This cohort study examines whether psoriasis severity is a factor in quality of life among children with a psoriasis diagnosis in the Netherlands.
Key Points
Questions
What is the association between the degree of psoriasis clearance and quality of life (QOL) in pediatric patients with psoriasis, and is the type of treatment associated with QOL in a real-world setting?
Findings
In this cohort study of 319 children with psoriasis, highest improvements in QOL were associated with a reduction of 90% or greater of the Psoriasis Area Severity Index, a reduction of 90% or greater in body surface area, and with systemic treatments. The association between systemic therapy and improvement in QOL was independent of the Psoriasis Area Severity Index response.
Meaning
These findings suggest that a 90% or greater Psoriasis Area Severity Index response or a decrease in body surface area involvement of 90% or greater may be clinically meaningful therapeutic goals in pediatric psoriasis.
Abstract
Importance
Treatment of psoriasis is associated with improved quality of life (QOL) in those with the disease. However, in daily clinical practice, the association between the degree of psoriasis clearance and QOL has not been studied to date, especially in the pediatric population.
Objectives
To identify the association between the degree of psoriasis improvement (as measured by the Psoriasis Area Severity Index [PASI] and body surface area [BSA] response) and QOL (as measured by the Children’s Dermatology Life Quality Index [CDLQI]) in pediatric psoriasis, and to assess the association of treatment type with QOL, independent of psoriasis improvement.
Design, Setting, and Participants
Data used in this single-center cohort study were extracted from the Child-CAPTURE (Continuous Assessment of Psoriasis Treatment Use Registry), a prospective, observational, daily clinical practice cohort of all children (aged <18 years) with a psoriasis diagnosis who attended the outpatient clinic of the Department of Dermatology at the Radboud University Medical Center in Nijmegen, the Netherlands, between September 3, 2008, and May 4, 2018. All records of treatment episodes with CDLQI, PASI, and BSA scores were included in the analysis.
Exposures
Patients were treated according to daily clinical care. Treatments were clustered into topical, dithranol, conventional systemic, and biological treatments. Because of low numbers of UV-B phototherapy, this treatment was not assessed.
Main Outcomes and Measures
Primary outcomes were mean change of CDLQI scores per PASI and BSA response categories (0 to <50, 50 to <75, 75 to <90, and ≥90) and mean CDLQI change per treatment categories.
Results
In total, 319 patients (median [interquartile range] age, 10.0 [7.0] years; 183 female [57.4%]) were analyzed for PASI score improvement (399 treatment episodes) and improvement in BSA involvement (366 treatment episodes). The greatest improvements in CDLQI scores were seen in the PASI ≥90 response category, with an estimated marginal mean change in CDLQI score of −6.6 (95% CI, –7.5 to –5.7). The greatest improvements in CDLQI scores were also observed in the BSA ≥90 response category, with an estimated marginal mean change in CDLQI score of −6.8 (95% CI, –7.5 to –6.1). Systemic treatment demonstrated a greater degree of improvement of CDLQI compared with topical treatment, independent of PASI response categories.
Conclusions and Relevance
This cohort study in a real-world setting found that the greatest improvements in QOL were associated with PASI 90 or greater, a decrease in BSA involvement of 90% or greater, and systemic treatments. These findings suggest that reaching PASI 90 or greater and decreasing BSA involvement by at least 90% may be clinically meaningful treatment goals that will help pediatric patients with psoriasis reach optimal QOL.
Introduction
Psoriasis has a negative association with quality of life (QOL) in adult and pediatric patients.1,2,3,4,5,6,7 Newer, more potent biological treatments have made the achievement of PASI 90 (a reduction of 90% of the Psoriasis Area Severity Index [PASI] score) more attainable, and data from randomized clinical trials in adult patients suggest the relevance of aiming for PASI 90, which has been associated with further improvement of QOL compared with lower clinical responses.8,9,10,11,12,13,14,15 Whether this suggestion holds true in daily clinical practice for pediatric patients with psoriasis has not been investigated to date.
In pediatric psoriasis, to our knowledge, only 1 randomized clinical trial has examined whether the degree of PASI response is associated with a greater degree of improvement in QOL.16 Langley et al16 evaluated the effect of etanercept on the QOL of 106 children with psoriasis and demonstrated that children treated with etanercept who achieved PASI 75 (a reduction of 75% of the PASI score) at week 12 had a greater percentage of improvements in Children’s Dermatology Life Quality Index (CDLQI) score compared with nonresponders. Furthermore, although treatments are known to improve psoriasis and thus the QOL, the type of treatment (eg, topical, systemic) might also have implications for the QOL of children.7 Whether the treatment itself is associated with QOL independent of the degree of psoriasis improvement has not been investigated to date.
In this cohort study of pediatric patients with psoriasis, we sought to identify the association between the degree of psoriasis improvement (as measured by PASI and body surface area [BSA] involvement) and QOL (as measured by CDLQI score). In addition, we examined the association of the type of treatment, independent of psoriasis improvement, with QOL.
Methods
Patient Selection and Study Design
Data for this single-center cohort study were extracted from the Child-CAPTURE (Continuous Assessment of Psoriasis Treatment Use Registry), a prospective, observational, daily clinical practice cohort of children and adolescents with psoriasis. The Child-CAPTURE includes all children (younger than 18 years at first visit) with the diagnosis of psoriasis who attended the outpatient clinic of the Department of Dermatology at the Radboud University Medical Center in Nijmegen, the Netherlands, between September 3, 2008, and May 4, 2018 (data lock). Patients were referred by general practitioners and dermatologists from across the Netherlands. This study was reviewed by the ethics committee of the region of Arnhem-Nijmegen and Radboud University Medical Center on the basis of the Dutch Code of Conduct for Health Research, the Dutch Code of Conduct for Responsible Use, the Dutch Personal Data Protection Act, and the Medical Treatment Agreement Act and was deemed by the ethics committee to not fall within the remit of the Medical Research Involving Human Subjects Act. Written informed consent was obtained from the parents or guardians and/or from the participating pediatric patients according to applicable rules. This study was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Outcome Measures and Treatments
At baseline and at every visit during follow-up, patient characteristics, psoriasis severity scores (PASI range 0-72; BSA; and Physician Global Assessment [PGA] range 0-5, with the highest score indicating severe psoriasis), and treatment characteristics were collected. To quantify the association of psoriasis with QOL, we collected the validated CDLQI score at every visit.17 The CDLQI total score ranges from 0 to 30, with a higher score indicating a greater influence on QOL. A CDLQI score of 0 or 1 represents no influence of psoriasis on a child’s QOL.18 Patients were treated according to daily clinical care. Treatment decisions were made by the treating physician. Type of treatment was recorded for each visit. Treatments were clustered into 4 categories: topical therapy (using only topical treatment, including topical corticosteroids, vitamin D analogues, and calcineurin inhibitors), dithranol (anthralin), conventional systemic therapy (methotrexate sodium and fumaric acid esters), and biological therapy (etanercept, adalimumab, or ustekinumab).
Treatment Episodes and Severity Response Categories
A treatment episode was defined as the period from the start to the end of treatment or follow-up. In systemic therapy, we considered episodes with treatment interruptions of up to 90 days as continuous episodes. Treatment episodes were included in the analysis if PASI, BSA, and CDLQI responses were available. The following were excluded from analysis: episodes with a duration less than 6 weeks, episodes with only 1 visit, and episodes with treatment other than those in the 4 categories. Given that the objective of this study was to identify the association between improvement of psoriasis and QOL, we excluded treatment episodes in which no improvement in PASI score or BSA involvement was achieved, except in the sensitivity analysis of all treatment episodes.
Clinical severity response was based on relative PASI and BSA responses, calculated using the lowest PASI and BSA scores for a treatment episode compared with the first PASI and BSA scores of that same episode. Corresponding CDLQI scores were used to assess the mean change in QOL. This approach (ie, using the lowest severity score during a treatment episode) was chosen over the approach of using the last severity scores of the treatment episode to establish an optimal severity response so that we could thoroughly analyze the association between severity score response and QOL response. Clinical severity responses were grouped into 4 response categories according to the relative PASI or BSA responses: PASI 0 to <50, 50 to <75, 75 to <90, or ≥90; or BSA 0 to <50, 50 to <75, 75 to <90, or ≥90.
Statistical Analysis
Patient characteristics were presented as medians (interquartile ranges [IQRs]) for continuous variables and numbers (percent) for categorical variables. Correlation coefficients of PASI, BSA, and PGA responses with the CDLQI score at the first visit in the Child-CAPTURE were calculated with Spearman rank-order correlation. Although analyses of patient characteristics and correlation coefficients were performed on patient level, all further analyses were conducted on treatment episode level. Consequently, a patient might have more than 1 treatment episode included in the analyses. To account for the correlations between treatment episodes of the same patient, we conducted analyses using linear mixed models and mixed-effects logistic regression models. All analyses were adjusted for the following confounders: CDLQI score or CDLQI domain scores at the start of treatment, PASI or BSA scores at the start of treatment, age, sex, psoriasis duration, and treatment duration (duration from the start of the episode to time of lowest severity score). Only confounders that altered the unadjusted exposure-outcome effect by 10% or more were retained in the models. We assessed 2 outcome measures: mean CDLQI change (assessed with linear mixed models) and percentage of CDLQI scores that reached 0 or 1 (assessed with mixed-effects logistic regression models).
In total, we performed 4 analyses to identify the (1) differences in CDLQI score changes between PASI response categories and BSA response categories; (2) differences in CDLQI domain score changes between PASI response categories; (3) differences in percentage of CDLQI 0 or 1 achievement between PASI response categories and BSA response categories; and (4) differences in CDLQI change between treatment categories. In addition, we performed a sensitivity analysis to assess the differences in CDLQI changes between PASI response categories for all treatment episodes, including episodes in which the psoriasis worsened.
Estimated marginal means were calculated with linear mixed models, and estimated percentages were calculated with mixed-effects logistic regression models. Statistical package SPSS, version 25 (IBM) and SAS, version 9.2 (SAS Institute Inc) were used to perform analyses. Two-sided P < .05 was considered statistically significant. All analyses were conducted from October 7, 2018, to December 13, 2018, and supervised by a senior statistician (H.M.M.G.) on our team.
Results
A total of 319 patients with 566 treatment episodes were selected; the selection of treatment episodes is shown in Figure 1. After treatment episodes with no improvement in PASI score or BSA involvement or with missing data were excluded, a total of 399 episodes were included for analysis per PASI response categories and 366 episodes were included for analysis per BSA response categories. Patient characteristics at first visit for 319 patients (median [IQR] age, 10.0 [7.0] years; 183 female [57.4%]) are presented in the Table.
Figure 1. Selection of Treatment Episodes.
BSA indicates body surface area; CDLQI, Children’s Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index.
Table. Characteristics of Pediatric Patients With Psoriasis at First Visit .
| Variable | No. (%) |
|---|---|
| Sex | |
| Male | 136 (42.6) |
| Female | 183 (57.4) |
| Family history | |
| Psoriasis in parents or siblings | 106 (33.2) |
| PsA in parents or siblings | 6 (1.9) |
| Type of psoriasisa | |
| Plaque | 280 (87.8) |
| Guttate | 29 (9.1) |
| Scalp | 268 (84.0) |
| Inverse | 115 (36.1) |
| Pustular | 4 (1.3) |
| Palmoplantar | 6 (1.9) |
| Joint involvementb | |
| PsA | 2 (0.6) |
| Arthralgia | 10 (3.1) |
| Age, median (IQR) [range], y | 10.0 (7.0) [0-17] |
| Psoriasis duration, median (IQR) [range], y | 1.5 (3.6) [0.0-13.0] |
| Psoriasis severity at baseline, median (IQR) [range] | |
| PASI (0-72) | 5.6 (5.0) [0.0-42.0] |
| BSA | 5.3 (8.0) [0.0-72.0] |
| PGA (0-5) | 2.0 (1.0) [0-5] |
| CDLQI score at baseline (0-30) | |
| No. | 309 |
| Median (IQR) [range] | 7.0 (8.0) [0-25] |
| CDLQI per severity category (0-30) | |
| PASI 0.0-4.9 | |
| No. (%) | 126 (39.6) |
| Median (IQR) [range] | 6.0 (6.0) [0-25] |
| PASI 5.0-9.9 | |
| No. (%) | 143 (44.9) |
| Median (IQR) [range] | 8.0 (7.0) [0-23] |
| PASI ≥10.0 | |
| No. (%) | 49 (15.4) |
| Median (IQR) [range] | 9.0 (7.0) [2-22] |
| CDLQI domain subscores, median (IQR) [range] | |
| Symptoms and feelings | 2.0 (3.0) [0-6] |
| Leisure | 1.0 (2.0) [0-9] |
| School or holidays | 1.0 (2.0) [0-3] |
| Personal relationships | 1.0 (2.0) [0-6] |
| Sleep | 1.0 (2.0) [0-3] |
| Treatment | 1.0 (2.0) [0-3] |
Abbreviations: BSA, body surface area; CDLQI, Children’s Dermatology Life Quality Index (where a higher score indicates a greater influence on quality of life); IQR, interquartile range; PASI, Psoriasis Area and Severity Index (where a higher score indicates more severe psoriasis); PGA, Physician’s Global Assessment (where the highest score indicates more severe psoriasis); PsA, psoriatic arthritis.
Total number of patients (n = 319) does not equal sum of patients reporting different types of psoriasis because more than 1 type of psoriasis can be reported in the same patient.
Arthralgia was defined as aching or pain in the joints without true arthritis.
A statistically significant moderate positive correlation between PASI and CDLQI scores was found at first visit (r = 0.47; P < .001). Similar correlations were found for BSA and CDLQI scores (r = 0.48; P < .001) as well as PGA and CDLQI scores (r = 0.50; P < .001).
Differences in CDLQI total score change between severity response categories are presented in Figure 2A and B. Analyses showed an increasing improvement in the CDLQI score, with higher relative PASI and BSA response categories. The highest CDLQI total score change was seen in the PASI ≥90 response category, with an estimated marginal mean (EMM) change in CDLQI score of −6.6 (95% CI, −7.5 to −5.7). The greatest CDLQI total score changes were also observed in the BSA ≥90 response category, with an EMM change in CDLQI score of −6.8 (95% CI, −7.5 to −6.1). The sensitivity analysis of all treatment episodes, including a PASI worsening category, revealed similar results: an increasing improvement in the CDLQI score with higher relative PASI response categories (eFigure in the Supplement).
Figure 2. Estimated Mean Children’s Dermatology Life Quality Index (CDLQI) Score Change and Estimated Percentages of CDLQI Score 0 or 1 Achievement by Psoriasis Area and Severity Index (PASI) and by Body Surface Area (BSA) Response Categories.
A and B, Linear mixed models were used for analyses. The following possible confounders were incorporated in the models: CDLQI, PASI, and BSA scores at the start of treatment; age; psoriasis duration; sex; treatment; and treatment duration. Only the starting CDLQI score, treatment, and psoriasis duration altered the unadjusted exposure outcome by 10% or higher and were kept in the models. Results are presented with a starting CDLQI score of 8.1 (the higher the score, the greater the influence on quality of life) and psoriasis duration of 3.7 years for PASI response categories, and with a starting CDLQI score of 8.2 and psoriasis duration of 3.8 years for BSA response categories. C and D, Mixed-effects logistic regression models were used for analyses. The following possible confounders were incorporated in the models: CDLQI, PASI, and BSA scores at the start of treatment; age; psoriasis duration; sex; treatment; and treatment duration. Only the starting CDLQI score and treatment altered the unadjusted exposure outcome of 10% or higher and were kept in the models. Results are presented with a starting CDLQI score of 8.1 and psoriasis duration of 3.7 years for PASI response categories, and with a starting CDLQI score of 8.2 and psoriasis duration of 3.8 years for BSA response categories. n Indicates number of treatment episodes; NS, not significant.
The estimated percentages of treatment episodes in which a CDLQI score of 0 or 1 (meaning no influence of psoriasis on QOL) was achieved are presented in Figure 2C and D. Overall, with increasing PASI and BSA response categories, higher estimated percentages of CDLQI 0 or 1 achievement were seen. Highest CDLQI 0 or 1 achievement was seen for PASI ≥90 response category, with an estimated percentage of 65.0% (95% CI, 47.6%-78.1%), and for BSA ≥90 response category, with an estimated percentage of 65.6% (95% CI, 53.6%-78.9%).
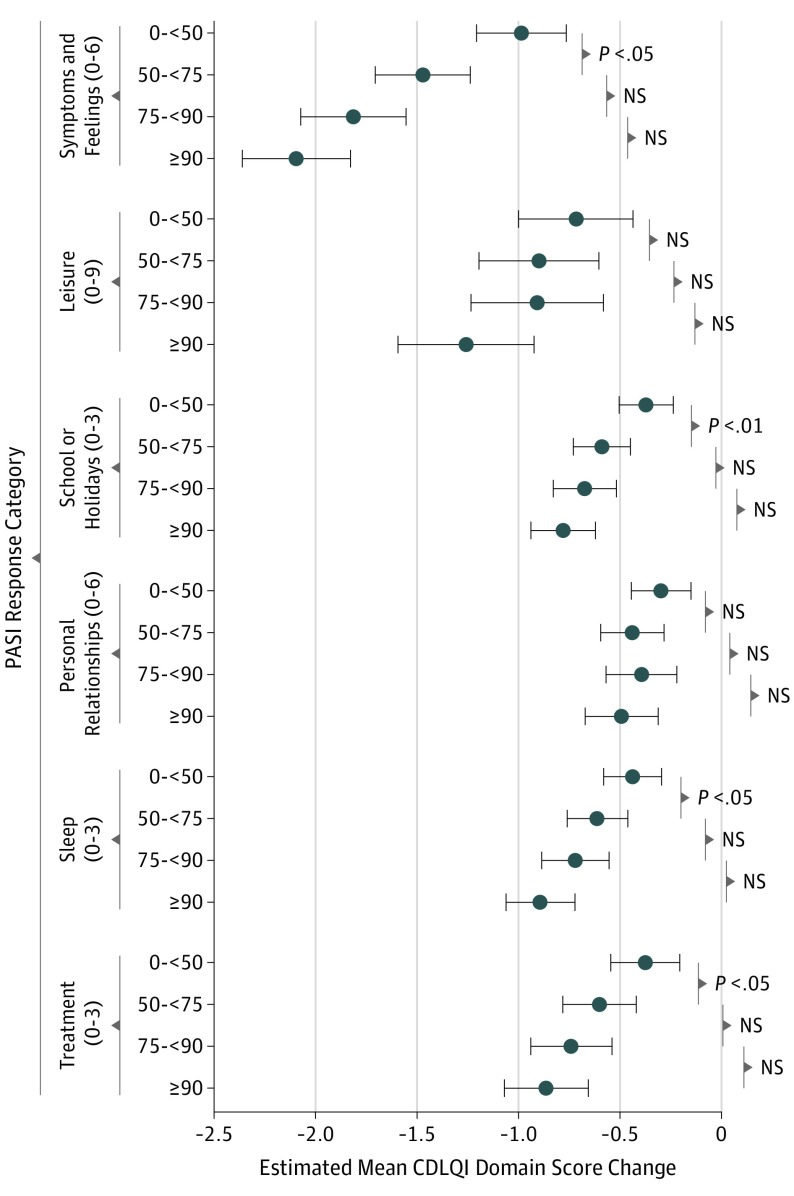
In the assessment of CDLQI domain score changes between PASI response categories, a trend comparable with the CDLQI total score analysis was found. Results are depicted in Figure 3. The symptoms and feelings domain showed the greatest improvement with higher PASI response categories. The personal relationships domain did not show any significant changes in CDLQI score with improving PASI response categories.
Figure 3. Estimated Mean Change of Children’s Dermatology Life Quality Index (CDLQI) Domain Changes by Psoriasis Area and Severity Index (PASI) Response Categories During Follow-up .
Linear mixed models were used for analyses. The following possible confounders were incorporated in the models: CDLQI and PASI scores at the start of treatment, age, psoriasis duration, sex, treatment, and treatment duration. Only the starting CDLQI domain score, treatment, and psoriasis duration altered the unadjusted exposure outcome effect by 10% or higher and were kept in the models. Results are presented with a psoriasis duration of 3.7 years and the following starting CDLQI domain scores (with higher scores indicating a greater influence on quality of life): symptoms and feelings, 2.5 (score range, 0-6); leisure, 1.6 (score range, 0-9); school or holidays, 0.9 (score range, 0-3); personal relationships, 0.9 (score range, 0-6); sleep, 1.0 (score range, 0-3); and treatment, 1.2 (score range, 0-3). The number of treatment episodes for PASI response categories are 0 to <50, 149; 50 to <75, 108; 75 to <90, 70; and ≥90, 72. Horizontal bars and whiskers represent 95% CIs. NS indicates not significant.
Differences in CDLQI total score change between treatment categories are presented in Figure 4. A greater improvement in CDLQI score was seen for systemic therapy (conventional systemic and biological) compared with topical treatment (topical and dithranol): EMM change in CDLQI scores of −4.3 (95% CI, −4.8 to –3.8), and −4.2 (95% CI, −4.9 to −3.5) for topical and dithranol, respectively, vs EMM change in CDLQI scores of −5.8 (95% CI, −6.7 to −4.9) and −5.8 (95% CI, −7.5 to −4.1) for conventional treatment and biological treatment, respectively. The analysis of CDLQI changes between treatment groups was adjusted for confounders, including the CDLQI score at the start of treatment and psoriasis duration. Because systemic treatments are more likely to reach higher PASI responses, analysis was further adjusted for PASI response categories. The higher improvement in QOL associated with systemic therapy was, therefore, independent from the PASI response.
Figure 4. Estimated Mean Children’s Dermatology Life Quality Index (CDLQI) Total Score Change by Treatment Categories During Follow-up .
Linear mixed models were used for analyses. The following possible confounders were incorporated in the models: age, sex, Psoriasis Area and Severity Index (PASI) score at the start of treatment, CDLQI total score at the start, PASI response categories, treatment duration, and psoriasis duration. Only PASI response categories, starting CDLQI total score, and psoriasis duration altered the unadjusted exposure outcome effect by 10% or higher and were kept in the models. Results are presented with the starting CDLQI total score of 8.1 (the higher the score, the greater the influence on quality of life) and psoriasis duration of 3.7 years. Vertical bars and whiskers represent 95% CIs.
Discussion
To our knowledge, this cohort study is the first to examine in a real-world setting the association between the degree of psoriasis improvement and QOL in pediatric patients with psoriasis. In pediatric psoriasis, only 1 randomized clinical trial to date has investigated the association between the degree of PASI response and improvement of QOL.16 That trial found that pediatric patients treated with etanercept who achieved PASI 75 had a higher percentage of improvement in CDLQI score compared with PASI 75 nonresponders at week 12 (75.1% vs 29.8%). However, the association of reaching PASI 90 or greater with the CDLQI score was not assessed. Previous studies have demonstrated a greater degree of improvements in Dermatology Life Quality Index in adult patients who achieved PASI 90 or greater compared with lower clinical responses.8,9,10,11,12,13,14,15 These results are in line with the findings in this present study that reaching PASI 90 or greater was associated with the highest improvement of QOL, which is reflected both in CDLQI score change and in percentage of CLDQI 0 or 1 achievement. A sensitivity analysis in which treatment episodes with worsening PASI scores were also included revealed similar results. Therefore, the results of this study seem to be robust. In addition, analysis of CDLQI domains demonstrated that the symptoms and feelings domain, which consisted of 2 items related to itch and embarrassment, seemed to be most closely associated with the improvement of PASI response. Further analysis of these 2 items showed greatest improvement in itch.
A decrease in BSA involvement of 90% or greater is also associated with a greater improvement in QOL, with similar improvement in CDLQI scores as PASI 90 or greater. We believe this result emphasizes the implication of the extent of affected area for the QOL. To our knowledge, the association between BSA decrease and QOL improvement has never been investigated in children and adults. Furthermore, a decrease in BSA involvement of 90% or greater was attained in more treatment episodes compared with PASI 90 or greater (117 episodes vs 72 episodes). These findings appear to support the use of BSA for assessing psoriasis in clinical practice.
Analysis of QOL per treatment category revealed that systemic treatments, including conventional systemic and biological therapies, are associated with greater improvements in QOL compared with topical and dithranol treatments. Because systemic treatments are more likely to reach higher PASI responses, we adjusted the analyses for PASI response categories. Thus, this positive association of systemic therapies with QOL appears to be independent of PASI score improvement. The weaker association between topical treatment and improvement in QOL might be attributed to several characteristics of topical agents, such as time taken to apply ointments, odors, and stickiness.19,20 However, we did not examine the factors associated with the advantages of systemic medication for QOL.
Strengths and Limitations
A strength of this study is its inclusion of 319 patients, which is a large number compared with the sample size in other pediatric psoriasis studies. In addition, all analyses were adjusted for possible confounders, including CDLQI score at the start of treatment, PASI or BSA scores at the start (to adjust for many patients who had mild-to-moderate psoriasis), age, sex, psoriasis duration, treatment, and treatment duration, and the analyses accounted for the fact that more than 1 treatment episode per patient was included. Furthermore, this study included several antipsoriatic treatments, including topical, dithranol, conventional systemic, and biological agents, rather than only biological agents, which was the case in previous (mainly adult) studies. Although UV-B phototherapy is a well-known therapy in pediatric psoriasis, we were unable to assess its association with QOL owing to low numbers. In this study, we used the CDLQI, a validated instrument for assessing the influence of psoriasis on QOL. Although the CDLQI might not capture all aspects of psychological well-being, it is commonly used in pediatric psoriasis studies and randomized clinical trials.18
This study also has limitations. The results are limited by the single-center study design. Furthermore, although in all analyses we adjusted for PASI, BSA, and CDLQI scores at the start of treatment, most participants had mild-to-moderate psoriasis and mild influence on QOL even before the start of treatment. Nevertheless, given that patients were referred by general practitioners and dermatologists from across the Netherlands, we believe this study cohort represents a regular pediatric psoriasis population.
Conclusions
This daily clinical practice cohort study in pediatric psoriasis demonstrates that QOL improvement is associated with higher PASI responses and improvement in BSA involvement. The greatest improvements in QOL were seen with the achievement of PASI 90 or greater and a decrease in BSA involvement of 90% or greater. Furthermore, systemic treatments are associated with a greater degree of improvement of QOL compared with topical treatments independent of PASI response. These findings suggest that a PASI 90 or greater and a decrease in BSA involvement of at least 90% may be clinically meaningful treatment goals that will help pediatric patients with psoriasis reach optimal QOL.
eFigure. Estimated Mean CDLQI Change per PASI Response Categories for All Treatment Episodes (Including Treatment Episodes With Worsening of Psoriasis)
References
- 1.Augustin M, Radtke MA. Quality of life in psoriasis patients. Expert Rev Pharmacoecon Outcomes Res. 2014;14(4):559-568. doi: 10.1586/14737167.2014.914437 [DOI] [PubMed] [Google Scholar]
- 2.Bronckers IMGJ, van Geel MJ, van de Kerkhof PCM, de Jong EMGJ, Seyger MMB. A cross-sectional study in young adults with psoriasis: potential determining factors in quality of life, life course and work productivity. J Dermatolog Treat. 2019;30(3):208-215. doi: 10.1080/09546634.2018.1506077 [DOI] [PubMed] [Google Scholar]
- 3.Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol. 2004;51(5):704-708. doi: 10.1016/j.jaad.2004.04.014 [DOI] [PubMed] [Google Scholar]
- 4.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136-139. doi: 10.1046/j.1087-0024.2003.09102.x [DOI] [PubMed] [Google Scholar]
- 5.de Jager ME, van de Kerkhof PC, de Jong EM, Seyger MM. A cross-sectional study using the Children’s Dermatology Life Quality Index (CDLQI) in childhood psoriasis: negative effect on quality of life and moderate correlation of CDLQI with severity scores. Br J Dermatol. 2010;163(5):1099-1101. doi: 10.1111/j.1365-2133.2010.09993.x [DOI] [PubMed] [Google Scholar]
- 6.Gånemo A, Wahlgren CF, Svensson Å. Quality of life and clinical features in Swedish children with psoriasis. Pediatr Dermatol. 2011;28(4):375-379. doi: 10.1111/j.1525-1470.2010.01292.x [DOI] [PubMed] [Google Scholar]
- 7.Oostveen AM, de Jager ME, van de Kerkhof PC, Donders AR, de Jong EM, Seyger MM. The influence of treatments in daily clinical practice on the Children’s Dermatology Life Quality Index in juvenile psoriasis: a longitudinal study from the Child-CAPTURE patient registry. Br J Dermatol. 2012;167(1):145-149. doi: 10.1111/j.1365-2133.2012.10996.x [DOI] [PubMed] [Google Scholar]
- 8.Elewski BE, Puig L, Mordin M, et al. Psoriasis patients with Psoriasis Area and Severity Index (PASI) 90 response achieve greater health-related quality-of-life improvements than those with PASI 75-89 response: results from two phase 3 studies of secukinumab. J Dermatolog Treat. 2017;28(6):492-499. doi: 10.1080/09546634.2017.1294727 [DOI] [PubMed] [Google Scholar]
- 9.Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645-648. doi: 10.1111/jdv.12817 [DOI] [PubMed] [Google Scholar]
- 10.Puig L, Thom H, Mollon P, Tian H, Ramakrishna GS. Clear or almost clear skin improves the quality of life in patients with moderate-to-severe psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2017;31(2):213-220. doi: 10.1111/jdv.14007 [DOI] [PubMed] [Google Scholar]
- 11.Revicki DA, Willian MK, Menter A, Saurat JH, Harnam N, Kaul M. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate to severe plaque psoriasis. Dermatology. 2008;216(3):260-270. doi: 10.1159/000113150 [DOI] [PubMed] [Google Scholar]
- 12.Strober B, Papp KA, Lebwohl M, et al. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol. 2016;75(1):77-82.e7. doi: 10.1016/j.jaad.2016.03.026 [DOI] [PubMed] [Google Scholar]
- 13.Torii H, Sato N, Yoshinari T, Nakagawa H; Japanese Infliximab Study Investigators . Dramatic impact of a Psoriasis Area and Severity Index 90 response on the quality of life in patients with psoriasis: an analysis of Japanese clinical trials of infliximab. J Dermatol. 2012;39(3):253-259. doi: 10.1111/j.1346-8138.2011.01459.x [DOI] [PubMed] [Google Scholar]
- 14.Edson-Heredia E, Banerjee S, Zhu B, et al. A high level of clinical response is associated with improved patient-reported outcomes in psoriasis: analyses from a phase 2 study in patients treated with ixekizumab. J Eur Acad Dermatol Venereol. 2016;30(5):864-865. doi: 10.1111/jdv.13032 [DOI] [PubMed] [Google Scholar]
- 15.Viswanathan HN, Chau D, Milmont CE, et al. Total skin clearance results in improvements in health-related quality of life and reduced symptom severity among patients with moderate to severe psoriasis. J Dermatolog Treat. 2015;26(3):235-239. doi: 10.3109/09546634.2014.943687 [DOI] [PubMed] [Google Scholar]
- 16.Langley RG, Paller AS, Hebert AA, et al. Patient-reported outcomes in pediatric patients with psoriasis undergoing etanercept treatment: 12-week results from a phase III randomized controlled trial. J Am Acad Dermatol. 2011;64(1):64-70. doi: 10.1016/j.jaad.2010.02.060 [DOI] [PubMed] [Google Scholar]
- 17.Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132(6):942-949. doi: 10.1111/j.1365-2133.1995.tb16953.x [DOI] [PubMed] [Google Scholar]
- 18.Salek MS, Jung S, Brincat-Ruffini LA, et al. Clinical experience and psychometric properties of the Children’s Dermatology Life Quality Index (CDLQI), 1995-2012. Br J Dermatol. 2013;169(4):734-759. doi: 10.1111/bjd.12437 [DOI] [PubMed] [Google Scholar]
- 19.Bewley A, Burrage DM, Ersser SJ, Hansen M, Ward C. Identifying individual psychosocial and adherence support needs in patients with psoriasis: a multinational two-stage qualitative and quantitative study. J Eur Acad Dermatol Venereol. 2014;28(6):763-770. doi: 10.1111/jdv.12174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Augustin M, Holland B, Dartsch D, Langenbruch A, Radtke MA. Adherence in the treatment of psoriasis: a systematic review. Dermatology. 2011;222(4):363-374. doi: 10.1159/000329026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Estimated Mean CDLQI Change per PASI Response Categories for All Treatment Episodes (Including Treatment Episodes With Worsening of Psoriasis)