Abstract
Purpose of review:
This review aims to describe the clinical impact and assessment tools capable of identifying delirium in cardiac arrest survivors as well as providing strategies aimed at preventing and treating delirium.
Recent findings:
Patient factors leading to a cardiac arrest, initial resuscitation efforts, and post-resuscitation management all influence the potential for recovery as well as the risk for development of delirium. Data suggests that delirium in cardiac arrest survivors is an independent risk factor for morbidity and mortality. Recognizing delirium in post-cardiac arrest patients can be challenging, however, detection is not only achievable, but important as it may aid in predicting adverse outcomes. Serial neurologic examinations and delirium assessments, targeting light sedation when possible, limiting psychoactive medications, and initiating patient care bundles are important care aspects for not only allowing early identification of primary and secondary brain injury, but in improving patient morbidity and mortality.
Summary:
Developing delirium after cardiac arrest is associated with increased morbidity and mortality. The importance of addressing modifiable risk factors, recognizing symptoms early, and initiating coordinated treatment strategies can help to improve outcomes within this high risk population.
Keywords: delirium, neurologic injury, cardiac arrest
Introduction
Cardiac arrest is the most common cause of hypoxic-ischemic brain injury,1,2 with neurologic injury accounting for the majority of post-resuscitation morbidity and mortality.3,4 Outcomes of cardiac arrest survivors are dependent on the effectiveness of immediate resuscitation, transfer, and post-resuscitation management in the ICU.1 Delirium, a syndrome of acute brain injury, has been described in critically ill patients for decades, including in patients who suffer from cardiac arrest. The first mention of delirium in cardiac arrest survivors described “organic brain syndrome” as a constellation of symptoms including disorientation, disorganized thinking, restlessness, or agitation.5 Evolving nomenclature and diagnostic criteria have since been instituted that better characterize delirium as a syndrome of acute brain dysfunction, unifying its description across multiple specialties.
Delirium has already been recognized as an ubiquitous problem affecting up to 80 % of medical and surgical intensive care unit (ICU) patients.6–9 Recognition and diagnosis are important to aid in early interventions as increasing duration of delirium is associated with prolonged time on mechanical ventilation, longer ICU and hospital stays, greater long-term disability, and worse cognitive dysfunction.10–13 Of growing interest is the prevalence and impact of delirium within post-cardiac arrest patients. Are they affected at the same rate? In the same way? And by the same adverse outcomes? These questions are important in guiding clinicians to target optimal prevention and treatment strategies focused on improving outcomes within this clinically challenging group of patients. Unfortunately, patients who suffer from cardiac arrest and anoxic brain injury are often excluded from studies of delirium, but data suggest delirium in cardiac arrest survivors is an independent risk factor for morbidity and mortality.14 This chapter will review the definition of delirium in critically ill patients, discuss risk factors associated with delirium, and provide the basis for clinicians to develop strategies aimed at preventing and treating delirium in the post-cardiac arrest patient.
Definition of Delirium and Motoric Subtypes
Delirium is a syndrome that develops over a short period of time and includes a disturbance of consciousness, inattention, and a change in cognition. It can be a direct result of a general medical condition, substance, medication, or a combination of causes.15 It has been further differentiated according to the level of arousal and motor activity into three motoric subtypes: hyperactive, hypoactive, and mixed delirium.16 Hypoactive delirium is characterized by a flat affect, withdrawal, apathy, or lethargy. The hyperactive subtype includes agitation, restlessness, emotional lability, or violence. Patients with mixed delirium experience periods of both hypoactive and hyperactive delirium. The distribution of subtypes across medical and surgical ICU patients shows the majority of patients experience hypoactive delirium, which is classically more difficult to identify and, therefore, largely under-reported.16–18 It is unclear whether or not the predominance of hypoactive delirium persists in post-cardiac arrest patients as only two studies have described motoric subtypes within this subgroup with varying distributions.19,20 Also unclear is whether patients with hypoactive delirium after cardiac arrest are at increased risk of death compared to their hyperactive or mixed counterparts, as has been seen in other medical and surgical populations.17,21
Risk Factors
The post-cardiac arrest population is unique from other ICU patients due to the severity of the acute brain insult and, often, significant underlying comorbidities. Patient factors leading to a cardiac arrest, initial resuscitation efforts, and post-resuscitation management all influence the potential for recovery1 as well as the risk for development of delirium. Risk factors for the development of delirium, broken down by these stages, are listed in Table 1. It is important to note while discussing risk factors for development of delirium in this population that cardiogenic shock, or cardiac arrest in itself, is a risk factor for delirium— with increased duration of arrest associated with longer duration of delirium following ROSC.14,19,20 Although not shown at this time to be an independent risk factor, the incidence of delirium in receiving targeted temperature management (TTM) was found to be 100% in a cohort of greater than 100 patients surviving and awakening following cardiac arrest.19 It is unclear how the extent of brain injury, sedation practices, temperature targets, paralytics, and other factors influenced this high prevalence, and it remains an exciting area for further research.
Table 1:
Modifiable and Non-Modifiable Risk Factors for Development of Delirium
| Baseline | Intra-Cardiac Arrest | Post-Resuscitation |
|---|---|---|
| Age (> 65 years) | Compliance with ACLS Guidelines | Acidosis |
| Alcoholism | Duration of Resuscitation | Anticholinergic drugs |
| Chronic Obstructive Pulmonary Disease (COPD) | Location (in-hospital vs community) | Alcohol or drug withdrawal |
| Cognitive impairment or Dementia | Quality of CPR | Anemia |
| Depression | Fever, infection, sepsis | |
| Gender (Male) | Hypotension | |
| History of delirium | Metabolic disturbances (e.g., sodium, calcium, blood urea nitrogen, bilirubin, albumin) | |
| Hypertension | Sedation (deep versus moderate/light) | |
| Smoking | Sleep disturbances | |
| Vision or hearing impairment | Targeting Temperature Management* |
Key:
indicates potential risk factor
Potentially modifiable risk factors for the clinician include obtaining ROSC as quickly as possible with continuous and adequate cardiopulmonary resuscitation (CPR) and defibrillation when indicated, correction of hypoxia, metabolic and electrolyte disturbances, prevention of hyperthermia, and treatment of underlying infection.22–24 In patients previously taking statin medications, continued use is recommended as discontinuation has been associated with increased delirium.25,26 In patients who progress to surgery, cardiac surgery without cardiopulmonary bypass appears to confer an advantage in decreasing delirium in some studies,27,28 but multiple other patient factors should be considered in deciding the best surgical approach for each patient.
Assessment Tools
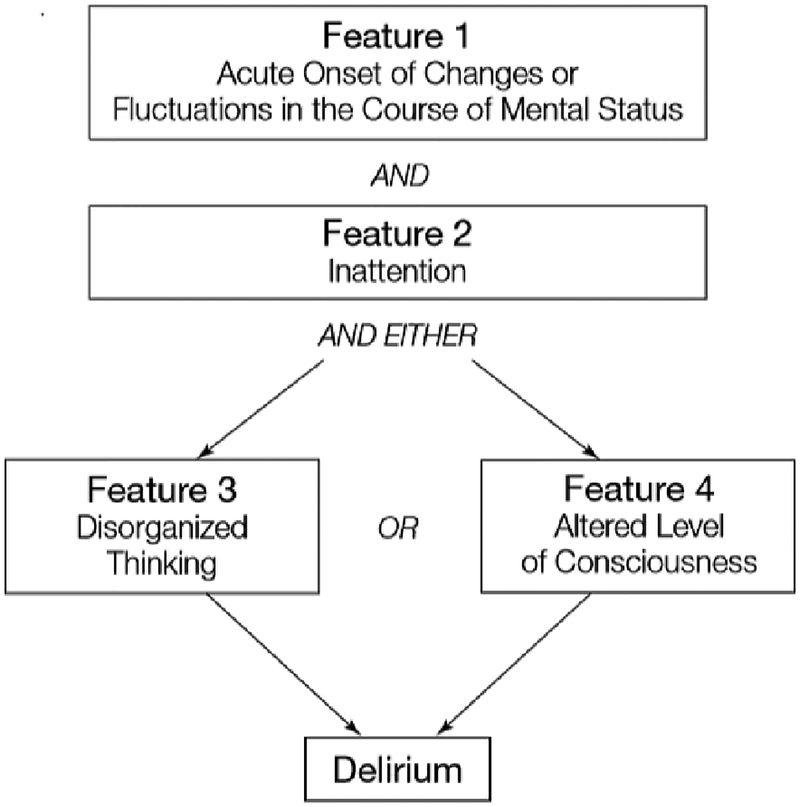
Early diagnosis of delirium is important for prognostication and avoiding exacerbating iatrogenic factors that will lengthen its course. Recognizing delirium is difficult in post-cardiac arrest patients as differentiating between encephalopathy, primary neurologic injury, and delirium can be challenging.29 Detection, however, is not only achievable among neurologically critically ill patients, but identification of delirious features may aid in predicting adverse outcomes for these patients.30 Assessment should first include establishing level of arousal through use of the Ramsay Sedation Scale,31 the Riker Sedation-Agitation Scale,32 or the Richmond Agitation-Sedation Scale (RASS).33,34 Once level of arousal has been determined and the patient is deemed responsive to voice, the clinician can proceed with assessing for the presence of delirium using one of two validated instruments in the ICU setting: the Intensive Care Delirium Screening Checklist (ICDSC)35 or the Confusion Assessment Method for the ICU (CAM-ICU).36,37 Evaluation for delirium using the ICDSC involves scoring of symptom presence over the course of a nursing shift, including altered level of consciousness, inattention, disorientation, hallucinations/delusions, psychomotor retardation/agitation, inappropriate speech or mood, sleep disturbances, and symptom fluctuations, each valued at one point. Patients scoring 4 or more points screen positive for delirium. The ICDSC has also been used to classify patients as having subsyndromal delirium if they have some signs of delirium but do not meet full criteria.38 Evaluation using the CAM-ICU is performed at a point in time and assesses for both an acute change in mental status and inattention plus either disorganized thinking or altered level of consciousness (Figure 1). The CAM-ICU has also been developed into a valid and reliable delirium severity scale, termed the Confusion Assessment Method for the ICU-7.39
Figure 1:
Confusion Assessment Method for the Intensive Care Unit (CAM-ICU): CAM-ICU tool can be used to determine the presence or absence of delirium after the level of sedation has been assessed and determined to be greater than −3 using the Richmond Agitation-Sedation Scale (RASS).
(Previously published from Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit [CAM-ICU]. JAMA. 2001;286:2703–2710.) reference 37
There has been question as to whether patients should be evaluated for delirium while receiving sedation. A small subset of patients will screen positive secondary to sedation-related delirium that rapidly clears following discontinuation of sedatives and may not carry the same long-term risks as more persistent delirium.40,41 For this reason, sedation should be paused whenever feasible for evaluations. If this is not safely possible, delirium evaluations should be continued with sedation (as the patient is able to participate), as failing to diagnose and intervene early on delirium far outweighs the risk of over-diagnosing a small group of patients.42
Patient Management
Sedation
Patients suffering from a cardiac arrest will often arrive to the ICU intubated with or without sedation. Serial neurologic examinations are especially important for both treatment and prognostication. Changes in neurologic exam should trigger a clinical response in the form of early involvement of a consultation service (e.g., neurology), repeat imaging, electroencephalography (EEG) placement, or family discussion of goals of care given the high level of morbidity and mortality associated with neurologic injury in these patients.43–45 General sedation practices associated with improved outcomes while on mechanical ventilation are the same across all ICUs: target lower sedation levels and perform daily pauses in sedation in conjunction with daily spontaneous ventilator weaning trials for appropriate patients.46–48 The notion that these patients require deeper levels of sedation as a way to decrease cerebral metabolic rate and injury has not been shown49 and is discouraged. Deeper sedation has not been associated with any benefit in neurologic outcomes and carries increased risks including prolonged ventilator support, pneumonia, delayed wakening, delayed mobility, increased delirium, and can confound neurologic prognostication.50 Further, deep sedation has been associated with increased delirium and decreased survival.51–54 These general sedation recommendations are modified for patients with severe neurologic injury when TTM is indicated for optimization of hypoxic-ischemic encephalopathy. These patients, with or without use of neuromuscular blockade, are not candidates for light sedation. Moderate sedation is recommended to prevent awareness and recall while avoiding adverse effects associated with deep sedation. To our knowledge there are no prospective studies looking at outcomes between moderately versus deeply sedated patients during targeted temperature management periods, but retrospective review has shown moderate sedation to be safe and feasible.55 Processed EEG monitoring to guide depth of sedation should be considered, especially in patients treated with neuromuscular blockade,56,57 though the authors recognize its use during TTM has not been studied.
Given the lack of evidence within the distinct population of cardiac arrest survivors, outside of targeting light sedation whenever possible, there are no strong recommendations for or against specific sedation strategies. Limited data for patients treated with TTM have shown a potential protective effect of propofol administration during rewarming.19 Benzodiazepines are commonly administered in the post-cardiac arrest population given the high incidence of hemodynamic instability and treatment of seizures in the sub-acute period. Although benzodiazepines are heavily implicated in both development and duration of delirium in critically ill medical and surgical populations,58–62 these effects are not as strongly associated within this sub-group.19 While this may be due to the lack of adequately powered studies (since most delirium studies exclude patients after cardiac arrest), it is also possible that this patient population is unique in their response to sedatives and other centrally active medications due to the extent of neurologic injury, degree of multi-organ dysfunction, drug metabolism, individual seizure risk, and subsequent cerebral hyper-metabolism associated with ROSC or rewarming after cooling. Although midazolam infusions appear to contribute to delayed awakening in patients following TTM,63 patient outcomes including mortality and long-term neurologic function do not appear altered by its use.64
In studies excluding patients with active myocardial ischemia, dexmedetomidine (alpha-2 agonist) compared to benzodiazepine infusions showed that the former reduced the burden of brain dysfunction with the most notable adverse side effect being bradycardia.65,66 This might be of particular concern in hypothermic patients who are already at increased risk for bradycardia but may benefit most from dexmedetomidine’s potential neuroprotective effects.67 There is further suggestion that for patients requiring surgical intervention, the use of prophylactic dexmedetomidine is preventative in the development of delirium.68,69 The beneficial mechanism of dexmedetomidine is likely multifactorial and may include a decrease in administration of opioids due to analgesic and subsequent opioid-sparing properties, better quality of sleep, lack of anticholinergic effects, and its association with decreased inflammatory biomarkers.69,70
Within the ICU, patients commonly receive sedative or analgesic medications. In the non-critically ill patient population, both the number of medications administered22 and their psychoactive effects71 have been suggestive of precipitating delirium. Hepatic and renal function has been found to be impaired in greater than 50% of post-cardiac arrest patients; pharmacokinetic alterations of administered medications can be profound.72 Further, hypothermia—either targeted or unintentional—can increase plasma concentrations of commonly administered medications such as propofol by approximately 30%,73 fentanyl by approximately 25%,74 and midazolam by approximately 10% for each degree Celsius (C) decrease from core temperature of 36.5 degrees C.75 Regardless of the use of targeted temperature management in these patients, continuous infusion of sedative and analgesic medications leads to accumulation and tolerance over time. Practices to reduce exposure to psychoactive medications are an important component of ICU care and strategies need to be employed to reduce the exposure in patients as early as possible.47
Prevention Strategies
There has been increasing evidence in critical care literature illustrating the benefit of patient care bundles in not only reducing the incidence of delirium, but decreasing mechanical ventilation use, coma, restraint use, and ICU readmissions while improving survival and post-ICU discharge disposition.48 To optimize critical care management, the Society of Critical Care Medicine (SCCM) recommends the ABCDEF bundle be applied to all ICU patients, including those post-cardiac arrest, as part of their ICU Liberation initiative. Components of the ABCDEF bundle include: Assessing and treating pain, Both Awakening and Breathing Trials, Choice of appropriate sedation, Delirium monitoring and management, Early mobility and Exercise, and Family Engagement.76
Indeed the best way to treat delirium is to prevent it from happening in the first place. There are currently no recommended prophylactic medications or pharmacologic protocols for the prevention of delirium, although there is suggestion that use of low-dose dexmedetomidine in elderly post-operative patients69 or via nocturnal administration77 may decrease incidence. Additionally, low-dose ketamine has recently been shown in a randomized placebo-controlled trail of medical and surgical ICU patients to decrease both delirium incidence and duration. Further studies are required before ketamine for delirium prophylaxis can be routinely recommended. Although statin use within the ICU has been shown to be associated with less delirium in prospective cohort studies,25,26 randomized controlled trials comparing statins to placebo have not shown decreased risk of delirium.78,79
The strongest prevention practice under current guidelines remains early mobility. Initiation generally progresses from passive range of motion to active range of motion, exercise in bed, sitting, standing, and ambulation depending on patient’s sedation level, neurologic function, and physical abilities. Early initiation of physical therapy, even when limited to once daily sessions coordinated with pauses in sedation, has been shown to decrease delirium, polypharmacy and hospital length of stay in addition to improving functional outcomes at discharge.80–82 Recognizing that there are potential obstacles to participation in the immediate post-cardiac arrest patient, physicians and clinicians should be continually assessing patients for appropriateness and initiation of physical therapy at the earliest possible time.
Delirium Management
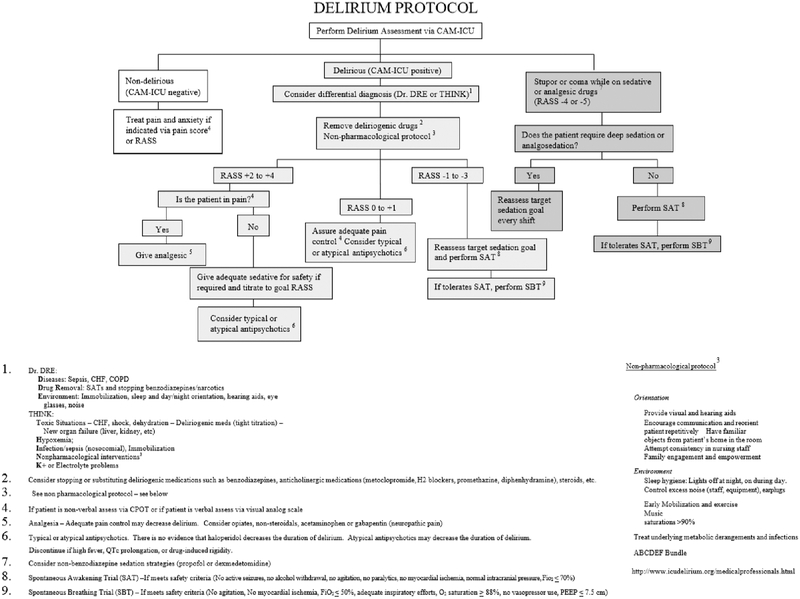
Once diagnosis of delirium is made, clinicians must attempt to identify and exclude all reversible causes. As mentioned previously, reversible causes include hypoxia, hypercarbia, hypoglycemia, metabolic derangements, infection, or continued shock. An empiric protocol based on current practice guidelines for all ICU patients is suggested in Figure 2. Institutional protocols may vary but should broadly align with recommendations from the SCCM.
Figure 2:
Delirium Protocol for recommended use in the intensive care unit setting.
(CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; CHF, congestive heart failure; CPAP, continuous positive airway pressure; dx, diagnosis; PEEP, positive end-expiratory pressure; RASS, Richmond Agitation-Sedation Scale)
(Previously published with permission to use courtesy Dr. E. W. Ely, (www.icudelirium.org).)
Specifically regarding treatment strategies in the agitated, delirious patient, dexmedetomidine may be superior in management to benzodiazepines, propofol, and placebo, particularly when used for liberation of mechanical ventilation.83,84 Antipsychotic medications, either typical or atypical, have increasingly become utilized in management, particularly for hyperactive delirium. Although shown not to be effective in prevention of delirium,85 there has been mixed evidence about the efficacy of antipsychotics in decreasing duration of symptoms in several small studies.86,87 A recent large randomized placebo-controlled trial of typical and atypical antipsychotics versus placebo, however, found no effect in decreasing duration of delirium;88, thus, evidence does not support the use of antipsychotics for the treatment of delirium. When these medications are used for control of agitated symptoms, they should be used with caution. Adverse reactions including dystonias, neuroleptic malignant syndrome, and extrapyramidal effects are all possible. Haloperidol use in the post-cardiac arrest patient should include daily monitoring of QT interval measurements as QT prolongation and arrhythmias deteriorating to torsades de pointes are known complications. Once initiated, clinicians should be mindful to create discontinuation strategies as antipsychotics initiated within the ICU are frequently inappropriately continued at hospital discharge and could pose long-term risks to patients.89
The treatment strategies for delirium are sparse, and the evidence is lacking for a single pharmacologic approach. Prevention with non-pharmacologic means, therefore, remains the best course of action.
Conclusion
Developing delirium after cardiac arrest is associated with increased morbidity and worse outcomes. The importance of addressing modifiable risk factors, recognizing symptoms early, and initiating coordinated treatment strategies can help to improve outcomes within this high risk population. Research investigating optimal targeted temperature management protocols, sedation depth, sedative medication choice, and other prevention strategies are required to further advance care.
Key Points:
Patient factors leading to a cardiac arrest, initial resuscitation efforts, and post-resuscitation management all influence the potential for recovery as well as the risk for development of delirium
Recognizing delirium in neurologically ill or post-cardiac arrest patients is difficult, but it is important in helping to identify patients at increased risk for adverse outcomes
Once diagnosis of delirium is made, clinicians must attempt to identify and exclude all reversible causes including hypoxia, hypercarbia, hypoglycemia, metabolic derangements, sedative medication effects, infection, or continued shock
Financial Support and sponsorship:
This work was supported by the Department of Anesthesiology, Critical Care Division, Vanderbilt University Medical Center, Nashville, TN, USA.
Disclosures of Funding: Dr. Boncyk receives support under the T32 research training grant from the National Institute of Health. Dr. Pandharipande is supported by a research grant from Pfizer (Hospira) Inc. in collaboration with the National Institutes of Health. Dr. Hughes is supported by an American Geriatrics Society Jahnigen Career Development Award and National Institutes of Health HL111111, R03AG045085 (Bethesda, Maryland, USA).
Footnotes
Conflict of Interest: none
References:
- 1.Howard RS, Holmes PA, Koutroumanidis MA. Hypoxic-ischaemic brain injury. Practical neurology. 2011;11(1):4–18. [DOI] [PubMed] [Google Scholar]
- 2.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. [DOI] [PubMed] [Google Scholar]
- 3.Madl C, Holzer M. Brain function after resuscitation from cardiac arrest. Curr Opin Crit Care. 2004;10(3):213–217. [DOI] [PubMed] [Google Scholar]
- 4.Stiell IG, Wells GA, Field B, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. The New England journal of medicine. 2004;351(7):647–656. [DOI] [PubMed] [Google Scholar]
- 5.Druss RG, Kornfeld DS. The survivors of cardiac arrest. A psychiatric study. Jama. 1967;201(5):291–296. [PubMed] [Google Scholar]
- 6.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297–1304. [DOI] [PubMed] [Google Scholar]
- 7.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Medicine. 2001;27(12):1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Archives of internal medicine. 2007;167(15):1629–1634. [DOI] [PubMed] [Google Scholar]
- 9.Spronk PE, Riekerk B, Hofhuis J, Rommes JH. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. The New England journal of medicine. 2013;369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. Jama. 2004;291(14):1753–1762. [DOI] [PubMed] [Google Scholar]
- 12.Pisani MAK SYJ; Kasl SV; Murphy TE; Araujo KLB; Van Ness PH Days of Delirium Are Associated with 1-Year Mortality in an Older Intensive Care Unit Population. American Journal of Respiratory and Critical Care Medicine. 2009;180(11):1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. [DOI] [PubMed] [Google Scholar]
- 14.Pauley E, Lishmanov A, Schumann S, Gala GJ, van Diepen S, Katz JN. Delirium is a robust predictor of morbidity and mortality among critically ill patients treated in the cardiac intensive care unit. Am Heart J. 2015;170(1):79–86, 86.e71. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th ed Washington, DC: 2013. [Google Scholar]
- 16.Pandharipande P, Cotton BA, Shintani A, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Medicine. 2007;33(10):1726–1731. [DOI] [PubMed] [Google Scholar]
- 17.Robinson TN, Raeburn CD, Zung VT, Brenner LA, Moss M The Motor Subtypes of Post-Operative Delirium in the Elderly. Archives of surgery (Chicago, Ill: 1960). 2011;146(3):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farina N, Smithburger P, Kane-Gill S. Screening and Management of Delirium in Critically Ill Patients. Hosp Pharm. 2015;50(8):667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollock JS, Hollenbeck RD, Wang L, et al. Delirium in Survivors of Cardiac Arrest Treated With Mild Therapeutic Hypothermia. American journal of critical care: an official publication, American Association of Critical-Care Nurses. 2016;25(4):e81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uguz F, Kayrak M, Cicek E, Kayhan F, Ari H, Altunbas G. Delirium following acute myocardial infarction: incidence, clinical profiles, and predictors. Perspectives in psychiatric care. 2010;46(2):135–142. [DOI] [PubMed] [Google Scholar]
- 21.Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62(2):174–179. [DOI] [PubMed] [Google Scholar]
- 22.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. Jama. 1996;275(11):852–857. [PubMed] [Google Scholar]
- 23.McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591–598. [DOI] [PubMed] [Google Scholar]
- 24.Lin SM, Huang CD, Liu CY, et al. Risk factors for the development of early-onset delirium and the subsequent clinical outcome in mechanically ventilated patients. J Crit Care. 2008;23(3):372–379. [DOI] [PubMed] [Google Scholar]
- 25.Page VJ, Davis D, Zhao XB, et al. Statin use and risk of delirium in the critically ill. Am J Respir Crit Care Med. 2014;189(6):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morandi A, Hughes CG, Thompson JL, et al. Statins and delirium during critical illness: a multicenter, prospective cohort study. Crit Care Med. 2014;42(8):1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucerius J, Gummert JF, Borger MA, et al. Predictors of delirium after cardiac surgery delirium: effect of beating-heart (off-pump) surgery. The Journal of thoracic and cardiovascular surgery. 2004;127(1):57–64. [DOI] [PubMed] [Google Scholar]
- 28.O’Neal JB, Billings FTt, Liu X, et al. Risk factors for delirium after cardiac surgery: a historical cohort study outlining the influence of cardiopulmonary bypass. Canadian journal of anaesthesia = Journal canadien d’anesthesie. 2017;64(11):1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frontera JA. Delirium and sedation in the ICU. Neurocrit Care. 2011;14(3):463–474. [DOI] [PubMed] [Google Scholar]
- *30.Patel MB, Bednarik J, Lee P, et al. Delirium Monitoring in Neurocritically Ill Patients: A Systematic Review. Crit Care Med. 2018;46(11):1832–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]; This systematic review showed that not only was it possible to measure delirium in neurocritically ill patients, but that it is also important. Delirium is considered a manifestation of secondary brain injury-- recognition of which can identify patients at increased risk for adverse outcomes.
- 31.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2(5920):656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329. [DOI] [PubMed] [Google Scholar]
- 33.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale - Validity and reliability in adult intensive care unit patients. American Journal of Respiratory and Critical Care Medicine. 2002;166(10):1338–1344. [DOI] [PubMed] [Google Scholar]
- 34.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). Jama. 2003;289(22):2983–2991. [DOI] [PubMed] [Google Scholar]
- 35.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–864. [DOI] [PubMed] [Google Scholar]
- 36.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. [DOI] [PubMed] [Google Scholar]
- 37.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). Jama. 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 38.Ouimet S, Riker R, Bergeron N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med. 2007;33(6):1007–1013. [DOI] [PubMed] [Google Scholar]
- 39.Khan BA, Perkins AJ, Gao S, et al. The Confusion Assessment Method for the ICU-7 Delirium Severity Scale: A Novel Delirium Severity Instrument for Use in the ICU. Crit Care Med. 2017;45(5):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189(6):658–665. [DOI] [PubMed] [Google Scholar]
- 41.Haenggi M, Blum S, Brechbuehl R, Brunello A, Jakob SM, Takala J. Effect of sedation level on the prevalence of delirium when assessed with CAM-ICU and ICDSC. Intensive Care Med. 2013;39(12):2171–2179. [DOI] [PubMed] [Google Scholar]
- *42.Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. The Lancet Respiratory medicine. 2018;6(3):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study divided delirium into non-mutually exclusive phenotypes including hypoxia, sepsis, sedative exposure, or metabolic dysfunction. Although they commonly occur together, sedative-associated, hypoxic, and septic delirium were shown to be distinct indicators of acute brain injury. Iatrogenic and modifiable factors, such as sedation, should be examined in these patients as an area to intervene and decrease risk.
- 43.Friberg H, Rundgren M, Westhall E, Nielsen N, Cronberg T. Continuous evaluation of neurological prognosis after cardiac arrest. Acta Anaesthesiol Scand. 2013;57(1):6–15. [DOI] [PubMed] [Google Scholar]
- 44.Oddo M, Rossetti AO. Predicting neurological outcome after cardiac arrest. Curr Opin Crit Care. 2011;17(3):254–259. [DOI] [PubMed] [Google Scholar]
- 45.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–210. [DOI] [PubMed] [Google Scholar]
- 46.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. [DOI] [PubMed] [Google Scholar]
- **47.Devlin JW, Skrobik Y, Gelinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. [DOI] [PubMed] [Google Scholar]; The most current pain, agitation/sedation, delirium, immobility, and sleep practice guidelines produced by the Society of Critical Care Medicine. There recommendations are updates from prior 2013 guidelines to include targetted light sedation, enhanced mobility, and emphasis on improved sleep quality for critically ill patients.
- **48.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit Care Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; The largest collaboration to date illustrating population effects of initiation of ABCDEF bundle within ICUs. Results showed adherence to the bundle produced clinical improvements including improved survival and decreased mechanical ventilation use, restraint use, ICU readmissions, and improved ICU discharge disposition.
- 49.Randomized clinical study of thiopental loading in comatose survivors of cardiac arrest. The New England journal of medicine. 1986;314(7):397–403. [DOI] [PubMed] [Google Scholar]
- 50.Riker RR, Gagnon DJ, May T, Seder DB, Fraser GL. Analgesia, sedation, and neuromuscular blockade during targeted temperature management after cardiac arrest. Best practice & research Clinical anaesthesiology. 2015;29(4):435–450. [DOI] [PubMed] [Google Scholar]
- *51.Shehabi Y, Bellomo R, Kadiman S, et al. Sedation Intensity in the First 48 Hours of Mechanical Ventilation and 180-Day Mortality: A Multinational Prospective Longitudinal Cohort Study. Crit Care Med. 2018;46(6):850–859. [DOI] [PubMed] [Google Scholar]; Although deep versus light sedation has no clear definition, the practice of targetting lighter sedation when possible is a large component of the current SCCM practice guidelines for pain, agitation/sedation, delirium, immobility, and sleep disruption. This study is important in defining that benefit by showing an ascending relationship between sedation intentisty and increased risk of death, delirium, and time to extubation.
- *52.Stephens RJ, Dettmer MR, Roberts BW, et al. Practice Patterns and Outcomes Associated With Early Sedation Depth in Mechanically Ventilated Patients: A Systematic Review and Meta-Analysis. Crit Care Med. 2018;46(3):471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]; Expanding on the data surrounding sedation depth, this systematic review and meta-analysis included nine randomised controlled and observational trials including over 4500 patients. Deeper sedation, although heterogeneously defined, was associated with increased mortality and length of stay.
- 53.Shehabi Y, Chan L, Kadiman S, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. 2013;39(5):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balzer F, Weiss B, Kumpf O, et al. Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Critical care (London, England). 2015;19:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.May TL, Seder DB, Fraser GL, Stone P, McCrum B, Riker RR. Moderate-dose sedation and analgesia during targeted temperature management after cardiac arrest. Neurocrit Care. 2015;22(1):105–111. [DOI] [PubMed] [Google Scholar]
- 56.Chamorro C, Borrallo JM, Romera MA, Silva JA, Balandin B. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Analg. 2010;110(5):1328–1335. [DOI] [PubMed] [Google Scholar]
- 57.Leary M, Fried DA, Gaieski DF, et al. Neurologic prognostication and bispectral index monitoring after resuscitation from cardiac arrest. Resuscitation. 2010;81(9):1133–1137. [DOI] [PubMed] [Google Scholar]
- 58.McPherson JA, Wagner CE, Boehm LM, et al. Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med. 2013;41(2):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. The Journal of trauma. 2008;65(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. [DOI] [PubMed] [Google Scholar]
- 61.Hughes CG, Morandi A, Girard TD, et al. Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology. 2013;118(3):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanders RD. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Medical hypotheses. 2011;77(1):140–143. [DOI] [PubMed] [Google Scholar]
- 63.Paul M, Bougouin W, Dumas F, et al. Comparison of two sedation regimens during targeted temperature management after cardiac arrest. Resuscitation. 2018;128:204–210. [DOI] [PubMed] [Google Scholar]
- 64.Bjelland TW, Dale O, Kaisen K, et al. Propofol and remifentanil versus midazolam and fentanyl for sedation during therapeutic hypothermia after cardiac arrest: a randomised trial. Intensive Care Med. 2012;38(6):959–967. [DOI] [PubMed] [Google Scholar]
- 65.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. Jama. 2007;298(22):2644–2653. [DOI] [PubMed] [Google Scholar]
- 66.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. Jama. 2009;301(5):489–499. [DOI] [PubMed] [Google Scholar]
- 67.Ezzati M, Broad K, Kawano G, et al. Pharmacokinetics of dexmedetomidine combined with therapeutic hypothermia in a piglet asphyxia model. Acta Anaesthesiol Scand. 2014;58(6):733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Serafim RB, Bozza FA, Soares M, et al. Pharmacologic prevention and treatment of delirium in intensive care patients: A systematic review. J Crit Care. 2015;30(4):799–807. [DOI] [PubMed] [Google Scholar]
- 69.Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. [DOI] [PubMed] [Google Scholar]
- 70.Wu M, Liang Y, Dai Z, Wang S. Perioperative dexmedetomidine reduces delirium after cardiac surgery: A meta-analysis of randomized controlled trials. J Clin Anesth. 2018;50:33–42. [DOI] [PubMed] [Google Scholar]
- 71.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. Jama. 1994;272(19):1518–1522. [PubMed] [Google Scholar]
- 72.MacLaren R, Gallagher J, Shin J, Varnado S, Nguyen L. Assessment of adverse events and predictors of neurological recovery after therapeutic hypothermia. The Annals of pharmacotherapy. 2014;48(1):17–25. [DOI] [PubMed] [Google Scholar]
- 73.Leslie K, Sessler DI, Bjorksten AR, Moayeri A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80(5):1007–1014. [DOI] [PubMed] [Google Scholar]
- 74.Fritz HG, Holzmayr M, Walter B, Moeritz KU, Lupp A, Bauer R. The effect of mild hypothermia on plasma fentanyl concentration and biotransformation in juvenile pigs. Anesth Analg. 2005;100(4):996–1002. [DOI] [PubMed] [Google Scholar]
- 75.Hostler D, Zhou J, Tortorici MA, et al. Mild hypothermia alters midazolam pharmacokinetics in normal healthy volunteers. Drug metabolism and disposition: the biological fate of chemicals. 2010;38(5):781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *76.Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF Bundle in Critical Care. Crit Care Clin. 2017;33(2):225–243. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence-based guide for clinicians describing key clinical implementations including Assess, prevent, and manage pain, Both spontaneous awakening trials and spontaneous breathing trials, Choice of analgesia and sedation, Delirium: assess, prevent, and manage, Early mobility and exercise, and Familiy engagement and empowerment.
- 77.Skrobik Y, Duprey MS, Hill NS, Devlin JW. Low-Dose Nocturnal Dexmedetomidine Prevents ICU Delirium A Randomized, Placebo-controlled Trial. American Journal of Respiratory and Critical Care Medicine. 2018;197(9):1147–1156. [DOI] [PubMed] [Google Scholar]
- 78.Needham DM, Colantuoni E, Dinglas VD, et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. The Lancet Respiratory medicine. 2016;4(3):203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Page VJ, Casarin A, Ely EW, et al. Evaluation of early administration of simvastatin in the prevention and treatment of delirium in critically ill patients undergoing mechanical ventilation (MoDUS): a randomised, double-blind, placebo-controlled trial. The Lancet Respiratory medicine. 2017;5(9):727–737. [DOI] [PubMed] [Google Scholar]
- 80.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. [DOI] [PubMed] [Google Scholar]
- 81.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016;388(10052):1377–1388. [DOI] [PubMed] [Google Scholar]
- 83.Reade MCE GM; Bellomo R; Bailey M; Bersten A; Cheung B; Davies A; Delaney A; Ghosh A; van Haren F; Harley N; Knight D; McGuiness S; Mulder J; O’Donoghue S; Simpson N; Young P; Dah LI A. Investigators Australian. Effect of Dexmedetomidine Added to Standard Care on Ventilator-Free Time in Patients With Agitated Delirium A Randomized Clinical Trial. Jama-Journal of the American Medical Association. 2016;315(14):1460–1468. [DOI] [PubMed] [Google Scholar]
- 84.Ng KT, Shubash CJ, Chong JS. The effect of dexmedetomidine on delirium and agitation in patients in intensive care: systematic review and meta-analysis with trial sequential analysis. Anaesthesia. 2018. [DOI] [PubMed] [Google Scholar]
- 85.van den Boogaard M, Slooter AJC, Bruggemann RJM, et al. Effect of Haloperidol on Survival Among Critically Ill Adults With a High Risk of Delirium: The REDUCE Randomized Clinical Trial. Jama. 2018;319(7):680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesthesia and Intensive Care. 2007;35(5):714–719. [DOI] [PubMed] [Google Scholar]
- 87.Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. The Lancet Respiratory medicine. 2013;1(7):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *88.Girard TD, Exline MC, Carson SS, et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. The New England journal of medicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Despite the near-ubiquitous use of antipsychotic medications in delirium management for ICU patients, there is poor quality evidence in the form of large RCTs supporting its use. This largest study to date compared haloperidol or ziprasidone to placebo for management of hypoactive or hyperactive ICU delirium. They were unable to find a significant difference intreatment groups regarding duration of delirium symptoms. Data to come on long-term outcomes is expected in the future.
- 89.Morandi AV E; Pandharipande PP; Girard TD; Solberg LM; Neal EB; Koestner T; Torres RE; Thompson JL; Shintani AK; Han JH; Schnelle JF; Fick DM; Ely EW; Kripalani S Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. Journal of the American Geriatrics Society. 2013;61(7):1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]