turkey. Pak J Med Sci. 2015;31(3):626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inomata T, Shiang T, Iwagami M, et al. Changes in distribution of dry eye disease by the new 2016 diagnostic criteria from the Asia Dry Eye Society. Sci Rep. 2018;8(1):1918. doi: 10.1038/s41598-018-19775-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-283. doi: 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 7.Tsubota K, Yokoi N, Shimazaki J, et al. ; Asia Dry Eye Society . New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65-76. doi: 10.1016/j.jtos.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 8.Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1(2):51-57. doi: 10.1007/s40135-013-0009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada M, Mizuno Y, Shigeyasu C. Impact of dry eye on work productivity. Clinicoecon Outcomes Res. 2012;4:307-312. doi: 10.2147/CEOR.S36352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inomata T, Iwagami M, Hiratsuka Y, et al. Maximum blink interval is associated with tear film breakup time: a new simple, screening test for dry eye disease. Sci Rep. 2018;8(1):13443. doi: 10.1038/s41598-018-31814-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inomata T, Nakamura M, Iwagami M, et al. Risk factors for severe dry eye disease: crowdsourced research using DryEyeRhythm. Ophthalmology. 2019;126(5):766-768. doi: 10.1016/j.ophtha.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 12.Basatneh R, Najafi B, Armstrong DG. Health sensors, smart home devices, and the internet of medical things: an opportunity for dramatic improvement in care for the lower extremity complications of diabetes. J Diabetes Sci Technol. 2018;12(3):577-586. doi: 10.1177/1932296818768618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong DG, Najafi B, Shahinpoor M. Potential applications of smart multifunctional wearable materials to gerontology. Gerontology. 2017;63(3):287-298. doi: 10.1159/000455011 [DOI] [PubMed] [Google Scholar]
- 14.Miller JD, Najafi B, Armstrong DG. Current standards and advances in diabetic ulcer prevention and elderly fall prevention using wearable technology. Curr Geriatr Rep. 2015;4(3):249-256. doi: 10.1007/s13670-015-0136-7 [DOI] [Google Scholar]
- 15.Dimitrov DV. Medical internet of things and big data in healthcare. Healthc Inform Res. 2016;22(3):156-163. doi: 10.4258/hir.2016.22.3.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94-101. doi: 10.1001/archophthalmol.2009.356 [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 18.Zung WWK. A Self-Rating Depression Scale. Arch Gen Psychiatry. 1965;12(1):63-70. doi: 10.1001/archpsyc.1965.01720310065008 [DOI] [PubMed] [Google Scholar]
- 19.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615-621. doi: 10.1001/archopht.118.5.615 [DOI] [PubMed] [Google Scholar]
- 20.Kitamura T, Shima S, Sugawara M, Toda MA. Temporal variation of validity of self-rating questionnaires: repeated use of the General Health Questionnaire and Zung’s Self-Rating Depression Scale among women during antenatal and postnatal periods. Acta Psychiatr Scand. 1994;90(6):446-450. doi: 10.1111/j.1600-0447.1994.tb01622.x [DOI] [PubMed] [Google Scholar]
- 21.Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-Rating Depression Scale. Br J Psychiatry. 1978;132:381-385. doi: 10.1192/bjp.132.4.381 [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 23.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334-365. doi: 10.1016/j.jtos.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 24.McConnell MV, Shcherbina A, Pavlovic A, et al. Feasibility of obtaining measures of lifestyle from a smartphone app: the MyHeart Counts Cardiovascular Health Study. JAMA Cardiol. 2017;2(1):67-76. doi: 10.1001/jamacardio.2016.4395 [DOI] [PubMed] [Google Scholar]
- 25.Zens M, Woias P, Suedkamp NP, Niemeyer P. “Back on Track”: a mobile app observational study using Apple’s ResearchKit Framework. JMIR Mhealth Uhealth. 2017;5(2):e23. doi: 10.2196/mhealth.6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan YY, Wang P, Rogers L, et al. The Asthma Mobile Health Study, a large-scale clinical observational study using ResearchKit. Nat Biotechnol. 2017;35(4):354-362. doi: 10.1038/nbt.3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujibayashi K, Takahashi H, Tanei M, Uehara Y, Yokokawa H, Naito T. A new influenza-tracking smartphone app (Flu-Report) based on a self-administered questionnaire: cross-sectional study. JMIR Mhealth Uhealth. 2018;6(6):e136. doi: 10.2196/mhealth.9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radin JM, Steinhubl SR, Su AI, et al. The Healthy Pregnancy Research Program: transforming pregnancy research through a ResearchKit app. NPJ Digit Med. 2018;1:45. doi: 10.1038/s41746-018-0052-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Paiva CS. Effects of aging in dry eye. Int Ophthalmol Clin. 2017;57(2):47-64. doi: 10.1097/IIO.0000000000000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Zheng K, Deng Z, et al. Prevalence and risk factors of dry eye disease among a hospital-based population in southeast China. Eye Contact Lens. 2015;41(1):44-50. doi: 10.1097/ICL.0000000000000064 [DOI] [PubMed] [Google Scholar]
- 31.Uchino M, Schaumberg DA, Dogru M, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008;115(11):1982-1988. doi: 10.1016/j.ophtha.2008.06.022 [DOI] [PubMed] [Google Scholar]
- 32.Roszkowska AM, Colosi P, Ferreri FM, Galasso S. Age-related modifications of corneal sensitivity. Ophthalmologica. 2004;218(5):350-355. doi: 10.1159/000079478 [DOI] [PubMed] [Google Scholar]
- 33.Yang AY, Chow J, Liu J. Corneal innervation and sensation: the eye and beyond. Yale J Biol Med. 2018;91(1):13-21. [PMC free article] [PubMed] [Google Scholar]
- 34.Ayaki M, Kawashima M, Uchino M, Tsubota K, Negishi K. Possible association between subtypes of dry eye disease and seasonal variation. Clin Ophthalmol. 2017;11:1769-1775. doi: 10.2147/OPTH.S148650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the united states among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90-98. doi: 10.1016/j.ajo.2017.06.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Survey Questions
eTable 2. Daily Subjective Symptom Questions
eTable 3. Classification of Dry Eye
eFigure. Age Distribution Based on Subgroup Analysis